Abstract
Belatacept-based immunosuppression in kidney transplantation confers fewer off-target toxicities compared to calcineurin inhibitors but comes at a cost of increased incidence and severity of acute rejection, potentially due to its deleterious effect on both the number and function of Foxp3+ Treg. TIGIT is a CD28 family coinhibitory receptor expressed on several subsets of immune cells including Treg. We hypothesized that coinhibition through TIGIT signaling could function to ameliorate costimulation blockade-resistant rejection. Results demonstrate that treatment with an agonistic anti-TIGIT antibody, when combined with costimulation blockade by CTLA-4Ig, can prolong allograft survival in a murine skin graft model compared to CTLA-4Ig alone. Further, this prolongation of graft survival is accompanied by an increase in the frequency and number of graft-infiltrating Treg and a concomitant reduction in the number of CD8+ T cells in the graft. Through the use of Treg-specific TIGIT conditional knockout animals, we demonstrate that the TIGIT-mediated reduction in the graft-infiltrating CD8+ T cell response is dependent on signaling of TIGIT on Foxp3+ Treg. Our results highlight both the key functional role of TIGIT on Foxp3+ Treg under conditions in which CTLA-4 is blocked and the therapeutic potential of TIGIT agonism to optimize costimulation blockade-based immunosuppression.
1. Introduction
Calcineurin inhibitors, the current standard of care immunosuppression for organ transplant recipients, are effective but associated with numerous off target toxicities1–4. Belatacept, a CTLA-4Ig fusion protein, inhibits cell-mediated graft rejection by binding CD80 and CD86 on antigen presenting cells (APC), subsequently preventing costimulatory signaling through CD28 on T cells5, and has been associated with fewer off-target toxicities and improved long-term graft survival and kidney function compared to calcineurin inhibitors 1–4. Despite these benefits, belatacept is associated with higher incidence and severity of acute cellular rejection following transplantation 6.
Alterations in two main immune cell subsets have been implicated in belatacept-resistant rejection: Foxp3+ Treg and CD8+ memory T cells. First, numerous studies have shown that the CTLA-4-mediated suppressive function of Treg is compromised in the setting of belatacept7. Second, studies in mice, non-human primates, and humans have identified CD8+ alloreactive memory T cells as mediating costimulation blockade-resistant rejection8, demonstrated by the findings that distinct CD8+ memory T cell populations exhibit reduced requirements for CD28 costimulation8–10. As such, identifying alternate pathways to both augment Treg-mediated suppression and inhibit CD28-independent memory CD8+ T cell populations is a clinically relevant goal in transplantation.
TIGIT is a T cell immune receptor with immunoglobulin and ITIM motifs with inducible expression on subsets of NK cells, CD4+ and CD8+ T cells11, and B cells12,13, and constitutive expression on a subset of regulatory T cells (Treg) 11,14. Analogous to the CTLA-4/CD28 checkpoint axis, coinhibitory TIGIT competes with the costimulatory adhesion molecule DNAM-1 (CD226) for binding to the ligands CD155 and CD112 on APC. TIGIT was first described to function by binding to its ligands and inducing the secretion of suppressive cytokines by APC, secondarily resulting in inhibition of T cell responses 11. However, both Treg-mediated 14–17 and CD8+ T-cell autonomous inhibitory mechanisms of TIGIT have recently been described 18. Both antagonistic and agonistic αTIGIT antibodies have been shown to modulate this immune checkpoint in vitro and in vivo in mice 15,19. In models of cancer, blocking TIGIT revived cytokine production in CD8+ T cells18, while TIGIT agonism promoted exhaustion of CD8+ T cells in models of cancer and chronic infection18–20. Recently, our group has shown that in vitro TIGIT agonism induces apoptosis of human memory T cell subsets associated with costimulation blockade-resistant rejection (CoBRR) in a Treg-dependent manner21.
Given its reported ability to both enhance Treg function and inhibit memory CD8+ T cell function, we aimed to determine the potential role of TIGIT agonism in ameliorating CoBRR in transplantation. Our results highlight the therapeutic potential of TIGIT agonism to function in combination with CTLA-4Ig to inhibit alloimmune responses within the graft and prolong allograft survival.
2. Methods
2.1. Mice
Male C57BL/6 (H-2b), BALB/c (H-2d), and B6/Ly5.2 mice (H-2b) (all 6–8 weeks) were obtained from the National Cancer Institute (Frederick, MD). OT-I 22 and OT-II 23 transgenic mice were purchased from Taconic Farms (Germantown, NY) and bred to Thy1.1+ mice at Emory. Transgenic mice expressing membrane-bound chicken ovalbumin under the control of the beta-actin promoter (C57BL/6 background, H-2b)24 were a generous gift from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN). Female B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr mice were purchased from Jackson Laboratories 25 (Stock No: 016959, Farmington, CT) and bred to TIGITfl/fl mice, a generous gift from Dr. Jane Grogan (Genentech). Recipients were either male or female, and donor animals for adoptive transfers and skin transplantation were sex matched to the recipient. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. The protocol (PROTO201700558) was approved by the Institutional Animal Care and Use Committee of Emory University.
2.2. Adoptive cell transfers and skin grafts
Thy1.1+ congenic OVA-specific OT-I (CD8+) and OT-II (CD4+) TCR transgenic cells were harvested from spleen and mesenteric lymph nodes. Cells were counted using a Nexcelom Cellometer Auto T4 (Nexecelom Bioscience, Lawrence, MA) and stained with CD8, CD4, Thy1.1, Vα2, and Vβ5 antibodies (Supplemental Table 1). Frequencies of OT-I and OT-II T cells were determined via Vα2 and Vβ5 TCR co-expression. 1e6 OT-I and 1e6 OT-II cells were resuspended in 1X PBS and co-adoptively transferred into naïve hosts 24 h prior to skin transplantation. Skin transplantation was performed using 1cm × 1cm full-thickness ear and tail skin from OVA expressing mice (or fully allogenic BALB/c skin where indicated) on the dorsal thorax and wrapped with bandages for 7 days 26. Where indicated, mice were treated intraperitoneally with 250ug CTLA-4Ig (abatacept, Bristol Myers-Squibb) on days 0, 2, 4, and 6; 250ug of anti-TIGIT antibody (Clone: 1G9, BioXCell, West Lebanon, NJ) on days 0, 2, 4, and 6; or the two combined. Grafts were considered rejected when less than 10% of viable graft remained.
2.3. Flow cytometry
Where indicated, OT-I T cells were labeled with 5uM CellTrace Violet (CTV) dye (Life Technologies, Invitrogen) and then adoptively transferred into naïve hosts. Spleens and draining lymph nodes (DLN, axial and brachial) were homogenized into single cell suspensions prior to antibody staining (Supplemental Table 1). The entire remaining skin grafts were removed from the mouse and cut into 2mm pieces. The tissue was then digested using 2 mg/mL collagenase-P in HBSS + Ca2+ and Mg2+ for 30 minutes at 37°C prior to homogenization and antibody staining. Capturing the entire area of the remaining graft permits assessment of the absolute number of lymphocytes infiltrating what was initially a 1cm2 size-standardized graft. For transcription factor staining, cells were fixed and permeabilized using the Foxp3/transcription factor staining kit from eBiosciences. For active caspase 3/7 and 7-AAD staining, CellEvent Caspase kit (Thermofisher) was used following the manufacturer’s instructions. All flow cytometry samples were acquired on a Fortessa cytometer (BD Biosciences), and data were analyzed using FlowJo (v10.8.1 Tree Star, San Carlos, CA) and Prism (v9 for Mac, GraphPad Software). Absolute cell numbers were calculated using CountBright Beads (Life Technologies).
2.4. RNA sequencing
Fully MHC-mismatched (Balb/c, H-2d) skin was grafted onto H-2b B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr xTIGITfl/fl recipients or wild type littermate controls. Animals were treated on days 0, 2, 4, and 6 with CTLA-4Ig + TIGIT agonist and sacrificed on day 10. DLN were recovered, homogenized and CD4+ T cells were negatively selected for using MACS enrichment (Miltenyi) prior to sorting on CD25+YFP+GITR+ (conditional knock-out) or CD25+YFP−GITR+ (WT) Treg. 5,000 purified Treg from 7 cKO and 7 WT animals were lysed using QIAshredder columns (Qiagen) and mRNA was extracted (RNeasy Micro extraction kit, Qiagen). Library preparation (TakaraBio’s SMART-Seq v4 Ultra Low Input RNA Kit with Illumina’s Nextera XT DNA Library Preparation Kit) and sequencing (Illumina NovaSeq 6000 and Illumina MiSeq) were performed by the Genomics Core at Emory University. Data was analyzed in R using the DESeq2 package from Bioconductor27, Gene Set Enrichment Analysis (v4.2.3 for Mac GSEA) and Morpheus (both publicly available from the Broad Institute). Gene ontology analysis of differentially expressed genes was performed using PANTHER28,29 and GORILLA30 software.
2.5. In vitro Treg suppression assays
Treg were isolated from naïve wildtype C57BL/6 mice or Foxp3tm4(YFP/icre)Ayr xTIGITfl/fl mice using the Miltenyi CD4+CD25+ regulatory T cell isolation kit (product # 130-091-041) following manufacturer’s instructions, purity of enrichment was confirmed by flow cytometry. Splenocytes were obtained from naïve OT-I mice and labeled with Cell Trace Violet according to manufacturer’s instructions. The proportion of CD8+ OT-I T cells of total splenocytes was determined by cell counting and flow cytometry, this number was used to determine the appropriate number of Treg to plate in each well with 3e5 total OT-I splenocytes. Treg/OT-I co-cultures were stimulated with the OVA peptide SIINFEKL (10nM) in the presence of 100μg/mL TIGIT agonist (clone 1G9), CTLA-4Ig, or CTLA-4Ig+TIGIT agonist for 3 days at 37°C. Flow cytometry was used to assess OT-I T cell proliferation and quantify Treg after culture (antibodies in Supplemental Table 1). Percent suppression was calculated using the following equation:
3. Results
3.1. TIGIT agonism alleviates costimulation blockade-resistant rejection and attenuates graft-infiltrating CD8+ T cell responses
We assessed the ability of TIGIT agonism to attenuate costimulation blockade-resistant allograft rejection in a minor antigenic mismatch model of murine skin transplantation. To do this, we performed an adoptive transfer of 1e6 Thy1.1+ TCR transgenic CD8+ (OT-I) and CD4+ (OT-II) T cells that specifically recognize OVA peptides presented on MHC class I and class II respectively one day prior to giving full thickness ear and tail skin grafts from donor mice expressing the membrane-bound OVA protein (mOVA) under the control of the β-actin promoter. Recipient mice were then treated with an agonistic anti-TIGIT antibody either alone or in combination with the costimulation blocker CTLA-4Ig (abatacept) on days 0, 2, 4, and 6 after grafting (Figure 1A). TIGIT agonism alone had no influence on graft survival compared to the PBS treated control group (median survival time, MST = 20 days), while CTLA-4Ig treatment modestly extended the MST to 30 days. In contrast, the combination of CTLA-4Ig and the TIGIT agonist significantly prolonged graft survival compared to either treatment alone, with 50% of mice in the study experiencing long-term graft survival extending beyond 80 days (log-rank Mantel-Cox P value of 0.0007, Figure 1B).
Figure 1: TIGIT agonism combined with CTLA-4Ig prolongs allograft survival, increases graft-infiltrating Treg while reducing graft-infiltrating CD8+ T cells.
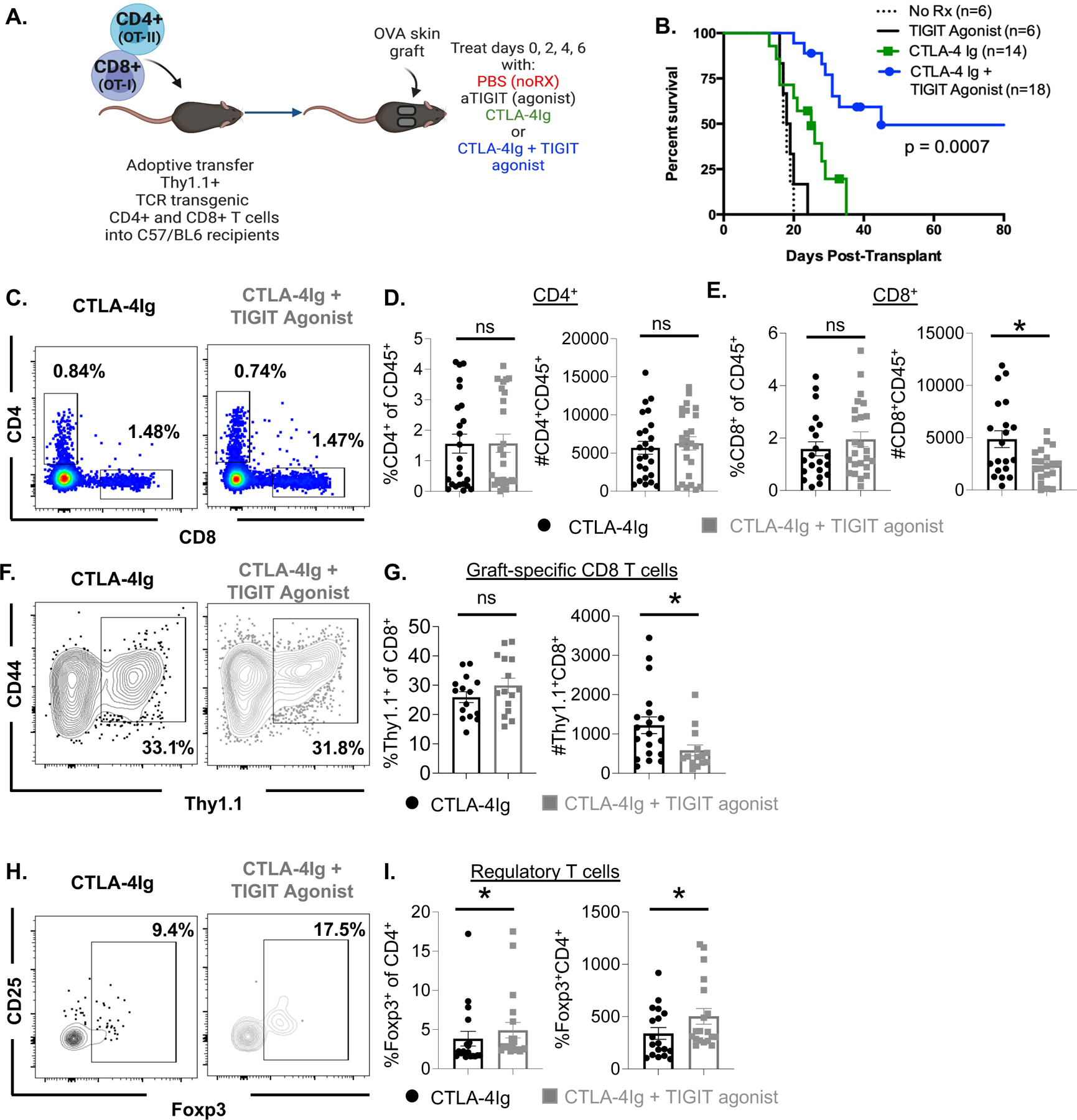
. (A) Schematic of experimental design. Thy1.1+ TCR transgenic OT-I and OT-II T cells are adoptively transferred into naive C57BL/6 hosts 24 h prior to grafting with OVA expressing skin. Animals are subsequently treated with PBS, TIGIT agonist (250ug), CTLA-4Ig (250ug) or a combination of CTLA-4Ig + TIGIT agonist on days 0, 2, 4, and 6 after transplant. Allograft survival was monitored after skin graft and cessation of immunosuppression (B). Skin graft tissue was also collected 10 days after allograft challenge and digest to determine the infiltrating lymphocyte populations (C-I). Representative flow cytometry plots and summary data for the bulk CD4+ T cell population (C, D left panel) bulk CD8+ T cell population (C, E right panel) from skin graft tissue are shown. Thy1.1+ OT-I T cells within the CD8+ T cell compartment were detected and quantified (representative flow F, summarized G). Treg were detected within the CD4+ T cell compartment off the grafted tissue (represented in H, summarized I). Summary of graft survival data pooled from three independent experiments. Flow cytometry data are representative of 3 independent experiments (n=20 per group) mean ± SEM is shown. Non-parametric Mann-Whitney T tests were performed (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
To understand the cellular mechanisms underlying the prolonged graft survival observed in animals treated with the combination of CTLA-4Ig and TIGIT agonist compared to CTLA-4Ig treatment alone, we assessed T cell populations within the grafted tissue 10 days post-transplant and treatment with CTLA-4Ig or CTLA-4Ig + TIGIT agonist as described above. Results showed no difference in the frequency or absolute number of graft-infiltrating CD4+ T cells between CTLA-4Ig-treated animals and animals treated with CTLA-4Ig + TIGIT agonist (Figure 1C, D). However, there was a significant reduction in the absolute number of graft-infiltrating CD8+ T cells in animals treated with the TIGIT agonist compared to animals treated with CTLA-4Ig alone (p=0.0326, Figure 1C, E). The number of graft-infiltrating donor-specific Thy1.1+ OT-I T cells in CTLA-4Ig+TIGIT agonist-treated animals was also significantly reduced compared to animals treated with CTLA-4Ig alone (p=0.0173, Figure 1F, G). Interestingly, higher frequencies (p=0.0136) and numbers (p=0.0046) of Foxp3+ Treg cells were detected in skin grafts of animals treated with CTLA-4Ig + TIGIT agonist compared to animals treated with CTLA-4Ig alone (Figure 1H, I). Surprisingly, these data were not recapitulated in the graft draining lymph nodes (Supplemental Figure 1A–I). Because naïve CD8+ T cell responses to allograft are primed in the graft draining lymph node31,32, and no difference in the magnitude of the antigen-specific response in the lymph nodes was observed, our data suggest that TIGIT agonism does not affect the T cell priming event. Instead, these data suggest that the combination of CTLA-4Ig + TIGIT agonist attenuates the immune response in the graft by simultaneously decreasing infiltration of donor-reactive CD8+ T cells and increasing infiltration of Treg within the graft.
Experiments to test the impact of global TIGIT knockout on graft survival were performed and revealed that there was no impact of global TIGIT deficiency on graft survival in the context of CTLA-4Ig. Likewise, global TIGIT deficiency in the absence of immunosuppression had no impact on graft survival (data not shown). From these experiments we concluded that during normal rejection responses, insufficient TIGIT coinhibitory signals are received to negatively regulate alloreactive T cells and the use of a pharmacologic TIGIT agonist can therefore function to provide these signals and synergize with CD28 blockade in prolonging allograft rejection (Figure 1B).
3.2. TIGIT on Treg is required for prolonged allograft survival and reduction of graft-infiltrating CD8+ T cells afforded by TIGIT agonism
Because TIGIT is expressed on convention CD4+ and CD8+ T cells as well as Treg (Supplemental Figure 2A–D) and is well-known to be expressed on NK cells11,33–35. We first determined that the magnitude of the NK cell population was not affected by treatment with the TIGIT agonist in our model, in that no significant change in the quantity of NK cells between CTLA-4Ig-treated and CTLA-4Ig+TIGIT agonist-treated animals was observed (Supplemental Figure 3A–B). Further, NK cells were not required for TIGIT agonist-induced graft survival, in that no significant difference in graft survival was observed between NK cell-depleted vs. non-depleted mice in the context of CTLA-4Ig+ TIGIT agonist (Supplemental Figure 3C). Thus, the ability of TIGIT agonism to prolong graft survival and reduce CD8+ graft-infiltrating T cells could be a direct effect of TIGIT on CD8+ effectors, or an indirect effect via a TIGIT+ Treg-dependent mechanism. Therefore, to determine whether the observed effect of TIGIT agonism on donor-reactive CD8+ T cells is Treg-dependent, we generated Treg-specific TIGIT conditional knockout mice. Foxp3Cre mice 25 were crossed with TIGITfl/fl mice, thus generating progeny in which TIGIT is knocked out on Treg. TIGIT expression remains intact on other lymphocyte populations (Supplemental Figure 4A–B). Genetic deletion of TIGIT from Treg had no effect on the size of the CD8+, CD4+, or Treg population in naïve mice (Supplemental Figure 4C–D). Foxp3CreTIGITfl/fl (cKO) mice and wildtype (WT) littermate controls adoptively transferred with 1e6 donor-reactive Thy1.1+ OT-I T cells, grafted with OVA-expressing skin grafts, and treated with CTLA-4Ig+TIGIT agonist (Figure 2A). Results indicated that CTLA-4Ig+TIGIT agonist-treated Foxp3CreTIGITfl/fl cKO animals exhibited accelerated allograft rejection compared to WT controls (Figure 2B, MST for WT animals >100 days compared to MST of 60 days in cKO animals, p=0.0107). Moreover, analysis of donor-reactive CD8+ T cells in these animals showed that TIGIT agonism failed to reduce the accumulation of donor-reactive Thy1.1+ T cells in the graft when TIGIT is not expressed by Treg (Figure 2C, D). These data show that the ability of TIGIT agonism to limit CD8+ T cell infiltration into the graft is dependent on the presence of TIGIT+ Treg.
Figure 2: TIGIT on Treg is required for prolonged allograft survival and the cellular responses conferred by treatment with CTLA-4Ig + TIGIT agonist.
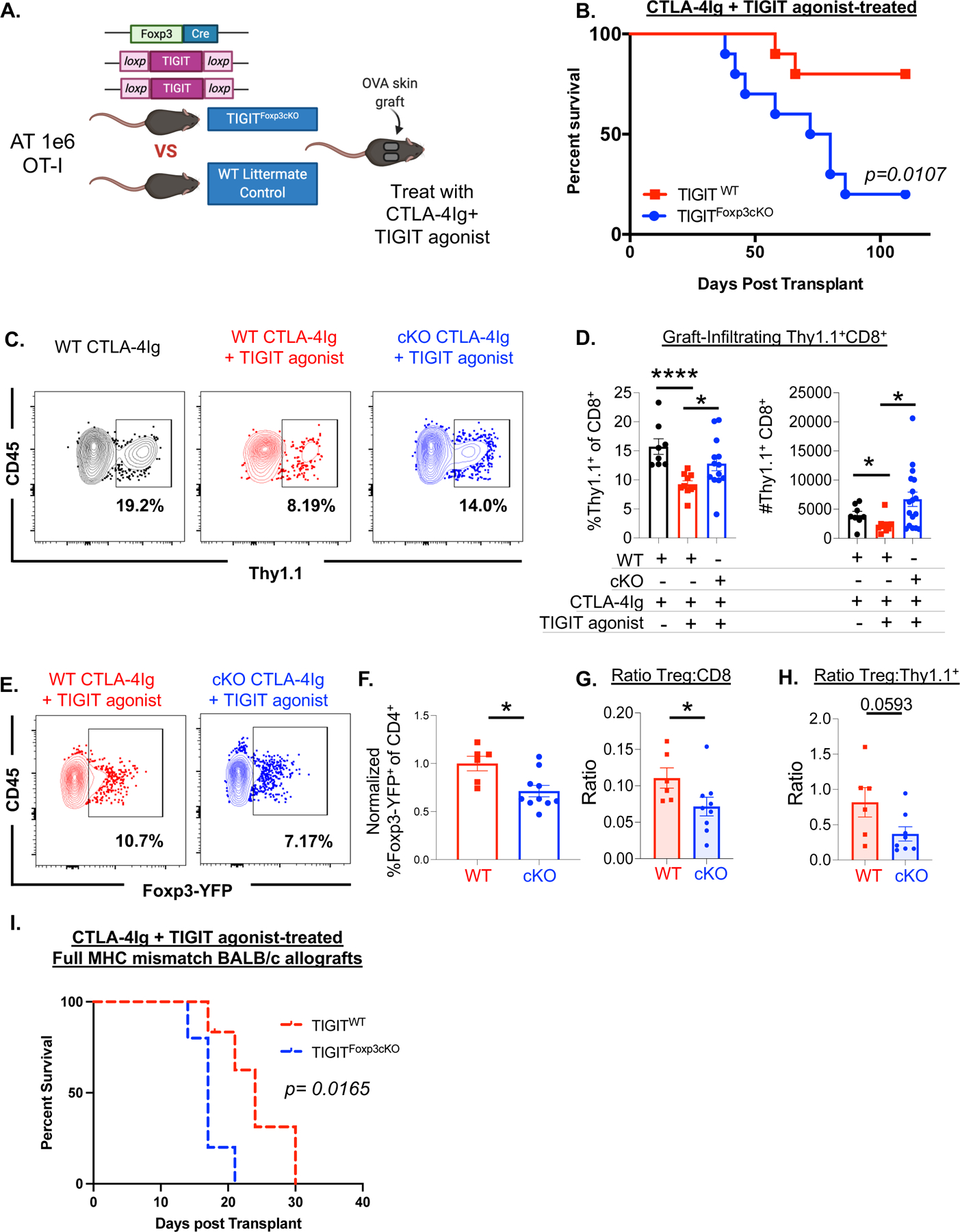
Foxp3tm4(YFP/icre)Ayr mice were crossed to TIGITfl/fl mice prior to adoptive transfer of OT-I T and OT-II T cells and OVA allograft challenge (schematic, A) and treatment CTLA-4Ig + TIGIT agonist. Skin graft survival was assessed comparing TIGITfl/fl WT littermate controls to Foxp3-Cre × TIGITfl/fl mice, all treated with the combination of CTLA-4Ig + TIGIT agonist (B). (C) Graft infiltrating lymphocytes were assessed 10 days after adoptive transfer of OT-I T cells and allograft challenge and treatment with CTLA-4Ig (Foxp3-Cre × TIGITWT/WT WT littermate controls) or CTLA-4Ig + TIGIT agonist (Foxp3-Cre × TIGITfl/fl cKO and Foxp3-Cre × TIGITWT/WT WT littermate controls). The frequency and number of Thy1.1+ cells within the CD8+ T cell compartment (C) were determined. Graft-infiltrating Treg were measured by gating on Live, CD45+CD4+ T cells and determining the Foxp3+ cell population within the CD4+ parent gate (E). Summary data is representative of 2-independent replicates (F) mean ± SEM is shown. The ratio of Treg: graft-infiltrating CD8+ T cells (G) and Treg: OT-I T cells (H) was calculated and is summarized for two independent replicates. (I) Foxp3-Cre × TIGITfl/fl cKO and Foxp3-Cre × TIGITWT/WT WT littermate controls were grafted with BALB/c skin and treated with 4 doses of CTLA-4Ig + TIGIT agonist, grafts were monitored for survival (n=5 per group, p=0.0165). Non-parametric Mann-Whitney T tests were performed (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
We also assessed the frequency of graft-infiltrating Treg by quantifying YFP expression by flow cytometry since both TIGIT cKO animals and wildtype littermate controls expressed YFP under the Foxp3 promotor in these experiments. We observed that TIGIT cKO mice exhibited a decrease in the frequency of graft-infiltrating Treg compared to wildtype mice after treatment with CTLA-4Ig+TIGIT agonist (p=0.0357, Figure 2E, F). Taken together, TIGIT cKO animals experience a significant reduction in the Treg: CD8 ratio and a modest reduction in the Treg: OT-I ratio within the graft tissue compared to WT animals after treatment with CTLA-4Ig+TIGIT agonist (p=0.036 Figure 2G, 2H).
To corroborate these results obtained in a minor mismatch model in a model of full MHC disparity, TIGIT cKO mice and WT littermate controls were grafted with fully MHC- mismatched BALB/c skin grafts and graft survival was assessed following treatment with CTLA-4Ig+ TIGIT agonist (Figure 2I). Like observations in the minor antigen mismatch model, WT recipients of a fully MHC-mismatched BALB/c allograft exhibited a significant graft-survival advantage (MST= 24 days) over TIGIT cKO recipients of a BALB/c graft (MST = 17 days, p=0.0165). Together with the data in the minor mismatch model, these data confirm that TIGIT expression on Treg is required to achieve the prolonged allograft survival afforded by TIGIT agonist treatment in the context of CTLA-4Ig.
3.3. WT Treg treated with TIGIT agonist express a gene profile enriched with a Treg phenotypic profile
Given that we observed a Treg-dependent reduction of donor-reactive Thy1.1+ CD8+ T cells in the skin graft tissue after treatment with CTLA-4Ig+TIGIT agonist, we sought to interrogate qualitative, transcriptomic differences between WT and TIGIT cKO Treg. BALB/c skin was transplanted onto Foxp3CreTIGITfl/fl cKO mice vs. WT littermate controls. Mice were treated with CTLA-4Ig+TIGIT agonist on days 0, 2, 4, and 6 and sacrificed on day 10. Treg were isolated from the draining lymph nodes by first enriching for CD4+ T cells using negative selection magnetic-activated cell sorting followed by FACS sorting for CD25+YFP+GITR+ (cKO) or CD25+YFP−GITR+ (WT) Treg (Figure 3A, B). Flow cytometry analysis confirmed that TIGIT cKO Treg exhibit reduced CD25 expression relative to WT Treg (p=0.035 Figure 3B). mRNA was then isolated from sorted cells and Illumina sequencing was performed. Over 14,000 genes were identified in the sorted Treg; of those, 67 were differentially expressed between TIGIT cKO Treg and WT Treg with an adjusted p value <0.05 (Figure 3C). Gene set enrichment analysis (GSEA) was performed on the entire gene set using the ImmuneSigDB database; of the 20 established gene sets with the highest enrichment scores within our data, 6 associated our data with a Treg (vs. Tconv) lineage in WT vs. cKO Treg. For example, GSE7852_Treg_vs_Tconv_LN_UP is a dataset curated by Feuerer et al. that is enriched in our transcriptome data (Figure 3D, normalized enrichment score= 2.26, p<0.01)36. Leading edge genes that contributed to this phenotype enrichment in WT vs cKO Treg treated with TIGIT agonist +CTLA-4Ig include genes related to Treg survival and stability (Figure 3E). These include IL2ra and IL2rb37, the SOCS family genes Socs538 and Cish39, as well as Dusp440,41, Penk42, and others. We also saw differential mRNA expression of genes related to cell survival between WT and TIGIT cKO Treg. Fam129a is a gene that has been shown to regulate protein translation and promote cell survival43 and is significantly enriched in WT Treg after treatment with CTLA-4Ig+TIGIT agonist compared to cKO Treg (p=0.019, Figure 3F). Only 25 genes were significantly upregulated in TIGIT cKO Treg compared to WT Treg. Of these, Brap44, Cables245, Dnaj346, and Usp447,48 are all reported to be involved in preventing cell cycle progression or promoting apoptosis (Figure 3F). We also quantified Foxp3 protein expression by flow cytometry in the grafts and draining lymph nodes of WT and TIGIT cKO mice after BALB/c allograft challenge and treatment with CTLA-4Ig + TIGIT agonist. Foxp3 expression was significantly higher in WT Treg compared to TIGIT cKO Treg in the graft (p=0.0286 Figure 3G), LAG3 was minimally expressed on Treg in the dLN and graft tissue, and expression within the graft was not different between the two groups (Figure 3H). The discrepancy between RNA and protein level data for LAG3 could be due to the reported cleavage of LAG3 from the cell surface as well as mechanisms of recycling and endosomal storage of the molecule49. We next performed gene ontology (GO) analysis on genes that were differentially expressed (DEG) between WT vs. cKO Treg. Genes that were upregulated in WT Treg relative to TIGIT cKO Treg were significantly enriched in pathways related to negative regulation of the adaptive immune response (Figure 3I, Supplemental Figure 5, Supplemental Table 2). These data demonstrate that Treg receiving TIGIT signaling in the context of CTLA-4Ig exhibit increased expression of transcripts associated with increased stability and survival of Treg compared to Treg where TIGIT has been genetically deleted.
Figure 3: Expression profile of WT vs. TIGIT cKO Treg after treatment with CTLA-4Ig + TIGIT agonist.
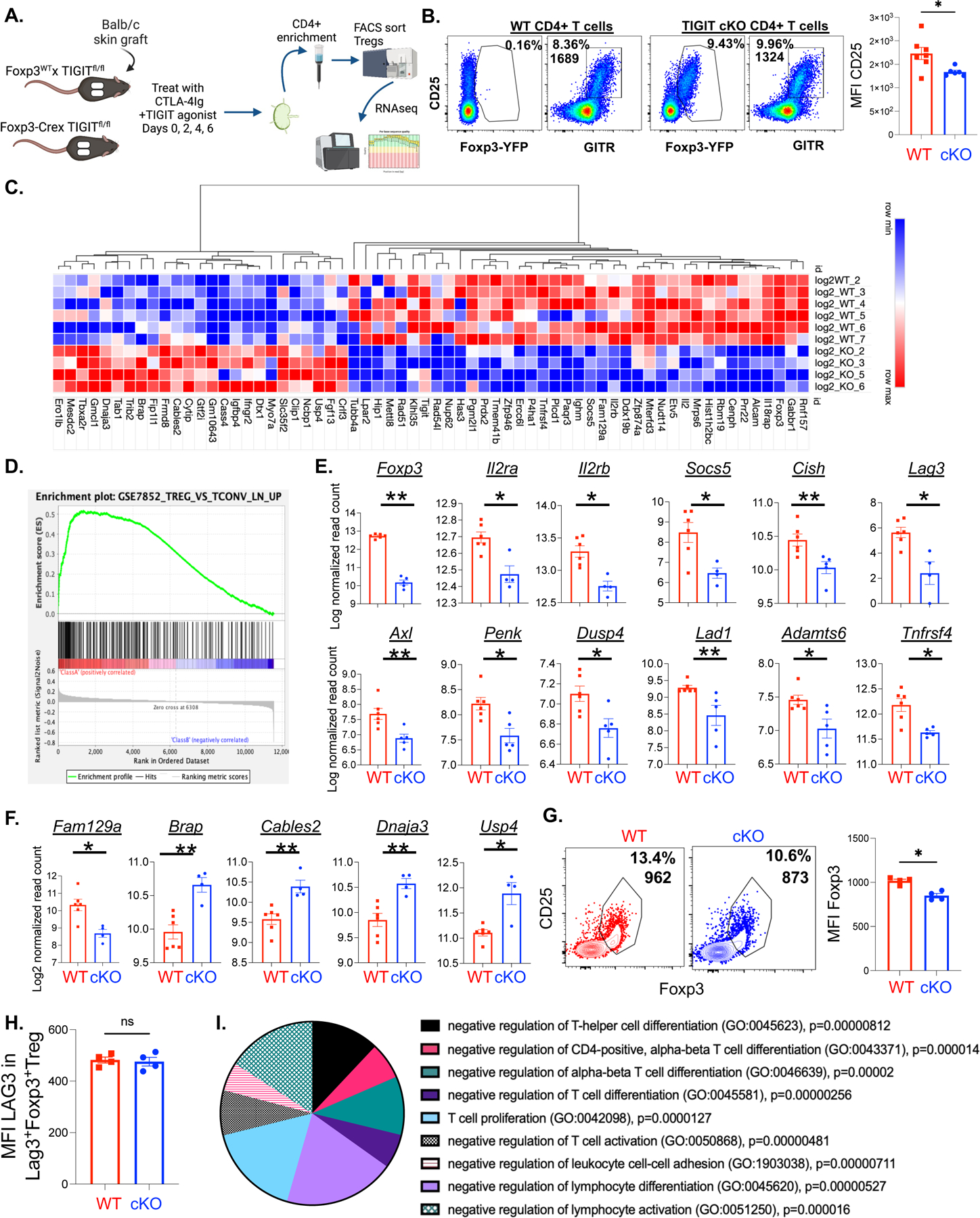
(A)Treg were isolated from the draining lymph nodes of WT (TIGITfl/fl) or TIGIT cKO (Foxp3-Cre × TIGITfl/fl) mice 10 days after fully allogenic mismatch skin graft challenge and treatment with CTLA-4Ig + TIGIT agonist. Cells were sorted (gating scheme and CD25 expression quantification, B) and mRNA from purified Treg was prepared for Next-Gen RNA sequencing analysis. 64 of the identified genes were significantly differentially expressed between WT and cKO Treg with an adjusted p value < 0.05 (represented in the heatmap, C). Differential expression by RNAseq of genes with the highest enrichment scores contributing to the leading edge within gene set GSE7852 are shown (D, E). Differentially expressed genes relating to cell survival, apoptosis, and cell cycle progression are highlighted in (F). Foxp3 protein expression was quantified using the mean fluorescence intensity (MFI) in the graft-infiltrating Treg population in of WT (Foxp3-Cre × TIGITWT/WT) and TIGIT cKO (Foxp3-Cre × TIGITfl/fl) mice after Balb/c allograft challenge and treatment with CTLA-4Ig+ TIGIT agonist (G, n=4 per group, p=0.0286). LAG3 protein expression was quantified by MFI in LAG3+ Treg population of the grafts of these animals (H, n=4 per group, p=0.886). Log2 normalized read counts, p values, and adjusted p values were calculated using DESeq2 from Bioconductor (C-F).
We next assessed the expression of genes associated with Treg function in WT vs. TIGIT cKO Treg. No difference in the mRNA expression of Ctla4, Tfgb, Fgl2, Helios, 4–1BB, Cd28, Icos, Il10, or Cd39 between WT and TIGIT cKO Treg was observed (Figure 4A), suggesting that the suppressive function of TIGIT cKO Treg was not demonstrably impaired as compared to WT Treg. To confirm these transcriptomic findings at a functional level, we performed Treg suppression assays using Treg from WT vs. TIGIT cKO mice, stimulated in the presence of CTLA-4Ig, TIGIT agonist, or the combination of CTLA-4Ig+TIGIT agonist. While each of the immunosuppressive treatments impaired in vitro proliferation of OT-I T cells compared to untreated OT-I responder cells (Supplemental Figure 6A), there was no difference in the percent suppression between WT and TIGIT cKO Treg in any of the treatment conditions (Figure 4C). This is consistent with our RNAseq findings that there were no differences in the expression of genes related to Treg function. Moreover, we did not observe an impact of either CTLA-4Ig, TIGIT agonist, or the combination on the suppressive capacity of Treg (Supplemental Figure 6B). However, we did measure a significant increase in the number of Foxp3+ Treg in cell culture wells after treatment with CTLA-4Ig+TIGIT agonist compared to CTLA-4Ig alone (p=0.0045). Together, these data demonstrate that in the context of CTLA-4Ig, TIGIT agonism does not affect the functional suppressive capacity of Treg on a per cell basis, but instead suggest that TIGIT agonism in the context of CTLA-4Ig improves Treg survival and/or differentiation.
Figure 4: TIGIT on Treg does not affect Treg suppressive function in the context of CTLA-4Ig.
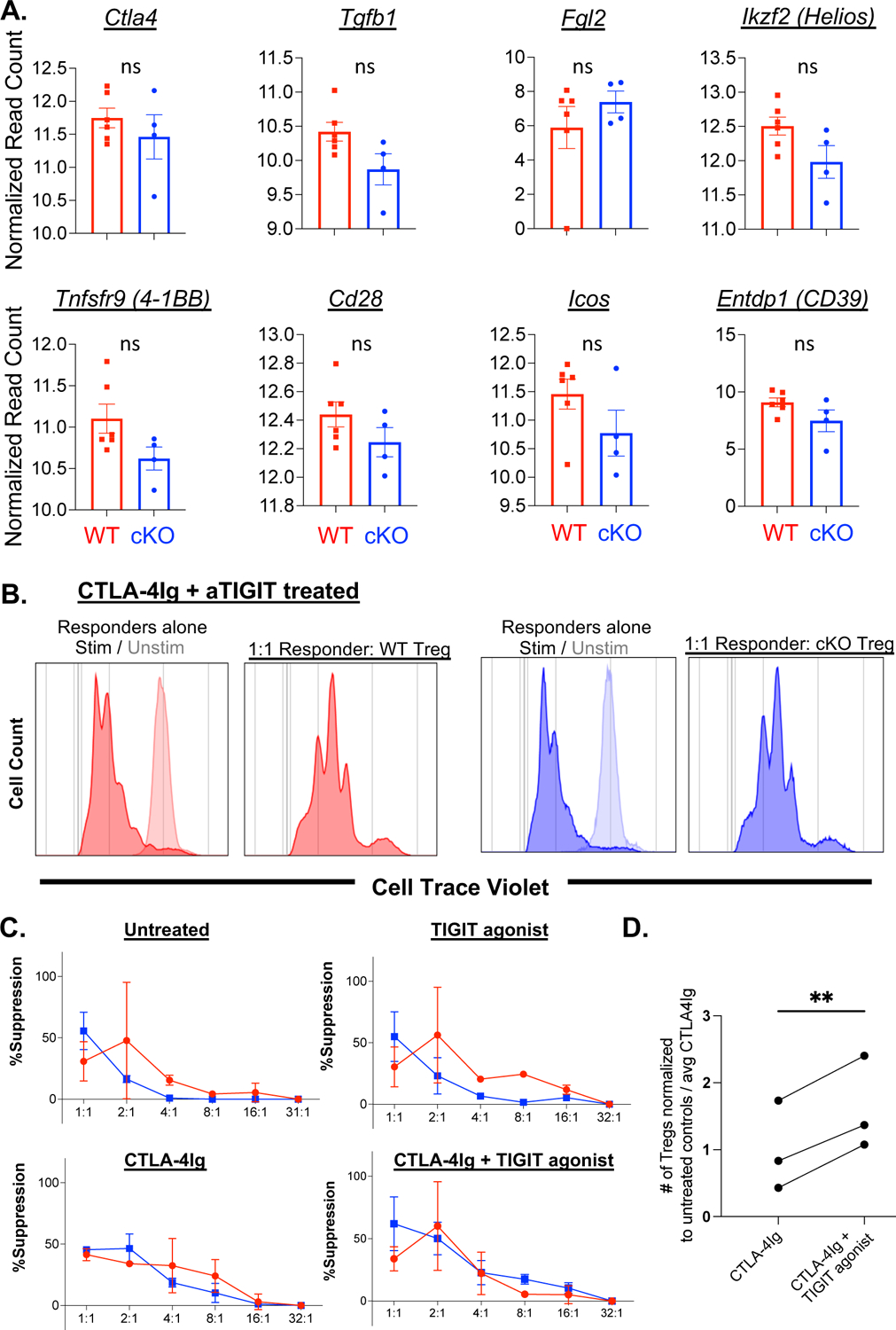
Differential gene expression was calculated for genes related to Treg function (A). Treg suppressor function was measured by Treg suppression assays where Treg were isolated from spleens of naïve WT (Foxp3-Cre × TIGITWT/WT) or TIGIT cKO (Foxp3-Cre × TIGITfl/fl) mice by MACS enrichment prior to plating with Cell Trace Violet (CTV) labeled splenocytes from OT-I mouse spleens. Cells were stimulated with SIINFEKL peptide and treated with 100μg/mL CTLA-4Ig, 100 μg/mL TIGIT agonist, or CTLA-4Ig+TIGIT agonist in culture for 3 days. Dilution of CTV was measured and the percent suppression was calculated (representative flow B, summarized for 2 independent experiments in C). Treg were cultured with naïve splenocytes and quantified by flow cytometry after 3 days in culture (summarized D), data are from 2 independent experiments. Non-parametric Mann Whitney T-tests were performed to determine p values in C, D. Non-parametric paired t-test was performed in E.
4. Discussion
The data presented here demonstrate that an agonistic anti-TIGIT antibody improves allograft survival in the setting of CTLA-4Ig costimulation blockade as compared to CTLA-4Ig alone. This increase in graft survival is underpinned by an increase in graft-infiltrating Treg, a cell subset well known for its ability to regulate alloimmune responses and create a tolerogenic immune landscape after transplantation50,51. However, most of the strategies for immunosuppression that are used clinically have a detrimental effect on the survival and function of Treg6,52,53. The data we present describe a potentially therapeutic strategy to promote the ability of the Treg that remain after costimulation blockade therapy to survive and access the allograft.
Our data show that TIGIT agonism in combination with CTLA-4Ig resulted in a decrease in graft-infiltrating CD8+ T cells and an increase in graft-infiltrating Treg compared to CTLA-4Ig treatment alone. This resulted in a 2.4-fold increase in the Treg:CD8+Teff cell ratio in the grafted tissue compared to CTLA-4Ig treatment alone. The long held notion has been that during the alloimmune response, Treg migrate to the draining lymph nodes and execute their suppression function on the antigen-specific response within the node7. However, our data suggest that a favorable Treg: Teff ratio within the graft may also contribute to the suppression of alloresponses. This is consistent with recent studies showing that Treg infiltration into allograft tissue can be a biomarker of rejection-free survival54–56.
Our data reveal that TIGIT expression and signaling is important for regulating gene expression in Treg. Foxp3 was a highly expressed gene in the Treg isolated from both the TIGIT cKO mice and WT littermate Treg, indicating that both groups of animals have Treg that express high amounts of the Foxp3 gene. Despite high expression in WT and cKO Treg, Foxp3 was the most significantly differentially expressed gene between WT and cKO Treg after allograft challenge and treatment with CTLA-4Ig + TIGIT agonist. This is consistent with recent literature showing that blocking CD226, the costimulatory counterpart to TIGIT, increased the expression of Foxp3 by providing more access for TIGIT to bind their shared ligand, CD15557. Our baseline analysis of the TIGIT conditional knockout mice compared to WT littermate controls showed that these animals possess similar frequencies and numbers of Treg in the blood, spleen and mesenteric lymph nodes at 6–8 weeks of age (Supplemental Figure 2G). Genes related to defects in cell cycle progression and mitochondrial regulation are upregulated in the TIGIT cKO Treg based on our RNA sequencing data, and experiments in WT mice +/−TIGIT agonism revealed no effects of TIGIT agonism on pro- or anti-apoptotic activity (Supplemental Figure 6C–E). Together, these data show that TIGIT cKO animals do not have a defect in the frequency and number of Treg at baseline or an increased propensity for apoptosis, but TIGIT signaling induced by the TIGIT agonist increases the expression of Foxp3 and supports Treg survival. Prior studies evaluating the function of TIGIT agonism have shown that TIGIT agonism leads to the production of IL-10 and the suppressive molecule Fgl2 by Treg14. Our RNA seq and in vitro ELISA data (data not shown) did not support this. Further, our in vitro assays show that there is no difference in the ability of WT or TIGIT cKO Tregs to suppress OT-I T cell proliferation in the presence of CTLA-4Ig+TIGIT agonist. While TIGIT agonism had a mild effect on Treg suppression compared to untreated wildtype T cells in culture (data not shown), this effect was not present between cells treated with CTLA-4Ig vs CTLA-4Ig+TIGIT agonist. Together, these data suggest that agonistic anti-TIGIT in the absence of CTLA-4 and CD28 signaling, as is the case in our studies, may not function through an IL-10 or Fgl2-dependent mechanism.
While we have identified a T cell intrinsic effect of TIGIT signaling on Treg, TIGIT is also expressed activated CD8+ T cells, and others have reported a CD8+ T cell intrinsic effect of TIGIT agonism20. Our study used novel Foxp3-Cre × TIGITfl/fl conditional knockout animals to show that reduction of graft-infiltrating CD8+ T cells was dependent on the presence of TIGIT+ Treg, but this does not eliminate the possibility of a CD8+ T cell-dependent role for TIGIT agonism For example, the median graft survival time for the TIGIT cKO mice treated with CTLA-4Ig + TIGIT agonist is >10 days longer than the MST observed when WT mice are treated with CTLA-4Ig alone (Figure 1B-green, Figure 4B-blue). However, the reduction of donor-specific graft-infiltrating CD8+ T cells we measured as a result of TIGIT agonism + CTLA-4Ig treatment are ablated when TIGIT is not expressed on Treg. Overall, these data further illuminate the Treg-autonomous role of TIGIT signaling in reducing graft-associated CD8+ T cell responses and improving allograft survival. These results provide a foundation for exploring TIGIT agonism as a therapeutic strategy to supplement belatacept treatment for transplant patients to reduce the incidence of CoBRR.
Supplementary Material
Acknowledgements
This work was supported by NIH/ NIAID AI154895, AI164716, and AI070081 to MLF.
Abbreviations:
- CoBRR
Costimulation blockade-resistant rejection
- Treg
Regulatory T cells
- NK
natural killer cells
- APC
Antigen presenting cells
- WT
Wild type
- cKO
conditional knock-out
- GSEA
Gene set enrichment analysis
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. MLF has received speaking honoraria from Veloxis Pharmaceuticals, Inc. The other authors have nothing to disclose.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Jardine AG. Assessing the relative risk of cardiovascular disease among renal transplant patients receiving tacrolimus or cyclosporine. Transpl Int. Apr 2005;18(4):379–84. doi: 10.1111/j.1432-2277.2005.00080.x [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. Aug 27 2004;78(4):557–65. doi: 10.1097/01.tp.0000128636.70499.6e [DOI] [PubMed] [Google Scholar]
- 3.Vanrenterghem Y Post-transplant diabetes mellitus and renal function with tacrolimus: A decade of use, a decade of evidence. Foreword. Nephrol Dial Transplant. Dec 2004;19 Suppl 6:vi1–vi2. doi: 10.1093/ndt/gfh1061 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CM, Arons RR. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation. Nov 15 2004;78(9):1351–5. doi: 10.1097/01.tp.0000140848.05002.b8 [DOI] [PubMed] [Google Scholar]
- 5.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. Mar 2006;24(3):233–8. doi: 10.1016/j.immuni.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. Jan 28 2016;374(4):333–43. doi: 10.1056/NEJMoa1506027 [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. May 30 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 8.Hartigan CR, Sun H, Ford ML. Memory T-cell exhaustion and tolerance in transplantation. Immunol Rev. Nov 2019;292(1):225–242. doi: 10.1111/imr.12824 [DOI] [PubMed] [Google Scholar]
- 9.Espinosa J, Herr F, Tharp G, et al. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. Am J Transplant. Apr 2016;16(4):1102–12. doi: 10.1111/ajt.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes-Cerisuelo M, Laurie SJ, Mathews DV, et al. Increased Pretransplant Frequency of CD28(+) CD4(+) TEM Predicts Belatacept-Resistant Rejection in Human Renal Transplant Recipients. Am J Transplant. Sep 2017;17(9):2350–2362. doi: 10.1111/ajt.14350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. Jan 2009;10(1):48–57. doi: 10.1038/ni.1674 [DOI] [PubMed] [Google Scholar]
- 12.Hasan MM, Nair SS, O’Leary JG, et al. Implication of TIGIT(+) human memory B cells in immune regulation. Nat Commun. Mar 9 2021;12(1):1534. doi: 10.1038/s41467-021-21413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao S, Bod L, Pochet N, et al. Checkpoint Receptor TIGIT Expressed on Tim-1(+) B Cells Regulates Tissue Inflammation. Cell Rep. Jul 14 2020;32(2):107892. doi: 10.1016/j.celrep.2020.107892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. Apr 17 2014;40(4):569–81. doi: 10.1016/j.immuni.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joller N, Hafler JP, Brynedal B, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. Feb 1 2011;186(3):1338–42. doi: 10.4049/jimmunol.1003081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucca LE, Axisa PP, Singer ER, Nolan NM, Dominguez-Villar M, Hafler DA. TIGIT signaling restores suppressor function of Th1 Tregs. JCI Insight. Feb 7 2019;4(3)doi: 10.1172/jci.insight.124427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Liang S, Jin J, et al. CD226 attenuates Treg suppressive capacity via CTLA-4 and TIGIT during EAE. Immunol Res. Dec 2019;67(6):486–496. doi: 10.1007/s12026-019-09112-9 [DOI] [PubMed] [Google Scholar]
- 18.Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. Dec 8 2014;26(6):923–937. doi: 10.1016/j.ccell.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 19.Dixon KO, Schorer M, Nevin J, et al. Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity. J Immunol. Apr 15 2018;200(8):3000–3007. doi: 10.4049/jimmunol.1700407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schorer M, Rakebrandt N, Lambert K, et al. TIGIT limits immune pathology during viral infections. Nat Commun. Mar 9 2020;11(1):1288. doi: 10.1038/s41467-020-15025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun H, Hartigan CR, Chen CW, et al. TIGIT regulates apoptosis of risky memory T cell subsets implicated in belatacept-resistant rejection. Am J Transplant. Oct 2021;21(10):3256–3267. doi: 10.1111/ajt.16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. Jan 14 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- 23.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. Feb 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x [DOI] [PubMed] [Google Scholar]
- 24.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. Nov 2003;3(11):1355–62. doi: 10.1046/j.1600-6135.2003.00246.x [DOI] [PubMed] [Google Scholar]
- 25.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. Apr 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 26.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. Dec 1999;104(12):1715–22. doi: 10.1172/JCI8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. Jan 8 2021;49(D1):D325–d334. doi: 10.1093/nar/gkaa1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. May 2000;25(1):25–9. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. Feb 3 2009;10:48. doi: 10.1186/1471-2105-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. Jun 2000;6(6):686–8. doi: 10.1038/76267 [DOI] [PubMed] [Google Scholar]
- 32.He C, Heeger PS. CD8 T cells can reject major histocompatibility complex class I-deficient skin allografts. Am J Transplant. May 2004;4(5):698–704. doi: 10.1111/j.1600-6143.2004.00416.x [DOI] [PubMed] [Google Scholar]
- 33.He Y, Peng H, Sun R, et al. Contribution of inhibitory receptor TIGIT to NK cell education. J Autoimmun. Jul 2017;81:1–12. doi: 10.1016/j.jaut.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 34.Li M, Xia P, Du Y, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem. Jun 20 2014;289(25):17647–57. doi: 10.1074/jbc.M114.572420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu F, Sunderland A, Zhou Y, Schulick RD, Edil BH, Zhu Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol Immunother. Oct 2017;66(10):1367–1375. doi: 10.1007/s00262-017-2031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. Aug 2009;15(8):930–9. doi: 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. Nov 2016;17(11):1322–1333. doi: 10.1038/ni.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knosp CA, Johnston JA. Regulation of CD4+ T-cell polarization by suppressor of cytokine signalling proteins. Immunology. Feb 2012;135(2):101–11. doi: 10.1111/j.1365-2567.2011.03520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Wu X, Wu D, et al. Treg expression of CIS suppresses allergic airway inflammation through antagonizing an autonomous TH2 program. Mucosal Immunol. Mar 2020;13(2):293–302. doi: 10.1038/s41385-019-0236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan D, Farache J, Mingueneau M, Mathis D, Benoist C. Imbalanced signal transduction in regulatory T cells expressing the transcription factor FoxP3. Proc Natl Acad Sci U S A. Dec 1 2015;112(48):14942–7. doi: 10.1073/pnas.1520393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsiao WY, Lin YC, Liao FH, Chan YC, Huang CY. Dual-Specificity Phosphatase 4 Regulates STAT5 Protein Stability and Helper T Cell Polarization. PLoS One. 2015;10(12):e0145880. doi: 10.1371/journal.pone.0145880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shime H, Odanaka M, Tsuiji M, et al. Proenkephalin(+) regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc Natl Acad Sci U S A. Aug 25 2020;117(34):20696–20705. doi: 10.1073/pnas.2000372117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun GD, Kobayashi T, Abe M, et al. The endoplasmic reticulum stress-inducible protein Niban regulates eIF2alpha and S6K1/4E-BP1 phosphorylation. Biochem Biophys Res Commun. Aug 17 2007;360(1):181–7. doi: 10.1016/j.bbrc.2007.06.021 [DOI] [PubMed] [Google Scholar]
- 44.D’Amora DR, Hu Q, Pizzardi M, Kubiseski TJ. BRAP-2 promotes DNA damage induced germline apoptosis in C. elegans through the regulation of SKN-1 and AKT-1. Cell Death Differ. Jul 2018;25(7):1276–1288. doi: 10.1038/s41418-017-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuoka M, Sudo H, Tsuji K, et al. ik3-2, a relative to ik3-1/Cables, is involved in both p53-mediated and p53-independent apoptotic pathways. Biochem Biophys Res Commun. Dec 12 2003;312(2):520–9. doi: 10.1016/j.bbrc.2003.10.142 [DOI] [PubMed] [Google Scholar]
- 46.Chao CN, Lo JF, Khan FB, et al. Tid1-S attenuates LPS-induced cardiac hypertrophy and apoptosis through ER-a mediated modulation of p-PI3K/p-Akt signaling cascade. J Cell Biochem. Oct 2019;120(10):16703–16710. doi: 10.1002/jcb.28928 [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Hao Q, Luo J, et al. USP4 inhibits p53 and NF-kappaB through deubiquitinating and stabilizing HDAC2. Oncogene. Jun 2 2016;35(22):2902–12. doi: 10.1038/onc.2015.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou X, Wang L, Zhang L, Pan X, Zhao W. Ubiquitin-specific protease 4 promotes TNF-alpha-induced apoptosis by deubiquitination of RIP1 in head and neck squamous cell carcinoma. FEBS Lett. Feb 14 2013;587(4):311–6. doi: 10.1016/j.febslet.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 49.Graydon CG, Mohideen S, Fowke KR. LAG3’s Enigmatic Mechanism of Action. Front Immunol. 2020;11:615317. doi: 10.3389/fimmu.2020.615317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaban E, Bayliss G, Malhotra DK, et al. Targeting Regulatory T Cells for Transplant Tolerance: New Insights and Future Perspectives. Kidney Dis (Basel). Nov 2018;4(4):205–213. doi: 10.1159/000490703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riella LV, Liu T, Yang J, et al. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. Apr 2012;12(4):846–55. doi: 10.1111/j.1600-6143.2011.03929.x [DOI] [PubMed] [Google Scholar]
- 53.Segundo DS, Ruiz JC, Izquierdo M, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. Aug 27 2006;82(4):550–7. doi: 10.1097/01.tp.0000229473.95202.50 [DOI] [PubMed] [Google Scholar]
- 54.Hu M, Wang C, Zhang GY, et al. Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. Am J Transplant. Nov 2013;13(11):2819–30. doi: 10.1111/ajt.12445 [DOI] [PubMed] [Google Scholar]
- 55.Singh A, Goerlich CE, Braileanu G, et al. PRESENCE OF GRAFT-INFILTRATING REGULATORY T CELLS ARE ASSOCIATED WITH LONG TERM CARDIAC XENOGRAFT SURVIVAL IN NON-HUMAN PRIMATE. Transplantation. 2020;104(S3):S641. doi: 10.1097/01.tp.0000702068.56688.88 [DOI] [Google Scholar]
- 56.Pilat N, Wiletel M, Weijler AM, et al. Treg-mediated prolonged survival of skin allografts without immunosuppression. Proc Natl Acad Sci U S A. Jul 2 2019;116(27):13508–13516. doi: 10.1073/pnas.1903165116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato K, Yamashita-Kanemaru Y, Abe F, et al. DNAM-1 regulates Foxp3 expression in regulatory T cells by interfering with TIGIT under inflammatory conditions. Proc Natl Acad Sci U S A. May 25 2021;118(21)doi: 10.1073/pnas.2021309118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


