Mesenchymal-epithelial transition (MET) exon 14 skipping mutations occur in ∼3% of patients with non-small-cell lung cancer (NSCLC), the latter accounting for 85% of all lung cancers [1]. In February 2021, the US Food and Drug Administration (FDA) granted accelerated approval to tepotinib (Tepmetko, EMD Serono, Rockland, MA, USA), a type 1b MET tyrosine kinase inhibitor, for its use in metastatic NSCLC with MET exon 14–skipping mutations. Mohan and Hermann [2] recently reported on a case of pseudo-acute kidney injury (AKI) following capmatinib, another type 1b MET inhibitor. They suggested competitive inhibition of creatinine secretion in the renal tubules to be a class effect of MET inhibitors, resulting in a distinct increase in serum creatinine and finally a falsely low glomerular filtration rate (GFR).
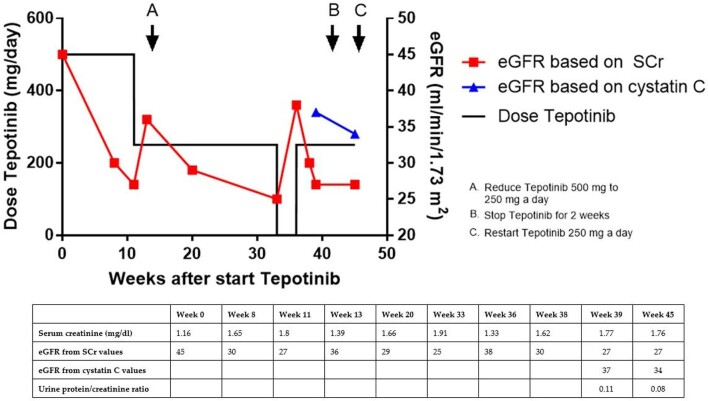
We herein report the case of a 79-year-old Caucasian female who was treated with tepotinib during 9 months for NSCLC. Past medical history included chronic kidney disease (CKD) stage 3 with a baseline serum creatinine (SCr) concentration at the start of tepotinib treatment of 1.16 mg/dl (Figure 1) and a corresponding estimated GFR (eGFR) of 45 ml/min/1.73 m2 (calculated by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation). Eleven weeks after the start of tepotinib 500 mg/day, her SCr had increased to 1.8 mg/dl (eGFR 27 ml/min/1.73 m2) (Figure 1). The tepotinib dose was reduced by 50% and 2 weeks later her SCr had improved to 1.39 mg/dl (eGFR 36 ml/min/1.73 m2). However, her SCr increased again to 1.91 mg/dl (eGFR 25 ml/min/1.73 m2) 20 weeks later, at week 33 after the start of tepotinib. Tepotinib was withheld for 2 weeks with a subsequent decrease in SCr to 1.33 mg/dl (eGFR 38 ml/min/1.73 m2) at week 36. The medication was restarted at its 50% reduced dose (250 mg/day). Three weeks later the patient presented at the outpatient nephrology clinic. Besides fatigue and peripheral oedema, known as side effects of tepotinib, the patient reported no nephrological complaints. She was normotensive and clinically euvolemic at physical examination. Ultrasound showed no evidence of hydronephrosis or other causes of AKI. Urinalysis showed an inactive sediment and revealed a normal protein:creatinine ratio of 0.112 mg/g.
FIGURE 1:
The eGFR calculated by SCr (in red) and by cystatin C (in blue) plotted against time from the start of treatment with tepotinib. Laboratory values during treatment are shown in the table.
As the deterioration in kidney function was definitely correlated with the introduction of tepotinib and resolved with reduction and even removal of the drug, the question arose if this could also be a pseudo-AKI related to MET inhibitors [2]. Therefore both SCr and cystatin C were measured. After resuming the tepotinib at a dose of 250 mg/day, SCr was again elevated to 1.77 mg/dl (eGFR 27 ml/min/1.73 m2) (Figure 1), whereas cystatin C measurements showed a distinctly better result: 1.58 mg/dl (eGFR 37 ml/min/1.73 m2). A pseudo-AKI due to tepotinib was diagnosed and tepotinib was continued at a dose of 250 mg/day. After 6 weeks, 45 weeks after the start of tepotinib, measurements showed similar results: the SCr was 1.76 mg/dl (eGFR 27 ml/min/1.73 m2) and cystatin C was 1.67 mg/dl (eGFR 34 ml/min/1.73 m2). The urinalysis continued to be bland throughout and the patient experienced no complaints and stayed normovolemic.
To the best of our knowledge, we herein report the first case of tepotinib-induced pseudo-AKI. Our finding further supports the hypothesis that competitive inhibition of creatinine secretion in the renal tubules could be a plausible class effect of MET inhibitors, leading to an increase in SCr and finally a falsely low GFR [2]. To further corroborate this hypothesis, one could use both in vivo and in vitro experiments. Creatinine is actively secreted in the renal tubules via several transporters, such as organic anion transporter 2 (OAT2). If one wants to study the possible inhibitory effect of tepotinib on tubular creatinine secretion via OAT2 in vitro, the OAT2-mediated uptake of creatinine on OAT2-overexpressing cell cultures in the absence versus presence of tepotinib could be evaluated. Furthermore, in vivo one could prospectively monitor both SCr and cystatin C levels in MET inhibitor–treated patients. Meanwhile, physicians should be aware of the possible side effect of MET inhibitors causing pseudo-AKI and use non-creatinine-based methods to distinguish a true AKI from pseudo-AKI. This could spare patients the redundant discontinuation of prognostically important cancer therapies.
Contributor Information
Veerle Wijtvliet, Department of Nephrology and Hypertension, Antwerp University Hospital, Antwerp, Belgium; Laboratory of Experimental Medicine and Pediatrics and member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Laurence Roosens, Department of Clinical Biology, Antwerp University Hospital, Antwerp, Belgium.
Charlotte De Bondt, Department of Thoracic Oncology, Antwerp University Hospital, Antwerp, Belgium.
Annelies Janssens, Department of Thoracic Oncology, Antwerp University Hospital, Antwerp, Belgium.
Daniel Abramowicz, Department of Nephrology and Hypertension, Antwerp University Hospital, Antwerp, Belgium; Laboratory of Experimental Medicine and Pediatrics and member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
AUTHORS’ CONTRIBUTIONS
All authors critically reviewed and approved the manuscript in its final form.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest. Furthermore, this article has not been published previously in whole or part.
REFERENCES
- 1. Socinski MA, Pennell NA, Davies KD. MET exon 14 skipping mutations in non-small-cell lung cancer: an overview of biology, clinical outcomes, and testing considerations. JCO Precis Oncol 2021;5:653–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohan A, Herrmann SM. Capmatinib-induced pseudo-acute kidney injury: a case report. Am J Kidney Dis 2022;79:120–4 [DOI] [PubMed] [Google Scholar]