Abstract
Objective:
Resolution of impaired microvascular flow may lag the normalization of macrocirculatory parameters. The significance of microcirculatory dysfunction in critically ill children and neonates is unknown, but microcirculatory variables can be measured using doppler or videomicroscopy imaging techniques. We outline the current understanding of the role of the microcirculation in critical illness, review methods for its assessment and perform a systematic review of how it has been monitored in critically ill neonates and children.
Design:
Systematic review (PROSPERO CRD42019117993)
Setting:
N/A
Subjects:
N/A
Interventions:
None
Methods:
We systematically searched MEDLINE, EMBASE, PubMed and Web of Science. We included studies of critically ill patients 0 to 18 years old investigating microcirculatory blood flow. Two reviewers analyzed abstracts and articles. Results were qualitatively analyzed due to study heterogeneity.
Results:
A total of 2559 abstracts met search criteria, of which 94 underwent full text review. Of those, 36 met inclusion criteria. Seven studies investigated microcirculatory changes in critically ill children. Twenty studies investigated the microcirculatory changes in neonates with variable diagnoses compared to a diverse set of clinical end points. Nine studies assessed the effects of age, sex and birth weight on microvascular flow in neonates. Across all studies, microcirculatory dysfunction was associated with poor outcomes and may not correlate with observed macrovascular function.
Conclusions:
Assessment of microvascular flow in critically ill children and neonates is possible, though significant challenges remain. In many such patients, microvascular blood flow is disrupted despite medical management targeting normalized macrovascular parameters. Future studies are needed to define normal pediatric microvascular flow parameters and to assess the impact of patient and treatment factors on its function.
Keywords: Microcirculation, critical illness, children, shock, endothelial dysfunction, capillary leak
Introduction
The microcirculation is a critically important and potentially underappreciated component of the cardiovascular system. This dynamic vascular segment resides between the arterioles and venules and is comprised of pre-capillary arterioles, capillaries and post-capillary venules(1,2). These vessels are less than 100 μm in diameter, smaller than a human hair. The microcirculation consists of endothelial cells and associated supporting cells. Pre-capillary arterioles are surrounded by smooth muscle cells (SMCs), which act to regulate blood flow to tissue. Capillaries and post-capillary venules are supported by pericytes and less frequent SMCs(3, 4). Capillaries, the smallest of these vessels range between 3 and 6 μm in diameter, are the most numerous and dynamic part of the microcirculation(5, 6). These tiny vessels are responsible for the delivery of nutrients and signaling molecules, removal of waste products, flux of intravascular fluid and heat exchange at the level of individual cells(7, 8). These processes require intimate contact, and consequently a single capillary may be responsible for supporting a layer of only two or three parenchymal cells(9, 10).
All blood flow is dictated by pressure gradients. In the microcirculation more specifically, the difference between pre-capillary hydrostatic pressure (Pcap) and post-capillary venular pressure (mean systemic filling pressure, Pmsf) drives flow. Under normal conditions, Pcap is dictated by precapillary SMC sphincter tone, subject to tissue specific autoregulation which is, in part, regulated by capillary pericytes(11). Regulation of Pmsf is dictated by venous volume and compliance of the larger venules and veins(12). Normal microcirculatory flow is characterized by homogenous red blood cell (RBC) density and velocity throughout the length of the capillary, with pre-capillary oxygen tension (PO2) of 90 mmHg and post-capillary PO2 of 45 mmHg. The transit time for a RBC in capillaries is highly variable across different organs(13).
In times of physiologic stress, the microcirculation adapts to meet increased tissue metabolism by three mechanisms. First, oxygen extraction is increased throughout the length of the capillary. This adaptation is passive and results in decreased central venous oxygen saturation. Second, greater cardiac output augments microvascular flow decreasing RBC transit time and boosting the total number of RBCs crossing the capillary per unit time. Lastly, most tissues have excess capillaries to augment perfusion during times of greater need. The relative perfusion of specific capillary networks is controlled by autonomic nervous system activity (neurogenic), small molecule autocrine signaling (metabolic) and pressure dependent autoregulation (myogenic) of the pre-capillary SMCs and pericytes(3). Microvascular pericytes in particular, are essential to regulating capillary diameter(14), barrier function(15), basement membrane properties(16) and endothelial sprouting(17), all essential functions to maintain adequate flow to support parenchymal cells. Increased capillary recruitment reduces the distance between perfused capillaries and lessens the distance for O2 to diffuse. These compensatory mechanisms vary by organ. For example, the heart and diaphragm have no recruitable capillaries, whereas this is the predominate adaptive method in skeletal muscle(18).
In times of pathophysiologic stress, such as shock, microvascular flow may be disrupted with a number of deleterious consequences. Although confirmatory evidence is limited, there are several proposed mechanisms by which the microcirculation may become altered(19). Since the microvasculature is responsible for oxygen and nutrient delivery, perturbations in capillary flow result in organ hypoperfusion. In late stages of shock, cellular hypoxia is evidenced by increased markers of perfusion inadequacy, such as increasing serum lactate or organ-specific enzymes. Blockage of capillaries results in an inappropriately heterogeneous microvascular flow (Figure 1, #1), with flow diverted through pathologically dilated capillaries, leading to microvascular shunt(20, 21). Hemodilution, which may result from volume resuscitation, reduces the number of RBCs in each capillary decreasing oxygen content (Figure 1, #2). In the setting of venous congestion, such as occurs in cardiogenic shock, Pmsf rises and impairs flow and oxygen delivery (Figure 1, #3). Finally, tissue edema, in the setting of capillary leak, expands the distance that oxygen must diffuse to reach cells, producing cellular dysoxia and dysfunction (Figure 1, #4). Tissue-specific capillaries have different responses to the above pathophysiologic processes. This heterogeneity of function makes it impossible to extrapolate findings from a single capillary to the system as a whole and thus undermines our understanding of global microvascular function(22).
Figure 1:
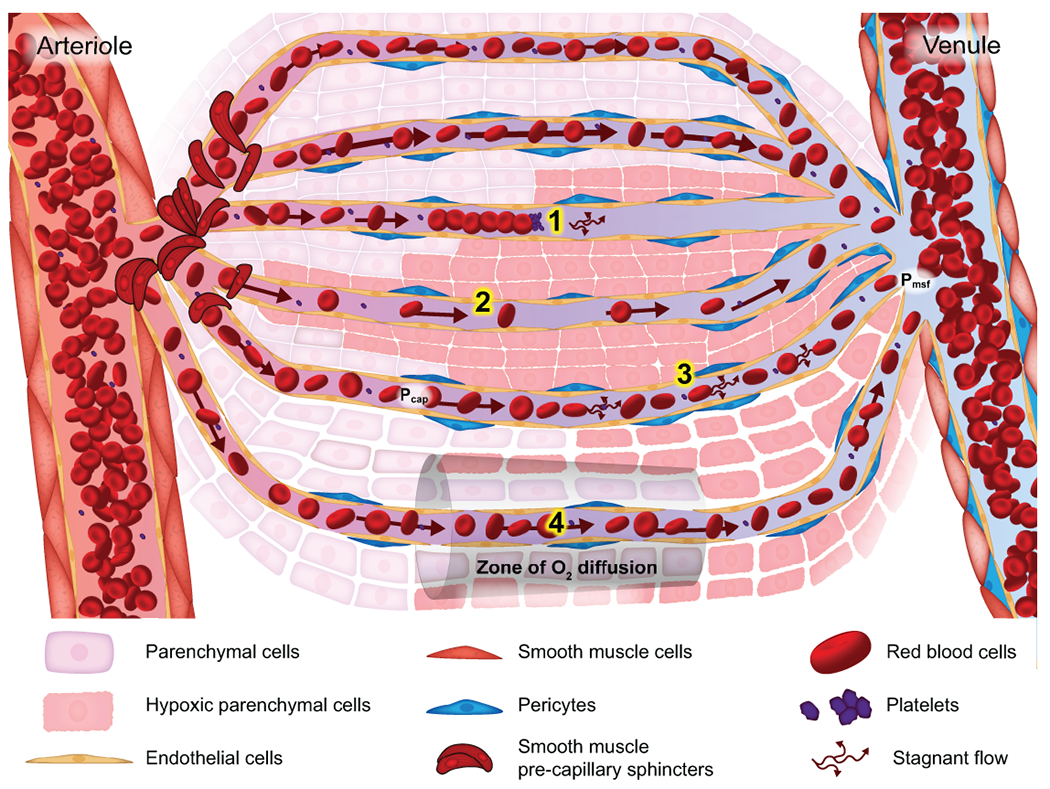
Microcirculatory blood flow and its pathologic alterations. Normal microvascular blood flow is determined by pre-capillary smooth muscle cell tone. Physiologic flow, illustrated by the block arrows, provides oxygen tension sufficient for cellular homeostasis along the entire capillary, as shown in the top 2 capillaries. Pathophysiologic microvascular flow, resulting in inadequate cellular oxygen delivery, may occur through 4 proposed mechanisms. (1) Capillaries may be blocked by platelet microthrombi, leukocyte plugs, non-deformable RBC or pre-capillary SMC dysfunction. Obstruction produces blockage to flow, resulting in cellular hypoxia distal to the obstruction and increased flow heterogeneity, as blood flow will be redirected to open capillaries, creating tissue-level shunting. (2) Hemodilution as seen after cardiopulmonary bypass or massive volume resuscitation, may result in cellular hypoxia despite appropriate flow. (3) Increased venous pressures, from rising CVP, or post-capillary tamponade from increased tissue hydrostatic pressure. This produces and increase in mean systemic filling pressure (Pmsf) with little change in the capillary hydrostatic pressure (Pcap) thereby decreasing driving pressure for capillary flow (Pcap- Pmsf). This results in rapid decrease is O2 tension and cellular hypoxia. (4) Tissue edema, from capillary leak or massive volume resuscitation, increases the distance for O2 to diffuse resulting in cellular hypoxia despite adequate flow. Multiple problems may be present in a single capillary network and their effects may overlap, exacerbating organ dysfunction.
Monitoring of microvascular function is challenging since the common macrovascular variables followed (e.g. blood pressure (BP), heart rate (HR), central venous pressure (CVP) and cardiac index) may have limited relevance. Hemodynamic coherence is a term that describes the extent to which normal macrovascular function correlates with appropriate microvascular function(23). Multiple studies in adults have demonstrated that hemodynamic coherence may be lost in critically ill humans(23, 24). Furthermore, persistent microvascular dysfunction has been associated with organ dysfunction and death in adults(25). Nonetheless, goal directed resuscitation of children in shock typically targets macrovascular hemodynamic goals, assuming that hemodynamic coherence is intact. Yet, specific treatment strategies, such as fluid resuscitation or vasopressor therapy, may improve macrovascular variables (such as CVP) to the detriment of microvascular flow (by decreasing Pcap-Pmsf)(26).
Microcirculatory function can be directly assessed in several ways. Videomicroscopy permits the observation of the number of RBCs and their velocity through individual capillaries (Table 1). These devices utilize dark-field microscopy where polarized light at 550 nm is preferentially scattered by the hemoglobin in RBCs. The scattered light is captured for analysis and produces short video clips of illuminated capillary networks on dark backgrounds. Recent advances in optics have resulted in more precise instruments, from orthogonal polarized spectral (OPS), side-stream dark field (SDF), and incident dark field (IDF) imaging. These devices, notably IDF, are state of the art for current research. Other technologies include laser doppler flowmetry (LDF) which measures the relative velocity of RBC flow through sections of tissue from the doppler shift of laser focused light (700 to 1000 nM). Finally, intravital microscopy and capillaroscopy utilize light microscopy to visualize flow velocity through capillary loops, although in humans, this technology is usually limited to the nailbed.
Table 1:
Types of microvascular imaging devices.
| Assessment technique and reference | Application and site of assessment | Variables measured | Limitations to use | Examples of commercial available devices |
|---|---|---|---|---|
| Videomicroscopy: OPS (27), SDF (28), IDF (29) | Direct viewing of buccal or sublingual microcirculation | TVD, FCD, PPV, PSPV, MFI, MHI | Image acquisition affected by operator skill Image analysis performed offline |
MicroScan CapiScope CytoCam |
| Doppler: LDF (30), LDPM (31) | Microcirculatory functional integrity assessment of superficial tissue | Relative flow, Microvascular Hgb content, Microvascular integrity | Does not differentiate between microvascular segments (i.e. arteriole, capillary and venule) | Blood FlowMeter Vein Finders |
| Microscopy: IVM (32) | Nailbed microscopy | Capillary density | Nailfold area only, unsuitable for critically ill patients | Capiscope |
OPS: Orthogonal polarization spectral imaging, SDF: Side-stream dark field imaging, IDF: Incident dark field imaging, LDF: Laser doppler flowmetry, LDPM: Laser doppler perfusion measurement, IVM: Intravital microscopy, TVD: Total vessel density, FCD: Functional capillary density, PPV: Proportion of perfused vessels, PSPV: Proportion of small perfused vessels, MFI: microcirculatory flow index, MHI: microcirculatory heterogeneity index, Hgb: Hemoglobin
Since the ultimate aim of resuscitation is to restore adequate cellular oxygen delivery, monitoring the microcirculation may provide the most pertinent measures (Table 2)(24). However, microcirculatory function is difficult to assess in critically ill children. A major challenge is clinical feasibility. OPS requires an external light source and is prone to blurring, blockage or difficulty positioning the device. SDF performs better but still requires manual intervention and scoring systems. Both of these methods are sensitive to motion artifacts. Some of the devices are quite bulky, and all require training and expertise to obtain and interpret measurements. Newer devices are smaller and more intuitive with the promise of automated data analysis that may be more amenable to pediatric studies. A more significant concern is the sampling location. Monitoring oral microcirculation may not provide information on perfusion of central, more essential organs(33). Obstructed or heterogeneous flow may be present in one organ and not in another. Another major impediment is the lack of normal pediatric values, complicating interpretation of image analysis in children and infants. Indirect assessments, such as near infrared spectroscopy (NIRS) or arterio-venous PCO2 differences, can provide insight into the global function of the microvasculature relative to metabolism.
Table 2:
Parameters that can be measured to describe the microcirculation. In general, the parameters targeted are assessments of vessel recruitment (FCD, PVD, PPV) or the character of flow through those vessels (MFI, HI, MFV). Not all devices can measure all parameters, and no pediatric normal values have been established.
| Microcirculatory variables | Units | Significance |
|---|---|---|
| Vessel diameter | μm | Small <10μm, Medium 10μm to 20μm, Large >20μm |
| Total vessel density (TVD) | mm/mm2 (1/mm) | Measure of all vessels in a field of view. Total length of vessels (small, medium or large) divided by total area of the field of view. |
| Perfused vessel density (PVD) | mm/mm2 (1/mm) | Proportion of perfused vessels multiplied by total vessel density. |
| Proportion of perfused vessels (PPV) | % vessels perfused | Percentage of perfused vessels in a given image quadrant obtained. Calculated by total length of perfused small vessels divided by total length of small vessels. |
| Microvascular flow index (MFI) | Continuous (normal), sluggish (slow), intermittent or absent (no flow) | Qualitative assessment of flow over quadrants. An average of images obtained. |
| Heterogeneity index (HI) | Arbitrary unit | Measure of flow heterogeneity. Maximum MFI quadrant minus Minimum MFI quadrant divided by mean MFI. |
| De Backer score | Vessels/mm | Alternate for total vessel density, using standard grid format with 3 equally spaced vertical and horizontal lines. Calculated as number of vessels crossing grid lines divided by the total length of the lines. |
| SO2 | % hemoglobin saturation | Tissue oxygenation for a specific organ studied, i.e. kidneys. |
| Microvascular flow velocity (MFV) | μm/sec | Can differentiate speeds between small, medium and large vessels |
FCD: functional capillary density, PVD: Perfused vessel density, PPV: proportion of perfused vessels, MFI: microcirculatory flow index, HI: microcirculatory heterogeneity index, MFV: microvascular flow velocity.
A better understanding of microcirculatory responses may result in more effective treatments for common conditions such as shock. The extent to which microvascular flow and hemodynamic coherence are disrupted by critical illness in children is much less well defined than in adults. The purpose of this review it to gain a deeper understanding of this issue. Therefore, we performed a systematic review to assess the current state of research into microvascular dysfunction in critically ill children and neonates.
Methods
Search Strategy.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(34). Our study protocol is registered in the PROSPERO database of systematic reviews, CRD42019117993 (Supplementary Table 1). We began our search with the Yale MeSH Analyzer(35), using key articles to refine the search strategy for the concepts critical illness, microcirculation, and children (Supplementary Table 2). In each database, we performed search queries and used an iterative process to translate and refine the searches. All searches were limited to the English language. Searches in Embase and MEDLINE were limited using the human filter. Additional articles were identified by examining other systematic reviews, reference lists, bibliographies, and pre-identified websites such as NIH reporter, conference abstracts (Web of Science), and publicly available internet searches (Google Scholar).
On December 6, 2018, the authors searched PubMed (conception through December 06, 2018), MEDLINE (Ovid MEDLINE ALL 1946 to December 06, 2018), Embase (Ovid Embase 1974 to 2018 December 07), and Web of Science (conception through December 06, 2018). On March 14, 2019, a second search was completed in Ovid MEDLINE ALL, Ovid Embase, PubMed, and Web of Science Core Collection. The search repeated the controlled vocabulary terms and free text terms. Manuscripts identified from these searches were de-duplicated in EndNote X8 (Clarivate Analytics) and uploaded to Covidence(36).
Study selection.
Two independent reviewers (LM, EN) completed title, abstract, and full text screens (Figure 2). The senior author (RP) mediated consensus meetings to resolve discrepancies. Studies were screened for meeting the inclusion criteria: (1) Observational case report, cohort, case control, or clinical trial studies; (2) Studies involving critically ill pediatric patients; and (3) Studies assessing microcirculatory flow. Since a continuum of disease states exist under the definition of critical illness, there is not one single condition that satisfies our query. Our search strategy (Supplemental Table 2) includes many terms associated with critical illness. For including or excluding studies in ambiguous cases, we relied on the primary authors definition or assessment of the study participants’ “critically ill status.” Studies investigating animal or human cellular models as well as those that did not specifically assess microcirculatory flow were excluded.
Figure 2:
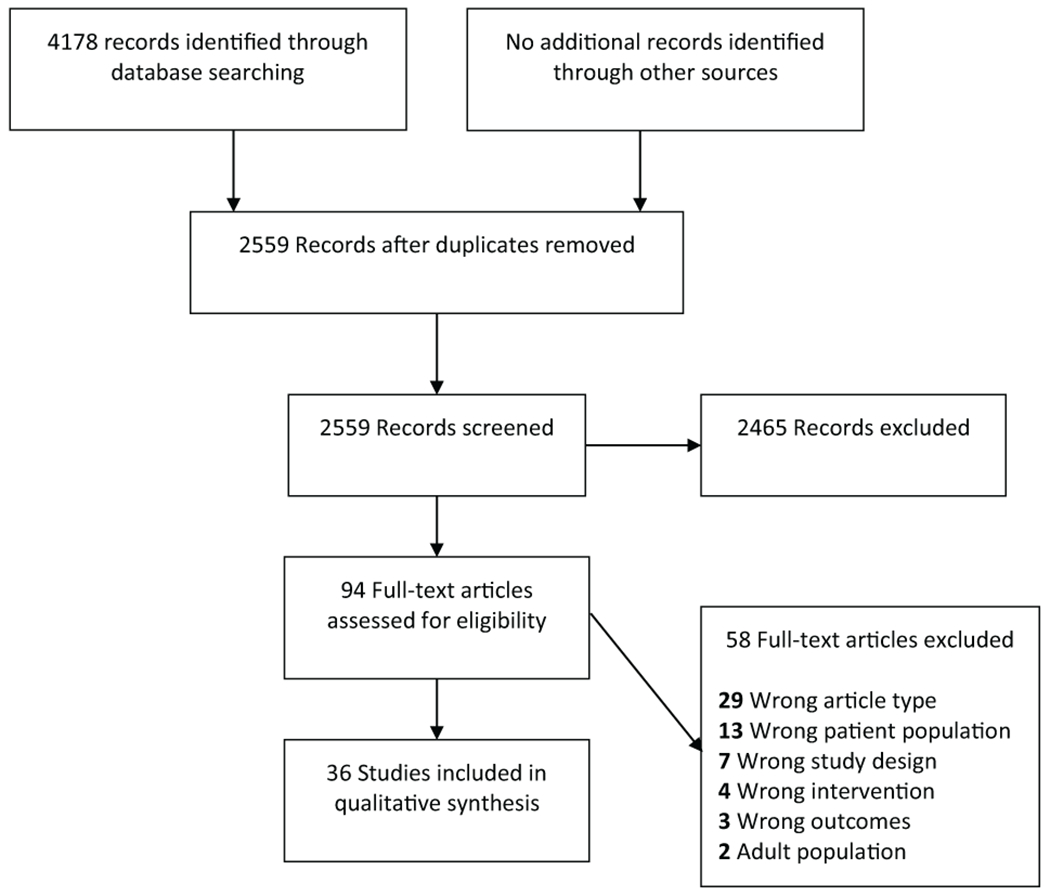
Study flow diagram and selection of eligible articles.
Assessment of the evidence and data abstraction.
All collected studies were reviewed using the QUIPS tool to assess risk of bias in prognostic factors studies from the Cochrane Review Group(37). A quantitative meta-analysis was not performed for several reasons. Relative estimates of effect could not be calculated for case series and studies that lacked control groups. Methodological heterogeneity, such as the type of assessment, the timing of assessment relative to disease onset, and the wide range of patient and treatment factors further prohibited quantification of results. Therefore, all studies were qualitatively analyzed.
Results
A total of 4178 citations were retrieved, pooled and de-duplicated to 2559. Of these, 94 articles met requirements for full text review, after which 36 studies were included in the investigation for evaluation of microcirculatory flow: 7 assessing critically ill children and 29 involving neonates.
Overall, the quality of the evidence for using microvascular assessment to predict outcomes is low. Of the 27 studies included, 8 rated as low, 18 as medium and 1 as high quality (Supplemental Table 3). One limitation of feasibility and generalizability was study design, as only a single randomized controlled trial involving 12 total neonates was identified(55). All other studies were observational trials or case reports. Studies assessed had low numbers of subjects, and most lacked control groups. Only 20 of the 36 studies included control patients, and 28 of the 36 studies involved 40 or less subjects. In all cases, primary data could not be completely reviewed. High subject dropout rate, lack of standardized of normal values, timing and method of assessment, parameters measured and comparators further downgraded the quality of the available evidence.
We identified 7 studies of microcirculation in critically ill children (Table 3). Of these, three investigated microcirculatory flow in sepsis and found decreased MFI and reduced functional capillary density (FCD) and proportion of perfused vessels (PPV), using OPS or SDF with sublingual or buccal mucosa sampling(38–40, 43). These variables correlated with increased inflammatory markers and improved over time with the patient condition. Two studies investigated the effects of cardiopulmonary bypass (CPB) on microcirculatory flow. CPB appeared to minimally alter baseline microvascular parameters during the procedure(42), but did negatively affect induced hyperemic response, while measuring microvascular vasodilation with cutaneous vascular conductance, intraoperatively(44). A single study investigated children after cardiac arrest and demonstrated decreased flow and perfused vessel density (PVD), which improved as patient condition recovered(41). Finally, a study using SDF sublingual sampling and investigating all-comers to the PICU found decreased MFI, PPV and PVD that correlated with low BP and mixed venous saturation and improved after macrocirculatory normalization(32).
Table 3:
Studies of microvascular function in critically ill children.
| Study | Design | Critically ill patients | Control patients | Method of assessment | Site of assessment | Quality Assessment | Major findings |
|---|---|---|---|---|---|---|---|
| Top, et al, 2011(38) | POC | 15 Survivors 3 Non-survivors |
0 | OPS | Buccal mucosa | Medium | Children who died from septic shock had initially increased then decreased FCD (3.2 cm/cm3, 0.8-3.8, to 1.9 cm/cm3, 1.0-2.1) compared to controls (1.7 cm/cm3, 0.8-3.4, to 4.3 cm/cm3, 2.1-6.9). FCD and MFI remained low despite correction of heart rate and blood pressure. |
| Paize, et al 2012(39) | POC | 20 | 40 | SDF | Sublingual mucosa | Medium | Children with severe meningococcal disease have significant reduction in MFI, PPV, & PVD that correlated with elevated markers of endothelial origin and predicted ventilatory support requirements. MFI and PPV normalized pre-extubation. |
| da Luz Caixeta, et al, 2013(40) | Case report | 2 | 0 | SDF | Sublingual mucosa | Low | Children with Dengue shock were found to have low capillary flow velocity, PVD, PPV and MFI. Patient 2 had increased PPV on day 2 but low flow resulting in persistent tissue hypoperfusion. |
| Buijs, et al, 2014(41) | POC | 11 Survivors 9 Non-survivors |
20 | SDF | Buccal mucosa | Medium | Children receiving hypothermia after cardiac arrest had decreased PVD NS, PPV NS, MFI NS, and MFI S that were associated with mortality risk. Perfusion improves after re-warming. Improvement in microcirculatory parameters coincided with improvements in arterial lactate and O2 tension. |
| Scolletta, et al, 2016(42) | POC | 17 Acyanotic 7 Cyanotic |
0 | SDF | Sublingual mucosa | Medium | Children undergoing congenital heart surgery with cardiopulmonary bypass had generally stable TVD, PVD, and PPV for acyanotic lesions and lower TVD, PVD and PPV for cyanotic lesions. There was weak inverse correlation between red blood cell storage time and MFI. |
| Gonzalez, et al, 2017(43) | POC | 18 | 0 | SDF | Sublingual mucosa | Medium | In children admitted to the intensive care unit for any reason, MFI, HI, PPV, & PVD correlate positively with systolic arterial pressure, arterial O2 tension, temperature and central venous saturation and negatively with central venous pressure and serum lactate. |
| Ugenti, et al, 2018(44) | Cross sectional observational study | 61 Cyanotic 39 Acyanotic |
0 | LDPM | Forehead skin | Low | Microvascular skin flow is similar in patients with cyanotic and acyanotic heart disease. Local thermal hyperemia response was blunted while patients were on cardiopulmonary bypass. |
POC: prospective observational cohort study, OPS: Orthogonal polarization spectral imaging, MFI: microvascular flow index, FCD: functional capillary density, SDF: Side-stream dark field imaging, CD: capillary density, PPV: proportion of perfused vessels, PVD: perfused vessel density, NS: Non-small vessels (Ø 11–100 μm), S: Small vessels (Ø ≤ 10 μm), HI: heterogeneity index, LDPM: laser Doppler perfusion monitoring.
See supplemental table 3 for details of quality assessment.
We identified, 29 studies involving neonates, with 20 studies relating microcirculatory flow to critical illness (Table 4) and 9 studies relating microcirculatory flow to gender or post-natal age (Supplementary Table 4). The 20 studies investigating microcirculatory changes in neonates included 300 unique patients of which 94 were full term, 82 were preterm and 124 had no gestational age reported. Included studies related flow to a variety of etiologies of critical illness, including acute respiratory distress syndrome (ARDS), sepsis and hypoxic ischemic encephalopathy (HIE, 4 studies each), cardiopulmonary bypass (3 studies) and congenital diaphragmatic hernia, anemia, hypotension and patent ductus arteriosus (a single study each).
Table 4:
Studies of microvascular function in critically ill neonates.
| Study | Design | Critically ill patients | Control patients | Method of assessment | Site of assessment | Quality assessment | Major findings |
|---|---|---|---|---|---|---|---|
| Pöschl, et al, 1994(45) | POC | 12 | 21 Healthy 7 Non-septic |
LDF | Back, thigh, heel skin | Medium | Infants with sepsis have reactive hyperemia compared to controls and changes occur before C-reactive protein or leukocyte count increase. |
| Martin, et al., 2001(46) | POC | 12 | 20 | LDF | Dorsal hand skin | Low | Infants with sepsis have reactive hyperemia (by post-occlusive perfusion measurement) that correlates with increased IL-6, IL-8 and TNF compared to controls. |
| Genzel-Boroviczény, et al., 2004(47) | POC | 13 | 0 | OPS | Upper arm skin | Low | Blood transfusion improved FVD in critically ill preterm anemic neonates but did not affect vessel diameter, RBC velocity or flow. |
| Top, et al., 2009(48) | POC | 14 | 10 | OPS | Buccal mucosa | Medium | FCD is significantly lower in neonates with ARDS before ECMO compared with control patients. FCD increased significantly after ECMO decannulation. |
| Weidlich, et al., 2009(49) | POC | 17 | 4 | OPS | Upper inner arm skin | Medium | FSVD declined in infants with infection before lab findings. |
| Hiedl, et al., 2010(50) | POC | 13 | 12 | SDF | Inner upper arm skin | Medium | FVD was lower in infants with hemodynamically significant PDA but same as control group after PDA closure. |
| Top, et al., 2011(51) | POC | 8 | 0 | OPS | Buccal mucosa | Low | Inhaled nitric oxide improves FCD in the buccal mucosa of infants with hypoxemic ARDS. |
| Top, et al, 2012(52) | POC | 21 | 7 | OPS | Buccal mucosa | Medium | FCD increased slightly but MFI and HI did not change after initiation of veno-arterial ECMO in neonates with cardiopulmonary failure in comparison to ventilated controls. |
| Ergenekon, et al., 2013(53) | POC | 7 | 7 | SDF | Axilla skin | Medium | Infants treated with TH had lower MFI and decreased flow velocity that normalized with re-warming. |
| Schwepcke, et al., 2013(54) | POC | 10 | 11 | SDF | Right arm skin | Low | Within the first 6 hours of life, hypotensive infants have higher FVD. FVD did not respond to medical management and normalized by 12 hours after birth. |
| Buijs, et al., 2014(55) | POC | 21 | 7 | SDF | Buccal mucosa skin | Low | Infants with CDH requiring dopamine had lower PPV and MFI. All microcirculatory variables in hypotensive neonates were lower than normal newborn controls. Inotropes corrected macrovascular but not microvascular indices. |
| Dehaes, et al., 2014(56) | POC | 10 | 17 | FDNIRS, DCS | Left, middle, and right frontal regions | High | Infants with HIE undergoing TH had a lower cerebral blood flow in comparison to control infants or post-TH. |
| Ishiguro, et al., 2014(57) | Case report | 1 | 0 | LDF | Forehead and foot skin | Low | A neonate in septic shock had decreased skin blood flow and MAP despite inotropes but flow and MAP did increase with blood transfusion. |
| Nussbaum, et al., 2015(58) | POC | 40 | 15 | SDF | Ear conch skin | Medium | Infants undergoing cardiopulmonary bypass had decreased MFI, PVD and vessel glycocalyx width. Values were similar to control infants within 24 hours. |
| Fredly, et al., 2016(59) | POC | 28 | 0 | LDPM, CAVM, DRS | Chest skin | Medium | Neonates with HIE and high C-reactive protein have lower FVD compared to infants with HIE and low C-reactive protein. Flow and heterogeneity were not significantly different. |
| Fredly, et al., 2016(60) | POC | 28 | 25 | LDPM, CAVM, DRS | Chest skin | Medium | Infants with HIE undergoing TH had a lower capillary flow velocity and increased FCD, HI and O2 extraction. Values were similar to control infants after re-warming. |
| Neunhoeffer, et al., 2016(61) | POC | 20 | 30 | LDF, tissue spectrometry | Placed above both kidneys | Medium | Infants undergoing cardiopulmonary bypass that developed AKI had decreased renal oxygen saturation and higher renal blood flow and renal resistive indices. |
| Troiani, et al., 2017(62) | POC | 12 ARDS 7 Cardiac |
28 | LDF | Back of forearm skin | Medium | Infants with ARDS had decreased skin microcirculatory flow velocity and reserve compared to infants with cardiac abnormalities or those without ARDS. |
| Puchwein-Schwepcke, et al, 2018(63) | RCT | 6 | 6 | SDF | Inner right arm skin | Medium | Infants randomized to high CO2 tension group had reduced FVD and increased medium/large versus small vessel distribution. |
| Neunhoeffer, et al., 2018(64) | POC | 14 HLHS 14 TGA |
0 | LDF, tissue spectrometry | Right forehead skin | Medium | Relative cerebral blood flow did not change in infants before and after heart surgery requiring cardiopulmonary bypass despite differences in cerebral oxygen extraction. |
POC: prospective observational cohort study, LDF: Laser doppler flowmetry, TNF: Tumor necrosis factor, OPS: Orthogonal polarization spectral imaging, FVD: Function vessel density, SDF: side-stream dark-field imaging, PDA: Patent ductus arteriosus, FCD: Functional capillary density, PARDS: Pediatric acute respiratory distress syndrome, ECMO: Extra-corporeal membranous oxygenation, FSVD: Functional small vessel density, MFI: microvascular flow index, HI: heterogeneity index, CDH: Congenital diaphragmatic hernia, FDNIRS: Frequency-domain near infrared spectroscopy, DCS: Diffuse correlation spectroscopy, HIE: hypoxic ischemic encephalopathy, TH: therapeutic hypothermia, LDPM: Laser doppler perfusion measurement, CAVM: computer-assisted video microscopy, DRS: Diffuse reflectance spectroscopy, AKI: Acute kidney injury, CHD: congenital heart disease, RCT: randomized clinical trial, HLHS: Hypoplastic left heart syndrome, TGA: Transposition of the great arteries.
See supplemental table 3 for details of quality assessment.
Neonates with sepsis demonstrated a decreased hyperemia response(45, 46), skin flow(57) and functional vessel density(49). Reactive hyperemia response refers to skin perfusion time, measuring velocity of red blood cells with laser doppler, pre and post-arterial occlusion. Vessels in neonates with sepsis took longer to establish perfusion in the venules post-occlusive stimulus as opposed to healthy newborns. Similarly, neonates with ARDS demonstrated decreased functional vessel density (FVD)(48, 51, 63) as evidenced by OPS and SDF imaging on skin or mucosal surfaces. Reduced flow and vessel density parameters were detected in neonates with congenital heart disease(50) and those undergoing CPB(58, 61), although the cerebral circulation was not affected allowing oxygen delivery to the brain to be preserved(64). Neonates undergoing therapeutic hypothermia for HIE demonstrated decreased flow parameters that improved with rewarming(53, 56, 60). Additionally, decreased vessel density was associated with a rise in systemic inflammatory markers(59). Among a general population of critically ill neonates, FVD improved after blood transfusion(47). However, neither flow nor vessel density was improved upon initiation of veno-arterial ECMO(52). Neonates who are hypotensive immediately after birth have altered vessel density that does not respond to resuscitation but improves within 12 hours(54). Finally, neonates with congenital diaphragmatic hernia had altered flow and vessel density that did not respond to inotrope therapy(55).
The 9 studies relating microcirculatory flow to gender or post-natal age are not discussed because they did not separate cohorts based on diagnosis or disease severity, rendering analysis on those parameters impossible(65–73).
Discussion
The vascular system is responsible for the delivery of O2 and nutrients to cells, a task so critical it has been dubbed “the organ of the intensivist”(74). Despite this significance, our review has revealed a paucity of studies on microcirculatory blood flow in critically ill children, with only 502 patients assessed across 27 studies. The settings of these studies and disease severity of their participants, demonstrate that assessment of microcirculatory function in critically ill children and neonates may be difficult but is possible. Analysis of the collected literature is challenging due to great variability in the patient populations, diagnoses, treatment factors and assessment timepoints. Despite these limitations, several important conclusions may be drawn on the nature and implications of microcirculatory changes in severely ill children and neonates.
The prevalence of microcirculatory dysfunction in children remains unknown. In adults, more than 15% of intensive care patients will have dysfunctional microcirculatory flow, and that its presence correlates with mortality(75, 76). Based on the studies we reviewed, microcirculatory irregularities appear to be more prevalent among critically ill pediatric patients despite a broad range of ages and conditions. Gonzalez and colleagues conducted the most comprehensive study of microvascular dysfunction in general PICUs(43). However, definitive conclusions from this study are difficult to draw due to low enrollment of only 18 eligible children, including the exclusion of multiple critically ill as well as generally well patients. The exact prevalence of microvascular dysfunction and its consequences is not known because studies lacked control groups, as well as the lack of pediatric normative data for microcirculatory variables and low PICU mortality rates(77). More worrisome, multiple studies demonstrated that microcirculatory dysfunction correlated with biochemical markers of tissue perfusion inadequacy, such as lactate and interleukins, but not with macrovascular parameters, implying loss of hemodynamic coherence(41, 43, 78). Across similar disease states, several studies of demonstrate more significant microcirculatory dysfunction in children and neonates compared to adults(38, 43), suggesting this may be a more significant problem in younger patients.
The effects of common therapies on microcirculatory dysfunction are more clear. Six studies in children and neonates demonstrated that interventions to normalize macrovascular parameters did not correct microvascular flow in the setting of inotropic therapy(38, 57), therapeutic hypothermia(41, 53) and ECMO(52). These findings are especially concerning since patients are considered for such therapies due to inadequate DO2, yet their application may not correct the underlying microvascular defects. Such differences are likely explained by variations in the device used and timing and location of assessments. In instances of rapid clinical decline, it is difficult to tease out the effects of multiple simultaneous interventions. Many of the studies in neonates used LDF instead of videomicroscopy, making direct comparisons between patient populations impossible, as in the comparison of inotrope use and blood transfusion with sampling via LDF versus SDF (47,49). The variable parameters assessed and outcome measures render more general conclusions untenable. These results are especially significant given the finding of the recent ANDROMEDA-SHOCK trial showing improved mortality in peripheral perfusion rather than lactate directed resuscitation in adults with septic shock(79). The collected literature indicate that microvascular dysfunction is common and likely underappreciated in the NICU and PICU and may have significant clinical consequences and treatment implications.
Few studies provide some insight into the effects of various therapies on the microcirculation. In neonates with respiratory distress syndrome, the application of inhaled nitric oxide improved perfused vessel density(51). Well known for its pulmonary vasodilatory effects, nitric oxide has multiple extra-pulmonary actions(80). In the only randomized control trial identified, permissive hypercapnia, a therapeutic approach commonly used in neonatal RDS, was shown to decrease vessel density(63). Similarly, neonates with congenital diaphragmatic hernia, treated with dopamine to correct HR and BP, continued to have reduced flow and vessel density(55). These findings have important clinical relevance since inotropic infusions commonly applied to treat shock may, in some instances, compromise end organ tissue perfusion. Defining how the microcirculation changes respond to common therapies and the ultimate impact on tissue perfusion and outcomes is an urgent research priority.
There are limitations to this investigation. As we have noted, there are multiple methods to assess the microcirculation that are not directly comparable. Standardization of techniques and measurements, as well an agreement on age-specific normal values, are essential to advancing our understanding of microvascular function. Without standard normal ranges for specific ages, body sizes and genders, the work done to assess the microcirculatory flow at the capillary level is not generalizable to the neonatal and pediatric ICU settings. More trials with greater subject numbers are needed to validate a method, timing and location of assessment. The limited number of studies, low quality of evidence and diagnostic and methodologic heterogeneity precluded a quantitative meta-analysis. Our review may further be limited by the potential impact of publication bias, as studies not finding a correlation between critical illness and disruption of microvascular flow are less likely to be published. Although we attempted to identify all relevant studies, it is possible that qualifying studies were inadvertently omitted from this systematic review. Despite these challenges, we felt it was important to try to understand the range of clinical settings in which microvascular monitoring is feasible and potentially informative. In fact, technology has sufficiently advanced to allow clinical researchers to begin to explore how patient and treatment factors alter the microcirculation of our patients. Multiple ongoing studies aim to address some of these concerns.
Conclusion
Adequate microvascular blood flow is essential for the maintenance of cells and organ function. Homeostasis relies on hemodynamic coherence, where microcirculatory flow is mirrored by macrovascular parameters. Despite logistic challenges, microvascular flow may be measured with laser Doppler flowmetry or videomicroscopy. In critically ill children, microvascular blood flow may be disrupted, and hemodynamic coherence may be lost. The timing, severity and clinical consequences of these changes are not well defined. More research is required to better understand how microvascular variables can be monitored in critically ill children, define age appropriate normal values, assess responses to common interventions and ultimately, define its impact on PICU morbidity and mortality.
Supplementary Material
Footnotes
This work was performed at Yale University
No authors have any financial disclosures.
No reprints will be ordered.
Copyright form disclosure: The authors have disclosed that they do not have any potential conflicts of interest
Article Tweet: #microcirculation in #PedsICU: can it be measured and is it relevant? A new #PedCritCareMed systematic review by @ricpierce @LMaitoza @EitanNee @YaleMed
Ow.ly/article link needed, Figure1 link needed
References
- 1.Huxley VH, Kemp SS. Sex-specific characteristics of the microcirculation. In: Advances in experimental medicine and biology; 2018. p. 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol 1968;37(2):244–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 2005;12(1):33–45. [DOI] [PubMed] [Google Scholar]
- 4.Krogh A The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 1919;52(6):457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Crit Care 2016;20(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotran RS, Majno G. A LIGHT AND ELECTRON MICROSCOPIC ANALYSIS OF VASCULAR INJURY*. Annals of the New York Academy of Sciences 1964;116(3):750–764. [DOI] [PubMed] [Google Scholar]
- 7.Pierce RW, Giuliano JS, Pober JS. Endothelial cell function and dysfunction in critically ill children. Pediatriacs 2017;140(1):e20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnovsky MJ. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol 1967;35(1):213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weibel ER. THE STRUCTURAL CONDITIONS FOR OXYGEN SUPPLY TO MUSCLE CELLS: THE KROGH CYLINDER MODEL. The Journal of Experimental Biology 2013;216(22):4135. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmann JA, Eid A, Spicer G, et al. Spectral contrast optical coherence tomography angiography enables single-scan vessel imaging. Light: Science & Applications 2019;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508(7494):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronicki RA, Penny DJ, Anas NG, et al. Cardiopulmonary Interactions. Pediatr Crit Care Med 2016;17(8 Suppl 1):S182–193. [DOI] [PubMed] [Google Scholar]
- 13.Lücker A, Secomb TW, Weber B, et al. The Relation Between Capillary Transit Times and Hemoglobin Saturation Heterogeneity. Part 1: Theoretical Models. Front Physiol 2018;9:420–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peppiatt CM, Howarth C, Mobbs P, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006;443(7112):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood–brain barrier. Nature 2010;468:557. [DOI] [PubMed] [Google Scholar]
- 16.Sava P, Cook IO, Mahal RS, et al. Human Microvascular Pericyte Basement Membrane Remodeling Regulates Neutrophil Recruitment. Microcirculation 2015;22(1):54–67. [DOI] [PubMed] [Google Scholar]
- 17.Chang WG, Andrejecsk JW, Kluger MS, et al. Pericytes modulate endothelial sprouting. Cardiovasc Res 2013;100(3):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Østergaard L, Granfeldt A, Secher N, et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiologica Scandinavica 2015;59(10):1246–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Top APC, Tasker RC, Ince C. The microcirculation of the critically ill pediatric patient. Critical care (London, England) 2011;15(2):213–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koning NJ, Simon LE, Asfar P, et al. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. American Journal of Physiology-Heart and Circulatory Physiology 2014;307(7):H967–H975. [DOI] [PubMed] [Google Scholar]
- 21.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Critical Care Medicine 1999;27(7):1369–1377. [DOI] [PubMed] [Google Scholar]
- 22.Kuiper JW, Tibboel D, Ince C. The vulnerable microcirculation in the critically ill pediatric patient. Critical Care 2016;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdem O, Kuiper JW, Tibboel D. Hemodynamic coherence in critically ill pediatric patients. Best Pract Res Clin Anaesthesiol 2016;30(4):499–510. [DOI] [PubMed] [Google Scholar]
- 24.Ince C Hemodynamic coherence and the rationale for monitoring the microcirculation. Critical care (London, England) 2015;19 Suppl 3(Suppl 3):S8–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakker J Lactate levels and hemodynamic coherence in acute circulatory failure. Best Pract Res Clin Anaesthesiol 2016;30(4):523–530. [DOI] [PubMed] [Google Scholar]
- 26.Bennett VA, Vidouris A, Cecconi M. Effects of Fluids on the Macro- and Microcirculations. Crit Care 2018;22(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groner W, Winkelman JW, Harris AG, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 1999;5(10):1209–1212. [DOI] [PubMed] [Google Scholar]
- 28.Ince C The microcirculation is the motor of sepsis. Crit Care 2005;9 Suppl 4:S13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchings S, Watts S, Kirkman E. The Cytocam video microscope. A new method for visualising the microcirculation using Incident Dark Field technology. Clin Hemorheol Microcirc 2016;62(3):261–271. [DOI] [PubMed] [Google Scholar]
- 30.Riva C, Ross B, Benedek GB. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Invest Ophthalmol 1972;11(11):936–944. [PubMed] [Google Scholar]
- 31.Micheels J, Alsbjorn B, Sorensen B. Laser doppler flowmetry. A new non-invasive measurement of microcirculation in intensive care? Resuscitation 1984;12(1):31–39. [DOI] [PubMed] [Google Scholar]
- 32.Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Practice and Research: Clinical Rheumatology 2005;19(3 SPEC. ISS.):437–452. [DOI] [PubMed] [Google Scholar]
- 33.Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: A quantitative analysis*. 2009;37(11):2875–2881. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementThe PRISMA Statement. Annals of Internal Medicine 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 35.HK GN, L. W The Yale MeSH Analyzer. 2019. [cited Available from: http://mesh.med.yale.edu/
- 36.Bramer W, Bain P. Updating search strategies for systematic reviews using EndNote. Journal of the Medical Library Association : JMLA 2017;105(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 1. Guidelines for guidelines. Health Research Policy and Systems 2006;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Top APC, Ince C, de Meij N, et al. Persistent low microcirculatory vessel density in nonsurvivors of sepsis in pediatric intensive care*. 2011;39(1):8–13. [DOI] [PubMed] [Google Scholar]
- 39.Paize F, Sarginson R, Makwana N, et al. Changes in the sublingual microcirculation and endothelial adhesion molecules during the course of severe meningococcal disease treated in the paediatric intensive care unit. Intensive Care Med 2012;38(5):863–871. [DOI] [PubMed] [Google Scholar]
- 40.Caixeta DM, Fialho FM, Azevedo ZM, et al. Evaluation of sublingual microcirculation in children with dengue shock. Clinics 2013;68(7):1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buijs EA, Verboom EM, Top AP, et al. Early microcirculatory impairment during therapeutic hypothermia is associated with poor outcome in post-cardiac arrest children: a prospective observational cohort study. Resuscitation 2014;85(3):397–404. [DOI] [PubMed] [Google Scholar]
- 42.Scolletta S, Marianello D, Isgrò G, et al. Microcirculatory changes in children undergoing cardiac surgery: a prospective observational study. British Journal of Anaesthesia 2016;117(2):206–213. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez R, Lopez J, Urbano J, et al. Evaluation of sublingual microcirculation in a paediatric intensive care unit: prospective observational study about its feasibility and utility. BMC Pediatr 2017;17(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ugenti V, Romano AC, Tibirica E. Microvascular endothelial dysfunction during cardiopulmonary bypass in surgery for correction of cyanotic and acyanotic congenital heart disease. Microvascular Research 2018;120:55–58. [DOI] [PubMed] [Google Scholar]
- 45.Pöschl JMB, Weiss T, Fallahi F, et al. Reactive hyperemia of skin microcirculation in septic neonates. Acta Paediatrica 1994;83(8):808–811. [DOI] [PubMed] [Google Scholar]
- 46.Martin H, Olander B, Norman M. Reactive Hyperemia and Interleukin 6, Interleukin 8, and Tumor Necrosis Factor-α in the Diagnosis of Early-Onset Neonatal Sepsis. Pediatrics 2001;108(4):e61–e61. [DOI] [PubMed] [Google Scholar]
- 47.Genzel-Boroviczény O, Christ F, Glas V. Blood Transfusion Increases Functional Capillary Density in the Skin of Anemic Preterm Infants. Pediatric Research 2004;56:751. [DOI] [PubMed] [Google Scholar]
- 48.Top AP, Ince C, van Dijk M, et al. Changes in buccal microcirculation following extracorporeal membrane oxygenation in term neonates with severe respiratory failure. Crit Care Med 2009;37(3):1121–1124. [DOI] [PubMed] [Google Scholar]
- 49.Weidlich K, Kroth J, Nussbaum C, et al. Changes in Microcirculation as Early Markers for Infection in Preterm Infants—An Observational Prospective Study. Pediatric Research 2009;66:461. [DOI] [PubMed] [Google Scholar]
- 50.Hiedl S, Schwepcke A, Weber F, et al. Microcirculation in Preterm Infants: Profound Effects of Patent Ductus Arteriosus. The Journal of Pediatrics 2010;156(2):191–196. [DOI] [PubMed] [Google Scholar]
- 51.Top AP, Ince C, Schouwenberg PH, et al. Inhaled nitric oxide improves systemic microcirculation in infants with hypoxemic respiratory failure. Pediatr Crit Care Med 2011;12(6):e271–274. [DOI] [PubMed] [Google Scholar]
- 52.Top AP, Buijs EA, Schouwenberg PH, et al. The Microcirculation Is Unchanged in Neonates with Severe Respiratory Failure after the Initiation of ECMO Treatment. Crit Care Res Pract 2012;2012:372956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ergenekon E, Hirfanoğlu I, Beken S, et al. Peripheral microcirculation is affected during therapeutic hypothermia in newborns. Archives of Disease in Childhood - Fetal and Neonatal Edition 2013;98(2):F155–F157. [DOI] [PubMed] [Google Scholar]
- 54.Schwepcke A, Weber FD, Mormanova Z, et al. Microcirculatory mechanisms in postnatal hypotension affecting premature infants. Pediatric Research 2013;74:186. [DOI] [PubMed] [Google Scholar]
- 55.Buijs EAB, Reiss IKM, Kraemer U, et al. Increasing Mean Arterial Blood Pressure and Heart Rate With Catecholaminergic Drugs Does Not Improve the Microcirculation in Children With Congenital Diaphragmatic Hernia: A Prospective Cohort Study. Pediatric Critical Care Medicine 2014;15(4):343–354. [DOI] [PubMed] [Google Scholar]
- 56.Dehaes M, Aggarwal A, Lin P-Y, et al. Cerebral Oxygen Metabolism in Neonatal Hypoxic Ischemic Encephalopathy during and after Therapeutic Hypothermia. Journal of Cerebral Blood Flow & Metabolism 2014;34(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishiguro A, Sakazaki S, Itakura R, et al. Peripheral blood flow monitoring in an infant with septic shock. Pediatrics International 2014;56(5):787–789. [DOI] [PubMed] [Google Scholar]
- 58.Nussbaum C, Haberer A, Tiefenthaller A, et al. Perturbation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. J Thorac Cardiovasc Surg 2015;150(6):1474–1481 e1471. [DOI] [PubMed] [Google Scholar]
- 59.Fredly S, Nygaard CS, Skranes JH, et al. Cooling Effect on Skin Microcirculation in Asphyxiated Newborn Infants with Increased C-Reactive Protein. Neonatology 2016;110(4):270–276. [DOI] [PubMed] [Google Scholar]
- 60.Fredly S, Fugelseth D, Nygaard CS, et al. Noninvasive assessments of oxygen delivery from the microcirculation to skin in hypothermia-treated asphyxiated newborn infants. Pediatric Research 2016;79:902. [DOI] [PubMed] [Google Scholar]
- 61.Neunhoeffer F, Wiest M, Sandner K, et al. Non-invasive measurement of renal perfusion and oxygen metabolism to predict postoperative acute kidney injury in neonates and infants after cardiopulmonary bypass surgery. British Journal of Anaesthesia 2016;117(5):623–634. [DOI] [PubMed] [Google Scholar]
- 62.Troiani S, Cardona A, Milioni M, et al. Evidence of impaired microvascular dilatation in preterms with acute respiratory distress syndrome. International Journal of Cardiology 2017;241:83–86. [DOI] [PubMed] [Google Scholar]
- 63.Puchwein-Schwepcke AF, Schottmayer K, Mormanova Z, et al. Permissive Hypercapnia Results in Decreased Functional Vessel Density in the Skin of Extremely Low Birth Weight Infants. Front Pediatr 2018;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neunhoeffer F, Hofbeck M, Schlensak C, et al. Perioperative Cerebral Oxygenation Metabolism in Neonates with Hypoplastic Left Heart Syndrome or Transposition of the Great Arteries. Pediatric Cardiology 2018;39(8):1681–1687. [DOI] [PubMed] [Google Scholar]
- 65.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating Pressure-Passivity Is Common in the Cerebral Circulation of Sick Premature Infants. Pediatric Research 2007;61:467. [DOI] [PubMed] [Google Scholar]
- 66.Stark MJ, Clifton VL, Wright IMR. Sex-Specific Differences in Peripheral Microvascular Blood Flow in Preterm Infants. Pediatric Research 2008;63:415. [DOI] [PubMed] [Google Scholar]
- 67.Stark MJ, Clifton VL, Wright IMR. Microvascular flow, clinical illness severity and cardiovascular function in the preterm infant. Archives of Disease in Childhood - Fetal and Neonatal Edition 2008;93(4):F271. [DOI] [PubMed] [Google Scholar]
- 68.Ishiguro A, Sekine T, Kakiuchi S, et al. Skin and subcutaneous blood flows of very low birth weight infants during the first 3 postnatal days. The Journal of Maternal-Fetal & Neonatal Medicine 2010;23(6):522–528. [DOI] [PubMed] [Google Scholar]
- 69.Ishiguro A, Sekine T, Suzuki K, et al. Changes in Skin and Subcutaneous Perfusion in Very-Low-Birth-Weight Infants during the Transitional Period. Neonatology 2011;100(2):162–168. [DOI] [PubMed] [Google Scholar]
- 70.Stark MJ, Hodyl NA, Wright IMR, et al. The influence of sex and antenatal betamethasone exposure on vasoconstrictors and the preterm microvasculature. The Journal of Maternal-Fetal & Neonatal Medicine 2011;24(10):1215–1220. [DOI] [PubMed] [Google Scholar]
- 71.Dyson RM, Palliser HK, Lakkundi A, et al. Early microvascular changes in the preterm neonate: a comparative study of the human and guinea pig. Physiological reports 2014;2(9):e12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dyson RM, Palliser HK, Latter JL, et al. A Role for H2S in the Microcirculation of Newborns: The Major Metabolite of H2S (Thiosulphate) Is Increased in Preterm Infants. PLOS ONE 2014;9(8):e105085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corbisier de Meautsart C, Dyson RM, Latter JL, et al. Influence of sympathetic activity in the control of peripheral microvascular tone in preterm infants. Pediatric Research 2016;80:793. [DOI] [PubMed] [Google Scholar]
- 74.Wetzel R The Intensivist’s System. Crit Care Med 1993;21(9):341–342. [DOI] [PubMed] [Google Scholar]
- 75.Vellinga NAR, Boerma EC, Koopmans M, et al. International Study on Microcirculatory Shock Occurrence in Acutely Ill Patients*. 2015;43(1):48–56. [DOI] [PubMed] [Google Scholar]
- 76.Scorcella C, Damiani E, Domizi R, et al. MicroDAIMON study: Microcirculatory DAIly MONitoring in critically ill patients: a prospective observational study. Annals of intensive care 2018;8(1):64–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burns JP, Sellers DE, Meyer EC, et al. Epidemiology of death in the PICU at five U.S. teaching hospitals*. Critical care medicine 2014;42(9):2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Top AP, Ince C, de Meij N, et al. Persistent low microcirculatory vessel density in nonsurvivors of sepsis in pediatric intensive care. Crit Care Med 2011;39(1):8–13. [DOI] [PubMed] [Google Scholar]
- 79.Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical TrialEffect on Septic Shock Mortality of Resuscitation Targeting Peripheral Perfusion vs Serum Lactate LevelsEffect on Septic Shock Mortality of Resuscitation Targeting Peripheral Perfusion vs Serum Lactate Levels. JAMA 2019;321(7):654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proceedings of the American Thoracic Society 2006;3(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


