ABSTRACT
Entry of influenza A viruses (IAVs) into host cells is initiated by binding to sialic acids (Sias), their primary host cell receptor, followed by endocytosis and membrane fusion to release the viral genome into the cytoplasm of the host cell. Host tropism is affected by these entry processes, with a primary factor being receptor specificity. Sias exist in several different chemical forms, including the hydroxylated N-glycolylneuraminic acid (Neu5Gc), which is found in many hosts; however, it has not been clear how modified Sias affect viral binding and entry. Neu5Gc is commonly found in many natural influenza hosts, including pigs and horses, but not in humans or ferrets. Here, we engineered HEK293 cells to express the hydoxylase gene (CMAH) that converts Neu5Ac to Neu5Gc, or knocked out the Sia-CMP transport gene (SLC35A1), resulting in cells that express 95% Neu5Gc or minimal level of Sias, respectively. H3N2 (X-31) showed significantly reduced infectivity in Neu5Gc-rich cells compared to wild-type HEK293 (>95% Neu5Ac). To determine the effects on binding and fusion, we generated supported lipid bilayers (SLBs) derived from the plasma membranes of these cells and carried out single particle microscopy. H3N2 (X-31) exhibited decreased binding to Neu5Gc-containing SLBs, but no significant difference in H3N2 (X-31)’s fusion kinetics to either SLB type, suggesting that reduced receptor binding does not affect subsequent membrane fusion. This finding suggests that for this virus to adapt to host cells rich in Neu5Gc, only receptor affinity changes are required without further adaptation of virus fusion machinery.
IMPORTANCE Influenza A virus (IAV) infections continue to threaten human health, causing over 300,000 deaths yearly. IAV infection is initiated by the binding of influenza glycoprotein hemagglutinin (HA) to host cell sialic acids (Sias) and the subsequent viral-host membrane fusion. Generally, human IAVs preferentially bind to the Sia N-acetylneuraminic acid (Neu5Ac). Yet, other mammalian hosts, including pigs, express diverse nonhuman Sias, including N-glycolylneuraminic acid (Neu5Gc). The role of Neu5Gc in human IAV infections in those hosts is not well-understood, and the variant form may play a role in incidents of cross-species transmission and emergence of new epidemic variants. Therefore, it is important to investigate how human IAVs interact with Neu5Ac and Neu5Gc. Here, we use membrane platforms that mimic the host cell surface to examine receptor binding and membrane fusion events of human IAV H3N2. Our findings improve the understanding of viral entry mechanisms that can affect host tropism and virus evolution.
KEYWORDS: influenza, sialic acid, Neu5Gc, Neu5Ac, virus entry, membrane fusion, single particle tracking, supported lipid bilayer, tropism, fusion kinetics, membranes
INTRODUCTION
Influenza A virus (IAV) is a negative-sense, single-stranded RNA virus which leads to respiratory infections in humans and other mammals. Such infections still pose a serious threat to human health, leading to over 300,000 deaths each year (1). During IAV infection, an essential early event is viral entry into the host cell, which includes two processes: receptor binding and membrane fusion. These two steps depend on interactions between the viral envelope glycoprotein hemagglutinin (HA) and the primary receptor of IAV, sialic acid (Sia) (2–4). Sias are derivatives of neuraminic acids that are linked to glycolipids and glycoproteins on the host cellular membrane and expressed ubiquitously on vertebrate cells and in mucus (5, 6). Importantly, Sias are a complex family of monosaccharides with multiple structural modifications that often play essential roles in virus-Sia interactions and host tropism (7, 8).
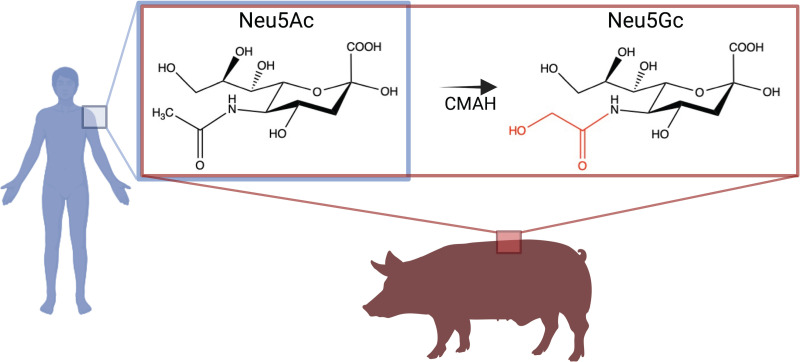
One of the most common Sia variants in mammalian species is N-glycolylneuraminic acid (Neu5Gc), which is formed by the hydroxylation of the N-acetyl group at the 5-C position of N-acetylneuraminic acid (Neu5Ac), the common Sias, which is the predominant form found in humans (9). Neu5Gc is essentially absent in humans due to the absence of a functional form of the enzyme CMP-N-acetylneuraminic acid hydroxylase (CMAH), which hydroxylates Neu5Ac to form Neu5Gc. Low levels of Neu5Gc in human tissues may be acquired from the diet; however, this Sia is highly abundant in other animal hosts for influenza, including pigs (10–12) (Fig. 1). Pigs express both Neu5Ac and Neu5Gc, and contain both of the common Sia linkages α2,3 and α2,6 that are expressed in birds and humans, respectively. Pigs are often characterized as “mixing vessels” of IAVs, because avian- and mammalian-adapted viruses may co-infect the same animal and undergo genetic reassortments, which can lead to new variants exhibiting a high transmission capability (13–15). Neu5Gc is often not specifically considered a receptor for IAVs in studies, or is seen to be nonbinding; therefore, more in-depth studies of how human IAVs interact with diverse Sia chemistry in nature (including the abundant Neu5Gc) are important to help provide a more complete understanding of the determinants of viral tropism and the factors that give rise to the emergence of new variants, particularly during host-switching events.
FIG 1.
The predominant types of sialic acids found in humans and pigs. Humans present Neu5Ac of Sias, whereas pigs present both Neu5Ac and Neu5Gc of Sias, due to the presence of the CMAH enzyme. This enzyme catalyzes the hydroxylation of the acetyl group on Neu5Ac. Image was created with BioRender.com.
Neu5Gc has been shown to affect viral functions in different ways; one example is to reduce the activities of IAV sialidase (also referred to as neuraminidase [NA]) (16). This enzyme cleaves off Sias from virion glycoproteins to facilitate viral egress. In addition to the release of progeny virions, NA activity is also suggested to correlate with the efficiency of viral entry (17, 18). However, the specific effects of Neu5Gc on the mechanisms of IAV viral entry are not well-documented. HA is proteolytically cleaved by host cell proteases into two subunits, HA1 and HA2, that are responsible for Sia binding and membrane fusion, respectively (19). During viral entry, the globular domain of the HA1 subunit binds to the host cell Sias, followed by the uptake of Sia-bound virions into endosomes. During the virions’ trafficking in the endocytic pathway, the low pH in the endosome triggers a conformational change in the stalk-like HA2 subunit of the cleaved HA, enabling the hidden fusion peptide to become exposed, and subsequently inserted into the host membrane. Successive conformational changes of HA2 brings the viral and cellular membranes together to complete membrane fusion and release the viral genome (2). In general, human IAVs show receptor specificity and preferentially bind to Neu5Ac over Neu5Gc (8, 20, 21). While the effect of Neu5Gc on the binding of different IAVs has been studied (20, 21), the effects of the Sia variation on subsequent membrane fusion steps is less clear. Here, we decouple the examination of the binding and fusion processes by using a single particle tracking (SPT) assay, which combines supported lipid bilayers (SLBs) derived from cell plasma membranes with microfluidics and total internal reflection microscopy (TIRFM). We are able to quantitatively study how Neu5Gc influences IAV viral entry processes at the membrane and provide insight into the effect of Neu5Gc on human IAV H3N2 (X-31) fusion kinetics.
We first used wild-type Neu5Ac-rich HEK293 (>95% Neu5Ac) and glycoengineered Neu5Gc-rich HEK-CMAH cells (95% Neu5Gc) to carry out standard infectivity assays with H3N2 (X-31). We show that H3N2 infection with Neu5Gc-rich HEK-CMAH cells is markedly reduced in the presence of Neu5Gc. To investigate the causes of the altered infectivity, we produced cell blebs that are chemically induced membrane vesicles derived from the HEK cells to establish the SLB platform for in vitro entry studies. By comparing the binding and fusion properties, we have found that H3N2 exhibits significantly reduced binding to Neu5Gc relative to Neu5Ac. Furthermore, for the membrane-bound particles, membrane fusion proceeds similarly regardless of the Sia type. The comparison of the binding and fusion on the cell-derived membranes from SLC35A1 knockout cells confirmed that the virus requires cell surface Sia to bind and fuse, as expected. Thus, the reduced infectivity we observed is likely due to differences in binding alone, suggesting that genome delivery is largely unimpeded once the virus is bound. This would enable the virus to adapt to this new host by evolving higher receptor affinity. With no additional fusion adaptation required to promote changes in host tropism, the barrier to reassortment and spillover to other hosts is lowered.
RESULTS
Generation and characterization of Neu5Gc-displaying and sialic acid-depleted HEK293 cell lines.
To investigate the impact of the modified Sia Neu5Gc on H3N2 infection, we first glycoengineered HEK293 cells to synthesize high levels of Neu5Gc (HEK-CMAH cells). HEK293 wild-type cells express Neu5Ac as the predominant Sia on their cell surface and do not produce Neu5Gc, as the human genome lacks a functional CMAH. We confirmed relative levels of Neu5Ac and Neu5Gc by HPLC analysis (methods described in Barnard et al. [8]), and showed that HEK293 wild-type cells display 95.8% Neu5Ac and 4.2% Neu5Gc (the latter likely derived from bovine serum used in culture medium) while HEK-CMAH cells display 4.2% Neu5Ac and 95.0% Neu5Gc. When stained with lectins Sambucus Nigra (SNA), the CMP-sialic acid transporter gene (SLC35A1) knockout cells (HEKΔSLC35A1 cells) showed no detectable level of Sia expression above background on the cell surface.
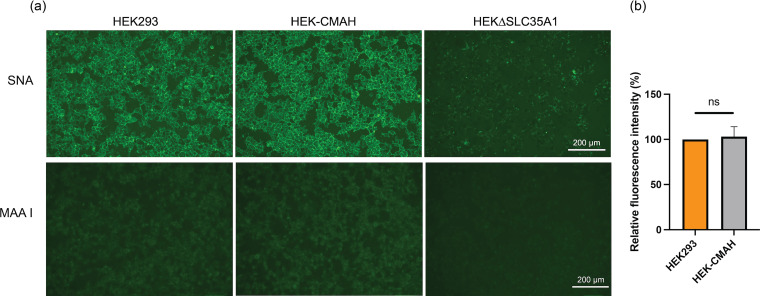
All cell lines were validated for their expected Sia composition (HEK-CMAH cells) or depletion (HEKΔSLC35A1 cells) in comparison to the parental HEK293 cell line. The fluorescein-labeled SNA was used to stain cells, as this lectin recognizes the α2,6 linkage of Neu5Ac and Neu5Gc equally (22). Lectin maackia amurensis I (MAA I) which recognizes the α2,3 linkage of Sias (23), was also used to characterize the cell lines produced. Following SNA staining, we observed that HEK-CMAH and HEK293 cells expressed predominantly α2,6 linkage of Sias, and that HEK-CMAH cells displayed comparable levels of cell surface lectins to HEK293 cells (Fig. 2). This confirms the minimal likelihood that H3N2 infection was altered due to different Sia levels and availability between these two cell lines. Conversely, HEKΔSLC35A1 cells had very low levels of lectin staining on their cell surfaces, indicating greatly reduced Sia levels (Fig. 2).
FIG 2.
The distribution of Sias in HEK293, HEK-CMAH, and HEKΔSLC35A1 cells. (a) Live cells were stained with 1:5,000 fluorescein-labeled SNA or MAA I for 30 min and then imaged using fluorescence microscopy. Both HEK293 and HEK-CMAH express substantial amounts of α2,6-Sias, including Neu5Ac and Neu5Gc, while HEKΔSLC35A1 cells show little to no expression of Sias. (b) Relative fluorescence level of SNA staining in each cell line was quantified using ImageJ, and data were analyzed using PRISM software. The fluorescence intensity in HEKΔSLC35A1 cells is not shown as the signal is below the detection limit.
Effects of Neu5Gc-presenting HEK cells on H3N2 (X-31) virus infection.
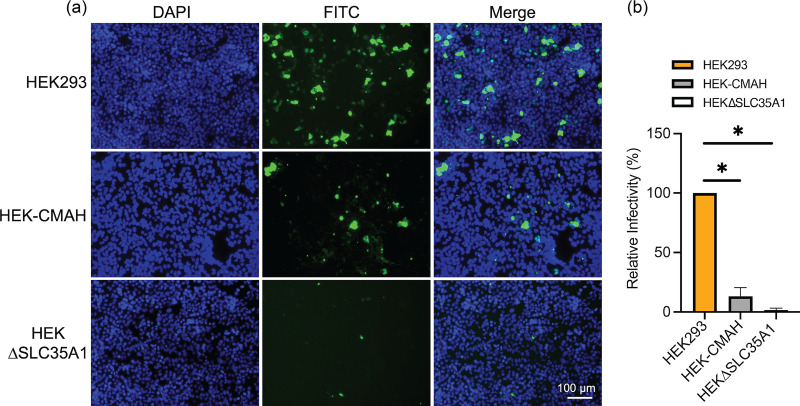
After validation of the cell lines, we carried out infectivity studies with H3N2 (X-31). Cells were inoculated at an MOI of 0.1 for 1 h, incubated in maintenance media for 18 h, then fixed and stained using an antibody against influenza nucleoprotein (NP) to identify infected cells. Many NP-positive cells are seen in HEK293 cells, which present more Neu5Ac than Neu5Gc (Fig. 3a). In contrast, there is a marked decrease in NP-positive cells observed in HEK-CMAH cells, suggesting that Neu5Gc does not effectively support H3N2 infection. As expected, HEKΔSLC35A1 cells show little NP signal, with mainly background fluorescence detected, indicating the decreased levels of Sias impair H3N2 infection. Quantification of the NP-positive cells across all cell lines reveals a 10-fold decrease in infectivity of H3N2 in HEK-CMAH cells in comparison to HEK293 cells (Fig. 3b), indicating that H3N2 virus infection is attenuated in cells possessing high levels of Neu5Gc.
FIG 3.
H3N2 (X-31) virus infection is reduced in Neu5Gc-rich HEK-CMAH cells. (a) The distribution of influenza virus NP proteins in Neu5Ac-rich HEK293, Neu5Gc-rich HEK-CMAH, and HEKΔSLC35A1 cells at 18 hpi was determined by immunofluorescence assay (IFA). (b) Relative infectivity was determined by quantifying the number of NP positive cells in relation to the total number of cells in the field of view using ImageJ. Data were analyzed using PRISM software.
Formation of bleb-derived supported lipid bilayers containing Sias.
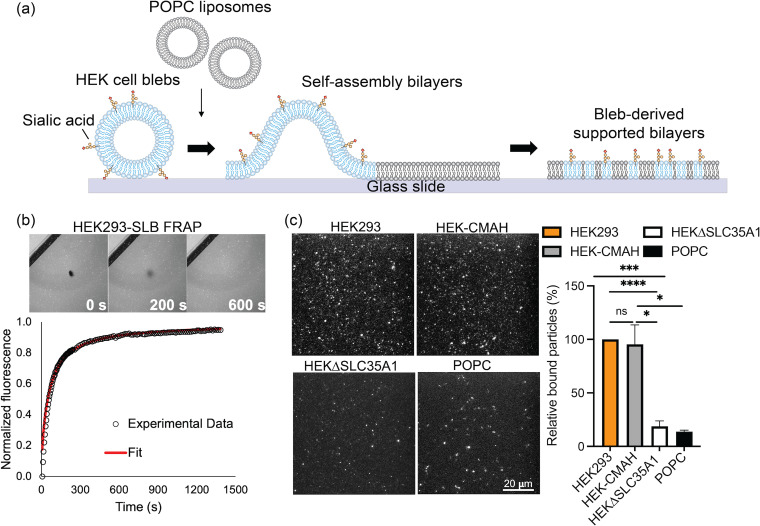
To examine the effect of Neu5Gc on H3N2 viral entry, we used an in vitro system using SLBs to directly quantify virion binding and membrane fusion. This simplified system mimics the plasma membrane and allows precise control and capture of the sequence of events that occur during viral entry. HEK-derived cell blebs used to form SLBs incorporate membrane proteins and lipids with their Sia modifications into membrane mimetic platforms. We chemically induced bleb formation from the previously described HEK cell lines using established procedures and combined the blebs with liposomes to induce rupturing onto the glass surface for SLB formation (24–27).
Fig. 4a demonstrates the process of bleb-SLB formation, where liposomes were used to rupture HEK293, HEK-CMAH, and HEKΔSLC35A1 blebs into planar SLBs containing the Sias of interest on the glass surface. SLBs were formed using approximately a 1:1 ratio of POPC liposomes to cell blebs. The mobility of the formed SLBs was verified using fluorescence recovery after photobleaching (FRAP), as this membrane property can affect interactions between membrane proteins and virus particles (27). Fig. 4b shows representative images of FRAP and a plot of the fluorescence recovery of HEK293-SLB over time. SLBs formed with HEK-CMAH and HEKΔSLC35A1 blebs show similar fluorescence recovery kinetics (Table 1). These data show that membrane fluidity in all the bled-derived SLBs was preserved, as the photobleached spot recovers with time. The resulting fluorescence intensity over time was plotted for each SLB and then fit to determine the diffusion coefficient (D) and mobile fraction (MF), confirming the high mobility of SLBs with few unruptured vesicles.
FIG 4.
HEK blebs-derived SLBs formed are mobile and retain available Sias. (a) Illustration of cell bleb-SLB formation. Chemically induced cell blebs are absorbed on a glass surface, then ruptured by fusogenic liposomes to form bilayers (created from Adobe illustrator). (b) The mobility of formed bleb-SLBs was determined by fluorescence recovery after photobleaching (FRAP). The images correspond to various times along the fluorescence recovery process of HEK293-SLB. (c) Left: Sias distribution on SLBs was determined by SNA lectin staining. SNA lectin, which recognizes α2-6 linked Sias, binds well to both Neu5Ac and Neu5Gc. Right: Sias incorporation was quantified by the number count of fluorescent particles in the field of view using ImageJ. Data were analyzed using PRISM software.
TABLE 1.
Diffusivity (D) and mobile fraction of bleb-SLBs (mean ± SD, n = 3)
| SLB type | D (μm2/s) | Mobile Fraction |
|---|---|---|
| HEK293 | 0.32 ± 0.04 | 0.98 ± 0.01 |
| HEK-CMAH | 0.26 ± 0.04 | 1.00 ± 0.02 |
| HEKΔSLC35A1 | 0.34 ± 0.02 | 0.94 ± 0.02 |
To determine if the Sias displayed on the cell surfaces were incorporated into the SLBs we formed with the cell blebs, we performed SNA lectin staining on the SLBs (Fig. 4c). Both HEK293- and HEK-CMAH-derived SLBs show appreciable amounts of bound SNA lectin with no significant difference in quantity, indicating that bleb-SLBs retain Sias from cell membranes after vesicles rupture and SLB formation (Fig. 4c). As expected, SLBs from HEKΔSLC35A1 cell blebs exhibited reduced SNA staining, indicating only very low levels of Sias present on these cells. We note that there was also a very low level of fluorescence signal detected in the control SLBs containing synthetic POPC liposomes only, suggesting that minor nonspecific binding of SNA lectin occurred. Nevertheless, these results verify that the bleb-SLBs incorporate Sias similarly to the live cells they are derived from, with Sias extensively expressed on both HEK293 and HEK-CMAH but not on HEKΔSLC35A1.
H3N2 virus binding to Neu5Ac- and Neu5Gc-containing SLBs.
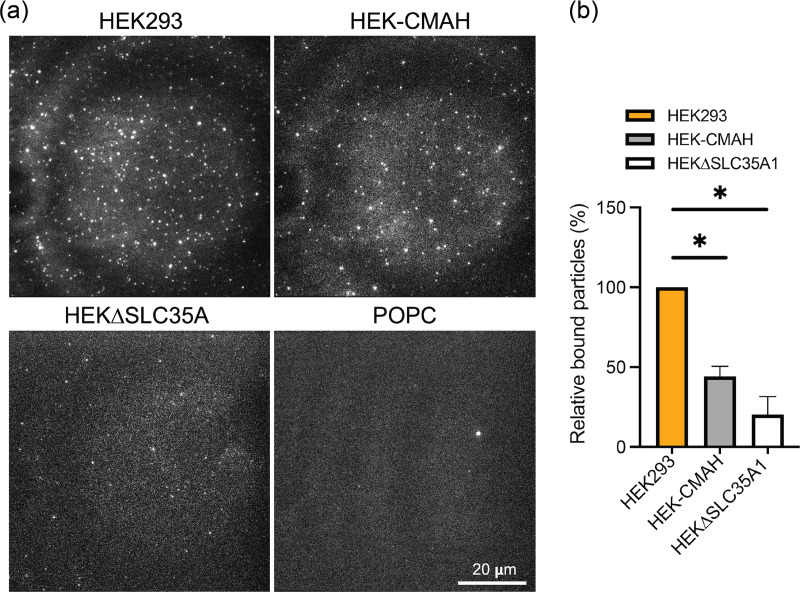
After verifying the successful formation of mobile bleb-SLBs containing Sias, we examined the effects of Neu5Ac and Neu5Gc on fluorescently labeled H3N2 virus binding to the SLB platform using TIRF microscopy (Fig. 5a). Bleb-SLBs were incubated with R18-labeled virions for 5 min, after which we observed fewer bound virions on HEK-CMAH-SLBs containing Neu5Gc in comparison to the Neu5Ac-containing HEK293-SLBs. Quantification of the particles in the field of view revealed an approximate 50% decrease in the number of bound particles on HEK-CMAH-SLBs compared to HEK293-SLB as shown in Fig. 5b. Even fewer particles (80% decrease compared to HEK293-SLB) were bound to the SLBs formed with HEKΔSLC35A1 blebs. These results suggest that the chemical modification of the Sia structure, from Neu5Ac to Neu5Gc, significantly reduces H3N2 binding efficiency, but does not completely eliminate it. Alternatively, the virions may bind to the remaining 10% of the Sia that was present as Neu5Ac. Few virions interact with the SLB when Sias are not expressed on the membrane surface. This observation of decreased virion binding to Neu5Gc-containing bilayers is consistent with the known human IAVs’ greater specificity for Neu5Ac (28). In addition, barely any virions were observed bound to the SLBs formed with only POPC liposomes, indicating that the specificity between this influenza virus and its host cell receptor is well preserved in our cell-free SLB system and that the liposomes themselves do not promote any significant nonspecific interactions.
FIG 5.
Decreased H3N2 (X-31) binding on HEK-CMAH bleb-derived SLBs containing predominantly Neu5Gc. (a) SLBs were incubated with R18-labeled virions and rinsed to remove unbound particles. Bound virus particles on HEK293, HEK-CMAH, and HEKΔSLC35A1 bleb-derived SLBs, as well as on POPC SLBs, were imaged using TIRF microscopy. (b) Quantitative particle counts were determined by ImageJ. Data were analyzed using PRISM software.
Measurement of H3N2 fusion kinetics between Neu5Ac- and Neu5Gc-containing SLBs using single-particle tracking.
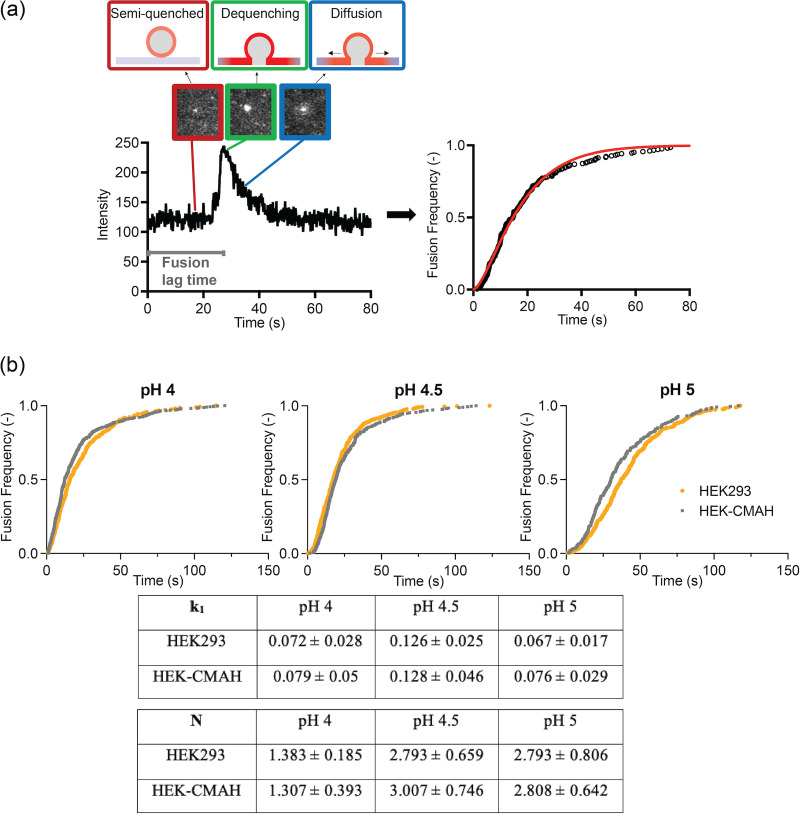
Next, we explored if the chemical modification of Neu5Ac to Neu5Gc affects H3N2 fusion behavior using SPT assay, which allows us to quantify the intermediate steps of viral and host membrane fusion by tracking and monitoring the fusion of single virions over time. H3N2 was labeled with a sufficiently high concentration of R18 so that it remained quenched in the viral membrane. Upon fusion, fluorescence dequenching signals that fusion between the virion and the SLB occurred. Bleb-SLBs were formed in microfluidic devices by rupturing the HEK cell-derived blebs with oregon green (OG)-labeled POPC liposomes at a flow rate of 100 μL/min. OG is a pH-sensitive fluorophore that reduces its emission intensity at low pH, and thus, it serves as a precise indicator of acidification (the fusion trigger) as it occurs in the microfluidic channels. This time point sets the start of the lag time quantification of HA-mediated fusion. Fig. 6a depicts the overview of the SPT assay, where we obtain the fluorescence intensity profile of each virion over time using TIRF microscopy. During the fusion event of a single virion with the SLB, the initially quenched R18 dequenches, leading to a spike in fluorescence intensity. This spike is followed by a decrease in the fluorescence intensity due to the radial diffusion of R18 away from the fusion site. The interval of time between the acidification of the system to trigger fusion and the spike of intensity is the fusion lag time. By collecting and plotting the lag time data, we are able to obtain cumulative distribution curves of fusion frequency and then fit them to the cumulative gamma distribution (equation shown below) to estimate the fusion kinetics:
FIG 6.
H3N2 (X-31) virus fusion kinetics using single particle tracking. (a) Overview of a single H3N2 influenza virus fusion event. The system is acidified to trigger fusion which leads to a spike in R18 intensity due to fluorescence dequenching. Fluorescence intensity then decreases as the fluorophores diffuse out from the site. The lag time between acidification and fusion is the fusion lag time, which is collected from multiple events and plotted as a cumulative distribution curve. Data are fitted to an equation to obtain fusion kinetics, k1 and N. (b) Cumulative distribution curves and fusion kinetics of H3N2 in HEK293- and HEK-CMAH-SLBs at a pH range from 4 to 5. Data were analyzed using MATLAB and Prism software.
In the equation, k1 is the fusion rate constant, t is the lag time, and N is an additional fit parameter often correlated with the number of steps in the reaction or the number of HA fusion proteins required to initiate fusion (29). A gamma distribution describes the multistep reaction and is used to fit the kinetics of single virion fusion here because individual fusion events are multistep processes that occur independently of each other.
We characterized H3N2 fusion behavior in HEK293- and HEK-CMAH-SLBs at pH 4, 4.5, and 5. We were unable to characterize HEKΔSLC35A1-SLBs, as there is a technical limitation to significant statistics with the limited number of binding and therefore fusion events. Fig. 6b illustrates the cumulative distribution curves and kinetics of H3N2 fusion in both SLBs at these three pHs. Interestingly, H3N2 shows similar fusion behavior in both SLBs, as the distribution curves of each condition nearly overlay each other; there is no significant difference between the calculated fusion rate constants (k1) or the reaction steps (N) between the HEK293- and HEK-CMAH-SLBs at pH 4, 4.5, and 5. These results suggest that although H3N2 virions bind less specifically to Neu5Gc, downstream fusion events are not influenced by this chemically different Sia. The requisite acidification of the environment to trigger fusion is confirmed by the control experiments where the pH 7 buffer was used as the trigger, leading to no fusion of H3N2 with the SLBs in the system (data not shown).
DISCUSSION
It is well-known that Sias are the host cell receptor for influenza viruses (30, 31). However, there is a lack of understanding of how structural and chemical variations in Sias affect the two main processes in virus host cell entry: receptor binding and, especially, membrane fusion. The most common modified form of Sia found in mammals is Neu5Gc, which is present primarily on the cells and the mucus of horses, mice, and pigs, but not humans. Although Neu5Gc exhibits lower specificity toward most IAVs, this Sia is highly biologically relevant, as it is expressed at high levels in a number of animal hosts that can be critical reservoirs of influenza viruses, including those involved in the emergence of new variants. A better understanding of this nonprimary receptor and how it might affect the entry and infection process will therefore provide insights into IAV host tropism. While there have been suggestions that some human influenza viruses show altered infectivity in cell lines containing modified Neu5Gc (8, 32, 33), a clear understanding of the basis for this difference is missing.
We tested the infectivity of IAV H3N2 (X-31) in our glycoengineered HEK cell lines expressing either Sia variant (Neu5Ac or Neu5Gc) and found a 10-fold decrease in H3N2 infectivity in the Neu5Gc-containing HEK-CMAH cells. H3N2 (X-31) is a recombinant strain with surface glycoprotein genes from H3N2 (A/Aichi/2/1968) and internal genes from H1N1 (A/PR/8/1934) (34). The attenuating effect of Neu5Gc on the infection of human IAVs varies by virus strain. pH1N1 (A/California/04/2009), H3N2 (A/Brazil/02/1999), and H1N1 (A/Brazil/1137/1999) have been reported to show reduced infectivity in cells enriched in Neu5Gc over Neu5Ac, while other strains, including PR8 H1N1 (A/Puerto Rico/8/1934), H3N2 (A/Victoria/361/2011), and H3N2 (A/Memphis/1/1971) show typical infectivity levels in host cells with a higher Neu5Gc content (8, 32, 33).
The infectivity patterns of these strains align with the specific binding properties of HA to the Neu5Ac. Binding of HA to Sias relies on the insertion of the carboxylate group of Sias into the carbohydrate-binding site of HA, resulting in the formation of hydrogen bonds (19, 35). In addition, the van der Waals contact between the methyl group of the N-acetyl chain and the hydrophobic pocket in the virus-binding site are also important to strengthen the binding (36, 37). Neu5Gc is modified from Neu5Ac, with a hydroxylated acetyl group, which introduces polarity that decreases the hydrophobic force. We believe that the attenuated binding of H3N2 (X-31) to Neu5Gc-containing SLBs is likely due to the absence of the hydrophobic methyl group in Neu5Gc. Additionally, the conversion to the hydroxyl group extends the side chain which may lead to steric hindrance (38). Therefore, it seems likely that the H3N2 (X-31) HA-Neu5Gc interaction is weakened such that it is more unlikely to maintain virion attachment to the host cell surface, commensurate with our observed ~50% reduction in bound virions. However, other H3N2 strains, including (A/Memphis/102/1972), (A/Victoria/3/1975), and (A/Texas/1/1977) show equivalent binding to Neu5Ac and Neu5Gc (39). A sequence alignment for the receptor binding domain of these virus strains suggests that Asn at amino acid 150, Tyr at amino acid 184, Asp at amino acid 217, and Lys at amino acid 236 could be critical to the acquisition of Neu5Gc binding, as they are shared only among the strains that show similar binding activity to Neu5Gc and Neu5Ac, but not other strains. More in-depth studies are required to confirm the importance of these residues/positions.
Sialic acids directly interact with the HA1 domain responsible for receptor binding, but not with the fusion domain, HA2. Therefore, it is reasonable to assume that once bound the host receptor modifications would not markedly influence the fusion mechanisms. Interestingly, it has been suggested that different receptor structures can result in different initial fusion activities (40). Further investigation into how Neu5Gc affects the fusion process is therefore needed. Given the difficulty in separating the binding and fusion processes in biochemical assays, it is not surprising that the relationship between infectivity and fusion is less understood, although it may also be an important aspect for consideration in host tropism. By using single particle tracking techniques to parse out binding from fusion, this study shows that although binding is influenced by the chemical modification of Sias from Neu5Ac to Neu5Gc, the subsequent viral membrane fusion is not affected by this change. Fusion kinetics of H3N2 (X-31) were similar between Neu5Ac- (HEK293) and Neu5Gc- (HEK-CMAH) enriched-SLBs, indicating that hydroxylation of the acetyl group of Neu5Ac by CMAH to produce Neu5Gc does not affect H3N2 virion-host cell membrane fusion. This finding is in line with the recent studies of H3N2/X-31, which showed that variations in receptor binding do not affect membrane fusion kinetics (41, 42). In addition, our results indicate that the infectivity of this virus is directly related only to the changes in receptor binding but not membrane fusion. Although in this case, fusion is not impacted by the receptor modification, a thorough understanding of the impact of receptor modifications on influenza viral entry warrants a more comprehensive study with other common types of Sia modifications to complement existing studies. The relationship between receptor binding and fusion is complex and incomplete and future studies will help identify the chemical interactions that mediate binding across different strains of influenza viruses and improve our understanding of the biochemical factors in viral entry that drive host tropism.
MATERIALS AND METHODS
Cells and virus.
HEK293 cells were obtained from the American Type Culture Collection (ATCC). HEK-CMAH cells were prepared by transfection of the chimpanzee cytidine CMAH gene in a plasmid under the control of the CMV promoter (pcDNA3.1, Invitrogen). Clones positive for Neu5Gc synthesis and display were determined by labeling released Sia with 1,2-diamino-4, 5-methylenedioxybenzene (DMB, Sigma-Aldrich) and high-performance liquid chromatography (HPLC) analysis as previously described (8). HEKΔSLC35A1 cells were generated by CRISPR-Cas9 editing. The SLC35A1 gene-knockout approach used was similar to the methods described in Han et al. (43). We used a Cas9 plasmid (PX459, Addgene plasmid #62988) that was engineered to target the human SLC35A1 gene through the single guide RNA (sgRNA) with the sequence TGAACAGCATACACTAACGATGG. Plasmids were transfected using TransIT-X2 (Mirus Bio LLC) (8). Transfected cells were selected with puromycin and single cell clones screened by SNA and MAA lectin staining to identify nonstaining variants. Edited sequences were confirmed by PCR amplification of the targeted regions, and sequencing the PCR product for each allele using primers (forward 5’CTGGTAGTCTGGGTAGATTCAAAGCATC and reverse 5’CAACTGGTAGGTCACCTGGTACACTGCT). Allele 1 contained both a 1 nt deletion (330 G) and a 6 nt deletion (359 to 364), and truncated the protein through a stop codon after aa 88. Allele 2 contained a 23 nt insertion, a 10 nt insertion, and an 83 nt insertion, and truncated the protein through a stop codon after aa 130. Edited cells were confirmed as having low levels of cell surface sialic acid using flow cytometry after incubating cells with fluorescein-labeled plant lectins Sambucus Nigra (SNA) or Maackia Amurensis I (MAA I) (Vector Laboratories). All cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (Cellgro) supplemented with 25 mM HEPES (Cellgro) and 10% HyClone FetalClone II (GE) at 37°C and 5% CO2. The human influenza A strain H3N2 (A/X-31) was obtained from Charles River.
Virus infection assay.
HEK293, HEK-CMAH, or HEKΔSLC35A1 cells were seeded in 24-well plates and then inoculated with H3N2 (X-31) at a multiplicity of infection (MOI) of 0.1 (proxy MDCK titer of H3N2 [X-31] for MOI of 0.1 in HEK cells) for 1 h. The inoculum was then removed, maintenance media with 2% HyClone and TPCK-trypsin (Sigma-Aldrich) at 0.4 μg/mL in DMEM was added, and the cells were incubated for 18 h. A time of 18 h was selected as a consistent postlogarithmic population growth time point for H3N2 (X-31) based on our preliminary time course results (data not shown). TPCK-trypsin was added to facilitate HA cleavage for priming infection. The cells were then fixed with 4% paraformaldehyde (PFA) at RT for 15 min, then blocked with 1× Carbo-free blocking buffer (Vector Laboratories) for 30 min. Cells were then incubated with mouse anti-NP antibody in permeabilization buffer (PBS, 0.5% BSA, 0.5% Triton X-100) for 60 min, rinsed with PBS, and further incubated with goat anti-mouse 488 antibody (Invitrogen) in permeabilization buffer for 45 min to detect viruses. Cells were also stained with 4’,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei. The cells were imaged using a fluorescence microscope, Zeiss AxioSkop HBO 50. Images were analyzed to obtain relative infected cell counts using ImageJ (NIH).
Preparation of cell blebs.
HEK293 and HEK-CMAH cells were seeded in 10-cm petri dishes at 8 × 105 cells/mL and grown until ~90% confluence. Cells were then rinsed with giant plasma membrane vesicle (GPMV) buffer (2 mM CaCl2, 10 mM HEPES, 150 mM NaCl, pH 7.4) twice, followed by an incubation with 4 mL of blebbing solution (GPMV buffer with 2 mM dithiothreitol [DTT] and 25 mM formaldehyde) at 37°C for 2 h. The media containing the cell blebs was collected into a 15-mL tube and placed on ice to settle any detached cells. The supernatant containing the cell blebs was then carefully transferred to a new tube to remove additional cell debris. The average diameter and concentration of cell blebs were determined by Malvern NS300 NanoSight at Cornell Nanoscale Science and Technology Facility (CNF).
Preparation of liposomes.
Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was purchased from Avanti Polar Lipids and used to make POPC liposomes used in this work. Texas Red-DHPE (TR) (Thermo Fisher Scientific) was used to label the POPC liposomes and served as a fluorescent probe during photobleaching experiments (described below in the FRAP section), and Oregon Green-DHPE (OG) (Invitrogen) was used as a pH sensor to mark acidification of the SLBs. First, 95% (mol/mol) POPC and 5% (mol/mol) TR or OG in chloroform were mixed in a scintillation vial. The solvent was then removed using a continuous stream of high purity nitrogen gas. Subsequently, the dried lipids were rehydrated by gently vortexing them in the presence of phosphate-buffered saline (PBS, pH 7.4). After several freeze-thaw cycles, the lipid solution was extruded 12 times through a membrane filter (Whatman Nuclepore) with a pore size 50 nm to form liposomes. The size and polydispersity of the liposomes were characterized using Zetasizer (Malvern).
Preparation of PDMS wells and microfluidic devices.
(i) Surface treatment of glass slides. Glass coverslip slides (25 mm × 25 mm, No. 1.5) from VWR were cleaned with piranha solution (45 mL of 50% hydrogen peroxide and 105 mL of 70% sulfuric acid) for 10 min, then rinsed with deionized water for 30 min. Prior to plasma surface treatment, glass slides were rinsed again with deionized water then dried with high purity nitrogen gas.
(ii) Fabrication of polydimethylsiloxane (PDMS) wells. 184 Sylgard silicone elastomer and its cross-linking agent were mixed thoroughly at a 10:1 ratio, then degassed for 10 min. The mixture was then poured into a petri dish and cured at 65°C for 4 h to form a thin sheet of PDMS. The PDMS was then cut into small squares and punctured at the center of each square. The PDMS squares were attached to the glass slides to form PDMS wells with an approximate volume of 120 μL for each well.
(iii) Fabrication of microfluidic devices. The methods of fabricating microfluidic devices were published previously by our group (24, 44). Briefly, we utilized a microchannel silicon mold that was developed using soft lithography at Cornell University Nanoscale Science and Technology Facility (CNF). Each mold contains six channels, each with the dimensions: 1 mm width, 1.5 cm length, and 70 μm depth. The microfluidic devices were formed using PDMS in the silicon mold. In order to separate PDMS from the master mold, the mold was coated with chlorotrimethylsilane (Sigma-Aldrich) in advance. As mentioned above, the mixture of elastomer and cross-linker was degassed and poured onto the mold, followed by curing at 65°C for 4 h. Both the piranha-cleaned glass slide used as the support and the cured PDMS released from the mold were plasma cleaned at a pressure of 700 millitorr for 20 s, then gently pressed together to form the microfluidic device.
Bleb-derived SLBs.
(i) Formation of bleb-SLBs. Different measurements require different fluorophores for fluorescence labeling of bilayers. Bleb-derived bilayers without fluorophores were formed following the steps below. A total of 100 μL of HEK293 or HEK-CMAH cell blebs in GPMV buffer were added into the PDMS well and incubated at RT for 10 min. HEKΔSLC35A1 cell blebs were also used to form SLBs and served as a negative control. Unbound cell blebs were rinsed away using GPMV buffer twice. Then, 100 μL of 2 mg/mL unlabeled fusogenic POPC liposomes were added to the well and incubated for 30 min to form bleb-derived supported bilayers.
(ii) Fluorescence recovery after photo-bleaching. Fluorescence recovery after photobleaching (FRAP) was performed to assess the mobility and uniformity of the Sias-containing SLBs using an inverted Zeiss Axio Observer.Z1 with a α Plan-Apochromat 20× objective. POPC liposomes were labeled with 0.5% (mol/mol) Texas red (TR) as the fluorescent probe during SLB formation following the procedure described above. A ~15 μm diameter spot was bleached on the SLB for 500 ms with a 150 mW, 561 nm semiconductor laser (Coherent Inc.). The fluorescence intensity of the bleached spot was recorded for 30 min, then normalized to a reference spot for each image. The data were fit to a Bessel function following the method of Soumpasis (45). The equation: D = w2/4t1/2 was used to calculate the diffusion coefficient (D), where w is the radius of the photobleached spot, and t1/2 is the time to reach half of the maximum recovery intensity. Mobile fraction (MF), which stands for the ratio of the recovered intensity over the initial fluorescence in the same area, was also calculated.
(iii) Lectin staining. To determine the distribution of Sias on bleb-SLBs, SLBs were blocked with 1× Carbo-free blocking buffer, which is free of glycoproteins, for 30 min. Blocked SLBs were then stained with SNA for 30 min. SNA is known to bind α2,6-linked Sias, including both α2,6-Neu5Ac and α2,6-Neu5Gc. The stained bleb-SLBs were then imaged using a fluorescence microscope.
SPT assay of membrane fusion event.
(i) Labeling and introducing virions to SLBs. H3N2 influenza virions were labeled with Octadecyl Rhodamine (R18) (Life Technologies) in a sonication bath at RT for 30 min. Excess R18 was removed by centrifuging the labeled virions through a microspin G-25 columns (Cytiva) for 2 min at 3,500 rpm. HEK293-, HEK-CMAH-, and HEKΔSLC35A1-SLBs formed in PDMS wells were incubated with labeled virions at RT for 5 min, followed by rinsing with PBS buffer to remove unbound virions. H3N2 binding on SLBs was imaged using TIRF microscopy, described in the section below. Images were processed using ImageJ for the quantification of bound virions.
(ii) Bleb-derived SLBs in microfluidic devices. SPT was used to follow individual virions as they bound and fused with the SLBs that were formed within the microfluidic devices. To generate the SLBs within the microfluidic devices, the inlets and outlets of the devices were connected with Tygon tubing (Cole-Parmer) and six syringes were attached to the end of the outlet tubing. Diluted cell blebs at a 1:6 ratio in GPMV buffer were drawn into each microchannel using a syringe pump at a flow rate of 100 μL/min and incubated at RT for 10 min to allow the binding of cell blebs to the glass surface. GPMV buffer was then used to rinse the microchannels at the same flow rate. OG-labeled POPC liposomes (0.5% OG [mol/mol]), served as a pH sensor to mark the acidification of the SLBs and were subsequently drawn into the microchannels and incubated for 2 h to allow bleb rupturing initiated by the fusion between liposomes and blebs. Every 30 min, more POPC liposomes diluted in PBS buffer were flowed through to avoid air bubbles and defects on the bilayers. Finally, PBS buffer was used to rinse the microchannels and remove unbound vesicles.
(iii) Total internal reflection fluorescence microscopy. The SPT assay was conducted using total internal reflection (TIRF) microscopy with an inverted Zeiss Axio Observer.Z1 and a α Plan-Apochromat 100× objective with a numerical aperture (NA) of 1.46. A drop of refractive index-matching oil (Carl Zeiss) was added onto the objective to enable viewing of the sample. We excited green (OG) and red (R18) fluorophores at the excitation wavelengths of 488 nm and 561 nm, respectively. The angles of incidence in the optical pathway were controlled by the Laser TIRF 3 slider (Carl Zeiss). Exceeding the critical angle of the glass/liquid interface (~62°) generates total internal reflection and an evanescent wave with a penetration depth of ~100 nm. Within this depth where virus binding and fusion occurs, the evanescent wave excites fluorophores on the SLB for the observation of bilayers and the bound R18-labeled virions. The acquired images from the fusion assay were processed using ImageJ (NIH) with the Time series analyzer V2.0 plugin to generate a table of averaged intensities of each fusion event with the corresponding fusion time. MATLAB was then used to process the data from ImageJ to calculate the fusion lag time and kinetic parameters (29).
Statistical analysis.
Variance analysis was performed using a t test with unequal variances to find significant differences between conditions using GraphPad Prism. All data were plotted using GraphPad Prism, and presented as the mean of at least three independent biological replicates for each experiment, with error bars representing the standard deviation (±SD). Statistical significance levels were determined as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
ACKNOWLEDGMENTS
This work was supported by Grant R21AI142297 from the National Institutes of Health (NIH). We thank Juliana Carten for the insightful review on the manuscript.
Contributor Information
Colin R. Parrish, Email: crp3@cornell.edu.
Susan Daniel, Email: sd386@cornell.edu.
Anice C. Lowen, Emory University School of Medicine
REFERENCES
- 1.Cozza V, Campbell H, Chang HH, Iuliano AD, Paget J, Patel NN, Reiner RC, Troeger C, Viboud C, Bresee JS, Fitzner J. 2021. Global seasonal influenza mortality estimates: a comparison of 3 different approaches. Am J Epidemiol 190:718–727. 10.1093/aje/kwaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skehel JJ, Wiley DC. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–569. 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 3.Luo M. 2012. Influenza virus entry, p 201–221. In Rossmann MG, Rao VB (eds), Viral molecular machines: advances in experimental medicine and biology, Springer US, Boston, MA, Vol 726. 10.1007/978-1-4614-0980-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Whittaker GR. 2006. Entry of influenza virus. In Pöhlmann S, Simmons G (eds), viral entry into host cells. Advances in Experimental Medicine and Biology, vol 790. Springer, New York, NY. 10.1007/978-1-4614-7651-1_4. [DOI] [PubMed] [Google Scholar]
- 5.Lewis AL, Chen X, Schnaar RL, et al. 2022. Sialic acids and other nonulosonic acids. In Varki A, Cummings RD, Esko JD, et al. Essentials of glycobiology [Internet]. 4th ed. Cold spring harbor (NY): Cold spring harbor laboratory press. Chapter 15. https://www.ncbi.nlm.nih.gov/books/NBK579976/. [Google Scholar]
- 6.Varki A. 1997. Sialic acids as ligands in recognition phenomena. FASEB J 11:248–255. 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 7.Wasik BR, Barnard KN, Parrish CR. 2016. Effects of sialic acid modifications on virus binding and infection. Trends Microbiol 24:991–1001. 10.1016/j.tim.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnard KN, Alford-Lawrence BK, Buchholz DW, Wasik BR, LaClair JR, Yu H, Honce R, Ruhl S, Pajic P, Daugherity EK, Chen X, Schultz-Cherry SL, Aguilar HC, Varki A, Parrish CR. 2020. Modified sialic acids on mucus and erythrocytes inhibit influenza A virus hemagglutinin and neuraminidase functions. J Virol 94. 10.1128/JVI.01567-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varki NM, Varki A. 2007. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest 87:851–857. 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. 1998. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem 273:15871. 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 11.Chou H-H, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the homo-pan divergence. Proc Natl Acad Sci USA 95:11751–11756. 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K, Kida H, Otsuki K, Kiso M, Ishida H, Kawaoka Y. 2000. Recognition of N -glycolylneuraminic acid linked to galactose by the Α2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J Virol 74:9300–9305. 10.1128/JVI.74.19.9300-9305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Couceiro JNSS, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72:7367–7373. 10.1128/JVI.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. 2010. Replication of Avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 7:38. 10.1186/1743-422X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang K-C. 2010. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res 6:4. 10.1186/1746-6148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corfield AP, Higa H, Paulson JC, Schauer R. 1983. The specificity of viral and bacterial sialidases for α(2–3)- and α(2–6)-linked sialic acids in glycoproteins. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 744:121–126. 10.1016/0167-4838(83)90080-8. [DOI] [PubMed] [Google Scholar]
- 17.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk H-D. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol 78:12665–12667. 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries E, de Vries RP, Wienholts MJ, Floris CE, Jacobs M-S, van den Heuvel A, Rottier PJM, de Haan CAM. 2012. Influenza A virus entry into cells lacking sialylated N-Glycans. Proc Natl Acad Sci USA 109:7457–7462. 10.1073/pnas.1200987109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiley DC, Shekel JJ. 1987. The structure and function of tile hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 56:365–394. 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 20.Spruit CM, Nemanichvili N, Okamatsu M, Takematsu H, Boons G-J, de Vries RP. 2021. N-glycolylneuraminic acid in animal models for human influenza A virus. Viruses 13:815. 10.3390/v13050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broszeit F, Tzarum N, Zhu X, Nemanichvili N, Eggink D, Leenders T, Li Z, Liu L, Wolfert MA, Papanikolaou A, Martínez-Romero C, Gagarinov IA, Yu W, García-Sastre A, Wennekes T, Okamatsu M, Verheije MH, Wilson IA, Boons G-J, de Vries RP. 2019. N-glycolylneuraminic acid as a receptor for influenza A viruses. Cell Rep 27:3284–3294.e6. 10.1016/j.celrep.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith D.F, Song X, Cummings RD. 2010. Use of glycan microarrays to explore specificity of glycan-binding proteins, p 417–444. In Methods in enzymology, Vol 480, Elsevier. 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 23.Geisler C, Jarvis DL. 2011. Letter to the glyco-forum: effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 21:988–993. 10.1093/glycob/cwr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costello DA, Millet JK, Hsia C-Y, Whittaker GR, Daniel S. 2013. Single particle assay of coronavirus membrane fusion with proteinaceous receptor-embedded supported bilayers. Biomaterials 34:7895–7904. 10.1016/j.biomaterials.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott RE. 1976. Plasma membrane vesiculation: a new technique for isolation of plasma membranes. Science 194:743–745. 10.1126/science.982044. [DOI] [PubMed] [Google Scholar]
- 26.Liu H-Y, Grant H, Hsu H-L, Sorkin R, Bošković F, Wuite G, Daniel S. 2017. Supported planar mammalian membranes as models of in vivo cell surface architectures. ACS Appl Mater Interfaces 9:35526–35538. 10.1021/acsami.7b07500. [DOI] [PubMed] [Google Scholar]
- 27.Richards MJ, Hsia C-Y, Singh RR, Haider H, Kumpf J, Kawate T, Daniel S. 2016. Membrane protein mobility and orientation preserved in supported bilayers created directly from cell plasma membrane blebs. Langmuir 32:2963–2974. 10.1021/acs.langmuir.5b03415. [DOI] [PubMed] [Google Scholar]
- 28.Long JS, Mistry B, Haslam SM, Barclay WS. 2019. Host and viral determinants of influenza A virus species specificity. Nat Rev Microbiol 17:67–81. 10.1038/s41579-018-0115-z. [DOI] [PubMed] [Google Scholar]
- 29.Floyd DL, Ragains JR, Skehel JJ, Harrison SC, van Oijen AM. 2008. Single-particle kinetics of influenza virus membrane fusion. Proc Natl Acad Sci USA 105:15382–15387. 10.1073/pnas.0807771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klenk E, Faillard H, Lempfrid H. 1955. Enzymatic effect of the influenza virus. Hoppe-Seylers Z Physiol Chem 301:235–246. 10.1515/bchm2.1955.301.1-2.235. [DOI] [PubMed] [Google Scholar]
- 31.Gottschalk A, Lind PE. 1949. Product of Interaction between influenza virus enzyme and ovomucin. Nature 164:232–233. 10.1038/164232a0. [DOI] [PubMed] [Google Scholar]
- 32.Bateman AC, Karamanska R, Busch MG, Dell A, Olsen CW, Haslam SM. 2010. Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells. J Biol Chem 285:34016–34026. 10.1074/jbc.M110.115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, Takano M, Kurebayashi Y, Masuda M, Kawagishi S, Takaguchi M, Yamanaka T, Minami A, Otsubo T, Ikeda K, Suzuki T. 2014. N-glycolylneuraminic acid on human epithelial cells prevents entry of influenza A viruses that possess N-glycolylneuraminic acid binding ability. J Virol 88:8445–8456. 10.1128/JVI.00716-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilbourne ED. 1969. Future influenza vaccines and the use of genetic recombinants Bull World Health Organ 41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 35.Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333:426–431. 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 36.Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS. 2014. The sweet spot: defining virus–sialic acid interactions. Nat Rev Microbiol 12:739–749. 10.1038/nrmicro3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauter NK, Bednarski MD, Wurzburg BA, Hanson JE, Whitesides GM, Skehel JJ, Wiley DC. 1989. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry 28:8388–8396. 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 38.Keppler OT, Herrmann M, von der Lieth CW, Stehling P, Reutter W, Pawlita M. 1998. Elongation of the N-acyl side chain of sialic acids in MDCK II cells inhibits influenza A virus infection. Biochem Biophys Res Commun 253:437–442. 10.1006/bbrc.1998.9650. [DOI] [PubMed] [Google Scholar]
- 39.Higa HH, Rogers GN, Paulson JC. 1985. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology 144:279–282. 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- 40.Ramalho-Santos O. 2004. The role of target membrane sialic acid residues in the fusion activity of the influenza virus: the effect of two types of ganglioside on the kinetics of membrane merging. Cell Mole Bio Letters 9:15. [PubMed] [Google Scholar]
- 41.Rawle RJ, Boxer SG, Kasson PM. 2016. Disentangling viral membrane fusion from receptor binding using synthetic DNA-lipid conjugates. Biophys J 111:123–131. 10.1016/j.bpj.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster ER, Liu KN, Rawle RJ, Boxer SG. 2022. Modulating the influenza A virus–target membrane fusion interface with synthetic DNA–lipid receptors. Langmuir 38:2354–2362. 10.1021/acs.langmuir.1c03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han J, Perez JT, Chen C, Li Y, Benitez A, Kandasamy M, Lee Y, Andrade J, tenOever B, Manicassamy B. 2018. Genome-wide CRISPR/Cas9 screen identifies host factors essential for influenza virus replication. Cell Rep 23:596–607. 10.1016/j.celrep.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu H-L, Millet JK, Costello DA, Whittaker GR, Daniel S. 2016. Viral fusion efficacy of specific H3N2 influenza virus reassortant combinations at single-particle level. Sci Rep 6:35537. 10.1038/srep35537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soumpasis DM. 1983. Theoretical analysis of fluorescence photobleaching recovery experiments. Biophys J 41:95–97. 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]