Abstract
Helical polymers with a defined main-chain atropoisomeric conformation are important materials in high value applications such as nonlinear optics and chiral separations. Currently, no methods exist for the cationic helix-sense-selective polymerization of prochiral vinyl monomers, which limits access to a number of potentially valuable optically active helical polymers. Here, we demonstrate the first stereoselective cationic helix-sense-selective polymerization of a prochiral vinyl monomer, which provides access to optically active helices of poly(N-vinylcarbazole). Chiral bis(oxazoline)-scandium Lewis acids serve as chiral counterions to polymerize N-vinylcarbazole into highly isotactic (up to 94% meso triads) polymers. Mechanistic investigations uncovered the distinct phenomenon that are responsible for independent control of conformational (i.e., helicity) and configurational (i.e., tacticity) stereochemistry. Polymer helicity was strongly influenced by the stereoselectivity of the first monomer propagation, whereas polymer tacticity was dictated by the thermodynamically controlled conformation of the growing polymer chain end. Overall, this method expands the suite of accessible helical polymers through helix-sense-selective polymerization and provides mechanistic insight into how polymer tacticity and helicity can be controlled independently.
Optically active helical polymers are an important class of materials that are currently used in commercial applications for chiral separations, biomimetics, and optoelectronics.1 The function of these materials is a direct result of their main-chain atropoisomeric conformation.1,2 The synthesis of optically active helical polymers from readily available vinyl monomers can be accomplished using helix-sense-selective polymerization (HSSP) strategies. Such methods have allowed for the simultaneous conformational (i.e., helicity) and configurational (i.e., tacticity) control of absolute polymer stereochemistry, which has parallels with modern methods in asymmetric catalysis that enable simultaneous control of product enantio- and diastereoselectivity.3–6
To date, HSSP methods have been limited to a few highly stereoselective asymmetric coordination–insertion, anionic, and radical approaches (Figure 1A).7–12 No examples of a stereoselective HSSP of a prochiral vinyl monomer that proceed through a cationic mechanism have been reported, presumably due to the inherent challenge of controlling enantiofacial addition of a vinyl monomer onto a prochiral sp2 hybridized carbenium ion.13 Asymmetric ion-pairing catalysis has proven to be a successful conceptual approach to control enantiofacial addition of nucleophiles to prochiral reactive intermediates in small molecule synthesis.14–16 Recently, asymmetric ion-pairing catalysis has demonstrated utility in cationic polymerization for the control of polymer tacticity, but has not achieved the ability to also control polymer helicity.13,17–20 The development of stereoselective HSSP cationic polymerizations remains a fundamental challenge that, if achieved, would expand the suite of accessible optically active helical polymers.
Figure 1.
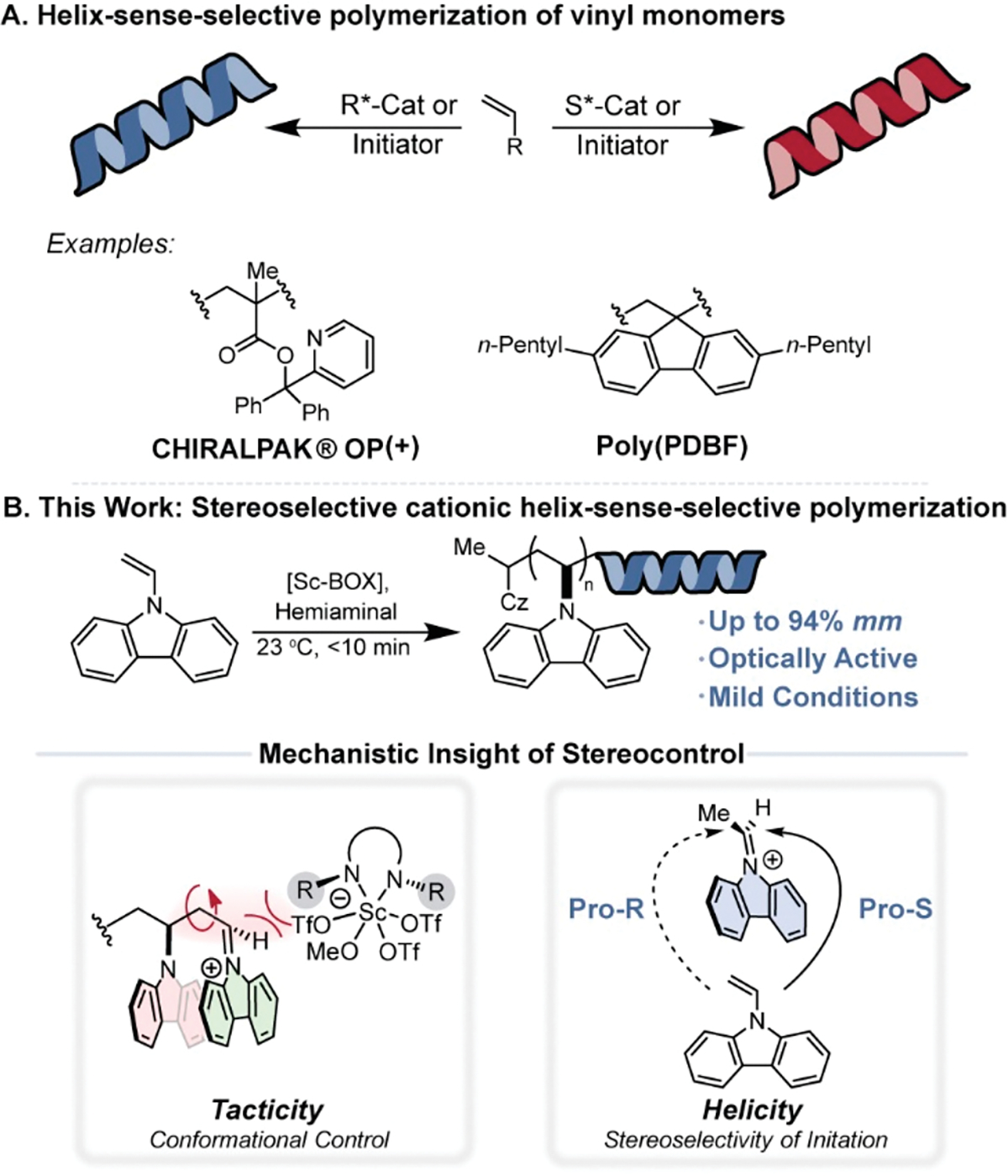
(A) Helix-sense-selective polymerizations of vinyl monomers. (B) Our approach for the stereoselective cationic helix-sense-selective polymerization of N-vinylcarbazole.
Atactic poly(N-vinylcarbazole) (poly(NVC)) is an industrially and academically relevant polymer made by either cationic or radical polymerization.21 The unique optical properties, hole-transport abilities, and high decomposition temperature of poly(NVC) enable applications in light emitting diodes and photorefractive materials.22–24 Recently, Aoshima and coworkers developed a Lewis acid catalyzed method to synthesize isotactic poly(NVC) with a percent meso triad (% mm) content up to 94%, but the method lacked the appropriate conformational control to form enantioenriched helical polymers and required extended reaction times at −78 °C to reach moderate molecular weights.25 Given these limitations, we identified the HSSP of poly(NVC) as an opportunity to solve an outstanding challenge in polymer synthesis while also generating new materials for structure–stereoselectivity–property evaluation. We hypothesized that asymmetric ion-pairing catalysis could serve as an overarching conceptual approach to mediate enantiofacial addition to the prochiral iminium ion chain end and control helicity and tacticity in tandem.
Herein, we report the first stereoselective cationic HSSP of a prochiral vinyl monomer, namely NVC. Bis(oxazoline) (BOX) ligands proved enabling for the identification of a chiral scandium Lewis acid that initiated cationic polymerization of NVC and served to mediate enantiofacial addition of subsequent monomers to yield highly isotactic poly(NVC) (up to 94% mm) under mild conditions. The chirality of the BOX ligand led to selective helix formation, but conditions which improved tacticity did not always improve helicity and vice versa, which contradicted previous observations for HSSP (Figure 1B).26 Mechanistic investigations uncovered the distinct phenomenon responsible for independent control of configurational and conformational stereochemistry. The mechanism of tacticity control was investigated computationally and suggested it was dictated by a conformational bias arising from π–π interactions between the last enchained, electron-rich carbazole ring and the prochiral, electron-poor carbazolium chain-end. Polymer helicity, conversely, was strongly influenced by the stereoselectivity of the first monomer propagation, which was confirmed through the synthesis of enantioenriched initiating species. Consequently, this method represents an HSSP where the helicity was decoupled from polymer tacticity, and the mechanistic insight provides a conceptual framework for how to control them independently.27
We began our studies by exploring the polymerization of NVC at room temperature. A control polymerization of NVC catalyzed by triflic acid (TfOH) generated poly(NVC) with 57% mm. Given the noncoordinating nature of the triflate counterion, the modest stereoselectivity observed is presumably due to chain-end control, whereby the last enchained monomer influences the stereochemistry of subsequent monomer addition. We identified substituted BOX ligand–metal complexes as promising chiral Lewis acids due to their significant precedent in asymmetric small molecule catalysis and their modular synthesis.28,29 Additionally, we hypothesized that hemiaminals of carbazole would be competent initiators for cationic polymerization.30–32
The use of strong Lewis acids (Figure 2, entries 5–9) facilitated reactivity to produce poly(NVC) with higher % mm compared to the triflic acid catalyzed polymerization, with ligated Sc(OTf)–L1 yielding 84% mm. Lowering the monomer concentration to 0.12 M enabled the synthesis of highly isotactic poly(NVC) with a tacticity of 92% mm at room temperature and a moderate molecular weight (Figure 2, entry 9). Lowering the temperature or decreasing the concentration further resulted in a decrease of isotacticity and a loss of molecular weight control (Table S1, entry 8).
Figure 2.
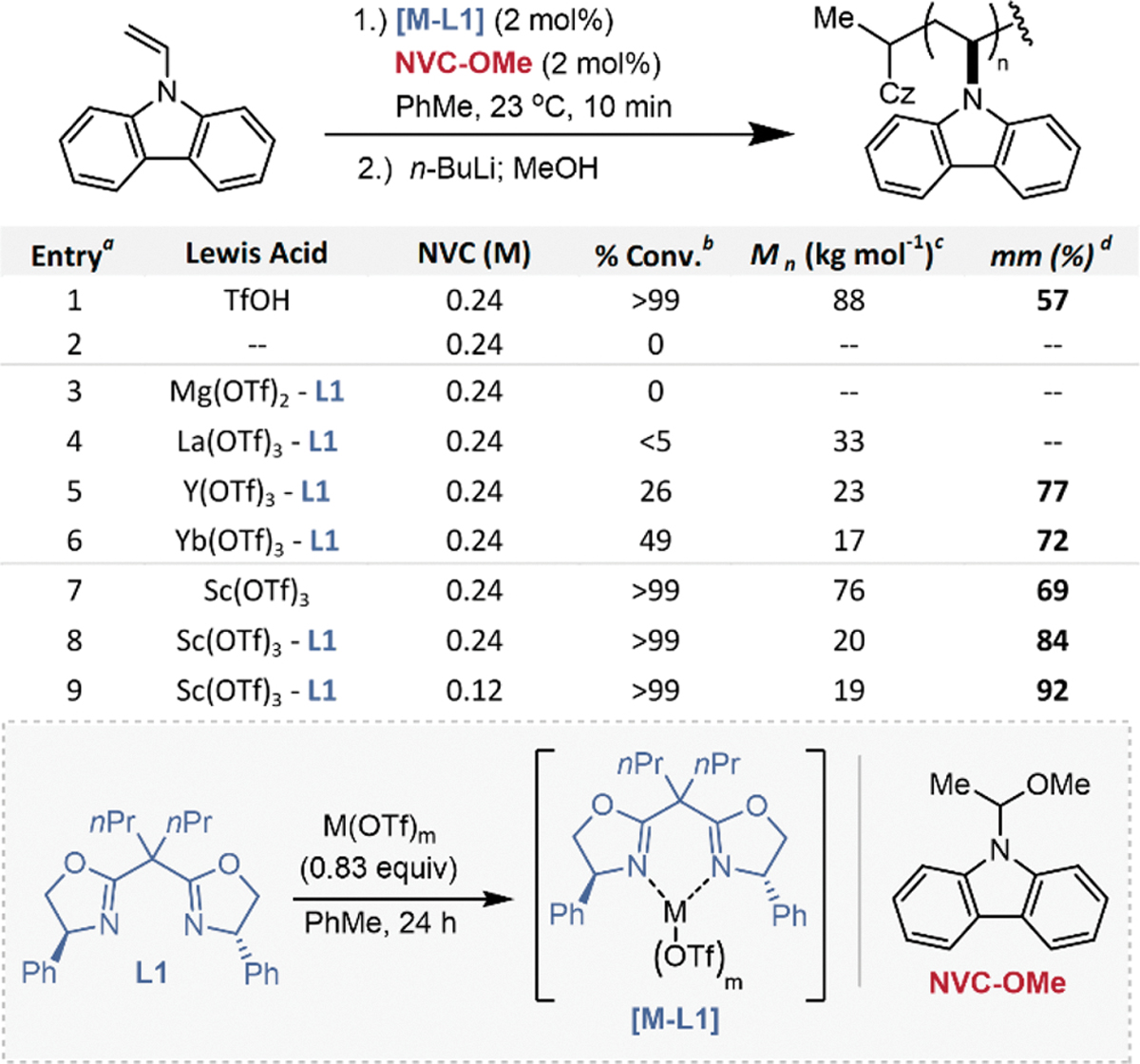
aAll polymerizations were run with 0.26 mmol of NVC and targeted an Mn of 9.7 kDa. bConversion into poly(NVC) was determined from crude 1H NMR. cMolar masses were determined by GPC in THF at 1 mg/mL and reported compared to polystyrene standards. dTacticity was determined by integrating the separated mm and mr/rr peaks by 1H NMR at 100 °C in C2D2Cl4.
To investigate the role the ligand played in influencing tacticity, BOX ligands with diverse steric properties and substitutions on the bridgehead position were evaluated (Figure 3). With the exception of L7, all BOX-ligated scandium Lewis acids polymerized NVC to complete conversion. Changing the bridgehead n-propyl groups of L1 to other alkyl or cyclic substituents universally decreased tacticity (Figure S3), which we attributed to poor solubility of the catalyst. Polymerization using the achiral BOX ligand L2 provided poly(NVC) with 70% mm. The addition of methylene spacers between the oxazoline and aryl substituent (L3 and L4), extending the size of the arene (L5), or an additional vicinal phenyl ring (L9), resulted in decreases in isotacticity compared to L1. Branched aliphatic substituents (S)-isopropyl (L6) and (S)-cyclohexyl (L8) yielded poly-(NVC) approaching the isotacticity provided by L1. The inclusion of a fused Indane ring on the BOX ligand framework (L10/L11) also resulted in high isotacticity (91% mm). Moreover, the polymerization of NVC with a racemic mixture of L10 and L11 produced a polymer with 91% mm, suggesting a lack of catalyst exchange between chain ends at room temperature, limited catalyst clustering, or a prevailing chain-end stereocontrol mechanism (Table S1, entry 18). Lowering the reaction temperature and concentration with L11 allowed the synthesis of poly(NVC) with 94% mm and greater than 120 kDa Mn (Table S1, entries 13–17).
Figure 3.
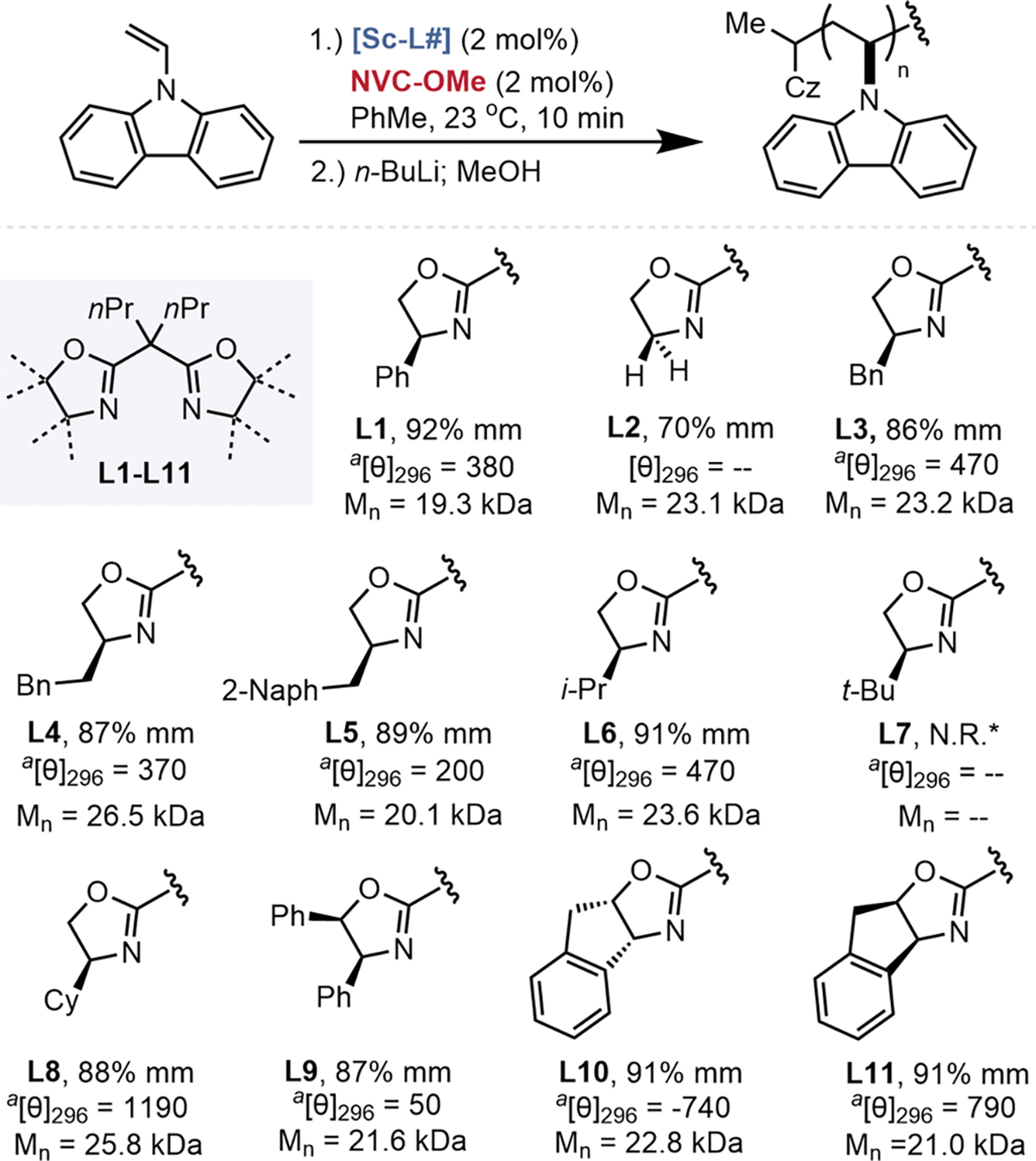
Derivatization of di-n-Pr-bis(oxazoline) ligands. 2.4 mol % of L1–11 were mixed with 2.0 mol % of Sc(OTf)3 at room temperature for 24 h. Polymerization were ran at 0.12 M. aUnits for molar ellipticity are deg·cm2·dmol−1.
The sense-selective formation of helices for samples of isotactic poly(NVC) was assessed by circular dichroism (CD) spectroscopy. Owing to the C2-symmetry of the monomer and pseudo mirror plane of the isotactic polymer, any optical activity would result from selective helix formation.33–35 Analysis with CD spectroscopy in the wavelength ranges generally associated with polymer tertiary structure (250–350 nm) displayed clear CD responses and inversion coinciding with absorbance of the carbazole motif and lacked optical activity from the enantioenriched alpha chain-end, thus revealing the first example of a stereoselective cationic vinyl HSSP (Figures S4–8, Tables S2–4). These helices of poly(NVC) were found to be stable in tetrahydrofuran solution without significant deterioration of optical activity, but did racemize upon extended heating in toluene (Figures S9–S10). Ligand identity and stereochemistry had a direct influence on the magnitude and sign of helicity ([θ]) (Figure 3). For example, NVC polymerization with L10 and its enantiomer, L11, produced samples of poly(NVC) with analogous isotacticities and magnitudes of helicity, but opposite CD responses, indicating that intentional ligand design can be used to tune helicity. To our surprise, and in contrast to previously reported examples of HSSP, there was no clear relationship between conditions which improved tacticity and those that improved helicity (Figure 3, Figure S5).26 For example, while poly(NVC) made using L8 demonstrated a lower isotacticity than that made with L1, it had a 3 times higher response in the CD spectrometer.
We sought to gain a mechanistic understanding for how tacticity and helicity arise independently. The implication that helicity is not directly tied to tacticity could allow conceptually different approaches toward catalysts design. We hypothesized that the high levels of isotacticity arise from conformational control of the propagating iminium and are derived from π-interactions between the electron-deficient carbazolium and the previously enchained monomer.36 Density functional theory (DFT) was utilized to investigate the chain-end reactive intermediate computationally. Ground state geometry optimizations using cpcm(toluene)/B3LYP-d3/def2-svp were performed on poly(NVC) after a single monomer addition in either the pro-meso or pro-racemo conformation. With no anion present, there was a 0.9 kcal/mol preference for the pro-meso conformation in the ground state, which we presume is due to unfavorable gauche interactions in the pro-racemo stereoisomer (Figure 4A).
Figure 4.
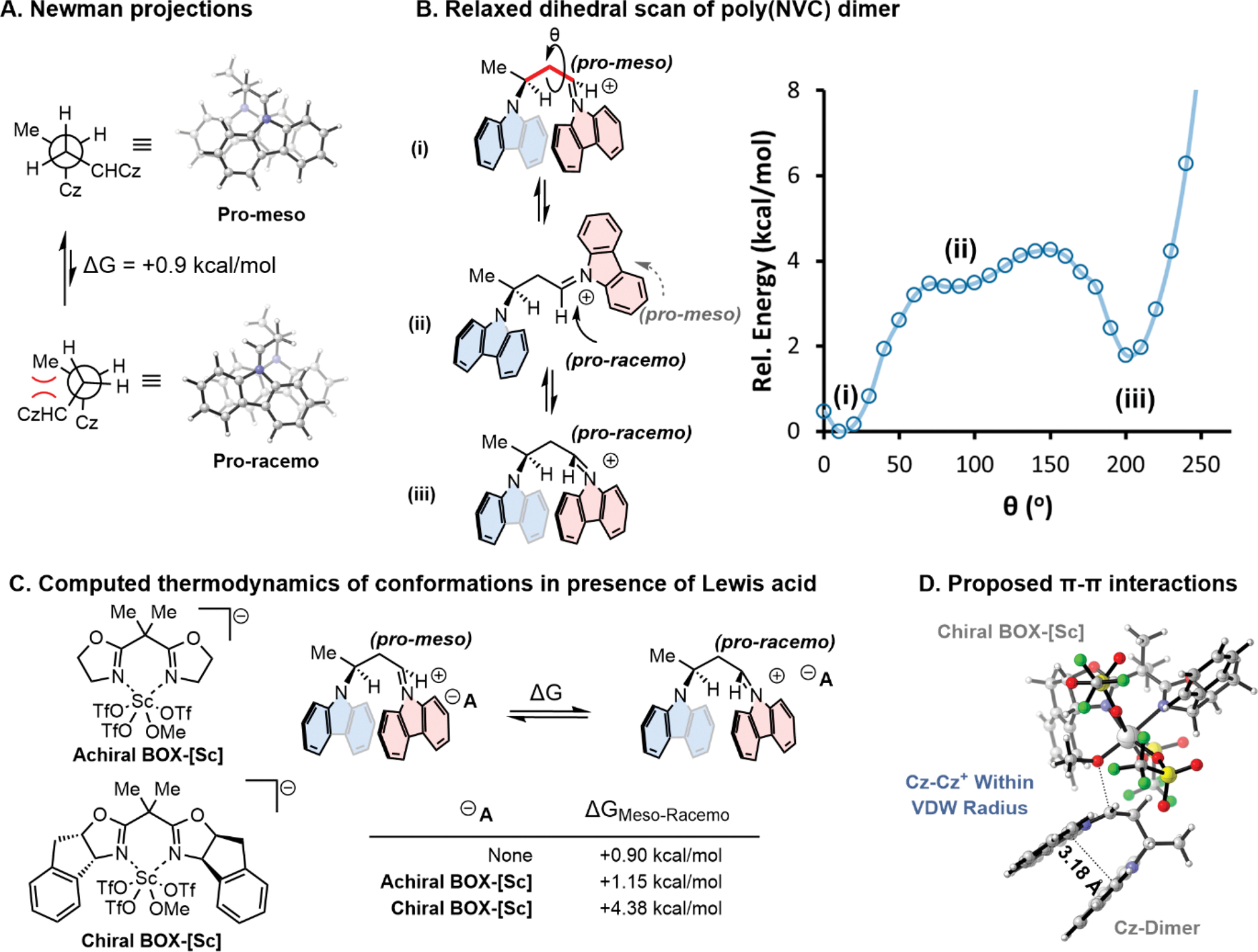
Computational study on the stereochemical conformational control of chain-end stereochemistry for investigating tacticity control.
A more in-depth analysis of the conformational energy landscape was accomplished using relaxed dihedral scans of the optimized pro-meso ground state structure (Figure 4B, (i)) at the cpcm(toluene)/B3LYP-d3/def2-tzvp level. To our surprise, intermediate structure (ii), which adopts a geometry to reduce A(1,3) strain and gauche interactions, is higher in energy compared to (i) and (iii) due to the strong orbital overlap between carbazole rings (π–π stacking) being ablated (Figures S18, S19). We hypothesize that the lower energy coplanar carbazole conformations (i) and (iii) hinder monomer attack from the posterior face; thus, the conformation of the propagating iminium (pro-meso or pro-racemo) would dictate facial selectivity for monomer addition and therefore the tacticity.
Thermodynamic data were extracted in the presence of the BOX-Sc(OMe)(OTf)3 counterion (Figure 4C). An unsubstituted, achiral BOX ligand provided a similar thermodynamic preference for the pro-meso conformation of the dimeric species compared a dimer with no associated counterion. However, calculation of the dimer with an indane-substituted chiral BOX complex as a counteranion favored the pro-meso conformer by 4.38 kcal/mol, indicating that the substitution of the ligand strongly influences ground state conformation through asymmetric ion-pairing interactions (Figure 4C, D).
The mechanistic understanding gained from DFT suggests tacticity is influenced by conformational control of the prochiral iminium chain-end, but the helicity-determining steps were still unclear. We hypothesized that the enantioselectivity of the first propagation event from the prochiral iminium ion may determine the magnitude and sign of helicity. This step would be stereochemically distinct from every other propagation due to the lack of π–π interactions with a previously enchained monomer. If operative, molar ellipticity and the percent enantiomeric excess (% ee) of the first stereocenter should be directly related.
To test this hypothesis, we sought to characterize how the enantioselectivity of only the first propagation event influenced the magnitude of helicity by initiating the polymerization of NVC using an enantioenriched initiating species, (+)-dCz (Figure 5A). Isotactic poly(NVC) of 91% mm was prepared using either chiral initiator (+)-dCz or NVC-OMe and catalyst [Sc-L11]. Initiating polymerization with (+)-dCz (69% ee) resulted in an amplification of CD response compared to initiation with NVC-OMe (Figure 5A, blue arrow). However, when the enantiomeric ligand L10 was used with initiator (+)-dCz, an inversion of CD response occurred compared to the polymerization initiated by NVC-OMe, indicating a mismatch between the ligand and initiator chirality—while maintaining absorbances within <5% of one another (Figure 5A red arrow, Figure S6). We then altered the magnitude of % ee of the chiral initiator and observed an accompanying linear increase in CD response for the absorbances at 296 and 265 nm (Figure 5B). The combination of these data indicate that the stereoselectivity (% ee) of the first propagation event strongly influences the sign and magnitude of helicity without affecting polymer tacticity, thus demonstrating an HSSP where the tacticity and helicity are decoupled.
Figure 5.
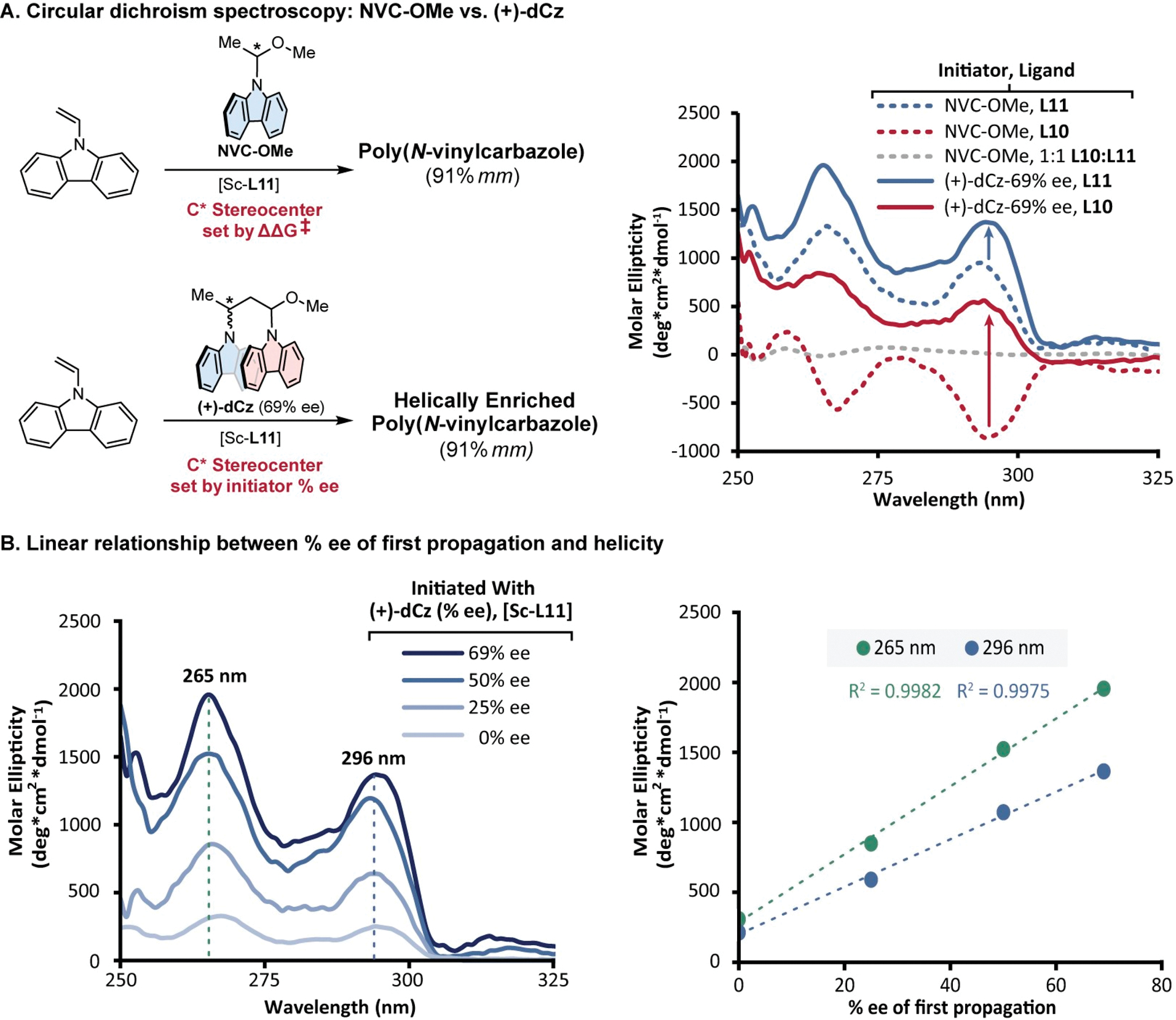
Mechanistic study of polymer helicity and its relationship to the stereoselectivity of the first monomer propagation event.
In summary, we have disclosed the first stereoselective cationic HSSP of a vinyl monomer. The optimized BOX-scandium Lewis acid catalyst allowed the synthesis of helical and highly isotactic poly(NVC) through the ionization of a simple hemiaminal initiator. Mechanistic investigations revealed that the stereoselective processes that endow tacticity and helicity are not directly related, which provides design parameters to rationally control helicity independently of tacticity for poly(NVC). During propagation, differences in pro-meso and pro-racemo conformations strongly influence the resulting tacticity, whereas the stereoselectivity of the first propagation determines the helicity of the polymer.
Supplementary Material
ACKNOWLEDGMENTS
This material is based upon work supported by the National Institute of General Medical Sciences of the National Institute of Health under Award Number 1-R35-GM142666–01 and the Arnold and Mable Beckman Foundation under the Beckman Young Investigator program. The UNC Department of Chemistry’s NMR Core Laboratory provided expertise and instrumentation that enabled this study with support from National Science Foundation (CHE-1828183 and CHE-0922858). We acknowledge the Macromolecular Interactions Facility (CD; supported by the National Cancer Institute of the National Institutes of Health under award number P30CA016086) for assistance with CD instrumentation.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c02738.
Synthesis, experimental details, characterization data, computational details, and supplementary figures (PDF)
Contributor Information
Cole C. Sorensen, Department of Chemistry, University of North Carolina, Chapel Hill, North Carolina 27599, United States
Frank A. Leibfarth, Department of Chemistry, University of North Carolina, Chapel Hill, North Carolina 27599, United States
REFERENCES
- (1).Ito S; Nozaki K Asymmetric Polymerization. Catal. Asymmetric Synth. Third Ed 2010, 931–985. [Google Scholar]
- (2).ckner S; Allegra G; Corradini P. Helix Inversions in Polypropylene and Polystyrene. Macromolecules 2002, 35, 3928–3936. [Google Scholar]
- (3).Krautwald S; Sarlah D; Schafroth MA; Carreira EM Enantio- and Diastereodivergent Dual Catalysis: α-Allylation of Branched Aldehydes. Science 2013, 340, 1065–1068. [DOI] [PubMed] [Google Scholar]
- (4).Krautwald S; Schafroth MA; Sarlah D; Carreira EM Stereodivergent α-Allylation of Linear Aldehydes with Dual Iridium and Amine Catalysis. J. Am. Chem. Soc. 2014, 136, 3020–3023. [DOI] [PubMed] [Google Scholar]
- (5).Shi SL; Wong ZL; Buchwald SL Copper-Catalysed Enantioselective Stereodivergent Synthesis of Amino Alcohols. Nature 2016, 532, 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Huo X; Zhang J; Fu J; He R; Zhang W Ir/Cu Dual Catalysis: Enantio- and Diastereodivergent Access to α,α-Disubstituted α-Amino Acids Bearing Vicinal Stereocenters. J. Am. Chem. Soc. 2018, 140, 2080–2084. [DOI] [PubMed] [Google Scholar]
- (7).Nakano T; Taniguchi K; Okamoto Y Asymmetric Polymerization of Diphenyl-3-Pyridylmethyl Methacrylate Leading to Optically Active Polymer with Helical Conformation and Chiral Recognition Ability of the Polymer. Polym. J. 1997, 29, 540–544. [Google Scholar]
- (8).Nakano T; Nakagawa O; Tsuji M; Tanikawa M; Yade T; Okamoto Y Poly(2,7-Di-n-Pentyldibenzofulvene) Showing Chiroptical Properties in the Solid State Based Purely on a Chiral Conformation. Chem. Commun. 2004, 4, 144–145. [DOI] [PubMed] [Google Scholar]
- (9).Hu W; Cao J; Huang Y -l.; Liang, S. Asymmetric Polymerization of N-Vinylcarbazole with Optically Active Anionic Initiators. Chin. J. Polym. Sci. (English Ed.) 2015, 33, 1618–1624. [Google Scholar]
- (10).Chu JH; Xu XH; Kang SM; Liu N; Wu ZQ Fast Living Polymerization and Helix-Sense-Selective Polymerization of Diazoacetates Using Air-Stable Palladium(II) Catalysts. J. Am. Chem. Soc. 2018, 140, 17773–17781. [DOI] [PubMed] [Google Scholar]
- (11).Yan X; Zhang S; Zhang P; Wu X; Liu A; Guo G; Dong Y; Li X [Ph3C][B(C6F5)4]: A Highly Efficient Metal-Free Single-Component Initiator for the Helical-Sense-Selective Cationic Copolymerization of Chiral Aryl Isocyanides and Achiral Aryl Isocyanides. Angew. Chemie - Int. Ed 2018, 57, 8947–8952. [DOI] [PubMed] [Google Scholar]
- (12).Zhou L; Xu XH; Jiang ZQ; Xu L; Chu BF; Liu N; Wu ZQ Selective Synthesis of Single-Handed Helical Polymers from Achiral Monomer and a Mechanism Study on Helix-Sense-Selective Polymerization. Angew. Chemie - Int. Ed 2021, 60, 806–812. [DOI] [PubMed] [Google Scholar]
- (13).Teator AJ; Varner TP; Knutson PC; Sorensen CC; Leibfarth FA 100th Anniversary of Macromolecular Science Viewpoint: The Past, Present, and Future of Stereocontrolled Vinyl Polymerization. ACS Macro Lett. 2020, 9, 1638–1654. [DOI] [PubMed] [Google Scholar]
- (14).Mahlau M; List B Asymmetric Counteranion-Directed Catalysis: Concept, Definition, and Applications. Angew. Chemie - Int. Ed 2013, 52, 518–533. [DOI] [PubMed] [Google Scholar]
- (15).Phipps RJ; Hamilton GL; Toste FD The Progression of Chiral Anions from Concepts to Applications in Asymmetric Catalysis. Nat. Chem. 2012, 4, 603–614. [DOI] [PubMed] [Google Scholar]
- (16).Brak K; Jacobsen EN Asymmetric Ion-Pairing Catalysis. Angew. Chemie - Int. Ed 2013, 52, 534–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Teator AJ; Leibfarth FA Catalyst-Controlled Stereo-selective Cationic Polymerization of Vinyl Ethers. Science 2019, 363, 1439–1443. [DOI] [PubMed] [Google Scholar]
- (18).Watanabe H; Yamamoto T; Kanazawa A; Aoshima S Stereoselective Cationic Polymerization of Vinyl Ethers by Easily and Finely Tunable Titanium Complexes Prepared from Tartrate-Derived Diols: Isospecific Polymerization and Recognition of Chiral Side Chains. Polym. Chem. 2020, 11, 3398–3403. [Google Scholar]
- (19).Zhang X; Yang Z; Jiang Y; Liao S Organocatalytic, Stereoselective, Cationic Reversible Addition–Fragmentation Chain-Transfer Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2022, 22, 144–679. [DOI] [PubMed] [Google Scholar]
- (20).Knutson PC; Teator AJ; Varner TP; Kozuszek CT; Jacky PE; Leibfarth FA Brønsted Acid Catalyzed Stereoselective Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2021, 143, 16388–16393. [DOI] [PubMed] [Google Scholar]
- (21).Okamoto K.-i.; Yamada M; Itaya A; Kimura T; Kusabayashi S. Polymerization of N-Vinylcarbazole, N-Vinyl-5H-Benzo[b]Carbazole, and N-Vinyl-7H-Benzo[c]Carbazole. Macromolecules 1976, 9, 645–649. [Google Scholar]
- (22).Kim W; Nishikawa Y; Watanabe H; Kanazawa A; Aoshima S; Fujii A; Ozaki M Stereoregularity Effect on Hole Mobility in Poly(N-Vinylcarbazole) Thin Film Evaluated by MIS-CELIV Method. Jpn. J. Appl. Phys. 2020, 59, SDDA01. [Google Scholar]
- (23).Kido J; Hongawa K; Okuyama K; Nagai K White Light-Emitting Organic Electroluminescent Devices Using the Poly(N-Vinylcarbazole) Emitter Layer Doped with Three Fluorescent Dyes. Appl. Phys. Lett. 1994, 64, 815–817. [Google Scholar]
- (24).Gong X; Robinson MR; Ostrowski JC; Moses D; Bazan GC; Heeger AJ High-Efficiency Polymer-Based Electrophosphorescent Devices. Adv. Mater. 2002, 14, 581–585. [Google Scholar]
- (25).Watanabe H; Kanazawa A; Aoshima S Stereospecific Living Cationic Polymerization of N-Vinylcarbazole through the Design of ZnCl2-Derived Counteranions. ACS Macro Lett. 2017, 6, 463–467. [DOI] [PubMed] [Google Scholar]
- (26).Nakano T; Okamoto Y Synthetic Helical Polymers: Conformation and Function. Chem. Rev. 2001, 101, 4013–4038. [DOI] [PubMed] [Google Scholar]
- (27).Nakano T; Okamoto Y; Hatada K Asymmetric Polymerization of Triphenylmethyl Methacrylate Leading to a One-Handed Helical Polymer: Mechanism of Polymerization. J. Am. Chem. Soc. 1992, 114, 1318–1329. [Google Scholar]
- (28).Nakafuku KM; Zhang Z; Wappes EA; Stateman LM; Chen AD; Nagib DA Enantioselective Radical C–H Amination for the Synthesis of β-Amino Alcohols. Nat. Chem. 2020, 12, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Connon R; Roche B; Rokade BV; Guiry PJ Further Developments and Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2021, 121, 6373–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Aoshima S; Higashimura T Living Cationic Polymerization of vinyl monomers by organoaluminum haliides. 3. Living polymerization of isobutyl vinyl ether by ethyldichloroaluminum in the presence of ester additives. Macromolecules 1989, 22, 1009–1013. [Google Scholar]
- (31).Katayama H; Kamigaito M; Sawamoto M; Higashimura T Living Cationic Polymerization of Isobutyl Vinyl Ether by the CF3CO2H–SnCl4–NBu4NCl System: In Situ Direct Analysis of the Growing Species by 1H, 13C and 19F NMR Spectroscopy. J. Phys. Org. Chem. 1995, 8, 282–292. [Google Scholar]
- (32).Ouchi M; Kammiyada H; Sawamoto M Ring-Expansion Cationic Polymerization of Vinyl Ethers. Polym. Chem. 2017, 8, 4970. [Google Scholar]
- (33).Okamoto Y; Nakano T Asymmetric Polymerization. Chem. Rev. 1994, 94, 349–372. [Google Scholar]
- (34).Mumby SJ; Beevers MS Electro-Optical Studies of Various Stereostructural Forms of Poly(N-Vinylcarbazole). Polymer (Guildf) 1989, 30, 860–865. [Google Scholar]
- (35).Crystal RG The Crystalline Morphology of Poly(N-Vinylcarbazole). Macromolecules 1971, 4, 379–384. [Google Scholar]
- (36).Ito S; Takami K; Tsujii Y; Yamamoto M Excimer Formation in Sterically Hindered Poly(9-Vinylcarbazole) and Its Dimer Model Compounds. Macromolecules 1990, 23, 2666–2673. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


