Abstract
Background
We assessed 10-year longitudinal associations between late-life social networks and incidence of all-cause dementia (ACD), Alzheimer’s disease (AD), and vascular dementia (VaD) in Japanese-American men.
Methods
We prospectively analyzed, from baseline (1991–1993) through 1999–2000, 2636 initially nondemented Kuakini Honolulu-Asia Aging Study participants who remained dementia-free during the first 3 years of follow-up. Global cognition was evaluated by the Cognitive Abilities Screening Instrument (CASI); depressive symptoms by the 11-item Center for Epidemiologic Studies Depression (CES-D) Scale; and social networks by the Lubben Social Network Scale (LSNS). Median split of LSNS scores defined weak/strong social network groups. A panel of neurologists and geriatricians diagnosed and classified dementia; AD and VaD diagnoses comprised cases in which AD or VaD, respectively, were considered the primary cause of dementia.
Results
Median (range) baseline age was 77 (71–93) years. Participants with weak (LSNS score ≤29) versus strong (>29) social networks had higher age-adjusted incidence (in person-years) of ACD (12.6 vs. 8.7; p = .014) and AD (6.7 vs. 4.0; p = .007) but not VaD (2.4 vs. 1.4; p = .15). Kaplan–Meier curves showed a lower likelihood of survival free of ACD (log-rank p < .0001) and AD (p = .0006) for men with weak networks. In Cox proportional hazards models adjusting for age, education, APOE ɛ4, prevalent stroke, depressive symptoms, and CASI score (all at baseline), weak networks predicted increased incidence of ACD (hazard ratio [HR] = 1.52, p = .009) and AD (HR = 1.67, p = .014) but not VaD (p > .2).
Conclusion
Weak social networks may heighten the risk of dementia and AD, underscoring the need to promote social connectedness in older adults.
Keywords: Cognition, Kuakini Honolulu Heart Program, Vascular dementia
In 2015 an estimated 47 million people worldwide were living with dementia (1). This number, doubling every 20 years, is projected to exceed 74 million in 2030 and 131 million by 2050 (1). AD is the most prevalent form of dementia, accounting for 50%–75% of cases (2), and is followed by vascular dementia (VaD) as the second most common category (3). In light of the current absence of pharmacological treatments which lead to a clinically meaningful reduction of symptoms associated with AD, preventive strategies focusing on primary preventions are urgently needed. Much research has therefore addressed aspects of lifestyle and environment that can be changed. Potentially modifiable risk factors include cognitive and physical inactivity (4), hearing loss (5), late-life depression (6), and vascular and behavioral risk factors (4). A third of dementia cases may be preventable (7). Even after genetic influences are taken into consideration, a substantial proportion of dementia risk remains unexplained, highlighting the importance of determining additional malleable factors (8).
One modifiable factor implicated in the development of dementia is social isolation (9,10). With longitudinal studies increasingly investigating the effects of lifestyle on cognitive decline, interest in the protective role of social networks has grown (9). Social networks attempt to capture the nature of the social environment by measuring the number, frequency, and closeness of interactions with others (9). Poor social relationships may increase the likelihood of developing dementia (10), although direct comparison of research findings is hampered by heterogeneous measures of social connection (11).One meta-analysis of longitudinal cohort studies concluded that incident dementia was linked to factors representing a paucity of social interaction (10); however, of the 8 studies that assessed social networks in relation to dementia risk, each defined social contact differently and only 2 detected statistically significant associations (10,11). Contradictory results exist regarding the impact of social network size and other aspects of social integration on incident dementia (10). While restricted social networks have been observed to augment dementia risk (12,13), 2 groups reported that social isolation, social engagement, and social support did not predict all-cause dementia and AD during 3 years of follow-up (14,15). The inconsistencies may be attributed in part to methodological issues other than dissimilar definitions of social contact: relatively few groups, for example, have considered the influence of APOE ɛ4 when examining social networks in relation to subsequent dementia (13,15–17). Moreover, many observational studies of social networks and dementia have involved follow-up periods of 5–6 years or less (10,18) and might suffer from reverse causation.
The highest rates of social isolation and dementia are found among the “oldest-old”, usually defined as ≥ 85 years of age (19). Investigations of social networks and incident dementia in this age group are still few (20) and may overlook the fact that risk factors are different for the oldest–old than for younger individuals (19). In the current study, social networks in late life were tested as predictors of incident AD, VaD, and all-cause dementia among Japanese-American men, participants of the Kuakini Honolulu-Asia Aging Study (Kuakini HAAS), over a follow-up period of 10 years. We hypothesized that weaker social networks would be associated with a greater incidence of all-cause dementia, AD, and VaD.
Materials and Methods
Study Population
The Kuakini Honolulu Heart Program (Kuakini HHP) began in 1965 as a longitudinal study of cardiovascular disease (CVD) in 8006 middle-aged Japanese-American men living on the island of Oahu, Hawaii (21). In 1991 the HHP was extended to the Kuakini Honolulu-Asia Aging Study (Kuakini HAAS), designed to investigate aging-related neurodegenerative diseases (22). The fourth Kuakini HHP examination (Kuakini HAAS baseline) was performed during 1991–1993. Of the 4671 surviving Kuakini HHP cohort members, almost 80% (N = 3734), who were by then 71–93 years old, participated in the Kuakini HAAS and were subsequently examined every 2–3 years. Our study analyzed data from 4 study visits; i.e., through Kuakini HHP exam 7 (1999–2000), with exam 4 serving as the baseline. Interviews were conducted in English or Japanese according to participant preference. The Kuakini HAAS was approved by the institutional review board of the Kuakini Medical Center (Honolulu, Hawaii). All participants and/or a responsible family member provided written informed consent at each examination.
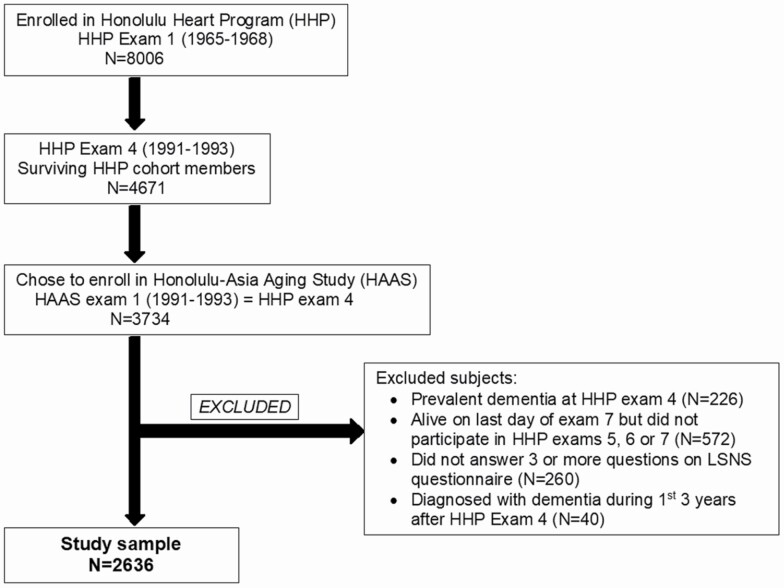
The current study investigated Kuakini HAAS participants who were free of dementia at exam 4 and who underwent at least 1 follow-up in-person assessment. Our analytical sample comprised 2636 of the 3734 Kuakini HAAS participants who underwent the baseline examination. A flow chart of the selection process is presented in Figure 1. Excluded were men with prevalent dementia, incomplete Lubben Social Network Scale (LSNS) responses (missing answers to >2 questions), and those who were still alive on the last day of exam 7 (October 16, 2000) but did not participate in follow-up exams 5, 6, or 7 and whose dementia status was therefore unknown. To reduce potential bias due to reverse causation, we also excluded the 40 individuals who were diagnosed with all-cause dementia during the first three years (<3 years) of follow-up. Participants were followed up for nearly 10 years (discussed later in more detail).
Figure 1.
Selection of our analytic sample from the Kuakini Honolulu Heart Program/Kuakini Honolulu Asia-Aging Study participants. LSNS = Lubben Social Network Scale.
Data Collection
Predictor variable: Social network
Data on social networks were collected using the 10-item LSNS, which was developed for use in an older adult population as a modification of the Berkman-Syme Social Network Index (23). By assessing social network size and the frequency of social contact with network members, the LSNS provides an objective measure of social interaction. In brief, 3 questions related to size of the active family network and the frequency of contact with the family member with whom the participant is most often in touch; 3 questions capture the same information for friends; 2 items concern confidant relationships; 1 item inquires about the frequency of helping others; and one question addresses the participant’s living situation. Each item is scored from 0 to 5, and the total LSNS score is an equally weighted sum of the 10 answers so that scores range from 0 to 50. Higher scores indicate stronger social networks; i.e., larger social networks and/or more frequent social contact. Our study sample was dichotomized by a median split of LSNS total baseline scores into groups with stronger and weaker social networks (>29 and ≤29, respectively). Additionally, as scores below 20 may identify older individuals at risk of extremely limited social networks (23), we created a second dichotomous variable for an exploratory analysis that used total LSNS scores <20 to differentiate the socially isolated from those with stronger social networks (≥20).
Outcome variables: Incident AD, VaD, and all-cause dementia
All participants underwent cognitive testing at baseline and at every follow-up visit. Global cognitive functioning was evaluated by the 100-point Cognitive Abilities Screening Instrument (CASI), on which scores range from 0 (worst) to 100 (best) (24). The CASI has been validated for use in studies of dementia in the United States and Japan (24).
Participants were selected for further neurological and neuropsychological evaluation based on CASI score cutpoints or specified drop in CASI score (25). These participants were further evaluated by neuropsychological test batteries developed by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (26), together with a neurologic exam and proxy interview. To facilitate further subclassification, those diagnosed with dementia underwent laboratory tests and either brain magnetic resonance imaging or computed tomography. Diagnoses of all-cause dementia, AD, and VaD were adjudicated by a consensus panel consisting of the study neurologist and at least 1–2 other physicians with expertise in geriatrics and dementia. Based on the results of the neuropsychological tests, neurological examination, brain imaging, and blood tests, final diagnoses were assigned according to criteria defined by: (a) the Diagnostic and Statistical Manual of Mental Disorders-III–Revised (DSM-III–R) for all-cause dementia, (b) the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) for AD, and (c) the California Alzheimer’s Disease Diagnostic and Treatment Centers (CADDTC) for VaD. Diagnoses of Alzheimer’s disease or VaD comprised cases in which AD or VaD, respectively, were considered the sole or primary cause of dementia.
The dementia case-finding procedure has previously been described in detail (25). At baseline (exam 4), the CASI was administered at phases 1 and 2 and the neurological examination at phase 3. Follow-up examinations were conducted in 2 phases with the CASI at phase 1 and neurological examination at phase 2. Most participants underwent all phases within 12 weeks (25).
Covariates
Adjustment covariates were selected according to their clinical relevance and probable associations with incident dementia and social network size. Two well-established risk factors for dementia onset are advanced age and the apolipoprotein E (APOE) ɛ4 allele, the major genetic contributor to Alzheimer’s disease (AD). APOE ɛ4 carriers are susceptible to cognitive decline and may be more vulnerable to detrimental effects of behaviors such as smoking and heavy use of alcohol or drugs (27). Covariates in our Cox regression models included baseline demographic and clinical characteristics such as education, APOE e4 carrier status, prevalent stroke, and depressive symptoms. APOE genotyping was performed at Duke University’s Bryan Alzheimer’s Disease Research Center (Durham, NC) by restriction enzyme isoform genotyping (restriction isotyping) using polymerase chain reaction amplification (28). Information on prevalent stroke was detected through a comprehensive surveillance system of all hospital discharge records from the island of Oahu, adjudicated by expert physicians using standard criteria. Years of formal education were obtained by self-report.
Depressive symptoms were measured by an 11-item version of the Center for Epidemiologic Studies Depression (CES-D) Scale (29). As the CES-D questionnaire contains an item on feeling lonely (29), perceived loneliness was implicitly included in the adjustment for depressive symptoms. The presence of depressive symptoms was defined by CES-D scores ≥9 (30). (Scores on the 11-item CES-D Scale range from 0 to 33 (29). Presence of depressive symptoms does not imply a clinical diagnosis of depression.) Only CES-D scores with fewer than 3 missing answers were considered valid for inclusion in statistical analyses, consistent with earlier studies of Kuakini HAAS participants (30). Participants who did not satisfy this criterion were removed from analyses involving depressive symptoms.
Hypercholesterolemia, hypertension, and other vascular conditions contribute to the pathogenesis of dementia, especially of the vascular type (31). In the Kuakini HAAS cohort, a healthful lifestyle at midlife was previously associated with lower late-life risk of AD and overall dementia (32). Most epidemiologic studies have identified CVD risk factors (eg, diabetes mellitus, elevated systolic blood pressure, high cholesterol, and moderate/heavy smoking) as posing a risk for subsequent cognitive decline and dementia only when measured in middle age, as the effects are cumulative over the years (33). A number of associations between midlife factors and risk for cognitive impairment or dementia no longer persist into later life (34); hypertension may even confer protective benefits when developed in old age (35). Given that our study participants were already in late life at baseline, we did not adjust for CVD risk factors in the statistical models. However, in sensitivity analyses, we tested the effects of total cholesterol (mg/dL), fasting plasma glucose (mg/dL), presence of hypertension (systolic blood pressure [BP] ≥140 mmHg, diastolic BP ≥90 mmHg, or history of antihypertensive medication use) (32), presence of diabetes mellitus (history of diabetes or use of insulin or oral medications for diabetes) (32), and smoking (pack-years).
Statistical Methods
General linear models adjusting for age compared baseline dementia risk factors between participants with stronger (LSNS score > 29) and weaker (LSNS score ≤ 29) social networks. Age-adjusted 10-year incidence rates of incident AD, VaD, and all-cause dementia were computed per 1000 person-years of follow-up and compared between the social network groups. Group comparisons of dementia-free survival were done using the Kaplan–Meier method with log-rank tests. Survival time, or time-to-event, was the period from exam 4 to the onset of dementia (defined as the midpoint of the interval between the last examination without dementia and the first follow-up examination with dementia). Participants who died were censored at the time of the last in-person assessment, because dementia status could not be confirmed between the last evaluation and death in our study. The final year of follow-up in our analyses was 2000.
Multivariable Cox proportional hazards models estimated the relative risks of incident AD, VaD, and all-cause dementia for the weak social network group, using the strong network group as a reference. Follow-up time was treated as a continuous variable; the Breslow method was used to handle ties; and the proportionality hazards assumption was tested by inspection of Kaplan–Meier survival curves. The Cox models controlled for the potentially confounding effects of the following variables at baseline: age (in years), education (in years), APOE ɛ4 carrier status (at least one e4 allele vs. none), prevalent stroke, presence of depressive symptoms (i.e., CES-D score as a categorical variable), and CASI score. Marital status was not included as a covariate because of its overlap with the LSNS. For each outcome (all-cause dementia, AD, and VaD), the hazard ratio was calculated using 3 Cox models: one unadjusted and 2 adjusted for age and other relevant covariates as described in the next section. Although we present p values that are uncorrected for multiple comparisons, associations meeting the Bonferroni criterion for significance (p < .017=.05/3) are noted.
To reduce the possibility of reverse causation, participants diagnosed with all-cause dementia within the first 3 years after baseline were excluded from the study sample. Sensitivity analyses that included those individuals were conducted to evaluate the robustness of our results. In additional sensitivity analyses, Cox regression models controlling for depressive symptoms tested the CES-D score as a continuous variable. We also investigated the sensitivity of the fully adjusted Cox model to effects of midlife and late-life CVD risk factors as adjustment covariates. Secondary analyses examined participants aged 85 and older.
For all comparisons, statistical significance was defined by a 2-tailed p < .05. Analyses were performed with the Statistical Analysis System, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Sample Characteristics
At baseline, the participants had a median (range) age of 76 (71–93) years, 12 (2–24) years of education, CES-D score of 3 (0–26), and CASI score of 88.9 (74.0–100.0). All participants were male; 84% were married, 18.5% had at least one APOE ɛ4 allele, and 8.7% had depressive symptoms (CES-D score ≥9). Valid baseline CES-D data were available for 2631 of the 2636 participants. According to the clinical cutpoint of 20 proposed by Lubben (23) to identify socially isolated individuals, 302 men (11.5%) whose baseline LSNS scores were below 20 could be considered at risk for severely limited social networks. LSNS data are summarized in Supplementary Table 1.
Table 1 presents mean baseline characteristics, as well as data on unadjusted dementia incidence, for the entire cohort (N = 2636) and for participant groups with stronger (N = 1295) and weaker (N = 1341) social networks. General linear models compared baseline dementia risk factors between these 2 groups. Weaker social networks were significantly associated with older age, fewer years of education, lower CASI score, higher CES-D score, and greater proportions of individuals who were unmarried or had depressive symptoms.
Table 1.
Baseline Characteristics and Dementia Incidence of the Entire Study Population and by Social Network Group
| Characteristics | Total Cohort (N = 2636) |
Social Network Strength | p Value | |
|---|---|---|---|---|
| Weak (N = 1341) | Strong (N = 1295) | |||
| Baseline | ||||
| Age (years) | 76.7 ± 3.9 | 77.3 ± 4.1 | 76.2 ± 3.6 | <.0001 |
| Education (years) | 11.0 ± 3.1 | 10.7 ± 3.0 | 11.2 ± 3.1 | <.0001 |
| Not married (%)a | 15.9 | 23.4 | 8.1 | <.0001 |
| With APOE ɛ4 allele (%)b | 18.5 | 17.4 | 19.6 | .149 |
| Prevalent stroke (%) | 2.47 | 2.76 | 2.16 | .323 |
| CES-D score | 3.54 ± 3.51 | 3.87 ± 3.57 | 3.20 ± 3.40 | <.0001 |
| Depressive symptoms (CES-D ≥ 9; %)c | 8.7 | 10.2 | 7.1 | .0053 |
| CASI score | 88.2 ± 6.0 | 87.6 ± 6.1 | 88.7 ± 5.8 | <.0001 |
| Cognitively impaired (CASI score < 74; %) | 0 | 0 | 0 | — |
| Total cholesterol (mg/dL)d | 193.01 ± 31.94 | 193.56 ± 32.19 | 192.48 ± 31.69 | .391 |
| Fasting glucose (mg/dL)e | 113.36 ± 27.76 | 112.63 ± 26.07 | 114.12 ± 29.38 | .171 |
| Diabetes mellitus (%)f | 29 | 30 | 27 | .038 |
| Hypertension (%)f | 75.0 | 76.0 | 74.8 | .489 |
| Smoking (pack-years)g | 25.8 | 26.2 ± 34.3 | 25.4 ± 34.4 | .578 |
| During follow-up | ||||
| Incident ACD (N, %) | 173 (6.6) | 110 (8.2) | 63 (4.9) | .0005 |
| Incident AD (N, %) | 104 (4.0) | 68 (5.1) | 36 (2.8) | .0025 |
| Incident VaD (N, %) | 38 (1.4) | 24 (1.8) | 14 (1.1) | .1270 |
Notes: Values are given as mean ± SD or median (range) for continuous variables, and as percentages for categorical variables. p Values were computed by t test or chi-square test, as appropriate. CES-D Scale = Center for Epidemiologic Studies Depression (CES-D) 11-item Scale; CASI = Cognitive Abilities Screening Instrument; ACD = all-cause dementia; AD = Alzheimer’s disease; VaD = vascular dementia; SD = standard deviation. The weak social network group is defined by Lubben social network scale (LSNS) score ≤29, and strong social networks by LSNS score >29.
a N = 2635.
b N = 2583.
c N = 2631 (valid CES-D, with < 3 missing answers).
d N = 2603.
e N = 2602.
f N = 2604.
g N = 2596.
Participants were followed for up to 9.8 years, with a median follow-up time of 8.2 years (range: 2.4–9.8 years). During the course of follow-up, 173 men (6.6%) were diagnosed with all-cause dementia. Of these cases, 104 (4.0% of the total cohort) were classified as AD and 38 (1.4%) as VaD. The median (range) time from baseline to the diagnosis of all-cause dementia was 6.3 (3.0–8.9) years, with participants aged 83.9 (75.3–97.8) years when diagnosed. Table 2 compares baseline characteristics between the men who developed incident all-cause dementia and those who remained dementia-free. Participants diagnosed with incident dementia were about 2 years older (78.5 ± 4.4 vs. 76.6 ± 3.9 years; p < .0001) and had more depressive symptoms (CES-D scores: 4.2 ± 4.0 vs. 3.5 ± 3.5; p = .0007) and lower CASI scores (84.8 ± 6.1 vs. 88.4 ± 5.9; p < .0001). This group also had greater proportions of men who were unmarried (23.1% vs. 15.4%; p = .007) or had depressive symptoms (15.6% vs. 8.2%; p = .0008) compared to the individuals who did not become demented. Prevalent stroke and the presence of the APOE ɛ4 allele did not differ significantly between participants who were diagnosed with all-cause dementia and those who were not.
Table 2.
Baseline Characteristics of Study Population by Participants Diagnosed With All-Cause Dementia During Follow-Up and Those Who Remained Dementia-Free
| Characteristics | Incident All-Cause Dementia | p Value | |
|---|---|---|---|
| Yes (N = 173) | No (N = 2463) | ||
| Age (years) | 78.5 ± 4.4 | 76.6 ± 3.9 | <.0001 |
| Education (years) | 10.7 ± 3.1 | 11.0 ± 3.1 | .219 |
| Not married (%)a | 23.1 | 15.4 | .007 |
| With APOE ɛ4 allele (%)b | 20.9 | 18.3 | .397 |
| Prevalent stroke (%) | 2.9 | 2.4 | .710 |
| CES-D score | 4.2 ± 4.0 | 3.5 ± 3.5 | .014 |
| Depressive symptoms (CES-D ≥ 9; %)c | 15.6 | 8.2 | .0008 |
| CASI score | 84.8 ± 6.1 | 88.4 ± 5.9 | <.0001 |
| LSNS score | 27.6 ± 7.5 | 29.0 ± 7.7 | .014 |
| LSNS score ≤ 29 (%) | 63.6 | 50.0 | .0005 |
| Total cholesterol (mg/dL)d | 189.2 ± 30.2 | 193.3 ± 32.0 | .10 |
| Fasting glucose (mg/dL)e | 114.1 ± 24.3 | 113.3 ± 28.0 | .73 |
| Diabetes mellitus (%)f | 30.6 | 28.8 | .61 |
| Hypertension (%)f | 79.2 | 75.2 | .23 |
| Smoking (pack-years)g | 22.9 ± 31.7 | 26.0 ± 34.5 | .25 |
Notes: Values are given as mean ± SD for continuous variables and as percentages for categorical data. CES-D Scale = Center for Epidemiologic Studies Depression (CES-D) 11-item Scale; CASI = Cognitive Abilities Screening Instrument; LSNS = Lubben Social Network Scale; SD = standard deviation.
a N = 2635.
b N = 2583.
c N = 2631.
d N = 2603.
e N = 2602.
f N = 2604.
g N = 2596.
Dementia Incidence
When age-adjusted rates of incident dementia per 1000 person-years of follow-up were compared between the strong and weak social network groups, weaker social networks at baseline were associated with a greater likelihood of subsequent all-cause dementia (10.8 vs. 6.7 per 1000 person-years; p = .002) and AD (6.7 vs. 4.0 per 1000 person-years; p = .007). Social network strength showed no significant relationship to age-adjusted rates of incident VaD, which were 2.4 and 1.4 per 1000 person-years, respectively, for participants with weak and strong social networks (p = .15).
Kaplan–Meier Survival Curves
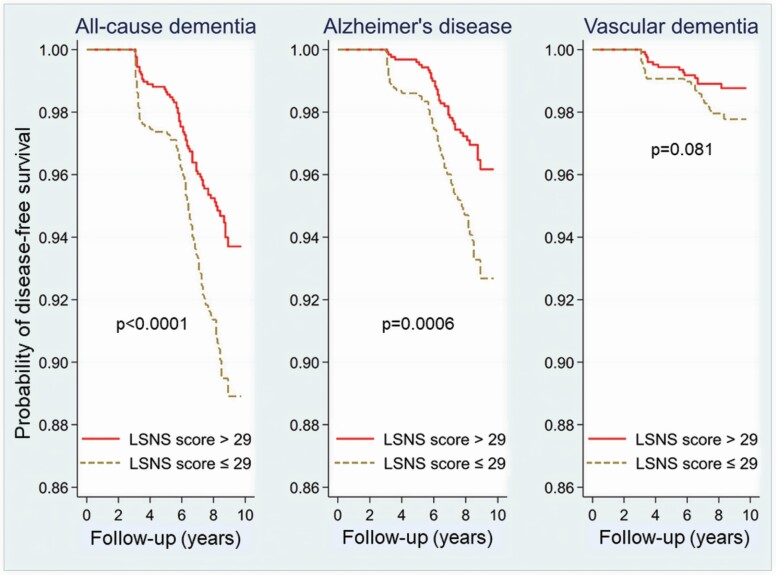
Figure 2 displays Kaplan–Meier curves for 10-year survival free of incident all-cause dementia, AD, and VaD by the weak and strong social network groups. When the log-rank test was used to assess statistical differences, individuals with strong versus weak social networks differed significantly in the incidence of all-cause dementia (p < .0001) and AD (p = .0006), but not VaD (p = .081). As shown by the survival curves, participants with weak social networks were more likely to develop all-cause dementia and AD. The curves diverged with time so that differences between groups became greater with longer follow-up. The probability of survival that was free of overall dementia at almost 10 years was approximately 93.8% for individuals who had strong social networks; for those with weak social networks, this probability was 89.0% (11% incidence). Curves for both groups exhibited the steepest declines after about 6 years of follow-up when the median participant age was approximately 82. The same pattern, less pronounced, was seen in the curves for AD-free survival.
Figure 2.
Kaplan–Meier survival curves for all-cause dementia (left), Alzheimer’s disease (middle), and vascular dementia (right) in groups stratified by median split of baseline Lubben Social Network Scale (LSNS) scores. LSNS scores > 29 are indicated by the solid red line, and LSNS scores ≤ 29 by the dashed brown line. p Values were determined by log-rank tests.
Multivariable Cox Regression
The impact of social networks on the 10-year incidence of all-cause dementia, AD, and VaD was evaluated using separate Cox proportional hazards models that included adjustment for confounding baseline variables (Table 3). The proportionality hazards assumption was met for all regressions. Model 1 was unadjusted; Model 2 adjusted for age, education, APOE ɛ4 allele status and prevalent stroke; and Model 3 added depressive symptoms (as a dichotomized variable) and CASI score to the covariates. Weak social networks at baseline were significantly associated with an increased risk of all-cause dementia and AD in all models. As shown by Model 2, weak social networks predicted a higher incidence of all-cause dementia (hazard ratio [HR] = 1.64, 95% confidence interval [CI]: 1.20–2.24; p = .002) and AD (HR = 1.79, 95% CI: 1.19–2.70; p = .005) compared to strong social networks independently of age, education, APOE ɛ4, and prevalent stroke. These relationships persisted when the fully adjusted model (Model 3) controlled for baseline CASI score and depression in addition to the other variables: weak social networks were associated with greater incident all-cause dementia (HR = 1.52, 95% CI: 1.11–2.08; p = .009) and AD (HR = 1.67, 95% CI: 1.11–2.51; p = .014). The results for AD and all-cause dementia survived Bonferroni correction for multiple comparisons (p < .017). Social networks were not linked to the incidence of VaD.
Table 3.
Hazard Ratios of Incident Dementia (With 95% CI) for Participants With Weak Social Networks (LSNS score ≤ 29), Computed by Cox Proportional Hazards Models for the First 10 Years of Follow-Up
| Model | N | Baseline Covariates | Hazard Ratio (95% Confidence Interval) and p Value | |||||
|---|---|---|---|---|---|---|---|---|
| All-Cause
Dementia (N = 173) |
p Value | Alzheimer’s Disease (N = 104) |
p Value | Vascular
Dementia (N = 38) |
p Value | |||
| 1 | 2636 | None (unadjusted) | 1.85 (1.36–2.52) | <.001 | 2.01 (1.34–3.01) | <.001 | 1.78 (0.920–3.45) | .086 |
| 2 | 2583 | Age, education, APOE ɛ4, and prevalent stroke | 1.64 (1.20–2.24) | .002 | 1.79 (1.19–2.70) | .005 | 1.50 (0.77–2.93) | .238 |
| 3 | 2578 | Age, education, APOE ɛ4, prevalent stroke, depressive symptoms, and CASI score | 1.52 (1.11–2.08) | .009 | 1.67 (1.11–2.51) | .014 | 1.34 (0.68–2.64) | .392 |
Notes: The group with strong social networks is used as a reference. Results are shown for unadjusted models and with adjustment for baseline covariates (age, education, APOE ɛ4 status, prevalent stroke, and depressive symptoms [CES-D score ≥ 9]), as indicated. p Values shown in bold indicate associations that are significant after Bonferroni correction for multiple comparisons. LSNS = Lubben Social Network Scale; CES-D Scale = Center for Epidemiologic Studies Depression (CES-D) 11-item Scale; CASI = Cognitive Abilities Screening Instrument.
Sensitivity Analyses
When the Cox models considered CES-D score as a continuous instead of categorical variable, the results (data not shown) were nearly identical to those presented earlier. Qualitatively similar though less striking results were produced by sensitivity analyses that included the individuals with dementia onset within 3 years post-baseline (total N = 2676). Participants with weak social networks (relative to strong) had significantly greater age-adjusted rates of all-cause dementia (12.6 vs. 8.7 per 1000 person-years of follow-up; p = .014) and a trend toward increased risk of AD (7.4 vs. 5.4 per 1000 person-years of follow-up; p = .078). Kaplan–Meier survival curves (data not shown) and log-rank tests demonstrated that weak social networks, compared to strong, were significantly associated with a higher incidence of all-cause dementia (p = .0007) and AD (p = .010). In multivariable Cox regression models adjusted for baseline age, education, prevalent stroke, and presence of an APOE ɛ4 allele, weak social networks were significantly related to increased risk of all-cause dementia (HR = 1.44, 95% CI: 1.09–1.91; p = .010) and AD (HR = 1.48, CI: 1.03–2.13; p = .036). When baseline CASI score and depression were added as covariates, these relationships lost significance (p = .052 for all-cause dementia; p = .10 for AD).
Finally, we performed sensitivity analyses that added major CVD risk factors as covariates to the fully adjusted Cox model (Model 3). Fasting plasma glucose, diabetes mellitus, hypertension, total cholesterol, and smoking were considered, using data available at our study baseline (HHP exam 4, or late life) as well as at HHP exam 1 (mean age = 51.7 ± 4.0 years, closer to midlife). Because of the collinearity between CVD variables at midlife and at baseline, the effects of CVD risk at these 2 timepoints were examined in separate Cox regression models. Controlling for baseline CVD risk (in addition to age, education, APOE ɛ4 allele status, prevalent stroke, depressive symptoms, and CASI score), weak social networks predicted higher incidence of all-cause dementia (HR = 1.58, 95% CI: 1.15–2.17; p = .005) and AD (HR = 1.72, 95% CI: 1.14–2.60; p = .009), but not VaD (HR = 1.44, 95% CI: 0.71–2.91; p = .309) relative to strong social networks. The inclusion of baseline CVD risk did not appreciably change the results of Model 3. Similarly, when Model 3 adjusted for midlife CVD factors (with baseline age replaced by age at HHP exam 1), weak social networks (compared to strong) predicted greater risk of all-cause dementia (HR = 1.49, 95% CI: 1.08–2.04; p = .015) and AD (HR = 1.56, 95% CI: 1.03–2.36; p = .035), but did not relate to incident VaD (HR = 1.35, 95% CI: 0.69–2.66; p = .39). Thus, although midlife CVD risk factors slightly attenuated social network effects on incident dementia and AD, there was no substantial change in the findings.
Secondary Analyses
Only 138 men in our study sample were aged ≥ 85 years at baseline, making meaningful stratified analyses of dementia incidence in the oldest–old impossible. To compare the effects of social network groups (LSNS score >29 and ≤29) on incident AD between the younger–old (age <85) and oldest–old (≥85), we modeled age at follow-up timepoints as a time-dependent covariate in Cox regression analyses. Defining the binary variable age_85 = baseline age + years of follow-up as 1 if age_85 ≥ 85 and 0 if age_85 <85, we tested social network group × age_85 interaction effects in the fully adjusted Cox model (Model 3 in Table 3). The interaction was not significant (p = .73), so the effects of weak social networks on AD incidence did not differ between the oldest–old and younger–old individuals; i.e., weak networks conferred the same AD risk in both age groups. Similar results were obtained for all-cause dementia (p = .23) and VaD (p = .14).
Exploratory Cox regression analyses that used Lubben’s cutpoint for social isolation (23) to define weak and strong social network groups by LSNS score <20 (N = 302) and ≥20 (N = 2 334), respectively, produced nonsignificant results (data not shown): social network strength did not relate to the risk of all-cause dementia, AD, or VaD (all p ≥ .3 in fully adjusted models).
Discussion
Weaker social networks in late life independently predicted higher incident all-cause dementia and AD after controlling for confounders, but showed no association with the development of VaD. The results of this study are supported by prior research. Larger social networks have been related to better global cognition in late life (36); despite measurement uncertainties (37), systematic reviews and meta-analyses confirm a risk-attenuating effect of large, robust late-life social networks on dementia (10,37,38). Our observation that weaker social networks in late life increased the risk of all-cause dementia is also in line with a previous examination of Kuakini HAAS participants. In that study, social engagement was assessed by summing 5 indicators to generate an index representing the number of social ties, a measure more limited than the LSNS score (13). Limited late-life social engagement was related to an elevated risk of all-cause dementia over an average period of 4.6 years (13). Similar results were observed for both incident AD and VaD (13), in partial agreement with our findings. We have extended the work to show that a weak social network in late life may predict the development of AD and overall dementia up to a decade later.
Converging data suggest that social relationships may modify associations of neuropathological changes with cognitive decline in preclinical AD. Loneliness correlated with brain amyloid burden in cognitively normal older adults (39), corroborating an observed link between loneliness and the risk of AD (40). Whereas social isolation is the objective condition of having few social contacts, loneliness is a subjective sense of distress stemming from a perceived deficiency in the number or quality of social relationships (41). Social isolation can contribute to loneliness, although each may occur without the other (40). In dementia-free individuals, the availability of social listening (a form of social support) has been associated with total cerebral volume, a neuroanatomical measure of early vulnerability to AD (42). Participants who had high listener availability showed smaller decreases in global cognition with lower cerebral volume (42). Social support was concluded to reduce AD risk by enhancing cognitive resilience so that cognitive performance is better than would be predicted by brain structure (42).
The processes which enhance cognitive resilience are not well understood. Perhaps the most compelling model of how socialization modulates dementia risk is that of cognitive reserve (38). Cognitive reserve provides resilience to brain pathology (43), may account for different trajectories of cognitive decline over time (43), and in particular, may explain how malleable risk factors affect the clinical onset of AD (44). The Rush Memory and Aging Project reported that social network size modified the relationship between AD pathology and cognitive loss; that is, cognitive function correlated inversely with pathology measures but remained higher in participants who had larger social networks (9). Cognitive resilience is likely a consequence of biological mechanisms that regulate synaptic plasticity and neurogenesis. Greater emotional support was cross-sectionally associated with higher serum levels of brain-derived neurotrophic factor (BDNF) in the Framingham Heart Study (FHS) (16). BDNF stimulates neurogenesis (45), induces neuronal repair and survival (46), and promotes synaptic growth, function, and plasticity (46). Not surprisingly, it may play a key role in AD neuropathology, which is characterized by widespread synaptic and neuronal loss (47). BDNF crosses the blood–brain barrier with ease (48) and its fluctuations in peripheral circulation parallel those in the central nervous system (16). Post-mortem analyses have revealed decreased brain BDNF concentrations in neurodegenerative diseases, including AD (49). In the FHS cohort, high serum BDNF was a longitudinal predictor of reduced risk of dementia and AD (50). Notably, social interaction has reversed memory deficits by increasing hippocampal BDNF and neurogenesis in a mouse model of AD (51). Similar mechanisms, by which neurotrophic factors mediate the beneficial action of strong social networks (or conversely, the exacerbating effects of weak social networks) on aging-related brain changes, may underlie the findings of our study. Such processes do not exclude other neurobiological pathways through which the social environment may influence the brain and cognitive health, as social relationships may work through various mechanisms (52). A rich social network can improve lifestyle and behavior, thereby reducing vascular risk (eg, through participation in physical activities) (18); it may also provide a buffer against stress, which is associated with hippocampal damage and greater AD risk (18).
The lack of a significant relationship between social networks and incident VaD in our cohort may be attributed to insufficient statistical power due to the low number of VaD cases. Yet it is also possible that differences between the 2 forms of dementia give rise to the association observed between social networks and incident AD but not VaD. VaD has a variable course and several clinically identifiable subtypes corresponding to different underlying vascular factors (53). Cognitive impairment in AD progresses in a more predictable manner with neuronal injury and death (53). Our findings are difficult to compare with published results as most prior studies of social interaction and dementia did not examine VaD separately. However, frequent social activity (>once/week) has been correlated with decreased risk of AD but not of VaD (54), and loneliness was identified as a risk factor for all-cause dementia and AD, but not for VaD (40).
For reasons that are obscure, AD may be more sensitive than VaD to the ameliorating effects of cognitive stimulation through social interaction. Both conditions can affect every cognitive domain, but VaD primarily impairs executive functioning and attention, whereas episodic memory decline is more prominent in AD (55). AD and VaD are characterized by distinct cognitive profiles and by different brain structural changes associated with the patterns of impairment; for instance, hippocampal atrophy is more pronounced in AD than in VaD and correlates with dementia severity (56). Patterns of correlation between gray matter and white matter degeneration are distinct in these two disorders, suggesting different underlying pathomechanisms (57). Myelin loss in the frontal lobes is more severe in VaD compared to AD (58), so cellular pathology may contribute to varied effects of supportive social interactions on functional resilience. In contrast to AD, VaD is generally not classified as a neurodegenerative disorder. Differences between the two dementias (or differences in aging-related cognitive decline) may be explained by different roles played by the frontal and medial-temporal systems (59). At least two mechanisms have been proposed as contributing to brain aging, one driven by AD-type pathology (longitudinal shrinkage of the entorhinal cortex, a medial-temporal structure), and the other associated with VaD risk and with reductions of prefrontal but not entorhinal volumes (59). Individuals with “mixed” dementia due to concomitant vascular and neurodegenerative pathologies exhibit aspects of both disorders (60). Unlike individuals in early old age who are likely to manifest a pattern of cognitive impairment resembling that of AD (61), those over 80 years old typically have a range of cognitive deficits across domains, corresponding to the mixed pathology that is more common in later old age than in younger (62). Our results for all-cause dementia may reflect associations among cases of mixed dementia; i.e., those not diagnosed as pure AD or VaD.
Strengths of the present study are the well-characterized Kuakini HAAS cohort; the decade-long follow-up period; our use of a standardized and validated scale, the LSNS, to assess social networks; and our analysis of incident AD and VaD in addition to all-cause dementia. Other strengths include the use of accurate, reliable dementia case-finding methods and good retention rates at follow-up visits. Data on social contact and dementia risk in Asians are relatively sparse because the majority of studies have been conducted in largely Caucasian populations. Our work provides evidence of similar relationships in a large sample that is racially and ethnically Japanese. The participants were older at baseline than those in many investigations of social network effects. Our study addresses a gap in the literature on the oldest–old, the fastest-growing segment of the population throughout much of the world (19). Identification of a weak social network in later life as a risk factor for dementia and AD is timely, especially because the risk of isolation for this age group has been heightened by restrictions imposed during the recent COVID-19 pandemic (63) over and above the shrinking social networks that accompany aging (64). While social isolation in older adults may signal prodromal dementia (14), the association between social networks and incident dementia found in this study is unlikely to be due to reverse causation given our exclusion of individuals who became demented in the first 3 years. If confirmed, the deleterious effect of restricted social networks in late life may have practical implications for interventions designed to increase social interaction (65).
This work also has limitations. The cohort of Japanese-American men was not representative of the older population racially, culturally, or by sex. Older men and women tend to have different patterns of social networks, and the effects of those networks on dementia may be gender-specific (17). We did not control for indices of physical ill-health (eg, vitamin D deficiency, which is linked to an elevated risk of AD and all-cause dementia (66)). Mortality as a competing risk for dementia was not taken into account in survival analyses. Our cutoff for weak social networks was not motivated by prior studies but was defined by median split of LSNS scores. We did not examine satisfaction with social relationships, and we utilized a composite measure of social networks rather than distinguishing among domains. Furthermore, with its focus on family and friends, the LSNS overlooks peripheral or weak ties, although social interactions with “consequential strangers” may complement close ties in their enhancement of quality of life (67).
An important step for future research is the inclusion of nonlinear effects in predictive models of social networks and dementia. Nonlinear associations between social contact and psychological well-being have identified a moderate level of socializing as optimal, beyond which outcomes do not improve (68). A focus on social network size and frequency may mask the adverse effects of toxic relationships (20), so qualitative aspects of social networks should be carefully considered. Further studies must determine which social network features afford the greatest protection against specific forms of dementia or cognitive decline, paving the way for clinical trials to test the efficacies of different social interventions in preventing or slowing AD, VaD, and other dementia types.
Conclusion
Weak social networks in late life were associated with an elevated risk of incident AD and all-cause dementia. Preventive strategies against social disengagement, even when implemented at an older age, may prevent or delay the onset of these diseases.
Supplementary Material
Acknowledgments
We thank our study participants, without whom this work would not have been possible. We also thank the anonymous reviewers for their insightful comments and suggestions.
Contributor Information
Kalpana J Kallianpur, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA; Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaii, Honolulu, Hawaii, USA.
Kamal H Masaki, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA; Department of Geriatric Medicine, University of Hawaii, Honolulu, Hawaii, USA.
Randi Chen, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA.
Bradley J Willcox, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA; Department of Geriatric Medicine, University of Hawaii, Honolulu, Hawaii, USA.
Richard C Allsopp, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA.
Philip Davy, Kuakini Center for Translational Research on Aging, Kuakini Medical Center, Honolulu, Hawaii, USA.
Hiroko H Dodge, Department of Neurology, Oregon Health & Science University, Portland, Oregon, USA; Layton Aging and Alzheimer’s Disease Center, Oregon Health & Science University, Portland, Oregon, USA.
Funding
This work was supported by the Kuakini Medical Center, the US National Institutes of Health (contract N01-AG-4-2149, Grants 5 U01 AG019349-05, 5R01AG027060 [Kuakini Hawaii Lifespan Study], 5R01AG038707 [Kuakini Hawaii Healthspan Study]), 1P20GM125526-01A1 [Kuakini Center of Biomedical Research Excellence for Clinical and Translational Research on Aging], and contract N01-HC-05102 from the National Heart Lung and Blood Institute.
Conflict of Interest
None declared.
Author Contributions
The manuscript was primarily written and revised by K.J.K., who also interpreted the data. H.H.D. and K.H.M. conceived the idea and contributed significantly to data interpretation and drafting the manuscript. R.C. performed the statistical analyses and participated in interpreting the data. All authors critically reviewed and approved the manuscript.
References
- 1. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M.. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 2. Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- 3. Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134:226–239. doi: 10.1016/j.neuropharm.2017.12.030 [DOI] [PubMed] [Google Scholar]
- 4. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 5. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diniz BS, Butters MA, Albert SM, Dew MA, ReynoldsCF, 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 8. Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16:175. doi: 10.1186/s12877-016-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pillai JA, Verghese J. Social networks and their role in preventing dementia. Indian J Psychiatry. 2009;51(suppl 1):S22–S28. [PMC free article] [PubMed] [Google Scholar]
- 10. Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 11. Desai R, John A, Stott J, Charlesworth G. Living alone and risk of dementia: a systematic review and meta-analysis. Ageing Res Rev. 2020;62:101122. doi: 10.1016/j.arr.2020.101122 [DOI] [PubMed] [Google Scholar]
- 12. Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 13. Saczynski JS, Pfeifer LA, Masaki K, et al. The effect of social engagement on incident dementia: the Honolulu-Asia aging study. Am J Epidemiol. 2006;163:433–440. doi: 10.1093/aje/kwj061 [DOI] [PubMed] [Google Scholar]
- 14. Holwerda TJ, Deeg DJ, Beekman AT, et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the amsterdam study of the elderly (Amstel). J Neurol Neurosurg Psychiatry. 2014;85:135–142. doi: 10.1136/jnnp-2012-302755 [DOI] [PubMed] [Google Scholar]
- 15. Heser K, Wagner M, Wiese B, et al. Associations between dementia outcomes and depressive symptoms, leisure activities, and social support. Dement Geriatr Cogn Dis Extra. 2014;4:481–493. doi: 10.1159/000368189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salinas J, Beiser A, Himali JJ, et al. Associations between social relationship measures, serum brain-derived neurotrophic factor, and risk of stroke and dementia. Alzheimers Dement (N Y). 2017;3:229–237. doi: 10.1016/j.trci.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J, Hasselgren C, Zettergren A, et al. The impact of social networks and Apoe Epsilon4 on dementia among older adults: tests of possible interactions. Aging Ment Health. 2020;24:395–404. doi: 10.1080/13607863.2018.1531368 [DOI] [PubMed] [Google Scholar]
- 18. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- 19. Kawas CH, Legdeur N, Corrada MM. What have we learned from cognition in the oldest-old. Curr Opin Neurol. 2021;34:258–265. doi: 10.1097/WCO.0000000000000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rohr S, Lobner M, Guhne U, et al. Changes in social network size are associated with cognitive changes in the oldest-old. Front Psychiatry. 2020;11:330. doi: 10.3389/fpsyt.2020.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yano K, Reed DM, McGee DL. Ten-year incidence of coronary heart disease in the Honolulu Heart Program. Relationship to biologic and lifestyle characteristics. Am J Epidemiol. 1984;119:653–666. doi: 10.1093/oxfordjournals.aje.a113787 [DOI] [PubMed] [Google Scholar]
- 22. Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447 [DOI] [PubMed] [Google Scholar]
- 23. Lubben JE. Assessing social networks among elderly populations. Fam Community Health. 1988;11:42–52. doi: 10.1097/00003727-198811000-00008 [DOI] [Google Scholar]
- 24. Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (Casi): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58; discussion 62. doi: 10.1017/s1041610294001602 [DOI] [PubMed] [Google Scholar]
- 25. White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older japanese-american men in Hawaii: the Honolulu-Asia aging study. JAMA. 1996;276:955–960. doi: 10.1001/jama.1996.03540120033030 [DOI] [PubMed] [Google Scholar]
- 26. Morris JC, Heyman A, Mohs RC, et al. The Consortium to establish a registry for Alzheimer’s Disease (Cerad). Part I. clinical and neuropsychological assessment of alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 27. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E Epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hixson JE, Vernier DT. Restriction isotyping of human Apolipoprotein E by gene amplification and cleavage with Hhai. J Lipid Res. 1990;31:545–548. doi: 10.1016/s0022-2275(20)43176-1 [DOI] [PubMed] [Google Scholar]
- 29. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 30. Takeshita J, Masaki K, Ahmed I, et al. Are depressive symptoms a risk factor for mortality in elderly Japanese American men?: the Honolulu-Asia aging study. Am J Psychiatry. 2002;159:1127–1132. doi: 10.1176/appi.ajp.159.7.1127 [DOI] [PubMed] [Google Scholar]
- 31. Takeda S, Rakugi H, Morishita R. Roles of vascular risk factors in the pathogenesis of dementia. Hypertens Res. 2020;43:162–167. doi: 10.1038/s41440-019-0357-9 [DOI] [PubMed] [Google Scholar]
- 32. Gelber RP, Petrovitch H, Masaki KH, et al. Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc. 2012;60:118–123. doi: 10.1111/j.1532-5415.2011.03768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korczyn AD, Vakhapova V. The prevention of the dementia epidemic. J Neurol Sci. 2007;257:2–4. doi: 10.1016/j.jns.2007.01.081 [DOI] [PubMed] [Google Scholar]
- 34. Deckers K, Kohler S, van Boxtel M, Verhey F, Brayne C, Fleming J. Lack of associations between modifiable risk factors and dementia in the very old: findings from the Cambridge City over-75s cohort study. Aging Ment Health. 2018;22:1272–1278. doi: 10.1080/13607863.2017.1280767 [DOI] [PubMed] [Google Scholar]
- 35. Kuo CY, Stachiv I, Nikolai T. Association of late life depression, (Non-) modifiable risk and protective factors with dementia and Alzheimer’s disease: literature review on current evidences, preventive interventions and possible future trends in prevention and treatment of dementia. Int J Environ Res Public Health. 2020;17:7475. doi: 10.3390/ijerph17207475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J Alzheimers Dis. 2019;70:S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fratiglioni L, Marseglia A, Dekhtyar S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol. 2020;19:533–543. doi: 10.1016/S1474-4422(20)30039-9 [DOI] [PubMed] [Google Scholar]
- 38. Penninkilampi R, Casey AN, Singh MF, Brodaty H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2018;66:1619–1633. doi: 10.3233/JAD-180439 [DOI] [PubMed] [Google Scholar]
- 39. Donovan NJ, Okereke OI, Vannini P, et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 2016;73:1230–1237. doi: 10.1001/jamapsychiatry.2016.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sundstrom A, Adolfsson AN, Nordin M, Adolfsson R. Loneliness increases the risk of all-cause dementia and Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2020;75:919–926. doi: 10.1093/geronb/gbz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ong AD, Uchino BN, Wethington E. Loneliness and health in older adults: a mini-review and synthesis. Gerontology. 2016;62:443–449. doi: 10.1159/000441651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salinas J, O’Donnell A, Kojis DJ, et al. Association of social support with brain volume and cognition. JAMA Netw Open. 2021;4:e2121122. doi: 10.1001/jamanetworkopen.2021.21122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stern Y, Barnes CA, Grady C, Jones RN, Raz N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging. 2019;83:124–129. doi: 10.1016/j.neurobiolaging.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/jneurosci.21-17-06718.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. Bdnf-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505 [DOI] [PubMed] [Google Scholar]
- 47. Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5 [DOI] [PubMed] [Google Scholar]
- 49. Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846 [DOI] [PubMed] [Google Scholar]
- 50. Weinstein G, Beiser AS, Choi SH, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsiao YH, Hung HC, Chen SH, Gean PW. Social interaction rescues memory deficit in an animal model of Alzheimer’s disease by increasing Bdnf-dependent hippocampal neurogenesis. J Neurosci. 2014;34:16207–16219. doi: 10.1523/jneurosci.0747-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen S. Social relationships and health. Am Psychol. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676 [DOI] [PubMed] [Google Scholar]
- 53. O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3 [DOI] [PubMed] [Google Scholar]
- 54. Yang SY, Weng PH, Chen JH, et al. Leisure activities, Apolipoprotein E E4 status, and the risk of dementia. J Formos Med Assoc. 2015;114:1216–1224. doi: 10.1016/j.jfma.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 55. Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 56. Dolek N, Saylisoy S, Ozbabalik D, Adapinar B. Comparison of hippocampal volume measured using magnetic resonance imaging in Alzheimer’s disease, vascular dementia, mild cognitive impairment and pseudodementia. J Int Med Res. 2012;40:717–725. doi: 10.1177/147323001204000236 [DOI] [PubMed] [Google Scholar]
- 57. Jang H, Kwon H, Yang JJ, et al. Correlations between gray matter and white matter degeneration in pure Alzheimer’s disease, pure subcortical vascular dementia, and mixed dementia. Sci Rep. 2017;7:9541. doi: 10.1038/s41598-017-10074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ihara M, Polvikoski TM, Hall R, et al. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strub RL. Vascular dementia. Ochsner J. 2003;5:40–43. [PMC free article] [PubMed] [Google Scholar]
- 61. Bondi MW, Houston WS, Salmon DP, et al. Neuropsychological deficits associated with Alzheimer’s disease in the very-old: discrepancies in raw vs. standardized scores. J Int Neuropsychol Soc. 2003;9:783–795. doi: 10.1017/s1355617703950119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Reuck J, Maurage CA, Deramecourt V, et al. Aging and cerebrovascular lesions in pure and in mixed neurodegenerative and vascular dementia brains: a neuropathological study. Folia Neuropathol. 2018;56:81–87. doi: 10.5114/fn.2018.76610 [DOI] [PubMed] [Google Scholar]
- 63. Wu CY, Mattek N, Wild K, et al. Can changes in social contact (frequency and mode) mitigate low mood before and during the Covid-19 pandemic? The I-conect project. J Am Geriatr Soc. 2022;70:669–676. doi: 10.1111/jgs.17607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wrzus C, Hanel M, Wagner J, Neyer FJ. Social network changes and life events across the life span: a meta-analysis. Psychol Bull. 2013;139:53–80. doi: 10.1037/a0028601 [DOI] [PubMed] [Google Scholar]
- 65. Dodge HH, Zhu J, Mattek N, et al. Web-enabled conversational interactions as a means to improve cognitive functions: results of a 6-week randomized controlled trial. Alzheimers Dement (N Y). 2015;1:1–12. doi: 10.1016/j.trci.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/wnl.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fingerman KL. Consequential strangers and peripheral ties: the importance of unimportant relationships. J Fam Theory Rev. 2009;1:69–86. doi: 10.1111/j.1756-2589.2009.00010.x [DOI] [Google Scholar]
- 68. Ren D, Stavrova O, Loh WW. Nonlinear effect of social interaction quantity on psychological well-being: diminishing returns or inverted U? J Pers Soc Psychol. 2022;122:1056. doi: 10.1037/pspi0000373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.