Abstract
Coumarin is a naturally occurring bioactive pharmacophore with wide occurrence among central nervous system (CNS)-active small molecules. 8-Acetylcoumarin, one of the natural coumarins, is a mild inhibitor of cholinesterases and β-secretase, which are vital targets of Alzheimer’s disease. Herein, we synthesized a series of coumarin–triazole hybrids as potential multitargeted drug ligands (MTDLs) with better activity profiles. The coumarin–triazole hybrids occupy the cholinesterase active site gorge from the peripheral to the catalytic anionic site. The most active analogue, 10b, belonging to the 8-acetylcoumarin core, inhibits acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and β-secretase-1 (BACE-1) with IC50 values of 2.57, 3.26, and 10.65 μM, respectively. The hybrid, 10b, crosses the blood–brain barrier via passive diffusion and inhibits the self-aggregation of amyloid-β monomers. The molecular dynamic simulation study reveals the strong interaction of 10b with three enzymes and forming stable complexes. Overall, the results warrant a detailed preclinical investigation of the coumarin–triazole hybrids.
1. Introduction
Alzheimer’s disease (AD) is a multifaceted progressive neurodegenerative disease with a time lag between the onset of a responsible biochemical process and the first found symptom. The former begins to cause neuronal damage many years advance when the latter is found. The impaired cholinergic transmission, accumulation of amyloid-β (Aβ) plaques, and neurofibrillary tangles in the brain are major hallmarks of AD that gradually lead to the death of nerve cells and brain atrophy.1 Hampering of the cholinergic transmission results in memory and attention-deficit issues. This hampering is caused due to overactivity of acetylcholinesterase (AChE), which causes the breakdown of the somatic neurotransmitter acetylcholine (ACh).2 Donepezil and galantamine are selective Food and Drug Administration (FDA)-approved AChE inhibitors. In the normal brain, the loss of ACh is compensated by butyrylcholine (BCh), but in AD, the levels of BCh are also depleted due to overexpression of the butyrylcholinesterase (BChE).3 This makes the dual inhibition of AChE and BChE important enzymatic targets. Rivastigmine is the only FDA-approved dual cholinesterase (ChE) inhibitor.4
The Aβ accumulation is considered a major culprit in AD pathology. The cleavage of Aβ precursor protein (APP) is responsible for forming the Aβ oligomers and plaques in the amyloidogenic pathway. In the nonamyloidogenic pathway, α-secretase cleaves the APP into water-soluble fragments, which could be eliminated from the body. In the amyloidogenic pathway, the cleavage of APP is caused by β-secretase (BACE-1 or Aβ precursor cleaving enzyme), which leads to the formation of insoluble waxy fragments called Aβs.5 This makes BACE-1 a vital target in AD drug development. Besides, the amyloid β 1–42 (Aβ1–42) is a self-aggregatory protein;6 thus, inhibition of its spontaneous aggregation is also an important target.7 The recently approved aducanumab is an anti-amyloid monoclonal antibody, the only disease-modifying therapy for treating AD. In contrast, all other marketed drugs only provide temporary relief but do not cure or alter the underlying pathogenesis of the disease.8
Natural products (NPs) have been the basis of traditional medicines throughout history. NPs are recognized to have chemical diversity and biochemical specificity, rendering them promising leads as such or via vital synthetic modifications in drug discovery. The literature reveals that nearly 84% of the FDA-approved drugs for treating central nervous system (CNS) diseases belong to NPs.9 NPs with coumarin motifs have been extensively studied for drug discovery and development as it is a multitargeted ligand with minimal toxic side effects. Coumarins are the NPs containing benzopyrone nucleus, which occurs in over 1300 secondary metabolites from terrestrial and marine sources.10 The conjugated double-bond system of coumarin has been interestingly manifested in medicinal chemistry and chemical biology. Many synthetic coumarins exhibit a broad spectrum of biological activities for treating various ailments like cancer,11 infectious diseases,12 diabetes,13 and neurological disorders.14 The FDA-approved coumarin class of drugs comprises anticoagulants such as warfarin, acenocoumarin, and the antibiotic novobiocin.15 Coumarin pharmacophore has been documented with neuroprotective and anti-Alzheimer properties.16 Thus, its relative availability, low toxicity, and ethnopharmacological importance have made it prudent to explore new derivatives of coumarins for AD.17 The coumarin-based compounds show anti-cholinesterase activity. The 8-acetylcoumarin 1 is a moderate inhibitor of AChE and BACE-1. Its hybrid structure 2 with donepezil shows improvement in the AChE and BACE-1 inhibition activity.18 Similarly, Zhao’s group has recently reported another coumarin-donepezil hybrid, 3, with dual ChE/BACE-1 inhibition activity.19 In both these hybrid structures, incorporating benzyl-piperidine into the structure helps interact with the catalytic site of ChEs. Recently, we reported that the placement of benzyl triazole (e.g., compound 4) to the AChE pharmacophore also helps to gain the interaction at the catalytic site of ChEs.20 Besides, the triazole-based compounds are known to show inhibition of ChEs,21 BACE-1,22 and Aβ-aggregation.23 Thus, based on the literature precedence, we synthesized coumarin–triazole hybrids (general structure shown in Figure 1) as potential dual inhibitors of ChEs and BACE-1.
Figure 1.
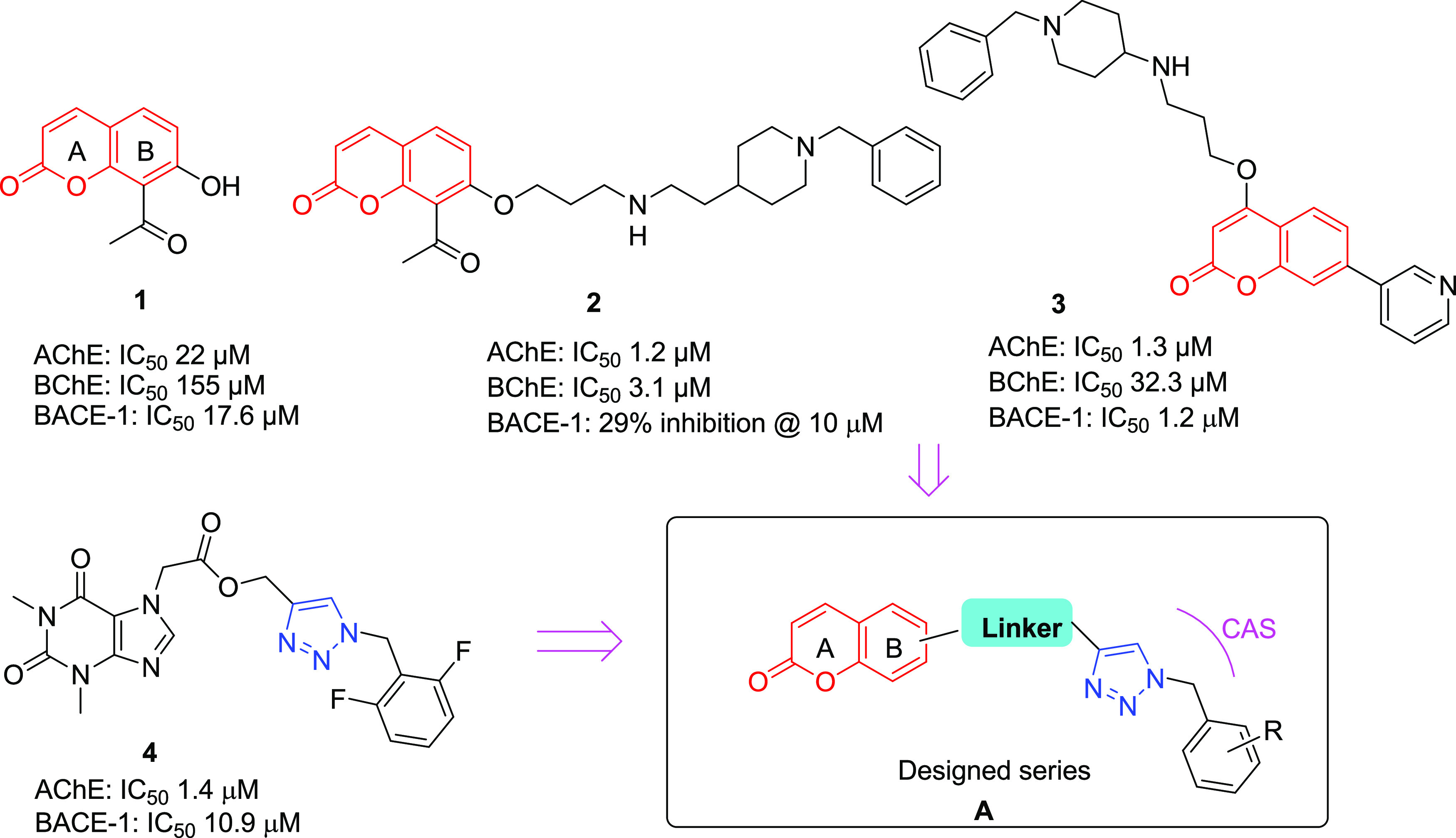
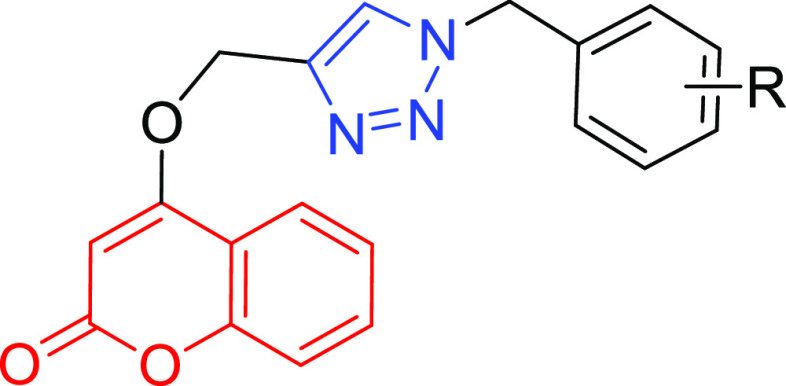
Chemical structures of coumarin derivatives 1–3 and triazole 4 that are reported to show dual ChE/BACE-1 inhibition. The proposed coumarin–triazole series is also shown with general structure A. Here, the coumarin may be connected to the triazole through ring A or B.
2. Results and Discussion
2.1. Synthesis and In Vitro Biological Evaluation
The first series of coumarin–triazole hybrids were synthesized with 4-hydroxycoumarin (5) as a precursor. The propargylation of 4-hydroxycoumarin yielded 4-O-propargylated coumarin 6. The click reaction of propargyl product with the benzyl bromide (7a) in the presence of sodium azide yielded the corresponding triazole, 8a, in 82% yield, as shown in Scheme 1. Various substituted benzyl bromides (7) also participated in the click reaction to produce corresponding triazoles, 8b–p, in good yields.
Scheme 1. Synthesis of Coumarin A-Ring Connected Triazole Hybrids, 8a–p.
Reagents and conditions: (a) propargyl bromide, K2CO3, dimethylformamide (DMF), 70 °C, 24 h, reflux, 95%; (b) TEA, sodium ascorbate, CuSO4·5H2O, room temperature (rt), 24 h, 54–85%.
All prepared coumarin triazoles 8a–p were screened for inhibition of ChEs and BACE-1 using the Ellman and fluorescence resonance energy transfer (FRET) assay, and results are summarized in Table 1. Cryptolepine and donepezil were used as positive controls for ChEs. Cryptolepine is a potent dual inhibitor of ChEs reported by us.24 Donepezil is an FDA-approved ChE inhibitor. The commercially available β-Secretase inhibitor IV was used as a positive control for BACE-1.25 The 4-hydroxycoumarin 5 is a poor inhibitor of AChE, BChE, and BACE-1. The condensation of 5 with the benzyl triazole did not significantly boost the inhibition of ChEs; however, a marginal improvement in BACE-1 inhibition was observed (5vs8a). However, placing various electron-withdrawing groups on the benzyl ring has helped dramatically increase the activity. The careful analysis of results provided structure–activity relationship (SAR) patterns. The boost in ChE inhibition by 2–3-fold was noticed simply by placing −Cl substitution on the benzyl ring (analogues, 8b, 8c, 8d). The −F substitution also boosted ChE inhibition, but only at the ortho-position (analogue 8e). The ortho-substitution with a powerful electron-withdrawing group, trifluoromethyl, yielded a compound 8i that inhibits AChE, BChE, and BACE-1 with IC50 values of 4.83, 7.04, and 10.17 μM, respectively. Similarly, the ortho-OCF3 substitution significantly boosted the inhibition of all three enzymes (analogue 8k). However, the analogues with −CF3 or −OCF3 at para-position were less effective than ortho-substituted analogues. Methoxy substitution provided the best results with better activity, particularly by disubstituted analogues. The 3,5-dimethoxy-substituted analogue, 8o, is the most active, with IC50 values of 2.76, 3.30, and 9.79 μM, respectively. Thus, it is clear that the substituents on the triazole benzyl ring play a vital role in the modulation of the activity of all three enzymes. The 2-trifluoromethyl-, 2-trifluoromethoxy-, 3-methoxy-, and 3,5-dimethoxy-substituted analogues were the most active in the coumarin A-ring connected triazole hybrid series.
Table 1. Cholinesterase and BACE-1 Inhibition Results for Coumarin A-Ring Connected Triazole Hybrids, 8a–p.
| IC50 (μM) ± SD |
||||
|---|---|---|---|---|
| entry | R | EeAChE | eqBChE | hBACE-1 |
| 5 | 138.56 ± 1.85 | 167.06 ± 5.63 | 168.63 ± 7.67 | |
| 8a | H | 150.70 ± 3.14 | 212.4 ± 2.55 | 72.90 ± 2.40 |
| 8b | 2-Cl | 68.71 ± 0.71 | 42.22 ± 0.78 | 186.6 ± 1.21 |
| 8c | 3-Cl | 62.64 ± 2.40 | 61.9 ± 3.66 | 24.82 ± 1.49 |
| 8d | 2,6-Cl | 59.41 ± 2.71 | 40.29 ± 0.93 | 105.83 ± 1.38 |
| 8e | 2-F | 89.23 ± 1.88 | 62.32 ± 1.20 | 111.23 ± 0.63 |
| 8f | 3-F | 232.76 ± 2.31 | 235.8 ± 3.60 | 22.32 ± 1.54 |
| 8g | 4-F | 193.6 ± 2.19 | 249.4 ± 3.1 | 24.22 ± 1.98 |
| 8h | 2,6-F | 73.89 ± 0.63 | 66.66 ± 1.37 | 134.53 ± 1.90 |
| 8i | 2-CF3 | 4.83 ± 0.11 | 7.04 ± 0.77 | 10.17 ± 0.79 |
| 8j | 4-CF3 | 23.26 ± 2.86 | 209.13 ± 2.11 | 19.54 ± 0.66 |
| 8k | 2-OCF3 | 9.13 ± 0.73 | 9.95 ± 0.89 | 17.98 ± 1.59 |
| 8l | 4-OCF3 | 45.39 ± 1.40 | 157.5 ± 1.98 | 132.9 ± 1.08 |
| 8m | 2-CH3 | 210.7 ± 1.83 | 171.6 ± 1.98 | 161.5 ± 1.85 |
| 8n | 3-OCH3 | 11.39 ± 1.16 | 139.7 ± 6.25 | 125.7 ± 4.18 |
| 8o | 3,5-di-OCH3 | 2.76 ± 0.04 | 3.30 ± 0.01 | 9.79 ± 1.36 |
| 8p | 4-NO2 | 200.4 ± 0.85 | 219.8 ± 3.04 | 156.03 ± 1.09 |
| cryptolepine | 0.33 ± 0.2 | 0.61 ± 0.3 | nta | |
| donepezil | 0.025 ± 0.005 | 5.72 ± 0.59 | nt | |
| β-secretase inhibitor IV | nt | nt | 0.019 ± 0.006 | |
nt: not tested.
Next, we synthesized a series of coumarin–triazole hybrids with triazole connected via the B-ring of coumarin. For this, we employed four different hydroxy coumarins, namely, 8-acetylcoumarin (1), 4-methyl-6,7-dihydroxycoumarin (11), 4-methyl-6-methoxy-7-hydroxycoumarin (14), and 7-hydroxycoumarin (umbelliferone, 17) as coumarin precursors. Based on the biological results of the 8a–p series, 2-trifluoromethyl, 2-trifluoromethoxy, 3-methoxy, and 3,5-dimethoxybenzyl substitutions were used for synthesizing coumarin B-ring connected triazole hybrids. Using a similar synthetic strategy, four series of coumarin–triazole hybrids, 10a–d, 13a–d, 16a–d, and 19a–d, were synthesized as depicted in Scheme 2.
Scheme 2. Synthesis of Coumarin B-Ring Connected Triazole Hybrids, 10a–d, 13a–d, 16a–d, and 19a–d.
Reagents and conditions: (a) propargyl bromide, K2CO3, DMF, 70 °C, 24 h, reflux, 75–91%; (b) TEA, NaN3, sodium ascorbate, CuSO4·5H2O, rt, 24 h, 50–80%; (c) methyl iodide, NaH, DMF, 0 °C, 90%.
The biological screening results of coumarin B-ring-connected triazole hybrids are provided in Table 2. In the acetylcoumarin series, the hybrids with methoxy substitution were better than parent coumarin; however, trifluoromethyl and trifluoromethoxy substitutions were unfavorable as they resulted in the loss of activity against all three targets. The 4-methylcoumarin-based hybrids 13a–d and 16a–d were ineffective with all four benzylic substitutions. The unsubstituted coumarin-based hybrids, 19a–d, were better inhibitors of ChEs than 4-methyl-substituted coumarin analogues. The 3-methoxybenzyl-containing coumarin–triazole 19a displayed significant inhibition of ChEs with IC50 values of 3.6 and 2.8 μM, respectively. However, its dimethoxy analogue 19b was not equally effective. Overall, the 3,5-dimethoxy analogue 10b from the acetylcoumarin series has potent inhibition of AChE and BChE and also BACE-1. However, the corresponding 3,5-dimethoxy analogues from other series, 13b, 16b, and 19b, were not active to the level of 10b. This probably indicates some role of the C8-acetyl group in the activity. Interestingly, compound 13b is a selective BChE inhibitor with IC50 of 4.7 μM and shows poor activity against AChE and BACE-1. The dimethoxy substitution with the bis-triazole unit in 13b probably fits appropriately in a wider BChE active site gorge but not in AChE active site gorge, which is comparatively narrower.
Table 2. Cholinesterase and BACE-1 Inhibition Results for Coumarin B-Ring Connected Triazole Hybrids, 10a–d, 13a–d, 16a–d, and 19a–d.
| IC50 (μM) ± SD |
||||
|---|---|---|---|---|
| entry | R | EeAChE | eqBChE | hBACE-1 |
| 1 | 23.71 ± 3.21 | 155.03 ± 3.09 | 18.42 ± 1.04 | |
| 11 | 68.14 ± 4.05 | 147.70 ± 5.46 | 104.8 ± 3.48 | |
| 14 | 89.09 ± 3.51 | 146.07 ± 4.91 | 91.09 ± 2.41 | |
| 17 | 164.46 ± 5.22 | 180.33 ± 4.58 | 174.53 ± 4.37 | |
| 10a | 3-OCH3 | 8.36 ± 1.29 | 7.40 ± 0.94 | 118.8 ± 2.49 |
| 10b | 3,5-di-OCH3 | 2.57 ± 0.31 | 3.26 ± 0.13 | 10.65 ± 0.47 |
| 10c | 2-CF3 | 173.06 ± 1.89 | 44.60 ± 1.84 | 138.8 ± 1.47 |
| 10d | 2-OCF3 | 139.20 ± 1.64 | 170.10 ± 1.76 | 141.9 ± 1.59 |
| 13a | 3-OCH3 | 130.70 ± 0.45 | 51.70 ± 1.42 | 128.6 ± 1.49 |
| 13b | 3,5-di-OCH3 | 167.53 ± 6.70 | 4.7 ± 0.67 | 115.2 ± 3.86 |
| 13c | 2-CF3 | 154.70 ± 1.42 | 82.54 ± 1.41 | 139.4 ± 1.00 |
| 13d | 2-OCF3 | 176.0 ± 10.25 | 167.06 ± 5.63 | 20.72 ± 0.93 |
| 16a | 3-OCH3 | 164.2 ± 1.84 | 95.82 ± 1.12 | 138.4 ± 1.02 |
| 16b | 3,5-di-OCH3 | 150.96 ± 5.05 | 191.2 ± 1.13 | 9.06 ± 0.88 |
| 16c | 2-CF3 | 141.7 ± 0.89 | 57.45 ± 1.42 | 156.1 ± 1.42 |
| 16d | 2-OCF3 | 166.5 ± 2.45 | 141.03 ± 0.60 | 167.6 ± 1.40 |
| 19a | 3-OCH3 | 3.62 ± 0.33 | 2.80 ± 0.22 | 125.6 ± 3.92 |
| 19b | 3,5-di-OCH3 | 59.67 ± 0.62 | 63.24 ± 2.44 | 132.0 ± 2.02 |
| 19c | 2-CF3 | 20.64 ± 0.95 | 77.33 ± 6.38 | 17.71 ± 0.65 |
| 19d | 2-OCF3 | 52.54 ± 0.40 | 97.23 ± 1.29 | 172.8 ± 0.66 |
| cryptolepine | 0.33 ± 0.2 | 0.61 ± 0.3 | nta | |
| donepezil | 0.025 ± 0.005 | 5.72 ± 0.59 | nt | |
| β-secretase inhibitor IV | nt | nt | 0.019 ± 0.006 | |
nt, not tested.
2.2. Enzyme Kinetics and Molecular Modeling for 10b
The enzyme kinetics of 10b was carried out to assess the mode of inhibition for all three targets. The Lineweaver–Burk (LB) double-reciprocal plot for AChE indicates a noncompetitive mode of inhibition with a ki value of 0.27 μM (Figure 2A). However, for BChE and BACE-1, the depicted mode of inhibition is a mixed type with ki values of 3.5 and 7 μM, respectively (Figure 2C,E). Further, we studied the interaction pattern of 10b with each enzyme via molecular modeling studies. As depicted in Figure 2B, 10b interacts with peripheral anionic site (PAS) as well as catalytic anionic site (CAS) residues of AChE. The acetylcoumarin core stays at the entrance of the gorge, and benzyl triazole orients toward the bottom of the cavity. Both the rings of coumarin form π–π stacking with Trp 286 residue. Interestingly, the carbonyl oxygen of the acetyl group forms H-bonding with Arg 296 and Phe 296 residues, which could be the crucial factor for the superior activity of the acetylcoumarin series vs other series. The linker oxygen also forms H-bond with the Phe 295 residue. The triazole ring and aryl of coumarin form π–π stacking with the Tyr 341 residue. The benzyl ring forms a vital π–π stacking with Trp 86 residue of the anionic subsite of the CAS. The noncompetitive mode of inhibition, as seen in the kinetic study, could be because of the strong interaction of the 10b with PAS residues via multiple π–π stacking and H-bonds. The docking study of 10b with BChE (Figure 2D) shows π–π interactions of benzyl with Phe 329 but not with the Trp 82 of the anionic subsite. The triazole ring forms π–π stacking with Tyr 332. Like in the case of AChE, the acetyl group participated in vital H-bonding with the PAS residues of BChE. The carbonyl oxygen of acetyl forms H-bond with Ser 287, which is also H-bonded with a coumarin ring oxygen atom. Figure 2F shows the interactions of 10b with BACE-1 (PDB: 1W51). It shows H-bonding with Thr 232, Asn 233, Arg 235, Gly 13, and Trp 115 residues; however, the interaction with Asp 32 and Asp 228 was missing. The interaction with these aspartate residues is vital for the potent inhibition of BACE-1.26 This could be the reason for its low activity level against the BACE-1 enzyme.
Figure 2.
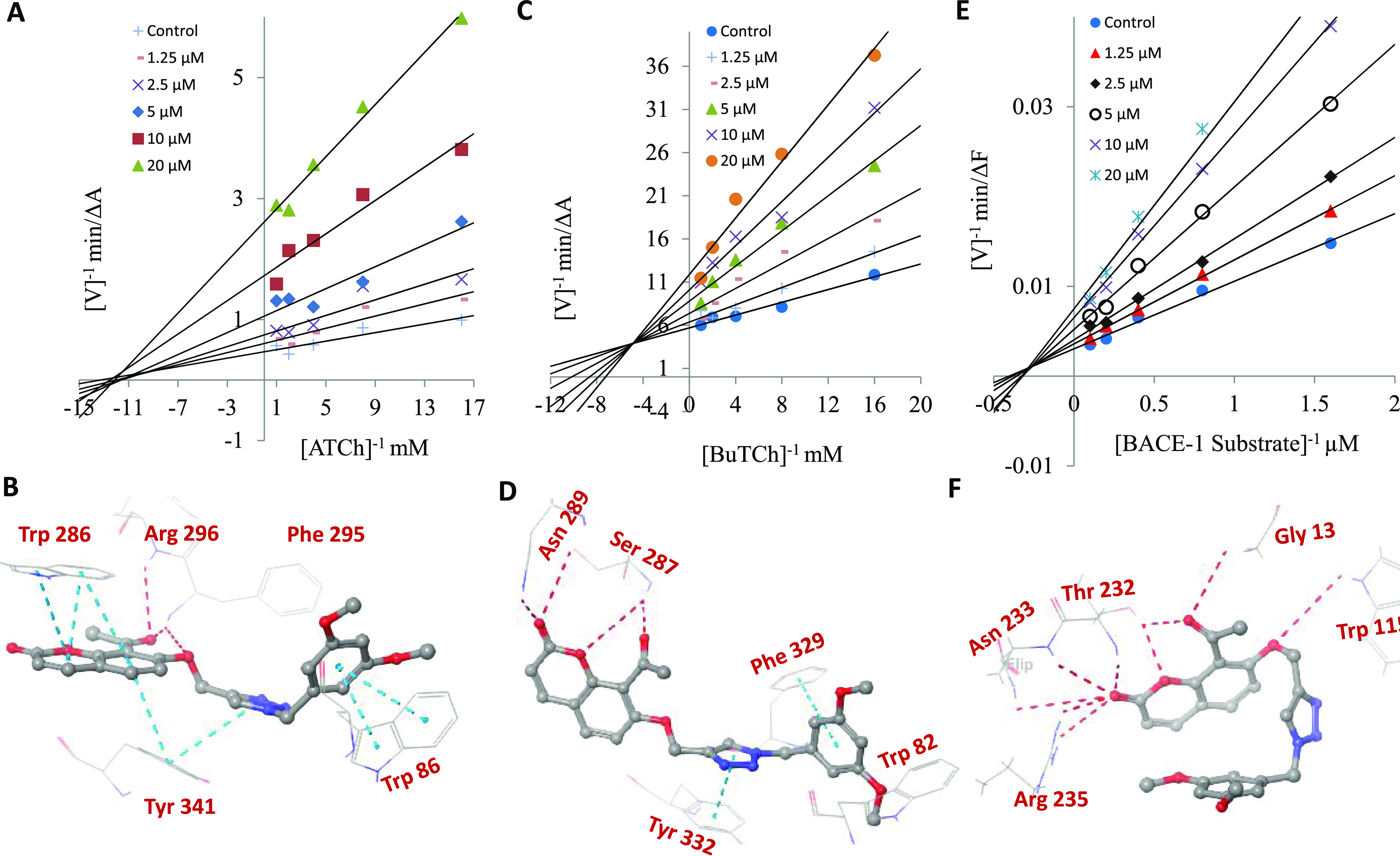
Enzyme kinetics and molecular modeling studies of 10b with AChE, BChE, and BACE-1. (A) LB plot for inhibition of AChE by 10b; (B) molecular docking of 10b with AChE (PDB: 4EY7); (C) LB plot for inhibition of BChE by 10b; (D) molecular docking of 10b with BChE (PDB: 6EP4); (E) LB plot for inhibition of BACE-1 by 10b; (F) molecular docking of 10b with the BACE-1 (PDB: 1W51). The light blue dotted lines indicate π–π interactions, and the dark red dotted lines indicate H-bonding interactions.
To understand the superiority of 8-acetyl-7-hydroxycoumarin framework vs 4-hydroxycoumarin and 7-hydroxycoumarin, we analyzed the binding pattern of 10b, 8o, and 19b in the AChE active site (Figure 3). These three analogues differ only via the coumarin framework; thus, their different activity is related to the coumarin–triazole linkage and substitution on coumarins. Both rings of coumarin core in 10b and 8o offered π–π stacking with Trp 286 at PAS, whereas in 19b, this π–π interaction is missing. Molecule 19b is flipped by 180° in the AChE active site, with the coumarin core oriented toward the bottom of the AChE active site gorge. The 3,5-dimethoxybenzyl unit of 19b did not show π–π stacking with Trp 286 though it offered H-bonding with Phe 295 residue. In particular, the bidentate H-bonding offered by acetyl carbonyl oxygen of 10b with Phe 295 and Arg 296 was missing in 19b, which could also be accounting for the 20-fold difference in AChE inhibition activity of 10bvs19b.
Figure 3.

Interaction pattern of 3,5-dimethoxybenzyl coumarin–triazole hybrids, 10b, 8o, and 19b with AChE (PDB: 4EY7).
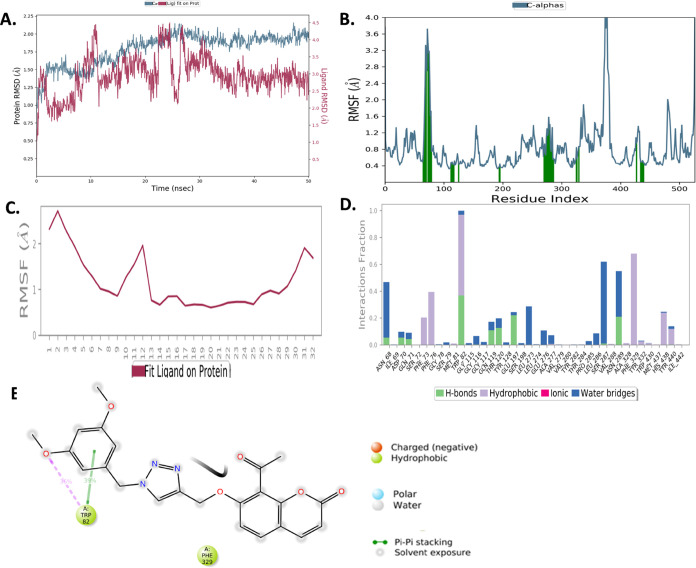
The 50 ns molecular dynamic simulation was carried out for enzyme–inhibitor complexes to determine the stability. The complex of compound 10b with AChE is stable throughout the study, as indicated by the root mean square deviation (RMSD) value below 3 Å for both protein and ligand (Figure 4A). The root mean square fluctuation (RMSF) plot shown in Figure 4B,C further indicates minimal fluctuations in the residues of protein and atom of the ligand (RMSF is below 4 Å) during the entire period of study. The orientation map of compound 10b is depicted in Figure 4D,E. The hydrophobic interaction with Tyr 341 was sustained for >56% and Trp 86 for more than 79% of the simulation time. Tyr 124 shows H-bonding with the triazole ring for >51% of the simulation run. Trp 286 offers hydrophobic interactions for >33% of the simulation run. Phe 295 depicts H-bonding >95% persistently throughout the simulation study (Figure 4D,E).
Figure 4.
MD simulation of 10b–AChE complex for 50 ns. (A) Protein–ligand RMSD during simulation; (B) RMSF of AChE; (C) RMSF of compound 10b; (D) interaction pattern of compound 10b with AChE during MD simulation; (E) two-dimensional (2D) diagram for the interaction of compound 10b with AChE.
As depicted in Figure 5A, the RMSD below 3 Å indicates the stability of the protein in the 10b–BChE complex throughout the study. The ligand slightly diffuses from the initial binding site; however, the overall orientation and interaction pattern is unaffected (Figure 5A). The RMSF depicts a value below the range of 4 Å, which indicates no significant conformational changes along the protein chain as the ligand fits in the protein (Figure 5B,C). The most preserved interactions during the simulation run were hydrophobic π–π stacking and H-bonding with Trp 82 for 39 and 36% of the run (Figure 5E).
Figure 5.
MD simulation of 10b–BChE complex for 50 ns. (A) Protein–ligand RMSD during simulation; (B) RMSF of BChE; (C) RMSF of compound 10b; (D) interaction pattern of compound 10b with BChE during MD simulation; (E) 2D diagram for compound 10b interactions with BChE.
The MD simulation of the 10b–BACE-1 complex also indicated the stability of the complex. The RMSD for the protein is below 3 Å, which indicates stability. In contrast, for the ligand, RMSD is slightly above 3 Å, which is conclusive that the ligand diffuses away from the protein (Figure 6A). The RMSF for the protein and the ligand fit is below 3.5 and 2 Å, respectively (Figure 6B,C). The H-bonding between the acetyl group of coumarin with Thr 72 is sustained for >89% of the simulation time. The triazole ring also maintained hydrophobic interaction with the Tyr 71 residue for >72% of the simulation run. The hydrophobic interaction with Trp 115 residue is also retained for a significant time (Figure 6D,E).
Figure 6.
MD simulation of 10b–BACE-1 complex for 50 ns. (A) Protein–ligand RMSD during simulation; (B) RMSF of BACE-1; (C) RMSF of compound 10b; (D) interaction pattern of compound 10b with BACE-1 during MD simulation; (E) 2D diagram for compound 10b interactions with BACE-1.
2.3. Inhibition and Aβ Self-Aggregation
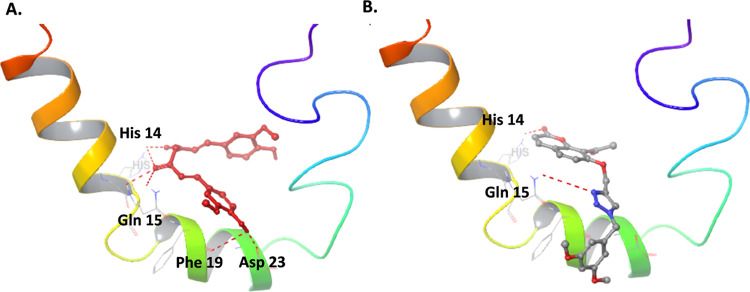
AD’s main hallmark is forming Aβ plaques, which occur via the aggregation of Aβ monomers.27 Thus, we evaluated the effect of compound 10b on the self-aggregation of Aβ1–42 monomers, which is one way to form oligomers and plaques. Analogue 10b displayed a 37% inhibition of self-aggregation of Aβ1–42 monomers, whereas the positive control, curcumin, showed 81% inhibition at 10 μM. The molecular docking of 10b with the Aβ-monomer revealed that this compound binds to the metal binding site residue His 14; however, it does not interact with the Asp 23, the key residue in forming a salt bridge between monomers during the aggregation process.28 Curcumin forms H-bonding with the metal binding site and the salt-bridge forming residue (Figure 7).
Figure 7.
Molecular docking of curcumin (A) and compound 10b (B) with the Aβ monomer (PDB: 1Z0Q).
2.4. Parallel Artificial Membrane Permeability Assay (PAMPA)–Blood–Brain Permeability (BBB) Permeability and In Silico Absorption Distribution Metabolism Elimination (ADME) Properties of 10b
Along with the activity profile of the compound against various targets, CNS blood–brain permeability (BBB) is also an indispensable factor for a drug with CNS disease on target. The blood–brain barrier restricts molecules’ passage from the brain vasculature to the brain due to its high trans-endothelial electrical resistance and low paracellular and transcellular permeability. Thus, the BBB permeability of 10b was experimentally determined using a PAMPA assay (Table 3).
Table 3. BBB Permeability of 10b Determined Using PAMPA–BBB Assay.
| compound | λmax (nm) | Pe × 10–6 cm/s ± SD | prediction of CNS permeation |
|---|---|---|---|
| 10b | 261 | 5.68 ± 1.1 | CNS+ |
| donepezil | 270 | 13.32 ± 1.82 | CNS+ |
| theophylline | 272 | 1.22 ± 1.38 | CNS– |
The ADME properties determine whether a compound has druglike properties. It is included at a very early stage of drug discovery. QikProp module of the Schrodinger molecular modeling software computationally determines the number of descriptors for small molecules, predicting the ADME properties.29 These properties include total solvent-accessible surface area (SASA), the hydrophobic component of the SASA (FOSA), the hydrophilic component of the SASA (FISA), and the estimated number of H-bond donors (donorHB) and acceptors (accptHB) in aqueous solution. Table 4 also shows that the predicted ranges for the calculated partition coefficients, which are octanol/water (QP log Po/w), aqueous solubility (QP log S), and skin permeability (QP log Kp) fall within the acceptable range. The computed IC50 for blockage of HERG K+ channels (QP log HERG), Caco-2 permeability, and Madin–Darby canine kidney (MDCK) permeability values are also within the normal limits. The BBB is also predicted computationally. The results show that QPlog BB also falls within the recommended range. This complies with the experimental results of the PAMPA assay, as shown in Table 3. ADME properties were also estimated for donepezil and β-secretase inhibitor IV and are listed in Table 4.
Table 4. ADME Properties for 10b and Positive Controlsa.
| property | reference range | 10b | donepezil | β-secretase inhibitor IV |
|---|---|---|---|---|
| molecular weight | 300–1000 | 435.435 | 379.498 | 578.725 |
| SASA | 0–750 | 734.966 | 726.298 | 860.774 |
| FOSA | 7–330 | 324.954 | 410.311 | 391.518 |
| FISA | 0–6 | 139.219 | 58.288 | 123.351 |
| donorHB | 2–20 | 0 | 0 | 4 |
| accptHB | 2–20 | 9.25 | 5.5 | 12.7 |
| QP log Po/w | –2 to 6.5 | 2.77 | 4.436 | 3.543 |
| QP log S | –6.5 to 0.5 | –3.99 | –4.904 | –4.698 |
| QP log HERG | concern below—5 | –6.178 | –6.868 | –7.634 |
| QPPCaco | <25 poor, >500 great | 473.899 | 691.924 | 167.134 |
| QP log BB | –3.0 to 1.2 | –1.396 | –0.021 | –1.597 |
| QPP MDCK | <25 poor, >500 great | 220.701 | 367.58 | 40 |
ADME properties were determined using the QikProp module of Schrodinger 10.2 software. Properties: total solvent-accessible surface area (SASA), the hydrophobic component of the SASA (FOSA), the hydrophilic component of the SASA (FISA), the estimated number of hydrogen-bond donor (donorHB) acceptor (accptHB) in aqueous solution. Also, QP log Po/w is the octanol/water partition coefficient, QP log S is aqueous solubility (mol/L), QP log HERG is IC50 value for blockage of HERG K+ channels, QPPCaco is Caco-2 permeability (nm/s), QP log BB is brain/blood partition coefficient, and QPPMDCK is apparent MDCK cell permeability (nm/s). MDCK cells are considered a good mimic for the blood–brain barrier.
3. Conclusions
We designed and synthesized coumarin–triazole hybrid 10b, displaying a multitargeted effect against AD targets, AChE, BChE, BACE-1, and Aβ-aggregation. It is predicted to cross the BBB based on the experimental PAMPA results and in silico QikProp prediction. The structure–activity relationship has been established for this scaffold, indicating that 3,5-dimethoxy substitution on the benzyl group along with the coumarin core with acetyl substitution is important for activity. The study also further validated the capability of triazole to interact at the CAS of the ChEs. The results presented herein warrant further investigation of 10b in preclinical studies.
4. Experimental Section
4.1. General
The local suppliers of Sigma-Aldrich, TCI chemicals, and CDH have provided the chemicals, solvents, reagents, and the required glassware and plasticware for the study. The enzymes, substrates, and positive controls for the biological studies are also procured from them. NMR spectra were recorded on a Bruker-Avance DPX FT-NMR 400 MHz instrument. IR spectra were recorded on a PerkinElmer IR spectrophotometer. High-resolution electrospray ionization mass spectra (HR-ESIMS) were obtained from an Agilent HR-ESIMS-6540-UHD machine. Absorbance and fluorescence readings were recorded on Molecular Devices and Biotage microplate readers, respectively. The absorbance readings in Ellman and PAMPA assays were recorded on SpectraMax Plus 384 plate reader. The fluorescence for the FRET assay was recorded on the Synergy H1 Hybrid Reader (Biotek). Melting points were recorded on a Buchi digital melting point apparatus.
4.2. Synthesis of 7-Hydroxy-6-methoxy-4-methyl-2H-chromen-2-one (14)30
The mixture of compound 11 (0.52 mmol) and NaH (1.05 mmol) in 2 mL of DMF was stirred at 0 °C for half an hour. After half an hour, methyl iodide (0.52 mmol) was added, and the reaction was further stirred for 5 h at rt. After 5 h, the reaction mixture was added to 50 mL of ice-cold water to remove DMF efficiently. Extraction was done with EtOAc (3 × 50 mL). The EtOAc layer was concentrated on the vacuum evaporator to obtain 14 (1.2 g, yield: 90%). Brown powder; m.p. 130–131 °C; IR (CHCl3) νmax (cm–1): 1710 (C=O), 1423 (C–O); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.09 (s, 1H), 6.83 (s, 1H), 6.17 (s, 1H), 3.97 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 162.90, 153.90, 151.29, 150.02, 143.95, 114.82, 114.03, 109.71, 100.82, 57.82, 20.22; ESIMS: m/z 207.1 [M + H]+; HR-ESIMS: m/z 207.0667 [M + H]+ calcd for C11H10O4 + H+ (207.0657).
4.3. Propargylation of Coumarin Hydroxyl Group: Synthesis of 6, 9, 12, 15, and 18
The coumarin 1, 5, 11, 14, or 17 (1 equiv) was treated with potassium carbonate (4 equiv) in DMF for 30 min at 70 °C. After 30 min, propargyl bromide (1.5 equiv) was added, and the reaction was continued for 24 h. After 24 h, the reaction was cooled and poured into ice-cold water to remove the DMF. The extraction was done with EtOAc (3 × 50 mL). The combined ethyl acetate layer was concentrated on a rotary evaporator, and the residue was purified over silica gel (solvent system: 40% EtOAc in hexane) to obtain respective propargylated compounds, 6, 9, 12, 15, and 18.
4.3.1. 4-(Prop-2-yn-1-yloxy)-2H-chromen-2-one (6)31
White fluffy powder; yield, 95%; m.p.153–154 °C; IR (CHCl3) νmax (cm–1): 1719 (C=O), 1251 (C–O); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.85 (dd, J = 7.9, 1.5 Hz, 1H), 7.59–7.55 (m, 1H), 7.34–7.31 (m, 1H), 7.29 (d, J = 7.3 Hz, 1H), 5.84 (s, 1H), 4.88 (d, J = 16 Hz, 2H), 2.69 (t, J = 2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.29, 162.66, 153.52, 132.61, 125.004, 123.27, 116.69, 91.72, 78.20, 75.58, 56.87; ESIMS: m/z 201 [M + H]+; HR-ESIMS: m/z 201.0557 [M + H]+ calcd for C12H8O3 + H+ (201.0552).
4.3.2. 8-Acetyl-7-(prop-2-yn-1-yloxy)-2H-chromen-2-one (9)
White powder; yield, 79%; m.p. 155–157 °C; IR (CHCl3) νmax (cm–1): 1722 (C=O), 1122 (C–O), 1324 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.67 (d, J = 8.7 Hz, 1H), 7.50 (d, J = 8.7 Hz, 1H), 7.05 (d, J = 8.6 Hz, 2H), 6.31 (d, J = 8.8 Hz, 1H), 4.82 (s, 2H), 2.62 (s, 3H), 2.58 (t, J = 2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 198.92, 159.57, 156.19, 151.43, 143.37, 129.62, 120.47, 114.42, 113.41, 109.38, 56.56, 32.37; ESIMS: m/z 243.06 [M + H]+; HR-ESIMS: m/z 243.0657 [M + H]+ calcd for C14H11O4 + H+ (243.0669).
4.3.3. 4-Methyl-6,7-bis(prop-2-yn-1-yloxy)-2H-chromen-2-one (12)
Light brown powder; yield, 77%; m.p. 183–184 °C; IR (CHCl3) νmax (cm–1): 1714 (C=O), 1183 (C–O), 1H NMR (400 MHz, CDCl3): δ (ppm) 7.23 (s, 1H), 7.05 (s, 1H), 6.20 (s, 1H), 4.84 (d, J = 2.4 Hz, 2H), 4.82 (d, J = 2.3 Hz, 2H), 2.59 (t, J = 2.5 Hz, 1H), 2.57 (t, J = 2.4 Hz, 1H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.02, 152.53, 151.57, 149.80, 144.13, 113.58, 113.05, 110.11, 102.66, 78.08, 57.46, 56.50, 18.50; ESIMS: m/z 269.08 [M + H]+; HR-ESIMS: m/z 269.0830 [M + H]+ calcd for C16H12O4 + H+ (269.0814).
4.3.4. 6-Methoxy-4-methyl-7-(prop-2-yn-1-yloxy)-2H-chromen-2-one (15)
Light brown powder; m.p. 177–179 °C; IR (CHCl3) νmax (cm–1): 1723 (C=O), 1159 (C–O); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.03 (s, 1H), 6.97 (s, 1H), 6.20 (s, 1H), 4.84 (d, J = 2.4 Hz, 2H), 3.94 (s, 3H), 2.60 (t, J = 2.4 Hz, 1H), 2.42 (d, J = 3.4 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.87, 152.51, 150.43, 149.11, 146.76, 113.79, 113.10, 106.03, 102.66, 56.92, 55.93, 18.52; ESIMS: m/z 245.2 [M + H]+; HR-ESIMS: m/z 245.0825 [M + H]+ calcd for C14H12O4 + H+ (245.0814).
4.3.5. 7-(Prop-2-yn-1-yloxy)-2H-chromen-2-one (18)
Yellowish brown powder; yield, 91%; m.p. 114–115 °C; IR (CHCl3) νmax (cm–1): 1726 (C=O), 1673, 1160 (C–O), 1H NMR (400 MHz, CDCl3): δ (ppm) 7.67 (d, J = 14.7 Hz, 1H), 7.42 (d, J = 13.7 Hz, 1H), 6.94–6.90 (m, 2H), 6.29 (d, J = 12.4 Hz, 1H), 4.77 (d, J = 2.0 Hz, 2H), 2.59 (t, J = 2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.18, 160.18, 155.81, 143.29, 128.85, 113.67, 113.20, 113.06, 102.35, 56.22; ESIMS: m/z 201.05 [M + H]+; HR-ESIMS: m/z 201.0561 [M + H]+ calcd for C12H8O3 + H+ (201.0552).
4.4. General Procedure for the Synthesis of Triazoles 8a–o, 10a–d, 13a–d, 16a–d, and 19a–d
To a round-bottom flask, substituted benzyl bromide (7) (1.5 equiv), triethylamine (0.3 equiv), and sodium azide (1.5 equiv) were taken in tert-butanol/water (1:1). This mixture was stirred at room temperature for half an hour. After half an hour, propargylated coumarin 6, 9, 12, 15, or 18 (1.5 equiv) was added along with sodium ascorbate (0.2 equiv) and copper sulfate (0.1 equiv), and the reaction was stirred for 24 h. Then, 30 mL water was added and extraction was done with EtOAc (3 × 50 mL). The three layers are combined and concentrated on a vacuum rotary evaporator. Thus, the residue was purified by column chromatography (solvent system: 40% EtOAc in hexane for 8a–o and 19a–d and 70% EtOAc in hexane for 10a–d, 13a–d, and 16a–d) to obtain final triazoles.
4.4.1. 4-((1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8a)
Off-white coarse powder; yield, 82%; m.p. 176–177 °C; IR (KBr) νmax (cm–1): 1715 (C=O), 1238 (C–O), 1376 (C–N); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.78 (s, 2H), 7.76 (d, J = 13 Hz, 1H), 7.56–7.53 (m, 1H), 7.43–7.37 (m, 1H), 7.31 (dd, J = 8.4, 1.4 Hz, 1H), 7.24–7.22 (m, 1H), 7.03–6.99 (m, 3H), 5.85 (s, 1H), 5.69 (s, 2H), 5.30 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 165.03, 162.46, 159.39, 153.23, 141.61, 132.57, 131.73, 124.31, 123.79, 122.75, 117.85, 116.28, 112.94, 91.24, 62.39, 41.98. ESIMS: m/z 334.11 [M + H]+; HR-ESIMS: m/z 334.1197 [M + H]+ calcd for C19H15N3O3 + H+ (334.1192).
4.4.2. 4-((1-(2-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8b)
White powder; yield, 67%; m.p. 162–163 °C; IR (CHCl3) νmax (cm–1): 1715 (C=O), 767 (C–Cl), 1187 (C–O), 1273 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.79 (s, 1H, triazole), 7.77 (d, J = 8.1 Hz, 1H), 7.55–7.51 (m, 1H), 7.45–7.44 (m, 1H), 7.37–7.33 (m, 1H), 7.31–7.29 (m, 3H), 7.25 (t, J = 4.3 Hz, 1H), 5.85 (s, 1H), 5.72 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.86, 162.65, 153.31, 142.01, 133.54, 132.40, 130.43, 129.86, 129.28, 127.32, 123.93, 123.65, 123.09, 117.13, 115.17, 91.16, 62.86, 51.87; ESIMS: m/z 368.08 [M + H]+; HR-ESIMS: m/z 368.0810 [M + H]+ calcd for C19H14ClN3O3 + H+ (368.0802).
4.4.3. 4-((1-(3-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8c)
White powder; yield, 71%; m.p. 198–199 °C; IR (CHCl3) νmax (cm–1): 1716 (C=O), 761 (C–Cl), 1214 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.78 (dd, J = 8.0, 4.0 Hz, 1H), 7.69 (s, 1H, triazole), 7.56–7.52 (m, 1H), 7.37–7.34 (m, 2H), 7.32–7.29 (m, 2H), 7.25 (d, J = 6.8 Hz, 1H), 7.21 (d, J = 6.5 Hz, 1H), 5.85 (s, 1H), 5.57 (s, 2H), 5.33 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.85, 162.45, 153.25, 142.17, 132.60, 130.59, 129.26, 128.25, 126.24, 123.95, 123.42, 123.17, 116.91, 115.46, 91.46, 62.60, 53.51; ESIMS: m/z 368.08 [M + H]+; HR-ESIMS: m/z 368.0809 [M + H]+ calcd for C19H14ClN3O3 + H+ (368.0802).
4.4.4. 4-((1-(2,6-Dichlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8d)
Yellowish orange powder; yield, 74%; m.p. 174–175 °C; IR (CHCl3) νmax (cm–1): 1716 (C=O), 761 (C–Cl), 1215 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (dd, J = 8.0, 4.0 Hz, 1H), 7.72 (s, 1H, triazole), 7.54–7.52 (m, 1H), 7.45 (d, J = 7.9 Hz, 1H), 7.43 (s, 1H), 7.35–7.30 (m, 2H), 7.25–7.21 (m, 1H), 5.92 (s, 1H), 5.85 (s, 2H), 5.29 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.92, 162.40, 153.83, 141.31, 136.83, 132.29, 131.05, 129.79, 129.53, 124.30, 123.46, 123.23, 122.99, 117.00, 115.47, 91.21, 62.81, 49.25; ESIMS: m/z 402.04 [M + H]+; HR-ESIMS: m/z 402.0415 [M + H]+ calcd for C19H13Cl2N3O3 + H+ (402.0412).
4.4.5. 4-((1-(2-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8e)
Pale white powder; yield, 65%; m.p. 182–183 °C; IR (CHCl3) νmax (cm–1): 1713 (C=O), 1452 (C–F), 1215 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (d, J = 8 Hz, 1H), 7.63 (s, 1H, triazole), 7.56 (t, J = 4 Hz, 1H), 7.34–7.32 (m, 3H), 7.24 (d, J = 8 Hz, 1H), 7.12 (t, J = 7.4 Hz, 2H), 5.84 (s, 1H), 5.56 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.97, 164.25 (d, 1JC–F = 279 Hz), 162.66, 153.35, 141.96, 132.61, 130.20 (d, 3JC–F = 9 Hz), 129.99, 123.94, 123.16, 116.82, 116.46 (d, 2JC–F = 21 Hz), 116.24, 115.41, 91.20, 62.61, 53.71; ESIMS: m/z 352.11 [M + H]+; HR-ESIMS: m/z 352.1107 [M + H]+ calcd for C19H14FN3O3 + H+ (352.1097).
4.4.6. 4-((1-(3-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8f)
White powder; yield, 83%; m.p. 173–175 °C; IR (CHCl3) νmax (cm–1): 1714 (C=O), 1761 (C–F), 1266 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (dd, J = 8.0, 4.4 Hz, 1H), 7.63 (s, 1H, triazole), 7.57–7.53 (m, 3H), 7.34–7.30 (m, 1H), 7.12–7.09 (m, 2H), 5.84 (s, 1H), 5.56 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.97, 164.24 (d, 1JC–F = 247 Hz), 162.66, 152.33, 141.96, 132.61, 130.20 (d, 3JC–F = 8 Hz), 130.03 (d, 4JC–F = 3 Hz), 123.94, 123.20, 123.15, 116.81, 116.45 (d, 2JC–F = 21 Hz), 115.41, 91.19, 62.61, 53.71; ESIMS: m/z 352.11 [M + H]+; HR-ESIMS: m/z 352.1103 [M + H]+ calcd for C19H14FN3O3 + H+ (352.1097).
4.4.7. 4-((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8g)
White powder; yield, 80%; m.p. 179–180 °C; IR (CHCl3) νmax (cm–1): 1714 (C=O), 761 (C–F), 1188 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (d, J = 4.7 Hz, 1H), 7.62 (s, 1H, triazole), 7.57–7.53 (m, 1H), 7.34–7.29 (m, 3H), 7.23–7.21 (m, 1H), 7.12–7.10 (m, 2H), 5.84 (s, 1H), 5.56 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.99, 164.23 (d, 1JC–F = 247 Hz), 162.70, 153.24, 141.93, 132.61, 130.20 (d, 3JC–F = 8 Hz), 130.05 (d, 4JC–F = 4 Hz), 123.96, 123.27, 123.16, 116.78, 116.43 (d, 2JC–F = 8 Hz), 91.64, 62.60, 53.60; ESIMS: m/z 352.11 [M + H]+; HR-ESIMS: m/z 352.1103 [M + H]+ calcd for C19H14FN3O3 + H+ (352.1097).
4.4.8. 4-((1-(2,6-Difluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8h)
White powder; yield, 70%; m.p. 176–178 °C; IR (CHCl3) νmax (cm–1): 1715 (C=O), 1452 (C–F), 1272 (C–O), 1376 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (dd, J = 8.0, 1.5 Hz, 1H), 7.64 (s, 1H), 7.56–7.52 (m, 1H), 7.41–7.38 (m, 2H), 7.33–7.30 (m, 2H), 7.24 (t, J = 7.6 Hz, 1H), 5.85 (s, 1H), 5.59 (s, 2H), 5.31 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 165.04, 162.76, 160.58 (d, 1JC–F = 243 Hz), 153.32, 141.81, 134.12, 132.60, 129.70, 129.07, 128.48,123.35, 123.16, 116.72, 115.42, 91.43, 62.64, 54.78; ESIMS: m/z 370.10 [M + H]+; HR-ESIMS: m/z 370.1010 [M + H]+ calcd for C19H13F2N3O3 + H+ (370.1003).
4.4.9. 4-((1-(2-(Trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8i)
Cream powder; yield, 78%; m.p. 185–186 °C; IR (CHCl3) νmax (cm–1): 1725 (C=O), 1454 (C–F), 1175 (C–O), 1334 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.76 (s, 1H, triazole), 7.74 (s, 2H), 7.60–7.56 (m, 1H), 7.54–7.48 (m, 2H), 7.31–7.28 (m, 2H), 7.25–7.19 (m, 1H), 5.86 (s, 1H), 5.80 (s, 2H), 5.33 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.96, 162.60, 153.34, 149.20, 138.10, 132.63, 131.48 (d, 3JC–F = 32 Hz), 128.38, 126.34 (q, 4JC–F = 7, 4 Hz), 125.06 (d, 1JC–F = 270 Hz), 123.95 (d, 2JC–F = 83 Hz), 123.44, 116.82, 115.39, 91.20, 62.59, 53.56; ESIMS: m/z 402.1 [M + H]+; HR-ESIMS: m/z 402.1073 [M + H]+ calcd for C20H14F3N3O3 + H+ (402.1066).
4.4.10. 4-((1-(4-(Trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8j)
White powder; yield, 69%; m.p. 188–189 °C; IR (CHCl3) νmax (cm–1): 1714 (C=O), 761 (C–F), 1280 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (d, J = 8.4 Hz, 1H), 7.63 (s, 1H, triazole), 7.57–7.53 (m, 1H), 7.34–7.30 (m, 3H), 7.24–7.21 (m, 1H), 7.13–7.07 (m, 2H), 5.84 (s, 1H), 5.56 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.98, 162.67, 161.78, 153.34, 141.95, 132.62, 130.20 (d, 4JC–F = 8 Hz), 129.99, 123.94, 123.17, 123.15, 116.82 (d, 2JC–F = 36 Hz), 116.24, 115.41, 91.20, 62.61, 53.71; ESIMS: m/z 402.10 [M + H]+; HR-ESIMS: m/z 402.1071 [M + H]+ calcd for C20H14F3N3O3 + H+ (402.1066).
4.4.11. 4-((1-(2-(Trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8k)
Light yellow powder; yield, 75%; m.p. 154–155 °C; IR (CHCl3) νmax (cm–1): 1726 (C=O), 1458 (C–F), 1248 (C–O), 1275 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.77 (d, J = 9.2 Hz, 1H), 7.72 (s, 1H, triazole), 7.57–7.52 (m, 1H), 7.47–7.43 (m, 1H), 7.35–7.30 (t, J = 7.4 Hz, 4H), 7.25 (t, J = 6.9 Hz, 1H), 5.85 (s, 1H), 5.68 (s, 2H), 5.32 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.96, 162.63, 153.33, 149.61, 142.07, 132.87, 132.63, 129.79, 130.79 (d, 3JC–F = 36 Hz), 129.72, 123.95 (d, 2JC–F = 81 Hz), 123.36, 121.70, 121.63 (d, 1JC–F = 256 Hz), 116.81, 115.39, 91.20, 62.59, 53.56; ESIMS: m/z 418.10 [M + H]+; HR-ESIMS: m/z 418.1021 [M + H]+ calcd for C20H14F3N3O4 + H+ (418.1015).
4.4.12. 4-((1-(4-(Trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8l)
White powder; yield, 78%; m.p. 165–167 °C; IR (CHCl3) νmax (cm–1): 1727 (C=O), 1452 (C–F), 1243 (C–O), 1278 (C–N); 1H NMR (400 MHz, dimethyl sulfoxide (DMSO)): δ (ppm) δ 8.49 (s, 1H, triazole), 7.74 (dd, J = 9.5, 4.5 Hz, 1H), 7.66–7.62 (m, 1H), 7.50–7.47 (m, 2H), 7.40–7.38 (m, 3H), 7.34–7.30 (m, 1H), 6.16 (s, 1H), 5.71 (s, 2H), 5.43 (s, 2H); 13C NMR (100 MHz, DMSO): δ (ppm) 164.81, 162.04, 153.21, 148.59, 148.57, 141.83, 135.80, 133.27, 130.54, 125.98, 124.66, 123.32, 121.86, 121.75 (d, 1JC–F = 255 Hz), 116.91, 115.50, 91.78, 63.20, 52.50; ESIMS: m/z 418.1 [M + H]+; HR-ESIMS: m/z 418.1015 [M + H]+ calcd for C20H15F3N3O4F3 + H+ (418.1015).
4.4.13. 4-((1-(2-Methylbenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8m)
Yellow powder; yield, 76%; m.p. 155–156 °C; IR (CHCl3) νmax (cm–1): 1715 (C=O), 1261 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.74 (d, J = 6.7 Hz, 1H), 7.59 (s, 1H, triazole), 7.54–7.50 (m, 1H), 7.32–7.29 (m, 2H), 7.25–7.20 (m, 4H), 5.83 (s, 1H), 5.60 (s, 2H), 5.28 (s, 2H), 2.31 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 165.03, 162.72, 153.25, 141.55, 136.98, 132.56, 132.13, 131.17, 129.55, 129.39, 126.80, 123.94, 123.43, 123.19, 116.70, 115.40, 91.09, 62.64, 52.58, 19.05, 18.83; ESIMS: m/z 348.1 [M + H]+; HR-ESIMS: m/z 348.1345 [M + H]+ calcd for C20H17N3O3 + H+ (348.1348).
4.4.14. 4-((1-(3-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8n)
White powder; yield, 60%; m.p. 127–129 °C; IR (CHCl3) νmax (cm–1): 1717 (C=O), 1159 (C–O), 1375 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.78 (dd, J = 7.4, 4.5 Hz, 1H), 7.64 (s, 1H), 7.56–7.52 (m, 1H), 7.34–7.30 (m, 2H), 7.27–7.21 (m, 1H), 6.92–6.88 (m, 2H), 6.84–6.83 (m, 1H), 5.84 (s, 1H), 5.55 (s, 2H), 5.31 (s, 2H), 3.80 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.95, 162.58, 159.88, 153.50, 142.37, 135.69, 132.57, 130.93, 124.23, 122.85, 120.17, 116.48, 115.08, 114.47, 114.09, 90.58, 62.66, 55.25, 54.24; ESIMS: m/z 364.1 [M + H]+; HR-ESIMS: m/z 364.1297 [M + H]+ calcd for C20H18FN3O4 + H+ (364.1303).
4.4.15. 4-((1-(3,5-Dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8o)
Off-white powder; yield, 72%; m.p. 159–160 °C; IR (CHCl3) νmax (cm–1): 1713 (C=O), 1226 (C–O), 1369 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.78 (d, J = 8.0 Hz, 1H), 7.65 (s, 1H, triazole), 7.57–7.53 (m, 1H), 7.32 (d, J = 5.0 Hz, 1H), 7.24 (d, J = 4.8 Hz, 1H), 6.45–6.43 (m, 3H), 5.84 (s, 1H), 5.50 (s, 2H), 5.33 (s, 2H), 3.78 (s, 6H); 13C NMR (100 MHz, CDCl3): δ (ppm) 165.01, 162.68, 161.41, 153.33, 141.83, 136.22, 132.58, 123.94, 123.38, 123.20, 116.79, 115.43, 106.25, 100.50, 91.16, 62.67, 55.48, 54.50; ESIMS: m/z 394.14 [M + H]+; HR-ESIMS: m/z 394.1414 [M + H]+ calcd for C21H19N3O5 + H+ (394.1403).
4.4.16. 4-((1-(4-Nitrobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (8p)
Light yellow powder; yield, 75%; m.p. 193–194 °C; IR (CHCl3) νmax (cm–1): 1731 (C=O), 1673, 1452 (NO2), 1187 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 8.28 (d, J = 1.8 Hz, 2H), 7.75 (dd, J = 7.4, 4.5 Hz, 1H), 7.71 (s, 1H), 7.60–7.54 (m, 1H), 7.46 (d, J = 1.9, 2H), 7.34–7.33 (m, 1H), 7.21–7.19 (m, 1H), 5.86 (s, 1H), 5.73 (s, 2H), 5.34 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.57, 162.52, 154.64, 153.26, 142.51, 141.21, 133.07, 124.90, 123.90, 123.62, 123.36, 116.78, 115.71, 90.76, 62.70, 52.69; ESIMS: m/z 379.10 [M + H]+; HR-ESIMS: m/z 379.1046 [M + H]+ calcd for C19H14N4O5 + H+ (379.1042).
4.4.17. 8-Acetyl-7-((1-(3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (10a)
Light orange powder; yield, 55%; m.p. 213–214 °C; IR (CHCl3) νmax (cm–1): 1731 (C=O), 1141 (C–O), 1391 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.64 (d, J = 7.7 Hz, 1H), 7.57 (s, 1H, triazole), 7.47 (d, J = 7.7 Hz, 1H), 7.30 (d, J = 7.7 Hz, 1H), 7.11 (d, J = 4.0 Hz, 2H), 6.90–6.83 (m, 2H), 6.77 (s, 1H), 6.31 (d, J = 4.3 Hz, 1H), 5.49 (s, 2H), 5.31 (s, 2H), 3.78 (s, 3H), 2.55 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 198.91, 160.19, 159.88, 151.13, 143.03, 142.36, 135.31, 130.22, 129.60, 122.54, 119.86, 114.10, 113.10, 112.79, 109.41, 63.32, 55.28, 54.29, 32.04.; ESIMS: m/z 406.14 [M + H]+; HR-ESIMS: m/z 406.1406 [M + H]+ calcd for C23H20N3O5 + H+ (406.1403).
4.4.18. 8-Acetyl-7-((1-(3,5-dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (10b)
White powder; yield, 54%; m.p. 232–234 °C; IR (CHCl3) νmax (cm–1): 1727 (C=O), 1142 (C–O), 1367 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.64 (d, J = 8.5 Hz, 1H), 7.58 (s, 1H, triazole), 7.45 (d, J = 8.7 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 6.43–6.41 (m, 1H), 6.38 (s, 2H), 6.30–6.27 (m, 1H), 5.42 (s, 2H), 5.29 (s, 2H), 3.76 (s, 6H), 2.56 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 199.32, 161.36, 159.73, 156.99, 151.56, 143.34, 142.82, 136.53, 129.71, 122.87, 120.15, 114.47, 113.34, 109.55, 106.24, 100.22, 62.87, 55.50, 54.66, 32.32.; ESIMS: m/z 436.15 [M + H]+; HR-ESIMS: m/z 436.1509 [M + H]+ calcd for C23H22N3O6 + H+ (436.1508).
4.4.19. 8-Acetyl-7-((1-(2-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (10c)
Orange sticky powder; yield, 51%; IR (CHCl3) νmax (cm–1): 1726 (C=O), 1185 (C–O), 1315 (C–N), 1255 (C–F); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.65 (d, J = 12 Hz, 2H), 7.46–7.41 (m, 2H), 7.33–7.30 (m, 2H), 7.28–7.27 (m, 1H), 7.11 (d, J = 12 Hz, 1H), 6.30 (d, J = 8 Hz, 1H), 5.62 (s, 2H), 5.32 (s, 2H), 2.56 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 198.93, 159.71, 157.17, 151.26, 143.38, 143.00, 130.91, 130.67, 130.49, 129.69, 128.82 (d, 1JC–F = 242 Hz), 127.49, 123.38, 120.63, 114.27, 113.37, 109.56, 63.16, 48.69, 32.43; ESIMS: m/z 444.1 [M + H]+; HR-ESIMS: m/z 444.1160 [M + H]+ calcd for C22H17N3O4F3 + H+ (444.1171).
4.4.20. 8-Acetyl-7-((1-(2-(trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (10d)
Reddish sticky powder; yield, 52%; IR (CHCl3) νmax (cm–1): 1728 (C=O), 1187 (C–O), 1312 (C–N), 1124 (C–F); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.64–7.62 (m, 2H), 7.46–7.40 (m, 2H), 7.32–7.30 (m, 2H), 7.26–7.24 (m, 1H), 7.11 (d, J = 7.7 Hz, 1H), 6.30 (d, J = 9.6 Hz, 1H), 5.62 (s, 2H), 5.32 (s, 2H), 2.56 (s, 3H). 13C NMR (100 MHz, CDCl3): δ (ppm) 198.95, 159.73, 157.16, 151.25, 143.37, 143.01, 130.67 (d, 3JC–F = 18 Hz), 129.69 (d, 1JC–F = 219 Hz), 126.39, 120.62, 120.01, 114.26, 113.37, 109.55, 63.15, 48.69, 32.44; ESIMS: m/z 460.1 [M + H]+; HR-ESIMS: m/z 460.1118 [M + H]+ calcd for C22H17N3O5F3 + H+ (460.1120).
4.4.21. 6,7-Bis((1-(3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-methyl-2H-chromen-2-one (13a)
Yellowish oil; yield, 64%; m.p. 129–131 °C; IR (CHCl3) νmax (cm–1): 1712 (C=O), (NO2), 1162 (C–O), 1388 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) δ 7.55 (d, J = 9.8 Hz, 2H), 7.23–7.21 (m, 1H), 7.19 (s, 1H), 7.17 (s, 1H), 6.87 (s, 2H), 6.83–6.78 (m, 3H), 6.75–6.72 (m, 2H), 6.69–6.68 (m, 1H), 6.08 (s, 1H), 5.41 (d, J = 9.1 Hz, 4H), 5.18 (d, J = 13.2 Hz, 4H), 3.70 (s, 6H), 2.28 (s 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.27, 160.12, 152.45, 152.08, 149.72, 144.67, 144.01, 135.92, 135.78, 130.28, 130.22, 123.42, 123.27, 120.35, 120.24, 114.29, 114.09, 113.83, 113.80, 113.35, 112.66, 111.01, 102.24, 64.21, 63.00, 55.33, 54.23, 18.86; ESIMS: m/z 595.2 [M + H]+; HR-ESIMS: m/z 595.2297 [M + H]+ calcd for C32H31N6O6 + H+ (595.2305).
4.4.22. 6,7-Bis((1-(3,5-dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-methyl-2H-chromen-2-one (13b)
Light yellow powder; yield, 61%; m.p. 124–126 °C; IR (CHCl3) νmax (cm–1): 1711 (C=O), 1159 (C–O), 1430 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.56 (d, J = 8.1 Hz, 2H), 7.17 (s,1H), 6.87 (s, 1H, triazole), 6.35–6.33 (m, 4H), 6.29 (d, J = 2.2 Hz, 2H), 6.08 (d, J = 1.1 Hz, 1H), 5.37 (d, J = 9.0 Hz, 4H), 5.19 (d, J = 11.5 Hz, 4H), 3.67 (s, 12H), 2.28 (d, J = 1.0 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.34, 161.31, 161.26, 152.44, 152.08, 149.72, 144.66, 144.02, 143.09, 136.60, 136.46, 123.43, 123.27, 113.34, 112.64, 110.97, 106.17, 106.12, 102.22, 100.48, 100.28, 64.21, 63.00, 55.44, 54.24, 18.85; ESIMS: m/z 655.2 [M + H]+; HR-ESIMS: m/z 655.2520 [M + H]+ calcd for C34H35N6O8 + H+ (655.2516).
4.4.23. 4-Methyl-6,7-bis((1-(2-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (13c)
Pale yellow powder; yield, 74%; m.p. 168–170 °C; IR (CHCl3) νmax (cm–1): 1728 (C=O), 1673, 1452 (NO2), 1163 (C–O), 1314 (C–N), 1062 (C–F); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.73 (d, J = 8.1 Hz, 2H), 7.65 (d, J = 8.2 Hz, 2H), 7.54–7.49 (m,2H), 7.46–7.44 (m, 3H), 7.24 (s, 1H), 7.20 (d, J = 1.2 Hz, 1H), 7.16 (d, J = 7.7 Hz, 1H), 6.96 (s, 1H), 6.16 (d, J = 1.2 Hz, 1H), 5.73 (d, J = 7.8 Hz, 4H), 5.28 (d, J = 9.8 Hz, 4H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.19, 152.34, 152.05, 149.75, 144.69, 144.16, 143.29, 132.79, 132.69, 130.27, 130.19, 128.89, 128.16 (d, 2JC–F = 31 Hz), 126.32 (q, 4JC–F = 6 Hz), 125.45 (d, 1JC–F = 272 Hz), 123.79 (d, 3JC–F = 15 Hz), 113.41, 112.75, 110.99, 102.39, 64.08, 63.02, 50.26, 18.81. ESIMS: m/z 671.18 [M + H]+; HR-ESIMS: m/z 671.1832 [M + H]+ calcd for C32H25N6O4F6 + H+ (671.1841).
4.4.24. 4-Methyl-6,7-bis((1-(2-(trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (13d)
Light brown powder; yield, 78%; m.p. 173–174 °C; IR (CHCl3) νmax (cm–1): 1724 (C=O), 1184 (C–O), 1339 (C–N) 1070 (C–F); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.67 (d, J = 8.0 Hz, 2H), 7.44–7.38 (m, 2H), 7.33–7.31 (m, 2H), 7.29 (s, 1H), 7.25 (s, 2H), 7.24–7.23 (m, 1H), 7.20–7.10 (m, 1H), 6.96 (s, 1H), 6.16 (d, J = 1.2 Hz, 1H), 5.63 (d, J = 7.8 Hz, 4H), 5.28 (d, J = 9.8 Hz, 4H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.25, 152.40, 152.01, 149.71, 146.94, 144.67, 144.06, 130.58, 130.55, 130.50 (d, 3JC–F = 11Hz), 130.39, 127.54 (d, 4JC–F = 8 Hz), 127.12 (d, 4JC–F = 8 Hz), 123.66 (d, 2JC–F = 16 Hz), 120.63, 113.38, 112.71, 110.88, 102.34, 64.01, 62.98, 48.49, 18.82; ESIMS: m/z 703.1 [M + H]+; HR-ESIMS: m/z 703.1733 [M + H]+ calcd for C32H25N6O4F6 + H+ (703.1740).
4.4.25. 6-Methoxy-7-((1-(3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-methyl-2H-chromen-2-one (16a)
Light yellow powder; yield, 70%; m.p. 159–161 °C; IR (CHCl3) νmax (cm–1): 1713 (C=O), 1275 (C–O), 1381 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.61 (s, 1H, triazole), 7.31–7.27 (m, 1H), 7.00 (s, 1H), 6.93 (s, 1H), 6.91–6.85 (m, 2H), 6.80–6.78 (m, 1H), 6.17 (s, 1H), 5.50 (s, 2H), 5.28 (s, 2H), 3.89 (s, 3H), 3.78 (s, 3H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.38, 161.34, 152.19, 151.20, 149.07, 146.45, 143.17, 136.36, 123.13, 113.11, 112.68, 106.20, 105.59, 101.94, 100.55, 63.07, 56.47, 55.46, 54.39, 18.91; ESIMS: m/z 408.1 [M + H]+; HR-ESIMS: m/z 408.1566 [M + H]+ calcd for C12H21N3O5 + H+ (408.1559).
4.4.26. 7-((1-(3,5-Dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-6-methoxy-4-methyl-2H-chromen-2-one (16b)
White powder; yield, 71%; m.p. 151–152 °C; IR (CHCl3) νmax (cm–1): 1728 (C=O), 1123 (C–O), 1350 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.61 (s, 1H, triazole), 7.03 (s, 1H), 6.93 (s, 1H), 6.43 (s, 1H), 6.37 (s, 2H), 6.18 (s, 1H), 5.45 (s, 2H), 5.29 (s, 2H), 3.89 (s, 3H), 3.76 (s, 6H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.23, 151.92, 151.16, 148.87, 146.62, 143.10, 136.03, 123.20, 113.07, 112.84, 106.56, 105.75, 101.49, 100.75, 63.17, 56.37, 55.63, 54.38, 19.08; ESIMS: m/z 438.1 [M + H]+; HR-ESIMS: m/z 438.1659 [M + H]+ calcd for C23H23N3O6 + H+ (438.1665).
4.4.27. 6-Methoxy-4-methyl-7-((1-(2-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (16c)
White powder; yield, 70%; m.p. 151–153 °C; IR (CHCl3) νmax (cm–1): 1727 (C=O), 1273 (C–O), 1427 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.67 (d, J = 8.5 Hz, 1H), 7.57 (s, 1H, triazole), 7.49–7.46 (m, 1H), 7.42–7.38 (m, 1H), 7.17 (s, 1H), 6.95 (s, 1H), 6.87 (s, 1H), 6.12 (s, 1H), 5.68 (s, 2H), 5.24 (s, 2H), 3.83 (s, 3H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.37, 152.16, 151.16, 149.06, 146.48, 143.44, 132.82, 130.39, 128.91, 123.16 (d, 1JC–F = 274 Hz), 113.21, 112.76, 105.65, 102.11, 63.09, 56.49, 49.68, 18.91; ESIMS: m/z 446.1 [M + H]+; HR-ESIMS: m/z 446.1330 [M + H]+ calcd for C22H18N3O4F3 + H+ (446.1328).
4.4.28. 6-Methoxy-4-methyl-7-((1-(2-(trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (16d)
White powder; yield, 79%; m.p. 164–166 °C; IR (CHCl3) νmax (cm–1): 1718 (C=O), 1275 (C–O), 1385 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.66 (s, 1H, triazole), 7.44–7.40 (m, 1H), 7.33–7.27 (m, 3H), 7.02 (s, 1H), 6.94 (s, 1H), 6.18 (s, 1H), 5.63 (s, 2H), 5.30 (s, 2H), 3.90 (s, 3H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.39, 152.21, 151.16, 149.05, 146.93, 146.46, 143.28, 130.60, 127.56, 127.03, 123.45 (d, 1JC–F = 257 Hz), 120.63, 113.16, 112.70, 105.61, 102.00, 63.03, 56.48, 48.47, 18.92. ESIMS: m/z 462.1 [M + H]+; HR-ESIMS: m/z 462.1265 [M + H]+ calcd for C22H19N3O5F3 + H+ (462.1277).
4.4.29. 7-((1-(3-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (19a)
White powder; yield, 70%; m.p. 137–138 °C; IR (CHCl3) νmax (cm–1): 1725 (C=O), 1156 (C–O), 1305 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.64 (d, J = 9.5 Hz, 1H), 7.60 (s, 1H, triazole), 7.38 (d, J = 8.4 Hz, 1H), 7.31 (d, J = 7.5 Hz, 1H), 6.92–6.86 (m, 4H), 6.81 (t, J = 4.5 Hz, 1H), 6.26 (d, J = 9.5 Hz, 1H), 5.52 (s, 2H), 5.22 (s, 2H), 3.78 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.67, 160.95, 160.19, 155.93, 143.75, 135.76, 130.78, 129.01, 123.01, 120.08, 114.27, 113.83, 113.44, 112.98, 112.77, 101.59, 62.23, 55.51, 53.83. ESIMS: m/z 364.1 [M + H]+; HR-ESIMS: m/z 364.1303 [M + H]+ calcd for C20H17N3O4 + H+ (364.1297).
4.4.30. 7-((1-(3,5-Dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (19b)
Light yellow powder; yield, 69%; m.p. 132–133 °C; IR (CHCl3) νmax (cm–1): 1728 (C=O), 1120 (C–O), 1277 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.65 (d, J = 9.5 Hz, 1H), 7.59 (s, 1H, triazole), 7.39 (d, J = 7.9 Hz, 1H), 6.93–6.90 (m, 2H), 6.44–6.43 (m, 1H), 6.41 (d, J = 7.8 Hz, 1H), 6.28 (d, J = 7.4 Hz, 1H), 5.47 (s, 2H), 5.23 (s, 2H), 3.76 (s, 6H); 13C NMR (100 MHz, CDCl3): δ (ppm) 161.37, 161.30, 161.09, 155.50, 143.38, 136.30, 128.91, 122.85, 113.49, 113.00, 112,78, 106.20, 102.08, 100.47, 61.94, 55.25, 54.25. ESIMS: m/z 394.14 [M + H]+; HR-ESIMS: m/z 394.1406 [M + H]+ calcd for C21H19N3O5 + H+ (394.1403).
4.4.31. 7-((1-(2-(Trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (19c)
Dark brown powder; yield, 67%; m.p. 119–121 °C; IR (CHCl3) νmax (cm–1): 1731 (C=O), 1673, 1452 (NO2), 1187 (C–O), 1377 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.74 (d, J = 7.8 Hz, 1H), 7.68 (s, 1H, triazole), 7.65 (d, J = 9.5 Hz, 1H), 7.56–7.53 (m, 1H), 7.49–7.46 (m, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.25 (d, J = 7.6 Hz, 1H), 6.93–6.91 (m, 1H), 6.89–6.88 (m, 1H), 6.26 (d, J = 9.5 Hz, 1H), 5.77 (s, 2H), 5.25 (s, 2H). 13C NMR (100 MHz, CDCl3): δ (ppm) 161.26, 161.05, 155.66, 143.51, 143.38, 132.79, 130.28, 128.95 (d,3JC–F = 3 Hz), 128.13 (d, 2JC–F = 30 Hz), 126.37 (q, 4JC–F = 12, 6 Hz), 125.44 (d, 1JC–F = 272 Hz), 123.54, 113.41, 113.00, 112.77, 102.02, 62.25, 50.32. ESIMS: m/z 402.1 [M + H]+; HR-ESIMS: m/z 402.1076 [M + H]+ calcd for C20H14N3O3F3 + H+ (402.1066).
4.4.32. 7-((1-(2-(Trifluoromethoxy)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (19d)
Pale white powder; yield, 62%; m.p. 123–124 °C; IR (CHCl3) νmax (cm–1): 1723 (C=O), 1124 (C–O), 1274 (C–N); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.78 (d, J = 7.9 Hz, 1H), 7.74 (s, 1H, triazole), 7.57–7.53 (m, 1H), 7.48–7.43 (m, 1H), 7.37–7.34 (m, 3H), 7.33 (d, J = 7.5 Hz, 1H), 7.27–7.23 (m, 1H), 5.87 (s, 1H), 5.69 (s, 2H), 5.34 (s, 2H); 13C NMR (100 MHz, CDCl3): δ (ppm) 164.99, 162.64, 153.32, 147.04, 141.89, 132.57, 130.77 (d, 4JC–F = 8.08 Hz), 127.60, 126.82, 123.93, 123.70, 123.14, 121.76 (d, 1JC–F = 259.76 Hz), 120.65, 116.76, 115.41, 91.17, 62.61, 48.70; ESIMS: m/z 418.1 [M + H]+; HR-ESIMS: m/z 418.1006 [M + H]+ calcd for C20H14N3O4F3 + H+ (418.1015).
4.5. In Vitro AChE and BChE Inhibition Assay
The cholinesterase inhibition experiments, including the enzyme kinetics, were evaluated by Ellman assay as described earlier.32
4.6. In Vitro BACE-1 Inhibition Assay
The fluorescence resonance energy transfer (FRET) assay was used to screen all synthesized compounds24 for BACE-1 inhibition and its enzyme kinetics.
4.7. Molecular Modeling
The Schrodinger software was used to carry out the molecular docking study. The crystal structures of human AChE (PDB ID: 4EY7),34 human BChE (PDB ID: 6EP4),35 and human BACE-1 (PDB ID: 1W51)36 were obtained from the RCSB Protein Data Bank. The protocols employed were as given under the default settings of glide. Further, MD simulation was carried out using Desmond software (v 3.8) for 50 ns by a similar protocol as used in the previous publication of our group.20
4.8. Aβ-42 Self-Aggregation Inhibition Assay
The fluffy white solid obtained by pretreatment of amyloid β 1–42 peptide (PP69, Sigma) was reconstituted to produce a 200 mM solution in a mixture of ACN/Na2CO3/NaOH in a ratio of 48.4:48.4:3.2. The Aβ aliquots with and without test inhibitors were incubated for 24 h at 30 °C. thioflavin T (ThT) fluorescence method was used to assess the aggregates.37
4.9. In Vitro PAMPA–BBB Assay
The samples were diluted with PBS (pH 7.4). The acceptor plate wells were filled with 300 μL of PBS. The 4 μL of 20 mg/mL porcine brain lipid (PBL) in dodecane was used to coat the permeable layer of the donor plate. The test compounds were added to the donor plate. The donor plate was kept on the acceptor plate like a “sandwich” and incubated at 25 °C for 18 h. UV absorbance was used to access the concentration of test samples in donor and acceptor wells. As described earlier, the absorbance was used to calculate the test compound’s effective permeability (Pe).38
4.10. In Silico ADME Properties
The ADME properties of ligands were determined using the QikProp module of the Schrodinger software. The descriptors and properties for reference ranges are for 95% of known drugs.
Acknowledgments
The authors thank the analytical department, IIIM, for analytical support. The financial support from CSIR YSA Grant (P90807) is gratefully acknowledged. IIIM Publication Number. CSIR-IIIM/IPR/00518.
Glossary
Abbreviations.
- AD
Alzheimer’s disease
- ACh
acetylcholine
- ADME
absorption distribution metabolism elimination
- BCh
butyrylcholine
- BBB
blood–brain barrier
- CAS
catalytic anionic site
- EeAChE
electric eel cholinesterase
- eqBChE
equine serum butyrylcholinesterase
- hBACE-1
human amyloid β precursor protein cleaving enzyme or β-secretase
- MD
molecular dynamics
- NP
natural product
- PDB
Protein Data Bank
- PAS
peripheral anionic site
- PAMPA
parallel artificial membrane permeability assay
- Pe
effective permeability coefficient
- RMSD
root mean square deviation
- SAR
structure–activity relationship
- ThT
thioflavin T
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07993.
Spectral data scans for all compounds (PDF)
Author Contributions
S.B.B. designed, executed, and coordinated this study. A.S. synthesized all compounds and performed biological assays and molecular modeling. A.S. and S.B.B. contributed to the manuscript writing.
The authors declare no competing financial interest.
Supplementary Material
References
- a Tatulian S. A. Challenges and hopes for Alzheimer’s disease. Drug Discovery Today 2022, 27, 1027–1043. 10.1016/j.drudis.2022.01.016. [DOI] [PubMed] [Google Scholar]; b Palmioli A.; Mazzoni V.; De Luigi A.; Bruzzone C.; Sala G.; Colombo L.; Bazzini C.; Zoia C. P.; Inserra M.; Salmona M.; De Noni I.; Ferrarese C.; Diomede L.; Airoldi C. Alzheimer’s Disease Prevention through Natural Compounds: Cell-Free, In Vitro, and In Vivo Dissection of Hop (Humulus lupulus L.) Multitarget Activity. ACS Chem. Neurosci. 2022, 13, 3152–3167. 10.1021/acschemneuro.2c00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra P. A. Impairments of attention in Alzheimer’s disease. Curr. Opin. Psychol. 2019, 29, 41–48. 10.1016/j.copsyc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Li Q.; Yang H.; Chen Y.; Sun H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. 10.1016/j.ejmech.2017.03.062. [DOI] [PubMed] [Google Scholar]
- Mehta M.; Adem A.; Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swahn B.-M.; Kolmodin K.; Karlström S.; Berg S.; Söderman P.; Holenz J.; Lindström J.; Sundström M.; Turek D.; Kihlström J.; Slivo C.; Andersson L.; Pyring D.; Rotticci D.; Ohberg L.; Kers A.; Bogar K.; von Kieseritzky F.; Bergh M.; Falting J.; et al. Design and Synthesis of β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1) Inhibitors with in Vivo Brain Reduction of β-Amyloid Peptides. J. Med. Chem. 2012, 55, 9346–9361. 10.1021/jm3009025. [DOI] [PubMed] [Google Scholar]
- Finder V. H.; Glockshuber R. Amyloid-beta aggregation. Neurodegener. Dis. 2007, 4, 13–27. 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- Nirmalraj P. N.; List J.; Battacharya S.; Howe G.; Xu L.; Thompson D.; Mayer M. Complete aggregation pathway of amyloid b (1-40) and (1-42) resolved on an atomically clean interface. Sci. Adv. 2020, 6, eaaz6014 10.1126/sciadv.aaz6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz M.; Silva V.; Monteiro C.; Silvestre S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interventions Aging 2022, 17, 797–810. 10.2147/CIA.S325026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bharate S. S.; Mignani S.; Vishwakarma R. A. Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis. J. Med. Chem. 2018, 61, 10345–10374. 10.1021/acs.jmedchem.7b01922. [DOI] [PubMed] [Google Scholar]; b Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]; c Dias D. A.; Urban S.; Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro A.; Matos M.; Uriarte E.; Santana L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501 10.3390/molecules26020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Küpeli Akkol E.; Genç Y.; Karpuz B.; Sobarzo-Sánchez E.; Capasso R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959 10.3390/cancers12071959. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Al-Warhi T.; Sabt A.; Elkaeed E. B.; Eldehna W. M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163 10.1016/j.bioorg.2020.104163. [DOI] [PubMed] [Google Scholar]; c Endo S.; Oguri H.; Segawa J.; Kawai M.; Hu D.; Xia S.; Okada T.; Irie K.; Fujii S.; Gouda H.; Iguchi K.; Matsukawa T.; Fujimoto N.; Nakayama T.; Toyooka N.; Matsunaga T.; Ikari A. Development of Novel AKR1C3 Inhibitors as New Potential Treatment for Castration-Resistant Prostate Cancer. J. Med. Chem. 2020, 63, 10396–10411. 10.1021/acs.jmedchem.0c00939. [DOI] [PubMed] [Google Scholar]
- a Sutar S. M.; Savanur H. M.; Malunavar S. S.; Pawashe G. M.; Aridoss G.; Kim K. M.; Lee J. Y.; Kalkhambkar R. G. Synthesis and Molecular Modelling Studies of Coumarin and 1-Aza-Coumarin Linked Miconazole Analogues and Their Antimicrobial Properties. ChemistrySelect 2020, 5, 1322–1330. 10.1002/slct.201903572. [DOI] [Google Scholar]; b Liu H.; Xia D. G.; Chu Z. W.; Hu R.; Cheng X.; Lv X. H. Novel coumarin-thiazolyl ester derivatives as potential DNA gyrase Inhibitors: Design, synthesis, and antibacterial activity. Bioorg. Chem. 2020, 100, 103907 10.1016/j.bioorg.2020.103907. [DOI] [PubMed] [Google Scholar]
- a Pan Y.; Liu T.; Wang X.; Sun J. Research progress of coumarins and their derivatives in the treatment of diabetes. J. Enzyme Inhib. Med. Chem. 2022, 37, 616–628. 10.1080/14756366.2021.2024526. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ranđelović S.; Bipat R. A Review of Coumarins and Coumarin-Related Compounds for Their Potential Antidiabetic Effect. Clin. Med. Insights: Endocrinol. Diabetes 2021, 14, 11795514211042023 10.1177/11795514211042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Muke S.; Kaikini A.; Peshattiwar V.; Bagle S.; Dighe V.; Sathaye S. Neuroprotective Effect of Coumarin Nasal Formulation: Kindling Model Assessment of Epilepsy. Front. Pharmacol. 2018, 9, 992 10.3389/fphar.2018.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rodríguez-Enríquez F.; Costas-Lago M. C.; Besada P.; Alonso-Pena M.; Torres-Terán I.; Viña D.; Fontenla J.; Sturlese M.; Moro S.; Quezada E.; Terán C. Novel coumarin-pyridazine hybrids as selective MAO-B inhibitors for the Parkinson′s disease therapy. Bioorg. Chem. 2020, 104, 104203 10.1016/j.bioorg.2020.104203. [DOI] [PubMed] [Google Scholar]
- Stefanachi A.; Leonetti F.; Pisani L.; Catto M.; Carotti A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mzezewa S. C.; Omoruyi S. I.; Zondagh L. S.; Malan S. F.; Ekpo O. E.; Joubert J. Design, synthesis, and evaluation of 3,7-substituted coumarin derivatives as multifunctional Alzheimer′s disease agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 1606–1620. 10.1080/14756366.2021.1913137. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Singh A.; Sharma S.; Arora S.; Attri S.; Kaur P.; Kaur Gulati H.; Bhagat K.; Kumar N.; Singh H.; Vir Singh J.; Mohinder Singh Bedi P. New coumarin-benzotriazole based hybrid molecules as inhibitors of acetylcholinesterase and amyloid aggregation. Bioorg. Med. Chem. Lett. 2020, 30, 127477 10.1016/j.bmcl.2020.127477. [DOI] [PubMed] [Google Scholar]; c Amin K. M.; Abdel Rahman D. E.; Abdelrasheed Allam H.; El-Zoheiry H. H. Design and synthesis of novel coumarin derivatives as potential acetylcholinesterase inhibitors for Alzheimer′s disease. Bioorg. Chem. 2021, 110, 104792 10.1016/j.bioorg.2021.104792. [DOI] [PubMed] [Google Scholar]; d Zhang J.; Li J. C.; Song J. L.; Cheng Z. Q.; Sun J. Z.; Jiang C. S. Synthesis and evaluation of coumarin/1,2,4-oxadiazole hybrids as selective BChE inhibitors with neuroprotective activity. J. Asian Nat. Prod. Res. 2019, 21, 1090–1103. 10.1080/10286020.2018.1492566. [DOI] [PubMed] [Google Scholar]
- a Anand P.; Singh B.; Singh N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. 10.1016/j.bmc.2011.12.042. [DOI] [PubMed] [Google Scholar]; b Bhatia R.; Chakrabarti S. S.; Kaur U.; Parashar G.; Banerjee A.; Rawal K. R. Multi-Target Directed Ligands (MTDLs): Promising Coumarin Hybrids for Alzheimer′s Disease. Curr. Alzheimer Res. 2021, 18, 802–830. 10.2174/1567205018666211208140551. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Nuthakki V. K.; Gairola S.; Singh B.; Bharate S. B. A Coumarin-Donepezil Hybrid as a Blood-Brain Barrier Permeable Dual Cholinesterase Inhibitor: Isolation, Synthetic Modifications, and Biological Evaluation of Natural Coumarins. ChemMedChem 2022, 17, e202200300 10.1002/cmdc.202200300. [DOI] [PubMed] [Google Scholar]
- Liu W.; Wu L.; Liu W.; Tian L.; Chen H.; Wu Z.; Wang N.; Liu X.; Qiu J.; Feng X.; Xu Z.; Jiang X.; Zhao Q. Design, synthesis and biological evaluation of novel coumarin derivatives as multifunctional ligands for the treatment of Alzheimer′s disease. Eur. J. Med. Chem. 2022, 242, 114689 10.1016/j.ejmech.2022.114689. [DOI] [PubMed] [Google Scholar]
- Sharma M.; Sharma A.; Nuthakki V. K.; Bhatt S.; Nandi U.; Bharate S. B. Design, synthesis, and structure-activity relationship of caffeine-based triazoles as dual AChE and BACE-1 inhibitors. Drug Dev. Res. 2022, 83, 1803–1821. 10.1002/ddr.21998. [DOI] [PubMed] [Google Scholar]
- a Najafi Z.; Mahdavi M.; Saeedi M.; Karimpour-Razkenari E.; Asatouri R.; Vafadarnejad F.; Moghadam F. H.; Khanavi M.; Sharifzadeh M.; Akbarzadeh T. Novel tacrine-1,2,3-triazole hybrids: In vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur. J. Med. Chem. 2017, 125, 1200–1212. 10.1016/j.ejmech.2016.11.008. [DOI] [PubMed] [Google Scholar]; b Le-Nhat-Thuy G.; Nguyen Thi N.; Pham-The H.; Dang Thi T. A.; Nguyen Thi H.; Nguyen Thi T. H.; Nguyen Hoang S.; Nguyen T. V. Synthesis and biological evaluation of novel quinazoline-triazole hybrid compounds with potential use in Alzheimer′s disease. Bioorg. Med. Chem. Lett. 2020, 30, 127404 10.1016/j.bmcl.2020.127404. [DOI] [PubMed] [Google Scholar]; c Mohammadi-Khanaposhtani M.; Saeedi M.; Zafarghandi N. S.; Mahdavi M.; Sabourian R.; Razkenari E. K.; Alinezhad H.; Khanavi M.; Foroumadi A.; Shafiee A.; Akbarzadeh T. Potent acetylcholinesterase inhibitors: design, synthesis, biological evaluation, and docking study of acridone linked to 1,2,3-triazole derivatives. Eur. J. Med. Chem. 2015, 92, 799–806. 10.1016/j.ejmech.2015.01.044. [DOI] [PubMed] [Google Scholar]
- Monceaux C. J.; Hirata-Fukae C.; Lam P. C. H.; Totrov M. M.; Matsuoka Y.; Carlier P. R. Triazole-linked reduced amide isosteres: an approach for the fragment-based drug discovery of anti-Alzheimer′s BACE1 inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3992–3996. 10.1016/j.bmcl.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Kaur A.; Narang S. S.; Kaur A.; Mann S.; Priyadarshi N.; Goyal B.; Singhal N. K.; Goyal D. Multifunctional Mono-Triazole Derivatives Inhibit Aβ42 Aggregation and Cu2+-Mediated Aβ42 Aggregation and Protect Against Aβ42-Induced Cytotoxicity. Chem. Res. Toxicol. 2019, 32, 1824–1839. 10.1021/acs.chemrestox.9b00168. [DOI] [PubMed] [Google Scholar]
- Nuthakki V. K.; Mudududdla R.; Sharma A.; Kumar A.; Bharate S. B. Synthesis and biological evaluation of indoloquinoline alkaloid cryptolepine and its bromo-derivative as dual cholinesterase inhibitors. Bioorg. Chem. 2019, 90, 103062 10.1016/j.bioorg.2019.103062. [DOI] [PubMed] [Google Scholar]
- Raghuvanshi R.; Jamwal A.; Nandi U.; Bharate S. B. Multitargeted C9-substituted ester and ether derivatives of berberrubine for Alzheimer′s disease: Design, synthesis, biological evaluation, metabolic stability, and pharmacokinetics. Drug Dev. Res. 2023, 84, 121–140. 10.1002/ddr.22017. [DOI] [PubMed] [Google Scholar]
- Hamed M. Y. Prediction of Drug Potencies of BACE1 Inhibitors: A Molecular Dynamics Simulation and MM_GB(PB)SA Scoring. Computation 2020, 8, 106 10.3390/computation8040106. [DOI] [Google Scholar]
- Bharadwaj P. R.; Dubey A. K.; Masters C. L.; Martins R. N.; Macreadie I. G. Abeta aggregation and possible implications in Alzheimer′s disease pathogenesis. J. Cell. Mol. Med. 2009, 13, 412–421. 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs T.; Ritter C.; Adrian M.; Riek-Loher D.; Bohrmann B.; Dobeli H.; Schubert D.; Riek R. 3D structure of Alzheimer′s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 17342–17347. 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari S.; Kaynak F. B.; Dalkara S. Synthesis and anticonvulsant screening of 1,2,4-triazole derivatives. Pharmacol. Rep. 2018, 70, 1116–1123. 10.1016/j.pharep.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Xia Y.-L.; Wang J.-J.; Li S.-Y.; Liu Y.; Gonzalez F. J.; Wang P.; Ge G.-B. Synthesis and structure-activity relationship of coumarins as potent Mcl-1 inhibitors for cancer treatment. Bioorg. Med. Chem. 2021, 29, 115851 10.1016/j.bmc.2020.115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Rojas P.; Janeczko M.; Kubiński K.; Amesty Á.; Masłyk M.; Estévez-Braun A. Synthesis and Antimicrobial Activity of 4-Substituted 1,2,3-Triazole-Coumarin Derivatives. Molecules 2018, 23, 199 10.3390/molecules23010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin N.; Nuthakki V. K.; Abdullaha M.; Hassan Q. P.; Gandhi S. G.; Bharate S. B. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus jacq as a dual cholinesterase inhibitor. ACS Omega 2020, 5, 1616–1624. 10.1021/acsomega.9b03693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J.; Rudolph M. J.; Burshteyn F.; Cassidy M. S.; Gary E. N.; Love J.; Franklin M. C.; Height J. J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L.; Brazzolotto X.; Macdonald I. R.; Wandhammer M.; Trovaslet-Leroy M.; Darvesh S.; Nachon F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098 10.3390/molecules22122098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.; Vuillard L.; Cleasby A.; Murray C. W.; Yon J. Apo and inhibitor complex structures of BACE (beta-secretase). J. Mol. Biol. 2004, 343, 407–416. 10.1016/j.jmb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Abdullaha M.; Nuthakki V. K.; Bharate S. B. Discovery of methoxy-naphthyl linked N-(1-benzylpiperidine) benzamide as a blood-brain permeable dual inhibitor of acetylcholinesterase and butyrylcholinesterase. Eur. J. Med. Chem. 2020, 207, 112761 10.1016/j.ejmech.2020.112761. [DOI] [PubMed] [Google Scholar]
- Nuthakki V. K.; Yadav Bheemanaboina R. R.; Bharate S. B. Identification of aplysinopsin as a blood-brain barrier permeable scaffold for anti-cholinesterase and anti-BACE-1 activity. Bioorg. Chem. 2021, 107, 104568 10.1016/j.bioorg.2020.104568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.