Abstract
Nonalcoholic steatohepatitis (NASH) is a common chronic liver disease that may advance to fibrosis and lead to mortality; however, no pharmacotherapy is currently available. We tested the hypothesis that inhibition of both the sodium–glucose cotransporters 1 and 2 with licogliflozin would lead to improvement in NASH. A total of 107 patients with phenotypic or histologic NASH were randomized (1:2:2) to receive oral administration of either placebo (n = 21), licogliflozin 30 mg (n = 43) or 150 mg (n = 43) once daily for 12 weeks. Licogliflozin 150 mg showed a significant 32% (80% confidence interval (CI): 21–43%; P = 0.002) placebo-adjusted reduction in serum alanine aminotransferase after 12 weeks of treatment, the primary endpoint of the study. However, the 30 mg dose of licogliflozin did not meet the primary endpoint (placebo-adjusted reduction 21% (80% CI: 7–32%; P = 0.061)). Diarrhea occurred in 77% (33 of 43), 49% (21 of 43) and 43% (9 of 21) of patients treated with licogliflozin 150 mg, 30 mg and placebo, respectively, which was mostly mild in severity. No other major safety concerns were identified. Treatment with 150 mg licogliflozin led to reductions in serum alanine aminotransferase in patients with NASH. Studies of longer duration and in combination with drugs that have different mechanisms of action are needed to validate these findings and to define a role of licogliflozin as a therapeutic option for NASH. ClinicalTrials.gov identifier: NCT03205150.
Nonalcoholic fatty liver disease (NAFLD) is a common chronic liver disease affecting nearly 25% of adults globally1. Nonalcoholic steatohepatitis (NASH) is a more severe form of NAFLD characterized by hepatic steatosis and inflammation and occurs in up to 30% of patients with diagnosed NAFLD2,3. Moreover, NASH can progress to advanced fibrosis, leading to cirrhosis and/or hepatocellular carcinoma and can result in the need for liver transplantation or in death4,5.
Metabolic disorders such as obesity and type 2 diabetes (T2D), are associated with a high risk of developing NAFLD and NASH6. Previous studies focused on lifestyle modification or bariatric surgery have shown that weight loss improves NASH, including its histologic characteristics7,8. In addition, the presence of obesity and insulin resistance is associated with progression of fibrotic disease5. Currently, there are no approved therapies for the treatment of NAFLD and/or NASH, and management usually involves weight loss through dietary modification and bariatric surgery as a second-line option2,9. Although behavioral interventions remain a cornerstone for the management of NASH, few patients are able to attain and sustain weight loss of the magnitude associated with regression of fibrosis10.
Studies in patients with T2D and NAFLD suggest a potential role for sodium–glucose cotransporter 2 (SGLT2) inhibitors in NASH, based on improvement in serum markers of liver injury, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT), as well as reduction of hepatic fat content11,12. Only a few randomized controlled studies have studied the effect of SGLT2 inhibitors on hepatic steatosis and fibrosis. Shimizu and colleagues13 reported that treatment with dapagliflozin significantly reduced hepatic steatosis over 24 weeks (P = 0.042) compared with the active control group. Improvement in hepatic fibrosis was observed in a subgroup of patients with advanced fibrosis at baseline. Furthermore, a randomized, double-blind, placebo-controlled study evaluated the effect of empagliflozin in patients with NAFLD and without T2DM over 24 weeks. Compared with placebo, reduction in hepatic steatosis was significant only in subgroup of patients with advanced steatosis at baseline (P = 0.035)14.
Licogliflozin is a selective and potent inhibitor of both SGLT1 and SGLT2. Binding of licogliflozin to these transporters leads to blockade of glucose absorption in the intestine (mediated by SGLT1) and reabsorption in the kidney (90% mediated by SGLT2 and to a smaller extent by SGLT1)15,16. We have previously shown that in patients with obesity and T2DM, licogliflozin causes significant weight loss9. Additionally, we noted reductions in glycated hemoglobin (HbA1c) and improved insulin sensitivity, as well as increases in the plasma levels of incretin hormones (glucagon-like peptide and peptide YY), which might be driven by increased luminal abundance of unabsorbed glucose in the distal gastrointestinal tract15. The potential for a higher magnitude of weight loss driven by combined renal and gastrointestinal caloric loss, as well as by the increase in incretin levels, suggests that dual inhibition of SGLT1/2 might provide improved efficacy in NASH when compared with SGLT2 inhibitors alone. We hypothesized that the use of licogliflozin in patients with NASH would lead to decreased liver fat content, hepatocyte injury and fibrogenesis. This phase 2a study was performed to evaluate the efficacy and safety of 12 weeks of treatment with licogliflozin in patients with NASH.
Results
Patient population.
A total of 107 patients were enrolled between 13 November 2017 and 7 August 2019, and randomized to receive daily oral doses of placebo (n = 21), licogliflozin 30 mg (n = 43) and licogliflozin 150 mg (n = 43) (Extended Data Fig. 1). Overall, 96 of 107 (89.7%) patients completed the study. A proportion of patients (two in placebo, two in licogliflozin 30 mg and seven in licogliflozin 150 mg arm) discontinued the study due to protocol deviation, patient and/or guardian decision, adverse events (AEs) and loss to follow up. Two patients in the licogliflozin 150 mg arm were excluded from the pharmacodynamic analysis set due to <80% compliance in study treatment administration or nonadherence to time of study-drug administration; all randomized patients were considered for the safety analysis set (Fig. 1).
Fig. 1: Patient disposition.
Two patients in the licogliflozin 150 mg treatment group were excluded from the PD analysis set: one patient was excluded due to <80% compliance in study treatment administration, and the second patient because study treatment was administered in the evening, instead of at lunchtime, throughout the study. BL, baseline; n, number of patients in each treatment group; PD, pharmacodynamics; PK, pharmacokinetic; SAF, safety analysis set.
Demographics and baseline characteristics were mostly comparable between the placebo and active cohorts. The mean age was 48.0, 53.1 and 49.5 years in the placebo, licogliflozin 30 mg and 150 mg cohorts, respectively. The proportion of women was 12 (57%), 25 (58%) and 22 (51%), respectively, in placebo, licogliflozin 30 mg and 150 mg cohorts. The majority of the patients were White (17 (81%), 34 (79%) and 35 (81%)) and the mean body mass index (BMI) was 35.1 kg m−2, 34.3 kg m−2 and 35.4 kg m−2 in the placebo, licogliflozin 30 mg and 150 mg cohorts, respectively. The mean baseline ALT and AST were higher in the cohort receiving licogliflozin 30 mg dose compared with the placebo and 150 mg dose cohorts (Table 1).
Table 1.
Baseline characteristics of patients
| Parameter, mean (s.d.)a | Placebo N = 21 | Licogliflozin 30 mg N = 43 |
Licogliflozin 150 mg N = 43 |
|---|---|---|---|
| Age, years | 48.0 (11.16) | 53.1 (12.57) | 49.5 (11.10) |
| Sex, female, n (%) | 12 (57) | 25 (58) | 22 (51) |
| Race, n (%) | |||
| White | 17 (81) | 34 (79) | 35 (81) |
| Asian | 3 (14) | 8 (19) | 4 (9) |
| Otherb | 1 (5) | 1 (2) | 4 (9) |
| Type 2 diabetes | 5 (24) | 10 (23) | 7 (16) |
| Weight (kg) | 98.8 (16.20) | 95.1 (18.09) | 100.2 (21.97) |
| BMI (kg m−2) | 35.1 (5.49) | 34.3 (4.40) | 35.4 (6.56) |
| Waist circumference (cm) | 113.3 (13.00) | 110.7 (11.99) | 114.0 (15.46) |
| ALT (U l−1) | 60.1 (29.08) | 80.6 (43.66) | 70.3 (25.94) |
| AST (U l−1) | 40.8 (12.60) | 54.3 (24.58) | 47.4 (20.05) |
| GGT (U l−1) | 69.1 (50.19) | 66.7 (60.40) | 61.4 (39.29) |
| HbA1c (%) | 7.3 (1.23) | 7.0 (1.32) | 7.4 (1.74) |
| HOMA-IRc | 11.2 (8.05) | 8.7 (7.68) | 6.7 (3.66) |
| Liver fat content (%) | 23.3 (9.42) | 18.2 (7.46) | 21.1 (8.99) |
| ELF (%) | 9.2 (1.14) | 9.7 (1.02) | 9.2 (0.80) |
| NAFLD fibrosis score | −1.4 (1.69) | −1.2 (1.37) | −1.3 (1.27) |
| FIB4 score | 1.1 (0.57) | 1.3 (0.83) | 1.2 (0.53) |
| APRI score | 0.5 (0.23) | 0.6 (0.38) | 0.6 (0.32) |
| Pro-C3 (ng ml−1) | 14.7 (6.42) | 17.2 (11.18) | 14.7 (8.59) |
| Antidiabetic medication, n (%) | |||
| Biguanides | 12 (57) | 22 (51) | 20 (47) |
| Combinations of oral blood-glucose-lowering drugsd | 2 (10) | 3 (7) | 6 (14) |
| DPP-4 inhibitors | 3 (14) | 5 (12) | 5 (12) |
| Insulins | 3 (14) | 5 (12) | 2 (5) |
| Sulfonylureas | 4 (19) | 4 (9) | 6 (14) |
Data are mean (s.d.) unless specified.
Other includes Black or African American.
Patients on insulin were excluded (placebo (n = 3), licogliflozin 30 mg (n = 5) and licogliflozin 150 mg (n = 2)).
Includes Eucreas, Jentadueto, Kazano, Kombiglyze, Metformin with Vildagliptin, Ristfor.
DPP-4, dipeptidyl peptidase 4; s.d., standard deviation.
Effect of licogliflozin on serum alanine aminotransferase.
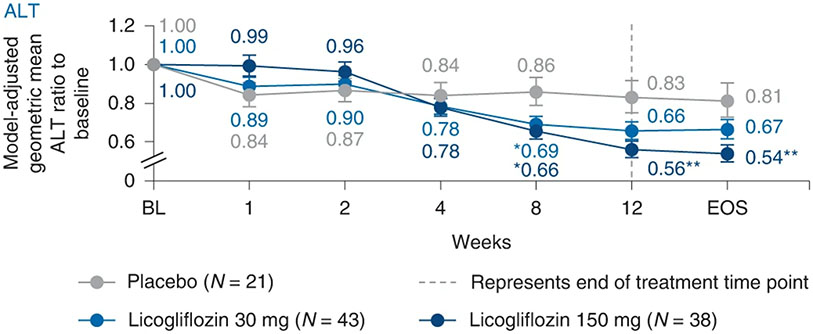
At week 12, a significant decrease (32%; 80% CI: 21–43%; P = 0.002) in plasma ALT (primary endpoint) was observed with licogliflozin 150 mg compared with placebo (Fig. 2). Treatment with licogliflozin 30 mg did not demonstrate a statistically significant ALT decrease (21%; 80% CI: 7–32%; P = 0.061) compared with placebo. A least-squares (LS) mean absolute decrease in ALT of 8.77 U l−1, 22.06 U l−1 (P = 0.075) and 30.41 U l−1 (P = 0.005) was shown for the placebo, licogliflozin 30 mg and 150 mg dose cohorts, respectively.
Fig. 2: Changes in levels of serum alanine aminotransferase from baseline up to week 12.
Data are presented as geometric mean ratio of ALT to baseline. Baseline is defined as the mean of measurements taken at the screening and baseline visits. A repeated-measures ANCOVA was performed for the log-transformed ratio to baseline. P values were based on two-sided ANCOVA test: *P < 0.05, **P < 0.01. Error bars represent s.e.; n for placebo, licogliflozin 30 mg and licogliflozin 150 mg are 21, 42, 38 (day 7); 21, 43, 36 (day 14); 21, 42, 34 (day 28); 20, 42, 34 (day 56); 20, 40, 34 (day 84); 19, 39, 34 (EOS). n, number of patients in each treatment group; s.e., standard error.
Liver fat content.
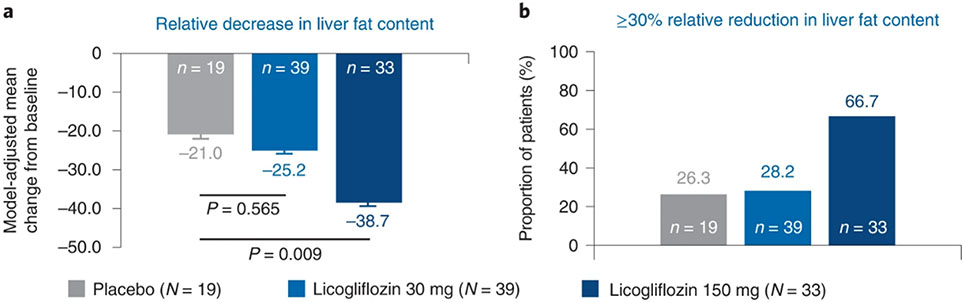
The mean relative reduction in liver fat content in those dosed with licogliflozin 150 mg (−38.7% (P = 0.009)) exceeded that observed with placebo (−21.0%). However, the relative reduction in liver fat content did not reach statistical significance with licogliflozin 30 mg (−25.2% (P = 0.565)). The proportion of patients achieving at least 30% relative reduction in liver fat content was 26.3% with placebo, 28.2% with licogliflozin 30 mg and 66.7% with 150 mg (Fig. 3a,b).
Fig. 3: Change in relative liver fat content after 12 weeks of treatment with licogliflozin.
a, Relative decrease in liver fat content (mean change from baseline). b, Proportion of patients (%) with ≥30% relative reduction in liver fat content. Baseline is defined as the last available measurement before the first dose. P values based on two-sided ANCOVA test. Error bars represent s.e. n, number of patients in each treatment group.
The mean absolute reduction from baseline liver fat content was 6.9% (P = 0.004 versus placebo) with licogliflozin 150 mg, 4.4% (P = 0.235 versus placebo) with licogliflozin 30 mg and 2.7% for placebo (Extended Data Fig. 2a). The proportion of patients achieving at least 5% absolute reduction in liver fat content was 5 of 19 (26.3%), 12 of 39 (30.8%) and 21 of 33 (63.6%) for the placebo, licogliflozin 30 mg and licogliflozin 150 mg dose cohorts, respectively (Extended Data Fig. 2b).
Entry into the study did not require a specified magnitude of liver fat content; however, the effect of licogliflozin on liver fat content was analyzed in patients using a prespecified baseline liver fat content of ≥10%, a threshold frequently used for inclusion in many NASH phase 2a trials17,18. The results were similar to those of the entire study population. Compared with placebo, licogliflozin at 150 mg resulted in a statistically significant reduction in mean absolute (3.0% versus 7.5%, respectively; P = 0.006) and mean relative liver fat content (21% versus 38%, respectively; P = 0.023) at week 12. Changes observed in the licogliflozin 30 mg group did not reach statistical significance in comparison with placebo for either mean absolute (4.8%; P = 0.256) or mean relative (27%; P = 0.492) fat content (Extended Data Fig. 3).
Serum aspartate aminotransferase and gamma-glutamyl transferase.
In addition to reductions in ALT, there were also improvements in AST (secondary endpoint) and GGT (exploratory endpoint) (Extended Data Fig. 4). At week 12, LS mean AST decreased by −2.30 U l−1, −13.45 U l−1 (P = 0.013) and −17.01 U l−1 (P = 0.001) with placebo, 30 mg and 150 mg doses, respectively. There were statistically significant placebo-adjusted reductions in serum AST levels at both the 30 mg (21% (P = 0.024)) and 150 mg dose (32% (P < 0.001)) at week 12. Similarly, LS mean GGT increased in the placebo group by 6.05 U l−1 but decreased in the 30 mg dose (−12.35 U l−1, P = 0.02) and 150 mg dose (−20.81 U l−1, P = 0.001) arms. The placebo-adjusted decrease in GGT was 24% (P = 0.008) and 36% (P < 0.001) for the 30 mg and 150 mg doses, respectively, at week 12.
Anthropometric parameters.
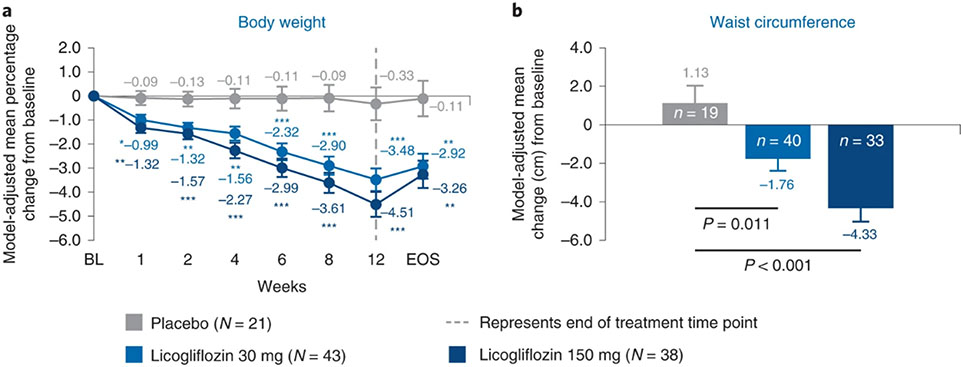
After 12 weeks of treatment, licogliflozin significantly reduced bodyweight, with placebo-adjusted bodyweight loss of 3.15% (P < 0.001) and 4.18% (P < 0.001) for the 30 mg and 150 mg doses, respectively. Reduction of bodyweight was dose dependent and noted as early as week 1 after treatment (Fig. 4a). There was also a reduction in waist circumference after 12 weeks of treatment; waist circumference increased by 1.13 cm in the placebo arm compared with a decrease of −1.76 cm and −4.33 cm in the 30 mg and 150 mg arms, respectively (Fig. 4b). This resulted in a waist circumference difference of −2.9 cm (P = 0.010) and −5.5 cm (P < 0.001) for the 30 mg and 150 mg arms, respectively, compared with placebo.
Fig. 4: Change in anthropometric parameters from baseline up to week 12.
a, Mean percentage change in bodyweight from baseline. b, Mean change in waist circumference from baseline. *P < 0.05, **P < 0.01 and ***P < 0.001. P values based on two-sided ANCOVA test. Baseline is defined as the last available measurement before the first dose. n for placebo, licogliflozin 30 mg and licogliflozin 150 mg for bodyweight are 21, 42, 38 (day 7); 21, 43, 36 (day 14); 20, 42, 30 (day 28); 20, 42, 34 (day 42); 20, 42, 33 (day 56); 19, 40, 34 (day 84); 19, 40, 34 (EOS), respectively. n for placebo, licogliflozin 30 mg and licogliflozin 150 mg for waist circumference are 19, 40 and 33, respectively. Error bars represent s.e. n, number of patients in each treatment group.
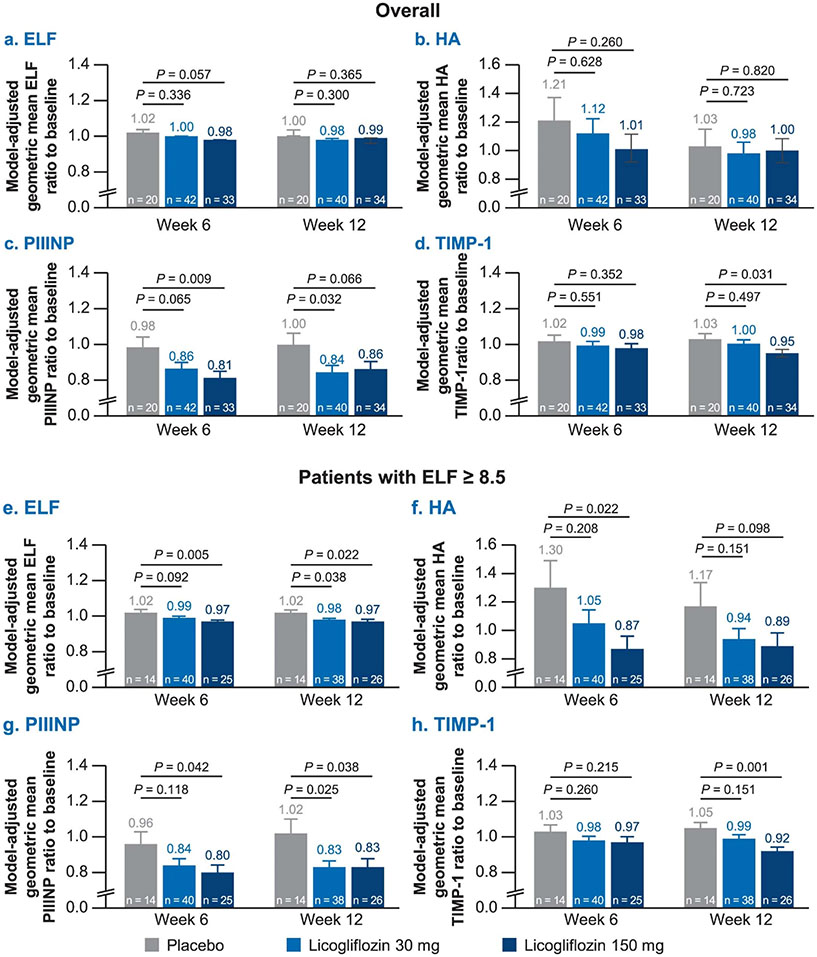
Enhanced liver fibrosis score and its components.
The effect of licogliflozin on biomarkers of liver fibrosis was assessed on the premise that improvement in lipotoxic-driven injury should result in an improvement of markers of fibrogenesis. First, the enhanced liver fibrosis (ELF) score, including the individual components (hyaluronic acid (HA), amino-terminal propeptide of procollagen type III (PIIINP) and tissue inhibitor of matrix metalloproteinase-1 (TIMP1)), was evaluated as a secondary endpoint. Because histologic confirmation of fibrosis was not a requirement for study entry, we prospectively proposed a subgroup analysis of the effect of licogliflozin on patients who entered the study with ELF scores of ≥8.5, a value that has been proposed correlating with the presence of advanced liver fibrosis19. Treatment with licogliflozin led to minor nonsignificant decreases in ELF and its components (Extended Data Fig. 5a-d), which were primarily driven by changes in PIIINP with licogliflozin 30 mg and TIMP1 with licogliflozin 150 mg (Extended Data Fig. 5c,d). Among the protocol-specified subgroup of patients with ELF ≥ 8.5, licogliflozin treatment resulted in a statistically significant reduction in ELF (geometric mean ratio to baseline) of 4% (P = 0.038) with licogliflozin 30 mg and 5% (P = 0.022) with licogliflozin 150 mg when compared with placebo. Similar decreases were noted in PIIINP with 30 mg (19%; P = 0.025) and 150 mg (19%; P = 0.038) compared with placebo. Likewise, the TIMP1 decrease for the 30 and 150 mg dose was 5% (P = 0.151) and 12% (P = 0.001), respectively, compared with placebo (Extended Data Fig. 5e-h).
Safety outcomes.
Licogliflozin was generally safe and well tolerated in NASH patients at both 30 mg and 150 mg doses. Incidence of AEs was lower in the licogliflozin 30 mg cohort (n (%): 31 (72.1%)) compared with placebo (18 (85.7%)) and licogliflozin 150 mg (36 (83.7%)) treatment cohorts at week 12. Incidence of AEs related to study drug were lower with licogliflozin 30 mg (23 (53.5%)) and placebo (11 (52.4%)) compared with licogliflozin 150 mg (33 (76.7%)). One patient in each arm discontinued the treatment due to AEs. The only severe AE in the study was in the placebo group as a result of a gastrointestinal illness, which led to discontinuation of the patient from the study. One patient in the 30 mg group discontinued due to an increase in ALT at screening through baseline which was greater than seven times the upper limit of normal. This patient was found on review to have a history of high liver enzymes, prior positive antinuclear antibodies and corticosteroid treatment for presumed autoimmune hepatitis. Despite lack of symptoms, the abnormally high baseline ALT and prior history suggesting a potentially concomitant non-NASH liver disease led to discontinuation. One patient in the 150 mg arm discontinued as a result of diarrhea.
About 33 (77%) of patients in the licogliflozin 150 mg group experienced diarrhea, while only 9 (43%) and 21 (49%) of patients experienced diarrhea in the placebo and licogliflozin 30 mg groups, respectively. In the majority of patients, diarrhea was mild in all arms (n (%) placebo: 8 (38.1%) licogliflozin 30 mg: 16 (37.2%); and licogliflozin 150 mg: 24 (55.8%)), while the incidence of moderate and severe diarrhea, respectively, was low in the placebo (1 (4.8%) and 0), licogliflozin 30 mg (3 (7.0%) and 2 (4.7%)) and licogliflozin 150 mg arms (6 (14.0%) and 3 (7.0)) (Supplementary Table 1). The incidence of flatulence, abdominal distension and fatigue was more in the licogliflozin 150 mg arm compared with placebo. Diarrhea, flatulence and abdominal distension occurred in 33 of 43 (76.7%), 8 of 43 (18.6%) and 6 of 43 (14.0%) patients, respectively, in the licogliflozin 150 mg cohort. No deaths were reported during the study (Table 2).
Table 2.
Safety outcomes up to week 12
| Incidence, n (%) | Placebo N = 21 | Licogliflozin 30 mg N = 43 |
Licogliflozin 150 mg N = 43 |
|---|---|---|---|
| Number of patients with at least 1 AE | 18 (85.7) | 31 (72.1) | 36 (83.7) |
| Number of patients with at least 1 SAE | 1 (4.8)a | 0 | 0 |
| AEs leading to discontinuation of study treatment | 1 (4.8) | 1 (2.3) | 1 (2.3) |
| Study-drug-related AEs leading to discontinuation of study treatment | 0 | 1 (2.3) | 1 (2.3) |
| Most frequent AEs with incidence ≥5% in any arm | |||
| Diarrhea | 9 (42.9) | 21 (48.8) | 33 (76.7) |
| Flatulence | 2 (9.5) | 2 (4.7) | 8 (18.6) |
| Headache | 3 (14.3) | 2 (4.7) | 5 (11.6) |
| Nausea | 3 (14.3) | 4 (9.3) | 3 (7.0) |
| Vomiting | 2 (9.5) | 5 (11.6) | 2 (4.7) |
| Abdominal pain | 2 (9.5) | 1 (2.3) | 5 (11.6) |
| Abdominal distension | 0 | 2 (4.7) | 6 (14.0) |
| Dizziness | 3 (14.3) | 0 | 4 (9.3) |
| Constipation | 1 (4.8) | 2 (4.7) | 3 (7.0) |
| Upper abdominal pain | 2 (9.5) | 0 | 3 (7.0) |
| Fatigue | 0 | 2 (4.7) | 3 (7.0) |
| Influenza | 0 | 4 (9.3) | 0 |
| Nasopharyngitis | 1 (4.8) | 0 | 3 (7.0) |
| Upper respiratory-tract infection | 1 (4.8) | 3 (7.0) | 0 |
| Muscle spasm | 2 (9.5) | 0 | 1 (2.3) |
One event of viral gastroenteritis.
SAEs, serious adverse events.
Metabolic parameters.
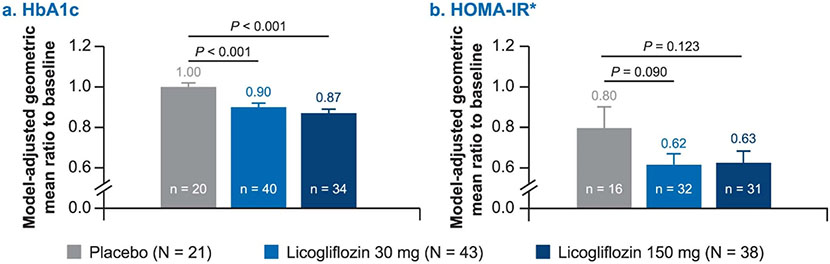
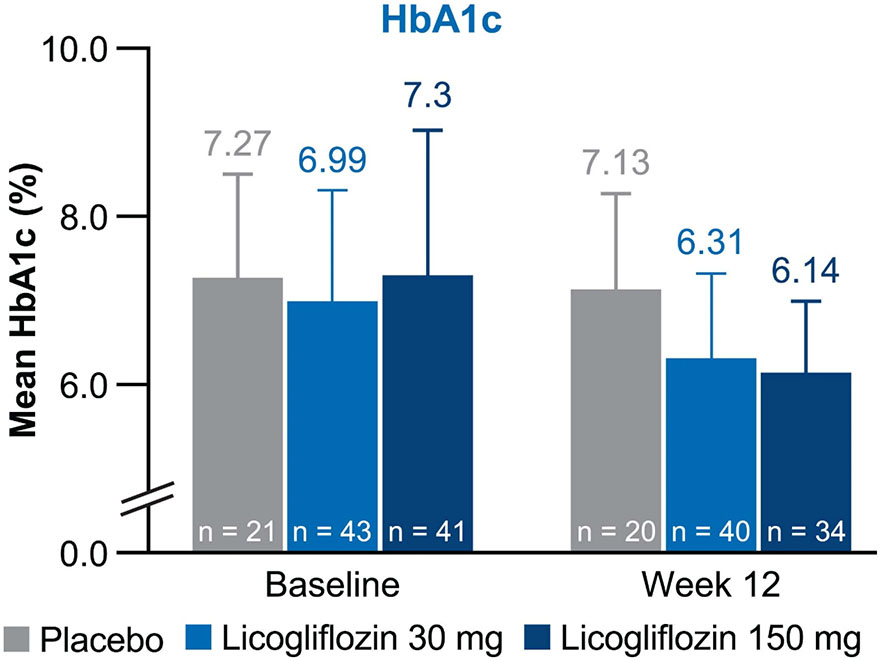
Treatment with licogliflozin also led to statistically significant reductions in glycated hemoglobin (HbA1c) at week 12 (Extended Data Fig. 6a). Placebo-adjusted absolute reductions in HbA1c were statistically significant (P < 0.001) at week 12 with both licogliflozin doses (−0.72% (s.e. = 0.20%) for 30 mg, −0.98% (s.e. = 0.21%) for 150 mg). Mean absolute values of HbA1c in all treatment groups at baseline and week 12 are presented in Extended Data Fig. 7. Changes in homeostatic model assessment of insulin resistance (HOMA-IR) were not statistically significant, 23% (s.e. = 16%, P = 0.090) and 22% (s.e. = 17%, P = 0.123) with the 30 mg and 150 mg doses, respectively, when compared with placebo (Extended Data Fig. 6b). Analysis of HOMA-IR excluded ten patients who received exogenous insulin (placebo (n = 3), licogliflozin 30 mg (n = 5) and licogliflozin 150 mg (n = 2)).
Lipid profile.
Treatment with licogliflozin had no significant effect on plasma lipids including total cholesterol, triglycerides (TGs), low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol when compared with placebo over the 12 weeks of treatment (Supplementary Table 2).
Effect on other liver fibrosis markers.
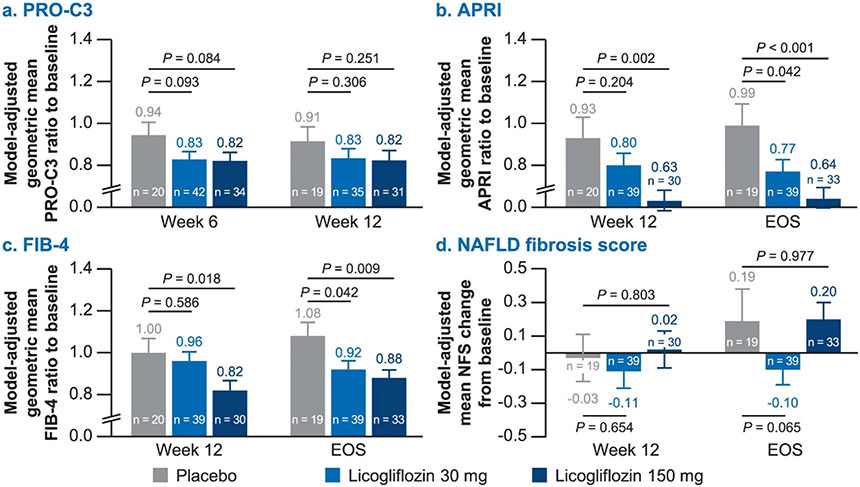
Exploratory endpoints for liver fibrosis including N-terminal neoepitope of procollagen type III (Pro-C3) and algorithmically derived scores, fibrosis-4 index (FIB4), AST-to-platelet ratio index (APRI) and NAFLD fibrosis scores were evaluated. Licogliflozin (150 mg) led to a significant reduction in FIB4 (−18%, s.e. = 8%, P = 0.018) and APRI scores (−32%, s.e. = 13%, P = 0.002) as compared with placebo at week 12. Interestingly, reductions in FIB4 and APRI scores persisted through end of study (EOS) (4 weeks after the drug treatment was discontinued). Changes in Pro-C3 and NAFLD fibrosis scores were not statistically significant with either dose of licogliflozin (Extended Data Fig. 8).
Subanalysis by presence or absence of diarrhea.
A post-hoc subgroup analysis was performed to assess the difference between treatment groups for bodyweight, ALT and liver fat content in patients with and without diarrhea. Patients with or without diarrhea as an AE showed a statistically significant placebo-adjusted percentage reduction in bodyweight (licogliflozin 30 mg: −3.35% with, versus −2.96% without; licogliflozin 150 mg: −4.11% with, versus −4.28% without) (Extended Data Fig. 9a) A similar placebo-adjusted reduction in ALT in the licogliflozin 150 mg arm was noted, whether patients had diarrhea (geometric mean ratio: 0.647; P = 0.0037) or not (0.643; P = 0.0091) (Extended Data Fig. 9b). Also, a statistically significant placebo-adjusted percentage reduction from baseline in liver fat content among those with diarrhea (24.4%; P = 0.035) and those without diarrhea (32.1%; P = 0.022) was noted with the 150 mg arm (Extended Data Fig. 9c).
Discussion
We evaluated the role of a dual inhibitor of SGLT1 and SGLT2 cotransporters in patients with NASH in a multicenter, randomized, double-blind, placebo-controlled study. Treatment with licogliflozin at doses of 150 mg and 30 mg showed placebo-adjusted reductions in serum ALT, with only the 150 mg dose meeting the primary endpoint at 12 weeks. Licogliflozin was generally well tolerated with a similar frequency of AEs across different arms. The most common AE in all groups was identified as diarrhea, affecting 42.9% placebo, 48.8% licogliflozin 30 mg and 76.7% licogliflozin 150 mg. This effect is likely due to SGLT1 inhibition within the gut and was tolerated, as in the active arms, only one patient discontinued the study drug (licogliflozin 150 mg) due to this AE.
In this study, we observed significant reductions in liver enzymes, including ALT, AST and GGT over a 12-week period during treatment with licogliflozin 150 mg in patients with NASH. SGLT2 inhibitors have been shown to reduce ALT, although not always AST and GGT, in NAFLD patients. In one randomized, single-center study in patients with NAFLD and T2DM, 20 weeks of treatment with empagliflozin led to lower ALT levels, but no significant effect on the circulating AST and GGT levels was observed20. In another single-arm exploratory study in ten patients with T2DM and NASH, 12 weeks of treatment with canagliflozin resulted in a reduction in ALT similar to that observed in the current study21. In a randomized active-controlled study, treatment with dapagliflozin also caused a reduction in ALT levels over 24 weeks13.
Over the 12-week period of our study, patients treated with licogliflozin at both 30 mg and 150 mg experienced moderate weight loss without evidence of a plateau. Weight loss through lifestyle modification or bariatric surgery has multiple beneficial effects for those with NAFLD and remains the cornerstone for the treatment of NASH4,7. Vilar-Gomez et al followed patients with biopsy-confirmed NASH over 52 weeks and reported that a loss of ≥10% bodyweight over 52 weeks resulted in resolution of NASH in 90% of the patients, with 45% having at least a one-stage improvement in the resolution of NASH7. Weight loss was also shown to correlate with improvements in NASH histology. Furthermore, even weight reduction in the range of 3–7% has been shown to improve steatosis and inflammation4. Licogliflozin could potentially differentiate from SGLT2 inhibitors because of its additional gastrointestinal effects15, which are expected to lead to greater weight loss while maintaining similar or better antiglycemic effects compared with SGLT2 inhibitors. Since there are no head-to-head studies between licogliflozin and other SGLT inhibitors, it is difficult to compare weight loss data because of differences in study design, especially the use of placebo run-ins in the few studies available 15,20,21,22,23. An extended study with licogliflozin (ELIVATE; ClinicalTrials.gov identifier: NCT04065841) is currently underway and will determine the true magnitude of weight loss in patients with NASH over longer durations of treatment. In addition to bodyweight loss, licogliflozin, at both doses, improved HbA1c, which was greater than improvements reported with SGLT2 inhibitor, dapaglifozin23. The effects on obesity and glycemic control abrogate the role of metabolic dysregulation in the pathogenesis of NASH6,24.
In our study, the observed decreases in absolute and relative liver fat content with 150 mg compared with placebo suggest a role for dual SGLT inhibition in affecting hepatic steatosis and fibrogenesis in line with the literature (Extended Data Fig. 10). The lack of effect of licogliflozin 30 mg on liver steatosis might be because of the smaller effect size of the 30 mg dose or because of the unusually robust placebo effect on liver fat content that amounted to a 21% relative decrease. This exceeds placebo effects in other studies that were in the range of 3.0–8.4% and a relative decrease25,26. In other studies on SGLT2 inhibitors, hepatic steatosis measured using transient elastography decreased significantly with dapagliflozin13 and empagliflozin in a subgroup analysis of patients with advanced steatosis, compared with placebo14.
We observed improvements in markers of liver fibrosis, specifically FIB4 and APRI, which decreased over time in the active groups with a statistically significant difference at week 12 for the 150 mg dose when compared with the placebo. Changes in ELF score, a noninvasive measure of liver fibrosis calculated from three markers of fibrosis: HA, TIMP1 and PIIINP, were not significant in the overall population. However, in a predetermined analysis of those with baseline ELF ≥ 8.5, the reduction in ELF score was significant in the licogliflozin-treated patients when compared with placebo. Previous studies of SGLT2 inhibitors used transient elastography to evaluate the liver stiffness measurement (LSM) as a surrogate marker of liver fibrosis. Liver stiffness decreased significantly with dapagliflozin in a subgroup of patients with significant fibrosis at baseline indicated by LSM values ≥ 8.0 kPa, while there were no significant changes in fibrosis markers, HA, FIB4 or NAFLD fibrosis score with dapagliflozin over 24 weeks of treatment13. In a separate study, LSM was significantly decreased in the empagliflozin-treated group, while no change was found in the placebo group14. These effects on liver fat, liver enzymes and markers of fibrosis provide further evidence that correcting metabolic abnormalities has therapeutic effects on NASH.
Licogliflozin has demonstrated a favorable safety profile. Diarrhea was the most commonly observed AE with licogliflozin 150 mg at an incidence rate of 77% which is comparable with those (~69–90%) reported in previous publications in patients with or without diabetes15,27. The effect was relatively well tolerated, and only one patient from Taiwan in the 150 mg arm discontinued because of diarrhea. The diarrhea observed with high-dose licogliflozin has been attributed to the complete inhibition of SGLT1 in the gut, and reduction in dietary carbohydrate when taking licogliflozin could alleviate or eliminate diarrhea28. In addition, licogliflozin-related diarrhea had minimal impact on health-related quality-of-life questionnaire reports15. A post-hoc analysis showed that efficacy of licogliflozin was minimally impacted by the occurrence of diarrhea. No safety signals, other than those that have been previously reported9, were observed in our study.
There are a number of limitations in the present study. This was a small proof-of-concept study of short duration. Long-term clinical studies will be needed to confirm these findings. The study enrolled patients mostly on the basis of phenotypic and not histologic diagnosis of NASH. Histologic assessment of NASH was not performed. Based on the promising efficacy on multiple noninvasive markers of NASH, an ongoing study ELIVATE (ClinicalTrials.gov identifier NCT04065841), will assess the effects of licogliflozin as monotherapy, as well as in combination with the farnesoid X receptor (FXR) agonist tropifexor over a 48-week treatment period in patients with histologically confirmed NASH.
In conclusion, licogliflozin 150 mg met the primary endpoint of the study with a significant placebo-adjusted reduction in ALT over 12 weeks; however, no significant differences were found at the lower dose of 30 mg. Licogliflozin was generally safe and well tolerated at 30 mg and 150 mg dose levels. Expanded clinical trials of licogliflozin alone, and in combination with drugs that have different mechanisms of action, are warranted to further determine the therapeutic potential of licogliflozin as a new therapeutic option for the treatment of patients with NASH.
Methods
Study design
This study (ClinicalTrials.gov identifier NCT03205150) was a nonconfirmatory, multicenter, randomized, double-blind, parallel-group, placebo-controlled study in patients with histologically confirmed or phenotypic NASH conducted at 15 centers across Argentina, Canada, Israel, the Netherlands, Russia, Taiwan, Thailand and the United States from October 2017 (first patient first visit) to November 2019 (last patient last visit).
Eligible patients were randomized in a 1:2:2 ratio to receive treatment with placebo, licogliflozin 30 mg orally daily (q.d.) or licogliflozin 150 mg orally q.d. for a period of 12 weeks (day 1 to 84), with a follow-up period of 28 days after the last drug administration (day 112) (Extended Data Fig. 1). Patients were randomized in two phases. In the first phase, 33 patients received either placebo or licogliflozin 150 mg daily in a 1:2 ratio to ascertain nonfutility of the highest dose of licogliflozin in the study through a planned interim analysis, before enrolling the entire cohort. In the second phase, licogliflozin at 30 mg was added to the study and the remaining 74 patients were enrolled. The final randomization ratio for the entire study was maintained at the planned 1:2:2 for placebo, 30 mg and 150 mg cohorts, respectively.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The study was approved by all competent ethics committees and regulatory authorities. Informed consent was obtained by investigators from all patients enrolled in the study.
Randomization and blinding
Treatment was assigned to individual patients by means of a randomization number. Randomization numbers were assigned to eligible patients in ascending, sequential order using the interactive response technology (IRT). A subject randomization list was produced by the IRT provider using a validated system that automates the random assignment of randomization numbers to treatment arms, which in turn were linked to medication numbers. Stratification was done by BMI at baseline <30 kg m−2 or ≥30 kg m−2 (Asian race), or <35 kg m−2 or ≥35 kg m−2 (others). The randomization scheme for patients was reviewed and approved by a member of the randomization office.
This was a double-blind study, in which both patient and investigator, including the site staff, were concealed from study treatment. Both treatment drug and placebo were provided as double-blinded patient packs, and the identity of the treatments was concealed by making the identical packaging, labeling, schedule of administration, appearance and odor.
Participants and interventions, and study procedures.
Adult male or female patients (aged ≥ 18 years) who were histologically or phenotypically diagnosed with NASH were included in the study based on fulfillment of the following criteria: Either (1) presence of NASH based on liver biopsy done within 2 years of randomization, with fibrosis levels of F1, F2 or F3 in the absence of a histological diagnosis of alternative chronic liver disease, and ALT ≥ 50 U l−1 (males) or ≥35 U l−1 (females) at screening or (2) phenotypic diagnosis of NASH based on presence of ALT ≥ 50 U l−1 (males) or ≥35 U l−1 (females), BMI ≥ 23 kg m−2 in Asian-heritage patients or ≥27 kg m−2 in patients other than Asian race, and diagnosis of T2DM based on HbA1c levels ranging between 6.5% and 10%. Patients with a history or presence of concomitant liver diseases and cirrhosis, hepatic decompensation or severe liver impairment, type 1 diabetes and uncontrolled diabetes were excluded. All inclusion and exclusion criteria are summarized in Supplementary Table 3. All treatments, licogliflozin 30 mg or 150 mg and placebo were orally administered once daily for 12 weeks.
Drug administration.
Randomized patients were administered licogliflozin 30 mg or licogliflozin 150 mg or placebo orally once daily for 12 weeks. The first dose of study drug was administered under the supervision of site staff along with a meal on day 1. Patients were provided with blinded medication kit for self-administration once daily for 12 weeks (from day 2 to day 84) before lunch, as instructed by the investigator. Study drugs were administered on site on days 56 and 84. The following drugs were permitted during the study if the dose was stable (within 25% of the current dose) for the last 3 months before randomization: oral antidiabetics, insulin, beta-blockers, thiazide diuretics, fibrates, statins, vitamin E, thyroid hormone, phenothiazines or second-generation antipsychotics, and estrogen. Patients were advised to maintain usual physical activity and follow the recommended diet plan (for example, the American Heart Association diet or country-specific equivalent diet).
Magnetic resonance imaging.
Magnetic resonance imaging (MRI) was performed on GE, Philips and Siemens MRI instruments at magnetic field strengths of 1.5 T and 3 T, whichever was available. No contrast was administered. Liver fat content was calculated from a two-dimensional six-echo spoiled gradient-recalled-echo breath-hold sequence with the entire liver in the field of view. The radiologist identified a representative region in each of the nine liver segments (eight Couinaud segments, with segment 4 further subdivided into segment 4a and segment 4b). A fat fraction map was calculated from the six-echo sequence using a multi-interference technique29,30,31. The liver fat content was calculated as mean fat fraction across all nine user-defined regions of interest in the liver.
Study endpoints.
The primary objective of the study was to compare the effect of licogliflozin at 30 mg q.d. and 150 mg q.d. doses with placebo on change in serum ALT levels from baseline to week 12 in patients with NASH. The secondary objectives were to compare the effect of the same active treatments against placebo after 12 weeks on the reduction of parameters such as serum AST levels, percentage of bodyweight and waist circumference, percentage of liver fat content (measured by MRI using proton-density fat fraction (MRI-PDFF))30,31, ELF, as well as its components (PIIINP, TIMP1 and HA). In addition, exploratory endpoints included changes over the 12 weeks between the active treatments and placebo in GGT, HbA1c, HOMA-IR, fasting lipid profile (total cholesterol, HDL, LDL and TG), and other biomarkers of liver fibrosis including serum levels of Pro-C3. Algorithmically derived scores for FIB4, AST to APRI and NAFLD fibrosis score were also determined. Blood samples were drawn for assessment of liver function tests, lipids, HbA1c, HOMA-IR and markers of fibrosis. The sampling for insulin, glucose, and lipid evaluation was conducted in the morning after overnight fasting.
Statistical analysis.
The sample size of 88 completers in the study (35 in each active treatment arm and 18 in placebo) provides a 67% power based on a one-sided test at the 0.1 significance level to detect a 19 U l−1 placebo-adjusted reduction from baseline in ALT with a standard deviation of 37.5 U l−1 in both active groups. This cut-off was chosen, as it corresponded to a 30 U l−1 decrease in ALT assuming a mean reduction of 11 U l−1 by placebo, which was observed in the FLINT study32. The PD analysis set was used for efficacy assessment and included all patients with baseline and at least one posttreatment PD measurement and no data-impacting protocol deviations. The safety analysis set included all patients who were randomized and received any study treatment.
The change in serum ALT, AST and GGT levels from baseline to week 12 was analyzed using a repeated-measures analysis of covariance (ANCOVA). P values calculated from a two-sided test within the ANCOVA framework compared each licogliflozin treatment versus placebo. The model included effects for treatment, visit, treatment by visit interaction, stratification factor (BMI group), baseline and baseline-by-visit interaction. An unstructured variance–covariance matrix was used to account for variance heterogeneity and correlation among multiple measurements from the same patient. Additionally, the log-transformed ratio to baseline ALT, AST and GGT levels was also analyzed using the aforementioned model with log-transformed baseline as a covariate. Other parameters, including bodyweight, waist circumference, percentage liver fat content, biomarkers of fibrosis, lipid profile, HbA1c and HOMA-IR were analyzed using either the ANCOVA (if there was only one scheduled posttreatment measurement for a parameter, such as percentage liver fat) or the repeated-measures ANCOVA. Two subgroup analyses in patients with liver fat ≥ 10% or ELF ≥ 8.5 at baseline were conducted to evaluate the treatment differences in reduction of liver fat and fibrosis, respectively. Furthermore, diarrhea (present/absent) and its interaction with treatment were added to the original ANCOVA model for analysis of percent change from baseline in weight, log-transformed ratio to baseline ALT and log-transformed ratio to baseline liver fat content to assess the impact of diarrhea on these key parameters. All statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC). Clinical data were collected with Timaeus (T5R3 version) and Cognos (version 10) systems.
Extended Data
Extended Data Fig. 1: Overview of the study design.
Patients were screened during a 28-day window, followed by a baseline run-in period of 14-days prior to the day of first treatment. Patients were then randomized in a 1:2:2 ratio to receive a placebo or licogliflozin at 30 mg or 150 mg daily. The study drug was stopped after 12-weeks of treatment and patients were subsequently followed up for 28-days.
Extended Data Fig. 2: Change in absolute liver fat content after 12 weeks of treatment with licogliflozin.
a, Absolute decrease in liver fat content (mean change from baseline); b, proportion of patients (%) with an ≥5% absolute reduction in liver fat content. Baseline is defined as the last available measurement prior to the first dose. P values based on two-sided ANCOVA test. Error bars represent s.e. n, number of patients in each treatment group.
Extended Data Fig. 3: Liver fat in subgroup of patients with baseline percentage liver fat content ≥10%.
a, Absolute reduction in liver fat content (mean change from baseline); b, relative reduction in liver fat content (% change from baseline). Baseline is defined as the last available measurement prior to the first dose. P values based on two-sided ANCOVA test. n, number of patients in each treatment group.
Extended Data Fig. 4: Change in serum AST and GGT levels after 12 weeks of treatment with licogliflozin.
a, AST; b, GGT. Data presented as geometric mean ratio to baseline. Baseline is defined as the mean of measurements taken at the screening and baseline visits. A repeated-measures ANCOVA was performed for the log-transformed ratio to baseline. P values based on two-sided ANCOVA test: *P < 0.05, **P < 0.01, and ***P < 0.001. Error bars represent s.e. n for placebo, licogliflozin 30 mg, and licogliflozin 150 mg for AST: 21, 42, 38 (day 7); 21, 43, 36 (day 14); 21, 41, 33 (day 28); 20, 40, 33 (day 56); 20, 40, 34 (day 84); 19, 39, 34 (EOS); GGT: 21, 42, 38 (day 7); 21, 43, 36 (day 14); 21, 42, 34 (day 28); 20, 42, 34 (day 56); 20, 40, 34 (day 84); 19, 39, 34 (EOS). Dotted gray line represents end-of-treatment time point.
Extended Data Fig. 5: Changes in ELF panel and its components after 12 weeks of treatment.
a, ELF; b, HA; c, PIIINP; d, TIMP1; e, ELF (patient ≥ 8.5); f, HA (patient ≥ 8.5); g, PIIINP (patient ≥ 8.5); h, TIMP1 (patient ≥ 8.5). All data presented as geometric mean ratio to baseline. Baseline is defined as the last available measurement prior to the first dose. P values based on two-sided ANCOVA test. N for placebo, licogliflozin 30 mg, and 150 mg are 18, 34, and 29 (ELF, HA, PIIINP, TIMP1); 14, 40, and 26 (ELF ≥ 8.5, HA ≥ 8.5, PIIINP ≥ 8.5, TIMP1 ≥ 8.5). n, number of patients in each treatment group. Error bars represent s.e.
Extended Data Fig. 6: Change in metabolic parameters from baseline up to week 12.
a, HbA1c; b, HOMA-IR. *Ten patients who received exogenous insulin were excluded from HOMA-IR calculation (placebo (n = 3), licogliflozin 30 mg (n = 5), and licogliflozin 150 mg (n = 2)). Data presented as geometric mean ratio to baseline. P values based on two-sided ANCOVA test. Baseline is defined as the last available measurement prior to the first dose. n, number of patients in each treatment group. N for placebo, licogliflozin 30 mg, and licogliflozin 150 mg for HbA1c are 20, 40, and 34; N for HOMA-IR are placebo (n = 16), licogliflozin 30 mg (n = 32), and licogliflozin 150 mg (n = 31). Error bars represent s.e.
Extended Data Fig. 7: Mean HbA1c by treatment at baseline and week 12.
Data presented as mean (%). Error bars represent SD. n for placebo, licogliflozin 30 mg, and licogliflozin 150 mg for baseline: 21, 43, 41; week 12: 20, 40, 34.
Extended Data Fig. 8: Changes in biomarkers of liver fibrosis after 12 weeks of treatment with licogliflozin.
Data presented in subfigures (a–c) as geometric mean ratio to baseline, and in subfigure d, as mean change from baseline. a, PRO-C3; b, APRI; c, FIB4; d, NAFLD fibrosis score. Baseline is defined as the last available measurement prior to the first dose. P values based on two-sided ANCOVA test. N for placebo, licogliflozin 30 mg, and 150 mg are 21, 43, and 37 (APRI, FIB4, NAFLD fibrosis score) 20, 42, and 34 (Pro-C3). Error bars represent s.e.
Extended Data Fig. 9: Study outcomes with licogliflozin treatment in patients with or without diarrhea.
a, Bodyweight; b, ALT; c, placebo-adjusted decrease in LFC. P values based on two-sided ANCOVA test: *P < 0.05, **P < 0.01, and ***P < 0.001. Dotted gray line represents end of treatment time point. Data in subfigure a presented as mean % change from baseline, b, as geometric mean ratio to baseline, and c, as mean (%) reduction. Error bars represent s.e. n for placebo, licogliflozin 30 mg, and licogliflozin 150 mg for bodyweight in subgroup with diarrhea: 12, 21, 8 (day 7); 12, 22, 8 (day 14); 11, 21, 5 (day 28); 11, 21, 7 (day 42); 11, 21, 7 (day 56); 10, 19, 7 (day 84); 10, 19, 7 (EOS); bodyweight in subgroup without diarrhea: 9, 21, 30 (day 7); 9, 21, 28 (day 14); 9, 21, 25 (day 28); 9, 21, 27 (day 42); 9, 21, 26 (day 56); 9, 21, 27 (day 84); 9, 21, 27 (EOS). ALT in subgroup with diarrhea: 12, 21, 8 (day 7); 12, 22, 8 (day 14); 12, 21, 7 (day 28); 11, 21, 7 (day 56); 11, 19, 6 (day 84); 10, 18, 7 (EOS); ALT in subgroup without diarrhea: 9, 21, 30 (day 7); 9, 21, 28 (day 14); 9, 21, 27 (day 28); 9, 21, 27 (day 56); 9, 21, 26 (day 84); 9, 21, 27 (EOS). LFC in subgroup with diarrhea: 11, 18, 7 (day 84) and in subgroup without diarrhea: 8, 21, 26 (day 84). LFC, liver fat content.
Extended Data Fig. 10: Summary of findings of licogliflozin in NASH.
1He, Y.L. et al. Diabetes Obes. Metab. 21, 1311–1321 (2019). 2Current study data. ALT, alanine aminotransferase; GLP-1, glucagon-like peptide 1; NASH, nonalcoholic steatohepatitis; SGLT, sodium–glucose cotransporters; wt, weight.
Supplementary Material
Acknowledgments
We thank the patients for their participation in the study. The senior scientific writers, S. Kaur, Z. Birajdar and A. Meka of Novartis, provided medical writing and editorial support, which was funded by Novartis Institutes for BioMedical Research, Cambridge, MA, USA, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The study and these analyses were funded by Novartis Institutes for BioMedical Research, Cambridge, MA, USA, which was the sponsor of the study.
Footnotes
Competing interests
S.A.H. reports advisory board fees, consultation and research grants from Akero, Altimmune, Axcella, CirU/Ls, Cymabay, Galectin, Genfit, Hepion, Hightide, Intercept, Madrigal, Metacrine, NGM and Northsea; consultation and research grants from CIVI, ENYO, Gilead, Novartis and Viking; also consultation for Canfite, Echosens, Foresite, Fortress, Kowa, Medpace, Prometic and Ridgeline. W.B.S. is an employee of Alliance for Multispecialty Research. S.K. reports advisory board fees and consultation work for Boehringer Ingelheim, Novo Nordisk and Merck, also grants and research support from Johnson & Johnson. E.R. reports funding from Novartis for the present study. E.S., F.P.M., D. Alpenidze, D. Aizenberg, N.K., C.Y.C., E.Z., P.C., P.N.C., H.K. and Z.B.A. have nothing to disclose. A.M., Y.Z., M.M., S.M., S.K., M.K.B. and Y.L.H. are employees of Novartis. A.P. and C.U. were employees of Novartis until August 2021 and February 2021, respectively. S.T. was an employee of Novartis until 2021.
Data availability
The authors declare that all data supporting the findings of this analysis are available within the article and its appendix. Requests for access to aggregate data and supporting clinical documents will be reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Availability of trial data is according to the criteria and process described at www.clinicalstudydatarequest.com.
References
- 1.Younossi Z et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol 15, 11–20 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Williams CD et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Wong VW et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol. Hepatol 1, 56–67 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol 70, 531–544 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL, Neuschwander-Tetri BA, Rinella M & Sanyal AJ Mechanisms of NAFLD development and therapeutic strategies. Nat. Med 24, 908–922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilar-Gomez E et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378 e365 (2015). quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 8.Lassailly G et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 159, 1290–1301 e1295 (2020). [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver, European Association for the Study of Diabetes & European Association for the Study of Obesity EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol 64, 1388–1402 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Hallsworth K & Adams LA Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 1, 468–479 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj H et al. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: a systematic review. World J. Diabetes 10, 114–132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiang JC & Wong VW SGLT2 inhibitors in liver patients. Clin. Gastroenterol. Hepatol 18, 2168–2172 e2162 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Shimizu M et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab 21, 285–292 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Taheri H et al. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv. Ther 37, 4697–4708 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He YL et al. The effects of licogliflozin, a dual SGLT1/2 inhibitor, on body weight in obese patients with or without diabetes. Diabetes Obes. Metab 21, 1311–1321 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Poulsen SB, Fenton RA & Rieg T Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens 24, 463–469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison SA et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394, 2012–2024 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Lawitz EJ et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol 16, 1983–1991 e1983 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Xie Q et al. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS ONE 9, e92772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuchay MS et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes Care 41, 1801–1808 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Seko Y et al. Efficacy and safety of canagliflozin in type 2 diabetes mellitus patients with biopsy-proven nonalcoholic steatohepatitis classified as stage 1-3 fibrosis. Diabetes Metab. Syndr. Obes 11, 835–843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenlof K et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab 15, 372–382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson JW et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 61, 1923–1934 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GI et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest 130, 1453–1460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology 155, 1463–1473 e1466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison SA et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Bays HE, Kozlovski P, Shao Q, Proot P & Keefe D Licogliflozin, a novel SGLT1 and 2 inhibitor: body weight effects in a randomized trial in adults with overweight or obesity. Obes. (Silver Spring) 28, 870–881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y, Schofield J, Mahling P, Mendonza AE & Hinder M Investigation and management of stool frequency and consistency associated with SGLT1 inhibition by reducing dietary carbohydrate: a randomized trial. Clin. Pharmacol. Ther 108, 995–1002 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Yokoo T et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5T. Radiology 251, 67–76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoo T et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0T. Radiology 258, 749–759 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mashhood A et al. Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J. Magn. Reson. Imaging 37, 1359–1370 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this analysis are available within the article and its appendix. Requests for access to aggregate data and supporting clinical documents will be reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Availability of trial data is according to the criteria and process described at www.clinicalstudydatarequest.com.