Abstract
Genetic heterogeneity poses a great challenge to the understanding and management of acute myeloid leukemia (AML). Knowledge of the IKZF1 mutation in AML specifically is extremely limited. In a previous work, we described the distribution pattern of IKZF1 mutation in AML, but its clinical impact has remained undefined due to the limited number of cases. Herein, we attempt to answer this question in one relatively large cohort covering 522 newly diagnosed AML patients. A total of 26 IKZF1 mutations were found in 20 AML patients (20/522, 3.83%). This condition has a young median age of onset of morbidity (P = 0.032). The baseline characteristics of IKZF1-mutated and wild-type patients were comparable. IKZF1 mutation showed significant co-occurrences with CEBPA (P < 0.001), SF3B1 (P < 0.001), and CSF3R (P = 0.005) mutations, and it was mutually exclusive with NPM1 mutation (P = 0.033). Although IKZF1-mutated AML was more preferably classified into the intermediate-risk group (P = 0.004), it showed one inferior complete remission rate (P = 0.032). AML with high burden of IKZF1 mutation (variant allele frequency > 0.20) showed relatively short overall survival period (P = 0.012), and it was an independent factor for the increased risk of death (hazard ratio, 6.101; 95% CI 2.278–16.335; P = 0.0003). In subgroup analysis, our results showed that IKZF1 mutation conferred poor therapeutic response and prognosis for SF3B1-mutated AML (P = 0.0017). We believe this work improves our knowledge of IKZF1 mutation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-023-00398-y.
Keywords: Acute myeloid leukemia, IKZF1 mutation, Clinical impact
IKZF1 mutation is one rare but recurrent alteration in AML. In a previous work, we described its distribution pattern in AML [1], but the clinical impact of IKZF1 mutation on AML remains undefined. We here address this issue in a cohort of 522 newly diagnosed AML patients (Additional file 1: Fig S1, Patients and methods in supplementary information).
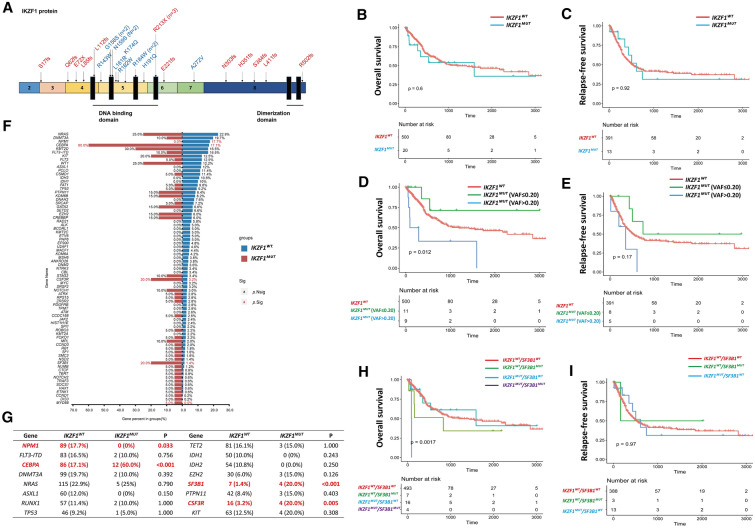
Recurrent IKZF1 mutation, including 12 missense mutations, 4 nonsense mutations, and 10 frame-shift mutations, was found in 20 patients (3.83%). Missense mutation preferred to localize at the exon 5 (91.67%), which mainly influences the DNA binding of IKZF1. A total of 35.7% of nonsense and frame-shift mutations were found to disrupt the DNA-binding domain and caused loss of the dimerization domain, while 64.3% of them only disrupted the dimerization domain (Fig. 1A, Additional file 4: Table S1). As indicated, IKZF1 mutation was recurrent in AML, but its role in AML pathogenesis needed further investigations.
Fig. 1.
IKZF1 mutation in AML. (A) The distribution of IKZF1 mutations, which were identified in our cohort, on the protein. The nonsense or frameshift mutation was marked as red, while the missense mutation was marked as blue. (B, C) The OS (B) and RFS (C) of IKZF1WT and IKZF1MUT groups in our AML cohort. (D, E) The influence of IKZF1 mutation burden on the prognosis of AML was studied, and the OS (D) as well as RFS (E) of IKZF1WT, IKZF1MUT with VAF > 0.20, and IKZF1MUT with VAF ≤ 0.20 groups are shown. (F) The difference of additional mutations distribution in IKZF1WT and IKZF1MUT groups, and the percentage of each gene mutation is exhibited. (G) The distribution of frequent AML-associated gene mutations in IKZF1WT and IKZF1MUT groups, and the count as well as percentage of each gene mutation are shown. (H, I) The prognostic role of combined IKZF1 and SF3B1 mutations on AML was investigated, and the OS (H) as well as RFS (I) of AML with different IKZF1 or SF3B1 mutated status are exhibited
To investigate the features of IKZF1MUT AML, we compared the baseline characteristics of the IKZF1MUT and IKZF1WT groups, and the only difference was found in median age. ELN 2017 prognostic stratification predicted the clinical outcome of AML patients well [2]. Compared to the IKZF1WT group, the IKZF1MUT group showed a higher frequency of patients in the ELN-intermediate-risk group and a lower frequency in the ELN-low-risk and ELN-high-risk groups, but the CR rate in the IKZF1MUT group was significantly lower than that in the IKZF1WT group under our treatment strategy (Table 1). More interestingly, IKZF1MUT patients showed similar OS and RFS with IKZF1WT patients (Fig. 1B, C). Though IKZF1 mutation conferred one disadvantaged therapeutic response for AML patients, overall, it finally did not influence their survival time.
Table 1.
Baseline characteristics of our AML cohort
| Characteristics | IKZF1WT group | IKZF1MUT group | P |
|---|---|---|---|
| N, % of total | 502, 96.2% | 20, 3.8% | |
| Age (years) | 50.0 (11.0–82.0) | 42.5 (15.0–66.0) | 0.032 |
| Gender | |||
| Male (N) | 220 (43.8%) | 10 (50%) | 0.585 |
| Female (N) | 282 (56.2%) | 10 (50%) | |
| Peripheral blood | |||
| White blood cells (109/L) | 11.30 (0.40–484.80) | 17.72 (1.67–120.00) | 0.533 |
| Hemoglobin (g/L) | 83.00 (20.00–204.00) | 92.00 (57.00–148.00) | 0.130 |
| Platelets (109/L) | 51.00 (2.00–565.00) | 63.00 (7.00–917.00) | 0.633 |
| Bone marrow blasts (%) | 59.5 (11.5–98.0) | 59.0 (20.0–96.0) | 0.559 |
| Diagnosis (N) | |||
| De novo AML | 481 (95.8%) | 19 (95.0%) | 0.584 |
| Secondary/therapy-related AML | 21 (4.2%) | 1 (5.0%) | |
| French-American-British (N) | |||
| M0 | 21 (4.2%) | 2 (10.0%) | 0.895 |
| M1 | 27 (5.4%) | 1 (5.0%) | |
| M2 | 170 (33.9%) | 7 (35.0%) | |
| M4 | 101 (20.1%) | 5 (25.0%) | |
| M5 | 161 (32.1%) | 5 (25.0%) | |
| M6 | 11 (2.2%) | 0 (0%) | |
| M7 | 1 (0.2%) | 0 (0%) | |
| Undefine | 10 (2.0%) | 0 (0%) | |
| Cytogenetics (N) | |||
| Normal karyotype | 247 (49.2%) | 10 (50.0%) | 0.944 |
| Complex karyotype | 44 (8.8%) | 2 (10.0%) | 0.693 |
| Monosomal karyotype | 16 (3.2%) | 0 (0%) | 1.000 |
| -5/5q-/monosomy 5 | 21 (4.2%) | 0 (0%) | 1.000 |
| -7/monosomy 7 | 17 (3.3%) | 0 (0%) | 1.000 |
| -17/17p abnormalities | 11 (2.2%) | 0 (0%) | 1.000 |
| Chromosome 3 abnormalities | 16 (3.2%) | 2 (10.0%) | 0.148 |
| Gene fusions (N) | |||
| RUNX1::RUNX1T1 | 65 (12.9%) | 1 (5.0%) | 0.494 |
| CBFB::MYH11 | 36 (7.2%) | 0 (0%) | 0.386 |
| BCR::ABL1 | 9 (1.8%) | 0 (0%) | 1.000 |
| KMT2A rearrangements | 19 (3.8%) | 0 (0%) | 1.000 |
| European Leukemia Net 2017 (N) | |||
| Low | 151 (30.1%) | 2 (10.0%) | 0.004 |
| Intermediate | 212 (42.2%) | 16 (80.0%) | |
| High | 139 (27.7%) | 2 (10.0%) | |
| Complete remission (N) | 394 (78.5%) | 13 (65.0%) | 0.032 |
| No complete remission (N) | 73 (21.5%) | 7 (35.0%) | |
To interpret the contrast phenomena and define the prognostic role of IKZF1 mutation more clearly, we analyzed the influence of its VAF, mutational type, and mutational count on the duration of survival. We performed maximally selective log-rank statistics in OS based on VAF and found that IKZF1MUT patients with a high IKZF1 VAF burden (VAF > 0.20) showed significantly poorer OS than those with low VAF or IKZF1WT, but the RFS did show any statistically significant difference (Fig. 1D, E, Additional file 5: Table S2). We found that neither the type nor the number of mutations influenced OS or RFS in IKZF1MUT patients (Additional file 1: Fig S1C–F). In this way, a high burden of IKZF1 mutation might predict poor prognosis in AML.
To exclude the impact of additional factors on OS, we performed univariate and multivariate analyses that included baseline characteristics and genetic alterations. In univariate analysis, we identified 20 factors that had a significant influence on OS in our AML cohort, including IKZF1 mutations with high VAF. In multivariate analysis, we strongly indicated that IKZF1 mutation with high VAF was one independent risk factor for the death of AML (HR, 6.101; 95% CI 2.278–16.335; P = 0.0003) (Additional file 6: Table S3).
We also analyzed the relationships among IKZF1 mutation and other gene mutations. IKZF1 mutation exhibited concurrences with CEBPA, SF3B1, and CSF3R mutations, but it was mutually exclusive with NPM1 mutation (Fig. 1F, G). We also performed subgroup survival analysis. The prognostic role of CEBPAbZIP−inf [3–5], SF3B1, and CSF3R mutations was revealed in our cohort (Additional file 2: Fig S2). IKZF1 mutation did not influence OS or RFS in CSF3RWT and CSF3RMUT (Additional file 3: Fig S3A, B, Additional file 7: Table S4). In IKZF1MUT patients, CEBPAbZIP−inf mutation (83.3%) was more common than non-CEBPAbZIP−inf mutation (16.7%). IKZF1 mutation conferred a relatively low CR in the CEBPAWT/non-CEBPAbZIP−inf−MUT group, but not in the CEBPAbZIP−inf−MUT group (Additional file 8: Table S5), and it influenced OS and RFS in the CEBPAWT/non-CEBPAbZIP−inf−MUT group but not in the CEBPAbZIP−inf−MUT group (Additional file 3: Fig S3C, D). IKZF1WT/SF3B1MUT AML patients exhibited a CR rate of 50%, and the therapeutic response was even worse in IKZF1MUT/SF3B1MUT AML. None of these patients achieved CR at any point during the regimen (Additional file 9: Table S6). IKZF1 mutation combined with SF3B1 mutation conferred extremely poor OS on AML, but the RFS of IKZF1MUT/SF3B1MUT AML patients was unavailable because no patient reached CR (Fig. 1H, I).
Compared with foreign cohorts (OHSU [6], 1.35%; TCGA [7], 0.5%; TARGET [8], 4.21%), the frequency of IKZF1 mutation was relatively high (3.83%). This may be because patients were of different races or it may be because of differences in sequencing depth. IKZF1 deletion, caused by -7/monosomy 7, was detected in 3.20% of our patients. Unlike in ALL [9], IKZF1 mutation and deletion were equally dominant in AML [10]. Missense mutation accounted for nearly half of IKZF1 mutations, and it almost affected the DNA-binding domain in AML, while its DNA-binding domain and dimerization domain involvement was relatively balanced in ALL [9]. IKZF1 aberration conferred poor prognosis in ALL [11], but only a high burden of IKZF1 mutation predicted poor OS in AML because IKZF1 mutation with VAF < 10% accounted for 35% of all IKZF1MUT patients, and IKZF1 mutation contributed less to disease than other mutations did in this group of patients. CEBPA mutation was the most common co-mutation that occurred alongside IKZF1 mutation in AML [1, 12].
Supplementary Information
Additional file 1: Fig S1. The mutational landscape of our AML cohort. (A) Frequent mutations with more than 10 counts in our cohort were showed. (B) The relationship between mutations was analyzed, concurrent and mutually-exclusive mutations were indicated. (C) The concurrent or mutually-exclusive mutations for common rearrangements in AML were exhibited.
Additional file 2: Fig S2. The prognostic role of CSF3R, CEBPAbZIP-inf, or SF3B1 mutation in AML. (A-B) The prognostic role of CSF3R mutation in AML, and OS (A) as well as PFS (B) were showed. (C-D) The OS (C) and PFS (D) of patients with CEBPAWT plus non-CEBPAbZIP-inf mutation or CEBPAbZIP-inf mutation in AML. (E-F) The OS (E) and RFS (F) of SF3B1WT and SF3B1MUT groups in our AML cohort.
Additional file 3: Fig S3. The prognostic role of IKZF1 mutation in the specific genetic AML subtype. (A-B) The influence of IKZF1 mutation on the OS (A) and PFS (B) of CSF3R-mutated AML. (C-D) The influence of IKZF1 mutation on the prognosis of the CEBPA-mutated AML was studied, and the OS (A) as well as RFS (B) of CEBPAWT plus non-CEBPAbZIP-inf-MUT and CEBPAbZIP-inf-MUT groups with or without IKZF1 mutation were showed.
Additional file 4: Table S1. IKZF1-mutated AML patients in our cohort.
Additional file 5: Table S2. The CR rate of AML with different burdens of IKZF1 mutation.
Additional file 6: Table S3. Univariate and multivariate analysis for overall survival duration.
Additional file 7: Table S4. The influence of IKZF1 mutation on AML with different CSF3R-mutated status.
Additional file 8: Table S5. The influence of IKZF1 mutation on AML with different CEBPA-mutated status.
Additional file 9: Table S6. The influence of IKZF1 mutation on AML with different SF3B1-mutated status.
Acknowledgements
We would like to thank all members of the Department of Hematology, the First Affiliated Hospital, Zhejiang University College of Medicine, and the Department of Hematology, Institute of Hematology, Changhai Hospital, for managing patients and supporting our work.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- CI
Confidence interval
- CR
Complete remission
- ELN
European Leukemia Net
- HR
Hazard ratio
- IKZF1MUT
IKZF1-mutated
- IKZF1WT
IKZF1-wild type
- CEBPAbZIP−inf−MUT
In-frame bZIP CEBPA-mutated
- OS
Overall survival
- RFS
Relapse-free survival
- VAF
Variant allele frequency
Author contributions
XZ designed this study. MY, YL, LW, XN, XH, GT, LM, JQ, WX, JW, GX, HM, WM, CY, and WY collected and integrated the clinical materials. LL, MZ, and SC conducted the sequencing experiments and mutational analysis. XZ, AH, LL, and CW displayed the data analysis. XZ wrote the manuscript. HZ, HT, JY, and JQ provided advice regarding this work. JW and JJ revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (81800199, 81670124, 81820108004, 81870143, 81530047, 81771779), and the Natural Science Foundation of Zhejiang Province (LY21H080003).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethical review committees of the First Affiliated Hospital of Zhejiang University School of Medicine (IIT20220659A) and Changhai Hospital (B2022-035). All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Consent for publication
Written informed consent was obtained from these patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Zhang and Aijie Huang are contributed equally to this work
Contributor Information
Lei Wang, Email: b_brendel@hotmail.com.
Wenjuan Yu, Email: drwjyu1977@zju.edu.cn.
Jianmin Wang, Email: jmwangch@139.com.
Jie Jin, Email: jiej0503@zju.edu.cn.
References
- 1.Zhang X, Zhang X, Li X, Lv Y, Zhu Y, Wang J, et al. The specific distribution pattern of IKZF1 mutation in acute myeloid leukemia. J Hematol Oncol. 2020;13(1):140. doi: 10.1186/s13045-020-00972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakita S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022;6(1):238–247. doi: 10.1182/bloodadvances.2021004292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube F, Georgi JA, Kramer M, Stasik S, Middeke JM, Rollig C, et al. CEBPA mutations in 4708 patients with acute myeloid leukemia: differential impact of bZIP and TAD mutations on outcome. Blood. 2022;139(1):87–103. doi: 10.1182/blood.2020009680. [DOI] [PubMed] [Google Scholar]
- 5.Tarlock K, Lamble AJ, Wang YC, Gerbing RB, Ries RE, Loken MR, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the children’s oncology group. Blood. 2021;138(13):1137–1147. doi: 10.1182/blood.2020009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526–531. doi: 10.1038/s41586-018-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolouri H, Farrar JE, Triche T, Jr, Ries RE, Lim EL, Alonzo TA, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2018;24(1):103–112. doi: 10.1038/nm.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchman ML, Low J, Qu C, Paietta EM, Kasper LH, Chang Y, et al. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell. 2015;28(3):343–356. doi: 10.1016/j.ccell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooij JD, Beuling E, van den Heuvel-Eibrink MM, Obulkasim A, Baruchel A, Trka J, et al. Recurrent deletions of IKZF1 in pediatric acute myeloid leukemia. Haematologica. 2015;100(9):1151–1159. doi: 10.3324/haematol.2015.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavallee VP, Gendron P, Lemieux S, D'Angelo G, Hebert J, Sauvageau G. EVI1-rearranged acute myeloid leukemias are characterized by distinct molecular alterations. Blood. 2015;125(1):140–143. doi: 10.1182/blood-2014-07-591529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig S1. The mutational landscape of our AML cohort. (A) Frequent mutations with more than 10 counts in our cohort were showed. (B) The relationship between mutations was analyzed, concurrent and mutually-exclusive mutations were indicated. (C) The concurrent or mutually-exclusive mutations for common rearrangements in AML were exhibited.
Additional file 2: Fig S2. The prognostic role of CSF3R, CEBPAbZIP-inf, or SF3B1 mutation in AML. (A-B) The prognostic role of CSF3R mutation in AML, and OS (A) as well as PFS (B) were showed. (C-D) The OS (C) and PFS (D) of patients with CEBPAWT plus non-CEBPAbZIP-inf mutation or CEBPAbZIP-inf mutation in AML. (E-F) The OS (E) and RFS (F) of SF3B1WT and SF3B1MUT groups in our AML cohort.
Additional file 3: Fig S3. The prognostic role of IKZF1 mutation in the specific genetic AML subtype. (A-B) The influence of IKZF1 mutation on the OS (A) and PFS (B) of CSF3R-mutated AML. (C-D) The influence of IKZF1 mutation on the prognosis of the CEBPA-mutated AML was studied, and the OS (A) as well as RFS (B) of CEBPAWT plus non-CEBPAbZIP-inf-MUT and CEBPAbZIP-inf-MUT groups with or without IKZF1 mutation were showed.
Additional file 4: Table S1. IKZF1-mutated AML patients in our cohort.
Additional file 5: Table S2. The CR rate of AML with different burdens of IKZF1 mutation.
Additional file 6: Table S3. Univariate and multivariate analysis for overall survival duration.
Additional file 7: Table S4. The influence of IKZF1 mutation on AML with different CSF3R-mutated status.
Additional file 8: Table S5. The influence of IKZF1 mutation on AML with different CEBPA-mutated status.
Additional file 9: Table S6. The influence of IKZF1 mutation on AML with different SF3B1-mutated status.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.