ABSTRACT
Background
Acute kidney injury (AKI) is often iatrogenic and potentially preventable. Reduced renal nicotinamide adenine dinucleotide (NAD+) is reported to increase the susceptibility of AKI. The present study explored the predictive value of urinary de novo NAD+ synthetic metabolites for AKI using two independent cohorts.
Methods
The expression of de novo NAD+ synthetic enzymes in human kidney was examined by immunohistochemistry and single-cell transcriptomes. Urine samples were collected from two independent cohorts: the methotrexate (MTX) cohort with high-dose MTX treatment for lymphoma (n = 189) and the liver transplantation cohort with orthotopic liver transplantation (n = 49). Urinary metabolomics study of NAD+de novo synthesis was performed by liquid chromatography with mass spectrometry, screening for AKI predictive biomarkers. Nephroseq database and immunohistochemistry were used to analyze kidney de novo NAD+ synthetic enzymes expression in AKI-susceptible conditions.
Results
Human proximal tubule was the main structure in the kidney that expressed the necessary enzymes for NAD+de novo synthesis. In the MTX cohort, the urinary quinolinic acid (QA)/3-hydroxyanthranilic acid (3-OH AA) ratio before chemotherapy was significantly lower in those who developed AKI after chemotherapy compared with those who did not. This finding was consistent in the liver transplantation cohort. The area under the receiver-operating characteristic curve (AUC) of urinary QA/3-OH AA for AKI prediction was 0.749 and 0.729 in two cohorts, respectively. 3-Hydroxyanthranilic acid dioxygenase (HAAO), the enzyme catalyzing QA synthesis from 3-OH AA, decreased in AKI-susceptible diabetic kidneys.
Conclusions
The human proximal tubules were important source of NAD+ from the de novo pathway. Reduced urinary QA/3-OH AA ratio, which possibly suggested decreased HAAO activity, could be a potential AKI predictive biomarker.
Keywords: acute kidney injury, 3-hydroxyanthranilic acid; nicotinamide adenine dinucleotide; quinolinic acid; renal tubules
INTRODUCTION
Acute kidney injury (AKI) is a global health burden with reported incidence ranging from 1.9% to 18% in hospitalized patients [1–4]. The mortality of AKI in the intensive care unit (ICU) is as high as 57.3% according to the Acute Kidney Injury–Epidemiologic Prospective Investigation (AKI-EPI) study [5]. Most studies demonstrate that the risk for end-stage renal disease after AKI increases significantly [6]. Iatrogenic factors such as nephrotoxic medicine or surgical procedures [7–10] are important causes of AKI. Variable AKI susceptibility has been observed. Aged, diabetes and chronic kidney disease (CKD) are reported to increase the susceptibility to AKI. Understanding the biological mechanism responsible for AKI risk is critical for the development of reliable biomarkers.
Our previous study and others have shown that declined nicotinamide adenine dinucleotide (NAD+) level was related to increased susceptibility to AKI, and NAD+ precursor protected mice from AKI [11–13]. NAD+ is known to be an indispensable cofactor for cellular redox reaction and is critical in energy supply. NAD+ has also been shown to be a substrate for certain important enzymes such as Sirtuins, poly-ADP-ribose-polymerases (PARPs) and CD38, and plays an important role in regulating aging and stress response [14]. These NAD+-dependent enzymes are the main consumers of NAD+. Cellular NAD+ levels are determined by a balance of its consumption and biosynthesis. Three biosynthetic pathways from different precursors contribute to NAD+ pool in mammals [15]. NAD+de novo biosynthesis is the most complicated one [15], in which conversion from tryptophan to NAD+ is catalyzed by eight enzymes, generating a series of intermediate metabolites. Manipulation of the de novo pathway has recently been shown to change NAD+ levels and resistance to AKI [12, 16].
In the present study, we characterized the expression and distribution of enzymes catalyzing the production of NAD+ from de novo pathway in the kidney. We then examined predictive value of urinary de novo NAD+ metabolites for AKI. Finally, we explored the potential role of 3-hydroxyanthranilic acid dioxygenase (HAAO), an enzyme catalyzing 3-hydroxyanthranilic acid (3-OH AA) to quinolinic acid (QA) in the de novo NAD+ pathway, in the susceptibility of AKI. The present study suggests that de novo NAD+ synthesis is associated with the susceptibility of AKI and the metabolites of this pathway are a potential indicator of AKI risk.
MATERIALS AND METHODS
Immunofluorescence/immunohistochemistry analyses
In order to characterize the localization of de novo NAD+ enzymes in human kidney, immunofluorescence and immunohistochemistry studies were performed on kidney biopsy specimen of patients from Huashan Hospital, Fudan University. Kidney biopsy samples from minimal change disease or immunoglobulin A nephropathy (Oxford classification 2016: M0E0S0T0-C0) were examined. These patients had normal serum creatinine levels and normal or very mild morphological changes in the glomeruli and tubules. The study was approved by the ethics committee of Huashan Hospital and written informed consent was obtained from all participants. Immunofluorescence and immunohistochemical staining were conducted on paraffin-embedded renal biopsy sections as previously described [17]. Primary antibodies and second antibody were described in Supplementary data description of Materials and methods. Negative controls were conducted using normal mouse or rabbit IgG instead of primary antibody (Supplementary data, Fig. S5).
Kidney samples of minimal change disease and diabetic kidney disease (DKD) were examined for HAAO expression. The HAAO staining was semi-quantitatively scored in a blinded manner. Five high-power fields were assessed for each slide. The score was based on positive stained area and staining intensity and details described in Supplementary data description of Materials and methods.
Prospective study design
The study was performed in accordance with the Declaration of Helsinki and the study protocol was approved by the ethics committee of Huashan Hospital, Fudan University. All participants gave their written informed consent to participate in the study. AKI was defined according to KDIGO criteria with an increase of serum creatinine by ≥26 μmol/L within 48 h as well as an increase to more than 1.5 times from baseline within 7 days as threshold for diagnosis [18].
Methotrexate cohort
We included 69 adult patients diagnosed with primary central nervous system lymphoma who were about to receive intravenous high-dose methotrexate (HDMTX) chemotherapy (>500 mg/m2 body surface area). The exclusion criteria were (i) CKD3–5 stage before treatment; (ii) AKI before treatment; (iii) renal malformations; (iv) medical history of nephrectomy or renal transplantation; (v) tumor invasion of kidney; and (vi) refusal to participate. All patients followed the same standard regimen that included fluid hydration, urine alkalinization and leucovorin rescue. Urine pH, blood MTX concentration and renal function were measured at 0, 24, 48 and 72 h after MTX infusion.
Liver transplantation cohort
We further enrolled 49 adult patients who were about to undergo orthotopic liver transplantation (OLT). The exclusion criteria were (i) CKD3–5 stage before surgery; (ii) hepatorenal syndrome or AKI due to other causes before surgery; (iii) renal malformations; (iv) medical history of nephrectomy or renal transplantation; (v) combined liver–kidney transplantation; (vi) secondary liver transplantation; (vii) died within 12 h after surgery; and (viii) refusal to participate. Standardized protocols of general anesthesia, surgical procedure, postoperative management and immunosuppressive therapy were applied by the liver transplantation team of Huashan Hospital, Fudan University, with minor modifications.
Urine sample collection
In the MTX cohort, urine samples of each treatment course were collected within 72 h before and 12 h after MTX infusion. In the liver transplantation cohort, urine samples were collected within 72 h before surgery. Approximately 10 mL of urine was centrifuged at 3000 r.p.m. for 10 min within 6 h after collection and the supernatant was stored at −80°C.
NAD+ metabolites measurements
Shimadzu LC30 UHPLC system (Shimadzu, consisting of a binary pump, a vacuum degasser, an autosampler and a column oven) and AB Sciex Triple Quadrupole 5500 Mass spectrometer (AB Sciex) were used for liquid chromatography with mass spectrometry (LC-MS) measurement. Isotopic standards (L-Tryptophan-2,3,3-d3 and Quinolinic acid-4,5,6-d3) and standards (Tryptophan, N′-Formylkynurenine, Kynurenin, Quinolinic acid, 3-Hydroxyanthranilic acid and Creatinine) were obtained from ANPEL Laboratory Technologies. Equivalent pure methanol with isotope standards was used for urine protein precipitation at a concentration of 5 μg/mL and mixed 1:1 with urine samples. LC-MS condition, quality control and method validation were detailed in the Supplementary data description of Materials and methods. Data were acquired and processed by software Analyst 1.6.3 (AB Sciex).
Single-cell transcriptomes
Sample source and methodologies are detailed in the Supplementary data description of Materials and methods.
Statistical analysis
SPSS version 25 (IBM, Armonk, NY, USA), MedCalc version 19.1 (Ostend, Belgium) and R version 3.6.2 (RStudio) were used for statistical analysis. In the MTX cohort, one patient might receive HDMTX treatment more than once. In consideration of repeated treatment courses of one patient, repeated measures logistic regressions by a generalized estimating equation (GEE) logistic regression model with robust variance estimators was applied. Univariate GEE analyses were used to assess the relationship between demographic or clinical characteristics and AKI as well as group differences in the biomarker levels. Multivariate GEE analyses were used to assess the adjusted odds ratio of AKI and probabilities needed for area under the receiver operating characteristic curve (AUC) calculation. In the liver transplantation cohort, comparisons between continuous characteristics of subject groups were analyzed with Mann–Whitney U tests or Student's t-test and dichotomous variables with the chi-square test or Fisher's exact test. Group differences in the biomarker levels were assessed with Mann–Whitney test. The odds ratio of AKI was determined with logistic regression. Diagnostic accuracies of urine biomarkers were assessed using receiver operating characteristic (ROC) curve analysis. AUCs were compared with the test developed by DeLong et al. [19]. The net reclassification index (NRI) and integrated discrimination index (IDI) indices were used to quantify the improvement of urinary biomarkers on AKI risk prediction. For NRI analyses, AKI risk categories were classified as low (<10%), medium (10%–20%) or high (>20%) risk. Spearman's rank correlation was used to identify the relationship between age and urinary biomarker. Two-tailed P-values <.05 were considered significant.
RESULTS
The de novo NAD+ biosynthetic pathway in the renal proximal tubule
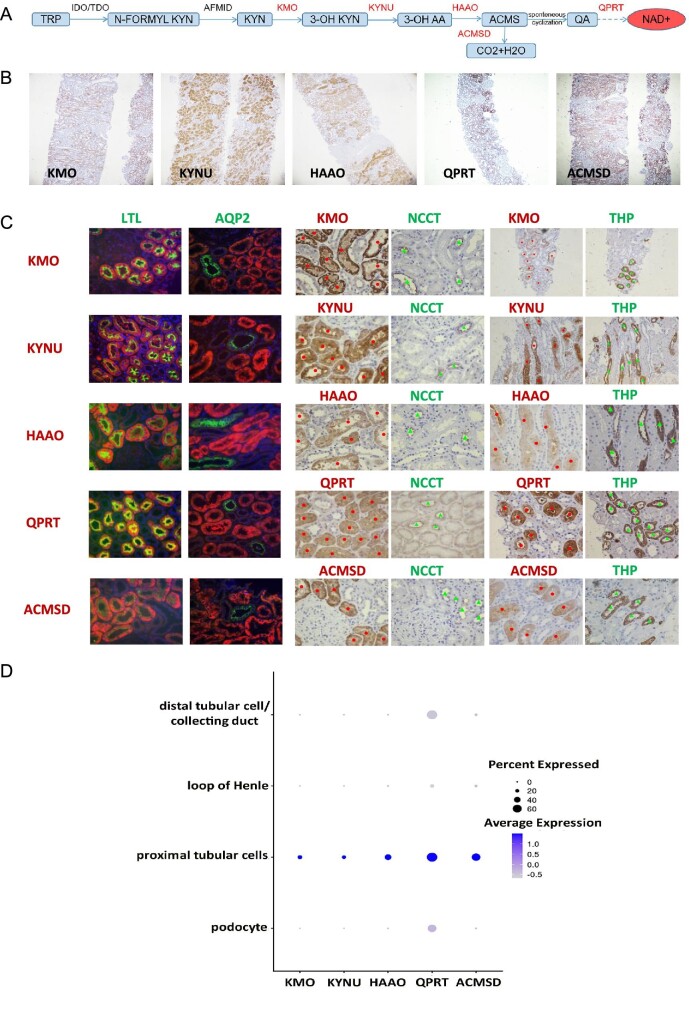
To determine whether the NAD+de novo pathway (Fig. 1A) was present in human kidney, we examined the expression of enzymes catalyzing NAD+de novo synthesis in human kidney tissue. Immunohistochemistry study revealed kynurenine monooxygenase (KMO), kynureninase (KYNU), HAAO, aminocarboxymuconic semialdehyde decarboxylase (ACMSD) and quinolinic acid phosphoribosyltransferase (QPRT) abundantly expressed in the cytoplasm of human renal tubular cells (Fig. 1B). To characterize the tubules that express these enzymes, specific markers for each segment were used. Immunofluorescence showed that all these enzymes were colocalized with lotus tetragonolobus lectin (LTL), a marker for proximal tubules, but not with aquaporin-2 (AQP2), a marker for collecting ducts. Immunohistochemistry staining of serial sections showed that these enzymes were not expressed in the segments labeled by thiazide sensitive Na-Cl cotransporter (NCCT, marker of distal convoluted tubules) or Tamm-Horsfall protein (THP, marker of thick ascending limb) (Fig. 1C). Single-cell RNA sequencing (scRNA-seq) was performed to further characterize the expression of these enzymes in human kidney. Kidney tissue was sampled from non-tumor part of a 54-year-old male patient who received radical nephrectomy for renal cell carcinoma. Six kidney cell types and four immune cell types were identified by unsupervised clustering (Supplementary data, Fig. S1). The predominant expression of NAD+de novo synthetic enzymes (KMO, KYNU, HAAO, QPRT and ACMSD) was found in the proximal tubular cells (Fig. 1D), consistent with immunohistochemistry/immunofluorescence staining. These results suggested that enzymes responsible for NAD+de novo synthesis primarily expressed in the proximal tubules of human kidney. We further examined the metabolites of the NAD+de novo pathway in the urine by LC-MS methods. Urinary tryptophan (TRP), N-formylkynurenine (N-formyl KYN), kynurenine (KYN), 3-OH AA and QA were detected (Fig. 2). Our data suggest that proximal tubular cells are a critical source of NAD+de novo synthesis in the kidney.
Figure 1:
Characterization of major enzymes responsible for NAD+de novo synthesis in human kidney. (A) De novo biosynthetic pathway of NAD+. (B) Representative immunohistochemical staining of NAD+de novo biosynthetic enzymes in human renal tissue specimens (magnification as 40×). (C) Immunofluorescence co-staining and immunohistochemical staining of serial sections of NAD+de novo biosynthetic enzymes (red dot) with renal tubular markers (green arrow). (Immunohistochemical staining for KMO/THP and KYNU/THP with magnification as 200× and others with magnification as 400×.) (D) A dot plot of single-cell RNA sequencing of KMO, KYNU, HAAO, QPRT and ACMSD genes in human kidney.
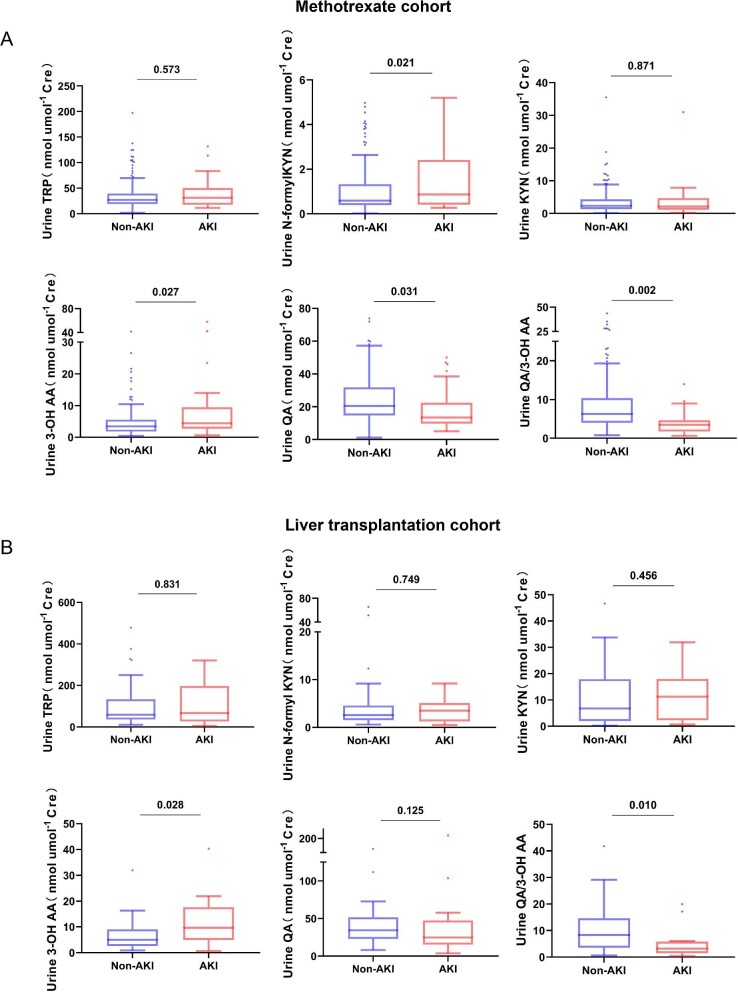
Figure 2:
Signature of urinary NAD+de novo metabolites in AKI cohorts. (A) In the MTX cohort, significantly higher concentration of urinary N-formyl KYN and 3-OH AA and lower urinary QA and QA/3-OH AA were exhibited in the AKI group (n = 35) compared with the non-AKI group (n = 154). No difference in urinary TRP or KYN between two groups was observed. (B) In the liver transplantation cohort, post-operative AKI patients (n = 16) had higher urinary 3-OH AA and lower urinary QA/3-OH AA than non-AKI patients (n = 33). No difference in urinary TRP, N-formyl KYN, KYN or QA between two groups was observed. All urine samples were collected before chemotherapy or surgery. Boxes show interquartile range; the horizontal lines are medians and the whiskers are plotted using Tukey method.
Clinical features of MTX cohort
To explore the association between urinary metabolites of the de novo NAD+ pathway and AKI risk, we performed a prospective study that enrolled patients with central nervous system lymphoma receiving HDMTX chemotherapy (MTX cohort). A total of 69 patients were recruited with 189 HDMTX treatment courses. The mean age was 58.82 ± 10.82 years and 68.11% of them were men (Supplementary data, Table S1). Among the 189 courses, 35 (18.52%) AKI incidents occurred after each HDMTX treatment. Considering that one patient might have repeated treatment courses, a GEE logistic regression model was applied (see statistical analysis of Materials and Methods). In the univariate GEE models, the relationships between characteristics of MTX treatment courses and AKI occurrence were assessed. The median baseline estimated glomerular filtration rate was 98.13 (88.93–105.57) mL/min/1.73 m2 with no significant difference between patients who developed and did not develop AKI. The patients who developed AKI received similar doses of MTX as those who did not develop AKI. The patients who developed AKI showed significantly higher comorbid diabetes prevalence and delayed MTX excretion compared with non-AKI group (Table 1).
Table 1:
Demographic and clinical characteristics of HDMTX treatment course in MTX cohort.
| Total (n = 189) | AKI (n = 35) | Non-AKI (n = 154) | P-value | |
|---|---|---|---|---|
| Age, years | 59.00 (51.00–65.00) | 59.00 (55.00–66.00) | 59.00 (50.25–65.00) | .259 |
| Male, n (%) | 128 (67.72) | 23 (65.71) | 105 (68.18) | .779 |
| BSA, m2 | 1.83 (1.70–1.89) | 1.85 (1.74–1.91) | 1.81 (1.70–1.89) | .437 |
| eGFR, mL/min/1.73 m2 | 98.13 (88.93–105.57) | 94.7 0(79.90–104.28) | 98.58 (89.46–105.59) | .158 |
| Hb, g/L | 125.95 ± 14.69 | 123.60 ± 15.29 | 126.49 ± 14.55 | .358 |
| WBC, ×109/L | 5.79 (4.50–7.34) | 5.77 (4.45–7.09) | 5.80 (4.50–7.45) | .923 |
| N% | 59.80 (52.55–67.45) | 62.20 (58.50–67.80) | 57.85 (51.90–67.32) | .355 |
| PLT, ×1012/L | 204.00 (162.50–249.00) | 187.00 (163.00–252.00) | 204.00 (161.00–249.00) | .816 |
| Diabetes, n (%) | 38 (20.10) | 15 (42.85) | 23 (14.93) | .006 |
| Hypertension, n (%) | 53 (28.04) | 14 (40.00) | 39 (25.32) | .134 |
| MTX dose, g/m2 | 4.56 (3.42–7.83) | 5.08 (3.49–7.83) | 4.13 (3.41–7.83) | .248 |
| Urine pH before MTX infusion | 7.50 (7.00–8.00) | 7.00 (7.00–8.00) | 7.50 (7.00–8.00) | .076 |
| Delayed MTX excretion, n (%) | 15 (7.93) | 12 (34.28) | 3 (1.94) | <.001 |
BSA, body surface area; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; WBC, white blood cell; N, neutrophil; PLT, platelet; MTX, methotrexate.
The association of urinary metabolites of the NAD+de novo pathway with HDMTX-induced AKI
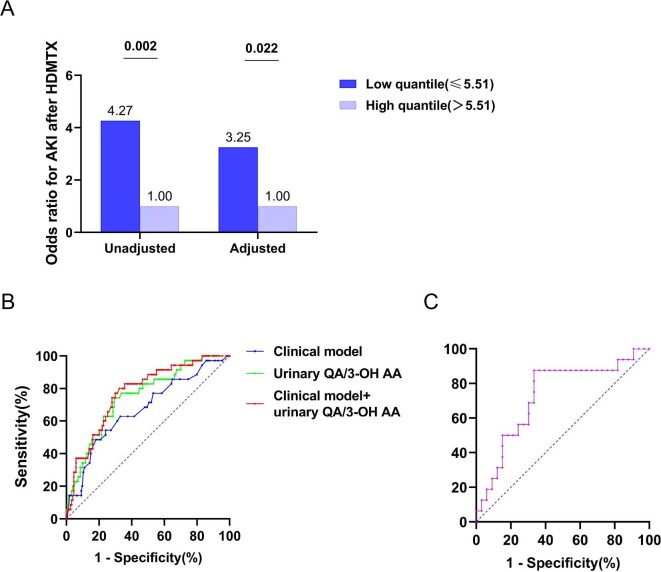
To examine the predictive value of metabolite(s) of the NAD+de novo pathway for AKI after HDMTX treatment, urine samples were collected 72 h before HDMTX treatment. The result showed that the pre-treatment urinary levels of N-formyl KYN and 3-OH AA were significantly higher and QA were significantly lower in those who developed AKI compared with those who did not develop AKI after treatment, while no difference in urinary TRP and KYN levels were observed between those who developed and those who did not develop AKI (adjusted by urinary creatinine level) (Fig. 2A). The association of urinary QA/3-OH AA ratio and the risk of AKI was then analyzed as shown in Fig. 3A. In the GEE model, the low quantile (lower than median) of pre-treatment QA/3-OH AA was associated with a 4.27-fold (95% confidence interval 1.67 to 10.69) odds for AKI compared with the high quantile (P = .002). After adjusting for age, sex and diabetes, the association between lower quantile of urinary QA/3-OH AA ratio and AKI risk still remained with an odds ratio of 3.25 (95% confidence interval 1.19 to 8.93) (P = .022).
Figure 3:
Predictive value of urinary QA/3-OH AA for AKI development. (A) Low quantile and high quantile are determined by the median of urinary QA/3-OH AA (5.51) in MTX cohort. In unadjusted model, the upper quantile of urinary QA/3-OH AA has 4.27 (95% confidence interval 1.67 to 10.69) odds for the development of AKI after HDMTX. After adjusting for age, sex and diabetes, the odds ratio is 3.25 (95% confidence interval 1.19 to 8.93). (B) ROC curves show the predictive performance for AKI after HDMTX. AUC for urinary QA/3-OH AA is 0.749(SEM 0.044). The clinical model is comprised of age, sex and diabetes and AUC for the clinical model is 0.672 (SEM 0.052). AUC for the combination of clinical model and urinary QA/3-OH AA is 0.772 (SEM 0.041). (C) ROC curves show the predictive performance for AKI after liver transplantation. AUC for urinary QA/3-OH AA is 0.729 (SEM 0.079).
A total of 90 samples of urine from 46 patients were collected 12 h after HDMTX treatment. Among them, 13 samples were collected after AKI development. There was no significant difference in urinary NAD+ metabolites levels between AKI and non-AKI group (Supplementary data, Fig. S2).
The predictive performance of urinary QA/3-OH AA ratio in HDMTX-induced AKI
The AUC for urinary QA/3-OH AA as AKI prediction was 0.749 [standard error of the mean (SEM) 0.044] (Fig. 3B), while AUC modeled using conventional variables including age, sex and diabetes (clinical model) was 0.672 (SEM 0.052), and inclusion of urinary QA/3-OH AA improved AUC to 0.772 (SEM 0.041) (P = .022) (Fig. 3B).
The NRI and the IDI were used to evaluate the improvement of risk prediction with addition of urinary QA/3-OH AA to the clinical model. We defined low AKI risk as <10%, intermediate AKI risk as 10%–20% and high AKI risk as >20% based on risk categories in the present study. Baseline urine QA/3-OH AA improved risk prediction over clinical model with NRI as 0.455 (SEM 0.128) (P =.004) and IDI as 0.065 (SEM 0.018) (P = .0003) (Supplementary data, Table S2).
Predictive value of urinary QA/3-OH AA for AKI in liver transplantation cohort
A total of 49 patients who were going to receive OLT were recruited. Sixteen of them developed AKI post-operatively (Table 2). Consistent with the MTX cohort, those patients who developed AKI had a lower pre-transplantation urinary QA/3-OH AA ratio and higher level of 3-OH AA compared with patients who did not develop AKI. The urinary levels of QA, TRP, N-formyl KYN and KYN were comparable between the two groups (Fig. 2B). AUC of pre-transplantation urine QA/3-OH AA ratio for AKI prediction was 0.729 (SEM 0.079) (Fig. 3C).
Table 2:
Demographic and clinical characteristics of liver transplantation cohort.
| Total (n = 49) | AKI (n = 16) | Non-AKI (n = 33) | P-value | |
|---|---|---|---|---|
| Age, years | 52.00 (46.00–55.50) | 52.50 (49.00–55.00) | 52.00 (44.00–56.50) | .677 |
| Male, n (%) | 40 (81.63) | 14 (87.50) | 26 (78.79) | .460 |
| BMI, kg/m2 | 23.71 ± 3.71 | 23.98 ± 3.71 | 23.58 ± 3.77 | .732 |
| eGFR, mL/min/1.73 m2 | 107.12 ± 15.40 | 102.44 ± 19.58 | 109.39 ± 12.65 | .140 |
| Hb, g/L | 109.30 ± 22.58 | 107.00 ± 18.32 | 110.42 ± 24.57 | .624 |
| WBC, ×109/L | 3.41 (2.37–4.82) | 3.38 (2.71–5.46) | 3.45 (2.25–4.68) | .449 |
| N, % | 63.90 (55.80–70.20) | 64.50 (57.30–69.08) | 63.30 (52.85–71.55) | .725 |
| PLT, ×1012/L | 53.00 (36.50–116.50) | 72.00 (50.25–97.75) | 48.00 (36.00–121.00) | .475 |
| ALT, U/L | 47.00 (22.50–75.50) | 38.50 (18.75–69.00) | 48.00 (24.00–84.50) | .624 |
| AST, U/L | 63.00 (30.50–126.50) | 63.00 (29.75–172.75) | 63.00 (30.50–111.00) | .475 |
| TB, μmol/L | 68.30 (28.05–206.85) | 67.70 (29.75–210.18) | 79.90 (27.05–206.85) | .798 |
| Albumin, g/L | 36.00 (31.00–37.50) | 33.50 (29.50–37.00) | 37.00 (32.50–40.50) | .242 |
| Pro-BNP, pg/mL | 76.90 (38.77–151.02) | 118.45 (69.22–197.55) | 62.80 (28.95–144.42) | .076 |
| INR | 1.52 (1.23–1.87) | 1.58 (1.38–1.91) | 1.47 (1.14–1.85) | .321 |
| MELD score | 17.27 ± 8.57 | 16.80 ± 7.37 | 17.50 ± 9.20 | .792 |
| Intraoperative blood loss, mL | 1000.0 (800.0–2000.0) | 1500.0 (1000.0–3000.0) | 1000.0 (700.0–1350.0) | .046 |
| HbA1c, % | 5.10 (4.43–5.78) | 5.05 (4.52–6.40) | 5.15 (4.30–5.50) | .434 |
| Diabetes, n (%) | 11 (22.44) | 6 (37.50) | 5 (15.15) | .141 |
| Hypertension, n (%) | 11 (22.44) | 3 (18.75) | 8 (24.24) | .666 |
| HBV, n (%) | 35 (71.42) | 14(87.50) | 21 (63.63) | .083 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; WBC, white blood cell; N, neutrophil; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; pro-BNP, pro-brain natural peptide; INR, International Normalized Ratio; MELD, the Model for End-stage Liver Disease; HBV, hepatitis B virus.
Association of urinary QA/3-OH AA ratio with age and diabetes
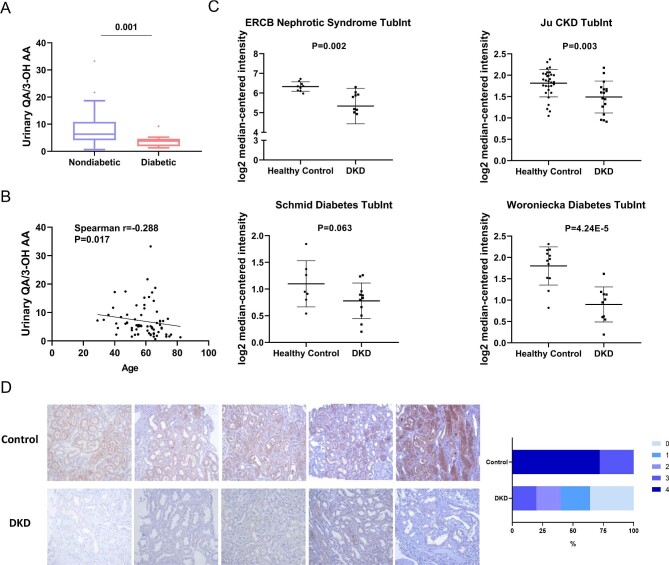
Aging and diabetes are two established risk factors for AKI. Further analysis exhibited significantly lower QA/3-OH AA ratio in diabetic patients than non-diabetic ones in MTX cohort (Fig. 4A). A negative correlation was found between urinary QA/3-OH AA and age (Fig. 4B).
Figure 4:
Urinary QA/3-OH AA and renal HAAO expression in AKI-susceptible subjects. (A) Diabetic patients (n = 16) had significantly lower urinary QA/3-OH AA than non-diabetic ones (n = 53). (B) Correlation analysis of urinary QA/-3-OH AA with age. The average urinary QA/3-OH AA of patients with multiple treatment courses in MTX cohort was used for analysis. (C) HAAO expression (log HAAO expression by microarray) in renal tubulointerstitial area of DKD patients compared with that of healthy controls (data from Nephroseq). (D) Representative immunohistochemical staining of HAAO in kidney biopsy from five patients diagnosed with DKD and five patients with minimal change disease (Control). Semi-quantification analysis suggested all slides from control groups had a staining intensity score more than 3 while 80% of slides from the DKD groups scored below 3.
HAAO expression in diabetic kidney
We examined whether the expressions of the enzymes in NAD+de novo synthesis pathway were altered in the kidney from patients with diabetes, a known high AKI risk population, using the Nephroseq dataset. A total of four datasets in which de novo NAD+ synthesis enzymes expression in renal tubulointerstitial areas of both DKD and healthy living controls were available were included for analysis. As shown in Supplementary data, Fig. S3, HAAO was remarkably repressed in renal tubulointerstitial areas of diabetic kidneys compared with healthy living donors. All four datasets showed down-regulation of HAAO expression in DKD patients (Fig. 4C). In the de novo NAD+ synthesis pathway, HAAO catalyzes QA synthesis from 3-OH AA. Decreased urinary QA/3-OH AA ratio may possibly result from reduced HAAO in the kidney. Decreased HAAO expression was further confirmed by immunohistochemistry study and single-cell transcriptomics. Immunohistochemistry staining of HAAO was performed on renal biopsy samples from five nondiabetic controls (minimal change disease patients with normal or very mild lesion under light microscopy) and five DKD patients. Figure 4D showed reduced HAAO expression in diabetic kidney compared with control. Decreased HAAO expression in diabetic kidney was also supported by scRNA-seq (Supplementary data, Fig. S4).
DISCUSSION
The present study reported that the enzymes of de novo NAD+ biosynthetic pathway were abundantly and differentially expressed in the human proximal tubules. Reduced urine QA/3-OH AA ratio had an ability to predict the development of AKI in patients receiving HDMTX. The predictive value of urine QA/3-OH AA ratio for AKI was validated in patients receiving liver transplantation. Furthermore, we showed that HAAO expression was dramatically decreased in diabetic kidneys, a known AKI-susceptible condition, which suggested a possible reason for declined urine QA/3-OH AA. Our study highlights the importance of de novo NAD+ metabolism in AKI susceptibility and urine QA/3-OH AA ratio as a potential biomarker for identifying patients at high risk of AKI.
NAD+ is not only vital for a variety of cellular events as an electron acceptor and regulator of mitochondrial function, but is also an essential substrate for several important enzymes, such as Sirtuins, PARPs and CD38 [15, 20]. Our previous study [11] and others [16, 21] have found that lower NAD+ levels were associated with compromised renal response to stress, and pharmaceutical restoration of NAD+ had a protective effect on the kidney from injury. Although the salvage pathway was once considered the dominant biosynthetic pathway responsible for NAD+ production, recent studies have revealed that in the liver and kidney, NAD+ can also be generated from tryptophan via the de novo pathway [22]. In the de novo pathway, tryptophan is converted to NAD+ through a series of enzymatic reactions (Fig. 1A). Blocking of crucial steps in the pathway has been shown to affect renal NAD+ volume and abate kidney health especially in response to stress [12, 16, 21, 23]. Katsyuba et al. [23] showed ACMSD inhibitor restored renal NAD+ levels and rescued mouse kidney from AKI induced by cisplatin and ischemic reperfusion. Tran et al. [16] found that AKI suppressed renal expression of a series of enzymes in the de novo pathway and upregulation of this pathway protected the kidney from acute injury. The same group later showed that in six AKI patients who received cardiac surgery, urine QA/tryptophan levels were elevated compared with those without AKI, indicating QPRT deficiency. The authors further showed that elevated QA/tryptophan ratio was associated with the development of AKI in an ICU cohort [12]. The present study using two independent cohorts demonstrated that lower urine QA/3-OH AA ratio predicted the occurrence of AKI after high-dose MTX or liver transplantation. High-dose MTX causes nephrotoxicity by precipitation of MTX leading to crystal nephropathy and direct toxic effect of MTX to renal tubules [24], and liver transplantation induces renal injury through ischemia reperfusion, hypoxia, the release of inflammatory cytokines from the allograft [25]. The consistent results in these two different cohorts of AKI suggested strong evidence for the predictive value of urine NAD+ metabolites for AKI. We then showed a repressed HAAO expression in diabetic kidney, which had high risk of AKI development, suggesting a possible reason for declined urine QA/3-OH AA ratio in AKI-susceptible individuals. Loss-of-function variants of HAAO was previously reported to result in NAD+ deficiency during embryogenesis and led to congenital renal defects, suggesting HAAO as an important factor in regulating NAD+ homeostasis and maintaining kidney integrity [26]. Therefore, the present study provides novel evidence suggesting that HAAO down-regulation may be associated with reduced NAD+ generation and increase the susceptibility to AKI after injury insults. The transcriptional regulation of HAAO in injury-susceptible kidney such as diabetic kidney remains to be explored. It is noteworthy that besides HAAO, QA/3-OH AA ratio could also possibly be affected by ACMSD and precise molecular mechanisms of de novo NAD+ enzymes changes in AKI-susceptible conditions are worth exploring in further studies.
Our study localized the enzymes for de novo NAD+ synthesis to the proximal tubules. Although indoleamine 2,3-dioxygenase (IDO) and formamidase (AFMID) were not examined in the present study, they have been nicely documented to be expressed universally, including in the kidney [27, 28]. The present study has suggested that the proximal tubule epithelium is a place where NAD+ is generated by de novo pathway from tryptophan in the kidney. The significance of de novo NAD+ generation in the proximal tubules is incompletely understood. The proximal tubules, which are enriched with mitochondria, consume more than two-thirds of oxygen in the kidney, rendering them the main target of ischemic or nephrotoxic insults [29]. However, whether the proximal tubules are source of NAD+ for other parts of the kidney [30] or other parts of our body remains to be further explored. In addition, liver has been demonstrated to be another source of NAD+ synthesis from de novo pathway [22]. Liver dysfunction is reported to be associated with increased tryptophan degradation via the NAD+de novo synthesis pathway, causing the changes of circulating metabolites of this pathway [31]. It remains unclear whether the circulating levels of these metabolites significantly affects their urine levels. In our study, lower urine QA/3-OH AA ratio was significantly associated with AKI occurrence in the liver transplantation cohort and also the MTX cohort, whose liver functions were normal. More studies are required to investigate the exact effect of liver function on urine levels of metabolites in the NAD+de novo synthesis pathway.
It has been well documented that aging, diabetes and CKD are risk factors for AKI [32]. It will be critical if we have biomarkers that can reliably predict the occurrence of AKI in high-risk patients. Prediction models based on clinical information have been reported, but failed to be practically implemented because of limitations to a very specific setting and were not based on the pathophysiology of AKI susceptibility [33, 34]. Several biomarkers have been developed, such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1(KIM-1), tissue inhibitor metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGFBF-7), interleukin-18 and liver fatty acid-binding protein (L-FABP) [35]. However, these are injury markers instead of predictive markers.
Limitations of our study include it being a single-center study with limited sample size. The association between urine QA/3-OH AA ratio and the severity of kidney injury or the long-term renal outcome are not investigated. Expanded sample volume and longer follow-up are necessary in any future study. Furthermore, the explicit mechanism regarding how repressed HAAO contributes to AKI susceptibility and whether therapeutic manipulation of HAAO can be kidney-beneficial remains to be explored.
In conclusion, major enzymes required for de novo synthesis of NAD+ were expressed by the human proximal tubular cells. Reduced urine QA/3-OH AA ratio was associated with occurrence of AKI and can be a potential predictive biomarker for AKI. Development of predictive biomarkers is important not only for identifying those patients who have increased susceptibility to AKI but also exploring potential intervention target to prevent AKI.
Supplementary Material
ACKNOWLEDGEMENTS
We thank H. Cai from Vanderbilt University School of Medicine for his advice on statistical strategy. We also thank L. Feng and Z.A. He from Instrumental Analysis Center, Shanghai Jiao Tong University for their expertise in LC-MS technique.
Contributor Information
Yujia Wang, Department of Nephrology, Huashan Hospital, Fudan University, Shanghai, China.
Yi Guan, Department of Nephrology, Huashan Hospital, Fudan University, Shanghai, China.
Qionghong Xie, Department of Nephrology, Huashan Hospital, Fudan University, Shanghai, China.
Weiyuan Gong, Department of Nephrology, Huashan Hospital, Fudan University, Shanghai, China.
Jianhua Li, Department of General Surgery, Huashan Hospital, Fudan University, Shanghai, China.
Tong Chen, Department of Hematology, Huashan Hospital, Fudan University, Shanghai, China.
Yanfang Xu, Department of Nephrology, Blood Purification Research Center, the First Affiliated Hospital, Fujian Medical University, Fuzhou, China.
Ning Xu, Department of Urology, Urology Research Institute, the First Affiliated Hospital, Fujian Medical University, Fuzhou, China.
Shaohao Chen, Department of Urology, Urology Research Institute, the First Affiliated Hospital, Fujian Medical University, Fuzhou, China.
Mo Chen, Department of Liver Surgery, Renji Hospital, Shanghai Jiao Tong University, Shanghai, China.
Zhengxin Wang, Department of General Surgery, Huashan Hospital, Fudan University, Shanghai, China.
Chuan-Ming Hao, Department of Nephrology, Huashan Hospital, Fudan University, Shanghai, China.
FUNDING
This work was supported by the Natural Science Foundation of China 81930120 to C.-M. H., 81700591 to Y.G. and 81520108006 and 81130075 to C.-M.H.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was performed in accordance with the declaration of Helsinki and the study protocol was approved by the ethics committee of Huashan Hospital, Fudan University (KY2020-061). All of the subjects gave their written informed consent.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Xu X, Nie S, Liu Zet al. . Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015;10:1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uchino S, Bellomo R, Goldsmith Det al. . An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006;34:1913–7. [DOI] [PubMed] [Google Scholar]
- 3. Liangos O, Wald R, O'Bell JWet al. . Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 2006;1:43–51. [DOI] [PubMed] [Google Scholar]
- 4. Wonnacott A, Meran S, Amphlett Bet al. . Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014;9:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoste EA, Bagshaw SM, Bellomo Ret al. . Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- 6. Forni LG, Darmon M, Ostermann Met al. . Renal recovery after acute kidney injury. Intensive Care Med 2017;43:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuhrman DY, Kellum JA. Epidemiology and pathophysiology of cardiac surgery-associated acute kidney injury. Curr Opin Anaesthesiol 2017;30:60–5. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberger C, Eckardt KU. Oxygenation of the transplanted kidney. Semin Nephrol 2019;39:554–66. [DOI] [PubMed] [Google Scholar]
- 9. Rossi AP, Vella JP. Acute kidney disease after liver and heart transplantation. Transplantation 2016;100:506–14. [DOI] [PubMed] [Google Scholar]
- 10. Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol 2018;13:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan Y, Wang SR, Huang XZet al. . Nicotinamide mononucleotide, an NAD(+) precursor, rescues age-Associated susceptibility to AKI in a sirtuin 1-dependent manner. J Am Soc Nephrol 2017;28:2337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poyan Mehr A, Tran MT, Ralto KMet al. . De novo NAD(+) biosynthetic impairment in acute kidney injury in humans. Nat Med 2018;24:1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faivre A, Katsyuba E, Verissimo Tet al. . Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol Dial Transplant 2021;36:60–8. [DOI] [PubMed] [Google Scholar]
- 14. Ralto KM, Rhee EP, Parikh SM. NAD(+) homeostasis in renal health and disease. Nat Rev Nephrol 2020;16:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 2015;22:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran MT, Zsengeller ZK, Berg AHet al. . PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016;531:528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He W, Wang Y, Zhang MZet al. . Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 2010;120:1056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 20. Chini CCS, Peclat TR, Warner GMet al. . CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat Metab 2020;2:1284–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faivre A, Katsyuba E, Verissimo Tet al. . Differential role of nicotinamide adenine dinucleotide deficiency in acute and chronic kidney disease. Nephrol Dial Transplant 2021;36:60–8. doi: 10.1093/ndt/gfaa124 [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Su X, Quinn WJ IIIet al. . Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 2018;27:1067–80.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsyuba E, Mottis A, Zietak Met al. . De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 2018;563:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howard SC, McCormick J, Pui CHet al. . Preventing and managing toxicities of high-dose methotrexate. Oncologist 2016;21:1471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong V, Nadim MK, Karvellas CJ. Post-liver transplant acute kidney injury. Liver Transpl 2021;27:1653–64. [DOI] [PubMed] [Google Scholar]
- 26. Shi H, Enriquez A, Rapadas Met al. . NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med 2017;377:544–52. [DOI] [PubMed] [Google Scholar]
- 27. Schefold JC, Zeden JP, Fotopoulou Cet al. . Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009;24:1901–8. [DOI] [PubMed] [Google Scholar]
- 28. Braidy N, Guillemin GJ, Mansour Het al. . Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J 2011;278:4425–34. [DOI] [PubMed] [Google Scholar]
- 29. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa K, Wakino S, Simic Pet al. . Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 2013;19:1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Claria J, Moreau R, Fenaille Fet al. . Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology 2019;69:1686–701. [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, James MT. Acute kidney injury. Ann Intern Med 2017;167:ITC66–80. [DOI] [PubMed] [Google Scholar]
- 33. Wilson T, Quan S, Cheema Ket al. . Risk prediction models for acute kidney injury following major noncardiac surgery: systematic review. Nephrol Dial Transplant 2016;31:231–40. [DOI] [PubMed] [Google Scholar]
- 34. Koyner JL, Adhikari R, Edelson DPet al. . Development of a multicenter ward-Based AKI prediction model. Clin J Am Soc Nephrol 2016;11:1935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kashani K, Al-Khafaji A, Ardiles Tet al. . Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.