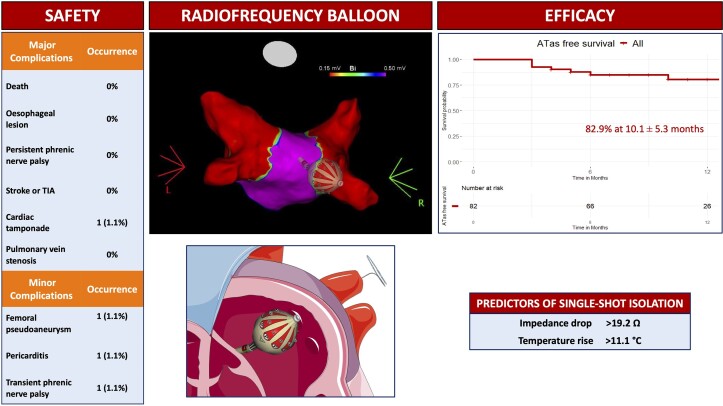
Abstract
Aims
The multielectrode radiofrequency balloon catheter (RFB) has been developed to achieve safe and effective pulmonary vein isolation (PVI) for atrial fibrillation (AF) ablation. This single-centre study aimed to evaluate the midterm clinical outcome and predictors of single-shot PVI with the novel RFB.
Methods and results
All consecutive patients with symptomatic paroxysmal or persistent AF undergoing first-time PVI with the RFB were prospectively included. Clinical and procedural parameters were systematically collected. The primary safety endpoint was defined as any major periprocedural complications. The primary efficacy endpoint consisted of freedom from any atrial tachyarrhythmias (ATas) lasting >30 s during the follow-up after a 3-month blanking period. Persistent single-shot PVI was defined as PVI achieved with a single RFB application without acute reconnection. A total of 104 consecutive patients (mean age 64.3 ± 11.4 years, 56.7% males) were included. 15 patients (14.4%) presented with persistent AF. The procedure time was 59.0 min with a dwell time of 20.0 min. One major complication occurred in one patient. At a mean follow-up of 10.1 ± 5.3 months, freedom from ATas was 82.9%. ATas occurred in 14 patients, 11/69 patients (15.9%) with paroxysmal AF and 3/13 (23.1%) with persistent AF. The best cut-offs to predict persistent single-shot PVI were impedance drop >19.2 Ω [area under the receiver operator characteristic curve (AUC) 0.74] and temperature rise >11.1° C (AUC 0.77).
Conclusion
In a large cohort of patients undergoing PVI with the RFB, the complication rate was 1%. At a mid-term follow-up of 10.1 ± 5.3 months, freedom from ATas was 82.9%. Specific cut-offs of impedance drop and temperature rise may be useful to predict persistent single-shot isolation.
Keywords: Atrial fibrillation, Radiofrequency balloon, Pulmonary vein isolation, Radiofrequency ablation, Catheter ablation, Single-shot predictors
Graphical Abstract
Graphical Abstract.
What’s new?
Single-shot ablation technology demonstrated equal efficacy to point-by-point ablation to achieve pulmonary vein isolation (PVI).
PVI with the multielectrode radiofrequency balloon catheter (RFB) is safe and effective, with a rate of major complications of 1%.
The RFB achieves fast and effective single-shot isolation, with a freedom from any atrial tachyarrhythmias of 82.9% after a mean follow-up of 10.1 ± 5.3 months.
Specific cut-offs of impedance drop (>19.2 Ω) and temperature rise (>11.1° C) demonstrated good predictive value for persistent single-shot isolation. These ablation parameters may be implemented during procedural workflow to determine the effectiveness of the ablation set.
Introduction
The multielectrode radiofrequency (RF) balloon catheter (RFB), HELIOSTAR, (Biosense Webster, CA, USA) has been recently launched on the market for pulmonary vein isolation (PVI) in the context of atrial fibrillation (AF) ablation. The RFB is a 28-mm compliant balloon compatible with a 3D electro-anatomical mapping system (CARTO 3, Biosense Webster, CA, USA). It has 10 flexible gold-plated electrodes arranged evenly on the surface, each capable of independently delivering irrigated unipolar RF energy, allowing tailored energy titration to anterior and posterior sites and focal, segmental, or circumferential ablation strategies.
The RADIANCE first-in-human multicentre trial was the first study to establish the feasibility of the RFB to achieve effective PVI with a favourable safety profile, without additional focal catheter touch-up ablation.1,2 The European multicentre SHINE study evaluated the safety and effectiveness of the second-generation RFB in combination with a circular multi-electrode diagnostic catheter (LASSOSTAR, Biosense Webster, Irvine, CA, USA) to perform PVI, with a 12-month follow-up.3 However, real-world data on a large population are lacking. Furthermore, no studies evaluated the role of an optimized workflow for PVI with the RFB as previously described by our group.4 Finally, there is a gap in the literature on specific ablation parameters predictive of single-shot PVI with the RFB.
The aim of this prospective single-centre study was to evaluate the clinical outcome on a mid-term follow-up of PVI with the RFB with an optimized workflow in a series of consecutive patients presenting with symptomatic AF.
Methods
Study population
The study was a prospective, single-centre study. All consecutive patients with symptomatic paroxysmal or persistent AF undergoing a first-time PVI-only procedure with the RFB (HELIOSTAR, Biosense Webster, CA, USA) between January 2021 and September 2022 were prospectively included. All procedures were performed at a single institution (Universitair Ziekenhuis Brussel, Brussels, Belgium) by two experienced operators in equal proportions. Patients were chronologically classified into two groups, early or late experience, if they were in the first or the second half of the whole cohort, respectively.
Paroxysmal and persistent AF was defined according to the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery.5 Clinical, procedural, and follow-up data were prospectively collected. The study protocol was conducted in accordance with the ethical principles established by the Declaration of Helsinki, as revised in 2013, and was approved by the local ethic committee of our institution. All patients provided written informed consent for the ablation procedure and data collection.
Ablation procedure
The procedural workflow used has been previously described elsewhere.4 Briefly, all procedures were performed under general anaesthesia and uninterrupted anticoagulation therapy. All antiarrhythmic drugs (AADs) were discontinued five half-lives before ablation. Before the procedure, a multielectrode oesophageal temperature probe (S-Cath, Circa Scientific LLC, Englewood, CO, USA) was positioned at the level of the left atrium (LA). Two femoral punctures were performed under echo guidance. A decapolar catheter was used for coronary sinus electrogram recordings and phrenic nerve pacing during ablation of the right pulmonary veins (PVs). A single transseptal puncture was then performed, followed by bolus dosing with unfractionated heparin to maintain activated clotting time at 300–350 s for the whole procedure. Pre-ablation 3D electro-anatomical map of the PVs and LA was created with a circular mapping catheter (LASSONAV, Biosense Webster, Irvine, CA, USA). The RFB equipped with the intraluminal circular diagnostic catheter (LASSOSTAR, Biosense Webster, Irvine, CA, USA) was then introduced into the LA through a dedicated deflectable sheath (GUIDESTAR, Biosense Webster, Irvine, CA, USA). Optimal RFB positioning, including correct alignment with the PVs and electrode-tissue contact, was assessed with fluoroscopy, mapping system visualization of the balloon, and according to the following baseline parameters: balloon inflation index >0.8, electrode impedance 90–120 Ω with a variability ≤20 Ω across electrodes, and electrode temperature ≤31° C with a variability ≤3° C between electrodes. PV occlusion assessment with contrast injection was performed according to the operator’s choice. After confirmation of optimal balloon positioning, ablation was performed in temperature-controlled mode with unipolar RF energy. Typically, two posterior electrodes were identified on the RFB with the 3D electro-anatomical map. The power setting was 15 W and the target electrode temperature was 55° C. The same energy was simultaneously delivered to all electrodes, with a duration of 20 s for the posterior and 60 s for the non-posterior electrodes. In the case of oesophageal temperature rise (defined as >39° C), a shorter application in the posterior electrodes was performed, according to the operator’s preference. During ablation, PVs potentials were monitored on the circular diagnostic catheter to evaluate real-time isolation. PVI achieved with a single RFB application was defined as ‘single-shot’ isolation, with or without early reconnection. After a 15-minute waiting time from the last application, remapping with the circular mapping catheter was performed to confirm PVI (Figure 1). In the case of PV acute reconnection, additional ‘reconnection’ RF applications were delivered to achieve durable PVI.
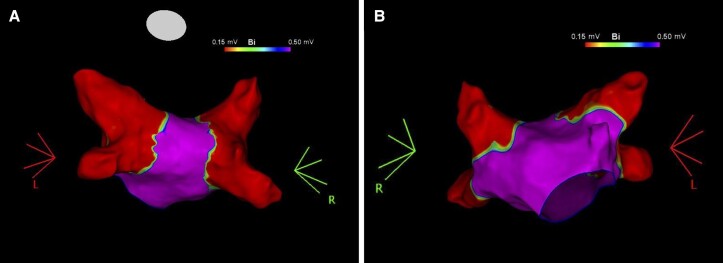
Figure 1.
Post-ablation voltage map of the left atrium. Voltage map of the left atrium in postero-anterior (Panel A) and antero-posterior (Panel B) views showing pulmonary vein isolation after ablation with the radiofrequency balloon catheter.
Post-procedural management and follow-up
All patients underwent continuous telemetry monitoring for at least 24 h after the procedure and were discharged after overnight observation if no complications occurred. Before discharge, transthoracic echocardiography and venous Doppler ultrasound were performed in all patients. Oral anticoagulation was started the same evening after ablation and continued for at least 2 months, thereafter it was prolonged according to the patient’s thromboembolic risk profile. AADs were continued for 2 months after ablation and then according to clinical judgment and patient preference.
Patients with oesophageal temperature rise ≥41° C for a cumulative time of ≥10 s during ablation were scheduled for oesophageal endoscopy within 5–8 days post-procedure.
Our institutional clinical follow-up strategy included in-person outpatient visits at 1, 3, 6, and 12 months after ablation for the first year, and then every 6 months. At each visit, a clinical examination and a 12-lead ECG were performed. Furthermore, a 7-day ECG Holter monitoring was recorded at 3, 6, and 12 months for the first year and then every 12 months.
Study endpoints and definitions
The primary safety endpoint included any major periprocedural complications [e.g. death, atrioesophageal fistula, stroke/transient ischaemic attack (TIA), pericardial effusion/tamponade with/without surgical treatment, myocardial infarction, and persistent phrenic palsy] occurring within 7 days post-procedure (except for atrioesophageal fistula). Minor complications were also reported, including vascular access complications requiring treatment, pericarditis, and transient phrenic palsy.
The primary efficacy endpoint was defined as arrhythmia-free survival during the follow-up. Arrhythmia recurrence was defined as any atrial tachyarrhythmias (ATas) ≥30 s after a 90-day post-ablation blanking period. Only patients with at least 3 months of follow-up were included for the survival analysis.
Procedural failure was defined as the inability to achieve PVI with the RFB and the need for additional focal RF catheter ablation. Device failure was defined as any dysfunction of the system (i.e. sheath or balloon catheter).
Total procedure time was defined as the time from the first femoral puncture to the time of the last catheter removal. Left atrial dwell time was considered as the time the RFB was left in the LA.
Ablation parameters among patients with persistent single-shot PVI, defined as single-shot isolation without acute reconnection, vs. patients without single-shot isolation or with acute PV reconnection were analysed and compared.
Statistical analysis
All variables were tested for normality with the Shapiro–Wilk test. Normally distributed variables were described as mean ± standard deviation and the groups were compared using ANOVA, paired or unpaired t-test as appropriate, while the non-normally distributed variables were described as median (Inter Quartile Range) and compared using the Mann–Whitney test or the Wilcoxon signed-rank test as appropriate. The categorical variables were described as frequencies (percentages) and compared using the χ2 test or Fisher’s exact test as appropriate.
A logistic regression analysis was performed to predict single-shot PVI, persistent at intra-procedural remapping after the first RF application. Predictors were chosen based on their predicted clinical role and included the following: mean impedance drop and mean temperature rise. Odds ratio (OR), relative 95% confidence interval (CI), and receiver operator characteristic (ROC) curve were calculated for each significant predictor. The best cut-off for each significant continuous variable was obtained using Youden’s method.6 Sensitivity and specificity were calculated at the best cut-off derived by Youden’s method. Discrimination was measured by area under the ROC curve measure (AUC).
Kaplan–Meier’s curves were drawn to describe patients’ freedom from ATas during the follow-up.
Survival analysis was performed with survival7 and survminer8 packages on R software.
A P value less than 0.05 was considered statistically significant.
The analysis was performed using R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
A total of 104 consecutive patients were included in the study. The mean age was 64.3 ± 11.4 years and 59 (56.7%) patients were males. The mean CHA2DS2-VASc score was 2.5 ± 1.6. Patients underwent AF ablation after a mean of 9.5 ± 16.0 months from the first diagnosis. At least one previous electrical cardioversion had been performed in 25 (24.0%) patients.
Fifteen (14.4%) patients had persistent AF and 89 (85.6%) patients presented with paroxysmal AF. Compared with patients with paroxysmal AF, patients with persistent AF had more dilated atria, as demonstrated by the mean left atrial volume index (47.5 ± 8.6 mL/m2 vs. 35.3 ± 9.9 mL/m2, P < 0.001).Complete patient characteristics are summarized in Table 1.
Table 1.
Baseline demographic and clinical characteristics
| Paroxysmal AF (n = 89) | Persistent AF (n = 15) | Total (n = 104) | P value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 63.9 ± 12.0 | 66.9 ± 6.3 | 64.3 ± 11.4 | 0.34 |
| Gender (male) | 47 (52.8%) | 12 (80.0%) | 59 (56.7%) | 0.06 |
| BMI (kg/m2) | 28.6 ± 5.1 | 30.9 ± 5.2 | 28.9 ± 5.2 | 0.11 |
| CHA2DS2-VASc score | 2.4 ± 1.7 | 2.7 ± 1.3 | 2.5 ± 1.6 | 0.58 |
| Hypercholesterolemia (n,%) | 43 (48.3%) | 11 (73.3%) | 54 (51.9%) | 0.09 |
| Diabetes (n,%) | 16 (18.0%) | 4 (26.7%) | 20 (19.2%) | 0.48 |
| Hypertension (n,%) | 53 (59.6%) | 12 (80.0%) | 65 (62.5%) | 0.16 |
| Heart failure (n,%) | 7 (7.9%) | 3 (20.0%) | 10 (9.6%) | 0.16 |
| Stroke or TIA (n,%) | 13 (14.6%) | 1 (6.7%) | 14 (13.5%) | 0.69 |
| CAD (n,%) | 12 (13.5%) | 3 (20.0%) | 15 (14.4%) | 0.45 |
| CKD (n,%) | 16 (18.0%) | 3 (20.0%) | 19 (18.3%) | 0.99 |
| LVEF (%) | 54.8 ± 5.9 | 54.5 ± 6.8 | 54.7 ± 6.0 | 0.89 |
| LAESVI (mL/m2) | 35.3 ± 9.9 | 47.5 ± 8.6 | 37.2 ± 10.6 | <0.001 |
| EHRA symptom score | 3.2 ± 0.9 | 3.0 ± 0.9 | 3.2 ± 0.9 | 0.32 |
| Prior cardioversion (n,%) | 19 (21.3%) | 6 (40%) | 25 (24.0%) | 0.26 |
| Time from AF diagnosis (months) | 9.4 ± 14.8 | 10.4 ± 23.6 | 9.5 ± 16.0 | 0.84 |
| Diuretics (n,%) | 16 (17.9%) | 5 (33.3%) | 21 (20.2%) | 0.32 |
| AADs Class Ic (n,%) | 15 (16.9%) | 3 (20.0%) | 18 (17.3%) | 1.00 |
| AADs Class III (n,%) | 18 (20.2%) | 6 (40.0%) | 24 (23.1%) | 0.21 |
| OAC (n,%) | 80 (89.9%) | 15 (100.0%) | 95 (91.3%) | 0.35 |
BMI, body mass index; TIA, transient ischaemic attack; CAD, coronary artery disease; CKD, chronic kidney disease; LVEF, left ventricle ejection fraction; LAESVI, left atrial end-systolic volume indexed; EHRA European Heart Rhythm Association; AF, atrial fibrillation; AADs, antiarrhythmic drugs; OAC, oral anticoagulation.
Procedural characteristics
The median procedure time was 59.0 (44.0–77.0) min, with a median dwell time of 20.0 (14.5–31.0) min and a median fluoroscopy time of 15.0 (9.0–22.5) min. Complete procedural characteristics, for the early and late experience groups (52 vs. 52 patients) are summarized in Table 2. Compared with the early experience group, in the late experience group, single-shot isolation was higher for LSPV [100% vs. 59.6%, P < 0.001] and LIPV [100% vs. 78.8%, P < 0.001]. Single-shot isolation was obtained in 89.4% of patients for the RIPV and 86.5% of patients for the RSPV, with no differences between the two groups. Oesophageal temperature rise occurred in 32 (30.8%) patients. Gastroscopy was performed in seven patients per study protocol and no procedural-related lesions were documented.
Table 2.
Procedural characteristics
| Early experience (n = 52) | Late experience (n = 52) | Total (n = 104) | P value | |
|---|---|---|---|---|
| Procedure time (min) | 62.0 (46.0–78.0) | 55.0 (43.0–75.0) | 59.0 (44.0–77.0) | 0.22 |
| Dwell time (min) | 20.0 (15.0–31.0) | 17.0 (15.0–31.0) | 20.0 (14.5–31.0) | 0.47 |
| Fluoroscopy time (min) | 17.0 (11.2–25.0) | 13.0 (6.8–20.2) | 15.0 (9.0–22.5) | 0.005 |
| LSPV single-shot (n,%) | 31 (59.6%) | 52 (100.0%) | 83 (79.8%) | <0.001 |
| LSPV applications (n,%) | 0.28 | |||
| 1 | 29 (55.8%) | 42 (80.8%) | 71 (68.2%) | |
| 2 | 18 (34.6%) | 6 (11.5%) | 24 (23.1%) | |
| >2 | 5 (9.6%) | 4 (7.7%) | 9 (8.7%) | |
| LSPV TTI (s) | 10.0 (9.0–11.8) | 10.0 (8.0–12.8) | 10 (8.0–12.0) | 0.78 |
| LSPV reconnection RF (n,%) | 7 (13.5%) | 2 (3.8%) | 9 (8.7%) | 0.09 |
| LIPV single-shot (n,%) | 41 (78.8%) | 52 (100%) | 93 (89.4%) | <0.001 |
| LIPV applications (n,%) | 0.72 | |||
| 1 | 41 (78.8%) | 45 (86.5%) | 86 (82.7%) | |
| 2 | 6 (11.5%) | 5 (9.6%) | 11 (10.6%) | |
| >2 | 5 (9.6%) | 2 (3.8%) | 7 (6.7%) | |
| LIPV TTI (s) | 10.0 (8.0–12.0) | 8.0 (7.2–10.0) | 9.0 (8.0–11.0) | 0.008 |
| LIPV reconnection RF (n,%) | 3 (5.8%) | 6 (11.5%) | 9 (8.7%) | 0.60 |
| RIPV single-shot (n,%) | 44 (84.6%) | 49 (94.2%) | 93 (89.4%) | 0.20 |
| RIPV applications (n,%) | 0.16 | |||
| 1 | 44 (84.6%) | 45 (86.5%) | 89 (85.6%) | |
| 2 | 4 (7.7%) | 4 (7.7%) | 8 (7.7%) | |
| >2 | 4 (7.7%) | 3 (5.8%) | 7 (6.7%) | |
| RIPV TTI (s) | 10.0 (8.0–11.0) | 8.0 (7.0–10.0) | 9.0 (8.0–11.0) | 0.009 |
| RIPV reconnection RF (n,%) | 2 (3.8%) | 2 (3.8%) | 4 (3.8%) | 0.99 |
| RSPV single-shot (n,%) | 43 (82.7%) | 47 (90.4%) | 90 (86.5%) | 0.20 |
| RSPV applications (n,%) | 0.62 | |||
| 1 | 38 (73.1%) | 44 (84.6%) | 82 (78.8%) | |
| 2 | 9 (17.3%) | 4 (7.7%) | 13 (12.5%) | |
| >2 | 4 (7.7%) | 4 (7.7%) | 8 (7.7%) | |
| RSPV TTI (s) | 9.0 (8.0–10.8) | 9.0 (7.0–10.0) | 9.0 (7.2–10.0) | 0.41 |
| RSPV reconnection RF (n,%) | 3 (5.8%) | 3 (5.8%) | 6 (5.8%) | 1.00 |
| Oesophageal temperature rise (n,%) | 12 (23.1%) | 20 (38.5%) | 32 (30.8%) | 0.09 |
LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; RF, radiofrequency application; TTI, time to isolation.
Primary safety and efficacy endpoints
Major periprocedural complications occurred in one (1.0%) patient. This complication was a cardiac tamponade that required thoracotomy for complete resolution. Three minor complications were also reported: one transient phrenic nerve palsy, one femoral pseudoaneurysm treated conservatively, and one pericarditis requiring pharmacological treatment.
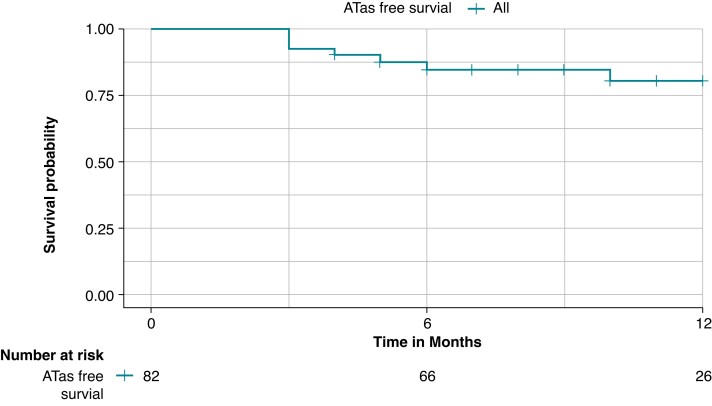
A total of 82 patients had a follow-up of at least 90 days (blanking period) and were included in the survival analysis. At a mean follow-up of 10.1 ± 5.3 months, ATas recurrence occurred in 14 patients (17.1%), Figure 2. A total of 28 patients (34.1%) were on AADs after the 3-month blanking period. ATas recurrence occurred in 11/69 patients (15.9%) with paroxysmal AF and 3/13 patients (23.1%) with persistent AF. ATas were adjudicated as AF in 11 patients (78.6%), including eight patients in the paroxysmal AF group and all three patients in the persistent AF group, and as atrial tachycardia in three patients (21.4%), all in the paroxysmal AF group. A redo procedure was performed in eight patients (61.5%) and at least one PV reconnection was observed in six patients (75%). A total of 11 reconnected veins were distributed as follows: LSPV in two patients (33.3%), LIPV in two patients (33.3%), RIPV in one patient (16.6%), and RSPV in six patients (100%). Re-PVI was performed in all cases with reconnected PVs. Additional anterior and roof lines were performed in one patient.
Figure 2.
Kaplan–Meier curve of any atrial tachyarrhythmias (ATas)-free survival during the follow-up; the overall freedom from ATas was 82.9% at a mean follow-up of 10.1 ± 5.3 months.
In 101 (97.1%) patients, PVI was achieved with only the RFB. Three (2.9%) patients required focal RF ablation because of RFB or sheath failure: one to achieve isolation in the left ridge and two for RIPV isolation.
Predictors of persistent single-shot isolation
To evaluate ablation parameters predictors of single-shot isolation, all first RF applications with impedance drop and temperature rise data available were included for the analysis.
A total of 416 first RF applications were examined, 353 (84.9%) with persistent single-shot isolation, confirmed at intra-procedural remapping, and 63 (15.1%) with no single-shot isolation or early (intra-procedural) reconnection. Overall, the mean impedance drop was 23.0 ± 5.7 Ω, and the mean temperature rise was 12.3 ± 3.4° C. RF applications associated with persistent single-shot isolation showed higher mean impedance drop [23.5 ± 5.6 Ω vs. 20.0 ± 4.9 Ω, P < 0.001] and temperature rise [12.5 ± 3.5° C vs. 10.6 ± 2.7° C, P < 0.001] compared with applications without single-shot isolation or with early reconnection.
Vein per vein analysis of ablation parameters in patients with and without persistent single-shot isolation is summarized in Table 3. RF applications resulting in persistent single-shot isolation showed higher values of mean impedance drop and temperature rise for all veins, except for LSPV.
Table 3.
Vein per vein analysis of impedance drop and temperature rise
| LSPV no single-shot/reconnection (n = 23) | LSPV persistent isolation (n = 81) | Total (n = 104) | P value | |
|---|---|---|---|---|
| Impedance drop (Ω) | 23.6 ± 3.9 | 25.4 ± 5.9 | 25.0 ± 5.5 | 0.21 |
| Temperature rise (°C) | 12.5 ± 2.8 | 13.0 ± 3.1 | 12.9 ± 3.0 | 0.50 |
| LIPV no single-shot/reconnection (n = 17) | LIPV persistent isolation (n = 87) | Total (n = 104) | P value | |
| Impedance drop (Ω) | 17.0 ± 4.1 | 22.0 ± 4.8 | 21.3 ± 5.0 | <0.001 |
| Temperature rise (°C) | 9.7 ± 1.5 | 12.2 ± 2.7 | 11.8 ± 2.7 | 0.001 |
| RIPV no single-shot/reconnection (n = 11) | RIPV persistent isolation (n = 93) | Total (n = 104) | P value | |
| Impedance drop (Ω) | 18.3 ± 4.9 | 23.3 ± 5.4 | 22.7 ± 5.5 | 0.006 |
| Temperature rise (°C) | 9.1 ± 1.9 | 12.7 ± 3.9 | 12.3 ± 3.9 | 0.005 |
| RSPV no single-shot/reconnection (n = 13) | RSPV persistent isolation (n = 91) | Total (n = 104) | P value | |
| Impedance drop (Ω) | 18.9 ± 3.9 | 23.5 ± 6.1 | 22.9 ± 6.0 | 0.013 |
| Temperature rise (°C) | 10.0 ± 2.5 | 12.4 ± 4.0 | 12.1 ± 3.9 | 0.05 |
LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
To evaluate predictors of persistent single-shot isolation confirmed at intra-procedural remapping, ablation parameters of all first RF applications in LIPV, RSPV, and RIPV were pooled for the analysis.
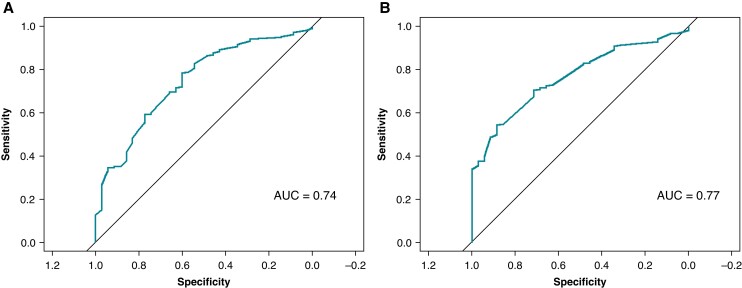
At univariate logistic regression analysis impedance drop (OR per 1 Ω increase, 1.13, 95% CI 1.07–1.2, P < 0.001) and temperature rise (OR per 1° C increase, 1.23, 95% CI 1.11–1.37, P < 0.001) were significant predictors of persistent single-shot isolation. The best cut-offs to predict persistent single-shot isolation were as follows: impedance drop >19.2 Ω (specificity 0.60, sensitivity 0.78, AUC 0.74) and temperature rise >11.1° C (specificity 0.89, sensitivity 0.54, AUC 0.77), Figure 3.
Figure 3.
ROC curves for univariate regression analysis of predictors of single-shot pulmonary vein isolation. Panel A: ROC curve for impedance drop (AUC = 0.74); panel B: ROC curve for temperature rise (AUC = 0.77).
Discussion
The results of this study can be summarized as follows: (1) in a large cohort of patients undergoing PVI with the RFB, the complication rate was as low as 1%; (2) at a mid-term follow-up of 10.1 ± 5.3 months the freedom from ATas recurrence was 82.9%; (3) the best cut-offs to predict persistent single-shot isolation were the following: impedance drop >19.2 Ω and temperature rise >11.1° C.
Safety of the radiofrequency balloon
In this prospective large single-centre study, only one patient experienced a major periprocedural complication, namely a cardiac tamponade, which necessitated cardiac surgery for full recovery. This complication occurred in a patient with persistent AF and a dilated left atrium. Invasive blood pressure drop was noticed at the end of post-ablation remapping, when the multipolar mapping catheter supported by the steerable sheath was at the level of the left atrial appendage. Although an ultrasound guided pericardiocentesis was immediately performed, the bleeding could not be controlled. This led to the decision to perform a surgical repair, which confirmed the presence of a defect at the roof of the left atrial appendage. Although, an enlarged left atrium may have misled the operator during mapping, the steerable sheath could have increased the support of the mapping catheter causing the perforation. This scenario has been previously described in a similar case.9
A few minor complications were also reported, including one transient phrenic nerve palsy, one femoral pseudoaneurysm treated conservatively, and one pericarditis requiring pharmacological treatment. Noticeably, no stroke or TIA, atrioesophageal fistulas, or PV stenosis were observed.
Gastroscopy was performed in seven patients with a temperature rise on the oesophageal probe >41° C for >10 s and no oesophageal lesions were reported. None of the patients had received oesophageal protection or deviation devices during the procedure. Compared to other technologies and ablation catheters, asymptomatic oesophageal lesions have been observed in 15–20% of patients with RF and cryoballoon ablation, whereas atrioesophageal fistulas have been reported in 0.02% to 0.11% of the patients.10–13
One transient phrenic nerve palsy was reported in the current study; the patient recovered phrenic function at the chest x-ray performed the day after the procedure. In this patient, the RF delivery was stopped immediately after the loss of capture of the phrenic nerve during RF application on the RSPV.
The hereby reported findings are consistent with those reported in the Radiance1 and Shine study.3 As a comparison, reported rates for phrenic nerve palsy, PV stenosis requiring intervention, and stroke or TIA with standard RF ablation are 0.4%, 0.29%, and 0.94%, respectively.14
Efficacy of the radiofrequency balloon
In the current study, at a mid-term follow-up of 10.1 ± 5.3 months the overall freedom from ATas recurrence was 82.9%. The arrhythmia recurrence rate was 15.9% in paroxysmal AF patients and 23.1% in persistent AF patients. The results on the subgroup of patients with paroxysmal AF are consistent with those reported by the previous multicentre studies on the RFB. In the first evaluation of the RFB, the Radiance study, the reported freedom from atrial arrhythmias at 12 months was 86.4% in a small cohort of 37 patients with paroxysmal AF.2 The subsequent multicentric study on the RFB, the Shine study, reported a freedom from ATas at 6 months of 81.0%, in a cohort of 84 patients with paroxysmal AF.3 Noticeably, the current study is the first to report outcomes of PVI with the RFB in a small cohort of patients with persistent AF (14.4% of the overall population). In this subgroup, the freedom from ATas was 76.9%, which is slightly higher than that reported on RF ablation15 or cryoballoon ablation16 in this population. A small sample size (13 patients) and a relatively shorter AF duration in our cohort (10.4 ± 23.6 months) might partly explain the results.
The procedure time was relatively short, with a median procedure time of 59 min, inclusive of 15–20 min of voltage mapping time before and after PVI. This is shorter than that reported in the Radiance and Shine studies (102 and 88 min, respectively) and it might reflect the use of an optimized workflow for PVI with the RFB.4 The reported procedure time is shorter compared with other technologies for PVI.17–20 It is reasonable to expect that with increased operator experience, improvement of the ablation system, and the use of a navigation-enabled mapping catheter procedure time may shorten.
Interestingly, the rate of single-shot isolation showed a significant improvement in the late experience group. This might be explained by the learning curve of the operators but also by the improved compatibility of the RFB with the 3D mapping system and the use of the third-generation RFB in the second phase of the reported clinical experience. The hereby presented clinical outcomes are consistent with single-centre reports and multicentre studies on RF point-by-point.10,19–21 Furthermore, considering other single-shot techniques, these results are consistent also with data on the cryoballoon,22–24 laserballoon,24,25 and pulsed field ablation.18,23,24
Predictors of persistent single-shot isolation
Following our previous report on optimal pre- and post-ablation parameters,4 a comprehensive RF ablation parameters analysis showed that post-ablation impedance drop >19.2 Ω (AUC 0.74) and temperature rise >11.1° C (AUC 0.77) are predictors of persistent single-shot isolation. Interestingly, these cut-offs could not be applied to the LSPV. The specificity of LSPV anatomy with its anterior ridge and the proximity of the left atrial appendage may partially explain the results. Moreover, a left common ostium was present in 5% of our cohort and might contribute to the absence of RF predictors in the LSPV, which is typically the first vein targeted during ablation of the left common ostium. However, with a larger number of patients and improved catheter manipulation, operators may yield a better positioning of the RFB, leading to increased stability and better lesion sets, with resulting different ablation parameters.
Along with optimal RFB positioning, a combination of these RF parameters is likely needed to achieve a high rate of persistent single-shot isolation. However, these results should be further investigated in a large subset of patients with prospective PV remapping.
Limitations
The main limitation of the study is its single-centre design. All ablations were performed in a tertiary centre with a large experience in single-shot PVI. The follow-up was relatively short. Continuous ECG monitoring was not performed in all patients and some subclinical ATas may have not been detected during the follow-up. Furthermore, 34.1% of the patients were on AADs after the blanking period and this could have led to a better primary efficacy outcome. Finally, adenosine or isoproterenol was not routinely used to assess PVI and dormant conduction. However, voltage remapping was performed in all patients as per protocol.
Conclusions
In a large cohort of patients undergoing PVI with the RFB, the complication rate is as low as 1%. At a mid-term follow-up of 10.1 ± 5.3 months, freedom from ATas recurrence was 82.9%. Specific cut-offs of impedance drop and temperature rise may be useful to predict persistent single-shot isolation.
Contributor Information
Alvise Del Monte, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Alexandre Almorad, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Luigi Pannone, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Domenico Giovanni Della Rocca, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Antonio Bisignani, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Cinzia Monaco, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Sahar Mouram, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Robbert Ramak, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Anaïs Gauthey, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Ingrid Overeinder, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Gezim Bala, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Antonio Sorgente, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Erwin Ströker, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Juan Sieira, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Pedro Brugada, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Mark La Meir, Cardiac Surgery Department, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, Laarbeeklaan 101, 1090, Brussels, Belgium.
Gian-Battista Chierchia, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Carlo de Asmundis, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090, Brussels, Belgium.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Reddy VY, Schilling R, Grimaldi M, Horton R, Natale A, Riva Set al. Pulmonary vein isolation with a novel multielectrode radiofrequency balloon catheter that allows directionally tailored energy delivery: short-term outcomes from a multicenter first-in-human study (RADIANCE). Circ Arrhythm Electrophysiol 2019;12:e007541. [DOI] [PubMed] [Google Scholar]
- 2. Dhillon GS, Honarbakhsh S, Di Monaco A, Coling AE, Lenka K, Pizzamiglio Fet al. Use of a multi-electrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol 2020;31:1259–69. [DOI] [PubMed] [Google Scholar]
- 3. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini Fet al. Safety, effectiveness, and quality of life following pulmonary vein isolation with a multi-electrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almorad A, Chierchia GB, Pannone L, Osorio TG, Sorgente A, Bisignani Aet al. The optimized clinical workflow for pulmonary vein isolation with the radiofrequency balloon. J Interv Card Electrophysiol 2022;64:531–8. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Hajian-Tilaki K. The choice of methods in determining the optimal cut-off value for quantitative diagnostic test evaluation. Stat Methods Med Res 2018;27:2374–83. [DOI] [PubMed] [Google Scholar]
- 7. Therneau TM. A package for survival analysis in R. R package version 238, 2021.
- 8. Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer’. Drawing survival curves using ‘ggplot2’. (R package version 0.3.1.). R Package version 0.4.3., 2017.
- 9. Guan F, Stähli BE, Jakob P, Wolber T. Perforation of multipolar electroanatomic mapping catheter in the left atrial appendage during left atrial mapping. HeartRhythm Case Rep 2022;8:615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips Tet al. Prospective randomized evaluation of high power during CLOSE-guided pulmonary vein isolation: the POWER-AF study. Circ Arrhythm Electrophysiol 2021;14:e009112. [DOI] [PubMed] [Google Scholar]
- 11. Singh SM, d'Avila A, Singh SK, Stelzer P, Saad EB, Sk Aet al. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10:1591–7. [DOI] [PubMed] [Google Scholar]
- 12. Fürnkranz A, Bordignon S, Böhmig M, Konstantinou A, Dugo D, Perrotta Let al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015;12:268–74. [DOI] [PubMed] [Google Scholar]
- 13. Yarlagadda B, Deneke T, Turagam M, Dar T, Paleti S, Parikh Vet al. Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm 2019;16:204–12. [DOI] [PubMed] [Google Scholar]
- 14. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J 2017;38:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira Jet al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: one-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 2015;12:60–6. [DOI] [PubMed] [Google Scholar]
- 17. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRet al. FIRE AND ICE investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 18. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 19. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht Set al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
- 20. Wintgens LIS, Klaver MN, Maarse M, Spitzer SG, Langbein A, Swaans MJet al. Efficacy and safety of the GOLD FORCE multicentre randomized clinical trial: multielectrode phased radiofrequency vs. irrigated radiofrequency single-tip catheter with contact force ablation for treatment of symptomatic paroxysmal atrial fibrillation. Europace 2021;23:1931–8. [DOI] [PubMed] [Google Scholar]
- 21. Kautzner J, Albenque JP, Natale A, Maddox W, Cuoco F, Neuzil Pet al. A novel temperature-controlled radiofrequency catheter ablation system used to treat patients with paroxysmal atrial fibrillation. JACC Clin Electrophysiol 2021;7:352–63. [DOI] [PubMed] [Google Scholar]
- 22. Bisignani A, Cecchini F, Mugnai G, Overeinder I, Sieira J, Osório TGet al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity-matched score comparison between different strategies. J Interv Card Electrophysiol 2022;64:9–16. [DOI] [PubMed] [Google Scholar]
- 23. Metzner A, Kuck KH, Chun JKR. What we have learned: is pulmonary vein isolation still the cornerstone of atrial fibrillation ablation? Europace 2022;24:ii8–ii13. [DOI] [PubMed] [Google Scholar]
- 24. Boersma L. New energy sources and technologies for atrial fibrillation catheter ablation. Europace 2022;24:ii44–51. [DOI] [PubMed] [Google Scholar]
- 25. Reynolds MR, Zheng Q, Doros G. Laser balloon ablation for AF: A systematic review and meta-analysis. J Cardiovasc Electrophysiol 2018;29:1363–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.