Abstract
Background
Optimal intraoperative positive end-expiratory pressure (PEEP) improves patient outcomes. Pulse oximetry has been used to determine the lung opening and closing pressures. Therefore, we hypothesized that intraoperative optimal PEEP obtained by titrating inspiratory oxygen fraction (FiO2) guided with pulse oximetry could improve perioperative oxygenation.
Methods
Forty-six males undergoing elective robotic-assisted laparoscopic prostatectomy were randomly assigned to either the optimal PEEP group (group O; n=23) or the fixed PEEP of 5 cmH2O group (group C; n=23). Optimal PEEP, defined as the PEEP with the lowest FiO2 or 0.21 to maintain SpO2 greater than or equal to 95%, was obtained in both groups after placing the patients in the Trendelenburg position and conducting intraperitoneal insufflation. Optimal PEEP was maintained for patients in group O. A PEEP of 5 cmH2O intraoperatively was maintained for patients in group C. Both groups were extubated in a semisitting position once the extubation criteria were met. The primary outcome was the arterial oxygen partial pressure (PaO2) divided by the inspiratory oxygen fraction (FiO2) prior to extubation. The secondary outcome was the incidence of postoperative hypoxemia (SpO2 less than 92% on room air after extubation) in the postanesthesia care unit (PACU).
Results
The median optimal PEEP was 16 cmH2O (IQR 12–18). The PaO2/FiO2 prior to extubation was significantly higher in group O than in group C (77.0±4.9 kPa vs. 60.6±5.9 kPa; P=0.04). PaO2/FiO2 was also significantly higher in group O 30 minutes after extubation (57.6±1.9 vs. 46.6±1.8 kPa; P=0.01). The incidence of hypoxemia on room air in the PACU was significantly lower in group O than in group C (4.3% vs. 30.4%; P=0.02).
Conclusions
Intraoperative optimal PEEP can be achieved by a titration of FiO2 guided with SpO2. Maintaining intraoperative optimal PEEP improves intraoperative oxygenation and reduces the incidence of postoperative hypoxemia.
Trial Registration
The study was prospectively registered on September 10, 2021, in the Chinese Clinical Trial Registry (identifier: ChiCTR2100051010).
Keywords: Positive end-expiratory pressure (PEEP), pulse oximetry, fraction of inspiratory oxygenation, oxygenation index, robot-assisted laparoscopic prostatectomy
Highlight box.
Key findings
• Individualized optimal positive end-expiratory pressure (PEEP) can be achieved with equipment available for anesthesia by titrating the PEEP level with FiO2 guided by SpO2. Maintaining the optimal PEEP level improves intraoperative oxygenation and reduces the incidence of hypoxemia immediately after surgery.
What is known and what is new?
• The optimal PEEP can improve the postoperative outcome. However, obtaining the optimal PEEP requires sophisticated equipment.
• The optimal PEEP can be achieved with FiO2 guided by SpO2 using existing equipment in any modern anesthesia site, which has significant translational value for daily anesthetic practice.
What is the implication, and what should change now?
• The method we used in this study to obtain the optimal PEEP was simple, practical, and improved the outcomes. Clinicians can adopt it to improve the quality of care.
Introduction
Optimal intraoperative positive end-expiratory pressure (PEEP) has been demonstrated to improve patient outcomes (1,2). However, the optimal PEEP differs among individuals, and an individual’s optimal PEEP is also affected by positioning, muscle paralysis, and several other factors (3,4). The common application of a fixed PEEP often leads to either lung overinflation or atelectasis. Therefore, optimal PEEP should be individualized and adjusted dynamically according to each patient’s needs (5). Several techniques have been used to determine the optimal PEEP (6-10). For example, electrical impedance tomography (EIT) can be performed at the bedside (5,11,12). However, the application of this technique requires special training, which increases the workload of the care team, and the cost efficiency of this procedure remains to be determined. Chest computed tomography (CT) is the gold-standard technique for assessing lung inflation (13). However, it is not feasible for use at the bedside, it exposes patients to X-rays, and its cost-effectiveness is unfavorable. Transpulmonary pressure is another alternative that can be used at bedside and is potentially cost-effective (14). However, it requires special training and additional equipment to measure transpulmonary pressure. In contrast, lung opening or closing pressure can be used to assess and calculate intrapulmonary shunts (15). Normally, the physiologic shunt is set at approximately 5%. If arterial blood oxygen saturation is greater than 97% on room air, the intrapulmonary shunt is estimated to be less than 7% (16). Therefore, this method can be used to assess the fraction of intrapulmonary shunts and subsequently estimate the optimal PEEP (17,18).
Recently, Ferrando et al. (19) reported that optimal PEEP could be obtained via titration by administering a minimal fraction of inspiratory oxygen (FiO2) with the guidance of pulse oximetry (SpO2) and measuring transpulmonary pressure in patients who were anesthetized. The authors found that the optimal PEEP values obtained using the two methods were comparable. Another study demonstrated that SpO2 could be used to determine the individualized lung opening and closing pressures in patients undergoing anesthesia and mechanical ventilation (20). We thus hypothesized that the optimal PEEP could be obtained with titration of the intraoperative PEEP levels and FiO2 with SpO2 guidance. Our secondary hypothesis was that the maintenance of the optimal PEEP derived from this method can improve intraoperative oxygenation and reduce the incidence of postoperative hypoxemia. We tested our hypothesis in patients undergoing robotic-assisted laparoscopic prostatectomy (RALP). We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/rc).
Methods
Ethics
This single-center, 2-arm, parallel, randomized controlled study was conducted from May 6, 2021, to October 10, 2021. It was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fudan University Shanghai Cancer Center (No. IRB2010225-11), and informed consent was obtained from all the patients. This study was also registered in the Chinese Clinical Trial Registry on September 10, 2021 (identifier: ChiCTR2100051010; principal investigator: JZ).
Inclusion and exclusion criteria
Between May 6, 2021, and October 10, 2021, adult patients aged 18 years or older who were scheduled for elective RALP under general anesthesia and who presented with an American Society of Anesthesiologists (ASA) physical status of I–III were recruited for this study. Patients with acute or chronic respiratory disorders, including chronic obstructive pulmonary disease and asthma, pulmonary hypertension, neuromuscular disease, and/or preoperative SpO2 less than 95% on room air were excluded. The patient enrollment process is illustrated in Figure 1.
Figure 1.
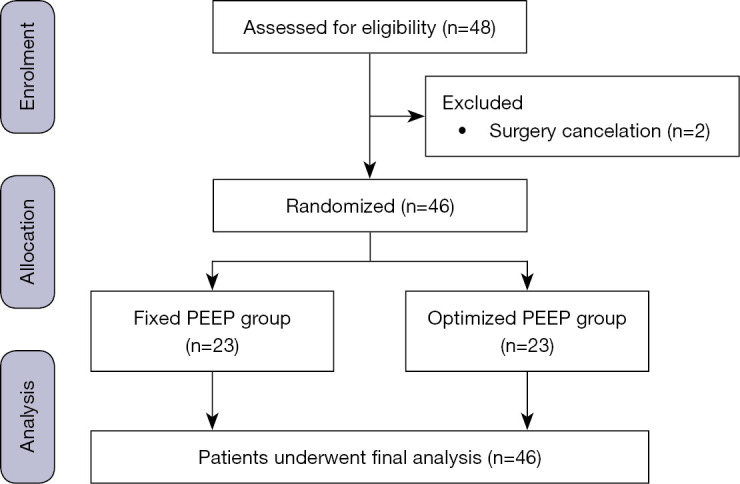
Flowchart of patient enrollment. PEEP, positive end-expiratory pressure.
Anesthesia management
Each patient’s general demographic and medical characteristics were abstracted from medical records. The characteristics investigated were sex, age, body mass index (BMI), predicted body weight (PBW), ASA classification, medical history, and preoperative SpO2 on room air. Intravenous access was established upon arrival at the operating room. Routine monitoring for general anesthesia was performed, including electrocardiography, noninvasive blood pressure, SpO2, capnography, and temperature. A radial arterial line was established to continuously measure arterial blood pressure and intermittent blood sampling for arterial blood gas (ABG) analysis. Patients were preoxygenated as usual at an O2 flow rate of 6 L·minute–1 until their expiratory oxygen concentration reached 80% or higher. Anesthetic induction was conducted with an intravenous targeted control infusion (TCI) of 4 µg·mL–1 of propofol (Marsh mode), 0.3 µg·kg–1 of sufentanil, and 0.6 mg·kg–1 of rocuronium (21). A 7.0-sized tracheal tube was inserted, and correct placement was confirmed with auscultation and the presence of bilateral equal breath sounds. General anesthesia was maintained with a continuous TCI infusion of 3 to 4 µg·mL–1 propofol and 1 to 2 ng·mL–1 remifentanil (Minto mode) as well as the intermittent administration of rocuronium to maintain adequate muscle paralysis.
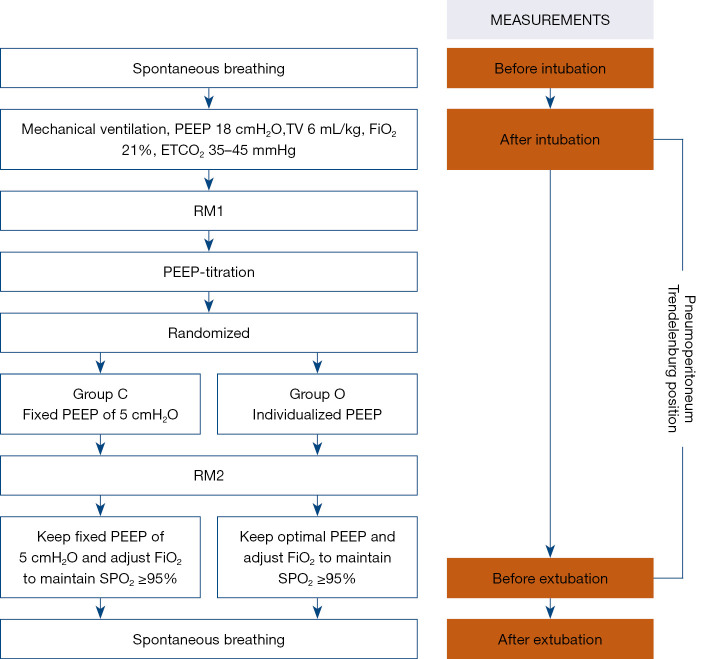
Study protocol
The study protocol is summarized in Figure 2. After tracheal intubation, mechanical ventilation was conducted with pressure-regulated volume-controlled ventilation using an operating room ventilator (Flow-I, Maquet Inc., Heidelberg, Germany). The ventilation was set at a tidal volume 6 mL·kg–1 and was initiated with a FiO2 of 1.0 to 0.21, a PEEP of 18 cmH2O, and a respiratory rate of 12–15 beats per minute to keep the end-tidal CO2 partial pressure between 35 and 45 mmHg. After the patients were placed in the Trendelenburg position and peritoneal insufflation was performed, they received the first recruitment maneuver (RM1) of 40 cmH2O for 15 seconds followed by PEEP at 18 cmH2O. This procedure was similar to that of a previous study demonstrating that the maximal optimal PEEP was not greater than 18 cmH2O (4). If the peak inspiratory pressure was greater than 40 cmH2O at a PEEP of 18 cmH2O, the participant’s study was terminated. The target SpO2 was 95–96%.
Figure 2.
Experimental protocol for PEEP titration. Group C, control group with a fixed PEEP of 5 cmH2O; group O, optimized PEEP group with an individualized PEEP at which SpO2 was maintained at 95–96% with minimal FiO2; TV, tidal volume; ETCO2, end-expiratory carbon dioxide partial pressure; SpO2, pulse oxygen saturation; RM, recruitment maneuver; PACU, postanaesthesia care unit. PEEP, positive end-expiratory pressure; PaO2, oxygen partial pressure in arterial blood; FiO2, fraction of inspired oxygen.
The PEEP titration process is shown in Figure S1. If the SpO2 was 95–96% with a FiO2 of 0.21 and a PEEP of 18 cmH2O, the optimal PEEP was 18 cmH2O; this was kept constant throughout the procedure until extubation. If the SpO2 was greater than 96%, the PEEP was reduced by 2 cmH2O stepwise, with each step lasting for 5 minutes until SpO2 dropped below 95%. Then, PEEP was increased up to 18 cmH2O in reverse order in the same stepwise manner until the intended SpO2 was reached and remained at a steady saturation of 95–96%. At a PEEP of 18 cmH2O, if the SpO2 was lower than 95%, the FiO2 was incrementally increased by 0.05 per step; each step lasted for 5 minutes in order to achieve an SpO2 of 95–96%. If PEEP was increased to 18 cmH2O and FiO2 was measured at 1.0 but the SpO2 remained lower than 95%, the study was terminated. The PEEP level at the minimal FiO2 necessary to maintain a SpO2 of 95–96% was considered the optimal PEEP. Once the optimal PEEP was achieved, patients randomized to group C received a PEEP of 5 cmH2O intraoperatively or were maintained within group O, thus maintaining optimal PEEP until extubation. Patients in both groups were extubated in the postanesthesia care unit (PACU) in the semisitting position once they met the criteria for extubation according to the judgment of their medical care team.
For both groups, intraoperative pulmonary dynamic compliance (Cdyn), PEEP, FiO2 (i.e., real-time FiO2 obtained from the gas analyzer within the anesthesia machine), driving pressure (defined as plateau pressure minus PEEP), and plateau pressure (determined as the pressure at the end of inspiration as displayed on the anesthesia machine) were recorded. Intermittent ABG analysis was performed in order to verify the accuracy of the SpO2 readings and to calculate the alveolar-arterial gradient [P(A-a)O2], while the respiratory rate was adjusted to maintain the PaCO2 in the range of 35–45 mmHg. In the PACU, vital signs and ABG results were recorded at 5, 10, and 30 minutes after extubation, and supplementary O2 was provided to the patients via a nasal cannula if the SpO2 was below 92%.
Statistical analysis
Intraoperative PaO2/FiO2 was reported as 55.7±10.9 kPa before extubation in patients undergoing RALP (4). We assumed that there were 10 kPa differences between the 2 groups, with a variance of 10.9 kPa, a statistical power of 80%, and a 2-sided α significance level of 0.05. A sample size of at least 18 patients in each arm was required to test our hypothesis. Considering a dropout rate of 30%, a total of 24 patients for each group and 48 patients in total were enrolled. Randomization was performed using a minimization randomization method as previously described (22). Patients were stratified by age (<65 vs. ≥65 years) and BMI (<24 vs. ≥24 kg·m–2) to test differences in age and BMI distribution. The randomization was performed via MinimPy2 version 2.0 (OSDN, Columbus, OH, USA). Randomization was performed the day before surgery by a research team member (LLG) who was blinded to the trial condition. The data were managed and analyzed by an independent researcher (PL).
Continuous variables are presented as means ± SD or medians with IQR according to whether the distribution was normal, while categorical variables are presented as counts and percentages. The χ2 test was used to compare differences in patient characteristics between the 2 groups. Unpaired t tests were used to compare differences in oxygen indices, driving pressure, and Cdyn at different time points. Repeated-measures analysis of variance (ANOVA) was used to compare differences in PaO2/FiO2, driving pressure, and Cdyn under mechanical ventilation prior to extubation. Differences in vital parameters, vasoactive medication dosage, and incidence of complications were tested via unpaired t tests. The Wilcoxon/Mann-Whitney test was used when the data were not normally distributed. Statistical analysis was performed using SPSS 24.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 8.0 (GraphPad Inc., San Diego, CA, USA). Statistical significance was set at P<0.05.
Results
Clinical characteristics
A total of 48 patients were initially enrolled in this study, and 2 patients were excluded from the study because their operations were canceled. Therefore, a total of 46 patients completed the study and underwent a final analysis (Figure 1). The clinical characteristics of the 2 groups are shown in Table 1, while the Perioperative data are shown in Table 2.
Table 1. Patient clinical characteristics.
| Characteristics | Group C (n=23) | Group O (n=23) |
|---|---|---|
| Age (years) | ||
| <65 | 10 (43.5) | 8 (34.8) |
| ≥65 | 13 (56.5) | 15 (65.2) |
| BMI (kg/m2) | ||
| <24 | 12 (52.2) | 13 (56.5) |
| ≥24 | 11 (47.8) | 10 (43.5) |
| ASA physical status | ||
| I | 2 (8.7) | 3 (13.0) |
| II | 21 (91.3) | 20 (87.0) |
| Smoking status | ||
| Never | 15 (65.3) | 14 (60.9) |
| Ever | 3 (13.0) | 4 (17.4) |
| Current | 5 (21.7) | 5 (21.7) |
| Comorbidity | ||
| Hypertension | 8 (34.8) | 6 (26.1) |
| Diabetes | 3 (13.0) | 4 (17.4) |
Data are presented as number (%). Group C, control group with a fixed PEEP of 5 cmH2O; group O, optimized PEEP group with an individualized PEEP at which SpO2 was maintained at 95–96% with minimal FiO2; BMI, body mass index; ASA, American Society of Anesthesiologists; PEEP, positive end-expiratory pressure.
Table 2. Intraoperative data.
| Variables | Group C (n=23) | Group O (n=23) | P value |
|---|---|---|---|
| Anesthesia duration (min) | 227.3±7.8 | 218.7±5.6 | 0.38 |
| Surgery duration (min) | 169.1±6.4 | 174.1±4.2 | 0.47 |
| Total amount of fluid infusion (mL) | 2,070±56.7 | 1,904±68.4 | 0.07 |
| Blood loss (mL) | 118.7±10.0 | 97.8±5.9 | 0.08 |
| Urinary output (mL) | 347.8±37.7 | 358.7±51.7 | 0.87 |
| Pneumoperitoneum pressure (mmHg) | 14 | 14 | – |
| Vasoactive injections | |||
| Received medication, n (%) | 21 (91.3) | 22 (95.7) | 0.55 |
| Ephedrine (mg) | 6.8±1.0 | 7.2±1.2 | 0.80 |
| Phenylephrine (μg) | 194.8±44.9 | 183.0±52.0 | 0.87 |
| P(A-a) O2 pre-extubation (kPa) | 9.0±2.0 | 3.62±0.9 | 0.01 |
| SpO2 <92% at PACU on room air | 7 (30.4) | 1 (4.3) | 0.02 |
Data are presented as mean ± SD or number (%). Group C, control group with a fixed PEEP of 5 cmH2O; group O, optimized PEEP group with an individualized PEEP at which SpO2 was maintained at 95–96% with minimal FiO2; P(A-a) O2, alveolar-arterial gradient; PACU, postanesthesia care unit.
Optimal PEEP level
Among all patients in both groups, the median optimal PEEP was 16 cmH2O (IQR 12–18 cmH2O). The FiO2 needed to obtain the optimal PEEP was 0.21±0.03. The details of the titration process are presented in Table S1. The time allotted to complete the titration of the optimal PEEP was half an hour or less.
Primary outcome
The PaO2/FiO2 was statistically significantly higher in group O than in group C prior to extubation (77.0±4.9 vs. 60.6±5.9 kPa; P=0.04; Figure 3A).
Figure 3.
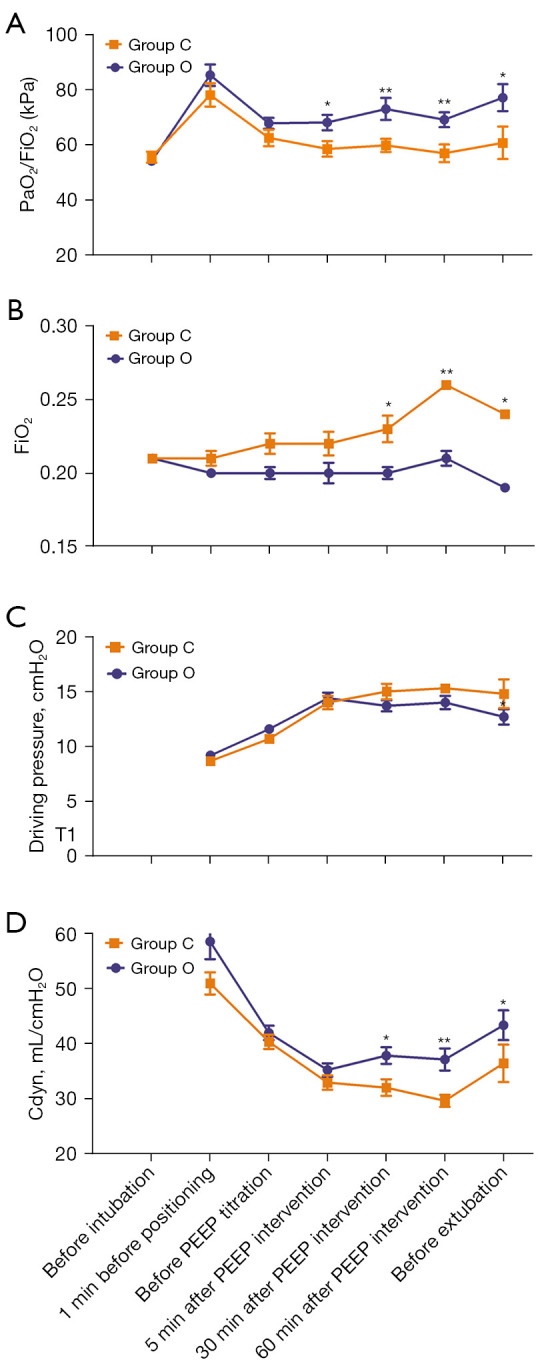
Time course of respiratory mechanics. (A) PaO2/FiO2. (B) Time course of FiO2. (C) Driving pressure. (D) Cdyn. *P<0.05; **P<0.01. Group C, control group with a fixed PEEP of 5 cmH2O; group O, optimized PEEP group with an individualized PEEP at which SpO2 was maintained at 95–96% with minimal FiO2; PEEP, positive end-expiratory pressure; PaO2, partial pressure of oxygen in arterial blood; FiO2, fraction of inspired oxygen; Cdyn, dynamic compliance.
Secondary outcomes
The respiratory mechanics corresponding to FiO2 are shown in Figure 3B. There was no statistically significant difference in the driving pressure between the 2 groups (Figure 3C). The Cdyn was higher in group O than in group C prior to extubation (43.4±2.7 mL·cmH2O–1 vs. 36.5±3.4 mL·cmH2O–1; P=0.032; Figure 3D). Intraoperative respiratory and blood parameters at 3 time points during the PEEP intervention are shown in Table S2.
Postoperative hypoxemia was defined as SpO2 less than 92% on room air detected within 30 min after extubation in the PACU. The incidence of hypoxemia was significantly lower in group O than in group C (4.3% vs. 30.4%; P=0.02; Table 2). The P(A-a) O2 in group C (9.0±2.0 kPa) was statistically significantly higher than that in group O (3.62±0.9 kPa; P=0.01). PaO2/FiO2 ratios at 3 time points in the PACU after extubation are shown in Figure S2.
Discussion
The main findings of this study are as follows: (I) the intraoperative optimal PEEP level could be achieved by titrating PEEP and FiO2 guided by the SpO2 readout in patients likely requiring a high PEEP; (II) maintaining optimal PEEP improved intraoperative oxygenation and reduced FiO2 to maintain normoxemia; and (III) the benefit of intraoperative optimal PEEP was sustained postoperatively in terms of reductions in the incidence of postoperative hypoxemia.
Our results confirmed the observations from a previous study and demonstrated that using equipment for routine anesthetic care could obtain an optimal PEEP level (4). This technique has the substantial advantage of being simple to use. In this study, the titration of PEEP and FiO2 was started simultaneously as the surgery progressed, and the duration of the PEEP titration was 20 to 30 minutes. Therefore, clinicians could obtain individualized optimal PEEP levels without interrupting or prolonging the surgery. This technique does not require additional training or equipment, such as an intraesophageal balloon to calculate transpulmonary pressure (23) or EIT to measure lung aeration (9,24). All the equipment needed to obtain and maintain the optimal PEEP is readily available in any modern operating room or anesthesia site. In addition, PEEP can be constantly reassessed and adjusted intraoperatively to maintain the optimal PEEP when respiratory mechanics change due to changes in the patient’s position or intra-abdominal insufflation pressure. A new algorithm may be developed using a closed-loop system to assess PEEP and automatically determine the individual’s optimal PEEP using this technique.
We tested our hypothesis in patients who underwent RALP because this population is more likely to require a high PEEP to minimize intraoperative atelectasis (25). RALP surgery is performed within the pneumoperitoneum with an intra-abdominal pressure of approximately 15 mmHg. During RALP surgery, the patient is placed in a steep Trendelenburg position (approximately 30 degrees) intraoperatively for over 3 to 4 hours (26,27). Therefore, patients are more prone to perioperative atelectasis formation if the PEEP is insufficiently high to counteract the reduction in functional residual capacity (28). However, in our institute, a PEEP of 5 cmH2O for patients undergoing RALP is a common practice. There are a few recommendations stating that the PEEP should be higher than 5 cmH2O if the patient is in the Trendelenburg position and/or if there is pneumoperitoneum (29). A PEEP of 5 cmH2O seems lower than that reported in the literature. However, guidelines for selecting the appropriate PEEP level for this patient population are unavailable because there is insufficient literature informing these criteria. Any fixed PEEP level would leave some patients either below or above the optimal PEEP level because of the variation in optimal PEEP is large (4). Furthermore, the optimal PEEP is likely not a constant but rather varies depending on the patient’s physiology and positioning as well as the specific surgical intervention (30). The range of the optimal PEEP observed in this study was between 2 and 18 cmH2O. We encountered a patient who could maintain an SpO2 greater than 95% with a FiO2 of 0.21 even when the PEEP was set at 2 cmH2O. We also performed ABG analysis and confirmed that the SpO2 and arterial hemoglobin oxygen saturation readouts were comparable. This finding indicates that, even with both pneumoperitoneum and a steep Trendelenburg position, this patient had an intrapulmonary shunt of less than 10% with a PEEP of 2 cmH2O (16).
A question remains about whether the optimal PEEP level we obtained was truly the optimal PEEP level because we did not have a means of validation via a chest CT scan or electric impedance tomography. However, although this is a scientifically important question, it may not be clinically important. Specifically, the approach employed in this study may not achieve a true PEEP (with no over or under PEEP). However, the PaO2/FiO2 improved by 27% (77.0/60.6 kPa) in group O vs. group C prior to extubation. Further studies are needed to determine the efficacy of this technique for achieving a true optimal PEEP compared to that obtained with EIT. Nevertheless, using this technique, we achieved clinically relevant improvements in intraoperative oxygenation compared with routine care. The mean FiO2 used to achieve optimal PEEP was 0.21, the SpO2 was 95–96% prior to extubation, and the intrapulmonary shunt was estimated to be less than 10% compared with that of the control group. We chose to titrate the optimal PEEP stepwise and bidirectionally; therefore, we were unlikely to inflate the lung at the optimal PEEP level. This finding suggests that even though the optimal PEEP in the present study may not be a true optimal PEEP, it is likely very close to the true optimal PEEP, and the difference between the two may not have clinical implications. Further studies are needed to assess the agreement of optimal PEEP obtained with the method used in this study as well as other well-established techniques, such as CT scans or EIT.
In our study, despite an observed average reduction of PaO2/FiO2 of about 16.4 kPa compared to the individualized PEEP group, we did not observe any intraoperative hypoxic events in the control group during general anesthesia. We believe this occurred because the participants we enrolled were nonobese patients with healthy lungs, among whom mild or moderate atelectasis might not have detrimental effects on oxygenation. We think this improvement in oxygenation may be significant in obese patients and those with impaired lung function.
A high PEEP level has been suggested to improve lung function and oxygenation (4). However, it also carries the risk of hemodynamic compromise. PEEP commonly affects cardiac function in a complex and often unpredictable fashion. PEEP usually does not change the heart rate; therefore, a decrease in cardiac output is a consequence of a reduction in left ventricular stroke volume (31). Particularly, a high PEEP level can restrict venous flow into the thorax by elevating lung volume and intrathoracic pressure, which reduces the filling of the right ventricle, thereby reducing the left ventricular stroke volume and cardiac output (32). A previous study showed that PEEP did not influence intrapulmonary shunt at a low FiO2 (0.2–0.3), which we set during titration, while it greatly decreased the shunt at an FiO2 higher than 0.3 (33). This finding can be explained by the fact that PEEP increases functional residual capacity (FRC), which can counteract an FRC reduction caused by high FiO2. Volume loading can be used to prevent circulatory depression and pulmonary shunt despite a high PEEP level (34). Therefore, we administered fluid loading to reduce the incidence of hypotension in this study.
It is important to note that the benefit of intraoperative optimal PEEP was sustained postoperatively. This is consistent with previous observations suggesting that an intraoperatively individualized PEEP can reduce postoperative atelectasis (2). However, a recent study showed that intraoperative PEEP only improved intraoperative but not postoperative oxygenation (35). This discrepancy among studies, including our current study, might be due to the different extubation approaches employed in the investigations. It is well known that a patient’s FRC depends on their sedation level, muscle tone, and position (36). In our institution, it is routine practice for patients to be extubated in a semisitting position. We observed the benefit of intraoperative PEEP on postoperative oxygenation when all patients were extubated in the semisitting position. Therefore, patients likely maintained a larger FRC (i.e., closer to the normal value) than did patients extubated in the supine position. This notion requires further validation. However, in a report by Simon et al. (35), the position of the patients during extubation was not stated. In our study, because there were no statistically significant differences between the 2 groups in terms of the consumption of intraoperative and postoperative narcotics and residual sedation levels in the PACU, the reduction in the incidence of postoperative hypoxemia on room air in group O was likely due to a reduction in postoperative atelectasis. Since the sample size was relatively small, we could not determine the effect of intraoperative PEEP on other outcomes, such as the incidence of postoperative pulmonary complications, including reintubation and postoperative pneumonia. This was a limitation of our study that should be addressed in further research and clinical practice. Nevertheless, the intrapulmonary shunt in these patients was an important factor because we chose an SpO2 of 92% or lower as the cutoff for the diagnosis of hypoxemia on room air. If we assume that the hypoxemia was due to the intrapulmonary shunt only and that no hypoxic vasoconstriction was involved, at an SpO2 of 92%, the intrapulmonary shunt was estimated to be approximately 24% using an equation described previously (16). Further studies should be conducted to assess the effect of intraoperative optimal PEEP on outcomes.
In addition to the substantial strengths of this investigation, this study had several limitations. First, we did not validate our observation that the optimal PEEP achieved with this technique was indeed the true optimal PEEP. Validation using EIT or transpulmonary pressure will be important for assessing the sensitivity and specificity of this method. Second, the American Food and Drug Administration (FDA) recently determined that pulse oximetry is inaccurate for reporting hemoglobin oxygen saturation. However, in this study, we validated SpO2 readings using ABG analysis. In addition, we used the same brand of oximetry in both groups of patients. Therefore, this potential inaccuracy did not affect our conclusions. Third, it is possible that the PEEP obtained in our study was above the true optimal PEEP. However, we allowed for the descending and ascending stepwise titration of FiO2 and PEEP. Therefore, the presence of an over and under PEEP at the level that would affect outcomes was possible, but unlikely. Although we may not achieve a perfectly individualized optimal PEEP, in practical terms, the PEEP value achieved in our study was likely close to the true value when the intrapulmonary shunt was less than 10%. Finally, the measure of static compliance (Cstat) would be better than that of Cdyn for our study. Cdyn describes the compliance measured during breathing, which involves a combination of lung compliance and airway resistance. In contrast, Cstat describes pulmonary compliance when there is no airflow, which is determined at the end of exhalation in conditions of a complete absence of flow in the airways. Therefore, the measurement of Cdyn is influenced by the resistance of airways an, compared with Cstat, reflects the extensibility of the lung tissue to a smaller degree.
Conclusions
Individualized optimal PEEP can be achieved with equipment available for anesthesia by titrating PEEP and FiO2 guided by SpO2. Maintaining an intraoperative optimal PEEP level improved intraoperative oxygenation and reduced the incidence of postoperative hypoxemia in patients likely to require a high intraoperative PEEP. Since the method we used in this study to obtain the optimal PEEP was simple and practical, hopefully, clinicians will be willing to adopt it and improve their quality of care.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank the Local Ethics Committee of the Fudan University Shanghai Cancer Center for their review and revision of important intellectual content. The authors would also like to thank the urologists at the Fudan University Shanghai Cancer Center for their help and support on this project.
Funding: This work was funded by a grant from the Shanghai Science and Technology Committee (No. 20Y11906200).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fudan University Shanghai Cancer Center (No. IRB2010225-11), and informed consent was obtained from all the patients.
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4357/coif). The authors have no conflicts of interest to declare.
(English Language Editors: C. Mullens and J. Gray)
References
- 1.Meininger D, Byhahn C, Mierdl S, et al. Positive end-expiratory pressure improves arterial oxygenation during prolonged pneumoperitoneum. Acta Anaesthesiol Scand 2005;49:778-83. 10.1111/j.1399-6576.2005.00713.x [DOI] [PubMed] [Google Scholar]
- 2.Pereira SM, Tucci MR, Morais CCA, et al. Individual Positive End-expiratory Pressure Settings Optimize Intraoperative Mechanical Ventilation and Reduce Postoperative Atelectasis. Anesthesiology 2018;129:1070-81. 10.1097/ALN.0000000000002435 [DOI] [PubMed] [Google Scholar]
- 3.Briassoulis G, Briassoulis P, Ilia S. The best PEEP or the optimal PEEP or the piece PEEP of the mechanical power puzzle?. Crit Care 2022;26:298. 10.1186/s13054-022-04162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girrbach F, Petroff D, Schulz S, et al. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: a prospective, randomised controlled clinical trial. Br J Anaesth 2020;125:373-82. 10.1016/j.bja.2020.05.041 [DOI] [PubMed] [Google Scholar]
- 5.Nestler C, Simon P, Petroff D, et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 2017;119:1194-205. 10.1093/bja/aex192 [DOI] [PubMed] [Google Scholar]
- 6.Grivans C, Stenqvist O. Gas distribution by EIT during PEEP inflation: PEEP response and optimal PEEP with lowest trans-pulmonary driving pressure can be determined without esophageal pressure during a rapid PEEP trial in patients with acute respiratory failure. Physiol Meas 2022. 10.1088/1361-6579/ac8ccc [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Xu F, Li W, et al. Driving Pressure-Guided Individualized Positive End-Expiratory Pressure in Abdominal Surgery: A Randomized Controlled Trial. Anesth Analg 2021;133:1197-205. 10.1213/ANE.0000000000005575 [DOI] [PubMed] [Google Scholar]
- 8.Girrbach F, Zeutzschel F, Schulz S, et al. Methods for Determination of Individual PEEP for Intraoperative Mechanical Ventilation Using a Decremental PEEP Trial. J Clin Med 2022;11:3707. 10.3390/jcm11133707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankman P, Shono A, Hermans BJ, et al. Detection of optimal PEEP for equal distribution of tidal volume by volumetric capnography and electrical impedance tomography during decreasing levels of PEEP in post cardiac-surgery patients. Br J Anaesth 2016;116:862-9. 10.1093/bja/aew116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian W, Chen W, Chao Y, et al. Application of dead space fraction to titrate optimal positive end-expiratory pressure in an ARDS swine model. Exp Ther Med 2017;13:1572-7. 10.3892/etm.2017.4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbas CSV, Amato MBP. Electrical Impedance Tomography to Titrate PEEP at Bedside in ARDS. Respir Care 2022;67:1061-3. 10.4187/respcare.10360 [DOI] [PubMed] [Google Scholar]
- 12.Frerichs I, Amato MB, van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017;72:83-93. 10.1136/thoraxjnl-2016-208357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malbouisson LM, Muller JC, Constantin JM, et al. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1444-50. 10.1164/ajrccm.163.6.2005001 [DOI] [PubMed] [Google Scholar]
- 14.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure-Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free From Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019;321:846-57. 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cove ME, Pinsky MR, Marini JJ. Are we ready to think differently about setting PEEP? Crit Care 2022;26:222. 10.1186/s13054-022-04058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roe PG, Jones JG. Analysis of factors which affect the relationship between inspired oxygen partial pressure and arterial oxygen saturation. Br J Anaesth 1993;71:488-94. 10.1093/bja/71.4.488 [DOI] [PubMed] [Google Scholar]
- 17.Mietto C, Malbrain ML, Chiumello D. Transpulmonary pressure monitoring during mechanical ventilation: a bench-to-bedside review. Anaesthesiol Intensive Ther 2015;47 Spec No:s27-37. 10.5603/AIT.a2015.0065 [DOI] [PubMed] [Google Scholar]
- 18.Lundin S, Grivans C, Stenqvist O. Transpulmonary pressure and lung elastance can be estimated by a PEEP-step manoeuvre. Acta Anaesthesiol Scand 2015;59:185-96. 10.1111/aas.12442 [DOI] [PubMed] [Google Scholar]
- 19.Ferrando C, Tusman G, Suarez-Sipmann F, et al. Individualized lung recruitment maneuver guided by pulse-oximetry in anesthetized patients undergoing laparoscopy: a feasibility study. Acta Anaesthesiol Scand 2018;62:608-19. 10.1111/aas.13082 [DOI] [PubMed] [Google Scholar]
- 20.Tusman G, Groisman I, Fiolo FE, et al. Noninvasive monitoring of lung recruitment maneuvers in morbidly obese patients: the role of pulse oximetry and volumetric capnography. Anesth Analg 2014;118:137-44. 10.1213/01.ane.0000438350.29240.08 [DOI] [PubMed] [Google Scholar]
- 21.Thomson AJ, Morrison G, Thomson E, et al. Induction of general anaesthesia by effect-site target-controlled infusion of propofol: influence of pharmacokinetic model and ke0 value. Anaesthesia 2014;69:429-35. 10.1111/anae.12597 [DOI] [PubMed] [Google Scholar]
- 22.Han B, Enas NH, McEntegart D. Randomization by minimization for unbalanced treatment allocation. Stat Med 2009;28:3329-46. 10.1002/sim.3710 [DOI] [PubMed] [Google Scholar]
- 23.Keller SP, Fessler HE. Monitoring of oesophageal pressure. Curr Opin Crit Care 2014;20:340-6. 10.1097/MCC.0000000000000092 [DOI] [PubMed] [Google Scholar]
- 24.Sella N, Pettenuzzo T, Zarantonello F, et al. Electrical impedance tomography: A compass for the safe route to optimal PEEP. Respir Med 2021;187:106555. 10.1016/j.rmed.2021.106555 [DOI] [PubMed] [Google Scholar]
- 25.Kalmar AF, Foubert L, Hendrickx JF, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth 2010;104:433-9. 10.1093/bja/aeq018 [DOI] [PubMed] [Google Scholar]
- 26.Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol 2012;78:596-604. [PubMed] [Google Scholar]
- 27.Awad H, Walker CM, Shaikh M, et al. Anesthetic considerations for robotic prostatectomy: a review of the literature. J Clin Anesth 2012;24:494-504. 10.1016/j.jclinane.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Yurtdaş G, Akdevelioğlu Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J Am Coll Nutr 2020;39:371-82. 10.1080/07315724.2019.1657515 [DOI] [PubMed] [Google Scholar]
- 29.Shono A, Katayama N, Fujihara T, et al. Positive End-expiratory Pressure and Distribution of Ventilation in Pneumoperitoneum Combined with Steep Trendelenburg Position. Anesthesiology 2020;132:476-90. 10.1097/ALN.0000000000003062 [DOI] [PubMed] [Google Scholar]
- 30.Sahetya SK, Goligher EC, Slutsky AS. Searching for the Optimal PEEP in Patients Without ARDS: High, Low, or in Between? JAMA 2020;324:2490-2. 10.1001/jama.2020.23067 [DOI] [PubMed] [Google Scholar]
- 31.Luecke T, Pelosi P. Clinical review: Positive end-expiratory pressure and cardiac output. Crit Care 2005;9:607-21. 10.1186/cc3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 1948;152:162-74. 10.1152/ajplegacy.1947.152.1.162 [DOI] [PubMed] [Google Scholar]
- 33.Oliven A, Taitelman U, Zveibil F, et al. Effect of positive end-expiratory pressure on intrapulmonary shunt at different levels of fractional inspired oxygen. Thorax 1980;35:181-5. 10.1136/thx.35.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erlandsson K, Odenstedt H, Lundin S, et al. Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery. Acta Anaesthesiol Scand 2006;50:833-9. 10.1111/j.1399-6576.2006.01079.x [DOI] [PubMed] [Google Scholar]
- 35.Simon P, Girrbach F, Petroff D, et al. Individualized versus Fixed Positive End-expiratory Pressure for Intraoperative Mechanical Ventilation in Obese Patients: A Secondary Analysis. Anesthesiology 2021;134:887-900. 10.1097/ALN.0000000000003762 [DOI] [PubMed] [Google Scholar]
- 36.Wahba RW. Perioperative functional residual capacity. Can J Anaesth 1991;38:384-400. 10.1007/BF03007630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as