Abstract
Background
A growing body of evidence shows differences in the prevalence of cardiometabolic syndrome (CMS) and dementia based on gender and ethnicity. However, there is a paucity of information about ethnic- and gender-specific CMS effects on brain age. We investigated the different effects of CMS on brain age by gender in Korean and British cognitively unimpaired (CU) populations. We also determined whether the gender-specific difference in the effects of CMS on brain age changes depending on ethnicity.
Methods
These analyses used de-identified, cross-sectional data on CU populations from Korea and United Kingdom (UK) that underwent brain MRI. After propensity score matching to balance the age and gender between the Korean and UK populations, 5759 Korean individuals (3042 males and 2717 females) and 9903 individuals from the UK (4736 males and 5167 females) were included in this study. Brain age index (BAI), calculated by the difference between the predicted brain age by the algorithm and the chronological age, was considered as main outcome and presence of CMS, including type 2 diabetes mellitus (T2DM), hypertension, obesity, and underweight was considered as a predictor. Gender (males and females) and ethnicity (Korean and UK) were considered as effect modifiers.
Results
The presence of T2DM and hypertension was associated with a higher BAI regardless of gender and ethnicity (p < 0.001), except for hypertension in Korean males (p = 0.309). Among Koreans, there were interaction effects of gender and the presence of T2DM (p for T2DM*gender = 0.035) and hypertension (p for hypertension*gender = 0.046) on BAI in Koreans, suggesting that T2DM and hypertension are each associated with a higher BAI in females than in males. In contrast, among individuals from the UK, there were no differences in the effects of T2DM (p for T2DM*gender = 0.098) and hypertension (p for hypertension*gender = 0.203) on BAI between males and females.
Conclusions
Our results highlight gender and ethnic differences as important factors in mediating the effects of CMS on brain age. Furthermore, these results suggest that ethnic- and gender-specific prevention strategies may be needed to protect against accelerated brain aging.
Keywords: Brain age, Cardiometabolic syndrome, Gender, Ethnicity
Background
Aging is an important risk factor for cognitive impairment and dementia [1]. As aging progresses, brain atrophy also occurs at a mean volume reduction rate of 0.5% per year after the age of 40 [2, 3]. Age-related brain atrophy is referred to as the brain age. Cardiometabolic syndrome (CMS), syndrome X, metabolic syndrome, and cardiometabolic dysfunction, composed of type 2 diabetes mellitus (T2DM), hypertension, and obesity, are critical modifiable risk factors for cognitive impairment. There is also a growing body of evidence that CMS has deleterious effects on brain atrophy [4] even in non-demented population [5, 6]. Previous studies have suggested that CMS may accelerate brain aging.
Several cross-sectional studies have shown differences in brain volume between males and females in cognitively unimpaired (CU) populations [7–9]. Previously, changes in brain age or atrophy were shown to occur differently depending on gender [3, 10, 11]. Additionally, previous studies based on Hispanic or Korean populations suggested that CMS-associated brain atrophy was more extensive or prominent in females than in males [6, 10]. However, considering the differences in the prevalence of CMS and dementia between Korean and European populations, it would be reasonable to hypothesize that there might be a difference in the gender-specific relationship between CMS and brain age between Koreans and Europeans.
Previous studies have analyzed various morphological features on brain magnetic resonance imaging (MRI), including cortical thickness [12], regional gray matter volume [11], and white matter hyperintensity [13] and integrity [14], and investigated the impact of CMS on brain structure in aging populations. Recently, various machine learning approaches have been developed to begin accurate prediction of brain age using the aforementioned brain imaging features [15–18] and provide a new metric called brain age index (BAI) to measure how old the brain age is compared to the chorological age at MRI scan. The difference between the predicted brain age using a deep learning-based algorithm and the chronological age is called the BAI, which explains how much older or younger an individual brain appears compared to the current age. A positive BAI is a novel marker of an older brain and has been shown to predict compromised brain health [19], earlier mortality [20], and cognitive impairment [21, 22].
In the present study, we investigated the different effects of CMS on BAI with respect to the sex of CU populations from Korea and United Kingdom (UK). Next, we determined whether CMS affects gender-specific BAI differently according to ethnicity. Considering that there are differences in incidence of CMS and cortical atrophy by gender and ethnicity, we hypothesized that there might be differences in the effects of CMS on the BAI in relation to gender and ethnicity.
Methods
Study populations
We enrolled CU participants aged ≥ 45 years from the Health Promotion Center of Samsung Medical Center (Seoul, Korea) who underwent a comprehensive health screening exam from September 1, 2008, to October 31, 2019. A total of 8227 eligible candidates underwent a full medical examination, which included cognitive assessment and 3.0-Tesla MRI, including high-resolution T1-weighted MRI, as part of a standard screening for dementia. The medical examination procedure used for the participants has been previously described [23]. We excluded participants who had any of the following conditions: 728 participants with missing data on years of education or Mini-Mental State Examination (MMSE) score [24]; 509 participants with significant cognitive impairment defined by MMSE scores below the 16th percentile in age-, gender-, and education-matched norms or through an interview conducted by a qualified neurologist; 312 participants with severe cerebral white matter hyperintensities (deep white matter ≥ 2.5 cm and caps or band ≥ 1.0 cm) or structural lesions such as territorial infarction, lobar hemorrhage, brain tumor, and hydrocephalus; 542 participants with missing information on DM, hypertension, or body mass index (BMI); and 377 participants with unreliable analyses of cortical thickness due to head motion, blurred MRI, inadequate registration to a standardized stereotaxic space, misclassification of tissue type, or inexact surface extraction. Finally, 5759 participants (3042 males and 2717 females) were included in this study.
Similar data for people of British ancestry was obtained from the UK Biobank (UKB, http://www.ukbiobank.ac.uk), a population-based prospective cohort study of approximately 500,000 people in the UK [https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001779, https://www.nature.com/articles/s41586-018-0579-z]. Of these participants, approximately 40,000 attended an additional visit during which MRI brain imaging data was collected in addition to other health-related data [https://www.nature.com/articles/s41467-020-15948-9]. We included non-Hispanic White adults only in the present study. We excluded participants with a self-reported or hospital record-based history of dementia, Parkinson’s disease, or other central nervous system-related diseases. Finally, 9903 (4736 males and 5167 females) UKB participants were included after applying the inclusion/exclusion criteria and after random selection of a smaller subset of participants for brain imaging data processing.
The institutional review board of the Samsung Medical Center approved this study and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants in the Health Promotion Center of Samsung Medical Center. Anonymous and deidentified data from the UKB was used for analysis and, therefore, the present study was exempted from obtaining informed consent.
Measurement of cardiometabolic syndrome
For populations from the Health Promotion Center at the Samsung Medical Center, a health screening exam was conducted by a well-trained medical professional using standardized protocols. Baseline workup included blood tests (complete blood cell count, liver/kidney/thyroid function test, and tumor markers), urine analyses, abdominal sonography, chest radiography, electrocardiogram, pulmonary function test, and gastroduodenoscopy. We classified each CMS component using the following criteria: T2DM was defined as a diagnostic history of T2DM or current use of any anti-diabetic medication; hypertension was defined as a diagnostic history of hypertension or current use of any antihypertensive medication; obesity and underweight were defined using the cut-off for BMI calculated by weight (kilograms)/height (meters) squared at the first visit. According to a previous study [10], populations with BMI < 18.5 kg/m2 were labeled as underweight, and those with BMI ≥ 27.5 kg/m2 were labeled as obese.
For populations from the UKB, the classification of T2DM, hypertension, and obesity was determined based on a combination of a touchscreen-based questionnaire, a verbal interview, and linked hospital records. Specifically, T2DM was defined as either self-reported T2DM, a doctor’s diagnosis of T2DM (Data Field 2443), patients who were taking insulin (Data Fields 6177, 6153), or a hospital data-linked record of an individual with a diagnosis of T2DM. Hypertension was defined as either self-reported hypertension (Data-Field 20,002) or a hospital data-linked record of having a primary or secondary diagnosis of hypertension (Data-Fields 41,202, 41,204, 41,203, and 41,205). BMI was calculated using weight and height measurements in the same way as the Samsung Medical Center data. Populations with a BMI < 18.5 kg/m2 were categorized as underweight, whereas those with BMI ≥ 35 kg/m2 were categorized as obese [25].
Acquisition of brain MRI
All Korean populations underwent a 3D volumetric brain MRI scan. An Achieva 3.0-Tesla MRI scanner (Philips, Best, the Netherlands) was used to acquire 3D T1 Turbo Field echo (TFE) MRI data using the following imaging parameters: sagittal slice thickness, 1.0 mm with 50% overlap; no gap; repetition time of 9.9 ms; echo time of 4.6 ms; flip angle of 8; and matrix size of 240 × 240 pixels reconstructed to 480 × 480 over a field view of 240 mm.
In the UKB populations, brain MRI scans were obtained at one of the three assessment sites using a 3.0 Tesla Siemens Skyra MRI Scanner. Among the six brain imaging modalities acquired was a T1-weighted, sagittal 3D magnetization prepared rapid gradient echo (MPRAGE) scan. The following imaging parameters were used in this T1-weighted acquisition: inversion time of 880 ms; repetition time of 2000 ms; 1 × 1 × 1 mm3 voxel size; 208 × 256 × 256 matrix size; and SENSE factor (R) of 2.0 [Miller, 2016: https://www.nature.com/articles/nn.4393].
Image processing and cortical surface extraction
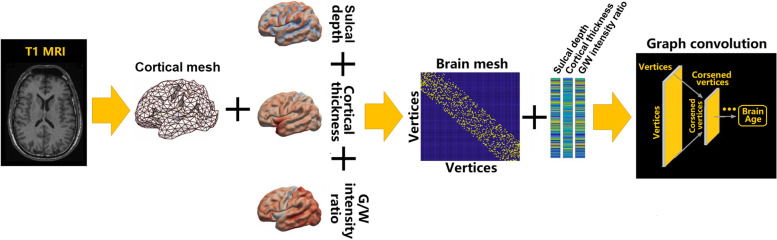
T1-weighted MRI scans from the Health Promotion Center in Korea and the UKB were used to reconstruct the inner and outer cortical boundaries using the CIVET pipeline developed at the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET). Cortical morphology was quantitatively characterized by measuring cortical thickness, sulcal depth, and gray/white intensity ratio [26] on the cortical surface at 81,924 vertices (163,840 polygons). These features were further resampled to the surface template using the transformation obtained in the surface registration to allow for inter-subject comparisons.
Development of prediction model for relative brain age
As illustrated in Fig. 1, we did not use topology-varying surfaces because of the nature of the graph convolutional networks (GCN) model used in this study. Rather, we considered the cortical morphological changes that occur in relation to brain size and gyrification using cortical thickness, volume, and sulcal depth. The GCN employed in our study requires identical graph/mesh structures for all individual inputs, whereas the features of the nodes/vertices can vary. Another advantage of the topology-kept surface model is that surface nodes are registered across all individuals such that anatomical information is shared.
Fig. 1.
The graph-based convolutional network for brain age prediction
Brain age index
After calculating the predicted brain age for each subject, we further calculated a metric that reflected the subject's relative brain health status, called the BAI. BAI was initially measured by subtracting the true brain age from the predicted brain age [27]. Due to regression dilution [28], however, it is also possible that regression models bias the predicted brain age toward the mean, underestimating the age of older subjects and overestimating the age of younger subjects [29]. When deriving the BAI, this bias must be corrected using a strategy introduced in other studies [15, 28]. We defined the new BAI as the difference between the individual BAI and the expected BAI (measurement fitted over the entire sample set using the regression model and leave-one-out cross-validation). The BAI was corrected such that the BAIs of the whole dataset analyzed became unbiased across all age ranges.
Propensity score matching
Propensity score matching was performed to minimize the differences in the demographics and cardiometabolic factors between the UK and KOR participants. The propensity score was obtained using multivariable logistic regression based on age, gender, T2DM, hypertension, and obesity. A total of 5541 KOR participants were matched with 9903 UK participants based on propensity scores using the 1:2 nearest-neighbor matching algorithm with caliper of 0.1. A good balance was achieved between the KOR and UK participants, with all standardized mean differences (age, gender, T2DM, hypertension, and obesity) below 0.1 after matching.
Statistical analysis
Independent t-tests and chi-squared tests were used to compare continuous and categorical variables, respectively.
To explore the association between the presence of each CMS component and brain age in females and males among the Korean and UK populations, we performed a linear regression analysis with the presence of T2DM, hypertension, obesity, and underweight as covariates. To assess whether the association between the presence of each CMS component and brain age might differ by gender in the Korean and the UK populations, we performed linear regression analyses by adding each two-way interaction term (the presence of each CMS component*gender) to covariates in Korean and UK populations after controlling for the other CMS components. To assess whether the association between the presence of each CMS component and brain age might differ by gender and ethnicity, we performed linear regression analyses with the addition of each three-way interaction term (the presence of each CMS component*gender*ethnicity) to covariates after controlling for the other CMS components.
False discovery rate (FDR) correction was conducted for all statistical analyses to control for p-values, and q-values were obtained after FDR correction. All reported p-values and q-values were two-sided and the significance level was set at 0.05. All analyses were performed using R version 4.3.0 (Institute for Statistics and Mathematics, Vienna, Austria; www.R-project.org).
Results
Demographics of cognitively unimpaired populations in the UK and Korea
After propensity score matching, the demographic characteristics of the two ethnic datasets were similar (Table 1). Among the 5541 Korean populations, there were 2599 (46.9%) females and 2942 (53.1%) males. Among the 9903 UK populations, there were 5167 (52.2%) females and 4736 (47.8%) males. There were some differences in mean age (64.0 and 63.6 years, p < 0.001), female ratio (46.9 and 52.2%, p < 0.001), and the presence of T2DM (17.3 and 9.8%, p < 0.001), hypertension (42.7% and 40.6%, p = 0.011), obesity (10.7 and 7.9%, p < 0.001), and underweight (1.8 and 0.6%, p < 0.001) between Koreans and participants from the UK.
Table 1.
Demographics of populations from UK Biobank and Health Promotion Center in Korea
| Variables | Korea | UK | p-value | ||||
|---|---|---|---|---|---|---|---|
| Females ( n = 2599) | Males ( n = 2942) | Total ( n = 5541) | Females ( n = 5167) | Males ( n = 4736) | Total ( n = 9903) | ||
| Age (years)a | 63.2 ± 6.9 | 64.7 ± 6.5 | 64.0 ± 6.7 | 63.4 ± 7.1 | 63.8 ± 7.4 | 63.6 ± 7.2 | 0.007 |
| Hypertension (n, %)b | 965 (37.1%) | 1402 (47.7%) | 2367 (42.7%) | 1813 (35.1%) | 2208 (46.6%) | 4021 (40.6%) | 0.1 |
| T2DM (n, %)b | 273 (10.5%) | 683 (23.2%) | 956 (17.3%) | 365 (7.1%) | 602 (12.7%) | 967 (9.8%) | < 0.001 |
| BMI (kg/m2)a | 23.5 ± 2.9 | 24.5 ± 2.6 | 24.0 ± 2.8 | 26.8 ± 5.2 | 27.7 ± 4.4 | 27.2 ± 4.9 | < 0.001 |
| Obesity (n, %) | 227 (8.7%) | 336 (12.4%) | 593 (10.7%) | 450 (8.7%) | 336 (7.1%) | 786 (7.9%) | < 0.001 |
| Underweight (n, %)b | 70 (2.7%) | 32 (1.1%) | 102 (1.8%) | 49 (0.9%) | 7 (0.1%) | 56 (0.6%) | < 0.001 |
Propensity score matching was performed to balance the age and gender between the Korean and UK populations, and 9903 out of 17,791 populations in the UK and 5541 out of 5759 populations in Korea were selected for the present study
Abbreviations: BMI, body mass index; T2DM, type2 diabetes mellitus; UK, United Kingdom
aDistribution of age and BMI was compared between the populations of UK and Korea using independent t tests
bDistribution of education level and presence of hypertension, T2DM, obesity, and underweight between the populations of UK and Korea were tested using the chi-squared test
Effects of cardiometabolic syndrome components on brain age index
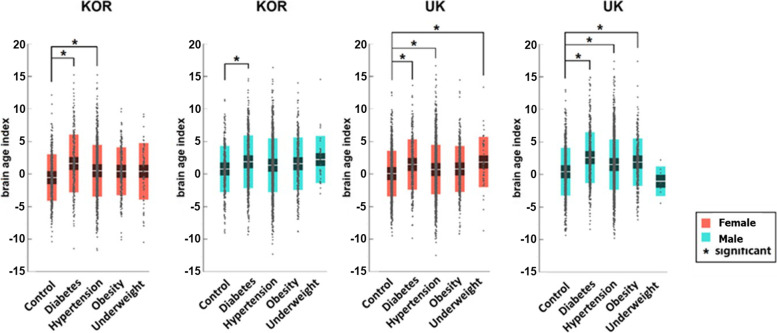
As shown in Fig. 2, DM was associated with increased BAI for all participants, regardless of gender and ethnicity (q < 0.001 in the four groups) (Table 2). Hypertension was associated with a significantly higher BAI for all participants (q < 0.001), except for Korean males (q = 0.309). Obesity significantly increased the BAI for UK males (q = 0.004). Being underweight increased the BAI significantly only for UK females (q = 0.002).
Fig. 2.
BAI distribution between groups regarding gender and ethnicity for healthy participants and participants with different CMS. BAI = 0 indicates that the chronological age is the same as the predicted brain age, with higher values indicating an older-appearing brain than chronological age. Asterisk symbol (*) indicates the following: q-values, FDR-corrected p-values, are lower than 0.05. BAI, brain age index; Kor, Korea; UK, United Kingdom; CMS, cardiometabolic syndrome
Table 2.
Brain age index in controls and four CMS component groups
| Ethnicity/gender | CMS | Age | BAI | β (SE) | q-value* |
|---|---|---|---|---|---|
| Korean females | Control | 61.6 ± 8.1 | − 0.67 ± 3.57 | ||
| T2DM | 66.9 ± 9.5 | 1.51 ± 4.44 | 1.73 (0.25) | < 0.001 | |
| Hypertension | 66.0 ± 8.0 | 0.39 ± 3.97 | 0.80 (0.20) | < 0.001 | |
| Obesity | 64.8 ± 8.5 | 0.31 ± 3.69 | 0.26 (0.94) | 0.347 | |
| Underweight | 62.8 ± 7.6 | 0.30 ± 4.34 | 0.77 (0.48) | 0.145 | |
| Korean males | Control | 64.1 ± 8.2 | 0.70 ± 3.55 | ||
| T2DM | 65.5 ± 8.6 | 1.82 ± 4.05 | 0.88 (0.19) | < 0.001 | |
| Hypertension | 65.3 ± 8.4 | 1.29 ± 4.13 | 0.19 (0.18) | 0.309 | |
| Obesity | 63.4 ± 7.7 | 1.52 ± 4.03 | 0.37 (0.23) | 0.144 | |
| Underweight | 67.5 ± 10.7 | 2.17 ± 3.63 | 1.24 (0.72) | 0.171 | |
| UK females | Control | 62.0 ± 7.2 | − 0.17 ± 3.50 | ||
| T2DM | 64.4 ± 7.4 | 1.22 ± 3.87 | 1.08 (0.20) | < 0.001 | |
| Hypertension | 65.2 ± 6.6 | 0.45 ± 3.79 | 0.49 (0.13) | < 0.001 | |
| Obesity | 61.5 ± 6.7 | 0.53 ± 3.54 | 0.32 (0.19) | 0.088 | |
| Underweight | 63.8 ± 7.0 | 1.60 ± 3.87 | 1.67 (0.53) | 0.002 | |
| UK males | Control | 61.5 ± 7.5 | 0.25 ± 3.66 | ||
| T2DM | 65.8 ± 6.8 | 2.42 ± 3.90 | 1.55 (0.17) | < 0.001 | |
| Hypertension | 65.6 ± 6.8 | 1.35 ± 3.86 | 0.73 (0.13) | < 0.001 | |
| Obesity | 62.9 ± 7.2 | 1.72 ± 3.63 | 0.65 (0.22) | 0.004 | |
| Underweight | 68.0 ± 6.3 | − 1.21 ± 2.28 | − 1.69 (1.42) | 0.235 |
Values of age and BAI are presented as mean ± standard deviation
Abbreviations: BAI, brain age index; CMS, cardiometabolic syndrome; T2DM, type2 diabetes mellitus; UK, United Kingdom
*q-values, FDR-corrected p-values, were obtained using a linear regression analysis with the presence of hypertension, T2DM, obesity, and underweight as covariates in each group (Korean females, Korean males, UK females and UK males)
Interactive effects of cardiometabolic syndrome components on brain age index in relation to gender and ethnicity
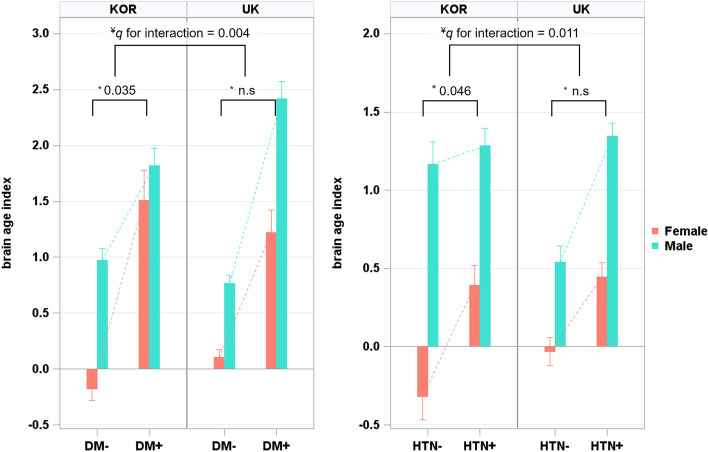
We also investigated the interaction of the presence of each CMS component and gender with BAI in Koreans and participants from the UK. Among Koreans, there were interactions between T2DM and gender with BAI (q = 0.035) and between hypertension and gender with BAI (q = 0.046), suggesting that the effects of T2DM and hypertension on BAI were more prominent in females than in males (Table 3, Fig. 3). Among British participants, however, there were no interactions of any CMSs and gender with BAI (q range 0.098 to 0.203, Table 3, Fig. 3). In fact, there were interactions between gender and ethnicity for T2DM (q = 0.004) and hypertension (q = 0.011, Table 3, Fig. 3).
Table 3.
Interaction effect on the difference in BAI between participants with each CMS and those with control
| Ethnicity | Two-way interaction (each CMS component*gender) | β (SE) | q-value* |
| Korea | T2DM*gender | 0.84 (0.32) | 0.035 |
| Hypertension*gender | 0.61 (0.27) | 0.046 | |
| Obesity*gender | − 0.11 (0.36) | 0.770 | |
| Underweight*gender | − 0.82 (0.85) | 0.450 | |
| UK | T2DM*gender | − 0.52 (0.26) | 0.098 |
| Hypertension*gender | − 0.26 (0.18) | 0.203 | |
| Obesity*gender | − 0.37 (0.28) | 0.191 | |
| Underweight*gender | 3.51 (1.50) | 0.076 | |
| Three-way interaction(each CMS component*gender*ethnicity) | β (SE) | q-value¥ | |
| Both | T2DM*gender*ethnicity | 1.36 (0.41) | 0.004 |
| Hypertension*gender*ethnicity | 0.89 (0.32) | 0.011 |
Abbreviations: BAI, brain age index; CMS, cardiometabolic syndrome; T2DM, type2 diabetes mellitus; UK, United Kingdom
*q-values, FDR-corrected p-values, were obtained using linear regression analyses with adding each two-way interaction term (the presence of each CMS component*gender) to covariates in Korean and UK populations after controlling for the other CMS components
¥q-values, FDR-corrected p-values, were obtained using linear regression analyses with additionally adding each three-way interaction term (the presence of each CMS component*gender*ethnicity) to covariates after controlling for the other CMS components
Fig. 3.
Ethnic- and gender-specific difference in BAI between participants with and without T2DM and HTN. Values depicted in the bar plot represent the mean of BAI, and values depicted in the error bar represent the standard error of mean. BAI = 0 indicates that the chronological age is the same as the predicted brain age, with higher values indicating an older-appearing brain than chronological age. Asterisk (*) symbol indicates the following: q-values, FDR-corrected p-values, were obtained using linear regression analyses with adding each two-way interaction term (the presence of each CMS component*gender) to covariates in Korean and UK populations after controlling for the other CMS components. Yen (¥) symbol indicates the following: q-values, FDR-corrected p-values, were obtained using linear regression analyses with additionally adding each three-way interaction term (the presence of each CMS component*gender*ethnicity) to covariates after controlling for the other CMS components. BAI, brain age index; Kor, Korea; UK, United Kingdom; T2DM, type 2 diabetes mellitus; HTN, hypertension
Discussion
In the present study, we systematically investigated the different effects of CMS on BAI in relation to gender and ethnic differences in a large sample of Korean and UK CU populations. Our major findings are as follows: first, among Koreans, the effects of DM and hypertension on BAI were higher in females than in males. This indicated interaction effects of gender and the presence of T2DM and hypertension on BAI in Korean population. Second, among the UK population, there were no differences in the effects of T2DM and hypertension on BAI between males and females. Overall, there was evidence that ethnicity modified the gender-specific relationship of T2DM and hypertension with BAI. Taken together, our findings suggest that CMS exerts different effects on brain age depending on gender and ethnicity. Therefore, ethnic- and gender-specific prevention strategies may be necessary to protect against accelerated brain aging.
We found that the presence of T2DM and hypertension was associated with a higher BAI regardless of gender and ethnicity, except for hypertension in Korean males. T2DM and hypertension are well-known risk factors for brain atrophy, which is an important indicator of brain age [30]. T2DM may have deleterious effects on the brain via various mechanisms, including cerebrovascular complications, glucose toxicity due to insulin resistance, and chronic inflammation [31]. Similarly, the positive association between hypertension and BAI may be due to several possible mechanisms including cerebral hypoperfusion, micro- and macrovascular damage in white matter, and cerebral microinfarcts [32, 33].
Our first major finding was that Korean females suffered more deleterious effects of T2DM and hypertension on brain age than Korean males. Although the underlying mechanisms for the gender-specific effects of T2DM and hypertension on brain age are not fully understood, our findings might be related to the complex effects of biological and socioeconomic differences [34]. Previous studies have suggested that hypertension exerts worse effects on multiple organs in females than in males. This was attributed to differences in sex hormones. There are stronger associations of hypertension with autonomic dysfunction in females than in males [35]. Similar associations are witnessed in the cases of microalbuminuria [36] and reduction of heart function [37]. In particular, females uniquely experience menopause transition, which might accelerate cardiometabolic syndrome, brain aging, or cognitive impairment via several mechanisms including changes in the availability of estrogen [38], estrogen receptor activity, and/or estrogen-regulated neural networks [39]. Specifically, estrogen deficiency in postmenopausal females leads to inflammatory process and vasoconstriction via the dysfunction of the renin-angiotensin system [40–42]. In fact, a growing body of evidence shows that menopause has a deleterious impact on cognitive function, which may contribute to the higher prevalence of dementia in females than in males [43–47]. Additionally, several studies have shown that females tend to maintain lifestyles that are more favorable for brain health, with overall lower drinking and smoking rates [48–51]. Therefore, our findings might be also related to differences in stress, alcohol consumption, smoking, and dietary habits according to gender.
Our second major finding was that there were interactive effects of the presence of T2DM and hypertension, gender, and ethnicity on BAI. That is, unlike Koreans, there were no differences in the effects of T2DM and hypertension on BAI between males and females in the UK population. A few studies have found that brain age differs depending on ethnicity [52, 53]. However, gender- and ethnicity-specific differences in the effects of T2DM and hypertension on brain age have not been extensively investigated. These differences might be related to the biological and socioeconomic differences between the Korean and UK populations. Previously, a higher frequency of CMS in Korean populations compared to Europeans has been explained by the fact that Asians have higher visceral fat and lower subcutaneous fat than Europeans with the same BMI [54]. This might increase the complication rate of CMS because visceral fat has more deleterious effects on arteriosclerosis and brain health than subcutaneous fat. In fact, Asians are more likely to develop CMS-related complications such as coronary artery disease [55], stroke [56], dementia [57–59], or mortality [60]. Another potential explanation is that there were fewer differences in socioeconomic status and years of education between males and females in the UK than in Korea. Further studies are needed to investigate the pathomechanism to explain gender differences according to ethnicity.
Limitations
The strengths of our study include a large sample size from two different cohorts, well-balanced clinical demographics between the two cohorts after propensity score matching, and a novel measurement of brain age that is sensitive to neurodegenerative changes in gray and white matter. However, our study had some limitations. First, owing to the cross-sectional study design, the causal or temporal relationship of the effects of CMS on brain aging was not determined. In addition, the study did not have information on exposure time or changes in the status of risk factors. Longitudinal studies are needed to identify whether there are dynamic differences from mid-adulthood to old age in the effects of risk factors on brain aging in elderly CU populations. Second, differences in subject selection methods between the two cohorts may have confounded the ethnic differences. Third, the presence of T2DM and hypertension was determined through the patient history of diagnosis or medications and not through clinical examinations including measurement of systolic blood pressure and fasting glucose. Fourth, obesity was defined using BMI only rather than waist circumstance, which has relevance to central obesity according to the International Diabetes Federation. We also used different criteria for diagnosing obesity according to ethnicity-specific BMI. This was, however, done to abide by a previous consensus on the definition of obesity according to ethnicity [25]. Finally, we did not consider the brain pathology markers of Alzheimer’s disease, lacunes, micro-cortical infarcts, and white matter hyperintensities, which can also be associated with brain age. Further studies are needed to identify the effects of CMS on brain aging in relation to the pathophysiological processes. Despite the aforementioned limitations, our study is the first report to compare the gender- and ethnicity-specific effects of CMS on brain age.
Conclusions
In the present study, we highlight gender and ethnic differences in the effects of CMS on brain age. Furthermore, our findings suggest that different measures may be needed to prevent accelerated brain aging by CMS in terms of gender and ethnic differences. In conclusion, CMS exerted different effects on brain age according to the gender and ethnicity of the individuals. Our study shows that it is important to control for T2DM and hypertension to prevent brain aging. Since the effects of T2DM and hypertension on brain age were the largest among Korean females, more careful treatment of these CMS components would be more effective to prevent or mitigate fast brain aging in Korean females. Therefore, ethnic- and gender-specific prevention strategies may be needed to protect against accelerated brain aging.
Acknowledgements
The UK Biobank Resource was accessed through Application Number 25641. We thank UK Biobank study participants and UK Biobank for enabling this resource.
Authors’ contributions
Concept and design: S.H. Kang, S.W. Seo, H. Kim. Acquisition of data: S.H. Kang, S.Y. Kim, Lee, Matloff, Zhao, H. Yoo, J.P. Kim, H.J. Kim, Jahanshad, Oh, Koh, Na, Gallacher, S.W. Seo, H. Kim. Analysis or interpretation of data: S.H. Kang, Liu, S.W. Seo, H. Kim. Drafting of the manuscript: S.H. Kang. Intellectual content: S.H. Kang, M. Liu, Gottesman, S.W. Seo, H. Kim. Statistical analysis: S.H. Kang, M. Liu, H. Yoo. Obtained funding: S.H. Kang, S.W. Seo, H. Kim. Administrative, technical, or material support: S.W. Seo, H. Kim. Supervision: S.W. Seo, H. Kim. The authors read and approved the final manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and Ministry of science and ICT, Republic of Korea (grant number: HU20C0111, HU22C0170); the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A5A2027340, NRF-2022R1F1A1063966); Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.2021–0-02068, Artificial Intelligence Innovation Hub); Future Medicine 20*30 Project of the Samsung Medical Center [#SMX1230081]; the “Korea National Institute of Health” research project(2021-ER1006-02); Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) and grant funded by Korea University Medicine (K2210201); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2022R1I1A1A01056956); the National Institutes of Health grants (P50NS035902, P01NS082330, R01NS046432, R01HD072074; P41EB015922; U54EB020406; U19AG024904; U01NS086090; 003585–00001); and HK funded by Bright Focus Foundation Award (A2019052S).
Availability of data and materials
Anonymized data for our analyses presented in this report are available upon request from the corresponding authors.
Declarations
Ethics approval and consent to participate
Approval was obtained from the ethics committee of Samsung Medical Centre. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients and caregivers.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sung Hoon Kang and Mengting Liu contributed equally to this work.
References
- 1.Wahl D, Solon-Biet SM, Cogger VC, Fontana L, Simpson SJ, Le Couteur DG, et al. Aging, lifestyle and dementia. Neurobiol Dis. 2019;130:104481. doi: 10.1016/j.nbd.2019.104481. [DOI] [PubMed] [Google Scholar]
- 2.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Kim S, Yoo H, Park S, Jang YK, Kim HJ, et al. Trajectories of physiological brain aging and related factors in people aged from 20 to over-80. J Alzheimers Dis. 2018;65:1237–1246. doi: 10.3233/JAD-170537. [DOI] [PubMed] [Google Scholar]
- 4.Söderlund H, Nyberg L, Nilsson LG. Cerebral atrophy as predictor of cognitive function in old, community-dwelling individuals. Acta Neurol Scand. 2004;109:398–406. doi: 10.1111/j.1600-0404.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 5.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotkowski E, Price LR, DeFronzo RA, Franklin CG, Salazar M, Garrett AS, et al. Metabolic syndrome predictors of brain gray matter volume in an age-stratified community sample of 776 Mexican-American adults: results from the genetics of brain structure image archive. Front Aging Neurosci. 2022;14:999288. doi: 10.3389/fnagi.2022.999288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lüders E, Steinmetz H, Jäncke L. Brain size and grey matter volume in the healthy human brain. NeuroReport. 2002;13:2371–2374. doi: 10.1097/00001756-200212030-00040. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, et al. Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cereb Cortex. 2018;28:2959–2975. doi: 10.1093/cercor/bhy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SE, Lee JS, Woo S, Kim S, Kim HJ, Park S, et al. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology. 2019;93:e1045–e1057. doi: 10.1212/WNL.0000000000008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Venkataraman AV, Bai W, Guitton F, Guo Y, Dehghan A, et al. Associations of regional brain structural differences with aging, modifiable risk factors for dementia, and cognitive performance. JAMA Netw Open. 2019;2:e1917257. doi: 10.1001/jamanetworkopen.2019.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi YY, Lee JJ, Choi KY, Seo EH, Choo IH, Kim H, et al. The Aging slopes of brain structures vary by ethnicity and sex: evidence from a large magnetic resonance imaging dataset from a single scanner of cognitively healthy elderly people in Korea. Front Aging Neurosci. 2020;12:233. doi: 10.3389/fnagi.2020.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharf EL, Graff-Radford J, Przybelski SA, Lesnick TG, Mielke MM, Knopman DS, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: the Mayo Clinic Study of Aging. Stroke. 2019;50:3037–3044. doi: 10.1161/STROKEAHA.119.025822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura Y, Shimoji K, Ishikawa J, Matsuo Y, Watanabe S, Takahashi H, et al. Subclinical atherosclerosis, vascular risk factors, and white matter alterations in diffusion tensor imaging findings of older adults with cardiometabolic diseases. Front Aging Neurosci. 2021;13:712385. doi: 10.3389/fnagi.2021.712385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–539. doi: 10.1016/j.neuroimage.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson BA, Bjornsdottir G, Thorgeirsson TE, Ellingsen LM, Walters GB, Gudbjartsson DF, et al. Brain age prediction using deep learning uncovers associated sequence variants. Nat Commun. 2019;10:5409. doi: 10.1038/s41467-019-13163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole JH, Leech R, Sharp DJ. Alzheimer's Disease Neuroimaging Initiative Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77:571–81. doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shokri-Kojori E, Bennett IJ, Tomeldan ZA, Krawczyk DC, Rypma B. Estimates of brain age for gray matter and white matter in younger and older adults: insights into human intelligence. Brain Res. 2021;1763:147431. doi: 10.1016/j.brainres.2021.147431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke K, Gaser C. Ten years of brainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol. 2019;10:789. doi: 10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle N, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23:1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liem F, Varoquaux G, Kynast J, Beyer F, KharabianMasouleh S, Huntenburg JM, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–188. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Franke K, Gaser C. Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease. GeroPsych. 2012;25:235–45. doi: 10.1024/1662-9647/a000074. [DOI] [Google Scholar]
- 23.Lee JS, Shin HY, Kim HJ, Jang YK, Jung NY, Lee J, et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci Rep. 2016;6:24284. doi: 10.1038/srep24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s continuum. Dement Neurocogn Disord. 2019;18:77–95. doi: 10.12779/dnd.2019.18.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JD, Evans AC, Tohka J. T1 white/gray contrast as a predictor of chronological age, and an index of cognitive performance. Neuroimage. 2018;173:341–350. doi: 10.1016/j.neuroimage.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 27.Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 28.Ning K, Zhao L, Matloff W, Sun F, Toga AW. Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep. 2020;10:10. doi: 10.1038/s41598-019-56089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CJ, Moriarty KP, Miller SP, Booth BG, Zwicker JG, Grunau RE, et al. Prediction of brain network age and factors of delayed maturation in very preterm infants. In: Descoteaux M, Maier-Hein L, Franz A, Jannin P, Collins DL, Duchesne S, et al., editors. Medical Image Computing and Computer-Assisted Intervention - MICCAI 2017: 20th International Conference, Quebec City, QC, Canada, September 11–13, 2017, Proceedings, Part I. Cham: Springer; 2017. pp. 84–91. [Google Scholar]
- 30.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7:373–380. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 31.Ninomiya T. Diabetes mellitus and dementia. Curr Diab Rep. 2014;14:487. doi: 10.1007/s11892-014-0487-z. [DOI] [PubMed] [Google Scholar]
- 32.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gąsecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15:547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca WA, Mielke MM, Vemuri P, Miller VM. Sex and gender differences in the causes of dementia: a narrative review. Maturitas. 2014;79:196–201. doi: 10.1016/j.maturitas.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, et al. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension. 2001;37:1351–1356. doi: 10.1161/01.HYP.37.6.1351. [DOI] [PubMed] [Google Scholar]
- 36.Palatini P, Mos L, Santonastaso M, Saladini F, Benetti E, Mormino P, et al. Premenopausal women have increased risk of hypertensive target organ damage compared with men of similar age. J Womens Health (Larchmt) 2011;20:1175–1181. doi: 10.1089/jwh.2011.2771. [DOI] [PubMed] [Google Scholar]
- 37.Lim JG, Shapiro EP, Vaidya D, Najjar SS, Turner KL, Bacher AC, et al. Sex differences in left ventricular function in older persons with mild hypertension. Am Heart J. 2005;150:934–940. doi: 10.1016/j.ahj.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Diaz Brinton R, et al. Female sex and Alzheime’'s risk: the menopause connection. J Prev Alzheimers Dis. 2018;5:225–230. doi: 10.14283/jpad.2018.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chedraui P, Jaramillo W, Pérez-López FR, Escobar GS, Morocho N, Hidalgo L. Pro-inflammatory cytokine levels in postmenopausal women with the metabolic syndrome. Gynecol Endocrinol. 2011;27:685–691. doi: 10.3109/09513590.2010.521270. [DOI] [PubMed] [Google Scholar]
- 41.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 42.Macova M, Armando I, Zhou J, Baiardi G, Tyurmin D, Larrayoz-Roldan IM, et al. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology. 2008;88:276–286. doi: 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2014;142:90–98. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilpi F, Soares ALG, Fraser A, Nelson SM, Sattar N, Fallon SJ, et al. Changes in six domains of cognitive function with reproductive and chronological ageing and sex hormones: a longitudinal study in 2411 UK mid-life women. BMC Womens Health. 2020;20:177. doi: 10.1186/s12905-020-01040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 48.Emslie C, Hunt K, Macintyre S. How similar are the smoking and drinking habits of men and women in non-manual jobs? Eur J Public Health. 2002;12:22–28. doi: 10.1093/eurpub/12.1.22. [DOI] [PubMed] [Google Scholar]
- 49.Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. Am J Drug Alcohol Abuse. 1999;25:239–256. doi: 10.1081/ADA-100101858. [DOI] [PubMed] [Google Scholar]
- 50.Thun M, Peto R, Boreham J, Lopez AD. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21:96–101. doi: 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 51.Cifkova R, Pitha J, Krajcoviechova A, Kralikova E. Is the impact of conventional risk factors the same in men and women? Plea for a more gender-specific approach. Int J Cardiol. 2019;286:214–219. doi: 10.1016/j.ijcard.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 52.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, et al. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Curr Alzheimer Res. 2015;12:632–639. doi: 10.2174/1567205012666150530203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- 55.McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol. 1989;42:597–609. doi: 10.1016/0895-4356(89)90002-4. [DOI] [PubMed] [Google Scholar]
- 56.Eastwood SV, Tillin T, Chaturvedi N, Hughes AD. Ethnic differences in associations between blood pressure and stroke in South Asian and European men. Hypertension. 2015;66:481–488. doi: 10.1161/HYPERTENSIONAHA.115.05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JE, Kim BS, Kim KW, Hahm BJ, Sohn JH, Suk HW, et al. Decline in the incidence of all-cause and Alzheimer’s disease dementia: a 12-year-later rural cohort study in Korea. J Korean Med Sci. 2019;34:e293. doi: 10.3346/jkms.2019.34.e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia. 2017;32:523–532. doi: 10.1016/j.nrl.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Jang J-W, Park JH, Kim S, Lee S-H, Lee S-H, Kim Y-J. Prevalence and incidence of dementia in South Korea: a nationwide analysis of the National Health Insurance Service senior cohort. J Clin Neurol. 2021;17:249–256. doi: 10.3988/jcn.2021.17.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001–2003. J Public Health (Oxf) 2007;29:191–198. doi: 10.1093/pubmed/fdm010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data for our analyses presented in this report are available upon request from the corresponding authors.