Abstract
Aims
The underlying mechanisms of atrial fibrillation (AF) are largely unknown. Inflammation may underlie atrial remodelling. Autoimmune diseases, related to increased systemic inflammation, may therefore be associated with new-onset AF.
Methods and results
Participants from the population-based UK Biobank were screened for rheumatic fever, gastrointestinal autoimmune diseases, autoimmune diseases targeting the musculoskeletal system and connective tissues, and neurological autoimmune diseases. Between 2006 and 2022, participants were followed for incident AF. Cox proportional hazards regression analyses were performed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to quantify associations. 494 072 participants free from AF were included (median age 58.0 years, 54.8% women). After a median of 12.8 years, 27 194 (5.5%) participants were diagnosed with new-onset AF. Rheumatic fever without heart involvement (HR, 95% CI: 1.47, 1.26–1.72), Crohn’s disease (1.23, 1.05–1.45), ulcerative colitis (1.17, 1.06–1.31), rheumatoid arthritis (1.39, 1.28–1.51), polyarteritis nodosa (1.82, 1.04–3.09), systemic lupus erythematosus (1.82, 1.41–2.35), and systemic sclerosis (2.32, 1.57–3.44) were associated with a larger AF risk. In sex-stratified analyses, rheumatic fever without heart involvement, multiple sclerosis, Crohn’s disease, seropositive rheumatoid arthritis, psoriatic and enteropathic arthropathies, systemic sclerosis and ankylosing spondylitis were associated with larger AF risk in women, whereas only men showed a larger AF risk associated with ulcerative colitis.
Conclusions
Various autoimmune diseases are associated with new-onset AF, more distinct in women. Our findings elaborate on the pathophysiological differences in autoimmunity and AF risk between men and women.
Keywords: Atrial fibrillation, Autoimmune disease, Inflammation, Sex differences, Risk factors
Graphical Abstract
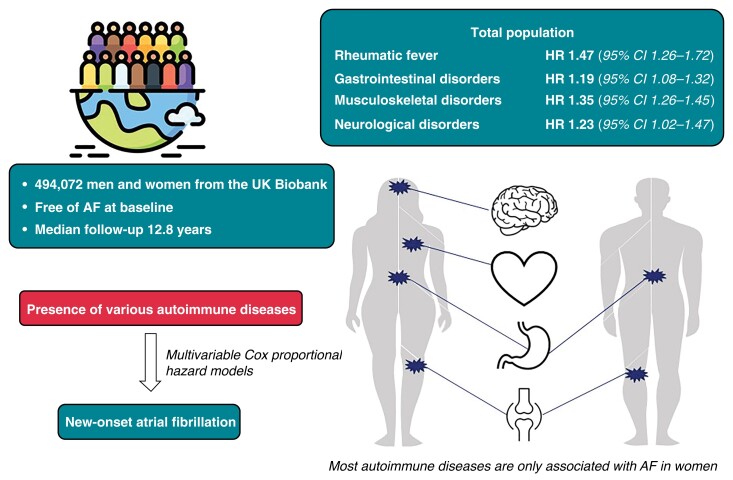
Graphical Abstract.
What’s New?
(Systemic) inflammation is suggested to be associated with atrial fibrillation development. Autoimmune diseases can be used to assess underlying (sub)clinical inflammation.
Various autoimmune diseases targeting different organs showed associations with new-onset AF in the general population.
For multiple autoimmune diseases, the impact on atrial fibrillation risk was higher in women. These differences may be due to underlying differences in autoimmunity and inflammation between men and women.
Introduction
Atrial fibrillation (AF) is a highly prevalent cardiac arrhythmia, associated with significant morbidity and mortality.1,2 While several AF risk factors have been identified, conclusive evidence on AF pathogenesis remains lacking.3,4
Inflammation is suggested to be associated with AF development through structural and electrical remodelling of the atria.5 Autoimmune diseases are accompanied by local or systemic inflammation, and may therefore be related to AF. A recent systematic review, indeed, suggested an association between rheumatoid arthritis and AF.6 However, conclusive evidence on the relation between autoimmunity and AF is lacking. This is, at least partly, due to the low prevalence of autoimmune diseases, resulting in a lack of power, short follow-up periods, and the inability to investigate multiple autoimmune diseases.7
Accumulating evidence suggests differences in AF aetiology and pathophysiology between men and women.8 Nearly all autoimmune diseases are more prevalent in women.9 While there could be various reasons for the higher prevalence, such as hormonal and genetic differences, an upcoming hypothesis is increased (re)activity of the innate immune system in women.9 This could imply that, besides a higher prevalence of autoimmune diseases, the accompanying immune response may lead to more complications in women. However, evidence regarding sex differences in cardiovascular complications of autoimmune diseases, in particular AF, is lacking.
Using data of almost half a million participants of the UK Biobank, we aimed to identify the association between a range of autoimmune diseases, including diseases targeting the metabolic and gastrointestinal (GI) systems, the musculoskeletal system and connective tissues (MSK), and the nervous system, with AF incidence. Additionally, we aimed to identify potential differences in the role of inflammation in AF pathogenesis between men and women.
Methods
Study population
For this study, we included participants from the UK Biobank. A detailed description of the aims of the study, study population, and methods of data collection has been published before.10 In short, the UK Biobank is a population-based cohort study following over 500 000 inhabitants from the UK since 2006. Through questionnaires, interviews, recurrent visits to assessment centres, and linkage to the health records, a wide range of psychosocial, sociodemographic, physical, and genetic data was collected. Participants without informed consent for follow-up data collection, either at baseline or during the follow-up, were excluded from this study.
Assessment of autoimmune diseases and atrial fibrillation
Participants were continuously monitored for disease occurrences through linkages with health-related medical records, including primary care data, hospital inpatient data, death register records, and self-reported medical conditions. All diseases were recorded based on ICD-10 codes. Included autoimmune diseases and their corresponding ICD-10 codes were: I00 (rheumatic fever without mention of heart involvement), G35 (multiple sclerosis), G70 (myasthenia gravis), K50 (Crohn’s disease), K51 (ulcerative colitis), M05 (seropositive rheumatoid arthritis), M06 (other rheumatoid arthritis), M07 (psoriatic and enteropathic arthropathies), M30 (polyarteritis nodosa), M32 (systemic lupus erythematosus; SLE), M33 (dermatopolymyositis), M34 (systemic sclerosis), M45 (ankylosing spondylitis), and M88 (Paget’s disease). While events may rely on self-report only, previous studies used similar methodology, and reported robust validity within the UK Biobank.11 Cases were defined as participants with a report of any autoimmune disease at inclusion. AF was defined as I48. Participants with at least one reported AF event before the first assessment date were excluded (n = 8342).
Assessment of cardiovascular risk factors
Data on age, sex, ethnicity, use of cholesterol-lowering medication, and use of blood pressure lowering medication were collected through questionnaire and interviews at the assessment centre. Furthermore, body mass index (BMI), defined as body weight divided by the square of height (kg/m2), systolic blood pressure, diastolic blood pressure, total cholesterol (in mmol/L), high-density lipoprotein (HDL) cholesterol (in mmol/L), and serum glucose (in mmol/L) were measured at the first assessment centre visit. If ICD-codes for hypertension (I10 or I15), heart failure (I50), or myocardial infarction (I21) were reported before the first assessment date, these were defined as prevalent cases.
Statistical analyses
The characteristics of the study population were presented as mean and standard deviation (SD), median and interquartile range (IQR), or counts and percentages, as appropriate. Sex differences were assessed through Student’s t-tests, Mann–Whitney U tests, or Pearson’s χ2 tests, as warranted by data and distribution type. Follow-up time was defined as the period between the first assessment centre visit and first AF event, mortality date, date of loss-to-follow-up, or 1 February 2022, whichever occurred first.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated through Cox proportional hazard regression analyses using two models: adjusted for age at baseline, sex, and ethnicity (model 1), and additionally adjusted for baseline BMI, total cholesterol, HDL-cholesterol, use of cholesterol-lowering medication, use of blood pressure lowering medication, smoking status, prevalent hypertension, prevalent type 2 diabetes (T2DM), prevalent heart failure, and prevalent myocardial infarction (model 2). To identify potential sex differences, we performed all analyses for the total population, included sex as an interaction term with the presence of the various autoimmune diseases, and performed all analyses in men and women separately. Under the assumption of missing at random, missing data (range of missingness: 0.0–14.5%) were imputed ten times using fully conditional specification and predictive mean matching methods, by using all available data as predictors. Finally, complete-cases analyses were performed as sensitivity analyses. In total, 14 278 (2.9%) participants were lost to follow-up. Additionally, 27 705 (5.6%) participants died before AF onset and were censored in the analyses.
Statistical significance was considered at two-tailed P-value ≤0.05. Data management, imputation of the missing values, and statistical analyses were performed in R: a language and environment for statistical computing, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, New York, USA).
Results
Baseline characteristics
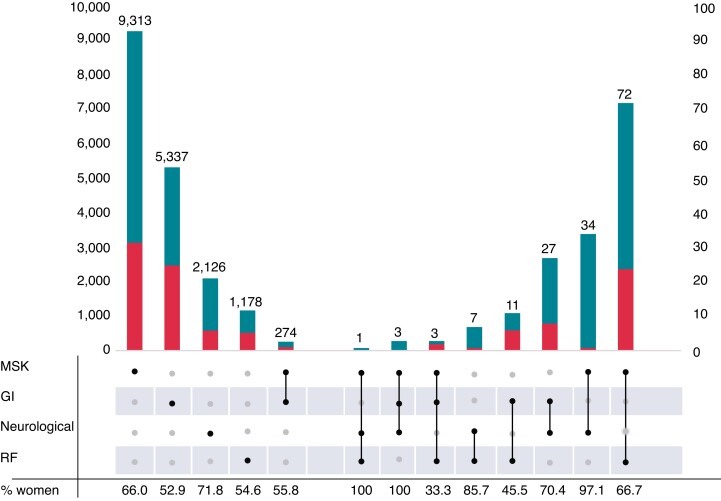
In total, we included 494 072 participants (median age 58.0, IQR 13.0), of whom 54.8% were women. Rheumatoid arthritis was the most prevalent autoimmune disease in our study population (n = 6572, 1.3%). All autoimmune diseases but ulcerative colitis (0.8% vs. 0.9%), and ankylosing spondylitis (0.2% vs. 0.4%) were more common in women than in men (Table 1). As depicted in Figure 1, the majority of our population had only one type of autoimmune disease. In both men and women, solitary MSK autoimmune diseases were the most common, followed by GI disorders, neurological disorders, and rheumatic fever without heart involvement. Only GI and MSK disorders were coexistent in over one hundred individuals.
Table 1.
Baseline characteristics of the study population
| Total population (n = 494 072) | Men (n = 223 268) | Women (n = 270 804) | P-value | |
|---|---|---|---|---|
| Age (years) | 58.0 (13) | 58.0 (13) | 57.0 (13) | <0.001 |
| Body mass index (kg/m2) | 27.4 ± 4.8 | 27.8 ± 4.2 | 27.1 ± 5.2 | <0.001 |
| Smoking status | <0.001 | |||
| ȃCurrent | 52 347 (10.6%) | 28 133 (12.6%) | 24 214 (8.9%) | |
| ȃFormer | 169 130 (34.2%) | 84 688 (37.9%) | 84 442 (31.2%) | |
| ȃNever | 269 712 (54.6%) | 109 051 (48.8%) | 160 661 (59.3%) | |
| Diastolic blood pressure (mmHg) | 82.2 ± 10.7 | 84.0 ± 10.5 | 80.7 ± 10.6 | <0.001 |
| Systolic blood pressure (mmHg) | 139.7 ± 19.7 | 142.8 ± 18.5 | 137.2 ± 20.3 | <0.001 |
| Cholesterol-lowering medication | 82 858 (16.8%) | 49 213 (22.0%) | 33 645 (12.4%) | <0.001 |
| Blood pressure medication | 99 685 (20.2%) | 52 897 (23.7%) | 46 788 (17.3%) | <0.001 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.5 ± 1.2 | 5.9 ± 1.1 | <0.001 |
| HDL-cholesterol (mmol/L) | 1.4 (0.5) | 1.2 (0.4) | 1.6 (0.5) | <0.001 |
| Glucose (mmol/L) | 4.9 (0.7) | 5.0 (0.8) | 4.9 (0.7) | <0.001 |
| Prevalent hypertension | 48 436 (9.8%) | 24 972 (11.2%) | 23 464 (8.7%) | <0.001 |
| Prevalent T2DM | 12 730 (2.6%) | 7829 (3.5%) | 4901 (1.8%) | <0.001 |
| Prevalent heart failure | 1806 (0.4%) | 1309 (0.6%) | 497 (0.2%) | <0.001 |
| Prevalent myocardial infarction | 10 520 (2.1%) | 8405 (3.8%) | 2115 (0.8%) | <0.001 |
| Prevalent coronary heart disease | 17 821 (3.6%) | 13 321 (6.0%) | 4500 (1.7%) | <0.001 |
| Gastrointestinal disorders | 5655 (1.1%) | 2649 | 3006 | 0.012 |
| ȃCrohn’s disease | 2061 (0.4%) | 911 (0.4%) | 1150 (0.4%) | 0.367 |
| ȃUlcerative colitis | 4067 (0.8%) | 1967 (0.9%) | 2100 (0.8%) | <0.001 |
| Musculoskeletal system and tissue disorders | 9710 (2.0%) | 3326 (1.5%) | 6384 (2.4%) | <0.001 |
| ȃRheumatoid arthritis | 6572 (1.3%) | 2019 (0.9%) | 4553 (1.7%) | <0.001 |
| ȃȃSeropositive rheumatoid arthritis | 376 (0.1%) | 110 (0.0%) | 266 (0.1%) | <0.001 |
| ȃȃOther rheumatoid arthritis | 6546 (1.3%) | 2008 (0.9%) | 4538 (1.7%) | <0.001 |
| ȃPsoriatic and enteropathic arthropathy | 395 (0.1%) | 188 (0.1%) | 207 (0.1%) | 0.337 |
| ȃPolyarteritis nodosa | 99 (0.0%) | 39 (0.0%) | 60 (0.0%) | 0.247 |
| ȃSystemic lupus erythematosus | 744 (0.2%) | 93 (0.0%) | 651 (0.2%) | <0.001 |
| ȃDermatopolymyositis | 152 (0.0%) | 57 (0.0%) | 95 (0.0%) | 0.057 |
| ȃSystemic sclerosis | 210 (0.0%) | 28 (0.0%) | 182 (0.1%) | <0.001 |
| ȃAnkylosing spondylitis | 1519 (0.3%) | 943 (0.4%) | 576 (0.2%) | <0.001 |
| ȃPaget’s disease | 392 (0.1%) | 77 (0.0%) | 315 (0.1%) | <0.001 |
| Neurological disorders | 2208 (0.4%) | 619 (0.3%) | 1589 (0.6%) | <0.001 |
| ȃȃMultiple sclerosis | 1983 (0.5%) | 521 (0.2%) | 1462 (0.5%) | <0.001 |
| ȃȃMyasthenia gravis | 230 (0.0%) | 99 (0.0%) | 131 (0.0%) | 0.513 |
Categorical data presented as count (%).
Continuous data presented as mean ± SD for normally distributed data, and median (IQR) for skewed data.
Significance of the differences between men and women was tested using Student’s t-tests for normally distributed continuous data, Mann–Whitney U tests for skewed continuous data, and Pearson’s χ2 test for categorical data.
HDL, high-density lipoprotein; T2DM, type 2 diabetes mellitus.
Figure 1.
Prevalence of (the combination of) various autoimmune diseases. Depicted are the prevalences of single autoimmune diseases (one dot) as well as combinations of those autoimmune diseases (multiple dots) in individuals for women (upper part of the bar) and men (lower part of the bar). The left Y-axis depicts the number of individuals for MSK, GI, neurological, RF, and the combination of MSK and GI. On the right Y-axis, the numbers are depicted for the other combinations of diseases. Rheumatic fever included rheumatic fever without heart involvement. Neurological disorders included multiple sclerosis and myasthenia gravis. Gastrointestinal disorders included Crohn’s disease and ulcerative colitis. MSK disorders included rheumatoid arthritis, psoriatic and enteropathic arthropathies, systemic lupus erythematosus, dermatopolymyositis, systemic sclerosis, ankylosing spondylitis, and Paget’s disease. GI, gastrointestinal disordes; MSK, musculoskeletal and connective tissue disorders; RF, rheumatic fever.
A significantly lower proportion of women used cholesterol-lowering medication (12.4% vs. 22.0%) and blood pressure lowering medication (17.3% vs. 23.7%). Women had lower prevalences at baseline of hypertension (8.7% vs. 11.2%), heart failure (0.2% vs. 3.8%), and acute myocardial infarction (0.8% vs. 3.8%), compared with men. All baseline characteristics are further depicted in Table 1.
Atrial fibrillation incidence
After a total follow-up time of 6 057 849 years (median 12.8 years, IQR 1.6), 16 804 men (7.5%) and 10 390 women (3.8%) developed new-onset AF. The incidence rates were 4.49/1000 person years (py) in the total population, 6.25/1000py in men, and 3.08/1000py in women.
As depicted in Table 2 and Figure 2, we found significant associations (HR, 95% CI) between the presence of rheumatic fever without heart involvement (1.47, 1.26–1.72). Autoimmune disease affecting the digestive system, including Crohn’s disease (1.23, 1.05–1.45) and ulcerative colitis (1.17, 1.05–1.31), were also significantly associated with incident AF. Autoimmune diseases targeting the MSK system also showed a larger risk for new-onset AF (1.35, 1.26–1.45). Looking at the specific autoimmune diseases, this association seemed to be mainly driven by rheumatic arthritis (1.39, 1.28–1.51), psoriatic and enteropathic arthropathies (1.38, 1.01–1.89), polyarteritis nodosa (1.79, 1.04–3.09), SLE (1.82, 1.41–2.35), and systemic sclerosis (2.32, 1.57–3.44). The other investigated diseases in this group, dermatopolymyositis, ankylosing spondylitis, and Paget’s disease, showed trends towards higher risk of incident AF, but the results were not significant after adjusting for cardiovascular risk factors. For the autoimmune diseases targeting the nervous system (multiple sclerosis and myasthenia gravis), we found a significant association with incident AF when combining the diseases (1.23, 1.02–1.47), but not when investigating the conditions separately. To identify the role of sex in the associations between the autoimmune diseases and AF, we included interaction terms between the autoimmune diseases and sex. These analyses showed a larger impact of rheumatic fever, all combined MSK diseases, rheumatoid arthritis, and ankylosing spondylitis on AF development in women. All other autoimmune disorders, excluding dermatopolymyositis, Paget’s disease, and myasthenia gravis, showed similar trends, albeit not statistically significant.
Table 2.
Associations of various autoimmune diseases with new-onset atrial fibrillation in the total population
| Model 1 | Model 2 | Sex interaction | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Rheumatic fever without heart involvement | 1.50 (1.28–1.76) | 1.47 (1.26–1.72) | 0.61 (0.44–0.84) |
| Gastrointestinal disorders | 1.24 (1.12–1.37) | 1.19 (1.08–1.32) | 0.98 (0.80–1.20) |
| ȃCrohn’s disease | 1.31 (1.11–1.54) | 1.23 (1.05–1.45) | 0.83 (0.59–1.15) |
| ȃUlcerative colitis | 1.21 (1.08–1.36) | 1.17 (1.05–1.31) | 0.97 (0.77–1.24) |
| All musculoskeletal diseases | 1.54 (1.44–1.65) | 1.35 (1.26–1.45) | 0.75 (0.65–0.86) |
| ȃAll rheumatoid arthritis | 1.60 (1.48–1.73) | 1.39 (1.28–1.51) | 0.79 (0.67–0.92) |
| ȃȃȃSeropositive rheumatoid arthritis | 2.09 (1.56–2.80) | 1.83 (1.36–2.45) | 0.60 (0.32–1.12) |
| ȃȃȃOther rheumatoid arthritis | 1.59 (1.47–1.73) | 1.39 (1.28–1.50) | 0.78 (0.66–0.92) |
| ȃȃPsoriatic and enteropathic arthropathy | 1.89 (1.38–2.58) | 1.38 (1.01–1.89) | 0.47 (0.25–0.88) |
| ȃȃPolyarteritis nodosa | 2.39 (1.39–4.12) | 1.79 (1.04–3.09) | 0.70 (0.24–2.08) |
| ȃȃSystemic lupus erythematosus | 2.10 (1.63–2.71) | 1.820 (1.41–2.35) | 0.96 (0.53–1.76) |
| ȃȃDermatopolymyositis | 1.21 (0.67–2.18) | 1.25 (0.69–2.25) | 1.25 (0.37–4.27) |
| ȃȃSystemic sclerosis | 2.52 (1.70–3.73) | 2.32 (1.57–3.44) | 0.57 (0.19–1.65) |
| ȃȃAnkylosing spondylitis | 1.24 (1.04–1.48) | 1.15 (0.96–1.38) | 0.64 (0.44–0.93) |
| ȃȃPaget’s disease | 1.36 (0.92–2.02) | 1.23 (0.83–1.82) | 1.74 (0.78–3.88) |
| Neurological diseases | 1.24 (1.04–1.49) | 1.23 (1.02–1.47) | 0.87 (0.60–1.26) |
| ȃȃMultiple sclerosis | 1.19 (0.97–1.45) | 1.20 (0.98–1.46) | 0.73 (0.48–1.12) |
| ȃȃMyasthenia gravis | 1.53 (1.01–2.33) | 1.35 (0.89–2.04) | 1.79 (0.66–4.86) |
Model 1 is adjusted for age, sex, and ethnicity
Model 2 is additionally adjusted for BMI, total cholesterol, HDL-cholesterol, use of cholesterol-lowering medication, use of blood pressure lowering medication, smoking status, prevalent hypertension, prevalent type 2 diabetes mellitus, prevalent heart failure, and prevalent acute myocardial infarction.
Presented are hazard ratios with corresponding 95% confidence intervals. Statistical significance is highlighted in bold.
BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HR, hazard ratio.
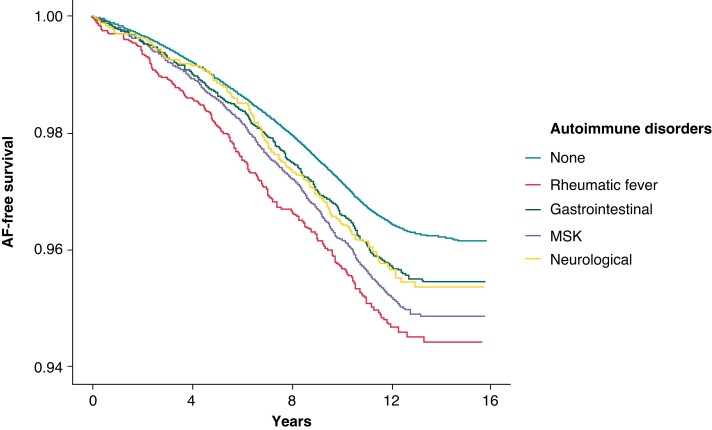
Figure 2.
The survival time free of atrial fibrillation per autoimmune disorder. Depicted is the probability of survival free of AF over time per autoimmune disorder group, adjusted for age, sex, ethnicity, BMI, total cholesterol, HDL-cholesterol, use of cholesterol-lowering medication, use of blood pressure lowering medication, smoking status, prevalent hypertension, prevalent type 2 diabetes, prevalent heart failure, and prevalent acute myocardial infarction. AF, atrial fibrillation; BMI, body mass index; HDL, high-density lipoprotein; MSK, musculoskeletal and connective tissue disorders.
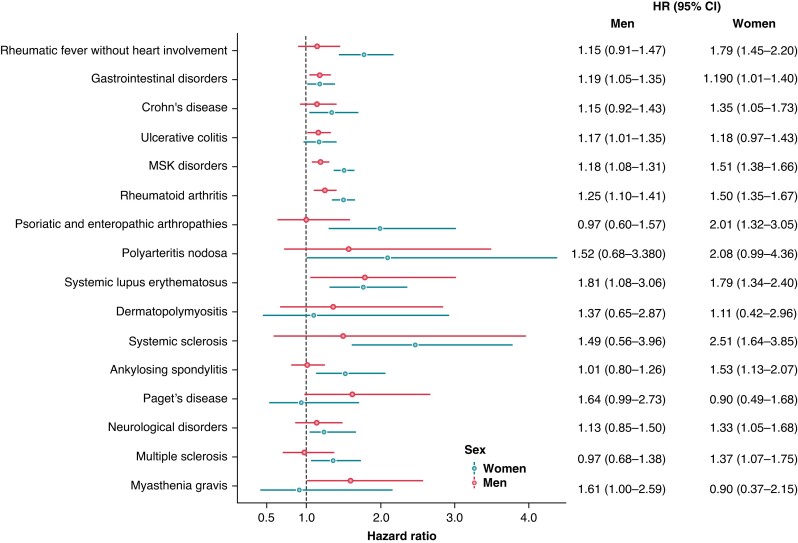
As visible in Figure 3 and Supplementary material online, Table S1, in men, we found significant associations between GI autoimmune diseases (1.19, 1.05–1.35) due to ulcerative colitis (1.17, 1.01–1.35), and MSK disorders (1.18, 1.07–1.31) due to rheumatoid arthritis (1.25, 1.10–1.41) and SLE (1.81, 1.07–3.06) also showed significant associations with new-onset AF. In contrast to the total population, we also found a distinct association between the presence of myasthenia gravis and new-onset AF (1.61, 1.00–2.59). Other autoimmune diseases, including rheumatic fever without heart involvement, polyarteritis nodosa, Paget’s disease, and neurological autoimmune diseases showed significant associations in univariable analyses, but these associations attenuated after adjusting for cardiovascular risk factors.
Figure 3.
Association of various autoimmune diseases with new-onset atrial fibrillation, stratified by sex. Depicted are hazard ratios with their corresponding 95% confidence intervals from model 2. Neurological disorders included multiple sclerosis and myasthenia gravis. Gastrointestinal disorders included Crohn’s disease, and ulcerative colitis. MSK disorders included rheumatoid arthritis, psoriatic and enteropathic arthropathies, systemic lupus erythematosus, dermatopolymyositis, systemic sclerosis, ankylosing spondylitis, and Paget’s disease. CI, confidence interval; HR, hazard ratio; MSK; musculoskeletal and connective tissue disorders.
We found that more autoimmune diseases were significantly associated with new-onset AF in women (see Supplementary material online, Table S2). We found significant associations for rheumatic fever without heart involvement (1.79, 1.45–2.20), Crohn’s disease (1.35, 1.05–1.73), MSK (1.51, 1.38–1.66), due to rheumatoid arthritis (1.50, 1.35–1.67), psoriatic and enteropathic arthropathies (2.01, 1.32–3.05), SLE (1.79, 1.34–2.40), systemic sclerosis (2.51, 1.64–3.85), and ankylosing spondylitis (1.53, 1.13–2.07) and new-onset AF. In contrast to men, multiple sclerosis (1.37, 1.07–1.75) was significantly associated with new-onset AF in women.
Sensitivity analyses adjusting for the use of medication affecting the immune system showed similar results (see Supplementary material online, Table S3).
Discussion
In the present study, we investigated the associations of multiple autoimmune diseases with new-onset AF in the population-based UK Biobank. We found significant associations between new-onset AF and rheumatic fever without heart involvement, GI (Crohn’s disease and ulcerative colitis), and MSK (rheumatoid arthritis, psoriatic and enteropathic arthropathies, polyarteritis nodosa, SLE, and systemic sclerosis) autoimmune diseases, with AF incidence. Moreover, we found evidence of significant differences between men and women in these associations.
Recent evidence suggests a role for inflammation and the innate immune system in cardiovascular disease development, including myocardial infarction, cerebrovascular events, and heart failure.12–15 However, evidence on the role of autoimmunity in AF development is scarce, at least partly due to the rarity of these disorders. In our present study, we identified a broad range of autoimmune diseases associated with incident AF in the general population.
We found that Crohn’s disease was significantly associated with AF in women, whereas ulcerative colitis was associated with AF solely in men. A systematic review of the limited available data suggested an association between GI disorders and AF presence.16 However, due to a lack of paucity of data, no robust conclusions could be made, and none of the studies evaluated potential sex differences. Our findings support the hypothesis that estrogen receptors have a paradoxical effect on GI inflammation, resulting in more severe colitis in men, but more ileitis in women.17,18
Rheumatoid arthritis is the most common autoimmune disease afflicting the MSK, mostly prevalent in women, and is suggested to increase the risk of cardiovascular disease, including AF.6,14,15,19,20 Our results are in line with the relatively large amount of evidence available on the relation between rheumatoid arthritis and AF. However, we were the first to investigate the risk in men and women separately in a large population. We found that the larger AF risk was mainly observed in women. Sex hormones may, at least partly, be the explanation for this. In patients with rheumatoid arthritis, elevated estrogen levels are found in the synovial fluid of the afflicted joints.21 Additionally, a decrease in disease activity was observed in patients undergoing androgen replacement therapy.21 However, while the mediating role of estrogen in autoimmune diseases may be part of the explanation, much remains unknown on the histopathology that causes these sex differences. This is further supported by the significant interactions we found between sex and autoimmune diseases targeting the MSK system.
In a similar way, psoriatic and enteropathic arthropathies, systemic sclerosis, and ankylosing spondylitis were associated with a higher AF risk in women only. While cardiac arrhythmias are relatively common in systemic sclerosis, data on the association between these diseases and AF is lacking.22 A Korean study found that individuals with ankylosing spondylitis had a 28% higher AF risk.23 We found a similar risk in the total population, attributed mainly to women. While this could be explained by a significant diagnostic delay and less targeted treatment in women, our findings support the notion that women with ankylosing spondylitis have a higher disease activity, and more extra-articular manifestations.24 In the same Korean population, patients with SLE had a doubled AF risk.25 We found a 82% larger risk, equal for men and women. SLE is a heterogeneous disease, targeting mostly the joints and hematological system in women, and the skin and internal organs in men.26 These different characterizations and localizations may imply different causes of atrial fibrosis and remodelling, resulting in a higher AF risk for both sexes.
We found a significantly higher AF risk in women, but not in men, in relation to multiple sclerosis. This contradicts a Swedish study, which found a lower AF risk in both men and women with multiple sclerosis.27 One explanation could be the lower number of events in our study. However, another explanation could be that, while we extensively adjusted for cardiovascular risk factors, Jadidi et al.27 only adjusted for age and country of birth.
Our results may, at least partly, be influenced by medication use. Certain autoimmune diseases can be treated with strong anti-inflammatory drugs, or even chemotherapy.28 The medication itself may give rise to AF through different pathways.29 While we performed sensitivity analyses adjusting for the use of numerous medications affecting the immune system, residual confounding due to medication use can not be ruled out. Similarly, autoimmune diseases may lead to lifestyle changes, such as lower physical activity due to joint pains, or an unhealthy diet due to GI diseases. While we adjusted for factors suggesting an unhealthy lifestyle, such as BMI, cholesterol, hypertension, and smoking status, other factors may underlie the associations. Shared genetic basis may reflect another potential underlying factor. It may be that the genetic preposition for the development of autoimmune disease is also associated with AF development. Future research, including Mendelian randomization studies, may shed light on this.
The strengths in our present study are the large population-based study population of almost 500 000 individuals, the median of over 12 years follow-up time, the continuous and careful assessment of both physical and social data, and the continuous linkage with multiple registries to confirm disease occurrences. In addition, we used robust statistical models to adjust for confounding, and used both multiple imputation and complete-case analyses to confirm our findings. However, our study also has some limitations. Due to the rarity of autoimmune disorders, some of our diseases of interest were low in prevalence. Moreover, due to the nature of data assessment in the UK Biobank, we were constricted to using ICD-10 codes. Using the ICD-10 codes carries various limitations, including the inability to investigate specific diseases, but rather the groups of autoimmune diseases. Moreover, this could have also influenced the relatively low number of participants with prevalent hypertension (9.8%), while 20.2% used blood pressure medication. Medication may be started due to comorbidity or prevention, while the ICD-10 diagnosis for hypertension is not registered. Also, due to this coding, we were unable to distinguish between the various AF patterns and were restricted to overall AF. As clinical trials in patients and are often expensive and time-consuming, we hope that our results open the door for future patient cohorts to further investigate differences in AF characteristics and prognosis between AF patients with and without autoimmune disorders. As AF can have an asymptomatic presentation, it is possible that AF patients free of symptoms, or who avoided visiting a healthcare professional, were missed. However, this would be similar to a real-world situation, in which individuals from the general population could also remain undiagnosed. Additionally, the UK Biobank comprises of mostly men and women from European heritage and of middle-to-older age. As all participants are volunteering to participate, healthy-volunteer bias is also a possibility. Lastly, in comparison to all other included autoimmune diseases, of which the vast majority is documented within the UK Biobank due to hospital or primary care registries, rheumatic fever was registered mainly due to self-report only. Recall bias or ambiguity in the questionnaires may have inflated the actual numbers of rheumatic fever events, as the prevalence within this population is higher than expected. Therefore, the results from this study may not be directly generalized to other populations.
Conclusion
Autoimmune diseases are significantly associated with the risk of new-onset AF in this prospective population-based study, comprising almost half a million participants. Our findings further elaborate on and contribute to the current knowledge of the pathophysiological differences in autoimmunity between men and women. This implies that various autoimmune diseases may modulate the propensity to develop AF, particularly in women. This information could also guide future preventive strategies. However, evidence on the role of autoimmunity in AF development is still scarce. Further evidence is required to support clinical translation of our findings.
Supplementary Material
Acknowledgements
The authors would like to thank the participants of the UK Biobank, the staff, and participating general practitioners and pharmacists.
Contributor Information
Martijn J Tilly, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
Sven Geurts, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
Fang Zhu, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
Maxime M Bos, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
M Arfan Ikram, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
Moniek P M de Maat, Department of Hematology, Erasmus MC University Medical Center Rotterdam, Rotterdam, The Netherlands.
Natasja M S de Groot, Department of Cardiology, Erasmus MC University Medical Center Rotterdam, Rotterdam, The Netherlands.
Maryam Kavousi, Department of Epidemiology, Erasmus MC University Medical Center Rotterdam, Office Na-2714, PO Box 2040, 3000 CA, Rotterdam, The Netherlands.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study has been supported by the Erasmus MC (Mrace) grant, and the Senior Scientist Grant from the Dutch Heart Foundation (03-004-2021-T050). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has also had funding from the Welsh Government, British Heart Foundation, Cancer Research UK and Diabetes UK. UK Biobank is supported by the National Health Service (NHS).
Data availability
The present study is conducted using the UK Biobank, application number 58237. The UK Biobank is an open-access data source.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol 2017;24:1555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol 2015;5:649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J 2005;26:2083–92. [DOI] [PubMed] [Google Scholar]
- 6. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis 2017;20:434–41. [DOI] [PubMed] [Google Scholar]
- 7. Gawalko M, Balsam P, Lodzinski P, Grabowski M, Krzowski B, Opolski Get al. Cardiac arrhythmias in autoimmune diseases. Circ J 2020;84:685–94. [DOI] [PubMed] [Google Scholar]
- 8. Westerman S, Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev 2019;15:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2001;2:777–80. [DOI] [PubMed] [Google Scholar]
- 10. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh Jet al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siebert S, Lyall DM, Mackay DF, Porter D, McInnes IB, Sattar Net al. Characteristics of rheumatoid arthritis and its association with major comorbid conditions: cross-sectional study of 502 649 UK Biobank participants. RMD Open 2016;2:e000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJet al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol 2020;4:100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015;12:230–43. [DOI] [PubMed] [Google Scholar]
- 14. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 15. Semb AG, Ikdahl E, Wibetoe G, Crowson C, Rollefstad S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol 2020;16:361–79. [DOI] [PubMed] [Google Scholar]
- 16. Zuin M, Zuliani G, Rigatelli G, Favero GD, Roncon L. Atrial fibrillation in patients with inflammatory bowel disease: a systematic review and meta-analysis. Eur J Intern Med 2020;76:120–2. [DOI] [PubMed] [Google Scholar]
- 17. De Simone V, Matteoli G. Estrogen-mediated effects underlie gender bias in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2018;5:638–9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rustgi SD, Kayal M, Shah SC. Sex-based differences in inflammatory bowel diseases: a review. Therap Adv Gastroenterol 2020;13:1756284820915043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carmona L, Cross M, Williams B, Lassere M, March L. Rheumatoid arthritis. Best Pract Res Clin Rheumatol 2010;24:733–45. [DOI] [PubMed] [Google Scholar]
- 20. Ma Y, Pan Z, Fan D, Xu S, Pan F. The increased risk of atrial fibrillation in inflammatory arthritis: a systematic review and meta-analysis of cohort studies. Immunol Invest 2022;51:1095–107. [DOI] [PubMed] [Google Scholar]
- 21. Kim JR, Kim HA. Molecular mechanisms of sex-related differences in arthritis and associated pain. Int J Mol Sci 2020;21:7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seferovic PM, Ristic AD, Maksimovic R, Simeunovic DS, Ristic GG, Radovanovic Get al. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology (Oxford) 2006;45:iv39–42. [DOI] [PubMed] [Google Scholar]
- 23. Moon I, Choi EK, Jung JH, Han KD, Choi YJ, Park Jet al. Ankylosing spondylitis: a novel risk factor for atrial fibrillation – a nationwide population-based study. Int J Cardiol 2019;275:77–82. [DOI] [PubMed] [Google Scholar]
- 24. Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim SY, Bae EH, Han KD, Jung JH, Choi HS, Kim CSet al. Systemic lupus erythematosus is a risk factor for atrial fibrillation: a nationwide, population-based study. Clin Exp Rheumatol 2019;37:1019–25. [PubMed] [Google Scholar]
- 26. Yacoub Wasef SZ. Gender differences in systemic lupus erythematosus. Gend Med 2004;1:12–7. [DOI] [PubMed] [Google Scholar]
- 27. Jadidi E, Mohammadi M, Moradi T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult Scler 2013;19:1336–40. [DOI] [PubMed] [Google Scholar]
- 28. Zack E. Chemotherapy and biotherapeutic agents for autoimmune diseases. Clin J Oncol Nurs 2012;16:E125–32. [DOI] [PubMed] [Google Scholar]
- 29. Yang X, Li X, Yuan M, Tian C, Yang Y, Wang Xet al. Anticancer therapy-induced atrial fibrillation: electrophysiology and related mechanisms. Front Pharmacol 2018;9:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The present study is conducted using the UK Biobank, application number 58237. The UK Biobank is an open-access data source.