Abstract
Aims
This post hoc analysis of the ATHENA trial (NCT00174785) assessed the effect of dronedarone on the estimated burden of atrial fibrillation (AF)/atrial flutter (AFL) progression to presumed permanent AF/AFL, and regression to sinus rhythm (SR), compared with placebo.
Methods and results
The burden of AF/AFL was estimated by a modified Rosendaal method using available electrocardiograms (ECG). Cumulative incidence of permanent AF/AFL (defined as ≥6 months of AF/AFL until end of study) or permanent SR (defined as ≥6 months of SR until end of study) were calculated using Kaplan–Meier estimates. A log-rank test was used to assess statistical significance. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were estimated using a Cox model, adjusted for treatment group. Of the 4439 patients included in this analysis, 2208 received dronedarone, and 2231 placebo. Baseline and clinical characteristics were well balanced between groups. Overall, 304 (13.8%) dronedarone-treated patients progressed to permanent AF/AFL compared with 455 (20.4%) treated with placebo (P < 0.0001). Compared with those receiving placebo, patients receiving dronedarone had a lower cumulative incidence of permanent AF/AFL (log-rank P < 0.001; HR: 0.65; 95% CI: 0.56–0.75), a higher cumulative incidence of permanent SR (log-rank P < 0.001; HR: 1.19; 95% CI: 1.09–1.29), and a lower estimated AF/AFL burden over time (P < 0.01 from Day 14 to Month 21).
Conclusion
These results suggest that dronedarone could be a useful antiarrhythmic drug for early rhythm control due to less AF/AFL progression and more regression to SR vs. placebo, potentially reflecting reverse remodeling.
Clinical trial registration
Keywords: Antiarrhythmic drug, Atrial fibrillation, Cardiovascular outcomes, Dronedarone, Progression
Graphical Abstract
Graphical Abstract.
What’s new?
Progression to permanent AF/AFL was less frequent and regression to permanent sinus rhythm (SR) was more frequent among patients with atrial fibrillation (AF)/atrial flutter (AFL) who received dronedarone vs. placebo, potentially reflecting reverse remodeling
Patients treated with dronedarone also had lower cumulative incidence of permanent AF/AFL, higher cumulative incidence of permanent SR, and lower estimated AF/AFL burden over time
Progression to permanent AF/AFL occurred later with dronedarone vs. placebo
To our knowledge, this post hoc analysis of the ATHENA trial is the first application of the Rosendaal method of linear interpolation to estimate AF/AFL burden
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with increased risk for stroke, heart failure, and cardiovascular death.1–3 AF is a progressive arrhythmia that can advance from paroxysmal (duration ≤7 days) to persistent (duration >7 days without spontaneous conversion to sinus rhythm [SR]) or to permanent AF.4,5 Results of a systematic review of use of AF ablation showed that in general population studies, progression to more persistent or permanent forms of AF occurred in 10%–20% of people with paroxysmal AF within 1 year of follow-up.6 Progression over time to more persistent forms of AF is associated with increased disease burden, hospitalization rates, and increased mortality4,7,8 and is related to structural and atrial electrical remodeling.9,10 The findings of several studies support the use of rhythm control in people with AF to slow disease progression and improve cardiovascular outcomes.11,12 Observational studies have shown that patients with non-permanent AF who received rhythm control were less likely to progress to permanent AF compared with those who received only rate control therapy,13 and that early rhythm control is associated with a lower risk of adverse cardiovascular outcomes.14 In the ATHENA trial, in which patients with paroxysmal or persistent AF were enrolled, treatment with dronedarone was associated with a significant reduction in the incidence of the primary composite endpoint of first cardiovascular hospitalization or death due to any cause compared with placebo.15 Additionally, in a post hoc analysis of the ATHENA population, dronedarone demonstrated efficacy vs. placebo regardless of duration of AF/atrial flutter (AFL), but was more robust in those with short (<3 months) or intermediate (3 to <24 months) AF/AFL history compared with those with an AF/AFL history ≥24 months.16 In the current post hoc analysis of ATHENA, we hypothesized that (i) there is a lower AF/AFL burden over time in patients receiving dronedarone compared with placebo, and (ii) there is less AF progression and more AF regression in dronedarone-treated vs. placebo-treated patients.
Methods
Overview of the ATHENA study
ATHENA was a double-blind, placebo-controlled randomized study (NCT00174785); design and primary results have been previously reported.15,17 The study evaluated outcomes among 4628 patients with paroxysmal or persistent AF/AFL (enrolled between June 2005 and December 2006) who received dronedarone (400 mg twice daily) or placebo. The mean follow-up period was 21 ± 5 months with a minimum follow-up of 12 months. All patients in the ATHENA study provided written informed consent; the study was approved by independent review boards at participating sites and was conducted according to the Declaration of Helsinki.
Outcomes
In the present analysis, the outcomes of interest were estimated AF/AFL burden over time, cumulative incidence of presumed permanent AF/AFL (defined as AF progression) and presumed permanent SR (defined as AF regression). To be classified as having presumed permanent AF/AFL, patients were required to have a period of ≥6 months with all available electrocardiograms (ECGs) showing AF/AFL until the end of study. To be classified as having permanent SR, patients were required to have a period of ≥6 months with all available ECGs showing SR until the end of study.
Statistical analysis
Demographic characteristics, clinical characteristics, and safety data were summarized using descriptive statistics. Cumulative incidence functions were calculated using the complement of Kaplan–Meier estimates. Comparison between treatment groups was assessed using a log-rank test. Hazard ratios (HRs) with corresponding 95% confidence interval (CIs) were estimated using a Cox model adjusted for treatment group. AF/AFL burden was estimated using the modified Rosendaal method18 to calculate percentage of time in AF/AFL assessed by available ECGs. The modified Rosendaal method18 uses linear interpolation to estimate the time a patient spends in AF/AFL. This method was adapted to apply to qualitative outcomes and to calculate the percentage of time each patient spent in AF/AFL using available ECG assessments. All tests were two-sided, and P-values of <0.05 were considered statistically significant. P-values are displayed for descriptive purpose only. Data were analyzed using SAS version 9.4 (Cary, NC, USA).
Results
Of the 4628 patients randomized in the ATHENA study, 4439 were included in the analysis (2208 in the dronedarone arm and 2231 in the placebo arm). A total of 189 patients with missing ECGs or undefined assessment at baseline were excluded from the analysis. Baseline and clinical characteristics were well balanced between groups (Table 1), as was the use of baseline medications (Table 2).
Table 1.
Baseline and clinical characteristics
| Patient characteristics | Progression to permanent AF/AFL | Regression to permanent SR | ||||||
|---|---|---|---|---|---|---|---|---|
| Dronedarone | Placebo | Dronedarone | Placebo | |||||
| Permanent AF/AFL | Non-permanent AF/AFL | Permanent AF/AFL | Non-permanent AF/AFL | Permanent SR | Not permanent SR | Permanent SR | Not permanent SR | |
| (n = 304) | (n = 1904) | (n = 455) | (n = 1776) | (n = 1149) | (n = 1059) | (n = 1021) | (n = 1210) | |
| Age, years mean (SD) | 70.2 (9.3) | 71.7 (8.8) | 71.7 (8.8) | 71.6 (9.0) | 71.5 (8.5) | 71.6 (9.3) | 70.8 (9.1) | 72.2 (8.8) |
| Male n (%) | 175 (57.6) | 942 (49.5) | 256 (56.3) | 979 (55.1) | 555 (48.3) | 562 (53.1) | 544 (53.3) | 691 (57.1) |
| Race n (%) | ||||||||
| ȃWhite | 269 (88.5) | 1713 (90.0) | 388 (85.3) | 1598 (90.0) | 1028 (89.5) | 954 (90.1) | 912 (89.3) | 1074 (88.8) |
| ȃAsian | 28 (9.2) | 117 (6.1) | 46 (10.1) | 103 (5.8) | 70 (6.1) | 75 (7.1) | 58 (5.7) | 91 (7.5) |
| ȃBlack | 0 (0.0) | 18 (0.9) | 5 (1.1) | 24 (1.4) | 8 (0.7) | 10 (0.9) | 11 (1.1) | 18 (1.5) |
| ȃOther | 7 (2.3) | 56 (2.9) | 16 (3.5) | 51 (2.9) | 43 (3.7) | 20 (1.9) | 40 (3.9) | 27 (2.2) |
| Weight, mean (SD), kg | 82.7 (17.5) | 80.1 (17.2) | 82.0 (18.9) | 80.2 (17.5) | 79.7 (16.9) | 81.2 (17.6) | 79.5 (16.1) | 81.4 (19.0) |
| CHA2DS2-VASc score mean (SD) | ||||||||
| ȃMale, n | 175 | 942 | 256 | 979 | 555 | 562 | 544 | 691 |
| ȃMean (SD) | 3.0 (1.5) | 3.1 (1.4) | 3.0 (1.4) | 3.1 (1.5) | 3.1 (1.4) | 3.1 (1.5) | 3.0 (1.4) | 3.1 (1.5) |
| ȃFemale, n | 129 | 962 | 199 | 797 | 594 | 497 | 477 | 519 |
| ȃMean (SD) | 4.5 (1.5) | 4.3 (1.4) | 4.5 (1.4) | 4.3 (1.4) | 4.3 (1.4) | 4.4 (1.4) | 4.2 (1.4) | 4.4 (1.5) |
| Cardiovascular comorbidities n (%) | ||||||||
| ȃStructural heart disease | 186 (61.4) | 1086 (57.5) | 270 (59.6) | 1074 (60.9) | 644 (56.6) | 628 (59.5) | 588 (58.0) | 756 (62.8) |
| ȃCoronary heart disease | 92 (30.3) | 534 (28.0) | 117 (25.7) | 574 (32.3) | 319 (27.8) | 307 (29.0) | 312 (30.6) | 379 (31.3) |
| ȃHypertrophic cardiomyopathy | 6 (2.0) | 38 (2.0) | 10 (2.2) | 36 (2.0) | 18 (1.6) | 26 (2.5) | 18 (1.8) | 28 (2.3) |
| ȃNon-ischaemic dilated cardiomyopathy | 12 (3.9) | 64 (3.4) | 21 (4.6) | 58 (3.3) | 30 (2.6) | 46 (4.3) | 26 (2.5) | 53 (4.4) |
| ȃIschaemic dilated cardiomyopathy | 10 (3.3) | 75 (3.9) | 24 (5.3) | 87 (4.9) | 37 (3.2) | 48 (4.5) | 36 (3.5) | 75 (6.2) |
| ȃHypertension | 261 (85.9) | 1656 (87.0) | 397 (87.3) | 1521 (85.6) | 1007 (87.6) | 910 (85.9) | 895 (87.7) | 1023 (84.5) |
| ȃAblation for AF/AFL | 11 (3.6) | 67 (3.5) | 20 (4.4) | 78 (4.4) | 33 (2.9) | 45 (4.2) | 34 (3.3) | 64 (5.3) |
| ȃPacemaker | 16 (5.3) | 142 (7.5) | 41 (9.0) | 147 (8.3) | 32 (2.8) | 126 (11.9) | 39 (3.8) | 149 (12.3) |
| ȃImplanted cardioverter defibrillator | 4 (1.3) | 32 (1.7) | 5 (1.1) | 30 (1.7) | 6 (0.5) | 30 (2.8) | 12 (1.2) | 23 (1.9) |
| ȃLone atrial fibrillation | 17 (5.6) | 120 (6.3) | 19 (4.2) | 115 (6.5) | 74 (6.5) | 63 (6.0) | 62 (6.1) | 72 (6.0) |
| CHF symptoms n (%) | 91 (29.9) | 562 (29.5) | 132 (29.0) | 538 (30.3) | 342 (29.8) | 311 (29.4) | 311 (30.5) | 359 (29.7) |
| NYHA class n (%) | ||||||||
| ȃI | 24 (7.9) | 180 (9.5) | 36 (7.9) | 135 (7.6) | 110 (9.6) | 94 (8.9) | 87 (8.5) | 84 (6.9) |
| ȃII | 54 (17.8) | 310 (16.3) | 75 (16.5) | 319 (18.0) | 189 (16.4) | 175 (16.5) | 190 (18.6) | 204 (16.9) |
| ȃIII | 13 (4.3) | 72 (3.8) | 21 (4.6) | 84 (4.7) | 43 (3.7) | 42 (4.0) | 34 (3.3) | 71 (5.9) |
| ȃNA | 213 (70.1) | 1342 (70.5) | 323 (71.0) | 1238 (69.7) | 807 (70.2) | 748 (70.6) | 710 (69.5) | 851 (70.3) |
| LVEF < 35% or NYHA class > 1 n | 302 | 1885 | 451 | 1757 | 1135 | 1052 | 1010 | 1198 |
| n (%) | 96 (31.8) | 578 (30.7) | 138 (30.6) | 561 (31.9) | 348 (30.7) | 326 (31.0) | 321 (31.8) | 378 (31.6) |
| Received concomitant oral anticoagulants n (%) | 280 (92.1) | 1257 (66.0) | 403 (88.6) | 1175 (66.2) | 650 (56.6) | 887 (83.8) | 570 (55.8) | 1008 (83.3) |
| eGFR category (CKD-EPI), n | 303 | 1898 | 453 | 1775 | 1146 | 1055 | 1021 | 1207 |
| ȃ<45 mL/min, n (%) | 35 (11.6) | 268 (14.1) | 63 (13.9) | 251 (14.1) | 134 (11.7) | 169 (16.0) | 138 (13.5) | 176 (14.6) |
| ȃ≥45 to <60 mL/min n (%) | 88 (29.0) | 536 (28.2) | 140 (30.9) | 510 (28.7) | 320 (27.9) | 304 (28.8) | 288 (28.2) | 362 (30.0) |
| ȃ≥60 mL/min n (%) | 180 (59.4) | 1094 (57.6) | 250 (55.2) | 1014 (57.1) | 692 (60.4) | 582 (55.2) | 595 (58.3) | 669 (55.4) |
| Time since first known AF/AFL episode n (%)a | ||||||||
| ȃ<12 months | 92 (51.4) | 868 (71.7) | 160 (58.6) | 759 (69.4) | 574 (76.3) | 386 (60.5) | 488 (73.9) | 431 (61.2) |
| ȃ≥12 months | 87 (48.6) | 344 (28.4) | 113 (41.4) | 334 (30.6) | 179 (23.7) | 252 (39.5) | 173 (26.1) | 274 (38.8) |
| Cardioversion n (%) | ||||||||
| ȃ≥ 1 cardioversion | 39 (12.8) | 282 (14.8) | 77 (16.9) | 392 (22.1) | 95 (8.3) | 226 (21.3) | 129 (12.6) | 340 (28.1) |
| ȃ≥ 1 successful cardioversion | 20 (6.6) | 268 (14.1) | 47 (10.3) | 372 (20.9) | 95 (8.3) | 193 (18.2) | 126 (12.3) | 293 (24.2) |
Percentages calculated on patients with known AF/AFL episode before study entry.
AF, atrial fibrillation; AFL, atrial flutter; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 and sex category (female); CHF, congestive heart failure; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtrate rate; LVEF; left ventral ejection fraction; NYHA, New York Heart Association; SD, standard deviation; SR, sinus rhythm; TEAE, treatment-emergent adverse-event.
Table 2.
Baseline cardiovascular medications
| Baseline medications n (%) | Progression to permanent AF/AFL | Regression to permanent SR | ||||||
|---|---|---|---|---|---|---|---|---|
| Dronedarone | Placebo | Dronedarone | Placebo | |||||
| Permanent AF/AFL | Non-permanent AF/AFL | Permanent AF/AFL | Non-permanent AF/AFL | Permanent SR | Not permanent SR | Permanent SR | Not permanent SR | |
| (n = 304) | (n = 1904) | (n = 455) | (n = 1776) | (n = 1149) | (n = 1059) | (n = 1021) | (n = 1210) | |
| Beta blocking agents (not including sotalol) | 215 (70.7) | 1348 (70.8) | 315 (69.2) | 1267 (71.3) | 836 (72.8) | 727 (68.6) | 724 (70.9) | 858 (70.9) |
| ȃACE inhibitors or AII receptor antagonist | 209 (68.8) | 1352 (71.0) | 321 (70.5) | 1228 (69.1) | 846 (73.6) | 715 (67.5) | 721 (70.6) | 828 (68.4) |
| Oral anticoagulant | 250 (82.2) | 1098 (57.7) | 351 (77.1) | 975 (54.9) | 564 (49.1) | 784 (74.0) | 461 (45.2) | 865 (71.5) |
| Diuretics | ||||||||
| ȃOther than spironolactone | 162 (53.3) | 979 (51.4) | 245 (53.8) | 929 (52.3) | 580 (50.5) | 561 (53.0) | 521 (51.0) | 653 (54.0) |
| ȃSpironolactone | 26 (8.6) | 113 (5.9) | 29 (6.4) | 101 (5.7) | 52 (4.5) | 87 (8.2) | 52 (5.1) | 78 (6.4) |
| Aspirin (<365 mg) | 96 (31.6) | 882 (46.3) | 138 (30.3) | 846 (47.6) | 581 (50.6) | 397 (37.5) | 558 (54.7) | 426 (35.2) |
| Statins | ||||||||
| ȃMetabolized by CYP3A4 | 99 (32.6) | 600 (31.5) | 148 (32.5) | 578 (32.5) | 343 (29.9) | 356 (33.6) | 320 (31.3) | 406 (33.6) |
| ȃNot metabolized by CYP3A4 | 22 (7.2) | 122 (6.4) | 27 (5.9) | 133 (7.5) | 73 (6.4) | 71 (6.7) | 64 (6.3) | 96 (7.9) |
| Calcium antagonist (with heart rate lowering effects) | 46 (15.1) | 274 (14.4) | 58 (12.7) | 232 (13.1) | 140 (12.2) | 180 (17.0) | 112 (11.0) | 178 (14.7) |
| Digitalis | 56 (18.4) | 252 (13.2) | 82 (18.0) | 211 (11.9) | 109 (9.5) | 199 (18.8) | 89 (8.7) | 204 (16.9) |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AFL, atrial flutter.
AF progression and regression
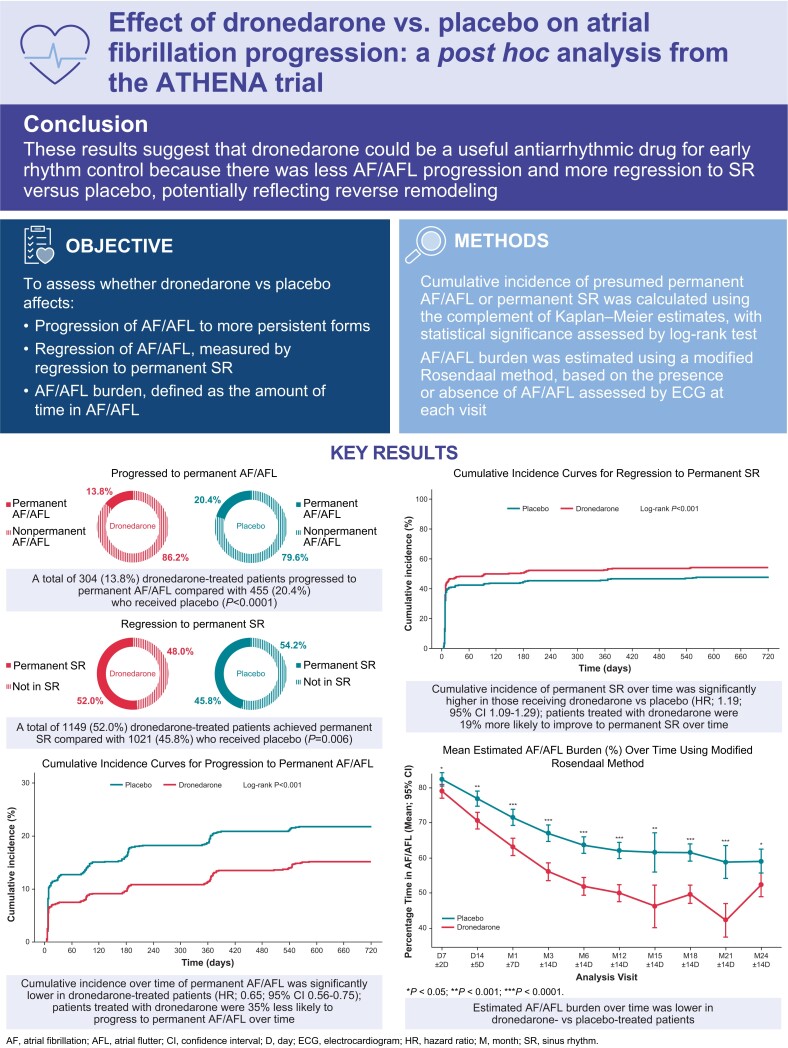
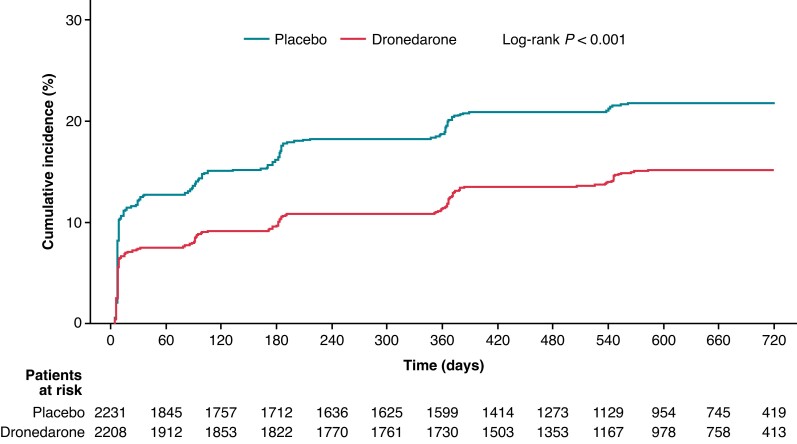
Overall, at the end of follow-up, 304 of 2208 dronedarone-treated patients had progressed to permanent AF/AFL compared with 455 of 2231 patients who received placebo (13.8% vs. 20.4%, respectively; P < 0.0001). A total of 1149 dronedarone-treated patients had achieved permanent SR compared with 1021 patients who received placebo (52.0% vs. 45.8%, respectively; P = 0.006). Irrespective of treatment group, the proportions of patients progressing to permanent vs. non-permanent AF/AFL were higher for those who had left atrial diameters >40 mm (dronedarone 85.7% vs. 67.3%; placebo 80.4% vs. 66.5%), for those with left ventricular ejection <50% (dronedarone 22.6% vs. 16.6%; placebo 21.3% vs. 17.3%) (Table 3). The reverse was observed for those achieving permanent SR (Table 3).
Table 3.
Baseline cardiovascular examinations
| Patient characteristics | Progression to permanent AF/AFL | Regression to permanent SR | ||||||
|---|---|---|---|---|---|---|---|---|
| Dronedarone | Placebo | Dronedarone | Placebo | |||||
| Permanent AF/AFL | Non-permanent AF/AFL | Permanent AF/AFL | Non-permanent AF/AFL | Permanent SR | Not permanent SR | Permanent SR | Not permanent SR | |
| (n = 304) | (n = 1904) | (n = 455) | (n = 1776) | (n = 1149) | (n = 1059) | (n = 1021) | (n = 1210) | |
| Baseline cardiovascular examination | ||||||||
| Left atrium diameter n | 300 | 1883 | 450 | 1748 | 1132 | 1051 | 1009 | 1189 |
| ȃMean (SD) | 46.53 (6.14) | 43.65 (6.77) | 46.35 (6.75) | 43.39 (7.01) | 42.88 (6.44) | 45.30 (6.87) | 42.37 (6.72) | 45.38 (7.04) |
| ȃ> 40 mm n (%) | 257 (85.7) | 1267 (67.3) | 362 (80.4) | 1162 (66.5) | 726 (64.1) | 798 (75.9) | 619 (61.3) | 905 (76.1) |
| Left ventricular ejection fraction % n | 301 | 1879 | 451 | 1748 | 1130 | 1050 | 1007 | 1192 |
| ȃMean (SD) | 56.01 (11.19) | 57.66 (10.82) | 56.70 (11.26) | 57.60 (11.20) | 58.33 (9.98) | 56.47 (11.71) | 58.68 (10.62) | 56.35 (11.59) |
| ȃ< 50% n (%) | 68 (22.6) | 312 (16.6) | 96 (21.3) | 303 (17.3) | 158 (14.0) | 222 (21.1) | 135 (13.4) | 264 (22.1) |
AF, atrial fibrillation; AFL, atrial flutter; SD, standard deviation.
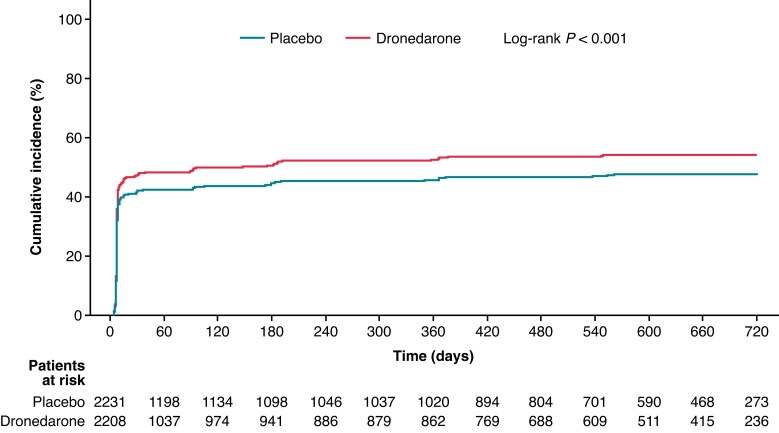
Progression of AF/AFL, as defined by the cumulative incidence of permanent AF/AFL over time, was lower in patients who received dronedarone vs. placebo (log-rank P < 0.001; HR: 0.65; 95% CI: 0.56–0.75; Figure 1). Regression of AF/AFL, as defined by the cumulative incidence of permanent SR over time, was higher in dronedarone-treated vs. placebo-treated patients (log-rank P < 0.001; HR: 1.19; 95% CI: 1.09–1.29; Figure 2). Estimated AF/AFL burden was significantly lower at each planned visit in patients who received dronedarone compared with those who received placebo (Figure 3).
Figure 1.
Cumulative incidence curves for progression to permanent AF/AFL. AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; HR, hazard ratio.
Figure 2.
Cumulative incidence curves for AF/AFL regression to permanent SR. AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; HR, hazard ratio; SR, sinus rhythm.
Figure 3.
Estimated mean AF/AFL burden (%) calculated using modified Rosendaal method.18 AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; D, day; M, month. Analysis performed using Student’s t-test: * P < 0.05; ** P < 0.001; *** P < 0.0001.
Safety
A summary of treatment-emergent adverse events (TEAEs) is included in Table 4. Rates of TEAEs leading to permanent discontinuation of study drug were higher with dronedarone vs. placebo in all comparison groups (Table 4), in line with the findings in the main ATHENA trial.15 Overall rates of discontinuation for dronedarone vs. placebo were 12.7% vs. 8.1%, respectively, with events of diarrhoea, nausea, prolongation of QT on ECG, and increased levels of serum creatinine contributing to the imbalance.
Table 4.
Treatment-emergent adverse events (TEAEs)
| Analyses by progression to presumed permanent AF/AFL | Analyses by regression to permanent SR | |||||||
|---|---|---|---|---|---|---|---|---|
| Dronedarone | Placebo | Dronedarone | Placebo | |||||
| Permanent AF/AFL | Non-permanent AF/AFL | Permanent AF/AFL | Non-permanent AF/AFL | Permanent SR | Not permanent SR | Permanent SR | Not permanent SR | |
| (n = 304) | (n = 1906) | (n = 455) | (n = 1774) | (n = 1150) | (n = 1060) | (n = 1020) | (n = 1209) | |
| Any TEAE n (%) | 231 (76.0) | 1356 (71.1) | 349 (76.7) | 1193 (67.2) | 779 (67.7) | 808 (76.2) | 663 (65.0) | 879 (72.7) |
| Serious TEAEan (%) | 67 (22.0) | 373 (19.6) | 105 (23.1) | 369 (20.8) | 197 (17.1) | 243 (22.9) | 203 (19.9) | 271 (22.4) |
| Deathsbn (%) | 5 (1.6) | 32 (1.7) | 8 (1.8) | 35 (2.0) | 8 (0.7) | 29 (2.7) | 7 (0.7) | 36 (3.0) |
| Permanently discontinued study drug for TEAE n (%) | 44 (14.5) | 236 (12.4) | 32 (7.0) | 147 (8.3) | 109 (9.5) | 171 (16.1) | 57 (5.6) | 122 (10.1) |
Including serious AEs leading to death.
Patients who died from first study drug intake up to last study drug intake plus 10 days.
Discussion
ATHENA is the largest clinical trial to date assessing clinical outcomes in AF patients using an antiarrhythmic drug (AAD). In this post hoc analysis, the effect of dronedarone on the progression of AF/AFL was evaluated using the cumulative incidence of presumed permanent AF/AFL and estimated AF/AFL burden using the modified Rosendaal method.18 To our knowledge, this is the first study to apply this methodology in the context of estimating AF/AFL burden.
A lower proportion of patients treated with dronedarone as compared with placebo progressed to permanent AF/AFL over time; a higher proportion of patients had AF regression to SR and, overall, had a lower estimated AF/AFL burden. At any time point during the study period, patients treated with dronedarone were 35% less likely to progress to permanent AF/AFL and 19% more likely to improve to permanent SR compared with patients who received placebo. The positive effects on progression, regression, and AF/AFL burden suggest that dronedarone may reverse atrial and ventricular remodeling. This suggestion is supported by results from animal studies of structural heart disease in which dronedarone was found to decrease remodeling through increased bioavailability of nitric oxide19 and to cause regression of myocardial remodeling.20–22 Moreover, dronedarone has previously been shown in the HESTIA study to reduce AF burden in patients with pacemakers who had paroxysmal or persistent AF.23 The protective effect of dronedarone in preventing recurrence of AF has also been recorded in a real world observational study (EFFECT-AF), with dronedarone having similar efficacy to other AADs (mainly amiodarone [46.5%], Class Ic drugs [42.1%], or sotalol [10.8%]) in preventing first AF recurrence.24 The positive effects on AF/AFL burden, and on AF progression and regression by dronedarone in the current study suggest dronedarone may be a preferred treatment option early in the course of AF, particularly given its low pro-arrhythmic risk.15 In a previous post hoc analysis of ATHENA that categorized patients into groups according to time since first-known AF/AFL episode (<3 months, 3 to <24 months, and ≥24 months),16 dronedarone was shown to be associated with improved primary outcome of cardiovascular hospitalization and death from any cause at all time points. The effects were, however, more pronounced in those with shorter vs. longer AF/AFL histories,16 suggesting that dronedarone should be used at an early stage of the AF disease, whereas factors such as age or sex do not impact the efficacy of dronedarone.25 Recently, the clinical importance of early rhythm control has been emphasized in a number of studies.11,12,26–28 The EAST-AFNET 4 trial demonstrated that early comprehensive rhythm control (using AADs and/or catheter ablation) was associated with a significant reduction in risk of adverse cardiovascular outcomes compared with the guideline-recommended standard of care, which was limited to the guideline-based management of AF-related symptoms.26,27 Additionally, the STOP AF First and EARLY AF trials support the use of ablation over AADs as initial early rhythm control therapy (dronedarone was used by 12% and 8.1% of patients included in the AAD groups in these studies, respectively).11,12
Patients with AF/AFL progression in this analysis tended to have larger left atrial diameters and lower left ventricular ejection fractions irrespective of treatment group, which is consistent with other reports.29 Additionally, in the dronedarone (as opposed to placebo) arm, patients who progressed to permanent AF/AFL were also more likely to have structural heart disease or coronary heart disease—all known factors related to the progression of atrial cardiomyopathy and to more persistent forms of AF.4,29 Although these differences were small, this post hoc analysis provides the incentive to further investigate whether treatment with the multi-channel blocker dronedarone can protect against atrial disease progression and promote AF regression in patients at different stages/durations of AF.
Limitations
This was a post hoc analysis, and as such, the results of this analysis should be considered hypothesis-generating. It was not known whether patients had paroxysmal or persistent forms of AF/AFL upon randomization or follow-up in the ATHENA trial. While the Rosendaal method has been previously used to estimate the percentage of time a continuous outcome remains within a therapeutic range, in this post hoc analysis, this method was adapted to estimate the percentage of time a patient remains in AF/AFL, a categorical outcome. ECG evaluations in this study were infrequent, as they were assessed at specific time points as per study protocol, limiting the accuracy of assessment of estimated AF/AFL burden, and AF/AFL progression and regression.
Conclusions
In this post hoc analysis of the ATHENA trial, the observation of a lower estimated AF/AFL burden, lower AF progression, and more AF regression to SR over time in patients receiving dronedarone compared with those receiving placebo suggests that dronedarone may potentially reverse cardiac remodeling and thereby be a useful early treatment for patients with AF.
Acknowledgements
The authors thank all the patients, their caregivers, and the investigators who took part in the ATHENA study. Sanofi was the study sponsor and was responsible for generating the data for this analysis. All authors contributed to the concept and design of the analysis, interpretation of the data, drafting/reviewing this paper, and confirming accuracy, and approved the final manuscript for submission. Under the direction of the authors and in accordance with GPP3 guidelines, medical writing and editorial support was provided by Barrie Anthony, PhD, CMPP, Evidence Medical Affairs (Philadelphia, PA, USA) and was funded by Sanofi US Inc. We thank Charlotte Singh, MD, CMPP (Sanofi) for coordinating the development, facilitating author discussions, and critical review of this manuscript.
Contributor Information
Carina Blomström-Lundqvist, Department of Medical Science, Uppsala University, Uppsala, 751 85, Sweden; Department of Cardiology, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, SE-701 82, Sweden.
Gerald V Naccarelli, Penn State University College of Medicine, Penn State Hershey Heart and Vascular Institute, 500 University Drive, Hershey, PA 17033, USA.
David S McKindley, Sanofi, Bridgewater, 55 Corporate Drive, NJ 08807, USA.
Gregory Bigot, IVIDATA Life Sciences, 79 Rue Baudin, Paris, 92300 Levallois-Perret, France.
Mattias Wieloch, Sanofi, Rue la Boetie 54-56, Paris 75008, France; Department of Coagulation Disorders, Lund University, Jan Waldenströms gata 14, Lund 20502, Sweden.
Stefan H Hohnloser, Division of Clinical Electrophysiology, Department of Cardiology, J.W. Goethe University, Theodor-Stern-Kai 7, Frankfurt D 60590, Germany.
Funding
This work was supported by Sanofi.
Data availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
References
- 1. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LHet al. ‘Real-world’ management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme-Atrial Fibrillation (EORP-AF) general pilot registry. Europace 2016;18:648–57. [DOI] [PubMed] [Google Scholar]
- 2. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann Met al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192–201. [DOI] [PubMed] [Google Scholar]
- 3. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MSet al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022;145:e153–639. [DOI] [PubMed] [Google Scholar]
- 4. De With RR, Marcos EG, Dudink E, Spronk HM, Crijns H, Rienstra Met al. Atrial fibrillation progression risk factors and associated cardiovascular outcome in well-phenotyped patients: data from the AF-RISK study. Europace 2020;22:352–60. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Proietti R, Hadjis A, AlTurki A, Thanassoulis G, Roux JF, Verma Aet al. A systematic review on the progression of paroxysmal to persistent atrial fibrillation: shedding new light on the effects of catheter ablation. JACC Clin Electrophysiol 2015;1:105–15. [DOI] [PubMed] [Google Scholar]
- 7. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJSet al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 8. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders Pet al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 9. Tieleman RG, Crijns HJ. The ‘second factor’ of tachycardia-induced atrial remodeling. Cardiovasc Res 2000;46:364–6. [DOI] [PubMed] [Google Scholar]
- 10. Wijffels MC, Kirchhof CJHJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 11. Andrade JG, Wazni OM, Kuniss M, Hawkins NM, Deyell MW, Chierchia GBet al. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:914–30. [DOI] [PubMed] [Google Scholar]
- 12. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani Set al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 13. Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JYet al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation: RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). J Am Coll Cardiol 2011;58:493–501. [DOI] [PubMed] [Google Scholar]
- 14. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HTet al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hohnloser SH, Crijns HJGM, van Eickels M, Gaudin C, Page RL, Torp-Pedersen Cet al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. [DOI] [PubMed] [Google Scholar]
- 16. Blomström-Lundqvist C, Marrouche N, Connolly S, Corp dit Genti V, Wieloch M, Koren Aet al. Efficacy and safety of dronedarone by atrial fibrillation history duration: insights from the ATHENA study. Clin Cardiol 2020;43:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hohnloser SH, Connolly SJ, Crijns HJGM, Page RL, Seiz W, Torp-Petersen C. Rationale and design of ATHENA: a placebo-controlled, double-blind, parallel arm trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. J Cardiovasc Electrophysiol 2008;19:69–73. [DOI] [PubMed] [Google Scholar]
- 18. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236–9. [PubMed] [Google Scholar]
- 19. Quintana-Villamandos B, Delgado-Martos MJ, Delgado-Baeza E. Impact of a multichannel blocker in attenuating intramyocardial artery remodeling in hypertensive rats through increased nitric oxide bioavailability. Biomed Res Int 2019;2019:6374582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chilukoti RK, Lendeckel J, Darm K, Bukowska A, Goette A, Suhling Met al. Integration of “omics” techniques: dronedarone affects cardiac remodeling in the infarction border zone. Exp Biol Med (Maywood) 2018;243:895–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quintana-Villamandos B, Gomez de Diego JJ, Delgado-Martos MJ, Muñoz-Valverde D, Soto-Montenegro ML, Desco Met al. Dronedarone produces early regression of myocardial remodelling in structural heart disease. PLoS One 2017;12:e0188442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quintana-Villamandos B, Pazó-Sayós L, Arribas SM, Rodríguez-Rodríguez P, Böger RH, González Net al. Dronedarone induces regression of coronary artery remodeling related to better global antioxidant status. Hypertens Res 2019;42:1485–94. [DOI] [PubMed] [Google Scholar]
- 23. Ezekowitz MD, Ellenbogen KA, DiMarco JP, Kaszala K, Boddy A, Geba GPet al. A placebo-controlled, double-blind, randomized, multicenter study to assess the effects of dronedarone 400 mg twice daily for 12 weeks on atrial fibrillation burden in subjects with permanent pacemakers. J Interv Card Electrophysiol 2015;42:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khachatryan A, Merino JL, de Abajo FJ, Botto GL, Kirchhof P, Breithardt Get al. International cohort study on the effectiveness of dronedarone and other antiarrhythmic drugs for atrial fibrillation in real-world practice (EFFECT-AF). Europace 2022;24:899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curtis AB, Zeitler EP, Malik A, Bogard A, Bhattacharyya N, Stewart Jet al. Efficacy and safety of dronedarone across age and sex subgroups: a post hoc analysis of the ATHENA study among patients with non-permanent atrial fibrillation/flutter. Europace 2022;24:1754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 27. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt Get al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021;144:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns Het al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian Pet al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm 2017;14:801–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and data set specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.