Abstract
Curcumin has been credited with a wide spectrum of pharmacological properties for the prevention and treatment of several chronic diseases such as arthritis, autoimmune diseases, cancer, cardiovascular diseases, diabetes, hemoglobinopathies, hypertension, infectious diseases, inflammation, metabolic syndrome, neurological diseases, obesity, and skin diseases. However, due to its weak solubility and bioavailability, it has limited potential as an oral medication. Numerous factors including low water solubility, poor intestinal permeability, instability at alkaline pH, and fast metabolism contribute to curcumin’s limited oral bioavailability. In order to improve its oral bioavailability, different formulation techniques such as coadministration with piperine, incorporation into micelles, micro/nanoemulsions, nanoparticles, liposomes, solid dispersions, spray drying, and noncovalent complex formation with galactomannosides have been investigated with in vitro cell culture models, in vivo animal models, and humans. In the current study, we extensively reviewed clinical trials on various generations of curcumin formulations and their safety and efficacy in the treatment of many diseases. We also summarized the dose, duration, and mechanism of action of these formulations. We have also critically reviewed the advantages and limitations of each of these formulations compared to various placebo and/or available standard care therapies for these ailments. The highlighted integrative concept embodied in the development of next-generation formulations helps to minimize bioavailability and safety issues with least or no adverse side effects and the provisional new dimensions presented in this direction may add value in the prevention and cure of complex chronic diseases.
1. Introduction
Chronic diseases including autoimmune diseases, cancer, cardiovascular diseases, diabetes, hepatocellular, neurological, and renal diseases have persistent high incidence and fatality rates worldwide.1,2 Finding feasible treatment strategies are challenging due to the high prevalence of these diseases and the involvement of several pathways in their development, including JAK/STAT3, JNK, NF-κB, MEK/ERK, p38/MAPK, and PI3K/Akt/mTOR, etc.1−10 Therefore, classical monotarget therapies are insufficient to treat these diseases. Besides, the cost of contemporary pharmaceuticals is high, and have several unfavorable side effects.7,10,11 Indeed, there is an increasing need for the development of safer, effective, multitargeted, and cost-effective therapeutic regimens to replace the current harmful and ineffective treatment approaches. A growing body of preclinical and clinical evidence suggests that natural substances derived from diverse plants are potential therapeutic candidates against a wide range of fatal chronic conditions, and their alternative formulations can be employed to boost the bioavailabilities of these substances.2,7,10,12−14
The perennial herb turmeric, Curcuma longa Linn. belongs to the Zingiberaceae family, is indigenous to South Asia’s tropical areas. The rhizomes of this plant have been used for centuries as a remedy for several diseases in the Indian (Ayurveda) and Chinese Medicinal Systems.15−17 Curcumin is a bioactive phytochemical derived from this rhizome. It has traditionally been used as a spice, food preservative, and coloring ingredient.15,18 The chemical name for curcumin is diferuloylmethane (C21H20O6) and the IUPAC name is (1E-6E)-1,7-bis(4-hydroxy-3-methoxy phenyl)-1,6-heptadiene-3,5-dione with a molecular weight of 368.37 g/mol and melting point of 183 °C. The two aryl rings in curcumin are symmetrically connected to a β-diketone moiety by ortho-methoxy phenolic groups.17−21 A pH-dependent keto-enol tautomerism appears in curcumin wherein the stable enol form predominates in an alkaline medium and a keto form in acidic and neutral conditions.17 In addition, curcumin’s color varies depending on the pH level, yielding a brilliant yellow solution between 2.5 and 7.0, and turning to dark red when the pH rises over that level.17,20
Currently, there are several curcumin-based products available in the market, including pills, ointments, capsules, and cosmetics.16,22−24 Turmeric and curcumin have been the established remedies for various ailments, primarily as antiatherosclerotic, antibacterial, anticancerous, antifungal, anti-inflammatory, antioxidant, antithrombotic, and antiviral agents.22,25,26 Additionally, a comprehensive analysis of the literature identified curcumin as one of the excellent natural compounds that exhibit analgesic, antirheumatic effects, hypoglycemia, hypolipidemia, hepatoprotective, nephron protective, pulmonoprotective, and cardioprotective activities.18,21,27−35 Besides, in vitro studies have shown that curcumin modulates several cell signaling pathways, upregulates p53, p21, and p27, downregulates cell survival gene products, and induces apoptosis.15,36−39 Numerous clinical studies have demonstrated its outstanding safety, tolerability, and effectiveness even at higher oral dosages, and is currently being sold as a dietary supplement in several countries across the world.27,28,40,41 Curcumin has not yet been authorized as a drug despite its excellent efficacy and safety, and a key issue for this is the relative bioavailability of curcumin. Research over the last three decades has revealed the poor gut absorption, rapid metabolism, and systemic elimination of curcumin that significantly restricts its bioavailability.18,23,42−44 Moreover, curcumin is a hydrophobic molecule with a logP of ∼3.2 (octanol-water partition coefficient), making it practically water-insoluble (with a water solubility of only 30 nM).21,45−47 Curcumin activity has a reported half-life of 10 min in a phosphate buffer of pH 7.4 which further limits its clinical use.46,47 Even after consuming high amounts of conventional curcumin, very low levels of plasma curcumin were detected. Hence, the overarching goal of all strategies is to increase curcumin’s solubility and bioavailability.48−52 Numerous approaches have been used to improve the solubility and subsequently the bioavailability of curcumin including curcumin-piperine complex, curcumin nanoparticles or nanomicelles, liposomal curcumin, phospholipidated curcumin, and phytosomal curcumin complex.18,42,44,47,53,54 Therefore, in the current review, we provide an overview of the bioavailability, safety, tolerability, and efficacy of various curcumin formulations in clinical trials. We have extensively reviewed the completed clinical trials on curcumin formulations of different generations and highlighted their efficacy in treating several chronic diseases. Significant variations in research design, volunteer race, dose, duration, and route of administration were noted. Moreover, we discussed the advantages and limitations of these formulations and highlighted the future perspectives from the podium to clinical practice.
2. Bioavailability of Conventional Curcumin
The major findings from curcumin research are the observation of noticeably low serum levels, limited tissue distribution, rapid metabolism, inactive metabolite formation, and rapid clearance/elimination from the body.18,42,47,48,55 Several studies have shown that administration of a large amount of pure curcumin yielded only a trace amount of serum levels of curcumin in rats owing to its poor absorption from the gut.55−60 Curcumin administered orally at 2 g/kg to rats showed a maximum serum concentration of only 1.35 ± 0.23 μg/mL at 0.83 h, whereas the same dosage showed undetectable or extremely low serum levels, i.e., 0.006 ± 0.005 μg/mL at 1 h.55 Similarly, in another clinical trial, it was shown that administration of 3.6 g of curcumin by the oral route generated serum levels of only 11.1 nmol/L after 1 h.51 More recently, Yang and colleagues demonstrated that curcumin given intravenously (10 mg/kg) produced a maximum serum level of 0.36 ± 0.05 g/mL, whereas a 50-fold increase in dosage of oral supplement produced only a maximum serum level of 0.06 ± 0.01 g/mL in rats.56 Besides, following oral treatment of 400 mg of curcumin in rats, Ravindranath et al. demonstrated that only residues of the unmodified substance were discovered in the liver and kidney.59 This study also showed that 90% of curcumin was noted in the stomach and small intestine at 30 min while only 1% of curcumin was present after 24 h.59 Another study revealed that administration of radiolabeled (tritium or H3) curcumin at 10, 80, and 400 mg doses resulted in the detection of a considerable amount of curcumin in tissues of rats administered with only 400 mg after 12 days.58 Also, the percentage of absorbed curcumin remained constant irrespective of the dosage indicating the dose-independent limitations to bioavailability in these animals.58 Similarly, supplementation of 450–3600 mg of curcumin daily for a week before surgery to patients with colorectal cancer metastases to liver showed no curcumin in their liver tissues.61 In phase II clinical trial on patients with advanced pancreatic cancer, an oral dose of 8 g/day curcumin resulted in only 22–41 ng/mL of plasma concentration.62 Further, orally given curcumin (2 g/kg) to rats had an absorption half-life of 0.31 ± 0.07 and elimination half-life of 1.7 ± 0.58 h, albeit in humans, the same dose did not enable the measurement of these shelf life values since most of the levels were below the detection limit at almost all the periods.55
These studies indicated that the method of administration (whether oral or intravenous) affects the serum levels of curcumin and further suggest that the serum achievable concentrations of curcumin in humans and rats are not exactly comparable. Hence, it is not only imperative to develop bioavailable curcumin but also equally important to find the safety and efficacy of these formulations in humans.
3. Methodology
A literature search was carried out using “curcumin and clinical trials” in two different databases, Pubmed and Scopus, until June 2022. Around 458 articles appeared in PubMed and 3622 articles appeared in Scopus for the mentioned keyword. The studies that appeared were analyzed thoroughly for the mentioned keywords.
The inclusion criteria applied to select the relevant studies were (a) clinical studies that have used various generations of curcumin formulation; (b) studies on human subjects (both healthy and diseased); (c) full-text manuscripts in English. The exclusion criteria were (a) preclinical studies; (b) studies on the pure form of curcumin; (c) full-text not in English; (d) in silico studies; (e) conference abstracts; (f) review articles; (g) meta-analysis; and (h) case reports. All the relevant articles as per these criteria are included in the table, figures, and text.
4. Curcumin Formulations
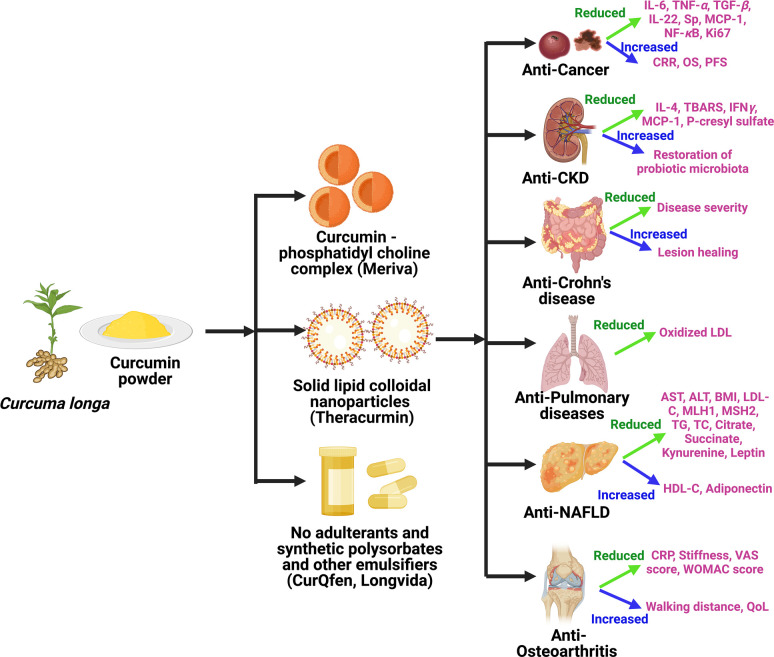
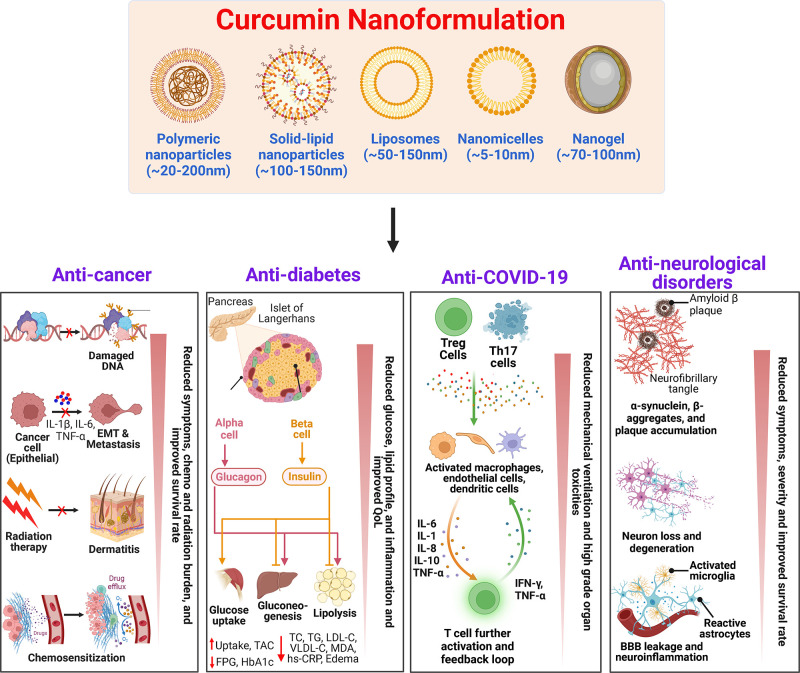
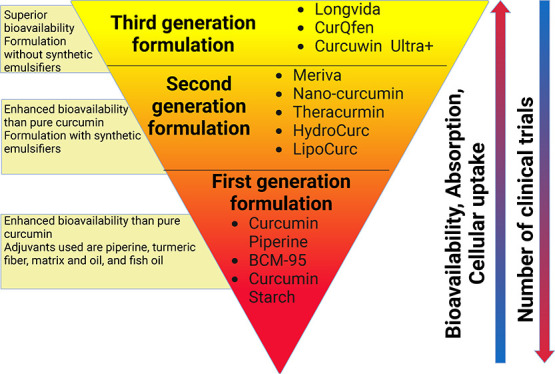
The straightforward ways to address the limitation(s) of curcumin are to enhance its bioavailability, shield it from oxidation and metabolism, and increase its ability to target diseased tissues and/or organs.18,47 One of the main strategies for increasing curcumin’s bioavailability is to utilize adjuvants that can inhibit or delay its metabolism.18,63 Other intriguing innovative formulations that appear to offer longer circulation, improved permeability, and resistance to metabolic processes include liposomes, micelles, nanoparticles, and phospholipid complexes.18,42,64 These bioavailable or bioenhanced formulations of curcumin are generally categorized into three different formulations. The classic example of first-generation formulation includes the use of significant amounts of adjuvants such as piperine from black pepper, turmeric oils, or any other natural compounds that were included to inhibit the essential detoxification enzymes such as hepatic aryl hydrocarbon hydroxylase, cytochrome P450, mixed-function oxygenases, and UDP-glucuronyltransferase.18,63,65,66 The first-generation formulation enhances the absorption time of curcumin by inhibiting or delaying its metabolism. The formulations such as curcumin–piperine, C3 complex–piperine (C3 complex/bioperine), turmeric fiber or oil with curcumin, BCM-95, and Cureit belong to the first-generation category.44 In the second-generation, emulsifiers such as carbohydrate complexes, polyethoxylated hydrogenated castor oil, lipid complexes, phospholipid complexes, polysorbates, water-dispersible nanopreparations, and spray drying were used to increase the solubility of curcumin. These included BioCurc, Cavacurcmin, CurcuWIN, Hydrocurc, Meriva, Nanocurcumin, Novasol, Theracurmin, and Turmipure Gold.44 Although increases in plasma curcuminoids levels occur primarily through their conjugated metabolites (glucuronides and sulfates), numerous studies have shown that these conjugated metabolites lack biologically significant effects because of the large size, quick renal elimination, limited membrane, and blood–brain barrier (BBB) permeability.44,67,68 For this reason, delivering curcumin in its free form (naturally unconjugated) is essential to maximize its therapeutic effects. The third-generation curcumin formulations including Longvida and CurQfen have solved the issue of “free” curcuminoids bioavailability, membrane permeability, and cellular uptake without the use of artificial emulsifiers like polysorbates.44 This section details the clinical safety and efficacy of all three generations of curcumin formulations. Different formulations and their composition are listed in Table 1.
Table 1. Composition of Various Curcumin Formulations That Are Tested Clinicallya.
| curcumin formulation | composition | ref |
|---|---|---|
| First-Generation Formulation | ||
| active ingredients formulated as soft gel capsules | fish oil 250 mg, phosphatidyl choline concentrated sunflower oil 150 mg, silymarin 75 mg, choline bitartrate 35 mg, curcumin 35 mg, d-α-tocopherol 10 mg for a total of 830 mg | (122) |
| ArtemiC oral spray (1 mL) | 6 mg artemisinin, 20 mg curcumin, 15 mg frankincense, 60 mg vitamin C | (127) |
| BCM-95 | Curcuma longa extract with essential oils from turmeric rhizome, rice flour, vegetable cellulose, vegetable stearate, silica | (116) |
| bioactive capsules | rutin (500 mg), 1.5 g fish oil (18% EPA and 7% DHA), 500 mg curcumin (95% curcuminoids) | (128) |
| C3 complex bioperine | curcuminoid extract containing curcumin, desmethoxycurcumin, bisdesmethoxycurcumin and piperine formulation | (51,61) |
| CartiJoint Forte | curcumin (BCM-95), chondroitin sulfate and glucosamine hydrochloride | (123) |
| Coltect tablet | curcumin 500 mg, green tea 250 mg, and selenium 100 μg | (130) |
| CUC-1 | 300 mg solution of curcumin | (129) |
| CuraMed | 552–578 mg of BCM-95 extracted in ethanol 99% (v/v) and 100% ethyl acetate +49–52 mg volatile oil from C. longa containing 22–23.4 mg aromatic turmerone + inactive excipients (120–140 mg) including phosphatidyl choline, medium chain TGs, glycerol, gelatin, yellow beeswax | (124) |
| Curamin | 350 mg BCM-95 + 150 mg of Boswellia serrata Roxb. ex Colebrgum resin extract corresponding to 75% boswellic acids and 10% 3-O-acetyl-11-keto-boswellic acid | (124) |
| Curcugreen | Dry rhizomes of turmeric extracted with ethyl acetate to form turmeric oleoresin, precipitated and combined with turmeric essential oil | (126) |
| Curcumall | A tincture of curcumin C3 95%, turmeric and ginger dissolved in glycerin and 0.4% alcohol | (131) |
| curcumin chitosan mouthwash | Purified curcuminoid powder 0.1 g (79:19:1 of curcumin/dimethoxycurcumin/bisdemethoxycurcumin) dissolved in 40 mL PEG, 25 mL of 2% low molecular weight chitosan | (87) |
| curcumin capsules from Theravalues Corporation, Tokyo, Japan | 10% curcumin, 2% other curcuminoids, 3.2% gum ghatti, 0.27% citric acid, 54.53% dextrin, and 30% maltose | (133) |
| curcumin forte | 95% curcumin plus 5% piperine | (86) |
| curcuminoid turmeric matrix formulation | 50% Total curcuminoids (41.2% curcumin, 7.3% desmethoxycurcumin, 1.5% bisdemethoxycurcumin), 3% essential oil, 2% protein, 40% total carbohydrate | (138) |
| curcuminoid turmeric oil formulation | 440 mg curcuminoid (347 mg curcumin, 84 mg desmethoxycurcumin, 9 mg bisdemethoxycurcumin), 38 mg of turmeric oil | (139,140) |
| Cureit/Acumin | 46.5% Total curcuminoids (36% curcumin, 9.0% desmethoxycurcumin, 1.5% bisdemethoxycurcumin), 43% total carbohydrates, 5% fiber, 2.4% proteins, 3.2% volatile oil containing aromatic turmerone, dihydroturmerone, turmeronol, curdione, bisacurone | (135) |
| Infla-Kine | Proprietary blend of Lactobacillus fermentum extract, burdock seed, zinc, lipoic acid, papaya enzyme, BCM-95 | (144) |
| Killox | 190 mg curcuminoids, 20 mg resveratrol, 100 mg NAC, 6 mg zinc with the formulation of enterosoma technology to obtain increased bioavailability | (146) |
| LCD capsule | Soft gel capsules containing lutein (20 mg), curcumin (200 mg total curcuminoids), zeaxanthin (4 mg) from marigold flower extract, algal source vitamin D3 (600 IU), medium chain triglyceride oil, linseed oil, olive oil, sunflower lecithin, tocopherol and thyme oil | (147) |
| natural product capsule by Vitacost | Each 500 mg capsule contain 150 mg curcumin, 75 mg resveratrol, 150 mg epigalloatechin-3-gallate, 125 mg soy isoflavone | (148) |
| Nutrafol women’s capsules | A proprietary blend of clinically tested and bio-optimized phytoactive extracts, vitamins, minerals and botanicals; major ingredients include standardized extracts of Ashwagandha, curcumin, piperine, capsiacin, hydrolyzed marine collagen, hyaluronic acid, organic kelp, saw palmetto, tocotrienol rich tocotrienol/tocopherol complex | (153) |
| PureVida | 460 mg of fish oil (DHA and EPA), 125 mg of Hytolive powder (12.5 mg of hydroxytyrosol), 50 mg of curcumin extract (47.5 mg of curcuminoids) | (150) |
| Reglicem | Chromium picolinate 100 μcg Cr, 200 mg curcumin dry extract, 200 mg berberine dry extract, 300 mg inositol, 40 mg banaba dry extract with 1% corosolic acid, silicon dioxide, magnesium stearate, dicalcium phosphate, microcrystalline cellulose | (151) |
| Turmix tablet | 300 mg curcumin plus 5 mg piperine | (113) |
| Turmix mouthwash | C. longa dry extract 0.1% w/v standardized to 95% curcumin (tetrahydrocurcumin) along with thymol, eucalyptol, clove oil, mentha oil, tea tree oil | (113) |
| Volatile oil formulation of curcumin | 85.9% curcuminoids (70.2% curcumin, 14.3% demethoxycurcumin, 1.4% bisdemethoxycurcumin), 7–9% essential oil naturally present in turmeric | (135) |
| WEC (hot water extract of curcumin) | C. longa rhizomes were crushed and incubated with hot water. The supernatant was concentrated, mixed with dextrin and spray-dried to obtain powder. The powder was later dissolved in dimethyl sulfoxide | (152) |
| Second-Generation Formulation | ||
| Actbiome | curcumin and asafetida complex was incorporated on to turmeric dietary fiber by spray drying process with complete natural matrix via polar–nonpolar sandwich technology | (154) |
| Algocur (Meriva formulation) | each tablet contains 1 g of Meriva | (199) |
| BioCurc/CLDM | 85% curcumin, 13% demethoxycurcumin, 2% bisdemethoxycurcumin, lauryl macrogol-32 glycerides, polysorbate-20, dl-α-tocopherol, hydroxyprolyl cellulose | (155) |
| CartiJoint Forte | Chondroitin sulfate, glucosamine hydrochloride, BCM-95 | |
| CHC (curcumin formulation with hydrophilic carrier) | Novel water-soluble formulation containing turmeric extract 20–28%, a hydrophilic carrier 63–75%, cellulose derivatives 10–40%, natural antioxidants 1–3% | (157) |
| CSL | curcumin/soy lecithin/microcrystalline cellulose in the ratio of 1:2:2 | (137) |
| curcumin nanomicelle gel from Sina Pharmaceuticals | 1% curcumin nanomicelle gel | (264) |
| curcuminoid cream from GPO Thailand | Tetrahydrocurcuminoid in phosphatidyl choline liposomes | (202) |
| curcuminoid micelles | 7% native curcumin powder containing 82% curcumin, 16% demethoxycurcumin and 2% bisdemethoxycurcumin and 93% Tween-80 filled in Licaps; finally, each capsule contained 20.1 mg curcumin, 3.9 mg demethoxycurcumin, and 0.5 mg bisdemethoxycurcumin | (160,161,163) |
| Curserin | 200 mg curcumin, 120 mg phosphatidylserine, 480 mg phosphatidylcholine and 8 mg piperine from Piper nigrum L. dry extract | (159) |
| CW8 | curcumin in complex with γ-cyclodextrin | (137) |
| FLAVOMEGA | fructose, phospholipidic curcumin, acetyl carnitine-HCL, ascorbic acid, flavoring, coenzyme Q10, Skullcap, Baicalin, green tea catechins, antiagglomerant, acesulfame potassium, sucralose | (164) |
| Flexofytol | bio-optimized curcumin 42 mg mixed with polysorbate Tween-80 | (165,166) |
| HydroCurc | 80% curcumin, 17% demethoxycurcumin, 3% bisdemethoxycurcumin, entrapped in LipiSperse delivery system | (167) |
| Ialuril soft gel tablets | Oral food integrator containing curcumin, quercetin, hyaluronic acid and chondroitin sulfate | (145) |
| Meriva | curcumin complexed with phosphatidyl choline | (179) |
| NE65 | Lipoid S LPC65 (5% w/w), olive oil (20% w/w), potassium sorbate (0.1% w/w) and distilled water | (200) |
| NLC65 | Lipoid S LPC65 (5% w/w), olive oil (2.22% w/w), precirol ATO5 (7.77% w/w) and distilled water | (200) |
| NLC80 | Lipoid S LPC80 (5% w/w), olive oil (2.22% w/w), precirol ATO5 (7.77% w/w) and distilled water | (200) |
| phospholipid curcumin formulation | 19.8% curcuminoids (16.1% curcumin, 3.2% demethoxycurcumin, 0.5% bisdemethoxycurcumin), 40% phospholipids, 40% microcrystalline cellulose | (135) |
| phospholipidated curcumin | ∼20% curcumin and soy phosphatidyl choline in 1:2 weight ratio, 2 parts of microcrystalline cellulose | (204) |
| theracurmin | curcumin dispersed in colloidal nanoparticles- Gum ghatti obtained from exudation of ghatti trees was dissolved in water and mixed with curcumin powder and glycerin, wet grinded and dispersed as colloidal nanoparticle by a high-pressure homogenizer | (282,283) |
| theracurmin beverage | Water, sugar syrup (high-fructose corn syrup, sugar), cinnamon extract, ginger, alanine, acidulant, Theracurmin, vitamin C, flavor, sweetner (licorice, sucralose), niacinamide, calcium pantothenate, vitamin B6, vitamin B2, vitamin B1, vitamin B12 | (286) |
| Third-Generation Formulation | ||
| curcumagalactomannosides | Novel oral delivery for of curcumin prepared using noncovalent complex formation between curcumin and fenugreek galactomannans | (301) |
| curcuRouge | Amorphous formulation of curcumin, modified starch, corn-starch containing 37 w/w% of curcumin | (297) |
| Curcuwin Ultra+ | 63–75% polyvinyl pyrrolidine, 10–40% cellulosic derivatives, 1–3% natural antioxidants, 20–28% turmeric extract | (47,304) |
| Longvida | curcumin in solid lipid formulation containing proprietary blend of vegetable derived stearic acid dextrin, hydroxypropyl methylcellulose, soy lecithin, ascorbyl palmitate, silicon dioxide | (306,308) |
Abbreviation: CLDM, Curcumin liquid droplet micromicellar formulation; DHA, Docosahexaenoic acid; EPA, icosapentaenoic acid; HCl, Hydrogen chloride.
4.1. First-generation curcumin formulation
Early attempts to increase absorption of curcumin included the addition of turmeric oil (BCM-95; BioCurcumax; Curcugreen), a small amount of piperine (curcumin C3 complex) to stimulate the gastrointestinal system, prevent curcumin efflux and inhibit hepatic and intestinal glucuronidation, or as a turmeric oleoresin (Curcugen).18,44,55 All of these formulations have shown incremental improvement in curcumin absorption and efficacy clinically (Table 2, Figure 1). For instance, supplementation with curcumin/piperine (500 mg-2g/day curcumin plus 5–20 mg/day piperine) formulation resulted in a significant reduction in ubiquitin, muscle atrophy F box (MAFbx)/atrogin-1, chymotrypsin-like protease, interleukin 2 (IL-2), TNF-α, INF, IL-6, IL-10, and enhancement in bioavailability, safety, tolerability, and delayed onset of muscle soreness in healthy subjects without adverse side effects.55,69,70
Table 2. Effect of Curcumin Formulations on Various Human Diseasesa.
| curcumin formulation | disease/condition | no. of patients | duration | dose | outcome | adverse effect (if any) | ref |
|---|---|---|---|---|---|---|---|
| First-Generation Formulation | |||||||
| active ingredients formulated as soft gel capsules | NAFLD | 126 | 3 months | 2 capsules/day | ↑cholesterol, ↑glucose, ↓AST | safe, well-tolerated, no adverse side effects | (122) |
| ArtemiC oral spray | COVID-19 | 50 | day 1 and day 2 | twice daily | ↑clinical improvement, ↑SpO2 normalization, ↓O2 supplementation, ↓fever, ↓hospital stay | no adverse side effects, safe, well-tolerated | (127) |
| BCM-95 | healthy volunteers | 11 | 2g | ↑bioavailability and retention time compared to curcumin–lecithin and piperine formulation | safe, no adverse side effects | (117) | |
| multiple myeloma | 33 | 28 days | 8 g/day | ↑overall remission, ↓NFκB, ↓TNF-α, ↓VEGF, ↓IL-6 | (118) | ||
| multiple sclerosis | 80 | 24 months | 1 g/day | ↓combined unique active lesions | no serious adverse side effects | (119) | |
| NAFLD | 50 | 12 weeks | 1.5g/day | ↑physical activity, ↓hepatic fibrosis, ↓TNF-α, ↓NF-κB, ↓AST, ↓ALT | safe, well-tolerated, no adverse side effects | (120) | |
| prediabetes | 84 | 90 days | 500 mg/day | ↑HDL, ↓BMI, ↓weight, ↓TC, ↓TG, ↓LDL, ↓ non-HDL-C | no serious adverse side effects | (121) | |
| bioactive capsule + WPI | age-related sarcopenia | 41 | 12 weeks | 7 capsules + 20 g | ↑knee extension strength, ↑gait speed | no serious adverse side effects | (128) |
| C3 complex + bioperine | healthy volunteers | 10 | 12 g + 60 mg | no significant effect | safe | (40) | |
| MetS | 117 | 8 weeks | 1 g/day + 10 mg/day | ↓LDL-C, ↓non-HDL-C, ↓TC, ↓TG, ↓LPA, ↑HDL-C | safe, well-tolerated, no serious adverse side effects | (92) | |
| 117 | 8 weeks | 1 g/day + 10 mg/day | ↑SOD, ↓MDA, ↓CRP, ↓glucose, ↓HbA1c, ↓SBP, ↓DBP | safe | (93) | ||
| 117 | 8 weeks | 1 g/day + 10 mg/day | ↓TNF-α, ↓TGF-β, ↓IL-6, ↓MCP-1 | safe, well-tolerated, no serious adverse side effects | (94) | ||
| 117 | 8 weeks | 1 g/day + 10 mg/day | ↑adiponectin, ↓leptin | well-tolerated | (95) | ||
| NAFLD | 70 | 12 weeks | 500 mg + 5 mg | ↑TIBC, ↓hematocrit, ↓ESR, ↓AST, ↓ALT, ↓ALP, ↓TC, ↓LDL-C, ↓iron, ↓Hb | no adverse side effects | (96) | |
| 55 | 8 weeks | 500 mg + 5 mg | ↓weight, ↓severity, ↓TNF-α, ↓MCP-1, ↓EGF | no serious adverse side effects | (97) | ||
| 55 | 8 weeks | 500 mg/day + 50 mg/day | no significant on PAB | no serious adverse side effects | (112) | ||
| obesity | 30 | 30 days | 1 g/day + 10 mg/day | ↓TG | safe, well-tolerated, no serious adverse side effect | (98) | |
| 30 | 2 weeks | 1 g/day + 10 mg/day | ↓PAB | (105) | |||
| 30 | 4 weeks | 1 g/day + 10 mg/day | ↑Zn/Cu | (106) | |||
| 30 | 4 weeks | 1 g/day + 10 mg/day | ↓IL-1β, ↓IL-4, ↓VEGF | (107) | |||
| osteoarthritis | 40 | 6 weeks | 1.5 g/day + 15 mg/day | ↑SOD, ↑GSH, ↓MDA, ↓oxidative stress | (91) | ||
| 53 | 6 weeks | 1.5 g/day + 15 mg/day | ↓IL-4, ↓IL-6, ↓hs-CRP, ↓TGF-β | (108) | |||
| SM-induced chronic pruritus | 96 | 4 weeks | 1 g/day + 10 mg/day | ↑GPx, ↑SOD, ↑CAT, ↓Sp, ↓VAS, ↓pruritus severity, ↓DLQI scores | safe, no serious adverse side effects | (99) | |
| 96 | 4 weeks | 1 g/day + 10 mg/day | ↓IL-8, ↓hs-CRP, ↓CGRP | (109) | |||
| SM-intoxicated with pulmonary complications | 78 | 4 weeks | 1.5 g/day + 15 mg/day | ↓FEV1, ↓FVC, ↓IL-6, ↓IL-8, ↓TNF-α, ↓TGF-β, ↓MCP-1, ↓Sp, ↓hs-CRP, ↓CGRP | safe, well-tolerated, no serious adverse side effects | (89) | |
| 89 | 4 weeks | 1.5 g/day + 15 mg/day | ↑GSH, ↑CpAT score ↓MDA, ↓symptoms, ↓SGRQ | safe | (110) | ||
| T2D | 100 | 3 months | 500 mg/day + 5 mg/day | ↓glucose, ↓C-peptide, ↓HbA1c, ↓ALT, ↓AST | no adverse side effects | (90) | |
| 118 | 12 weeks | 1 g/day + 10 mg/day | ↑HDL-C, ↓TC, ↓non-HDL-C, ↓LPA, ↓weight, ↓BMI, ↓TG | no adverse side effects | (111) | ||
| TBI | 62 | 7 days | 500 mg/day + 5 mg/day | ↓leptin | well-tolerated, no serious adverse side effects | (100) | |
| 62 | 7 days | 500 mg/day + 5 mg/day | ↑GPx, ↓IL-6, ↓CRP, ↓MCP-1, ↓TNF-α, ↓SOFA score, ↓APACHE II, ↓NUTRIC score | safe, no adverse side effects | (101) | ||
| C3 complex + piperine | PMS | 76 | 10 days/3 CMC | 500 mg + 5 mg | ↑vitamin D, ↓AST, ↓DB | no serious adverse side effects | (102) |
| 124 | 10 days/3 CMC | 500 mg + 5 mg | ↓PSST score, ↓dysmenorrhea pain | no serious adverse side effects | (103) | ||
| T2D | 118 | 8 weeks | 1 g/day + 10 mg/day | ↑TAC, ↑SOD, ↓MDA | safe, no serious adverse side effects | (104) | |
| CartiJoint Forte | osteoarthritis | 53 | 6 weeks | 1.5g/day | ↓VAS score, ↓WOMAC score | no adverse side effects | (123) |
| CUC-1 paclitaxel | metastatic breast cancer | 150 | 12 weeks | 300 mg/week (i.v.) + 80 mg/m2 | ↑ORR, ↑physical performance | anemia, grade 3–4 side effects occurred in 5 patients | (129) |
| Coltect tablet | ulcerative colitis index | 20 | 8 weeks | 2 tablets/day | ↑remission rate, ↓Clinical activity | safe, tolerated | (130) |
| CuraMed | osteoarthritis | 201 | 12 weeks | 1.5 g/day | ↑Physical performance, ↑pain relief, ↑40 m walking speed ↓pain index, ↓stiffness, ↓degree of difficulty to move knee joint, ↓pain on standing from chair, ↓time taken to rise from chair, ↓time taken to ascend or descend from the stairs, ↓WOMAC index | safe, tolerated | (124) |
| periodontitis | 76 | 7 days | 200 mg | ↓postoperative discomfort, ↓pain | no adverse side effects | (125) | |
| CuraMin | osteoarthritis | 201 | 12 weeks | 1.5 g/day | ↑physical performance, ↑pain relief, ↑40 min walking speed, ↓WOMAC index, ↓pain index, ↓stiffness, ↓degree of difficulty to move knee joint, ↓pain on standing from chair, ↓time taken to rise from chair, ↓time taken to ascend or descend from the stairs | safe, tolerated | (124) |
| Curcugreen | obesity | 84 | 90 days | 500 mg/day | ↑physical activity, ↓BMI, ↓FPG, ↓HbA1c, ↓insulin | no serious adverse side effects | (126) |
| Curcugreen + zinc | obesity | 84 | 90 days | 500 mg/day + 30 mg/day | ↑Insulin sensitivity, ↓BMI, ↓FPG, ↓insulin resistance | no serious adverse side effects | (126) |
| Curcumall | OLP | 7 | 21 days | 20 drops/day | no adverse side effects | (131) | |
| curcumin amorphous formulation | NAFLD | 80 | 8 weeks | 500 mg/day | ↓liver fat content, ↓BMI, ↓TC, ↓LDL-C, ↓TG, ↓AST, ↓ALT, ↓glucose, ↓glycated Hb | safe, well-tolerated, no serious adverse side effects | (132) |
| curcumin capsule (from Theravalues Corporation) | exercise-induced oxidative stress | 10 | 2 h before exercise ±2 h after exercise | 90, 180 mg | ↑BAP, ↑GSH, ↑CAT, ↓d-ROMs | (133) | |
| curcumin forte (Solgar) | schizophrenia | 38 | 24 weeks | 3 g/day | ↑PANSS score, ↓CDSS scores | no adverse side effects | (86) |
| curcuminoid-chitosan mouthwash | denture stomatitis | 30 | 2 weeks | 3 × 10 mL/day | ↑anti-Candida activity, complete response in 80% patients | no adverse side effects | (87) |
| Cureit/Acumin | aged adults | 30 | 3 months | 500 mg | ↑handgrip strength, ↑weight-lifting capacity, ↑distance covered ↓time taken to walk the same distance | no serious adverse side effects | (134) |
| healthy volunteers | 45 | single dose | 500 mg | exhibited greater bioavailability than phospholipid formulation and volatile oil formulation | no adverse side effects | (135) | |
| 30 | single dose | 500 mg | ↑VO2 max, ↓CK, ↓VAS score, ↓DOMS occurrence | no adverse side effects | (136) | ||
| CEO (essential oil formulation) | healthy subjects | 12 | 376 mg | ↑absorption | no adverse side effects | (137) | |
| CW8 (γ-cyclodextrin formulation) | healthy subjects | 12 | 376 mg | ↑absorption | no adverse side effects | (137) | |
| curcuminoid turmeric matrix formulation | RA | 36 | 90 days | 500 mg/day 1000 mg/day | ↑ACR response, ↓VAS score, ↓DAS score ↓ESR, ↓CRP, ↓RF values, ↓swollen joints, ↓tender joints | no serious adverse side effects | (138) |
| curcuminoid turmeric oil formulation | T2D | 53 | 10 weeks | 1500 mg/day | ↑adiponectin, ↓TG, ↓hs-CRP | (139) | |
| 53 | 10 weeks | 1500 mg/day | ↓mean weight, ↓BMI, ↓waist circumference, ↓FBS | no adverse side effects | (140) | ||
| curcumin alcohol gel | psoriasis | 10 | 4 weeks | 1% gel | ↓PhK activity, ↓TRR, ↓severity of parakeratosis, ↓CD8+ T cells | (141) | |
| curcumin gel | OSF | 60 | 6 weeks | 3 or 4x 5 mg/day | ↓burning sensation, ↑mouth opening capacity | safe, nontoxic | (142) |
| OSF | 40 | 4 weeks | 2% gel | ↓burning sensation, ↓LDH, ↑mouth opening capacity | safe, noninvasive, no adverse side effects | (143) | |
| curcumin mucoadhesive patch | OSF | 40 | 4 weeks | 2% gel | ↓burning sensation, ↓LDH, ↑mouth opening capacity | safe, noninvasive, no adverse side effects | (143) |
| curcumin + Boswellia + spirulina | benign thyroid nodules | 34 | 12 weeks (3 visits with 6 week interval) | 800 + 100 + 100 mg/day | ↓benign thyroid nodules | no adverse side effects | (81) |
| CU-FEO (curcumin + fennel essential oil) | IBS | 121 | 30 days | 84 mg +50 mg | ↑symptom relief, ↑QoL ↓severity score, ↓abdominal pain | safe, well-tolerated, no adverse side effects | (82) |
| curcumin + piperine | healthy volunteers | 8 | single dose | 2 g+20 mg | ↑bioavailability no adverse side effects | safe, well-tolerated, | (55) |
| recreationally active subjects | 23 | 11 days | 2 g/day +20 mg/day | ↑DOMS time, ↓Ubiquitin, ↓MAFbx/atrogin-1, ↓chymotrypsin-like protease | (69) | ||
| healthy subjects | 16 | 7 days | 500 mg/day +20 mg/day | ↓IL-2, ↓TNF-α, ↓IFN, ↓IL-6, ↓IL-10 | (70) | ||
| arsenic-induced oxidative stress | 286 | 3 months | 1g/day | ↑antioxidant capacity, ↓DNA damage, ↓ROS generation, ↓lipid peroxidation | (71) | ||
| bronchial asthma | 40 | 2 months | 2 × 750 mg/day +5 mg/day | ↓IL-6, ↑FEV1, ↑FVC, ↑ACT score | (72) | ||
| COVID-19 | 140 | 14 days | 2x(525 mg+2.5 mg) | ↑O2 saturation, ↓symptoms, ↓deterioration, ↓hospitalized duration | safe, no serious adverse side effects | (73) | |
| COVID-19 | 46 | 14 days | 2x(500 mg+5 mg)/day | ↓weakness, ↓dry cough, ↓sore throat, ↓sputum cough, ↓ague, ↓muscular pain, ↓headache, ↓dyspnea | no serious adverse side effects | (74) | |
| OSF | 90 | 6 months | 600 mg/day | ↑mouth opening flexibility, ↑tongue protrusion, ↑cheek flexibility, ↓burning sensation | no adverse side effects | (75) | |
| pancreatitis | 20 | 6 weeks | 500 mg/day +5 mg/day | ↑GSH, ↓erythrocyte MDA levels | no adverse side effects | (76) | |
| T2D | 118 | 12 weeks | 1 g/day +10 mg/day | ↑adiponectin, ↓leptin, ↓TNF-α, ↓leptin/adiponectin ratio | (77) | ||
| curcumin + piperine + ginger | RA | 60 | 8 weeks | - | ↓TJC, ↓ESR, ↓SJC, ↓DAS score, ↓inflammation, ↓pain | no serious adverse side effects | (78) |
| curcuminoids + piperine + taurine | hepatocellular cancer | 20 | 3 cycles 30 days each | 4 g + 40 mg +500 mg/day | ↑OS, ↑albumin, ↓IL-10, ↓miR-21, ↓AST, ↓ALT, ↓AFU | (79) | |
| curcuminoids + piperine | healthy volunteers | 8 | 2 days | 16 g + 96 mg | no significant effect on paracetamol metabolization | (115) | |
| Oxy-Q (curcumin + quercetin) | FAP | 5 | 6 months | 1440 mg/day +60 mg/day | ↓polyp number, ↓polyp size | no serious adverse side effects | (83) |
| curcumin tablet lactoferrin + N-acetylcysteine + pantoprazole | H. pylori+ with dyspepsis | 25 | 7 days | 60 mg+200 mg+ 1200 mg +40 mg/day | ↑cure rate, ↓overall severity, ↓serum pepsinogens | (84) | |
| curcumin extract + Calendula extract | CP/CPPS III | 55 | 1 month | 350 mg +80 mg | ↓inflammation | no adverse side effects, well-tolerated | (85) |
| curcumin + propranolol + aliskiren + cilazapril + celecoxib + piperine + aspirin + metformin | glioblastoma | 10 | 10 weeks | - | ↑median survival | minimal adverse effects, safe | (80) |
| Ialuril soft gel tablets | endometriosis | 20 | 12 weeks | 2 pills/day | ↓dysmenorrhea, ↓chronic pelvic pain, ↓dysuria | no adverse side effects | (145) |
| Infla-Kine | healthy volunteers | 24 | 4 weeks | 2 capsules/day | ↑QoL, ↓IL-6, ↓IL-8, ↓NF-κB, ↓TNF-α | (144) | |
| Killox | TURP, TURB, and BPH | 80 | 10–60 days | Once/day | ↓postoperative and late complications, duration of irritation | well-tolerated, no adverse side effects | (146) |
| LCD capsule | dry eye syndrome | 60 | 8 weeks | 1 tablet/day | ↑Schirmer’s strip wetness length, ↑tear volume, ↑TBUT score, ↑SPEED score, ↓OSDI score, ↓corneal and conjunctival staining score, ↓tear osmolarity, ↓MMP-9 positive score | safe, no adverse side effects | (147) |
| MEC | RA with chronic periodontitis | 45 | 6 weeks | 2 × 10 mL/day | ↓ESR, ↓RF, ↓CRP, ↓ACPA, ↓PI, ↓PD, ↓CAL | well-tolerated | (88) |
| NAIOS | ME/CFS | 76 | 15.2 ± 4.81 months | - | ↓IgM-mediated autoimmune response to OSEs and NO- adducts, ↓FF score, ↓severity of illness | (149) | |
| NP capsule by Vitacost | healthy volunteers | 11 | 2 weeks | 1 g/day | ↓TNF-α induced NF-κB activation | safe, well-tolerated | (148) |
| Nutrafol women’s capsule | women with self-perceived hair thinning | 40 | 6 months | 4 capsules/day | ↑number of terminal and vellus hairs, ↑hair growth, ↑quality, ↑volume, ↑thickness | no serious adverse side effects, safe, well-tolerated | (153) |
| PureVida | breast cancer | 45 | 1 month | 3 capsules/day | ↓CRP, ↓pain score | no serious adverse side effects | (150) |
| Reglicem | fasting dysglycemia | 148 | 3 months | 1 tablet/day | ↓FBS, ↓PPBS, ↓HbA1c, ↓insulin, ↓HOMA-index, ↓TG, ↓TC, ↓CRP | no serious adverse side effects | (151) |
| Turmix tablet | OSF | 147 | 12 weeks | 3 times a day | ↑mouth opening flexibility, ↑tongue protruding capacity, ↓burning sensation | (113) | |
| OSF | 12 weeks | 900 mg/day | ↑mouth opening flexibility, ↓burning sensation | no adverse side effects | (114) | ||
| Turmix tablet + Turmix mouthwash | OSF | 147 | 12 weeks | 3 times a day +2 times a day | ↑tongue protruding capacity, ↑mouth opening flexibility, ↓burning sensation | (113) | |
| WEC | healthy subjects | 47 | 8 weeks | 0.75 g | ↑H2O content of the skin, ↓TEWL | no serious adverse side effects | (152) |
| WEC + curcumin | healthy subjects | 47 | 8 weeks | 0.75 g + 30 mg | ↑H2O content of the skin, ↓TEWL | no serious adverse side effects | (152) |
| Second-Generation Formulation | |||||||
| Actbiome | healthy subjects | 30 | 8 weeks | 2 × 250 mg/day | ↑fecal bifidobacteria, ↑fecal lactobacilli, ↑ideal stool form and frequency, ↓IL-10, ↓GSRS score | no adverse side effects | (154) |
| Algocur | men rugby players with osteo-muscular pain | 50 | 10 days | 2 tablets/day | ↑physical function, ↑adherence to treatment, ↓pain, ↓VAS score | safe, well-tolerated | (199) |
| BioCurc/CLDM | healthy volunteers | 15 | 48 h (14 days wash out period) | 6 capsules (64.6 mg) | ↑absorption, ↑bioavailability | safe, no adverse side effects | (155) |
| cavacurcumin + ω-3 FA + astaxanthin + GLA + tocotrienols + hydroxy tyrosol + vitamin D3 + potassium | healthy volunteers | 80 | 4 weeks | 500 mg +675 mg +3 mg+9.5 mg+ 12.5 mg+6.25 mg +1000 IU + 12.5 mg | ↑brachial flow mediated dilation, ↑EPA, ↑ω-3 FA index, ↓hs-CRP, ↓SBP | no adverse side effects, well-tolerated | (156) |
| cCHC | healthy volunteers | 12 | 4 trials separated by 7 days each | 376 mg | ↑absorption compared to CS, CP, CTR | no adverse side effects | (157) |
| diabetic macular edema | 73 | 6 months | 2 tablets/die | ↓CRT, ↓inner retinal layer thickness | safe, no adverse side effects | (158) | |
| CSL (phytosomal formulation) | healthy subjects | 12 | 376 mg | ↑absorption | no adverse side effects | (137) | |
| curcumin phosphatidyl choline + Irinotecan | solid tumors | 23 | 28 days | 1, 2, 3 and 4 g/day + 200 mg/m2 | ↑delay in disease progression | no toxicity, tolerated leukopenia, nausea, fatigue, diarrhea | (41) |
| Curserin | obesity | 80 | 8 weeks | 800 mg/day | ↑HDL-C, ↓FPG, ↓FPI, ↓GGT, ↓HOMA-IR, ↓GOT, ↓GPT, ↓LAP, ↓FLI, ↓TG, ↓non-LDL-C, ↓HSI | no adverse side effects | (159) |
| curcuminoid micelles | healthy subjects | 42 | 6 weeks (4 weeks wash out phase) | 294 mg/day | ↑Bioavailability | safe and well-tolerated GI side effects | (160) |
| MI | 110 | single dose | 480 mg | ↓raise of CK-MB | (163) | ||
| FLAVOMEGA | DMD, FSHD, LGMD | 29 | 24 weeks | 80 g/day | ↑muscle performance, ↑global strength, ↑lower limb strength, ↑isokinetic knee extension, ↑6 min walk distance, ↓CK, ↓ROS, ↓valine, ↓FFA | no adverse side effects, well-tolerated | (164) |
| Flexofytol | osteoarthritis | 22 | 3 months | 6 capsules/day | ↓Coll2–1, ↓CRP, ↓global disease assessment activity | well-tolerated, no serious side adverse | (165) |
| Flexofytol + Boswellia extract + pine bark extract + methylsulfonyl methane | osteoarthritis | 106 | 12 weeks | 168 mg/day +250 mg/day +100 mg/day +1500 mg/day | ↓activity impairment, ↓FIHOA score | no serious adverse side effects | (166) |
| iron + HydroCurc | healthy subjects | 155 | 6 weeks | 18 mg +500 mg/day 65 mg +500 mg/day | ↓TBARS, ↓TNF-α, ↓GI side effects, ↓fatigue, ↓IL-6 | no adverse side effects | (167) |
| HydroCurc + maltodextrin | healthy and young males | 28 | single dose | 500 mg+500 mg | ↑IL-6, ↑IL-10, ↓DOMS pain, ↓TC, ↓capillary lactate and LDH | (168) | |
| lecithinized curcumin | MetS | 120 | 6 weeks | 1 g/day | no effect on vitamin E, ↓vitamin E/LDL, ↓vitamin E/TC, ↓vitamin E/TG | no serious adverse side effects | (170) |
| Lipocurc | locally advanced or metastatic tumors | 32 | 8 weeks | 100, 300 mg/m2 | ↓PSA, ↓CEA, ↓CA 19–9 | well-tolerated, anemia, hemolysis | (169) |
| Meriva | healthy subjects | 9 | 209, 376 mg of curcuminoids | ↑absorption | (173) | ||
| healthy subjects | 12 | 7 days | 2 g/day | ↑absorption | no serious adverse side effects | (174) | |
| CKD | 24 | 3 or 6 months | 1000 mg/tablet | ↓MCP-1, ↓IL-4, ↓IFNγ, ↓TBARS, ↓P-cresyl sulfate, ↓carbohydrate intake, ↓protein intake, ↓total fiber intake, ↓phosphorus and potassium intake, ↓Escherichia-Shigella, ↓Enterobacter verrucomicrobia, ↓Firmicutes, ↑Lachnoclostridium spp., ↑Lachnospiraceae family, ↑Lactobacillaceae spp., ↑Prevotellaceae | no adverse side effects | (175) | |
| diabetes with microangiopathy | 50 | 4 weeks | 1 g/day | ↑PO2, ↓skin flux, ↓edema | well-tolerated | (176) | |
| 77 | 4 weeks | 1 g/day | ↑visual acuity, ↑microcirculation, ↓retinal edema, ↓peripheral edema | well-tolerated | (177) | ||
| diabetic macular edema | 11 | 3 months | 1 g/day | ↓macular edema | no adverse side effects | (195) | |
| Gulf War illness | 39 | every 30 ± 3 days four times | 1 or 4 g day | ↓symptom severity | no serious adverse side effects | (178) | |
| hypercholesterolemia | 76 | 4 weeks | 1 g/day | ↓TC, ↓LDL-C, ↓TC/HDL ratio | no adverse side effects | (179) | |
| MetS | 120 | 6 weeks | 1 g/day | ↑zinc, ↑zinc/copper ratio | (180) | ||
| NAFLD | 102 | 8 weeks | 1 g/day | ↓TC, ↓TG, ↓LDL-C, ↓non-HDL-C, ↓uric acid | safe, well-tolerated | (181) | |
| 102 | 8 weeks | 1 g/day | ↑hepatic vein flow, ↓portal vein diameter, ↓liver volume ↓BMI, ↓waist circumference, ↓AST, ↓ALT | safe, well-tolerated | (182) | ||
| 36 | 8 weeks | 1.5 g/day | ↑hepatic vein flow, ↓NAFLD severity, ↓BMI, ↓TC, ↓LDL-C, ↓non-HDL-C, ↓TG, ↓portal vein diameter, ↓Liver size, ↓AST, ↓ALT, ↓serum uric acid, ↓HDL | safe, well-tolerated | (183) | ||
| 58 | 8 weeks | 250 mg/day | ↓3-methyl-2-oxovaleric acid, ↓3-hydroxyisobutyrate, ↓citrate, ↓kynurenine, ↓succinate, ↓α-ketolgutarate, ↓methylamine, ↓yrimethylamine, ↓hippurate, ↓indoxyl sulfate, ↓taurocholic acid ↓chenodeoxy cholic acid, ↓lithocholic acid, | (193) | |||
| 65 | 8 weeks | 250 mg/day | ↑HDL-C, ↑adiponectin, ↓leptin | safe, no side effects | (194) | ||
| 54 | 8 weeks | 250 mg/day | ↓MLH1, ↓MSH2, ↓weight, ↓waist circumference, ↓hip circumference, ↓BMI | safe, well-tolerated, no serious adverse side effects | (184) | ||
| osteoarthritis | 50 | 12 weeks | 1 g/day | ↑walking distance in treadmill, ↓WOMAC score, ↓CRP, ↓distal edema, ↓hospitalization, ↓usage of anti-inflammatory drugs | no adverse side effects | (185) | |
| 100 | 8 months | 1 g/day | ↑Karnofsky scale score, ↓stiffness, ↓WOMAC score, ↓negative effects on social function, ↓IL-1β, ↓IL-6, ↓ESR, ↓sCD40L, ↓sVCAM-1, ↑distance covered on treadmill | excellent tolerability, safe | (186) | ||
| pancreatic cancer | 44 | until death | 2 g/day 28 days cycle | ↑response rate, ↑stable disease period, ↑OS, ↑PFS | safe | (187) | |
| prostatic hyperplasia | 61 | 24 weeks | 1 g/day | ↑QoL, ↓signs and symptoms, ↓urinary infections and block | no adverse side effects | (188) | |
| psoriasis | 63 | 12 weeks | 2 g/day | ↓IL-22, ↓PASI | safe and well-tolerated | (189) | |
| risk of T2D | 29 | 12 weeks | 1 g/day | ↓GSK-3β, ↓IAPP, ↓insulin resistance ↓risk of Alzheimer’s disease | no adverse side effects | (190) | |
| solid tumors | 96 | 8 weeks | 900 mg/day | ↑QoL, ↓IL-6, ↓TNF-α, ↓TGF-β, ↓Sp, ↓hs-CRP, ↓CGRP, ↓MCP-1 ↓IL-8 | safe and well-tolerated, no serious adverse side effects | (191) | |
| solid tumors with radio- and chemo-therapy-induced side effects | 158 | 4 months | 500 mg/day | ↓burden of side effects | no serious adverse side effects | (192) | |
| Meriva + fish oil | healthy subjects | 16 | 4 days separated by a week of out period | 180 mg +2 capsules | ↓PPBS, post- prandial insulin | (196) | |
| Meriva + anthocyanin | colorectal adenomatous polyposis | 35 | 4–6 weeks | 1 g/day +1g/day | ↓NFκB, ↓Ki67 | no serious adverse side effects | (198) |
| Meriva + phytosterol | hyper-cholesterolemia | 70 | 4 weeks | 200 mg/day +2 g/day | ↓TC, ↓LDL-C | no adverse side effects | (179) |
| 82 | 4 weeks | 228 mg/day +2.3 g/day | ↓TC, ↓LDL-C, ↓TC:HDL-C ratio, ↓CVD risk, ↓LDL-P number | safe | (197) | ||
| micellar curcumin formulation (beverage) | glioblastoma | 13 | 4 days | 3 × 70 mg | ↑bioavailability, ↑inorganic phosphate, ↓PCr/Pi ratio, ↑intratumoral pH | no serious adverse side effects | (162) |
| nanocurcumin (curcumin nanomicelle from Exir Nano Sina company) | amylotrophic lateral sclerosis | 54 | 12 months | 80 mg/day | ↑survival | safe, no adverse side effects | (218) |
| ankylosing spondylitis | 24 | 4 months | 80 mg/day | ↓RORγ t, ↓IL-17, ↓IL-23, ↓miR-141, ↓miR-155, ↓miR-200, ↓symptoms | (219) | ||
| Behcet’s disease | 36 | 8 weeks | 80 mg/day | ↑Treg cells, ↑RNAs of FOXP3, ↑TGF-β, ↑IL-10, ↑miR-25, ↑miR-106b | no adverse side effects | (237) | |
| bladder cancer | 26 | 4 weeks | 160 mg/day | ↑clinical response | well-tolerated | (246) | |
| CAD | 80 | 3 months | 80 mg/day | ↓MMP-9, ↓MMP-2 | safe | (220) | |
| COVID-19 | 40 | 14 days | 160 mg/day | ↓mRNA and serum IL-6, mRNA and serum IL-1β, ↓serum IL-18 | (238) | ||
| COVID-19 | 60 | 2 weeks | 4 soft gels/day | ↑lymphocyte count, ↓symptoms | no adverse side effects | (221) | |
| COVID-19 | 41 | 2 weeks | 160 mg/day | ↑oxygen saturation, ↓symptoms, ↓symptom resolution time, ↓lymphocyte count, ↓hospitalized duration | no serious adverse side effects | (243) | |
| COVID-19 | 80 | 21 days | 160 mg/day | ↑Treg cell frequency, ↑FOXP3, ↑IL-10, ↑IL-35, ↑TGF-β | (239) | ||
| COVID-19 | 40 | 2 weeks | 160 mg/day | ↑IL-4, ↑FOXP3, ↓IFNγ, ↓TBX21 | no adverse side effects | (240) | |
| COVID-19 | 80 | 21 days | 160 mg/day | ↓RORγ t, ↓IL-17, ↓IL-21, ↓IL-23, ↓GM-CSF, ↓symptoms, ↓Th17 count, ↓hospitalized duration ↓mortality rate, | (241) | ||
| COVID-19 | 60 | 7 days | 240 mg/day | ↓mortality rate, ↓IFNγ, ↓TNF-α ↓IL-6, ↓IL-1β | safe and tolerable | (242) | |
| COVID-19 | 48 | 6 days | 160 mg/day | ↑O2 saturation, ↓symptoms, ↓LOS | no adverse side effects | (222) | |
| diabetic foot ulcer | 60 | 12 weeks | 80 mg/day | ↑TAC, ↑Insulin sensitivity, ↑GSH, ↓FPG, ↓insulin, ↓TC, ↓LDL-C | safe, no serious adverse side effects | (248) | |
| diabetes on HD | 60 | 12 weeks | 80 mg/day | ↑TAC, ↑TN, ↑PPARγ, ↑LDLR, ↓TC, ↓LDL-C, ↓VLDL-C, ↓MDA, ↓TC/HDL-C, ↓hs-CRP, ↓insulin, ↓TG, ↓FPG, | no adverse side effects | (249) | |
| DSPN | 80 | 8 weeks | 80 mg/day | ↓HbA1c, ↓FBS, ↓total reflex score, ↓total neuropathy score, ↓waist circumference, ↓temperature, | safe, well-tolerated | (252) | |
| gingivitis | 50 | 4 weeks | 80 mg/day | ↓MGI, ↓PBI | no adverse side effects | (261) | |
| hemodialysis | 54 | 3 months | 120 mg/day | ↓serum IL-6 and TNF-α, ↓mRNA IL-6 and TNF-α | (291) | ||
| HNC | 32 | 6 weeks | 80 mg/day | ↑OM development duration, ↓OM severity | no adverse side effects | (223) | |
| infertility | 60 | 10 weeks | 80 mg/day | ↑sperm count, ↑sperm concentration, ↑sperm motility, ↑TAC, ↑testosterone, ↓MDA, ↓CRP, ↓TNF-α, ↓FSH, ↓LH, ↓PRL | no adverse side effects | (229) | |
| MetS | 50 | 12 weeks | 80 mg/day | ↓TG, ↓HOMA-β | (230) | ||
| 50 | 12 weeks | 80 mg/day | ↑adiponectin, ↑TAC, ↓MDA | no serious adverse side effects | (253) | ||
| migraine | 44 | 2 months | 80 mg/day | ↓MCP-1, ↓headache attack frequency, ↓headache severity and duration | no adverse side effects | (255) | |
| 100 | 8 weeks | 80 mg/day | ↓frequency, severity, duration of headache | no adverse side effects | (256) | ||
| 80 | 2 months | 80 mg/day | no significant effect on VCAM | (257) | |||
| 80 | 2 months | 80 mg/day | ↓headache frequency, ↓IL-1β | no adverse side effects | (258) | ||
| NAFLD | 84 | 3 months | 80 mg/day | ↑HDL, ↑QUICKI, ↑Nesfatin, ↓fatty liver degree, ↓AST, ↓ALT, ↓FBS, ↓FBI, ↓HbA1c, ↓TG, ↓TC, ↓LDL, ↓HOMA-IR, ↓TNF-α, ↓IL-6, ↓hs-CRP | no adverse side effects | (254) | |
| oral mucositis | 50 | 7 weeks | 160 mg/day | ↓pain score, ↓severity | (262) | ||
| OLP | 57 | 1 month | 80 mg/day | ↓pain, ↓lesion, ↓burning sensation | no adverse side effects | (247) | |
| osteoarthritis | 30 | 3 months | 80 mg/day | ↑Treg cells, ↓VAS score, ↓CRP, ↓CD4+ and CD8 T+ cells, ↓Th 17 cells, ↓B cells | no adverse side effects | (226) | |
| 30 | 3 months | 80 mg/day | ↓miR-155, ↓miR-138, ↓miR-16 | (236) | |||
| Parkinson’s disease | 60 | 9 months | 80 mg/day | ↓MDS-UPDRS part III score | well-tolerated, mild GI symptoms | (231) | |
| prostate cancer | 64 | 3 days before RT and during RT | 120 mg/day | ↓radiation-induced proctitis | well-tolerated, no serious adverse side effects | (224) | |
| RA | 65 | 12 weeks | 120 mg/day | ↓DAS score, ↓TJC, ↓SJC | no adverse side effects | (225) | |
| RRMS | 25 | 6 months | 80 mg/day | ↓Th17 cells, ↓RORγ t, ↓IL-17 | (259) | ||
| 50 | 6 months | - | ↑miR-15a, ↑miR-19b, ↑miR-106b, ↑miR-320a, ↑miR-363, ↑miR-31, ↑miR-181c, ↑miR-150, ↑miR-340, ↑miR-599, ↓miR-17-92, ↓miR-16, ↓miR-27, ↓miR-29b, ↓miR-126, ↓miR-128, ↓miR-132, ↓miR-155, ↓miR-326, ↓miR-550 | no systemic adverse effects | (232) | ||
| 50 | 6 months | 80 mg/day | ↑Treg cells frequency, ↑FOXP3, ↑IL-10, ↑TGF-β | (260) | |||
| schizophrenia | 64 | 16 weeks | 80, 160 mg/day | ↑response rate, ↓PANSS positive subscale, ↓PANSS negative subscale score, ↓CGI-S, ↓CGI-I, ↓PANSS general psychopathology subscale score, ↓total PANSS score | safe, no serious adverse side effects | (233) | |
| sepsis | 40 | 10 days | 160 mg/day | ↓PCT, ↓IL-6, ↓TNF-α, ↓duration of mechanical ventilation, ↓SOFA | (244) | ||
| 40 | 10 days | 160 mg/day | ↓MDA, ↓IL-18, ↓IL-1β, ↓ICAM-1, ↓TC, ↓VCAM-1, ↓ IL-6, ↓TLR-4, ↓Bax, ↓FBS, ↓TG, ↓ALT, ↓ALP, ↓GGT, ↓bilirubin, ↓creatinine, ↓prealbumin, ↓SOFA score, ↓duration of ventilation, ↑IL-10, ↑CAT, ↑SOD, ↑TAC, ↑Bcl-2, ↑Nrf-2, ↑TLC | (245) | |||
| 14 | 10 days | 160 mg | ↓ESR, ↓IL-8, ↓neutrophils, ↓platelets, ↓Presepsin, ↓WBCs | no adverse side effects | (227) | ||
| T2D | 40 | 8 weeks | 80 mg/day (with endurance training) | ↓FBG, ↓glycated Hb, ↓insulin | (250) | ||
| T2D associated polyneuropathy | 80 | 8 weeks | 80 mg/day | ↓depression, ↓anxiety | safe, well-tolerated | (251) | |
| thyroid cancer undergone thyroidectomy | 21 | 10 days | 160 mg/day | ↓micronuclei in lymphocyte | safe, no adverse side effects | (228) | |
| ulcerative colitis | 56 | 4 weeks | 240 mg/day | ↓score for urgency of defecation, ↓SCCAI score | no serious adverse side effects | (235) | |
| nanocurcumin (from Theravalues Corp. Japan) | MetS | 44 | 6 weeks | 80 mg/day | ↑IL-10, ↑BDNF, ↑TAC, ↓IL-6, ↓MDA, ↓hs-CRP | (265) | |
| nanocurcumin | breast cancer | 42 | 2 weeks | 80 mg/day | ↓RISR severity, ↓pain | (266) | |
| nanocurcumin | migraine | 38 | 2 months | 80 mg/day | ↓Pentraxin 3 | (267) | |
| 80 | 2 months | 80 mg/day | ↓IL-6 mRNA, ↓IL-6, ↓hs-CRP | no adverse side effects | (268) | ||
| 40 | 2 months | 80 mg/day | ↓IL-17, ↓IFNγ | no adverse side effects | (269) | ||
| nanocurcumin (prepared using wet milling technique) | RA | 10 | 8 months | 20 mg/L and 50 mg/L | ↓inflammation | (270) | |
| nanocurcumin + ω-3 fatty acids | migraine | 72 | 2 months | - | ↓attack frequency, ↓ICAM-1 | (271) | |
| 74 | 2 months | 80 mg/day +2.5 g/day | ↓TNF-α, ↓attack frequency | no adverse side effects | (272) | ||
| 80 | 2 months | 80 mg/day +2.5 g/day | ↓IL-6 mRNA, ↓IL-6, ↓hs-CRP | no adverse side effects | (268) | ||
| 74 | 2 months | 80 mg/day +1800 mg/day | ↓COX-2, ↓iNOS, ↓frequency, severity and duration of headache | (273) | |||
| 80 | 2 months | 80 mg/day | ↓VCAM, ↓headache severity and frequency | (257) | |||
| 80 | 2 months | 80 mg/day | ↓headache frequency, ↓IL-1β | no adverse side effects | (258) | ||
| nanocurcumin + acetretin | psoriasis | 15 | 12 weeks | 3 g/day +0.4 mg/kg/day | ↓PASI | no serious adverse side effects | (274) |
| nanocurcumin + coenzyme Q10 | migraine | 100 | 8 weeks | 80 mg/day +300 mg/day | ↑MSQ score, ↓frequency, severity, duration of migraine, ↓MIDAS score, ↓HIT-6 score | no adverse side effects | (256) |
| nanocurcumin + Nigella sativa oil | postmenopausal women | 120 | 6 months | 80 mg +1 g | ↑miR-21 | no serious adverse side effects | (275) |
| nanocurcumin mouthwash | HNC oral mucositis | 74 | 6 weeks | 0.1% | ↑delayed onset, ↓risk of OM, ↓severity | no adverse side effects | (276) |
| curcumin nanomicelle gel from Sina (Iran) | OLP | 31 | 4 weeks | 1% | ↑efficacy index, ↓REU score | well-tolerated, no adverse side effects | (263) |
| RAS | 48 | 1 week | 3 × 1%/day | ↑efficacy index, ↓lesion size, ↓pain score | no adverse side effects | (264) | |
| curcumin nanomicelle from Minoo Pharmaceuticals Co. with resistance training | NAFLD | 45 | 12 weeks | 80 mg/day | ↓AST, ↓ALT | (277) | |
| nanogel 2% curcumin | chronic periodontitis | 45 | 45 days | 2% gel | ↓Aggregatibacter actinomycetemcomitans, ↓Tannerella forsythia, ↓Porphyromonas gingivalis | (278) | |
| curcumin nanoparticle | periodontitis | 20 | 15 days | 50 μg | ↑Veillonella parvula, ↑Actinomyces spp., ↓PPD, ↓CAL, ↓BOP, ↓IL-6, ↓Porphyromonas gingivalis | no adverse side effects | (279) |
| NE 65 | healthy subjects | 15 | 4 weeks | 0.125 g | ↓skin surface permittivity, ↓NMF | (200) | |
| NLC 65 | healthy subjects | 15 | 4 weeks | 0.125 g | ↑TEWL, ↓NMF, ↓skin surface permittivity, ↓urea | (200) | |
| NLC 80 | healthy subjects | 15 | 4 weeks | 0.125 g | ↑TEWL, ↓skin hydration, ↓skin surface permittivity, ↓NMF, ↓urea | (200) | |
| phospholipidated curcumin | MetS | 120 | 6 weeks | 1 g/day | no significant improvement in pro- and antioxidant balance | (204) | |
| 120 | 6 weeks | 1 g/day | ↓saturated fatty acid intake | (205) | |||
| 120 | 6 weeks | 1 g/day | ↓severe anxiety | no serious adverse side effects | (206) | ||
| 80 | 6 weeks | 1 g/day | no significant effect on BMI, waist circumference, and serum cathepsin D levels | (207) | |||
| phospholipidated curcuminoids | MetS | 120 | 6 weeks | 1 g/day | no significant effect | (208) | |
| phytosomal curcumin | MetS | 81 | 6 weeks | 1 mg/day | no considerable effect on aryl esterase activities | (203) | |
| curcuminoid cream from GPO, Thailand | focal or generalized vitiligo | 10 | 12 weeks | Twice daily | ↑repigmentation | safe, well-tolerated, minor adverse side effects | (202) |
| Theracurmin | non demented adults | 46 | 18 months | 180 mg/daymonths | ↑verbal and visual memory, ↑attention, ↓depression, ↓FDDNP | Four subjects complained abdominal pain, gastritis, nausea and one subject complained heat and pressure in chest | (280) |
| aged adults | 40 | 18 months | 180 mg/day | ↑improved SRT, ↑visual memory ↑attention, ↓neurodegeneration | safe | (281) | |
| healthy subjects | 14 | single dose | 30 mg | ↑plasma concentration of curcumin, ↑alcohol intoxication, ↓acetaldehyde | no adverse side effects | (282) | |
| healthy subjects | 6 | 150 mg, 210 mg | ↑absorption, ↑bioavailability curcumin, ↑alcohol intoxication, ↓acetaldehyde | safe, no serious adverse side effects | (283) | ||
| healthy subjects | 9 | 182.4 ± 1.0 mg | ↑absorption and bioavailability than BCM-95 and Meriva | no adverse side effects | (116) | ||
| healthy subjects | 14 | 4 weeks | 300 mg/day | ↑MVC torque recovery, ↓CK | (284) | ||
| healthy subjects | 10 | 7 days before exercise | 180 mg/day | ↓IL-8, ↓inflammation | (288) | ||
| healthy subjects | 10 | 7 days after exercise | 180 mg/day | ↑MVC torque, ↑ROM, ↓muscle soreness, ↓CK activity | (288) | ||
| Crohn’s disease | 30 | 12 weeks | 360 mg/day | ↑clinical response rate, ↑lesion healing, ↓endoscopic disease severity | no serious adverse side effects | (290) | |
| COPD | 48 | 24 weeks | 180 mg/day | ↓AT-LDL | safe, no serious adverse side effects | (293) | |
| exercise-induced muscle soreness | 24 | 7 days before and 4 days after exercise | 180 mg/day | ↑ROM, ↓muscle soreness | (287) | ||
| osteochondral diseases | 50 | 12 months | 180 mg/day | ↑JOA score, ↑VAS, ↑JKOM, ↓roughness in lateral compartment of femur, ↓stiffness of knee cartilage | no serious adverse effects | (292) | |
| postmenopausal women | 56 | 8 weeks | 150 mg/day | ↓brachial SBP | no adverse side effects | (289) | |
| noninsulin dependent DM | 33 | 6 months | 180 mg/day | ↓rise in oxidized LDL, ↓TG, ↓γ-GTP | (294) | ||
| theracurmin + exercise | postmenopausal women | 45 | 8 weeks | 150 mg/day | ↓brachial and aortic SBP, ↓radial AIx, ↓DBP | no adverse side effects | (289) |
| theracurmin solution | pancreatic or biliary duct cancer | 16 | >9 months | 200–400 mg/day | no adverse side effects | (285) | |
| theracurmin beverage | healthy subjects | 24 | 30 mg/100 mL | ↑absorption efficiency | no adverse side effects | (286) | |
| valdone curcumin soft gel | ulcerative colitis | 69 | 6 weeks | 100 mg/day | ↑clinical response rate, ↑clinical remission rate | no serious adverse side effects | (201) |
| Third-Generation Formulation | |||||||
| curcumin galactomannan formulation | healthy subjects | 18 | 30 days | 1000 mg/day | ↑α- and β-waves of EEG, memory improvement, ↓α/β ratio, audio-reaction time, ↓choice based-visual reaction time | (302) | |
| osteoarthritis | 80 | 84 days | 400 mg/day | ↑walking performance, ↓VAS ↓stiffness score, ↓IL-1β, ↓VCAM | safe | (298) | |
| CurQfen | occupational stress | 60 | 30 days | 1000 mg/day | ↑QoL, ↑CAT, ↑SOD, ↑GPx, ↑GSH, ↓TBARS, ↓fatigue, ↓lipid peroxidation | safe, no serious adverse side effects | (299) |
| obesity | 22 | 12 weeks | 500 mg/day | ↑HDL, ↓homocysteine | no adverse side effects | (300) | |
| osteoarthritis | 84 | 6 weeks | 400 mg/day | ↑improvement in walking, ↑physical activity, ↓VAS score, ↓WOMAC score, ↓stiffness score, ↓hs-CRP, ↓IL-1β, ↓IL-6, ↓sVCAM | no serious adverse side effects | (301) | |
| curcuRouge | aged adults | 40 | 4 weeks | 180 mg/capsule | ↓WBC count, ↓neutrophil count, ↓neutrophil/lymphocyte | no safety issues | (303) |
| curcuwin Ultra+ | healthy subjects under fasting | 24 | 250, 500 mg | ↑bioavailability | no safety issues | (304) | |
| Longvida | aged adults | 60 | 12 weeks | 400 mg/day | ↑mood-related benefits, ↓fatigue ↑cognitive benefits | safe, well-tolerated | (305) |
| aged adults | 80 | 12 weeks | 400 mg/day | ↑working memory performance ↓fatigue score, ↓tension, ↓anger, effects | no serious adverse side | (306) | |
| middle aged and older adults | 39 | 12 weeks | 2000 mg/day | ↑vascular NO bioavailability, flow-mediated dilation, ↑NO-dependent dilation | safe, well-tolerated ↓oxidative stress, ↑brachial artery | (307) | |
| healthy subjects | 38 | 4 weeks | 80 mg/day | ↑CAT, ↑MPO, ↑NO scavenged radicals, ↓TG, ↓salivary amylase, ↓ALT, ↓Aβ protein, ↓ICAM | (308) | ||
| Alzheimer’s disease | 8 | 2 × 20 g/day | 2 days | ↑detection of amyloid spots in retina | (314) | ||
| obesity | 134 | 16 weeks | 160 mg/day | ↑cerebrovascular responsiveness | (309) | ||
| 152 | 16 weeks (160 mg/day curcumin) | 800 mg | no significant effect on arthritis | no serious adverse side effects | (310) | ||
| OSF | 30 | 3 months | 2 g/day | ↑mouth opening capacity, ↓burning sensation | (312) | ||
| osteoarthritis effects | 50 | 90 days | 2 × 400 mg/day | ↓VAS score, ↓WOMAC score | no serious adverse side | (313) | |
| Longvida + fish oil | obesity | 152 | 16 weeks | 800 mg/day + 400 mg/day EPA + 2 g/day DHA | ↑HDL-C, ↓HR, ↓TG ↓cerebral artery stiffness | no serious adverse side effects | (311) |
| obesity | 134 | 16 weeks | 800 mg/day + 400 mg/day EPA + 2 g/day DHA | ↑cerebrovascular responsiveness 400 mg/day EPA + 2 g/day DHA | (309) | ||
Abbreviations: Aa, Aggregatibacter actinomycetemcomitans; ACPA, Anticitrullinated protein antibody; ACR, American College of Rheumatology; ACT, Asthma control test; ADPKD, Autosomal dominant polycystic kidney disease; AFU, Alpha-L-fucosidase; ALDH, Aldehyde dehydrogenase; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AOPPs, Advanced oxidation protein products; APACHE II, Acute physiology, and chronic health 370 evaluation II; AST, Aspartate aminotransferase; BALP, Bone-specific alkaline phosphatase; BANA, N-benzoyl-dl-arginine-2-naphthylamide; BAP, Biological antioxidant potential; BAX, Bcl-2 associated X-protein; BCL-2, B-cell lymphoma 2; BDNF, Brain-derived neurotrophic factor; BMD, Bone mineral density; BMI, Body mass index; BOP, Bleeding on probing; BPH, Benign prostatic hyperplasia; BSE, Boswellia serra extract; BVAS, Birmingham vascular activity score; CA 19–9, Carbohydrate antigen 19–9; CAL, Clinical attachment level; CAT, Catalase; CD40L, Cluster of differentiation 40 ligand; CD133, Cluster of differentiation 133; CDAI, Crohn’s disease activity index; CDSS, Calgary depression scale for schizophrenia; CEA, Carcinoembryonic antigen; CFU, Colony forming unit; CGI-I, Clinical global impressions-improvement score; CGI-S, Clinical global impressions-severity score; CGRP, Calcitonin gene related peptide; CK, Creatinine kinase; CKD, Chronic kidney disease; CK-MB, Creatinine kinase, MB fraction; CLDM, Curcumin liquid droplet micellar formulation; CLDQ, Chronic liver disease questionnaire; CMC, Consecutive menstrual cycle; Coll2–1, Serum type 2 collagen peptide; COPD, Chronic obstructive pulmonary disease; COX-2, Cyclooxygenase 2; COVID-19, Coronavirus disease 2019; CP, Curcumin phytosome formulation; CpAT, COPD assessment test; CRP, C-reactive protein; CRT, Central retinal thickness; CS, Standardized curcumin; CTR, Curcumin formulation with volatile oils of turmeric rhizome; CTx, C-terminal cross-linking telopeptide of type I collagen; Cu, Copper; CUA, Combined unique activity; Cur, Curcumin; CVD, cadiovascular disease; CXCl1, CXC motif chemokine ligand 1; DAS, Disease activity score; DASS-21, Depression, anxiety, stress scale-21; DB, Direct bilirubin; DBP, Diastolic blood pressure; dFLC, Difference between clonal and nonclonal free-light chain; DFP, Deferiprone; DLQI, Dermatology life quality index; DMD, Duchenne muscular dystrophy; DNA, Deoxyribonucleic acid; DOMS, Delayed onset muscle soreness; DSPN, Diabetic sensorimotor polyneuropathy; EEG, Electroencephalogram; EGF, Epidermal growth factor; EPA, Eicosapentanoic acid; ESR, Erythrocyte sedimentation rate; FA, Fatty acid; FEV, Forced expiratory volume; FF score, Fibromyalgia and fatigue rating score; FFA, Free fatty acids; FDDNP, -(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile; FIHOA, Functional index for hand osteoarthritis; FLC, Free-light chain; FLI, fatty liver index; FLIP, FLICE inhibitory proteins; FMD, Brachial artery flow-mediated dilation; FOXP3, Forkhead box P3; FPG, Fasting plasma glucose; FPI, fasting plasma insulin; FSH, Follicular stimulating hormone; FSHD, Facioscapulohumeral dystrophy; FVC, Forced vital capacity; GGT, Gamma-glutamyl transferase; GI, Gingival index; GLA, Gamma linoleic acid; GM-CSF, Granulocyte-macrophage colony stimulating factor; GOT, Glutamate-oxaloacetate transaminase; GPT, Glutamate pyruvate transaminase; GPx, Glutathione peroxidase; GSH, Glutathione; GSK-3β, Glycogen synthase kinase-3 beta; GSRS, Gastrointestinal symptom rating scale; GTP, Guanosine triphosphate; H2O, Water; H2O2, Hydrogen peroxide; HAM/TSP, HTLV-1-associated myelopathy/tropical spastic paraparesis; Hb, Hemoglobin; HbA1c, Hemoglobin A1c; HDL, High density lipoprotein; HDL-C, HDL-cholesterol; HDR, Headache daily results; HIT-6, Headache impact test 6; HNC, Head and neck cancer; HOMA-β, Homeostatic model assessment for pancreatic beta cell function; HOMA-IR, Homeostatic model assessment for insulin resistance; hs-CRP, High-sensitivity C-reactive protein; HSC, Hematopoietic stem cell; HSI, Hepatic steatosis index; HTLV-1, Human lymphotropic virus type-1; IAPP; Islet amyloid polypeptide; IBD, Inflammatory bowel disease; IBS, Inflammatory bowel syndrome; ICAM, Intracellular adhesion molecule; IgM, Immunoglobulin M; IFN, Interferon; iFLC, Involved free-light chain ratio; IFNγ, Interferon gamma; IIEF-5, 5-item version of the international index of erectile function; IL, Interleukin; iNOS, Inducible nitric oxide synthase; IPSS, International prostate symptom score; IPSS-S, International prostate symptom score-storage sub score; IPSS-V, International prostate symptom score-voiding sub score; IR, Insulin resistance; JKOM, Japanese knee osteoarthritis measure; JOA, Japanese orthopedic association, LAP, Lipid accumulation, product; LDH, Lactate dehydrogenase; LDL, Low density lipoprotein; LDL-C, LDL cholesterol; LDLR, LDL receptor; LDSI, liver disease symptom index; LGMD, Limb girdle muscular dystrophy; LH, Leutinizing hormone; LNAA, Large neutral amino acids; LOS, Length of hospital stay; LPA, Lipoprotein A; LV, Left ventricular; MAFbx, Muscle atrophy F-box; ME/CSF, Myalgic encephalomyelitis/chronic fatigue syndrome; MCP-1, Monocyte chemoattractant protein-1; MDA, Malondialdehyde; MDS-UPDRS, Movement Disorder Society sponsored revision of the Unified Parkinson’s Disease Rating Scale; MELD, Model for end-stage liver disease; MetS, Metabolic syndrome; MGI, Modified gingival index; MHb, Methemoglobin; MI, Myocardial infarction; MIDAS, Migraine disability assessment; MIF, Monocyte inhibitory factor; miRNA, Micro RNA; MLH1, MutL homologue 1; MMP, Matrix metalloproteinase; MN, Micronuclei; MPO, Myeloperoxidase; MSM, Methylsulfonyl methane; MSH2, MutS homologue 2; MSQ, Migraine-specific quality of life; MVC, Maximal voluntary contraction; NAC, N-acetylcysteine; NAFLD, Nonalcoholic fatty liver disease; NAIOS, Nutraceuticals with anti-inflammatory, oxidative and nitrosative stress; NF-κB, Nuclear factor kappa B; NLC, Nanostructured lipid carriers; NMF, Natural moisturizing factor; NO, Nitric oxide; NO-adducts, Nitroso-adducts; NP, Nanoparticle; Nrf2, Nuclear factor erythroid 2-related factor 2; NTBI, Nontransferrin bound iron; NT-proBNP, N-terminal pro hormone B-type natriuretic peptide; NUTRIC, Nutrition risk in critically ill; OLP, Oral lichen planus; OM, Oral mucositis; ORR, Objective response rate; OS, Overall survival; OSDI, Ocular surface disease index; OSE, Oxidative specific epitopes; OSF, Oral submucous fibrosis; PAB, Pro-oxidant antioxidant balance; PANSS, Positive and negative symptoms scale; PASI, Psoriasis area severity index; PBE, Pine bark extract; PBI, Papillary bleeding index; PCI, percutaneous coronary intervention; PCOS, Polycystic ovary syndrome; PCr/Pi, Phosphocreatine to inorganic phosphate ratio; PCS, P-cresyl sulfate; PCT, Procalcitonin; PD, Pocket depth; PDT, Photodynamic therapy; PFS, Progress free survival; PGC-1α, Peroxisome proliferator and activated γ receptor coactivator 1 alpha; PGE2, Prostaglandin E2; PI, Plaque index; PhK, Phosphorylase kinase; Pg, Porphyromonas gingivalis; PMS, Premenstrual syndrome; PPARγ, Peroxisome proliferator-activated receptor gamma; PPBS, Postprandial blood sugar; PPD, Probing pocket depth; ppFEV1, Predicted forced expiratory volume in one second; PPFT, Periprostatic fat thickness; PON1, paraoxonase-1; PRL, Prolactin; PSA, Prostate-specific antigen; PSQI, Pittsburgh sleep quality index; PSST, PMS screening tool; PTH, Parathyroid hormone; PV, Prostatic volume; Qmax, maximum flow rate; QoL, Quality of life; QUICKI, quantitative insulin sensitivity check index; RA, Rheumatoid arthritis; RAS, Recurrent aphthous stomatitis; REEDA, Redness, edema, ecchymosis, discharge, approximation; REU, Reticular erosive ulcerative score; RF, Rheumatoid factor; rFLC, Free-light chain ratio; RISR, Radiation induced skin reactions; ROM, Range of motion; RORγ t, Retinoic-acid-receptor-related orphan nuclear receptor gamma; ROS, Reactive oxygen species; RT, radiotherapy; SBI, Sulcus bleeding index; SBP, Systolic blood pressure; SCCAI, Simple clinical colitis activity index; sCD40L, Cluster of differentiation 40 ligand; SGRQ, St. George respiratory questionnaire; SF-36, Short form healthy survey; SJC, Swelling joint count; SM, Sulfur-mustard; SMCs, Subjective memory complaints; SOD, Superoxide dismutase; SODA, Severity of dyspepsia assessment; SOFA, Sequential organ failure assessment; Sp, Substance P; SPEED, Standard patient evaluation of eye dryness; SRT, Selective reminding test; SSQOL, Stroke specific quality of life; sVCAM, Soluble vascular cell adhesion molecule; T2D, Type 2 diabetes mellitus; TAC, Total antioxidant capacity; TBARS, Thiobarbituric acid reactive substances; TBUT, Tear-film breakup time; TBX21, T-box transcription factor 21; TC, Total cholesterol; TEWL, Transepidermal water loss; Tf, Tannerella forsythia; TG, Triglyceride; TGF-β, Transforming growth factor-beta; TIBC, Total iron binding capacity; TJC, Tender joint count; TLC, Total lymphocyte count; TLR4, Toll-like receptor 4; TN, Total nitrite; TNF-α, Tumor necrosis factor alpha; TRP, Tryptophan; TRR, Transferrin receptor; TURB, Transurethral resection of bladder; TURP, Transurethral resection of prostate; UGT, Uridine diphosphate glucuronosyltransferase; uDPYD, Urinary deoxypyridinoline; UIBC, Unsaturated iron-binding capacity; VAS, Visual analog scale; VCAM, Vascular cell adhesion molecule; VEGF, Vascular endothelial growth factor; VO2 max, Maximal oxygen consumption; WBC, White blood cells; WEC, Hot water extract; WPI; Whey protein isolate; WOMAC, Western Ontario and McMaster Universities osteoarthritis index; Zn, Zinc
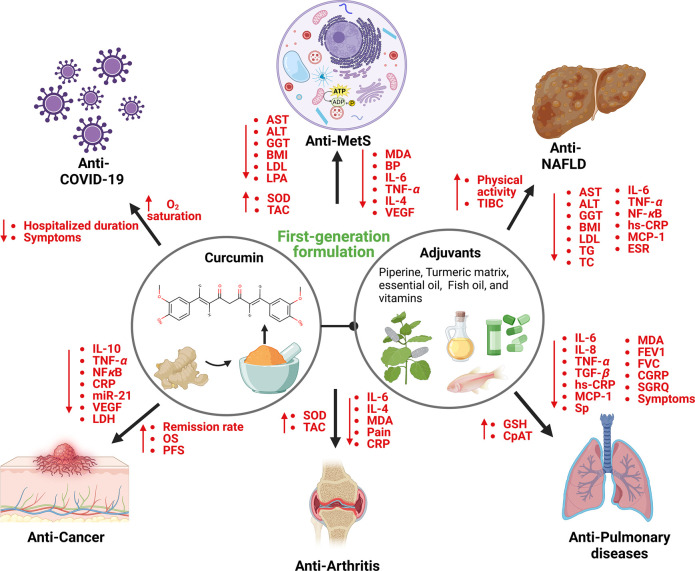
Figure 1.
Broad range of biological activities and molecular mechanisms of first-generation curcumin formulations. The first-generation formulations have shown excellent enhancement in the absorption and cellular uptake of curcumin. Various phase I/II clinical trials demonstrated that these formulations are effective against arthritis, cancer, COVID-19, MetS, NAFLD, and pulmonary diseases by modulating inflammatory cytokines, oxidative stress-related molecules, liver enzymes, and lipid profiles. The figure was created using BioRender.com.
In patients with arsenic-induced oxidative stress, this formulation effectively decreased DNA damage, ROS generation, lipid peroxidation, and improved antioxidant capacity.71 In another study, the administration of curcumin (1.5 mg/day) and piperine (5 mg/day) for 2 months resulted in the efficient alleviation of IL-6 and improvement in forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and asthma control test scores in bronchial asthma patients compared to those who received regular asthma drugs.72 Moreover, this formulation (1–1.5 g/day curcumin with 5 mg/day piperine) reduced the symptoms including weakness, dry cough, sore throat, sputum cough, ague muscular pain, headache, dyspnea, deterioration, and hospitalized duration in COVID-19 patients without side effects.73,74 Besides, the treatment with this formulation significantly improved mouth opening flexibility, cheek flexibility, and tongue protrusion capacity and suppressed burning sensation in oral submucous fibrosis (OSF) patients compared to placebo (starch and lactose capsules).75 In another randomized placebo-controlled trial, this formulation was shown to effectively augment GSH levels and decrease erythrocyte MDA levels in pancreatitis patients with no adverse side effects.76 In addition, it also reduced leptin and TNF-α levels and increased adiponectin levels in T2D patients over 12 weeks of treatment.77 Curcumin formulation with piperine and ginger ameliorated erythrocyte sedimentation rate (ESR), tender joint count (TJC), swelling joint count (SJC), disease activity score (DAS), and relieved pain and inflammation in rheumatoid arthritis patients.78 Another study demonstrated that curcumin along with piperine and taurine remarkably suppressed IL-10, AST, ALT, α-L-fucosidase, and miR-21 levels and improved overall survival in hepatocellular cancer patients.79 Another curcumin/piperine tablet containing other ingredients including propranolol, aliskiren, cilazapril, celecoxib, aspirin, and metformin for 10 weeks enhanced the median survival rate in glioblastoma patients.80 This treatment was also found to be safe with minimal side effects including indigestion and marginal bradycardia (propranolol effect).80 Another study employed two tablets of curcumin–spirulina–Boswellia extract (each tablet with 400 mg curcumin, 50 mg spirulina, and 50 mg Boswellia extract) to patients with benign thyroid nodules and reported the reduced nodule area without adverse side events.81 Also, curcumin and fennel essential oil (FEO) tablets (2 capsules, a total of 84 mg curcumin with 50 mg FEO) caused substantial relief in symptoms and improved quality of life in inflammatory bowel syndrome (IBS) patients.82 Moreover, the administration of three Oxy-Q tablets (each tablet containing 480 mg curcumin with 20 mg quercetin) repressed polyp size and number without side effects in familial adenomatous polyposis patients (FAP).83 In another study, a novel curcumin formulation administered as 2 capsules per day (each capsule containing 30 mg curcumin, 100 mg bovine lactoferrin, 15 mg zinc acetate, 100 mg lysolecithin), 600 mg N-acetylcysteine (NAC), and 20 mg pantoprazole inhibited serum pepsinogens, decreased disease severity and improved the cure rate in patients infected with Helicobacter pylori (H. pylori).84 In addition, rectal suppositories of 350 mg curcumin and 80 mg Calendula extract (1 suppository/die, for 1 month) significantly inhibited inflammation compared to those who received a placebo suppository (identical to treatment) without side effects.85 Yet another formulation, Curcumin Forte (95% curcumin plus 5% piperine formulation) remarkably increased positive and negative symptoms scale (PANSS) and reduced Calgary depression scale for schizophrenia (CDSS) scores in schizophrenic patients with no reported adverse side effects compared to identical colored and sized placebo tablets.86 Further, washing the mouth with curcumin and chitosan solution (10 mL) three times a day for 2 weeks inhibited Candida activity and achieved a complete response in 80% of denture stomatitis patients.87 In another study mouthwash containing essential oils and curcumin (MEC) effectively reduced ESR, rheumatoid factor (RF), CRP, anticitrullinated peptide antibody (ACPA), plaque index (PI), pocket depth (PD), clinical attachment level (CAL) and also was found to be well-tolerated in rheumatoid arthritis (RA) patients with periodontitis.88
C3 complex/bioperine, a curcuminoid extract containing curcumin, desmethoxycurcumin, and bisdemethoxycurcumin in combination with bioperine (piperine), has been demonstrated to be anti-inflammatory, antidiabetic, and antiarthritic agent.89−91 C3 complex/bioperine administered at the dose of 500 mg to 12 g per day for the duration of 7 days to months was safe, tolerated, and effective without any serious side effects.40,89,90,92−104 Moreover, randomized clinical trials have shown that administration of C3 complex/bioperine (1 g/day of C3 complex plus 10 mg/day of bioperine) in patients with metabolic syndrome effectively reduced C-reactive protein (CRP), glucose, glycated hemoglobin (HbA1c), lipoprotein a (LPA), low-density lipoprotein cholesterol (LDL-C), nonhigh-density lipoprotein cholesterol (non-HDL-C), malondialdehyde (MDA), total cholesterol (TC), triglycerides (TG), systolic blood pressure (SBP), diastolic blood pressure (DBP), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), leptin, tumor necrosis factor-alpha (TNF-α), and transforming growth factor-beta (TGF-β), and upregulated adiponectin, HDL-C, and superoxide dismutase (SOD) levels compared to placebo containing the same amount of lactose and bioperine in a matched shape, size and color.92−95 The treatment with this formulation was also shown to improve nonalcoholic fatty liver disease (NAFLD) by decreasing alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), hematocrit, erythrocyte sedimentation rate (ESR), iron, hemoglobin (Hb), LDL-C, TC, MCP-1, TNF-α, and epidermal growth factor (EGF).96,97 Besides, C3 complex/bioperine formulation remarkably reduced TG, interleukin 1 beta (IL-1β), interleukin 4 (IL-4), PAB and vascular endothelial growth factor (VEGF), and enhanced zinc/copper (Zn/Cu) ratio in obese subjects.98,105−107 In addition, in randomized double-blind clinical trials, oral intake of this formulation (1.5 g/day) for 6 weeks was shown to reduce MDA and oxidative stress levels and upregulated SOD and glutathione (GSH) levels in osteoarthritis patients.91,108 Interestingly, in clinical trials administration of C3 complex (1–1.5 g/day) with bioperine (10–15 mg/day) for 4 weeks showed increased levels of glutathione peroxidase (GPx), SOD, catalase (CAT), and decreased levels of substance P (Sp), visual analog scale (VAS), pruritus severity, dermatology life quality index (DLQI) scores, interleukin 8 (IL-8), high sensitivity CRP (hs-CRP), calcitonin-gene related peptide (CGRP), FEV1, FVC, IL-6, TNF-α, TGF-β, MCP-1, St. George respiratory questionnaire (SGRQ) score and increased glutathione and COPD assessment test (CpAT) scores in patients with sulfur-mustard induced chronic pruritis and pulmonary complications.89,99,109,110 Ingestion of this complex also showed antidiabetic effects by reducing glucose, C-peptide, HbA1c, ALT, Non-HDL-C, LPA, MDA, and AST and increasing total antioxidant capacity (TAC) and SOD levels in diabetic patients.90,104,111 Further, this regimen showed a promising effect in treating critically ill traumatic brain injury (TBI) patients by increasing GPx levels and suppressing leptin, IL-6, CRP, MCP-1, TNF-α, acute physiology, and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score, and nutrition risk in critically ill (NUTRIC) score.100,101 It was also shown to be effective in treating premenstrual syndrome (PMS) and dysmenorrhea with a remarkable reduction in AST, direct bilirubin, dysmenorrhea pain, and PMS screening tool (PSST) score and enhancement in vitamin D levels.102,103 This C3 and piperine formulation, however, showed no significant effect on pro-oxidant antioxidant balance (PAB) in NAFLD patients after 8 weeks treatment.112 Moreover, turmix tablet (300 mg curcumin plus 5 mg piperine) with or without turmix mouthwash for 12 weeks reduced burning sensation, improved mouth opening capacity, and tongue protruding ability in OSF patients.113,114 However, this combination was shown to provide no effects on paracetamol metabolization in healthy subjects.115
BCM-95 is a novel well established curcumin formulation wherein curcumin is complexed with essential oils from turmeric rhizome, rice flour, vegetable cellulose, vegetable stearate, and silica.116 BCM-95 has been shown to provide improved bioavailability and increased retention time of curcumin compared to curcumin-lecithin and curcumin-piperine formulations in healthy subjects.117 Several clinical trials have proven the safety, tolerability, and efficacy of BCM-95 in humans.117−121 BCM-95 is effective in treating multiple myeloma, multiple sclerosis, NAFLD, and prediabetic conditions by reducing BMI, weight, TC, TG, low-density lipoprotein (LDL), inflammatory molecules such as NF-κB, IL-6, TNF-α, and VEGF, liver enzymes including AST and ALT, diseases lesions, and hepatic fibrosis.118−121 BCM-95 treatment increased high-density lipoprotein (HDL), overall remission rate, and physical activity in these patients.118−121 In another study, oral intake of an active natural ingredient formulation (A total of 830 mg formulation consisting of fish oil 250 mg, phosphatidyl choline concentrated sunflower oil 150 mg, silymarin 75 mg, choline bitartrate 35 mg, curcumin 35 mg, D-α-tocopherol 10 mg) capsules (2 capsules/day) for 3 months was shown to be effective in decreasing liver enzymes such as AST, in patients with NAFLD compared to those who received tablet containing the same amount of choline and formulation excipients.122 This shows that efficacy is attributed to curcumin but not to choline and hence, the anti-NAFLD effect is a stand-alone effect of administered first-generation curcumin formulation.122 Although, ALT, and gamma-glutamyl transferase (GGT) levels were decreased in these patients after curcumin treatment the reduction was not found to be statistically significant. This formulation has also been shown to be safe and well-tolerated with no reported adverse side effects.122 It was also shown that administration of another dietary supplement product CartiJoint Forte, a formulation of BCM-95, chondroitin sulfate, and glucosamine hydrochloride, resulted in a significant reduction in VAS score and WOMAC score in osteoarthritis patients with no noticeable adverse events compared to placebo group.123 Another two novel BCM-95 formulations, CuraMed (552–578 mg of BCM-95 extracted in ethanol 99% (v/v) and 100% ethyl acetate, 49–52 mg volatile oil from C. longa containing 22–23.4 mg aromatic turmerone, and inactive excipients) and CuraMin (350 mg BCM-95, 150 mg of Boswellia serrata Roxb. ex Colebr gum resin extract corresponding to 75% boswellic acids and 10% 3-O-acetyl-11-keto-boswellic acid) have been demonstrated to ameliorate the pain, stiffness, degree of difficulty in moving the knee joint, and to enhance the physical performance in osteoarthritis patients. The placebo used in this study contained calcium phosphate, FD&C yellow 5, FD&C yellow 6, gelatin, magnesium stearate, maltodextrin, silica oxide, and titanium oxide. Both the formulations were found to be safe, well-tolerated, and did not show any serious adverse side effects on these patients.124 Moreover, CuraMed also reduced postoperative discomfort and pain in periodontitis patients compared to control group who received mefenamic acid.125 In another study, Curcugreen (dry turmeric rhizomes extracted with ethyl acetate called turmeric oleoresin, precipitated and combined with turmeric essential oil) alone or in combination with zinc was found to be effective in treating obesity by reducing body mass index (BMI), fasting plasma glucose (FPG), HbA1c, insulin, insulin resistance and increasing physical performance capacity compared to zinc with lactose as placebo tablets.126 Besides, oral spray formulation of curcumin, ArtemiC containing 12 mg artemisinin, 40 mg curcumin, 30 mg frankincense, and 120 mg vitamin C in 1 mL spray when used twice a day for 2 days enhanced the clinical improvement, oxygen saturation and decreased fever and hospitalized duration in coronavirus disease 19 (COVID-19) patients compared to placebo spray (containing the same solvent of ArtemiC except for the active ingredients).127 No reported adverse effects were observed in this trial.127 Moreover, curcumin bioactive capsules containing 500 mg/day rutin, 1.5 g/day fish oil (18% EPA and 7% DHA), 50 mg/day curcumin (95% curcuminoids) along with 20 g whey protein isolate (WPI) for 12 weeks has been shown to ameliorate age-related sarcopenia as evidenced by enhanced gait speed and knee extension strength without any serious side effects.128 In another study, CUC-1 formulation (curcumin with paclitaxel) administered intravenously (300 mg solution/week) increased physical performance and objective responsive rate (ORR) in metastatic breast cancer patients (MBC) (n = 150).129 However, this intravenous infusion resulted in anemia and hematological grade 3–5 side effects in a few patients (n = 5).129 Oral intake of two Coltect tablets (each tablet containing 500 mg curcumin, 250 mg green tea, and 100 μg selenium) per day for 8 weeks enhanced the remission rate and suppressed the clinical activity of ulcers in ulcerative colitis patients.130 This formulation was also found to be safe and well-tolerated among these patients.130
Another formulation, Curcumall (curcumin C3 95%, turmeric, and ginger dissolved in glycerin and 0.4% alcohol) was shown to have no adverse effects on patients with oral lichen planus (OLP).131 Additionally, curcumin dispersion amorphous formulation (500 mg/day) was reported to significantly reduce LDL-C, TG, AST, ALT, glucose, and HbA1c, and was safe, well-tolerated, and had no side effects in NAFLD patients.132 Another study showed that curcumin capsules (Theravalues Co. Tokyo, Japan) containing 0.27% citric acid, 10% Curcumin, 2% other curcuminoids, 54.53% dextrin, 3.2% gum ghatti, and 30% maltose enhanced biological antioxidant potential (BAP), GSH and CAT and suppressed derivatives of reactive oxygen metabolites in healthy subjects with exercise-induced oxidative stress.133 In addition, the novel curcumin formulation, Cureit/Acumin (46.5% total curcuminoids, 43% total carbohydrates, 5% fiber, 2.4% proteins, 3.2% volatile oil) exhibited enhanced bioavailability than phospholipid and volatile oil formulation of curcumin and was found to be safe without any side effects and improved handgrip strength, weight lifting capacity, walking distance and reduced the creatinine kinase (CK), muscle soreness and time taken to walk the same distance in healthy volunteers.134−136 Another randomized study tested the effectiveness of curcumin essential oil formulation, curcumin phytosomal formulation, and γ-cyclodextrin curcumin formulation on healthy volunteers and reported enhanced curcumin absorption without adverse events.137 Furthermore, curcuminoid turmeric matrix formulation (50% total curcuminoids, 3% essential oil, 2% protein, 40% total carbohydrate) suppressed CRP, rheumatoid factor (RF), SJC, TJC, ESR, and disease activity scores compared with food-grade starch as placebo in RA patients.138 In another study, curcumin turmeric oil formulation (440 mg curcuminoid, 38 mg of turmeric oil) reduced mean weight, BMI, waist circumference, FBS, TG, hs-CRP, and increased adiponectin levels in T2D patients compared to administration of the same amount of rice flour as placebo.139,140 Heng and colleagues showed that the application of curcumin alcohol gel reduced phosphorylase kinase activity, TRR, the severity of parakeratosis, and CD8+ T cells in psoriasis patients.141 Similarly, the application of curcumin gel or curcumin mucoadhesive patch formulation reduced the burning sensation and improved mouth opening capacity in patients with OSF without any adverse side effects.142,143
Another novel curcumin formulation, Infla-Kine containing a proprietary blend of Lactobacillus fermentum extract, lipoic acid, burdock seed, papaya enzyme, zinc, and BCM-95 downregulated inflammatory cytokines such as IL-6, IL-8, NF-κB, and TNF-α thereby improved quality of life in healthy volunteers.144 Iauril soft gels containing curcumin, quercetin, hyaluronic acid and chondroitin sulfate reduced dysmenorrhea, chronic pelvic pain, and dysuria in patients with endometriosis.145 Killox, another curcumin formulation, (190 mg curcuminoids, 20 mg resveratrol, 100 mg NAC, 6 mg zinc with the formulation of enterosoma technology) reduced postoperative irritation duration and complications in patients who underwent transurethral resection of prostate, transurethral resection of bladder and with benign prostate hyperplasia (BPH).146 The formulation did not induce any side effects and it was also found to be safe and well-tolerated in these patients.146 In addition, LCD capsules (soft gel capsules containing lutein 20 mg, curcumin 200 mg, zeaxanthin 4 mg from marigold flower extract, algal source vitamin D3 600 IU, medium chain TG oil, linseed oil, olive oil, sunflower lecithin, tocopherol and thyme oil) improved Schirmer’s strip wetness length, tear volume, TBUT score, SPEED score, OSDI score, corneal and conjunctival staining score, tear osmolarity, and MMP-9 positive score with comparative safety and no adverse side effects in patients with dry eye syndrome in contrast to soyabean oil as placebo.147 Moreover, natural product capsules manufactured by Vitacost consisting of 150 mg curcumin, 75 mg resveratrol, and 150 mg epigallocatechin-3-gallate for each 500 mg tablet was shown to reduce TNF-α induced NF-κB activation in healthy volunteers.148 In another study administration of nutraceuticals with anti-inflammatory, oxidative, and nitrosative stress (NAIOS) containing l-carnitine, coenzyme Q10, curcumin, lipoic acid, quercetin or NAC, glutamine, taurine, and zinc reduced IgM mediated autoimmune responses, fibromyalgia, and fatigue rating and severity of diseases in patients suffering from myalgic encephalomyelitis/chronic fatigue syndrome.149 Further, PureVida (460 mg of fish oil, 125 mg of Hytolive powder containing 12.5 mg of hydroxytyrosol, 50 mg of curcumin extract) formulation relieved pain and reduced CRP in breast cancer patients with no serious adverse side effects.150 Another study showed that Reglicem formulation (chromium picolinate 100 μcg Cr, 200 mg curcumin dry extract, 200 mg berberine dry extract, 300 mg inositol, 40 mg banaba dry extract with 1% corosolic acid, silicon dioxide, magnesium stearate, dicalcium phosphate, microcrystalline cellulose) reduced FBS, postprandial blood sugar (PPBS), HbA1c, insulin, homeostatic model assessment (HOMA)-index, TG, TC, and CRP levels in fasting dysglycemia patients.151 In another clinical trial, hot water extract of curcumin with or without pure curcumin powder for 8 weeks improved the water content of the skin and suppressed trans-epidermal water loss in healthy subjects.152 Furthermore, Nutrafol women’s capsule formulation (a proprietary blend of clinically tested and bio-optimized phytoactive extracts, vitamins, minerals, and botanicals including standardized extracts of ashwagandha, curcumin, piperine, capsaicin, hydrolyzed marine collagen, hyaluronic acid, organic kelp) augmented hair growth, quality, volume and thickness without any side effects in women with self-perceived hair thinning.153
Taken together, these results indicated that the first-generation curcumin formulations enhanced the absorption and bioavailability of pure curcumin and were effective against various ailments including autoimmune diseases, cancer, diabetes, hemoglobinopathies, oral diseases, and PMS.
4.2. Second-Generation Curcumin Formulation
Curcumin is readily soluble in fat. Hence, newer formulations have been developed to enhance the solubility of curcumin to enhance its absorption and bioavailability.18,48 Over the years, various technologies have been utilized to enhance its solubility including the usage of polysorbates, phospholipid complexes, liquid droplet nanomicelle, and spray drying.44 The novel curcumin second and third-generation formulations and their antichronic disease effects have been highlighted in Figure 2.
Figure 2.
Novel next-generation formulations of curcumin and their biological effects. The promising next-generation formulations of curcumin including Meriva, Theracurmin, Longvida, and CurQfen have shown various clinical benefits including anticancer, anti-CKD, anti-Crohn’s disease, anti-NAFLD, and antiosteoarthritis activities. The figure was generated using BioRender.com.
These second-generation formulations have shown excellent bioavailability and antiarthritic, anticancer, antidiabetic, and antiviral activities in numerous clinical trials (Table 2). For example, oral intake of 500 mg/day novel Actbiome formulation, (curcumin and asafetida complex was incorporated into turmeric fiber) for 8 weeks showed a reduction in IL-10 and gastrointestinal symptom rating scale (GSRS) score and increased fecal bifidobacteria, fecal lactobacilli, and ideal stool form and frequency without any side effects in healthy subjects.154 Another study showed that a polysorbate formulation of curcumin named BioCurc/CLDM (85% curcumin, 13% desmethoxycurcumin, 2% bisdemethoxycurcumin, lauryl macrogol-32 glycerides, polysorbate-20, dl-alpha-tocopherol, hydroxy prolyl cellulose) (6 tablets, cross-over study) possess excellent absorption and bioavailability and safety in healthy individuals.155 In addition, cyclodextrin formulation of curcumin known as Cavacurcumin along with omega-3 fatty acids (ω-3 FA), astaxanthin, gamma linoleic GLA, tocotrienols, hydroxy tyrosol, and vitamin D3 resulted in substantial reduction of hs-CRP, and SBP in healthy volunteers. This regimen was also well-tolerated without any adverse side effects.156 Additionally, curcumin has also been formulated with hydrophilic carriers (CHC) to suppress its hydrophobicity and to enhance its solubility. The CHC formulation has been shown to increase curcumin bioavailability compared to standardized curcumin mixture, phytosomal curcumin formulation, and curcumin-turmeric volatile oil formulation in healthy volunteers.157 This formulation has also been reported to be safe and did not cause any side effects in both healthy subjects and diabetic patients.157,158 Besides, curcumin phosphatidylcholine along with irinotecan treatment has been shown to delay the disease progression without serious toxicity in patients with solid tumors.41 Another phytosomal curcumin formulation, Curserin (200 mg curcumin, 480 mg phosphatidylcholine, 120 mg phosphatidylserine, and 8 mg piperine) increased HDL-C and decreased FPG, fasting plasma insulin (FPI), GGT, HOMA for insulin resistance (HOMA-IR), glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), lipid accumulation product (LAP), fatty liver index (FLI), TG, non-LDL-C, and hepatic steatosis index (HSI) in obese patients without adverse side effects.159 In another study, oral intake of curcuminoid micelles capsules containing 20.1 mg curcumin, 3.9 mg demethoxycurcumin, and 0.5 mg bisdemethoxycurcumin was shown to be safe, well-tolerated, and enhanced the bioavailability of curcumin in healthy subjects.160,161 Also, micellar curcumin formulation increased intratumor pH and inorganic phosphate levels in glioblastoma patients with minor side effects.162 In another study, this formulation was shown to reduce creatinine kinase MB (CK-MB) in myocardial infarction patients.163
Another phospholipidic formulation FLAVOMEGA containing acetylcarnitine, acesulfame potassium, antiagglomerant, ascorbic acid, baicalin, coenzyme Q10, fructose, green tea catechins, phospholipidic curcumin, skullcap, and sucralose improved muscle strength, performance, and isokinetic knee extension and suppressed CK, reactive oxygen species, valine and free fatty acids in patients with Duchenne muscular dystrophy (DMD), facioscapulohumeral muscular dystrophy (FSHD), and limb-girdle muscular dystrophy (LGMD).164 This formulation was also found to be safe, and well-tolerated without causing side effects.164 Moreover, curcumin polysorbate formulation Flexofytol remarkably reduced Coll2-1, CRP, and global disease assessment activity in osteoarthritis patients in 3 months.165 In another study, Flexofytol along with Boswellia extract pine bark extract, and methylsulfonyl methane for 12 weeks reduced activity impairment and FIHOA score without any significant adverse effects in osteoarthritis patients.166 Another formulation HydroCurc consists of 80% curcumin, 17% demethoxycurcumin, and 3% bisdemethoxycurcumin entrapped in a LipiSperse delivery system, was demonstrated to inhibit the formation of thiobarbituric acid reactive substances (TBARS), TNF-α, IL-6, and relieved fatigue.167 The formulation itself did not cause any side effects and further reduced the iron-induced gastrointestinal (GI) side effects.167 Also, a single dose of HydroCurc along with maltodextrin enhanced IL-6, and IL-10, and reduced TC, pain, and capillary lactate dehydrogenase during the postexercise period in healthy young men.168 In another study, Lipocurc formulation was shown to reduce PSA, CEA, and CA 19-9 in patients with advanced metastatic tumors without any side effects.169 Although, lecithinized curcumin did not affect vitamin E in metabolic syndrome patients, reduced ratio of vitamin E/LDL, vitamin E/TC, and vitamin E/TG were noticed.170
As mentioned curcumin is least soluble in water with an estimated solubility of 11 ng/mL in alkaline conditions while it is readily soluble in lipids or fats.43,171,172 Hence, efforts have been made to develop various lipid or phospholipid curcumin formulations and several of these formulations have shown tremendous potential as therapeutic agents.173 For example, Meriva, a lecithin delivery method for curcumin, has better tissue dispersion and bioavailability than the unformulated natural substance.64,173 This novel second-generation formulation has been shown to be safe and well-tolerated at a dose of 250 mg/day to 4 g/day for a period of 7 days to 8 months and did not cause any side effects both in healthy subjects and patients.173−192 Moreover, this formation has been shown to ameliorate metabolic disorders including diabetes-associated edema and microangiopathy, hypercholesterolemia, metabolic syndrome, and NAFLD.176,177,179−184,193−195 In various clinical trials, this formulation reduced skin flux, peripheral edema, retinal edema, LDL-C, TC, TG, LDL-C, non-HDL-C, uric acid, BMI, waist circumference, hip circumference, AST, ALT, portal vein diameter, liver size, 3-methyl-2-oxovaleric acid, 3-citrate, hippurate, hydroxyisobutyrate, indoxyl sulfate, α-ketoglutarate, kynurenine, methylamine, succinate, trimethylamine, chenodeoxy cholic acid, lithocholic acid, taurocholic acid, leptin, MutL homologue 1 (MLH1), and MutS homologue 2 (MSH2), and increased adiponectin levels, zinc levels, Zinc to copper ratio, PO2, visual acuity, microcirculation score, in patients.176,177,179−184,193,194 In another study, administration of Meriva (1 g/day) for 3 or 6 months caused a substantial reduction in MCP-1, IL-4, IFNγ, TBARS, p-cresyl sulfate, carbohydrate intake, protein intake, total fiber intake, phosphorus and potassium intake, and gut microbes such as Escherichia-Shigella, Enterobacter verrucomicrobia, Firmicutes, and improved other species of microbes including Lactobacillaceae spp., Lachnoclostridium spp., Lachnospiraceae family, and Prevotellaceae without side effects in patients suffering from chronic kidney diseases.175 In addition, this formulation showed potential anticancer activities against solid tumors with enhanced safety, tolerability, and minimal adverse side effects.187−189,191,192 It also improved response rate, stable disease period, inflammation, quality of life, and survival rate, and reduced the burden of chemotherapeutic side effects among these patients.187−189,191,192 In addition, Meriva mitigated inflammatory markers such as CRP, IL-1β, IL-6, ESR, sCD40L, and sVCAM-1, WOMAC score, Karnofsky scale score, stiffness, negative effects on social function, and boosted physical performance capacity in osteoarthritis with excellent safety and tolerability (Figure 2).185,186 It also reduced the risk of development of T2D and Alzheimer’s disease in adults of age between 30 and 70 years.190 Another study showed that this formulation (1 g or 4g/day) reduced the severity of gulf war illness disease without any serious side effects.178 Moreover, Meriva along with fish oil reduced postprandial insulin levels in healthy subjects whereas Meriva with phytosterol reduced cardiovascular disease (CVD) risk in hypercholesterolemia patients.179,196,197
In another study, Meriva with anthocyanin has shown improvement in colorectal adenomatous polypos symptoms and it reduced NF-κB and Ki67 levels.198 Further, another Meriva formulation called Algocur (each tablet contains 1g of Meriva) improved physical performance and reduced pain in men rugby players with osteo-muscular pain.199 This formulation also showed to be safe and well-tolerated among these men.199 Wolf and colleagues developed three different lipidated curcumin—NE65, NLC65, and NLC80—formulations reduced trans-epidermal water loss and modulated skin barrier functions in healthy subjects.200 Another study showed that supplementation of Valdone curcumin soft gel (utilized self-emulsifying drug delivery system) improved clinical response and remission rates in ulcerative colitis patients.201 Furthermore, topical application of curcuminoid-phosphatidyl choline formulated cream enhanced repigmentation in vitiligo patients.202 However, another phytosomal curcumin formulation was shown to possess no considerable effect on aryl esterase activities in MetS patients.203 Also, several studies have also revealed that phospholipidated formulations of both curcumin and curcuminoids were not considerably effective in treating patients with MetS.204−208
Nanoencapsulation or nanoformulation of curcumin is another promising strategy both to increase bioavailability and to decrease curcumin degradation rate in vivo.23,43 Several synthetic and natural polymers, such as chitosan, N-isopropylacrylamide (NIPAAM), N-vinyl-2- polyethylene glycol monoacrylate (NIPAAM [VP/PEG A]), poly(lactic-co-glycolic acid), pyrrolidone, poly(vinyl alcohol) (PVA), and silk fibroin have been developed for curcumin nanoencapsulation.43,209−212 Over the years, nanotechnology-based therapeutic delivery methods, including nanoparticles, liposomes, and nanoemulsions, have been developed.213,214 The use of biochemical changes at the tissue microenvironment level in diseased states to initiate and activate drug release has replaced more traditional drug release mechanisms with the controlled-release mechanisms by novel engineered nanoparticle drug delivery systems.214,215 Data indicated that these formulations increased treatment effectiveness while concurrently decreasing harmful side effects.211,212,216,217 The novel nanocurcumin formulation developed by Exir Nano Sina (Iran) has shown excellent therapeutic efficacy in various diseases such as amyotrophic lateral sclerosis (ALS), ankylosing spondylitis (AS), arthritis, cancer, COVID-19, CVDs, diabetes, infertility, MetS, NAFLD, neurological and psychological disorders without side effects (Figure 3).195,218−235 Administration of this formulation for 12 months increased the survival rate in ALS patients.218 It also reduced RORγ t, IL-17, IL-23, miR-141, miR-155, miR-200, and symptoms in AS patients.219 The antiarthritic potential of this formulation has been evidenced by its capacity in suppressing CRP, CD4+ and CD8 T+ cells, Th 17 cells, B cells, miRNA-155, miRNA-138, miRNA-16 and VAS score, and augmenting Treg cells without any adverse events in the clinical trials involving osteoarthritis and RA patients.225,226,236 It was also shown to be effective in treating Behcet’s disease where it improved Treg cells, FOXP3, TGF-β, IL-10, miRNA-25, and miRNA-106b.237
Figure 3.
Molecular targets of curcumin nanoformulations. Increasing lines of evidence suggest that nanoformulations of curcumin possess high bioavailability and safety and are effective against various ailments. These formulations have been shown to inhibit DNA damage, inflammatory cytokines, lipid profile, and reduce amyloid plaque formation in the central nervous system. The figure was created using BioRender.com.
Multiple clinical trials have shown its potential in treating COVID-19 disease with admirable safety and tolerability. Nanocurcumin formulation in COVID-19 patients led to reduced levels of GM-CSF, IFNγ, IL-1β, IL-6, IL-17, IL-18, IL-21, IL-23, RORγ t, T-box transcription factor 21 (TBX21), and TNF-α, and induced FOXP3, IL-4, IL-10, IL-35, and TGF-β levels.238−242 It also improved lymphocyte count, oxygen saturation levels, symptoms, and Treg cell frequency and reduced symptom resolution time, hospitalized duration, and mortality rate in COVID-19 patients.221,222,238−243 Several studies have also revealed the beneficial effects of nanocurcumin formulation in treating critically ill patients with sepsis. Nanocurcumin from Exir-Nano-Sina (Iran) suppressed Bcl-2, inflammatory molecules such as ICAM-1, IL-1β, IL-6, IL-18, TLR-4, TNF-α, and VCAM-1, creatinine, lipid profile, liver enzymes and reduced mechanical ventilation period in these patients.227,244,245 In addition, nanocurcumin treatment enhanced clinical response rate and ameliorated radiation-induced dermatitis in various cancers including bladder, head and neck, and prostate cancers.224,246,247 It also reduced DNA damage and micronuclei formation in lymphocytes of thyroid cancer patients.228 Moreover, the antidiabetic properties of nanocurcumin were attributed their capacity in reducing FBG, glycated Hb, insulin, hs-CRP, TC, TAC, TN, LDL-C, VLDL-C, TC/HDL-C, MDA, and augmented insulin sensitivity, TAC, peroxisome proliferator-activated receptor gamma (PPARγ), LDLR, and GSH levels in T2D patients.248−251 It also suppressed neuropathy, depression, and anxiety in T2D-associated peripheral neuropathy patients.251,252 Nanocurcumin supplementation for 10 weeks improved sperm count, sperm motility, and testosterone levels in patients with infertility complaints.229 This study also showed that nanocurcumin increased testosterone levels and reduced follicular stimulating hormone (FSH), luteinizing hormone (LH), and prolactin levels, although not statistically significant.229 This formulation has also been shown to decrease TG, HOMA-β, and MDA, and upregulate adiponectin levels and TAC in MetS patients.230,253 Further, treatment with this formulation remarkably reduced the degree of fatty liver, liver enzymes, lipid profile, and inflammatory mediators in patients with NAFLD.254 This formulation was also effective in treating neurological disorders such as migraine, multiple sclerosis, and Parkinson’s disease and was able to improve disease severity, symptoms, and deregulated miRNAs with no or mild GI side effects.231,232,255−260 It also reduced pain, severity, lesion area, and burning sensation in patients with various oral diseases including gingivitis, mucositis, and OLP.247,261−264 In another study, this formulation enhanced the responsive rate while reducing the positive and negative PANSS subscale score in schizophrenia patients.233 Further, nanocurcumin formulation from Theravalues Corp., Japan was demonstrated to downregulate IL-6, hs-CRP, MDA, and upregulate IL-10, brain-derived neurotrophic factor (BDNF), and TAC in MetS patients.265 Furthermore, several other nanocurcumin formulations have also been developed by various laboratories and these formulations have shown tremendous potential in helping healthy subjects and treating various chronic diseases such as arthritis, cancer, NAFLD, neurological disorders, oral diseases, and skin diseases.266−279 Thus, nanocurcumin formulation with enhanced bioavailability and safety has been promising in treating several human diseases.
A distinctive example of a submicron crystal dispersion of curcumin known as Theracurmin was reported to have 27-fold higher bioavailability in comparison with pure curcumin.49 Increasing lines of evidence also suggested its enhanced bioavailability with acceptable safety and mild side effects in healthy subjects and cancer patients (Figure 2).116,280−286 It has also been shown to reduce exercise-induced muscle soreness and increased the range of motion.287,288 In postmenopausal women, it reduced brachial SBP.289 This formulation also improved clinical response rate and lesion healing and reduced endoscopic disease severity in Crohn’s disease patients.290 In another study, Theracurmin significantly reduced mRNA expression of IL-6 in PBMCs and serum levels of IL-6 in hemodialysis patients.291 Another salient example of clinical benefits of Theracurmin comes from the trial on osteochondral diseases in which it reduced roughness in the femur bone and stiffness in the knee joint.292 It also inhibited the raise in oxidized LDL in both COPD and T2D patients.293,294
Collectively, these studies suggest that second-generation formulations of curcumin improved the bioavailability of curcumin and their significance drives the ancillary goal to develop them as therapeutic drugs.
4.3. Third-Generation Curcumin Formulation
An expansive frontier in nutraceuticals is unfolding third-generation curcumin formulation via increasing the bioavailability of “free” curcuminoids without using synthetic polysorbates and/or emulsifiers.44,295 These formulations are well established to have superior absorption, BBB-permeability, cellular uptake, and better tissue distribution.295,296 Based on the published literature, these formulations have greater than 100-fold higher bioavailability compared to pure curcumin.47 Besides, these formulations are devoid of adulterants and contaminants, making them safer and nongenotoxic and nonhepatotoxic for long-time clinical use.44 Indeed, third-generation formulations have been developed recently and these formulations include curcumin galactomannan formulation or CurQfen (noncovalent complex between curcumin and fenugreek galactomannans), curcuRouge (Starch and curcumin formulation), Curcuwin Ultra (cellulosic derivatives and curcumin formulation), and Longvida (soy lecithin and curcumin formulation) (Figure 2).47,295,297 CurQfen was shown to be safe and well-tolerated and had no adverse side effects have been reported in clinical trials.44,298−301 This formulation also improved α- and β-waves of EEG, memory improvement, and reduced choice-based-visual reaction time in healthy subjects.302 It also improved walking performance, VAS score, and WOMAC score, and inhibited the rise in hs-CRP, IL-1β, and IL-6 levels in osteoarthritis patients.298,301 In addition, its antiobesity and anti-CVD properties were attributed to its increased levels of HDL and reduced levels of homocysteine within 12 weeks of treatment.300 Besides, to a certain extent, this formulation relieved occupational stress as evidenced by improvement in QoL, SOD, GPx, GSH, and fatigue.299 In another study, curcuRouge was demonstrated to reduce the neutrophil to lymphocyte ratio without any safety issues in healthy subjects.303 Another formulation Curcuwin Ultra+ showed enhanced bioavailability and was found to be safe in healthy subjects.304 Another next-generation formulation with superior bioavailability, Longvida was also elucidated to reduce fatigue, and oxidative stress, tension, and anxiety, and improved mood-related issues, and cognitive functions in healthy individuals with excellent safety and no side effects.305−308 Moreover, clinical trials on obese patients have revealed that Longvida intake improved cerebral artery stiffness, cerebrovascular responsiveness, and lipid profile without side effects.309−311 It has also been shown to be beneficial in treating both OSF and osteoarthritis.312,313 Another illuminating clinical trial evidenced the use of Longvida in detecting amyloid spots in the retina of Alzheimer’s patients and reported that this formulation exhibits a greater capacity to identify these spots in positron emission tomography (PET) scanning compared to conventional amyloid PET in longitudinal evaluation of amyloid risk and neurodegeneration (LEARN) study.314
Certainly, these clues warrant further investigations on third-generation curcumin formulations as a novel nutraceutical formulation in diagnosing and treating various ailments.
5. Conclusion
Advances in chemistry and technologies have provided the versatility and tools to develop a range of innovative curcumin formulations with considerable improvement in oral bioavailability and safety. Decades of research on curcumin and its formulations resulted in the increased oral bioavailability of curcumin from 11 ng/mL to 626.98 μg/mL. These curcumin formulations were found to be safe and well-tolerated even at higher doses ranging from 2 g/day to 12 g/day and for a prolonged duration of 6 months to an year. The simplest first-generation formulation with adjuvants to second-generation with polysorbates to third-generation with only natural material have shown tremendous absorption capacity, cellular uptake, and safety not only in diseased but also in healthy subjects providing the evidence of disease prevention and treatment capability of these formulations. As we noted at this time, few of these regimens including curcumin plus piperine combination, BCM-95, nanocurcumin, Meriva, and Theracurmin have been tested clinically and are effective against chronic diseases such as arthritis, autoimmune diseases, cancer, diabetes, endometriosis, hemoglobinopathies, metabolic syndrome, neurological disorders, obesity, oral diseases, psychological disorders, and skin diseases. All the formulations have been shown beneficial effects compared to either placebo such as calcium phosphate, lactose, rice flour, and starch, or the standard care treatment. Major grade 3 side effects, GI intolerability, and hepatotoxicity were reported when curcumin was administered intravenously earlier. Nevertheless, the minor side effects in most of these trials with oral intake of curcumin formulations include cold, irritation, indigestibility, and nausea which in few cases might be attributed to adjuvants and emulsifiers. However, clinical studies are scarce at this time on upcoming and more promising third-generation formulations. Notably, it is advisable to opt for highly bioavailable curcumin formulations that have demonstrated their therapeutic efficacy at a relatively low dosage of 80–500 mg/day. Further, most of the clinical trials conducted were restricted to a small number of patient groups. However, more research is needed to examine the safety and effectiveness of curcumin formulations in both large and diverse patient populations with different phases of the disease. As such, all these formulations cannot be inherently compared due to dissimilarities in the dose, duration of treatment, clinical study design, formulation type, the method used for analysis, and population disparity. Recently, as detailed earlier, curcumin formulation was also used to diagnose the amyloid spots clinically. Therefore, curcumin formulations have significant potential to serve as preventive, diagnostic, and therapeutic entities.
Acknowledgments
Mangala Hegde acknowledges Science and Engineering Research Board (SERB)-National Post-Doctoral Fellowship (NPDF) (PDF/2021/004053). All the figures were created using BioRender.com.
Author Contributions
M.H. contributed to the initial drafting of the manuscript, review of literature, table preparation, visualization, and overall editing; S.G. contributed to the initial drafting of the manuscript and overall editing; B.B. and R.V. provided critical overall manuscript editing and revision; A.B.K. contributed to conceptualization, funding, overall supervision, and supported review development and overall editing.
This project was supported by BT/556/NE/U-Excel/2016 grant awarded to A.B.K. by Department of Biotechnology (DBT), Government of India.
The authors declare no competing financial interest.
References
- Tabas I.; Glass C. K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013, 339 (6116), 166–172. 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote B.; Elbarbry F.; Bui F.; Su J. W.; Seo K.; Nguyen A.; Lee M.; Rao D. A. Mechanistic Basis for the Role of Phytochemicals in Inflammation-Associated Chronic Diseases. Molecules 2022, 27 (3), 781. 10.3390/molecules27030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Sung B.; Ravindran J.; Diagaradjane P.; Deorukhkar A.; Dey S.; Koca C.; Tong Z.; Gelovani J. G.; Guha S.; et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int. J. Cancer 2012, 131 (3), E292–303. 10.1002/ijc.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Nair A. S.; Ahn K. S.; Pandey M. K.; Yi Z.; Liu M.; Aggarwal B. B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109 (12), 5112–5121. 10.1182/blood-2007-01-067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn B. J.; Dallos M.; Kitagawa H.; Kunnumakkara A. B.; Memmott R. M.; Hollander M. C.; Gills J. J.; Dennis P. A. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res. (Phila) 2013, 6 (8), 801–810. 10.1158/1940-6207.CAPR-13-0058-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmueller A.; Mueller A. L.; Kunnumakkara A. B.; Aggarwal B. B.; Shakibaei M. Multifunctionality of Calebin A in inflammation, chronic diseases and cancer. Front Oncol 2022, 12, 962066 10.3389/fonc.2022.962066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M.; Girisa S.; Naliyadhara N.; Kumar A.; Alqahtani M. S.; Abbas M.; Mohan C. D.; Warrier S.; Hui K. M.; Rangappa K. S.; et al. Natural compounds targeting nuclear receptors for effective cancer therapy. Cancer Metastasis Rev. 2022, 10.1007/s10555-022-10068-w. [DOI] [PubMed] [Google Scholar]
- Bordoloi D.; Banik K.; Padmavathi G.; Vikkurthi R.; Harsha C.; Roy N. K.; Singh A. K.; Monisha J.; Wang H.; Kumar A. P. TIPE2 Induced the Proliferation, Survival, and Migration of Lung Cancer Cells Through Modulation of Akt/mTOR/NF-kappaB Signaling Cascade. Biomolecules 2019, 9 (12), 836. 10.3390/biom9120836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Wu L.; Yan G.; Chen Y.; Zhou M.; Wu Y.; Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6 (1), 263. 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D.; Patni P.; Bishayee A.; Sah A. N.; Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022, 80, 1–17. 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Zhong Z.; Vong C. T.; Chen F.; Tan H.; Zhang C.; Wang N.; Cui L.; Wang Y.; Feng Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42 (3), 1246–1279. 10.1002/med.21876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla R. K.; De R.; Efferth T.; Mezzetti B.; Sahab Uddin M.; Sanusi; Ntie-Kang F.; Wang D.; Schultz F.; Kharat K. R.; et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through #INPST hashtag analysis. Phytomedicine 2023, 108, 154520 10.1016/j.phymed.2022.154520. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P.; Sreekala C.; Zhang Z.; Budhraja A.; Ding S.; Son Y. O.; Wang X.; Hitron A.; Hyun-Jung K.; Wang L.; et al. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anticancer Agents Med. Chem. 2012, 12 (10), 1159–1184. 10.2174/187152012803833035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti R.; Koshima C.; Forster-Carneiro T.; Gomes M.; Rostagno M.; Prado J.; Meireles M.. Uses and applications of extracts from natural sources. Natural Product Extraction; Royal Society of Chemistry, 2022; pp 1–65; DOI: 10.1039/9781839165894-00001. [DOI] [Google Scholar]
- Aggarwal B. B.; Kunnumakkara A. B.. Molecular Targets and Therapeutic Uses of Spices: Modern Uses for Ancient Medicine; World Scientific, 2009. [Google Scholar]
- Gupta S. C.; Sung B.; Kim J. H.; Prasad S.; Li S.; Aggarwal B. B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr Food Res. 2013, 57 (9), 1510–1528. 10.1002/mnfr.201100741. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Smart J. D.; Pannala A. S. Recent developments in formulation design for improving oral bioavailability of curcumin: a review. Journal of drug delivery science and technology 2020, 60, 102082 10.1016/j.jddst.2020.102082. [DOI] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. Bioavailability of curcumin: problems and promises. Mol. Pharmaceutics 2007, 4 (6), 807–818. 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Araiza-Calahorra A.; Akhtar M.; Sarkar A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends in Food Science & Technology 2018, 71, 155–169. 10.1016/j.tifs.2017.11.009. [DOI] [Google Scholar]
- Esatbeyoglu T.; Huebbe P.; Ernst I. M.; Chin D.; Wagner A. E.; Rimbach G. Curcumin--from molecule to biological function. Angew. Chem., Int. Ed. Engl. 2012, 51 (22), 5308–5332. 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- Zielinska A.; Alves H.; Marques V.; Durazzo A.; Lucarini M.; Alves T. F.; Morsink M.; Willemen N.; Eder P.; Chaud M. V. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina (Kaunas) 2020, 56 (7), 336. 10.3390/medicina56070336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu M. M.; Nagesh P. K.; Jaggi M.; Chauhan S. C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17 (6), 1341–1356. 10.1208/s12248-015-9811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu M. M.; Jaggi M.; Chauhan S. C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 2012, 17 (1–2), 71–80. 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C.; Patchva S.; Aggarwal B. B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013, 15 (1), 195–218. 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalraj A.; Sukumaran N. P.; Kunnumakkara A. B.; Gopi S.. The Chemistry and Biological Activities of Curcuminoids: Impacts on Neurological Disorders. Curcumin for Neurological and Psychiatric Disorders; Elsevier, 2019; pp 105–127. [Google Scholar]
- Kunnumakkara A. B.; Bordoloi D.; Padmavathi G.; Monisha J.; Roy N. K.; Prasad S.; Aggarwal B. B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174 (11), 1325–1348. 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisa S.; Kumar A.; Rana V.; Parama D.; Daimary U. D.; Warnakulasuriya S.; Kumar A. P.; Kunnumakkara A. B. From Simple Mouth Cavities to Complex Oral Mucosal Disorders-Curcuminoids as a Promising Therapeutic Approach. ACS Pharmacol Transl Sci. 2021, 4 (2), 647–665. 10.1021/acsptsci.1c00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Harsha C.; Parama D.; Girisa S.; Daimary U. D.; Mao X.; Kunnumakkara A. B. Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases. Phytother Res. 2021, 35 (12), 6768–6801. 10.1002/ptr.7264. [DOI] [PubMed] [Google Scholar]
- Shabnam B.; Harsha C.; Thakur K. K.; Khatoon E.; Kunnumakkara A. B.. Curcumin: a potential molecule for the prevention and treatment of inflammatory diseases. In The Chemistry and Bioactive Components of Turmeric; Royal Society of Chemistry, 2020; pp 150–171. [Google Scholar]
- Jacob J.; Amalraj A.; Raj K. K. J.; Divya C.; Kunnumakkara A. B.; Gopi S. A novel bioavailable hydrogenated curcuminoids formulation (CuroWhite) improves symptoms and diagnostic indicators in rheumatoid arthritis patients - A randomized, double blind and placebo controlled study. J. Tradit Complement Med. 2019, 9 (4), 346–352. 10.1016/j.jtcme.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiso Y.; Suzuki Y.; Watanabe N.; Oshima Y.; Hikino H. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983, 49 (3), 185–187. 10.1055/s-2007-969845. [DOI] [PubMed] [Google Scholar]
- Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br. J. Pharmacol. 1998, 124 (3), 425–427. 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N.; Punithavathi D.; Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br. J. Pharmacol. 2000, 129 (2), 231–234. 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J. Med. Sci. 1972, 26 (4), 269–270. [PubMed] [Google Scholar]
- Arun N.; Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr 2002, 57 (1), 41–52. 10.1023/A:1013106527829. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Bordoloi D.; Harsha C.; Banik K.; Gupta S. C.; Aggarwal B. B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci. (Lond) 2017, 131 (15), 1781–1799. 10.1042/CS20160935. [DOI] [PubMed] [Google Scholar]
- Bordoloi D.; Kunnumakkara A. B.. The potential of curcumin: a multitargeting agent in cancer cell chemosensitization. In Role of nutraceuticals in cancer chemosensitization; Elsevier, 2018; pp 31–60. [Google Scholar]
- Kocaadam B.; Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev. Food Sci. Nutr 2017, 57 (13), 2889–2895. 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- Yeung A. W. K.; Horbanczuk M.; Tzvetkov N. T.; Mocan A.; Carradori S.; Maggi F.; Marchewka J.; Sut S.; Dall’Acqua S.; Gan R. Y.; et al. Curcumin: Total-Scale Analysis of the Scientific Literature. Molecules 2019, 24 (7), 1393. 10.3390/molecules24071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickovic U.; Doberer D.; Gouya G.; Aschauer S.; Weisshaar S.; Storka A.; Bilban M.; Wolzt M. Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. Biomed Res. Int. 2014, 2014, 458592 10.1155/2014/458592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbolahan O. B.; O’Neil B. H.; McRee A. J.; Sanoff H. K.; Fallon J. K.; Smith P. C.; Ivanova A.; Moore D. T.; Dumond J.; Asher G. N. A phase I evaluation of the effect of curcumin on dose-limiting toxicity and pharmacokinetics of irinotecan in participants with solid tumors. Clin Transl Sci. 2022, 15 (5), 1304–1315. 10.1111/cts.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S. J.; Chen O.; Ray S. D.; Ji J.; Bucci L. R.; Preuss H. G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25 (6), 1397. 10.3390/molecules25061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H.; Loo C. Y.; Young P. M.; Traini D.; Mason R. S.; Rohanizadeh R. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv 2014, 11 (8), 1183–1201. 10.1517/17425247.2014.916686. [DOI] [PubMed] [Google Scholar]
- Pancholi V.; Smina T. P.; Kunnumakkara A. B.; Maliakel B.; Krishnakumar I. M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol Rep 2021, 8, 1255–1264. 10.1016/j.toxrep.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini K. I. The chemistry of curcumin: from extraction to therapeutic agent. Molecules 2014, 19 (12), 20091–20112. 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J.; Pan M. H.; Cheng A. L.; Lin L. I.; Ho Y. S.; Hsieh C. Y.; Lin J. K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed Anal 1997, 15 (12), 1867–1876. 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Jamwal R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr Med. 2018, 16 (6), 367–374. 10.1016/j.joim.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara A. B.; Harsha C.; Banik K.; Vikkurthi R.; Sailo B. L.; Bordoloi D.; Gupta S. C.; Aggarwal B. B. Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin Drug Metab Toxicol 2019, 15 (9), 705–733. 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- Imaizumi A. Highly bioavailable curcumin (Theracurmin): Its development and clinical application. PharmaNutrition 2015, 3 (4), 123–130. 10.1016/j.phanu.2015.08.002. [DOI] [Google Scholar]
- Lao C. D.; Ruffin M. T. t.; Normolle D.; Heath D. D.; Murray S. I.; Bailey J. M.; Boggs M. E.; Crowell J.; Rock C. L.; Brenner D. E. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006, 6, 10. 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. A.; Euden S. A.; Platton S. L.; Cooke D. N.; Shafayat A.; Hewitt H. R.; Marczylo T. H.; Morgan B.; Hemingway D.; Plummer S. M.; et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10 (20), 6847–6854. 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- Kanai M.; Yoshimura K.; Asada M.; Imaizumi A.; Suzuki C.; Matsumoto S.; Nishimura T.; Mori Y.; Masui T.; Kawaguchi Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 2011, 68 (1), 157–164. 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- Zheng B.; McClements D. J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25 (12), 2791. 10.3390/molecules25122791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S.; Tyagi A. K.; Aggarwal B. B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat 2014, 46 (1), 2–18. 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba G.; Joy D.; Joseph T.; Majeed M.; Rajendran R.; Srinivas P. S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64 (4), 353–356. 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Yang K. Y.; Lin L. C.; Tseng T. Y.; Wang S. C.; Tsai T. H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr B Analyt Technol. Biomed Life Sci. 2007, 853 (1–2), 183–189. 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Pan M. H.; Huang T. M.; Lin J. K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27 (4), 486–494. [PubMed] [Google Scholar]
- Ravindranath V.; Chandrasekhara N. Metabolism of curcumn-studies with [3H] curcumin. Toxicology 1981, 22 (4), 337–344. 10.1016/0300-483X(81)90027-5. [DOI] [PubMed] [Google Scholar]
- Ravindranath V.; Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16 (3), 259–265. 10.1016/0300-483X(80)90122-5. [DOI] [PubMed] [Google Scholar]
- Marczylo T. H.; Verschoyle R. D.; Cooke D. N.; Morazzoni P.; Steward W. P.; Gescher A. J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007, 60 (2), 171–177. 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Garcea G.; Berry D. P.; Jones D. J.; Singh R.; Dennison A. R.; Farmer P. B.; Sharma R. A.; Steward W. P.; Gescher A. J. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 2005, 14 (1), 120–125. 10.1158/1055-9965.120.14.1. [DOI] [PubMed] [Google Scholar]
- Dhillon N.; Aggarwal B. B.; Newman R. A.; Wolff R. A.; Kunnumakkara A. B.; Abbruzzese J. L.; Ng C. S.; Badmaev V.; Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14 (14), 4491–4499. 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Teiten M. H.; Dicato M.; Diederich M. Hybrid curcumin compounds: a new strategy for cancer treatment. Molecules 2014, 19 (12), 20839–20863. 10.3390/molecules191220839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H.; Shakeri A.; Rashidi B.; Jalili A.; Banikazemi Z.; Sahebkar A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother 2017, 85, 102–112. 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- DB M.; Sreedharan S.; Mahadik K. Role of piperine as an effective bioenhancer in drug absorption. Pharm. Anal Acta 2018, 9 (7), 1000591. 10.4172/2153-2435.1000591. [DOI] [Google Scholar]
- Volak L. P.; Ghirmai S.; Cashman J. R.; Court M. H. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab. Dispos. 2008, 36 (8), 1594–1605. 10.1124/dmd.108.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A.; Sung B.; Bhanu Prasad B. A.; Schuber P. T. Jr.; Prasad S.; Aggarwal B. B.; Bornmann W. G. Curcumin glucuronides: assessing the proliferative activity against human cell lines. Bioorg. Med. Chem. 2014, 22 (1), 435–439. 10.1016/j.bmc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S.; Ray S. Issues with human bioavailability determinations of bioactive curcumin. Biomedical Journal of Scientific & Technical Research 2019, 12 (4), 9417–9419. 10.26717/BJSTR.2019.12.002289. [DOI] [Google Scholar]
- Cardaci T. D.; Machek S. B.; Wilburn D. T.; Hwang P. S.; Willoughby D. S. Ubiquitin Proteasome System Activity is Suppressed by Curcumin following Exercise-Induced Muscle Damage in Human Skeletal Muscle. J. Am. Coll Nutr 2021, 40 (5), 401–411. 10.1080/07315724.2020.1783721. [DOI] [PubMed] [Google Scholar]
- Miranda-Castro S.; Aidar F. J.; de Moura S. S.; Marcucci-Barbosa L.; Lobo L. F.; de Assis Dias Martins-Junior F.; da Silva Filha R.; Vaz de Castro P. A. S.; Simoes e Silva A. C.; da Gloria de Souza D. The Curcumin Supplementation with Piperine Can Influence the Acute Elevation of Exercise-Induced Cytokines: Double-Blind Crossover Study. Biology (Basel) 2022, 11 (4), 573. 10.3390/biology11040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas J.; Sinha D.; Mukherjee S.; Roy S.; Siddiqi M.; Roy M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol 2010, 29 (6), 513–524. 10.1177/0960327109359020. [DOI] [PubMed] [Google Scholar]
- Khdair S. A.; Abdulridha M. K.; Shafek M. A. The effect of curcumin adjuvant therapy on pulmonary function and levels of interleukin-6 (IL-6) and superoxide dismutase-3 (EC-SOD3) in patients with chronic bronchial asthma. Indonesian Journal of Pharmacy 2021, 32, 232–240. 10.22146/ijp.1136. [DOI] [Google Scholar]
- Pawar K. S.; Mastud R. N.; Pawar S. K.; Pawar S. S.; Bhoite R. R.; Bhoite R. R.; Kulkarni M. V.; Deshpande A. R. Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front Pharmacol 2021, 12, 669362 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari G.; Sahebkar A.; Soleimani D.; Mahdavi A.; Rafiee S.; Majeed M.; Khorvash F.; Iraj B.; Elyasi M.; Rouhani M. H.; et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials 2022, 23 (1), 472. 10.1186/s13063-022-06375-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyush P.; Mahajan A.; Singh K.; Ghosh S.; Gupta S. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis 2019, 25 (1), 73–79. 10.1111/odi.12947. [DOI] [PubMed] [Google Scholar]
- Durgaprasad S.; Pai C. G.; Vasanthkumar; Alvres J. F.; Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005, 122 (4), 315–318. [PubMed] [Google Scholar]
- Panahi Y.; Khalili N.; Sahebi E.; Namazi S.; Atkin S. L.; Majeed M.; Sahebkar A. Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus. Curr. Clin Pharmacol 2018, 12 (4), 253–258. 10.2174/1574884713666180104095641. [DOI] [PubMed] [Google Scholar]
- Hemmati A. A.; Rajaee E.; Houshmand G.; Fakhroddin M.; Dargahi-MalAmir M.; Hesam S.; Maram N. Study the effects of anti-inflammatory curcumex capsules containing three plants (ginger, curcumin and black pepper) in patients with active rheumatoid arthritis. IIOAB Journal 2016, 7 (Suppl 5), 389–392. [Google Scholar]
- Hatab H. M.; Abdel Hamid F. F.; Soliman A. F.; Al-Shafie T. A.; Ismail Y. M.; El-Houseini M. E. A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: a pilot study. J. Gastrointest Oncol 2019, 10 (4), 766–776. 10.21037/jgo.2019.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rawe M.; Wickremesekera A. C.; Pandey R.; Young D.; Sim D.; FitzJohn T.; Burgess C.; Kaye A. H.; Tan S. T. Treatment of glioblastoma with re-purposed renin-angiotensin system modulators: Results of a phase I clinical trial. J. Clin Neurosci 2022, 95, 48–54. 10.1016/j.jocn.2021.11.023. [DOI] [PubMed] [Google Scholar]
- Stancioiu F.; Mihai D.; Papadakis G. Z.; Tsatsakis A.; Spandidos D. A.; Badiu C. Treatment for benign thyroid nodules with a combination of natural extracts. Mol. Med. Rep 2019, 20 (3), 2332–2338. 10.3892/mmr.2019.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portincasa P.; Bonfrate L.; Scribano M. L.; Kohn A.; Caporaso N.; Festi D.; Campanale M. C.; Di Rienzo T.; Guarino M.; Taddia M.; et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. J. Gastrointestin Liver Dis 2020, 25 (2), 151–157. 10.15403/jgld.2014.1121.252.ccm. [DOI] [PubMed] [Google Scholar]
- Cruz-Correa M.; Shoskes D. A.; Sanchez P.; Zhao R.; Hylind L. M.; Wexner S. D.; Giardiello F. M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2006, 4 (8), 1035–1038. 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Di Mario F.; Cavallaro L. G.; Nouvenne A.; Stefani N.; Cavestro G. M.; Iori V.; Maino M.; Comparato G.; Fanigliulo L.; Morana E.; et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure?. Helicobacter 2007, 12 (3), 238–243. 10.1111/j.1523-5378.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- Morgia G.; Russo G. I.; Urzi D.; Privitera S.; Castelli T.; Favilla V.; Cimino S. A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III. Arch Ital Urol Androl 2017, 89 (2), 110–113. 10.4081/aiua.2017.2.110. [DOI] [PubMed] [Google Scholar]
- Miodownik C.; Lerner V.; Kudkaeva N.; Lerner P. P.; Pashinian A.; Bersudsky Y.; Eliyahu R.; Kreinin A.; Bergman J. Curcumin as Add-On to Antipsychotic Treatment in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Clin Neuropharmacol 2019, 42 (4), 117–122. 10.1097/WNF.0000000000000344. [DOI] [PubMed] [Google Scholar]
- Mustafa M. W.; Ungphaiboon S.; Phadoongsombut N.; Pangsomboon K.; Chelae S.; Mahattanadul S. Effectiveness of an Alcohol-Free Chitosan-Curcuminoid Mouthwash Compared with Chlorhexidine Mouthwash in Denture Stomatitis Treatment: A Randomized Trial. J. Altern Complement Med. 2019, 25 (5), 552–558. 10.1089/acm.2018.0459. [DOI] [PubMed] [Google Scholar]
- Anusha D.; Chaly P. E.; Junaid M.; Nijesh J. E.; Shivashankar K.; Sivasamy S. Efficacy of a mouthwash containing essential oils and curcumin as an adjunct to nonsurgical periodontal therapy among rheumatoid arthritis patients with chronic periodontitis: A randomized controlled trial. Indian J. Dent Res. 2019, 30 (4), 506–511. 10.4103/ijdr.IJDR_662_17. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Ghanei M.; Bashiri S.; Hajihashemi A.; Sahebkar A. Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res. (Stuttg) 2015, 65 (11), 567–573. 10.1055/s-0034-1389986. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Khalili N.; Sahebi E.; Namazi S.; Simental-Mendia L. E.; Majeed M.; Sahebkar A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. (Stuttg) 2018, 68 (7), 403–409. 10.1055/s-0044-101752. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Alishiri G. H.; Parvin S.; Sahebkar A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J. Diet Suppl 2016, 13 (2), 209–220. 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Khalili N.; Hosseini M. S.; Abbasinazari M.; Sahebkar A. Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med. 2014, 22 (5), 851–857. 10.1016/j.ctim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Hosseini M. S.; Khalili N.; Naimi E.; Majeed M.; Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr 2015, 34 (6), 1101–1108. 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Hosseini M. S.; Khalili N.; Naimi E.; Simental-Mendía L. E.; Majeed M.; Sahebkar A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomedicine & pharmacotherapy 2016, 82, 578–582. 10.1016/j.biopha.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Hosseini M. S.; Khalili N.; Naimi E.; Soflaei S. S.; Majeed M.; Sahebkar A. Effects of supplementation with curcumin on serum adipokine concentrations: A randomized controlled trial. Nutrition 2016, 32 (10), 1116–1122. 10.1016/j.nut.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Valizadegan G.; Ahamdi N.; Ganjali S.; Majeed M.; Sahebkar A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell Biochem 2019, 120 (9), 15989–15996. 10.1002/jcb.28877. [DOI] [PubMed] [Google Scholar]
- Saberi-Karimian M.; Keshvari M.; Ghayour-Mobarhan M.; Salehizadeh L.; Rahmani S.; Behnam B.; Jamialahmadi T.; Asgary S.; Sahebkar A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement Ther Med. 2020, 49, 102322 10.1016/j.ctim.2020.102322. [DOI] [PubMed] [Google Scholar]
- Mohammadi A.; Sahebkar A.; Iranshahi M.; Amini M.; Khojasteh R.; Ghayour-Mobarhan M.; Ferns G. A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: a randomized crossover trial. Phytother Res. 2013, 27 (3), 374–379. 10.1002/ptr.4715. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Sahebkar A.; Amiri M.; Davoudi S. M.; Beiraghdar F.; Hoseininejad S. L.; Kolivand M. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2012, 108 (7), 1272–1279. 10.1017/S0007114511006544. [DOI] [PubMed] [Google Scholar]
- Shadnoush M.; Zahedi H.; Norouzy A.; Sahebkar A.; Sadeghi O.; Najafi A.; Hosseini S.; Qorbani M.; Ahmadi A.; Ardehali S. H.; et al. Effects of supplementation with curcuminoids on serum adipokines in critically ill patients: a randomized double-blind placebo-controlled trial. Phytother Res. 2020, 34 (12), 3180–3188. 10.1002/ptr.6749. [DOI] [PubMed] [Google Scholar]
- Zahedi H.; Hosseinzadeh-Attar M. J.; Shadnoush M.; Sahebkar A.; Barkhidarian B.; Sadeghi O.; Najafi A.; Hosseini S.; Qorbani M.; Ahmadi A.; et al. Effects of curcuminoids on inflammatory and oxidative stress biomarkers and clinical outcomes in critically ill patients: A randomized double-blind placebo-controlled trial. Phytother Res. 2021, 35 (8), 4605–4615. 10.1002/ptr.7179. [DOI] [PubMed] [Google Scholar]
- Arabnezhad L.; Mohammadifard M.; Rahmani L.; Majidi Z.; Ferns G. A.; Bahrami A. Effects of curcumin supplementation on vitamin D levels in women with premenstrual syndrome and dysmenorrhea: a randomized controlled study. BMC Complement Med. Ther 2022, 22 (1), 19. 10.1186/s12906-022-03515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A.; Zarban A.; Rezapour H.; Agha Amini Fashami A.; Ferns G. A. Effects of curcumin on menstrual pattern, premenstrual syndrome, and dysmenorrhea: A triple-blind, placebo-controlled clinical trial. Phytother Res. 2021, 35 (12), 6954–6962. 10.1002/ptr.7314. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Khalili N.; Sahebi E.; Namazi S.; Karimian M. S.; Majeed M.; Sahebkar A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology 2017, 25 (1), 25–31. 10.1007/s10787-016-0301-4. [DOI] [PubMed] [Google Scholar]
- Sahebkar A.; Mohammadi A.; Atabati A.; Rahiman S.; Tavallaie S.; Iranshahi M.; Akhlaghi S.; Ferns G. A.; Ghayour-Mobarhan M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother Res. 2013, 27 (12), 1883–1888. 10.1002/ptr.4952. [DOI] [PubMed] [Google Scholar]
- Mohajer A.; Ghayour-Mobarhan M.; Parizadeh S. M. R.; Tavallaie S.; Rajabian M.; Sahebkar A. Effects of supplementation with curcuminoids on serum copper and zinc concentrations and superoxide dismutase enzyme activity in obese subjects. Trace Elem Electrolytes 2015, 32, 16–21. 10.5414/TEX01363. [DOI] [Google Scholar]
- Ganjali S.; Sahebkar A.; Mahdipour E.; Jamialahmadi K.; Torabi S.; Akhlaghi S.; Ferns G.; Parizadeh S. M.; Ghayour-Mobarhan M. Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. ScientificWorldJournal 2014, 2014, 898361 10.1155/2014/898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimnia A. R.; Panahi Y.; Alishiri G.; Sharafi M.; Sahebkar A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. (Stuttg) 2015, 65 (10), 521–525. 10.1055/s-0034-1384536. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Sahebkar A.; Parvin S.; Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann. Clin Biochem 2012, 49, 580–588. 10.1258/acb.2012.012040. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Ghanei M.; Hajhashemi A.; Sahebkar A. Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Randomized Controlled Trial. J. Diet Suppl 2016, 13 (1), 93–105. 10.3109/19390211.2014.952865. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Khalili N.; Sahebi E.; Namazi S.; Reiner Z.; Majeed M.; Sahebkar A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement Ther Med. 2017, 33, 1–5. 10.1016/j.ctim.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Mirhafez S. R.; Farimani A. R.; Gholami A.; Hooshmand E.; Tavallaie S.; Nobakht M. Gh B. F. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Drug Metab Pers Ther 2019, 34 (2), 20180040. 10.1515/dmpt-2018-0040. [DOI] [PubMed] [Google Scholar]
- Rai A.; Kaur M.; Gombra V.; Hasan S.; Kumar N. Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis. J. Investig Clin Dent 2019, 10 (4), e12464 10.1111/jicd.12464. [DOI] [PubMed] [Google Scholar]
- Sharma N.; Jain A.; Bahudar S.; Oberoi S. S. Efficacy of Curcumin and Piperine as Antioxidant Adjuvant to Intralesional Dexamethasone Injection for Management of Oral Submucous Fibrosis: A Clinical Trial. Journal of Orofacial Sciences 2021, 13 (2), 129. 10.4103/jofs.jofs_218_21. [DOI] [Google Scholar]
- Volak L. P.; Hanley M. J.; Masse G.; Hazarika S.; Harmatz J. S.; Badmaev V.; Majeed M.; Greenblatt D. J.; Court M. H. Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br. J. Clin. Pharmacol. 2013, 75 (2), 450–462. 10.1111/j.1365-2125.2012.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa Y.; Hirano S.; Katanasaka Y.; Miyazaki Y.; Funamoto M.; Okamura N.; Hojo Y.; Suzuki H.; Doi O.; Yokoji T.; et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study. J. Nutr Sci. Vitaminol (Tokyo) 2015, 61 (1), 37–44. 10.3177/jnsv.61.37. [DOI] [PubMed] [Google Scholar]
- Antony B.; Merina B.; Iyer V. S.; Judy N.; Lennertz K.; Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm. Sci. 2008, 70 (4), 445–449. 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa D.; Suharti C.; Riwanto I.; Dharmana E.; Pangarsa E. A.; Setiawan B.; Suyono S.; Tobing M. L.; Suhartono S.; Hadisapurto S. Curcumin as adjuvant therapy to improve remission in myeloma patients: A pilot randomized clinical trial. Caspian J. Intern Med. 2022, 13 (2), 375–384. 10.22088/cjim.13.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracca M.; Quarantelli M.; Moccia M.; Vacca G.; Satelliti B.; D’Ambrosio G.; Carotenuto A.; Ragucci M.; Assogna F.; Capacchione A.; et al. ProspeCtive study to evaluate efficacy, safety and tOlerability of dietary supplemeNT of Curcumin (BCM95) in subjects with Active relapsing MultIple Sclerosis treated with subcutaNeous Interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial. Mult Scler Relat Disord 2021, 56, 103274 10.1016/j.msard.2021.103274. [DOI] [PubMed] [Google Scholar]
- Saadati S.; Sadeghi A.; Mansour A.; Yari Z.; Poustchi H.; Hedayati M.; Hatami B.; Hekmatdoost A. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol 2019, 19 (1), 133. 10.1186/s12876-019-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandish M.; Mozaffari-Khosravi H.; Mohammadi S. M.; Cheraghian B.; Azhdari M. Curcumin and zinc co-supplementation along with a loss-weight diet can improve lipid profiles in subjects with prediabetes: a multi-arm, parallel-group, randomized, double-blind placebo-controlled phase 2 clinical trial. Diabetol Metab Syndr 2022, 14 (1), 22. 10.1186/s13098-022-00792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti C.; Colucci M.; Storto M.; Semeraro F.; Ammollo C. T.; Incampo F.; Costanzo S.; De Bartolomeo G.; Portincasa P.; Barone M.; et al. Randomised trial of chronic supplementation with a nutraceutical mixture in subjects with non-alcoholic fatty liver disease. Br. J. Nutr. 2020, 123 (2), 190–197. 10.1017/S0007114519002484. [DOI] [PubMed] [Google Scholar]
- Sterzi S.; Giordani L.; Morrone M.; Lena E.; Magrone G.; Scarpini C.; Milighetti S.; Pellicciari L.; Bravi M.; Panni I.; et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur. J. Phys. Rehabil Med. 2016, 52 (3), 321–330. [PubMed] [Google Scholar]
- Haroyan A.; Mukuchyan V.; Mkrtchyan N.; Minasyan N.; Gasparyan S.; Sargsyan A.; Narimanyan M.; Hovhannisyan A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blind, placebo-controlled study. BMC Complement Altern Med. 2018, 18 (1), 7. 10.1186/s12906-017-2062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Askar M.; AlMubarak A. M.; Alqutub M. N.; Mokeem S.; Javed F.; Vohra F.; Abduljabbar T. Analgesic Efficacy of Curcuma longa (Curcumin) after Surgical Periodontal Therapy. Oral Health Prev Dent 2022, 20 (1), 19–26. 10.3290/j.ohpd.b2572979. [DOI] [PubMed] [Google Scholar]
- Karandish M.; Mozaffari-Khosravi H.; Mohammadi S. M.; Cheraghian B.; Azhdari M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother Res. 2021, 35 (8), 4377–4387. 10.1002/ptr.7136. [DOI] [PubMed] [Google Scholar]
- Hellou E.; Mohsin J.; Elemy A.; Hakim F.; Mustafa-Hellou M.; Hamoud S. Effect of ArtemiC in patients with COVID-19: A Phase II prospective study. J. Cell Mol. Med. 2022, 26 (11), 3281–3289. 10.1111/jcmm.17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry-Regard C.; Vinyes-Pares G.; Breuille D.; Moritani T. Supplementation with Whey Protein, Omega-3 Fatty Acids and Polyphenols Combined with Electrical Muscle Stimulation Increases Muscle Strength in Elderly Adults with Limited Mobility: A Randomized Controlled Trial. Nutrients 2020, 12 (6), 1866. 10.3390/nu12061866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan T.; Tananyan A.; Janoyan N.; Tadevosyan A.; Petrosyan H.; Hovhannisyan A.; Hayrapetyan L.; Arustamyan M.; Arnhold J.; Rotmann A. R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218 10.1016/j.phymed.2020.153218. [DOI] [PubMed] [Google Scholar]
- Shapira S.; Leshno A.; Katz D.; Maharshak N.; Hevroni G.; Jean-David M.; Kraus S.; Galazan L.; Aroch I.; Kazanov D.; et al. Of mice and men: a novel dietary supplement for the treatment of ulcerative colitis. Therap Adv. Gastroenterol 2018, 11, 1756283X1774186 10.1177/1756283X17741864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad S.; Meidan I.; Sellam G.; Simaan S.; Zeevi I.; Waldman E.; Weintraub M.; Revel-Vilk S. Topical curcumin for the prevention of oral mucositis in pediatric patients: case series. Altern Ther Health Med. 2013, 19 (3), 21–24. [PubMed] [Google Scholar]
- Rahmani S.; Asgary S.; Askari G.; Keshvari M.; Hatamipour M.; Feizi A.; Sahebkar A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother Res. 2016, 30 (9), 1540–1548. 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- Takahashi M.; Suzuki K.; Kim H. K.; Otsuka Y.; Imaizumi A.; Miyashita M.; Sakamoto S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35 (6), 469–475. 10.1055/s-0033-1357185. [DOI] [PubMed] [Google Scholar]
- Varma K.; Amalraj A.; Divya C.; Gopi S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24 (1), 40–49. 10.1089/jmf.2020.4778. [DOI] [PubMed] [Google Scholar]
- Gopi S.; Jacob J.; Varma K.; Jude S.; Amalraj A.; Arundhathy C. A.; George R.; Sreeraj T. R.; Divya C.; Kunnumakkara A. B.; et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study. Phytother Res. 2017, 31 (12), 1883–1891. 10.1002/ptr.5931. [DOI] [PubMed] [Google Scholar]
- Amalraj A.; Divya C.; Gopi S. The Effects of Bioavailable Curcumin (Cureit) on Delayed Onset Muscle Soreness Induced By Eccentric Continuous Exercise: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2020, 23 (5), 545–553. 10.1089/jmf.2019.4533. [DOI] [PubMed] [Google Scholar]
- Purpura M.; Lowery R. P.; Wilson J. M.; Mannan H.; Munch G.; Razmovski-Naumovski V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr 2018, 57 (3), 929–938. 10.1007/s00394-016-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalraj A.; Varma K.; Jacob J.; Divya C.; Kunnumakkara A. B.; Stohs S. J.; Gopi S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20 (10), 1022–1030. 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- Adibian M.; Hodaei H.; Nikpayam O.; Sohrab G.; Hekmatdoost A.; Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2019, 33 (5), 1374–1383. 10.1002/ptr.6328. [DOI] [PubMed] [Google Scholar]
- Hodaei H.; Adibian M.; Nikpayam O.; Hedayati M.; Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr 2019, 11, 41. 10.1186/s13098-019-0437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng M. C.; Song M. K.; Harker J.; Heng M. K. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J. Dermatol 2000, 143 (5), 937–949. 10.1046/j.1365-2133.2000.03767.x. [DOI] [PubMed] [Google Scholar]
- Nerkar Rajbhoj A.; Kulkarni T. M.; Shete A.; Shete M.; Gore R.; Sapkal R. A Comparative Study to Evaluate Efficacy of Curcumin and Aloe Vera Gel along with Oral Physiotherapy in the Management of Oral Submucous Fibrosis: A Randomized Clinical Trial. Asian Pac J. Cancer Prev 2021, 22 (S1), 107–112. 10.31557/APJCP.2021.22.S1.107. [DOI] [PubMed] [Google Scholar]
- Chandrashekar A.; Annigeri R. G.; Va U.; Thimmasetty J. A clinicobiochemical evaluation of curcumin as gel and as buccal mucoadhesive patches in the management of oral submucous fibrosis. Oral Surg Oral Med. Oral Pathol Oral Radiol 2021, 131 (4), 428–434. 10.1016/j.oooo.2020.12.020. [DOI] [PubMed] [Google Scholar]
- Mikirova N. A.; Kesari S.; Ichim T. E.; Riordan N. H. Effect of Infla-Kine supplementation on the gene expression of inflammatory markers in peripheral mononuclear cells and on C-reactive protein in blood. J. Transl Med. 2017, 15 (1), 213. 10.1186/s12967-017-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupi E.; Lazzeri L.; Centini G. Endometriosis and pain: postsurgical alternative treatment in patients desiring pregnancy. Journal of Endometriosis and Pelvic Pain Disorders 2015, 7 (3), 95–99. 10.5301/je.5000226. [DOI] [Google Scholar]
- Cosentino V.; Fratter A.; Cosentino M. Anti-inflammatory effects exerted by Killox(R), an innovative formulation of food supplement with curcumin, in urology. Eur. Rev. Med. Pharmacol Sci. 2016, 20 (7), 1390–1398. [PubMed] [Google Scholar]
- Radkar P.; Lakshmanan P. S.; Mary J. J.; Chaudhary S.; Durairaj S. K. A Novel Multi-Ingredient Supplement Reduces Inflammation of the Eye and Improves Production and Quality of Tears in Humans. Ophthalmol Ther 2021, 10 (3), 581–599. 10.1007/s40123-021-00357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak K.; McKinney J.; Heilbrun L. K.; Sarkar F. H. Critical need for clinical trials: an example of a pilot human intervention trial of a mixture of natural agents protecting lymphocytes against TNF-alpha induced activation of NF-kappaB. Pharm. Res. 2010, 27 (6), 1061–1065. 10.1007/s11095-010-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M.; Leunis J. C. Attenuation of autoimmune responses to oxidative specific epitopes, but not nitroso-adducts, is associated with a better clinical outcome in Myalgic Encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol Lett. 2014, 35 (7), 577–585. [PubMed] [Google Scholar]
- Martinez N.; Herrera M.; Frias L.; Provencio M.; Perez-Carrion R.; Diaz V.; Morse M.; Crespo M. C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: results of a pilot study. Clin Transl Oncol 2019, 21 (4), 489–498. 10.1007/s12094-018-1950-0. [DOI] [PubMed] [Google Scholar]
- Derosa G.; D’Angelo A.; Vanelli A.; Maffioli P. An Evaluation of a Nutraceutical with Berberine, Curcumin, Inositol, Banaba and Chromium Picolinate in Patients with Fasting Dysglycemia. Diabetes Metab Syndr Obes 2020, 13, 653–661. 10.2147/DMSO.S232791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K.; Ohara T.; Muroyama K.; Yamamoto Y.; Murosaki S. Effects of hot water extract of Curcuma longa on human epidermal keratinocytes in vitro and skin conditions in healthy participants: A randomized, double-blind, placebo-controlled trial. J. Cosmet Dermatol 2019, 18 (6), 1866–1874. 10.1111/jocd.12890. [DOI] [PubMed] [Google Scholar]
- Ablon G.; Kogan S. A Six-Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women With Self-Perceived Thinning Hair. J. Drugs Dermatol 2018, 17 (5), 558–565. [PubMed] [Google Scholar]
- Amalraj A.; Varma K.; Jacob J.; Kuttappan S. Efficacy and safety of a gut health product (Actbiome) prepared by incorporation of asafoetida-curcumin complex onto the turmeric dietary fiber in the management of gut health and intestinal microflora in healthy subjects: A randomized, double-blind, placebo controlled study. Bioactive Carbohydrates and Dietary Fibre 2021, 26, 100280 10.1016/j.bcdf.2021.100280. [DOI] [Google Scholar]
- Stohs S. J.; Ji J.; Bucci L. R.; Preuss H. G. A Comparative Pharmacokinetic Assessment of a Novel Highly Bioavailable Curcumin Formulation with 95% Curcumin: A Randomized, Double-Blind, Crossover Study. J. Am. Coll Nutr 2018, 37 (1), 51–59. 10.1080/07315724.2017.1358118. [DOI] [PubMed] [Google Scholar]
- Birudaraju D.; Cherukuri L.; Kinninger A.; Chaganti B. T.; Shaikh K.; Hamal S.; Flores F.; Roy S. K.; Budoff M. J. A combined effect of Cavacurcumin, Eicosapentaenoic acid (Omega-3s), Astaxanthin and Gamma -linoleic acid (Omega-6) (CEAG) in healthy volunteers- a randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN 2020, 35, 174–179. 10.1016/j.clnesp.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Jager R.; Lowery R. P.; Calvanese A. V.; Joy J. M.; Purpura M.; Wilson J. M. Comparative absorption of curcumin formulations. Nutr J. 2014, 13, 11. 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravano M.; Allegrini D.; Carnevali A.; Costanzo E.; Giannaccare G.; Giorno P.; Scorcia V.; Spedicato G. A.; Varano M.; Romano M. R. Effectiveness of a Hydrophilic Curcumin-Based Formulation in Coadjuvating the Therapeutic Effect of Intravitreal Dexamethasone in Subjects With Diabetic Macular Edema. Front Pharmacol 2022, 12, 726104 10.3389/fphar.2021.726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero A. F. G.; Sahebkar A.; Fogacci F.; Bove M.; Giovannini M.; Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. Eur. J. Nutr 2020, 59 (2), 477–483. 10.1007/s00394-019-01916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A.; Bohnert L.; Schiborr C.; Frank J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr Food Res. 2016, 60 (7), 1555–1563. 10.1002/mnfr.201501034. [DOI] [PubMed] [Google Scholar]
- Schiborr C.; Kocher A.; Behnam D.; Jandasek J.; Toelstede S.; Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr Food Res. 2014, 58 (3), 516–527. 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- Dutzmann S.; Schiborr C.; Kocher A.; Pilatus U.; Hattingen E.; Weissenberger J.; Gessler F.; Quick-Weller J.; Franz K.; Seifert V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr Cancer 2016, 68 (6), 943–948. 10.1080/01635581.2016.1187281. [DOI] [PubMed] [Google Scholar]
- Aslanabadi N.; Entezari-Maleki T.; Rezaee H.; Jafarzadeh H. R.; Vahedpour R. Curcumin for the prevention of myocardial injury following elective percutaneous coronary intervention; a pilot randomized clinical trial. Eur. J. Pharmacol. 2019, 858, 172471 10.1016/j.ejphar.2019.172471. [DOI] [PubMed] [Google Scholar]
- Sitzia C.; Meregalli M.; Belicchi M.; Farini A.; Arosio M.; Bestetti D.; Villa C.; Valenti L.; Brambilla P.; Torrente Y. Preliminary Evidences of Safety and Efficacy of Flavonoids- and Omega 3-Based Compound for Muscular Dystrophies Treatment: A Randomized Double-Blind Placebo Controlled Pilot Clinical Trial. Front Neurol 2019, 10, 755. 10.3389/fneur.2019.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y.; Gharbi M.; Dierckxsens Y.; Priem F.; Marty M.; Seidel L.; Albert A.; Heuse E.; Bonnet V.; Castermans C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement Altern Med. 2014, 14, 159. 10.1186/1472-6882-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Robbins S.; Eyles J.; Fedorova T.; Virk S.; Deveza L. A.; McLachlan A. J.; Hunter D. J. Efficacy and safety of a supplement combination on hand pain among people with symptomatic hand osteoarthritis an internet-based, randomised clinical trial the RADIANT study. Osteoarthritis Cartilage 2021, 29 (5), 667–677. 10.1016/j.joca.2021.01.011. [DOI] [PubMed] [Google Scholar]
- Tiekou Lorinczova H.; Begum G.; Temouri L.; Renshaw D.; Zariwala M. G. Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study. Nutrients 2022, 14 (3), 712. 10.3390/nu14030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard A. R.; Briskey D.; Richards A.; Rao A. Curcumin Improves Delayed Onset Muscle Soreness and Postexercise Lactate Accumulation. J. Diet Suppl 2021, 18 (5), 531–542. 10.1080/19390211.2020.1796885. [DOI] [PubMed] [Google Scholar]
- Greil R.; Greil-Ressler S.; Weiss L.; Schonlieb C.; Magnes T.; Radl B.; Bolger G. T.; Vcelar B.; Sordillo P. P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc()) in patients with locally advanced or metastatic cancer. Cancer Chemother Pharmacol 2018, 82 (4), 695–706. 10.1007/s00280-018-3654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi A.; Sadeghnia H. R.; Saberi-Karimian M.; Safarian H.; Ferns G. A.; Ghayour-Mobarhan M.; Sahebkar A. Effects of Curcumin on Serum Vitamin E Concentrations in Individuals with Metabolic Syndrome. Phytother Res. 2017, 31 (4), 657–662. 10.1002/ptr.5779. [DOI] [PubMed] [Google Scholar]
- Tonnesen H. H.; Masson M.; Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244 (1–2), 127–135. 10.1016/S0378-5173(02)00323-X. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Haddadi A.; Molavi O.; Lavasanifar A.; Lai R.; Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed Mater. Res. 2008, 86A (2), 300–310. 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]
- Cuomo J.; Appendino G.; Dern A. S.; Schneider E.; McKinnon T. P.; Brown M. J.; Togni S.; Dixon B. M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod 2011, 74 (4), 664–669. 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- Asher G. N.; Xie Y.; Moaddel R.; Sanghvi M.; Dossou K. S.; Kashuba A. D.; Sandler R. S.; Hawke R. L. Randomized Pharmacokinetic Crossover Study Comparing 2 Curcumin Preparations in Plasma and Rectal Tissue of Healthy Human Volunteers. J. Clin Pharmacol 2017, 57 (2), 185–193. 10.1002/jcph.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivari F.; Mingione A.; Piazzini G.; Ceccarani C.; Ottaviano E.; Brasacchio C.; Dei Cas M.; Vischi M.; Cozzolino M. G.; Fogagnolo P. Curcumin Supplementation (Meriva((R))) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14 (1), 231. 10.3390/nu14010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G.; Belcaro G.; Cornelli U.; Luzzi R.; Togni S.; Dugall M.; Cesarone M. R.; Feragalli B.; Ippolito E.; Errichi B. M.; et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011, 53 (3 Suppl 1), 43–49. [PubMed] [Google Scholar]
- Steigerwalt R.; Nebbioso M.; Appendino G.; Belcaro G.; Ciammaichella G.; Cornelli U.; Luzzi R.; Togni S.; Dugall M.; Cesarone M. R.; et al. Meriva(R), a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012, 54 (1 Suppl 4), 11–16. [PubMed] [Google Scholar]
- Donovan E. K.; Kekes-Szabo S.; Lin J. C.; Massey R. L.; Cobb J. D.; Hodgin K. S.; Ness T. J.; Hangee-Bauer C.; Younger J. W. A Placebo-Controlled, Pseudo-Randomized, Crossover Trial of Botanical Agents for Gulf War Illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French Maritime Pine Bark (Pinus pinaster). Int. J. Environ. Res. Public Health 2021, 18 (5), 2468. 10.3390/ijerph18052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J. A.; Stojanovski E.; MacDonald-Wicks L.; Garg M. L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 2018, 82, 22–35. 10.1016/j.metabol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Safarian H.; Parizadeh S. M. R.; Saberi-Karimain M.; Darroudi S.; Javandoost A.; Mohammadi F.; Moammeri M.; Ferns G. A.; Ghayour-Mobarhan M.; Mohebati M. The Effect of Curcumin on Serum Copper and Zinc and Zn/Cu Ratio in Individuals with Metabolic Syndrome: A Double-Blind Clinical Trial. J. Diet Suppl 2019, 16 (6), 625–634. 10.1080/19390211.2018.1472711. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Kianpour P.; Mohtashami R.; Jafari R.; Simental-Mendia L. E.; Sahebkar A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc Pharmacol 2016, 68 (3), 223–229. 10.1097/FJC.0000000000000406. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Kianpour P.; Mohtashami R.; Jafari R.; Simental-Mendia L. E.; Sahebkar A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. (Stuttg) 2017, 67 (4), 244–251. 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- Panahi Y.; Kianpour P.; Mohtashami R.; Soflaei S. S.; Sahebkar A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: a clinical study. J. Asian Nat. Prod Res. 2019, 21 (8), 798–805. 10.1080/10286020.2018.1505873. [DOI] [PubMed] [Google Scholar]
- Hariri M.; Gholami A.; Mirhafez S. R.; Bidkhori M.; Sahebkar A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complement Ther Med. 2020, 51, 102447 10.1016/j.ctim.2020.102447. [DOI] [PubMed] [Google Scholar]
- Belcaro G.; Cesarone M. R.; Dugall M.; Pellegrini L.; Ledda A.; Grossi M. G.; Togni S.; Appendino G. Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52 (2 Suppl 1), 55–62. [PubMed] [Google Scholar]
- Belcaro G.; Cesarone M. R.; Dugall M.; Pellegrini L.; Ledda A.; Grossi M. G.; Togni S.; Appendino G. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med. Rev. 2010, 15 (4), 337–344. [PubMed] [Google Scholar]
- Pastorelli D.; Fabricio A. S. C.; Giovanis P.; D’Ippolito S.; Fiduccia P.; Solda C.; Buda A.; Sperti C.; Bardini R.; Da Dalt G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79. 10.1016/j.phrs.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Ledda A.; Belcaro G.; Dugall M.; Luzzi R.; Scoccianti M.; Togni S.; Appendino G.; Ciammaichella G. Meriva(R), a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: a pilot, product evaluation registry study. Panminerva Med. 2012, 54 (1 Suppl 4), 17–22. [PubMed] [Google Scholar]
- Antiga E.; Bonciolini V.; Volpi W.; Del Bianco E.; Caproni M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. Biomed Res. Int. 2015, 2015, 283634 10.1155/2015/283634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thota R. N.; Rosato J. I.; Dias C. B.; Burrows T. L.; Martins R. N.; Garg M. L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3beta and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12 (4), 1032. 10.3390/nu12041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi Y.; Saadat A.; Beiraghdar F.; Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res. 2014, 28 (10), 1461–1467. 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- Belcaro G.; Hosoi M.; Pellegrini L.; Appendino G.; Ippolito E.; Ricci A.; Ledda A.; Dugall M.; Cesarone M. R.; Maione C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva(R)) to alleviate the adverse effects of cancer treatment. Phytother Res. 2014, 28 (3), 444–450. 10.1002/ptr.5014. [DOI] [PubMed] [Google Scholar]
- Chashmniam S.; Mirhafez S. R.; Dehabeh M.; Hariri M.; Azimi Nezhad M.; Nobakht M. G. B. F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Eur. J. Clin Nutr 2019, 73 (9), 1224–1235. 10.1038/s41430-018-0386-5. [DOI] [PubMed] [Google Scholar]
- Mirhafez S. R.; Farimani A. R.; Dehhabe M.; Bidkhori M.; Hariri M.; Ghouchani B. F.; Abdollahi F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointestin Liver Dis 2019, 28, 183–189. 10.15403/jgld-179. [DOI] [PubMed] [Google Scholar]
- Mazzolani F.; Togni S.; Giacomelli L.; Eggenhoffner R.; Franceschi F. Oral administration of a curcumin-phospholipid formulation (Meriva(R)) for treatment of chronic diabetic macular edema: a pilot study. Eur. Rev. Med. Pharmacol Sci. 2018, 22 (11), 3617–3625. 10.26355/eurrev_201806_15189. [DOI] [PubMed] [Google Scholar]
- Thota R. N.; Dias C. B.; Abbott K. A.; Acharya S. H.; Garg M. L. Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study. Sci. Rep 2018, 8 (1), 13679. 10.1038/s41598-018-32032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J. A.; Wolska A.; Remaley A. T.; Stojanovski E.; MacDonald-Wicks L.; Garg M. L. Bread enriched with phytosterols with or without curcumin modulates lipoprotein profiles in hypercholesterolaemic individuals. A randomised controlled trial. Food Funct 2019, 10 (5), 2515–2527. 10.1039/C8FO02512F. [DOI] [PubMed] [Google Scholar]
- Briata I. M.; Paleari L.; Rutigliani M.; Petrera M.; Caviglia S.; Romagnoli P.; Libera M. D.; Oppezzi M.; Puntoni M.; Siri G.; et al. A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps. Int. J. Mol. Sci. 2021, 22 (20), 11024. 10.3390/ijms222011024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro F.; Zacconi P.; Bertuccioli A.; Togni S.; Eggenhoffner R.; Giacomelli L.; Scaltrini S. A naturally-inspired, curcumin-based lecithin formulation (Meriva(R) formulated as the finished product Algocur(R)) alleviates the osteo-muscular pain conditions in rugby players. Eur. Rev. Med. Pharmacol Sci. 2017, 21 (21), 4935–4940. [PubMed] [Google Scholar]
- Wolf M.; Klang V.; Stojcic T.; Fuchs C.; Wolzt M.; Valenta C. NLC versus nanoemulsions: Effect on physiological skin parameters during regular in vivo application and impact on drug penetration. Int. J. Pharm. 2018, 549 (1–2), 343–351. 10.1016/j.ijpharm.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Pal P.; Penmetsa A.; Kathi P.; Girish G.; Goren I.; Reddy D. N. Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study. J. Clin Gastroenterol 2021, 55 (8), 702–708. 10.1097/MCG.0000000000001416. [DOI] [PubMed] [Google Scholar]
- Asawanonda P.; Klahan S. O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg 2010, 28 (5), 679–684. 10.1089/pho.2009.2637. [DOI] [PubMed] [Google Scholar]
- Dizaji B. F.; Rivandi M.; Javandoost A.; Saberi Karimian M.; Raei A.; Sahebkar A.; Ferns G.; Mobarhan M. G.; Pasdar A. Association of genetic polymorphisms of PON1 and CETP with the presence of metabolic syndrome; the effects of genotypes on their serum activity and concentrations. Egyptian Journal of Medical Human Genetics 2018, 19 (1), 43–48. 10.1016/j.ejmhg.2017.12.001. [DOI] [Google Scholar]
- Ghazimoradi M.; Saberi-Karimian M.; Mohammadi F.; Sahebkar A.; Tavallaie S.; Safarian H.; Ferns G. A.; Ghayour-Mobarhan M.; Moohebati M.; Esmaeili H.; et al. The Effects of Curcumin and Curcumin-Phospholipid Complex on the Serum Pro-oxidant-Antioxidant Balance in Subjects with Metabolic Syndrome. Phytother Res. 2017, 31 (11), 1715–1721. 10.1002/ptr.5899. [DOI] [PubMed] [Google Scholar]
- Mohammadi F.; Ghazi-Moradi M.; Ghayour-Mobarhan M.; Esmaeili H.; Moohebati M.; Saberi-Karimian M.; Safarian H.; Tavallaie S.; Ferns G. A.; Sahebkar A. The Effects of Curcumin on Serum Heat Shock Protein 27 Antibody Titers in Patients with Metabolic Syndrome. J. Diet Suppl 2019, 16 (5), 592–601. 10.1080/19390211.2018.1472710. [DOI] [PubMed] [Google Scholar]
- Saberi-Karimian M.; Parizadeh S. M. R.; Ghayour-Mobarhan M.; Salahshooh M. M.; Dizaji B. F.; Safarian H.; Javandoost A.; Ferns G. A.; Sahebkar A.; Ahmadinejad M. Evaluation of the effects of curcumin in patients with metabolic syndrome. Comparative Clinical Pathology 2018, 27 (3), 555–563. 10.1007/s00580-017-2624-y. [DOI] [Google Scholar]
- Shirmohammadi L.; Ghayour-Mobarhan M.; Saberi-Karimian M.; Iranshahi M.; Tavallaie S.; Emamian M.; Sahebkar A. Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome. Cardiovasc Hematol Disord Drug Targets 2020, 20 (2), 116–121. 10.2174/1871529X19666190919110652. [DOI] [PubMed] [Google Scholar]
- Saberi-Karimian M.; Ghazizadeh H.; Mohammadzadeh E.; Ferns G. A.; Ghayour-Mobarhan M.; Sahebkar A. Does curcumin have an effect on sleep duration in metabolic syndrome patients?. Avicenna J. Phytomed 2021, 11 (2), 190–198. [PMC free article] [PubMed] [Google Scholar]
- Bisht S.; Feldmann G.; Soni S.; Ravi R.; Karikar C.; Maitra A.; Maitra A. Polymeric nanoparticle-encapsulated curcumin (″nanocurcumin″): a novel strategy for human cancer therapy. J. Nanobiotechnology 2007, 5, 3. 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J.; Zhang Y.; Han S.; Chen Y.; Li B.; Liao M.; Chen W.; Deng X.; Zhao J.; Huang B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400 (1–2), 211–220. 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Nair K. L.; Thulasidasan A. K.; Deepa G.; Anto R. J.; Kumar G. S. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int. J. Pharm. 2012, 425 (1–2), 44–52. 10.1016/j.ijpharm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Mohanty C.; Sahoo S. K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31 (25), 6597–6611. 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- Bertrand N.; Wu J.; Xu X.; Kamaly N.; Farokhzad O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv Rev. 2014, 66, 2–25. 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M.; Islam M. R.; Akash S.; Harun-Or-Rashid M.; Ray T. K.; Rahaman M. S.; Islam M.; Anika F.; Hosain M. K.; Aovi F. I.; et al. Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance. Biomed Pharmacother 2022, 153, 113305 10.1016/j.biopha.2022.113305. [DOI] [PubMed] [Google Scholar]
- Kamaly N.; Yameen B.; Wu J.; Farokhzad O. C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116 (4), 2602–2663. 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosangari S. L.; Watkin K. L. Effect of preparation techniques on the properties of curcumin liposomes: characterization of size, release and cytotoxicity on a squamous oral carcinoma cell line. Pharm. Dev Technol. 2012, 17 (1), 103–109. 10.3109/10837450.2010.522583. [DOI] [PubMed] [Google Scholar]
- Lin H. Y.; Thomas J. L.; Chen H. W.; Shen C. M.; Yang W. J.; Lee M. H. In vitro suppression of oral squamous cell carcinoma growth by ultrasound-mediated delivery of curcumin microemulsions. Int. J. Nanomedicine 2012, 7, 941–951. 10.2147/IJN.S28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi M.; Agah E.; Nafissi S.; Jaafari M. R.; Harirchian M. H.; Sarraf P.; Faghihi-Kashani S.; Hosseini S. J.; Ghoreishi A.; Aghamollaii V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15 (2), 430–438. 10.1007/s13311-018-0606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajialilo M.; Dolati S.; Abdolmohammadi-Vahid S.; Ahmadi M.; Kamrani A.; Eghbal-Fard S.; Ghassembaglou A.; Valizadeh A.; Shenas M. H. M.; Aghebati-Maleki L.; et al. Nanocurcumin: A novel strategy in treating ankylosing spondylitis by modulating Th17 cells frequency and function. J. Cell Biochem 2019, 120, 12027. 10.1002/jcb.28488. [DOI] [PubMed] [Google Scholar]
- Mogharrabi M.; Rahimi H. R.; Hasanzadeh S.; Dastani M.; Kazemi-Oskuee R.; Akhlaghi S.; Soukhtanloo M. The effects of nanomicelle of curcumin on the matrix metalloproteinase (MMP-2, 9) activity and expression in patients with coronary artery disease (CAD): A randomized controlled clinical trial. ARYA Atheroscler 2020, 16 (3), 136–145. 10.22122/arya.v16i3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi R.; Salari S.; Sharifi M. D.; Reihani H.; Rostamiani M. B.; Behmadi M.; Taherzadeh Z.; Eslami S.; Rezayat S. M.; Jaafari M. R.; et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr 2021, 9 (8), 4068–4075. 10.1002/fsn3.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarkar Shafie E.; Taheri F.; Alijani N.; Okhovvat A. R.; Goudarzi R.; Borumandnia N.; Aghaghazvini L.; Rezayat S. M.; Jamalimoghadamsiahkali S.; Hosseinzadeh-Attar M. J. Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial. Phytother Res. 2022, 36 (2), 1013–1022. 10.1002/ptr.7374. [DOI] [PubMed] [Google Scholar]
- Delavarian Z.; Pakfetrat A.; Ghazi A.; Jaafari M. R.; Homaei Shandiz F.; Dalirsani Z.; Mohammadpour A. H.; Rahimi H. R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec Care Dentist 2019, 39 (2), 166–172. 10.1111/scd.12358. [DOI] [PubMed] [Google Scholar]
- Saadipoor A.; Razzaghdoust A.; Simforoosh N.; Mahdavi A.; Bakhshandeh M.; Moghadam M.; Abdollahi H.; Mofid B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother Res. 2019, 33 (2), 370–378. 10.1002/ptr.6230. [DOI] [PubMed] [Google Scholar]
- Javadi M.; Khadem Haghighian H.; Goodarzy S.; Abbasi M.; Nassiri-Asl M. Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial. Int. J. Rheum Dis 2019, 22 (10), 1857–1862. 10.1111/1756-185X.13688. [DOI] [PubMed] [Google Scholar]
- Atabaki M.; Shariati-Sarabi Z.; Tavakkol-Afshari J.; Mohammadi M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. Int. Immunopharmacol 2020, 85, 106607 10.1016/j.intimp.2020.106607. [DOI] [PubMed] [Google Scholar]
- Naeini F.; Tutunchi H.; Razmi H.; Mahmoodpoor A.; Vajdi M.; Sefidmooye Azar P.; Najifipour F.; Tarighat-Esfanjani A.; Karimi A. Does nano-curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial. J. Food Biochem 2022, 46 (5), e14093 10.1111/jfbc.14093. [DOI] [PubMed] [Google Scholar]
- Farhadi M.; Bakhshandeh M.; Shafiei B.; Mahmoudzadeh A.; Hosseinimehr S. J. The radioprotective effects of nano-curcumin against genotoxicity induced by iodine-131 in patients with differentiated thyroid carcinoma (DTC) by micronucleus assay. International Journal of Cancer Management 2018, 10.5812/ijcm.14193. [DOI] [Google Scholar]
- Alizadeh F.; Javadi M.; Karami A. A.; Gholaminejad F.; Kavianpour M.; Haghighian H. K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother Res. 2018, 32 (3), 514–521. 10.1002/ptr.5998. [DOI] [PubMed] [Google Scholar]
- Bateni Z.; Rahimi H. R.; Hedayati M.; Afsharian S.; Goudarzi R.; Sohrab G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother Res. 2021, 35 (7), 3945–3953. 10.1002/ptr.7109. [DOI] [PubMed] [Google Scholar]
- Ghodsi H.; Rahimi H. R.; Aghili S. M.; Saberi A.; Shoeibi A. Evaluation of curcumin as add-on therapy in patients with Parkinson’s disease: A pilot randomized, triple-blind, placebo-controlled trial. Clin Neurol Neurosurg 2022, 218, 107300 10.1016/j.clineuro.2022.107300. [DOI] [PubMed] [Google Scholar]
- Dolati S.; Aghebati-Maleki L.; Ahmadi M.; Marofi F.; Babaloo Z.; Ayramloo H.; Jafarisavari Z.; Oskouei H.; Afkham A.; Younesi V.; et al. Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double-blind, placebo-controlled trial. J. Cell Physiol 2018, 233 (7), 5222–5230. 10.1002/jcp.26301. [DOI] [PubMed] [Google Scholar]
- Hosseininasab M.; Zarghami M.; Mazhari S.; Salehifar E.; Moosazadeh M.; Fariborzifar A.; Babaeirad S.; Hendouei N. Nanocurcumin as an Add-on to Antipsychotic Drugs for Treatment of Negative Symptoms in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin Psychopharmacol 2021, 41 (1), 25–30. 10.1097/JCP.0000000000001324. [DOI] [PubMed] [Google Scholar]
- Maulina T.; Diana H.; Cahyanto A.; Amaliya A. The efficacy of curcumin in managing acute inflammation pain on the post-surgical removal of impacted third molars patients: A randomised controlled trial. J. Oral Rehabil 2018, 45 (9), 677–683. 10.1111/joor.12679. [DOI] [PubMed] [Google Scholar]
- Masoodi M.; Mahdiabadi M. A.; Mokhtare M.; Agah S.; Kashani A. H. F.; Rezadoost A. M.; Sabzikarian M.; Talebi A.; Sahebkar A. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: A randomized double-blind controlled trial. J. Cell Biochem 2018, 119 (11), 9552–9559. 10.1002/jcb.27273. [DOI] [PubMed] [Google Scholar]
- Atabaki M.; Shariati-Sarabi Z.; Tavakkol-Afshari J.; Taghipour A.; Jafari M. R.; Nikpoor A. R.; Mohammadi M. Curcumin as an effective suppressor of miRNA expression in patients with knee osteoarthritis. Avicenna J. Phytomed 2022, 12 (4), 346–356. 10.22038/AJP.2021.19380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasian S.; Soltani-Zangbar M. S.; Khabbazi A.; Farzaneh R.; Malek Mahdavi A.; Motavalli R.; Hajialilo M.; Yousefi M. Nanocurcumin supplementation ameliorates Behcet’s disease by modulating regulatory T cells: A randomized, double-blind, placebo-controlled trial. Int. Immunopharmacol 2021, 101, 108237 10.1016/j.intimp.2021.108237. [DOI] [PubMed] [Google Scholar]
- Valizadeh H.; Abdolmohammadi-Vahid S.; Danshina S.; Ziya Gencer M.; Ammari A.; Sadeghi A.; Roshangar L.; Aslani S.; Esmaeilzadeh A.; Ghaebi M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol 2020, 89, 107088 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S.; Saeed B. Q.; Temirgalieva E.; Yumashev A. V.; El-Esawi M. A.; Navashenaq J. G.; Valizadeh H.; Sadeghi A.; Aslani S.; Yousefi M.; et al. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. 2021, 276, 119437 10.1016/j.lfs.2021.119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassaniazad M.; Eftekhar E.; Inchehsablagh B. R.; Kamali H.; Tousi A.; Jaafari M. R.; Rafat M.; Fathalipour M.; Nikoofal-Sahlabadi S.; Gouklani H.; et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. 2021, 35 (11), 6417–6427. 10.1002/ptr.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi S.; El-Esawi M. A.; Mahmoud Z. H.; Timoshin A.; Valizadeh H.; Roshangar L.; Varshoch M.; Vaez A.; Aslani S.; Navashenaq J. G.; et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J. Cell Physiol 2021, 236 (7), 5325–5338. 10.1002/jcp.30233. [DOI] [PubMed] [Google Scholar]
- Asadirad A.; Nashibi R.; Khodadadi A.; Ghadiri A. A.; Sadeghi M.; Aminian A.; Dehnavi S. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res. 2022, 36 (2), 1023–1031. 10.1002/ptr.7375. [DOI] [PubMed] [Google Scholar]
- Saber-Moghaddam N.; Salari S.; Hejazi S.; Amini M.; Taherzadeh Z.; Eslami S.; Rezayat S. M.; Jaafari M. R.; Elyasi S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial. Phytother Res. 2021, 35, 2616. 10.1002/ptr.7004. [DOI] [PubMed] [Google Scholar]
- Karimi A.; Mahmoodpoor A.; Kooshki F.; Niazkar H. R.; Shoorei H.; Tarighat-Esfanjani A. Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: A pilot randomized clinical trial. European Journal of Integrative Medicine 2020, 36, 101122 10.1016/j.eujim.2020.101122. [DOI] [Google Scholar]
- Karimi A.; Naeini F.; Niazkar H. R.; Tutunchi H.; Musazadeh V.; Mahmoodpoor A.; Asghariazar V.; Mobasseri M.; Tarighat-Esfanjani A. Nano-curcumin supplementation in critically ill patients with sepsis: a randomized clinical trial investigating the inflammatory biomarkers, oxidative stress indices, endothelial function, clinical outcomes and nutritional status. Food Funct 2022, 13 (12), 6596–6612. 10.1039/D1FO03746C. [DOI] [PubMed] [Google Scholar]
- Sandoughdaran S.; Razzaghdoust A.; Tabibi A.; Basiri A.; Simforoosh N.; Mofid B. Randomized, Double-blind Pilot Study of Nanocurcumin in Bladder Cancer Patients Receiving Induction Chemotherapy. Urol J. 2020, 18 (3), 295–300. 10.22037/uj.v0i0.5719. [DOI] [PubMed] [Google Scholar]
- Kia S. J.; Basirat M.; Mortezaie T.; Moosavi M. S. Comparison of oral Nano-Curcumin with oral prednisolone on oral lichen planus: a randomized double-blinded clinical trial. BMC Complement Med. Ther 2020, 20 (1), 328. 10.1186/s12906-020-03128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari M.; Razzaghi R.; Momen-Heravi M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2021, 35 (4), 2099–2107. 10.1002/ptr.6957. [DOI] [PubMed] [Google Scholar]
- Shafabakhsh R.; Asemi Z.; Reiner Z.; Soleimani A.; Aghadavod E.; Bahmani F. The Effects of Nano-curcumin on Metabolic Status in Patients With Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran J. Kidney Dis 2020, 14 (4), 290–299. [PubMed] [Google Scholar]
- Zamani S. K.; Rezagholizadeh D. M. Effect of eight-week curcumin supplementation with endurance training on glycemic indexes in middle age women with type 2 diabetes in Iran, A preliminary study. Diabetes Metab Syndr 2021, 15 (3), 963–967. 10.1016/j.dsx.2021.04.002. [DOI] [PubMed] [Google Scholar]
- Asadi S.; Gholami M. S.; Siassi F.; Qorbani M.; Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2020, 34 (4), 896–903. 10.1002/ptr.6571. [DOI] [PubMed] [Google Scholar]
- Asadi S.; Gholami M. S.; Siassi F.; Qorbani M.; Khamoshian K.; Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement Ther Med. 2019, 43, 253–260. 10.1016/j.ctim.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Bateni Z.; Behrouz V.; Rahimi H. R.; Hedayati M.; Afsharian S.; Sohrab G. Effects of nano-curcumin supplementation on oxidative stress, systemic inflammation, adiponectin, and NF-κB in patients with metabolic syndrome: A randomized, double-blind clinical trial. Journal of Herbal Medicine 2022, 31, 100531 10.1016/j.hermed.2021.100531. [DOI] [Google Scholar]
- Jazayeri-Tehrani S. A.; Rezayat S. M.; Mansouri S.; Qorbani M.; Alavian S. M.; Daneshi-Maskooni M.; Hosseinzadeh-Attar M. J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond) 2019, 16, 8. 10.1186/s12986-019-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedighiyan M.; Abdolahi M.; Jafari E.; Vahabi Z.; Sohrabi Athar S.; Hadavi S.; Narimani Zamanabadi M.; Yekaninejad M. S.; Djalali M. The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: a double blind clinical trial study. BMC Res. Notes 2022, 15 (1), 189. 10.1186/s13104-022-06074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parohan M.; Sarraf P.; Javanbakht M. H.; Foroushani A. R.; Ranji-Burachaloo S.; Djalali M. The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: a randomized, placebo-controlled, double-blind trial. Nutr Neurosci 2021, 24 (4), 317–326. 10.1080/1028415X.2019.1627770. [DOI] [PubMed] [Google Scholar]
- Abdolahi M.; Karimi E.; Sarraf P.; Tafakhori A.; Siri G.; Salehinia F.; Sedighiyan M.; Asanjarani B.; Badeli M.; Abdollahi H.; et al. The omega-3 and Nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: a randomized clinical trial. BMC Res. Notes 2021, 14 (1), 283. 10.1186/s13104-021-05700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarvar N. M.; Soveid N.; Abdolahi M.; Djalali M.; Hatami M.; Karzar N. H. Anti-Neuroinflammatory Properties of n-3 Fatty Acids and Nano- Curcumin on Migraine Patients from Cellular to Clinical Insight: A Randomized, Double-Blind and Placebo-Controlled Trial. Endocr Metab Immune Disord Drug Targets 2021, 21 (2), 365–373. 10.2174/1871530320666200729144430. [DOI] [PubMed] [Google Scholar]
- Dolati S.; Ahmadi M.; Rikhtegar R.; Babaloo Z.; Ayromlou H.; Aghebati-Maleki L.; Nouri M.; Yousefi M. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int. Immunopharmacol 2018, 61, 74–81. 10.1016/j.intimp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Dolati S.; Babaloo Z.; Ayromlou H.; Ahmadi M.; Rikhtegar R.; Rostamzadeh D.; Roshangar L.; Nouri M.; Mehdizadeh A.; Younesi V.; et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol 2019, 327, 15–21. 10.1016/j.jneuroim.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Malekzadeh M.; Kia S. J.; Mashaei L.; Moosavi M. S. Oral nano-curcumin on gingival inflammation in patients with gingivitis and mild periodontitis. Clin Exp Dent Res. 2021, 7 (1), 78–84. 10.1002/cre2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia S. J.; Basirat M.; Saedi H. S.; Arab S. A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: a randomized clinical trial. BMC Complement Med. Ther 2021, 21 (1), 232. 10.1186/s12906-021-03400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M.; Gholami S.; Mahboubi A.; Jaafari M. R.; Namdari M. Combination Therapy with 1% Nanocurcumin Gel and 0.1% Triamcinolone Acetonide Mouth Rinse for Oral Lichen Planus: A Randomized Double-Blind Placebo Controlled Clinical Trial. Dermatol Res. Pract 2020, 2020, 4298193 10.1155/2020/4298193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M.; Mahboubi A.; Jaafari M. R.; Ebrahimi F.; Tofangchiha M.; Alizadeh A. Comparative Efficacy of 1% Curcumin Nanomicelle Gel and 2% Curcumin Gel for Treatment of Recurrent Aphthous Stomatitis: A Double-Blind Randomized Clinical Trial. J. Evid Based Dent Pract 2022, 22 (2), 101708 10.1016/j.jebdp.2022.101708. [DOI] [PubMed] [Google Scholar]
- Osali A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol Metab Syndr 2020, 12, 26. 10.1186/s13098-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talakesh T.; Tabatabaee N.; Atoof F.; Aliasgharzadeh A.; Sarvizade M.; Farhood B.; Najafi M. Effect of Nano-Curcumin on Radiotherapy-Induced Skin Reaction in Breast Cancer Patients: A Randomized, Triple-Blind, Placebo-Controlled Trial. Curr. Radiopharm 2022, 15, 332. 10.2174/1874471015666220623104316. [DOI] [PubMed] [Google Scholar]
- Djalali M.; Djalali M.; Abdolahi M.; Mohammadi H.; Heidari H.; Hosseini S.; Sadeghizadeh M. The Effect of Nano-Curcumin Supplementation on Pentraxin 3 Gene Expression and Serum Level in Migraine Patients. Rep. Biochem Mol. Biol. 2020, 9 (1), 1–7. 10.29252/rbmb.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolahi M.; Sarraf P.; Javanbakht M. H.; Honarvar N. M.; Hatami M.; Soveyd N.; Tafakhori A.; Sedighiyan M.; Djalali M.; Jafarieh A.; et al. A Novel Combination of omega-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study. CNS Neurol Disord Drug Targets 2018, 17 (6), 430–438. 10.2174/1871527317666180625101643. [DOI] [PubMed] [Google Scholar]
- Djalali M.; Abdolahi M.; Hosseini R.; Miraghajani M.; Mohammadi H.; Djalali M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement Ther Clin Pract 2020, 41, 101256 10.1016/j.ctcp.2020.101256. [DOI] [PubMed] [Google Scholar]
- Nivetha B.; Rahmathunisha A.; Lokeshwari K.; Kumaresan A.; Nikkitha S. K.; Yeseshivi L.; Janani R. Efficacy of nanocurcumin with application of iontophoresis on inflammatory arthritis patients. Research Journal of Pharmacy and Technology 2022, 15 (2), 825–829. 10.52711/0974-360X.2022.00137. [DOI] [Google Scholar]
- Soveyd N.; Abdolahi M.; Djalali M.; Hatami M.; Tafakhori A.; Sarraf P.; Honarvar N. M. The Combined Effects of omega −3 Fatty Acids and Nano-Curcumin Supplementation on Intercellular Adhesion Molecule-1 (ICAM-1) Gene Expression and Serum Levels in Migraine Patients. CNS Neurol Disord Drug Targets 2018, 16 (10), 1120–1126. 10.2174/1871527317666171213154749. [DOI] [PubMed] [Google Scholar]
- Abdolahi M.; Tafakhori A.; Togha M.; Okhovat A. A.; Siassi F.; Eshraghian M. R.; Sedighiyan M.; Djalali M.; Mohammadzadeh Honarvar N.; Djalali M. The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients. Immunogenetics 2017, 69 (6), 371–378. 10.1007/s00251-017-0992-8. [DOI] [PubMed] [Google Scholar]
- Abdolahi M.; Jafarieh A.; Sarraf P.; Sedighiyan M.; Yousefi A.; Tafakhori A.; Abdollahi H.; Salehinia F.; Djalali M. The Neuromodulatory Effects of omega-3 Fatty Acids and Nano-Curcumin on the COX-2/ iNOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocr Metab Immune Disord Drug Targets 2019, 19 (6), 874–884. 10.2174/1871530319666190212170140. [DOI] [PubMed] [Google Scholar]
- Bilia A. R.; Bergonzi M. C.; Isacchi B.; Antiga E.; Caproni M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol 2018, 70 (7), 919–928. 10.1111/jphp.12910. [DOI] [PubMed] [Google Scholar]
- Farshbaf-Khalili A.; Farajnia S.; Pourzeinali S.; Shakouri S. K.; Salehi-Pourmehr H. The effect of nanomicelle curcumin supplementation and Nigella sativa oil on the expression level of miRNA-21, miRNA-422a, and miRNA-503 gene in postmenopausal women with low bone mass density: A randomized, triple-blind, placebo-controlled clinical trial with factorial design. Phytother Res. 2021, 35 (11), 6216–6227. 10.1002/ptr.7259. [DOI] [PubMed] [Google Scholar]
- Shah S.; Rath H.; Sharma G.; Senapati S. N.; Mishra E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomised controlled trial. Indian J. Dent Res. 2020, 31 (5), 718–727. 10.4103/ijdr.IJDR_822_18. [DOI] [PubMed] [Google Scholar]
- Moradi Kelardeh B.; Rahmati-Ahmadabad S.; Farzanegi P.; Helalizadeh M.; Azarbayjani M. A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw Mov Ther 2020, 24 (3), 154–160. 10.1016/j.jbmt.2020.02.021. [DOI] [PubMed] [Google Scholar]
- Guru S. R.; Reddy K. A.; Rao R. J.; Padmanabhan S.; Guru R.; Srinivasa T. S. Comparative evaluation of 2% turmeric extract with nanocarrier and 1% chlorhexidine gel as an adjunct to scaling and root planing in patients with chronic periodontitis: A pilot randomized controlled clinical trial. J. Indian Soc. Periodontol 2020, 24 (3), 244–252. 10.4103/jisp.jisp_207_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pacheco C. G.; Fernandes N. A. R.; Primo F. L.; Tedesco A. C.; Bellile E.; Retamal-Valdes B.; Feres M.; Guimaraes-Stabili M. R.; Rossa C. Jr. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: Randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin Oral Investig 2021, 25 (5), 3217–3227. 10.1007/s00784-020-03652-3. [DOI] [PubMed] [Google Scholar]
- Small G. W.; Siddarth P.; Li Z.; Miller K. J.; Ercoli L.; Emerson N. D.; Martinez J.; Wong K. P.; Liu J.; Merrill D. A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr Psychiatry 2018, 26 (3), 266–277. 10.1016/j.jagp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Ross S. M. Curcuma longa (Theracumin(R)): A Bioavailable Form of Curcumin and Its Cognitive Benefits. Holist Nurs Pract 2018, 32 (4), 217–220. 10.1097/HNP.0000000000000281. [DOI] [PubMed] [Google Scholar]
- Sasaki H.; Sunagawa Y.; Takahashi K.; Imaizumi A.; Fukuda H.; Hashimoto T.; Wada H.; Katanasaka Y.; Kakeya H.; Fujita M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34 (5), 660–665. 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- Kanai M.; Imaizumi A.; Otsuka Y.; Sasaki H.; Hashiguchi M.; Tsujiko K.; Matsumoto S.; Ishiguro H.; Chiba T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol 2012, 69 (1), 65–70. 10.1007/s00280-011-1673-1. [DOI] [PubMed] [Google Scholar]
- Tanabe Y.; Maeda S.; Akazawa N.; Zempo-Miyaki A.; Choi Y.; Ra S. G.; Imaizumi A.; Otsuka Y.; Nosaka K. Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur. J. Appl. Physiol 2015, 115 (9), 1949–1957. 10.1007/s00421-015-3170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M.; Otsuka Y.; Otsuka K.; Sato M.; Nishimura T.; Mori Y.; Kawaguchi M.; Hatano E.; Kodama Y.; Matsumoto S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol 2013, 71 (6), 1521–1530. 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- Morimoto T.; Sunagawa Y.; Katanasaka Y.; Hirano S.; Namiki M.; Watanabe Y.; Suzuki H.; Doi O.; Suzuki K.; Yamauchi M.; et al. Drinkable preparation of Theracurmin exhibits high absorption efficiency--a single-dose, double-blind, 4-way crossover study. Biol. Pharm. Bull. 2013, 36 (11), 1708–1714. 10.1248/bpb.b13-00150. [DOI] [PubMed] [Google Scholar]
- Tanabe Y.; Chino K.; Sagayama H.; Lee H. J.; Ozawa H.; Maeda S.; Takahashi H. Effective Timing of Curcumin Ingestion to Attenuate Eccentric Exercise-Induced Muscle Soreness in Men. J. Nutr Sci. Vitaminol (Tokyo) 2019, 65 (1), 82–89. 10.3177/jnsv.65.82. [DOI] [PubMed] [Google Scholar]
- Tanabe Y.; Chino K.; Ohnishi T.; Ozawa H.; Sagayama H.; Maeda S.; Takahashi H. Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand J. Med. Sci. Sports 2019, 29 (4), 524–534. 10.1111/sms.13373. [DOI] [PubMed] [Google Scholar]
- Sugawara J.; Akazawa N.; Miyaki A.; Choi Y.; Tanabe Y.; Imai T.; Maeda S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am. J. Hypertens 2012, 25 (6), 651–656. 10.1038/ajh.2012.24. [DOI] [PubMed] [Google Scholar]
- Sugimoto K.; Ikeya K.; Bamba S.; Andoh A.; Yamasaki H.; Mitsuyama K.; Nasuno M.; Tanaka H.; Matsuura A.; Kato M.; et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn’s Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns Colitis 2020, 14 (12), 1693–1701. 10.1093/ecco-jcc/jjaa097. [DOI] [PubMed] [Google Scholar]
- Vafadar-Afshar G.; Rasmi Y.; Yaghmaei P.; Khadem-Ansari M. H.; Makhdoomi K.; Rasouli J. The effects of nanocurcumin supplementation on inflammation in hemodialysis patients: A randomized controlled trial. Hemodial Int. 2021, 25 (2), 232–239. 10.1111/hdi.12911. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y.; Mori K.; Yamada S.; Mukai S.; Hirose A.; Nakamura R. The Oral Administration of Highly-Bioavailable Curcumin for One Year Has Clinical and Chondro-Protective Effects: A Randomized, Double-Blinded, Placebo-Controlled Prospective Study. Arthrosc Sports Med. Rehabil 2022, 4 (2), e393–e402. 10.1016/j.asmr.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto M.; Sunagawa Y.; Katanasaka Y.; Miyazaki Y.; Imaizumi A.; Kakeya H.; Yamakage H.; Satoh-Asahara N.; Komiyama M.; Wada H.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron Obstruct Pulmon Dis 2016, 11, 2029–2034. 10.2147/COPD.S104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto M.; Shimizu K.; Sunagawa Y.; Katanasaka Y.; Miyazaki Y.; Kakeya H.; Yamakage H.; Satoh-Asahara N.; Wada H.; Hasegawa K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 8208237 10.1155/2019/8208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik K.; Khatoon E.; Hegde M.; Thakur K. K.; Puppala E. R.; Naidu V. G. M.; Kunnumakkara A. B. A novel bioavailable curcumin-galactomannan complex modulates the genes responsible for the development of chronic diseases in mice: A RNA sequence analysis. Life Sci. 2021, 287, 120074 10.1016/j.lfs.2021.120074. [DOI] [PubMed] [Google Scholar]
- Krishnakumar I.; Maliakel A.; Gopakumar G.; Kumar D.; Maliakel B.; Kuttan R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. Journal of functional foods 2015, 14, 215–225. 10.1016/j.jff.2015.01.049. [DOI] [Google Scholar]
- Sunagawa Y.; Miyazaki Y.; Funamoto M.; Shimizu K.; Shimizu S.; Nurmila S.; Katanasaka Y.; Ito M.; Ogawa T.; Ozawa-Umeta H.; et al. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, two-way crossover study. Journal of Functional Foods 2021, 81, 104443. 10.1016/j.jff.2021.104443. [DOI] [Google Scholar]
- Khanna A.; Das S. S.; Smina T. P.; Thomas J. V.; Kunnumakkara A. B.; Maliakel B.; Krishnakumar I. M.; Mohanan R. Curcumagalactomannoside/Glucosamine Combination Improved Joint Health Among Osteoarthritic Subjects as Compared to Chondroitin Sulfate/Glucosamine: Double-Blinded, Randomized Controlled Study. J. Altern Complement Med. 2020, 26 (10), 945–955. 10.1089/acm.2020.0128. [DOI] [PubMed] [Google Scholar]
- Pandaran Sudheeran S.; Jacob D.; Natinga Mulakal J.; Gopinathan Nair G.; Maliakel A.; Maliakel B.; Kuttan R.; Im K. Safety, Tolerance, and Enhanced Efficacy of a Bioavailable Formulation of Curcumin With Fenugreek Dietary Fiber on Occupational Stress: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin Psychopharmacol 2016, 36 (3), 236–243. 10.1097/JCP.0000000000000508. [DOI] [PubMed] [Google Scholar]
- Campbell M. S.; Ouyang A.; I M. K.; Charnigo R. J.; Westgate P. M.; Fleenor B. S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. 10.1016/j.nut.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Thomas J. V.; Smina T. P.; Khanna A.; Kunnumakkara A. B.; Maliakel B.; Mohanan R.; Krishnakumar I. M. Influence of a low-dose supplementation of curcumagalactomannoside complex (CurQfen) in knee osteoarthritis: A randomized, open-labeled, active-controlled clinical trial. Phytother Res. 2021, 35 (3), 1443–1455. 10.1002/ptr.6907. [DOI] [PubMed] [Google Scholar]
- Khanna A.; Das S. S.; Kannan R.; Swick A. G.; Matthewman C.; Maliakel B.; Ittiyavirah S. P.; Krishnakumar I. M. The effects of oral administration of curcumin-galactomannan complex on brain waves are consistent with brain penetration: a randomized, double-blinded, placebo-controlled pilot study. Nutr Neurosci 2022, 25 (6), 1240–1249. 10.1080/1028415X.2020.1853410. [DOI] [PubMed] [Google Scholar]
- Kishimoto A.; Imaizumi A.; Wada H.; Yamakage H.; Satoh-Asahara N.; Hashimoto T.; Hasegawa K. Newly Developed Highly Bioavailable Curcumin Formulation, curcuRouge(TM), Reduces Neutrophil/Lymphocyte Ratio in the Elderly: A Double-Blind, Placebo-Controlled Clinical Trial. J. Nutr Sci. Vitaminol (Tokyo) 2021, 67 (4), 249–252. 10.3177/jnsv.67.249. [DOI] [PubMed] [Google Scholar]
- Kothaplly S.; Alukapally S.; Nagula N.; Maddela R. Superior Bioavailability of a Novel Curcumin Formulation in Healthy Humans Under Fasting Conditions. Adv. Ther 2022, 39 (5), 2128–2138. 10.1007/s12325-022-02081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H.; Pipingas A.; Scholey A. B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol 2015, 29 (5), 642–651. 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Cox K. H. M.; White D. J.; Pipingas A.; Poorun K.; Scholey A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12 (6), 1678. 10.3390/nu12061678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker J. R.; Strahler T. R.; Bassett C. J.; Bispham N. Z.; Chonchol M. B.; Seals D. R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 2017, 9 (1), 187–208. 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSilvestro R. A.; Joseph E.; Zhao S.; Bomser J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012, 11, 79. 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszewski J. C.; Howe P. R. C.; Wong R. H. X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150 (12), 3190–3199. 10.1093/jn/nxaa299. [DOI] [PubMed] [Google Scholar]
- Kuszewski J. C.; Wong R. H. X.; Howe P. R. C. Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity. Rheumatol Adv. Pract 2020, 4 (2), rkaa036. 10.1093/rap/rkaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszewski J. C.; Wong R. H. X.; Wood L. G.; Howe P. R. C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr Metab Cardiovasc Dis 2020, 30 (4), 625–633. 10.1016/j.numecd.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Hazarey V. K.; Sakrikar A. R.; Ganvir S. M. Efficacy of curcumin in the treatment for oral submucous fibrosis - A randomized clinical trial. J. Oral Maxillofac Pathol 2015, 19 (2), 145–152. 10.4103/0973-029X.164524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte P. A.; Giramkar S. A.; Harke S. M.; Kulkarni S. K.; Deshmukh A. P.; Hingorani L. L.; Mahajan M. P.; Bhalerao S. S. Evaluation of the efficacy and safety of Capsule Longvida((R)) Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: a pilot clinical study. J. Inflamm Res. 2019, 12, 145–152. 10.2147/JIR.S205390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngolab J.; Donohue M.; Belsha A.; Salazar J.; Cohen P.; Jaiswal S.; Tan V.; Gessert D.; Korouri S.; Aggarwal N. T.; et al. Feasibility study for detection of retinal amyloid in clinical trials: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial. Alzheimers Dement (Amst) 2021, 13 (1), e12199 10.1002/dad2.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]