Abstract
Treatment of atrial fibrillation (AF) remains challenging despite significant progress in understanding its underlying mechanisms. The first detailed, quantitative theory of functional re-entry, the ‘leading circle’ model, was developed more than 40 years ago. Subsequently, in decades of study, an alternative paradigm based on spiral waves has long been postulated to drive AF. The rotor as a ‘spiral wave generator’ is a curved ‘vortex’ formed by spin motion in the two-dimensional plane, identified using advanced mapping methods in experimental and clinical AF. However, it is challenging to achieve complementary results between experimental results and clinical studies due to the limitation in research methods and the complexity of the rotor mechanism. Here, we review knowledge garnered over decades on generation, electrophysiological properties, and three-dimensional (3D) structure diversity of the rotor mechanism and make a comparison among recent clinical approaches to identify rotors. Although initial studies of rotor ablation at many independent centres have achieved promising results, some inconclusive outcomes exist in others. We propose that the clinical rotor identification might be substantially influenced by (i) non-identical surface activation patterns, which resulted from a diverse 3D form of scroll wave, and (ii) inadequate resolution of mapping techniques. With rapidly advancing theoretical and technological developments, future work is required to resolve clinically relevant limitations in current basic and clinical research methodology, translate from one to the other, and resolve available mapping techniques.
Keywords: Rotor, Atrial fibrillation, Spiral wave, Mapping, Ablation
Introduction
Re-entry is central to the maintenance of atrial fibrillation (AF). Advanced mapping methods allow identifying a unique spiral-wave generator re-entry mechanism known as the rotor, which is a critical driver of AF in both experimental and clinical models. However, the properties of rotors and the effect of rotor ablation need to be defined further. We review the history and current status of basic and clinical science approaches to rotor identification and ablation.
Evolution of the atrial fibrillation theory and a proposal of the rotor concept
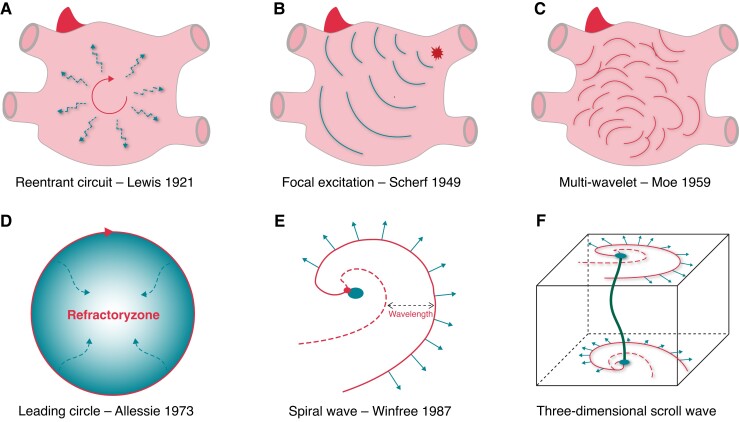
In 1913, Mines et al. first proposed a re-entrant excitation mechanism based on anatomical disorders.1 Then, Lewis et al. integrated such concepts into the hypothesis of ‘cyclic excitation caused by re-entry’2 (Figure 1A). The hypothesis states that the activity of the re-entrant circuit around the anatomical barrier may produce atrial flutter or cause fibrillation-like excitation depending on the different sizes of the re-entrant circuit and tissue refractory periods. In 1949, Scherf et al. found that a small dose of aconitine administered to the epicardium of the right atrial appendage in dogs induced atrial tachycardia or AF, with focal excitation as its underlying mechanism3 (Figure 1B). In 1959, Moe et al. proposed the multi-wavelet theory and published the classical computer-model study4,5 (Figure 1C), in which an estimated 15–30 electrical wavelets were required for AF maintenance. This theory was validated in animal models of AF more than 20 years later, thanks to the advent of high-density mapping. Unlike Moe’s results, Allessie et al. reported that in an in vivo canine model, AF was maintained by only 4–6 wavelets,6 and Wang et al. showed that class IC anti-arrhythmic drugs suppressed AF by reducing the number of wavelets.7 After that, the multi-wavelet mechanism was widely accepted.8–10 However, with only 4–6 wavelets in an AF model, both atria are likely to recover from the previous excitation, resulting in wavelet fusion and AF termination. Therefore, what are the structural and electrophysiological bases for maintaining these wavelets? Is it possible that a high-frequency ‘mother rotor’ forms multiple wavelets after collision and fragmentation?
Figure 1.
Types of maintenance mechanisms that drive AF. (A) Re-entry around a fixed anatomical obstacle leads to fibrillation-like excitation; (B) AF is driven by ectopic foci derived from PV; (C) AF maintenance at posterior left atrium that has been hypothesized to be driven by multiple wavelets; (D) Leading circle re-entry, in which activation propagates around a functionally refractory core; (E) The spiral wavefront revolves around an excitable, but nonexcited, core. The curvature of the active wavefront is greatest adjacent to the core, which is a phase singularity at the point in which the wavefront and the wavetail meet, thus a rotor has a highly variable wavelength and an often-undetectable excitable gap; and (F) A 3-dimensional scroll wave emanating from a filament, the identical or not identical activation patterns present at endo- and epicardial atrial surfaces.
In 1973, Allessie et al. (1973) documented functional re-entry independent of anatomical barriers in rabbit atria, which have the smallest-sized circuit, minimum re-excitation gap, and refractory centre.11 They developed the ‘leading circle hypothesis’ (Figure 1D) and posited that the shorter the wavelength and the larger the atria, the more simultaneous re-entry circuits can be accommodated and that their stable existence would underlie AF maintenance. However, AF suppression was independent of wavelength reduction in experimental models and patients with AF.12 Subsequently, studies on functional re-entrant rhythm led to the emergence of the concept of spiral waves (Figure 1E). The spiral wave was first discovered in 1990 in ex vivo sheep hearts, and spiral waves that moved rapidly in the ventricle were then shown to cause ventricular fibrillation.13,14 However, stable rotation-like excitation was not detected using isochronous excitation mapping in the ex vivo sheep AF model. The introduction of more sophisticated signal analysis methods, including optical phase mapping, allowed to define and description spiral waves in greater detail, and in 1998, using optical phase mapping, Gray et al. recorded the rotor-like activity of spiral waves, which were triggered by heterogeneity in tissue conduction and refractory periods.15
Electrophysiological mechanisms of rotors
Morphological characteristics of the rotor
The rotor, as a ‘spiral wave generator,’ is a curved ‘vortex’ formed by spin motion in the two-dimensional (2D) plane.16 Morphologically, the spiral wave is connected by a curved wavefront (solid red line in Figure 1E) and wavetail (red dashed line in Figure 1E). The wavefront represents the depolarized region, which continuously conducts excitations outward; the wavetail represents the cardiomyocytes that have completed depolarization and are recovering to a resting state, and the area between the wavefront and the tail represents cardiomyocytes in the absolute refractory period. The connection point between the wavefront and the tail is the tip of the spiral wave (red point in Figure 1E), at which all excitation states converge, shifting the cardiomyocytes to a non-excitable state; therefore, this connection point is also called a phase singularity (PS). During the spin motion of the spiral wave around the PS, the PS can meander to form the corresponding trajectory, and the region enclosed by this trajectory is the core of the spiral wave (blue circle in Figure 1E).17,18 At the PS, the shorter action potential duration and slower conduction velocity enable re-entry near the core, where the wavefront/tail meets and the excitable gap diminishes.12
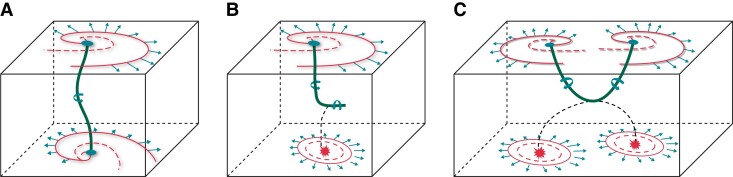
With the development of optical mapping technology, some studies hypothesized that the atrial re-entries recorded optically from the surfaces corresponded to three-dimensional (3D) scroll waves spanning the thickness of the wall (Figure 1F).19,20 Such a scroll wave’s behaviour is embodied in its organizing axis or filament (green line in Figure 1F), a largely quiescent tube about which the scroll rotates. The filament is not always ‘I-shaped’ and extends between the two surfaces; its tension and stability are determined by heterogeneous myocardial thickness, stretch, and remodelling (i.e. ionic and anatomic remodelling). The mathematical approach is to calculate the filament shape as a ‘minimal path,’ i.e. the scroll wave follows the ‘least resistance’ rule that yields the prediction for stationary filaments on a purely geometrical basis (Figure 2A).20In vitro AF model, scroll waves with a bent L-shaped or U-shaped filament were observed by combining endo-epicardial optical mapping (Figure 2B and C).19
Figure 2.
Three-dimensional structure of the scroll wave (rotor). (A) A steady-state scroll and filament under uniform left-handed twisted anisotropy in the mathematical model; (B) and (C) Examples of endo-epicardial re-entrant-breakthrough-dissociated activation patterns suggesting either an L-shaped filament (upper panel) or a U-shaped filament (lower panel) of scroll waves by a combination of endo-epicardial optical mapping.
Recently, Allessie and colleagues first proposed the presence of endocardial–epicardial dissociation (EED).21 After that, direct evidence of EED by simultaneous endo-epicardial mapping showed that EED was characterized by significant temporal heterogeneity,22 transitioning of preferential activation between the myocardial layers,22 and transmural conduction.23 With non-identical surface activation patterns on the endocardial and epicardial surfaces, the activity of scroll waves in the 3D myocardial wall thickness was hypothesized to conform to either ectopic discharge or scroll waves. Also, dyssynchronous activations on the endo- and epicardial surfaces further increased AF complexity, which may make endocardial mapping and ablation insufficient to address the AF mechanism.
Electrophysiological characteristics of the rotor
In high-density optical mapping and computer mathematical models,24,25 the activity of rotors is characterized by wavebreak, meandering, and variable wavelength. (i) Wavebreak develops when the rotor encounters an anatomical or functional barrier and splits into two or more daughter rotors, which rotate in opposing spirals around two new PSs.26 Most of these daughter rotors are unstable and will dissipate by colliding with each other or refractory tissue; in contrast, relatively stable daughter rotors can continue to generate new daughter rotors. Atrial fibrillation is maintained when the rate of wavebreak is greater than or equal to the rate of rotor extinction.27 This phenomenon also partly explains the difficulty in the self-termination of persistent AF. (ii) Meandering is when the PS of a rotor is mobile with a usually uncertain movement range and direction. Heterogeneity of cardiac ion channels influences the meandering trajectory. Calvo et al. found that the rotor meanders towards areas with lower rectifying potassium current (IK1) distribution, lower regional myocardial excitability, and more extended refractory periods.28 In some optical mapping experiments, the rotor was usually anchored in a region with significant heterogeneity in myocardial thickness and myocardial fibre alignment.19,29 Thus, rotor meandering is closely related to the electrical and anatomical heterogeneity of the myocardium (3). Rotor wavelength is variable, defined as the distance from the wavefront to the tail. Under different ion channel conditions, the rotor’s wavelength, core area, and excitable gap will change according to ion channel conditions. Nattel et al.27 summarized the effects of ion channels on the electrophysiological characteristics of the rotor: a reduction in sodium current leads to a reduction in rotor propagation and rotation speed, a decrease in wavefront curvature (WC), an increase in the core area, and an expansion of the meandering range. An increase in IK1 leads to a reduction in rotor wavelength, core area, and meandering range, while a decrease in outward IK1 leads to an increase in rotor wavelength, core area, and meandering range. Therefore, different ion channel states have different effects on the rotor wavelength and electrophysiological characteristics, which underlie the considerable complexity and uncertainty of rotor activity, rendering clinical mapping and identification of rotors more challenging.
Generation mechanism of the rotor
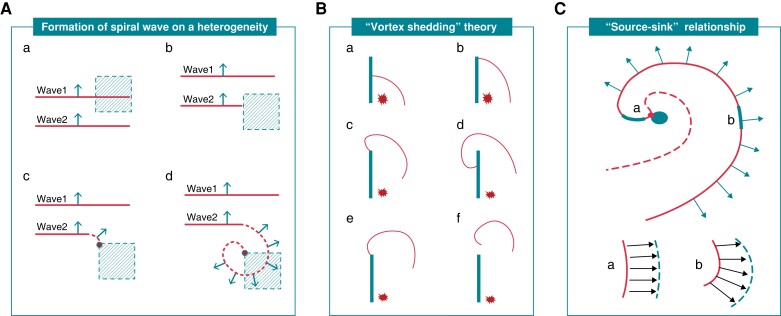
In 1978, Krinsky30 first hypothesized the process of spiral wave formation in a heterogeneous moiety as follows (Figure 3A): in the myocardium with a highly heterogeneous refractory period, a pair of activation waves randomly propagates into the 2D myocardial tissue, with the coupling interval of these two waves being infinitely close to the refractory period of the region with higher refractoriness. Under these conditions, refractoriness heterogeneities result in wavebreaks, and the edges of wavebreaks evolve into a spiral wave. However, this mathematical model has specific requirements for the heterogeneity of both tissue refractory period and coupling interval of the two pulses, limiting its reproduction in animal models of AF. In 1996, Cabo et al.31 proposed the ‘vortex shedding’ theory of spiral wave initiation (Figure 3B): in cardiac tissue, partial blockade of the membrane sodium channels or high-frequency (HF) excitation may result in an unexcitable obstacle with sharp edges, which may destabilize the propagation of electrical excitation waves, causing the formation of self-sustained vortices and turbulent cardiac electrical activity. The formation of such vortices, which visually resembles vortex shedding in hydrodynamic turbulent flows, is a potential mechanism leading to the spontaneous initiation of uncontrolled high-frequency excitation of the heart. This theory provides the experimental and theoretical bases for forming spiral waves in pathological conditions (e.g. post-myocardial infarction ventricular myocardium and atrial myocardium with persistent AF), which combine severe fibrosis and reduced Na+ current density and/or excitability.
Figure 3.
Mechanisms of rotor generation. (A) Formation of spiral wave is based on heterogeneity. (a) In the myocardium with high heterogeneity of refractory periods, a pair of wavefronts tandemly propagates into the myocardium, and the coupling interval of these two waves is infinitely close to the refractory period of the region with higher refractoriness. (b) Because this region has not recovered excitability, Wave 2 can only propagate forward along the edge of this region, but not invade it. (c) When forward propagation of Wave 2 is separated from the region with higher refractoriness, the wavefront near its edge becomes curved and subsequently ‘collides,’ and then forms PS. (d) As excitability recovers in the region with higher refractoriness, Wave 2 can invade this region and rotate completely around the nascent PS; (B) The ‘vortex shedding’ theory. (a–b) On a slice of sheep ventricular epicardium (∼ 0.5 mm thick), Cabo et al artificially etched a linear obstacle. A point stimulus was set on the right side of the obstacle, and the wavefront was unable to cross the obstacle and only spread outward along the right side until reaching the obstacle edge. (c–d) When myocardium excitability was normal, the wavefront curvature (WC) that reached the obstacle edge would significantly increase, further prompting the wavefront to detour over the obstacle without separating from it, and eventually extinguishing after activating the entire myocardium; (e–f) Use of the sodium channel blocker tetrodotoxin to reduce excitability was not enough to detour over the obstacle and separate from the obstacle although the WC increased. If the separated wavefront were further curled, a broken tip would form, and then the wavefront would go on spin motion around this broken tip, thus generating a ‘vortex'. (C) The ‘source-sink relationship' theory. The morphology of spiral waves suggests that the ‘source' and ‘sink' away from the core have a higher matching level and a small WC (a); in contrast, the wavefront closer to the core has a lower ‘source-sink' matching relationship and a larger WC (b), i.e. ‘source-sink’ mismatch.
Notably, in studies by Krinsky 30 and Cabo et al.,31 the WC would increase when the wavefront reached the edge of the refractory period region/obstacle. The latter phenomenon has been explained using the ‘source-sink relationship’ theory (Figure 3C),12 in which, during excitation conduction, the wavefront represents the depolarized region that is defined as the ‘source.’ In contrast, the cardiomyocytes in the resting state in front of the wavefront are defined as the ‘sink’. When the excitation conducts in a narrow region, the ‘source’ and the ‘sink’ activated in front of the wavefront are in a high matching state, and its WC is relatively small. When the wavefront propagates to the adjacent larger region, the ‘sink’ in front of the wavefront suddenly increases. At the same time, the ‘source’ does not change significantly. At this time, the matching level of the ‘source’ and ‘sink’ decreases, and the WC increases in order to facilitate excitation forward propagation. Administration of Na + channel blockers may decrease myocardial excitability and reduce the ‘source,’ which increases the source-sink-mismatch, further facilitating the formation of ‘vortex shedding.’ In addition, the morphology of spiral waves suggests that the ‘source-sink’ away from the core has a higher matching level and a small WC (wavefront at ‘a’ in Figure 3C); in contrast, the wavefront closer to the core has a lower ‘source-sink’ matching relationship and a larger WC (wavefront at ‘b’ in Figure 3C), i.e. a ‘source-sink’ mismatch.32 When this ‘source-sink’ mismatch in the wavefront reaches an extreme, the ‘source’ is insufficient to activate the ‘sink’ in front of it, eventually forming the PS. Therefore, a ‘source-sink’ mismatch has also been considered a critical mechanism for spiral wave formation.12
Optical mapping technology for the rotor
Principle of optical mapping technology
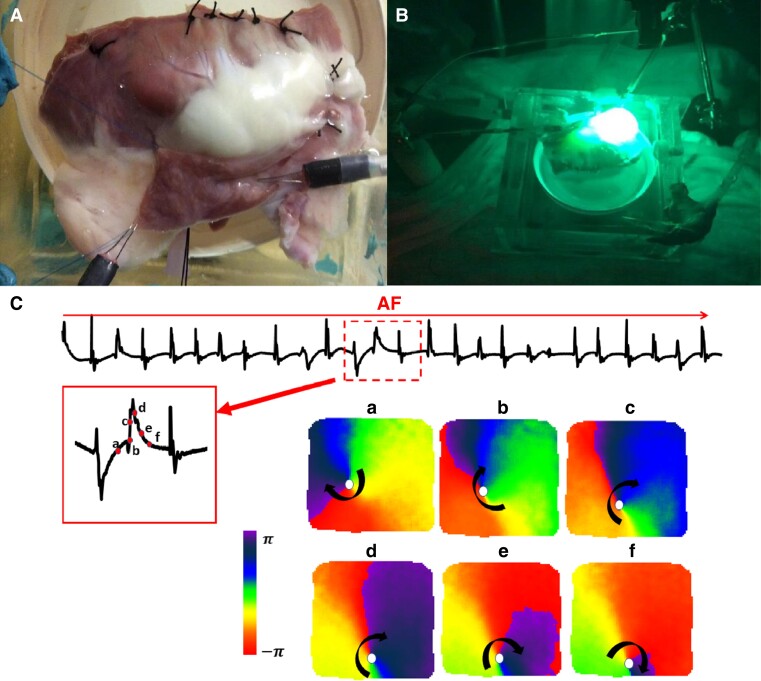
The optical mapping technology was first used to record action potentials in nerve cells in 1973.33 In 1976, Salama et al. first recorded the electrical activity of cardiomyocytes using optical mapping technology.34 Later in 1990, Davidenko et al. detected the spiral wave and demonstrated that it could induce ventricular tachycardia using optical mapping in animal hearts.35 The principle of optical mapping technology is based on wavelength-dependent light–tissue interactions, including photon scattering, absorption, reflection, and fluorescence effects. Optical mapping technology mainly uses a voltage-sensitive fluorescent dye as a marker, which allows the transmembrane potential change of cells to be manifested as a change in fluorescent substance brightness that an optical detection device can record. Voltage-sensitive fluorescent dyes are usually calcium chelators. After cardiomyocytes are marked with fluorescent dyes, intra-cellular calcium ions are combined with fluorescent dyes to allow immediate detection of intracellular calcium ion concentration changes by optical detection equipment, thus reflecting the process of cardiomyocyte depolarization and repolarization. Computer processing will enable us to understand the depolarization and repolarization of different regions of myocardial tissue and more intuitively perceive the electrical activity process of myocardial tissue over time, thereby rendering optical mapping techniques suitable for dynamic observation of the rotor. An example of optical mapping in a canine AF model from our previous research is presented in Figure 4.
Figure 4.
Example of optical mapping in a canine AF model (A) a canine right atrium was dissected and fluorescent dye was introduced via Langendorff perfusion of the coronary arteries. A pair of bipolar electrodes were positioned on the superior RA appendage. (B) The right atrium was excited with a laser. (C) AF was induced by pacing at 160ms cycle length. The subsequent panels show 6 snapshots of phase maps corresponding to the time points indicated in red points of the amplified ECG episode. Clockwise rotor activity was observed with phase singularities at the core tip of the rotor.
Application of optical mapping in atrial fibrillation models
In 1995, Gray et al. first recorded the activity of rotors on a ventricular fibrillation model using optical mapping.14 After that, this research group found rotors in an isolated sheep heart model for AF and indicated that rotors were formed based on the heterogeneity and refractory periods of tissue conduction, with temporal and spatial periodicity.24 Several years later, other researchers using a combination of endo-epicardial optical mapping reported that the patterns of rotor activation on the endocardial and epicardial myocardium surface could be identical or otherwise in the normal sheep heart.19,20 Various non-identical endo-epicardial activation patterns, such as multiple centrifugal breakthrough activations, wavebreaks, and short-lived re-entries, suggest the existence of transmural propagation of rotational activity throughout the 3D thickness myocardium in association with increased stability of AF. In 2015, Hansen and colleagues29 used optical mapping on both the endocardium and the epicardium of human hearts and demonstrated a delay in action potential during transmural conduction, and that the rotors mapped in the endo- or epicardium with multiple expression forms, such as a spiral-wave like a re-entry, stable foci or breakthrough, or spatially unstable breakthrough. The appearance of these activation forms depends on a variety of factors: (i) the spiral shape of the 3D scroll wave: I-shaped, L-shaped, or U-shaped, and the complexity of its shape largely depends on the thickness of the local myocardium; (ii) the conduction characteristics of the local myocardium, which determines the number of breakthroughs on the surface of the myocardium. Because the rotor has a complex spatiotemporal structure, attention is called to the different manifestations of rotors when mapping the endocardium.
Clinical mapping methods for the rotor: pros and cons
Panoramic mapping
Intracardiac panoramic mapping
The focal impulse and rotor modulation (FIRM) methodology was first used clinically in the CONFIRM study, published in 2012 by Narayan et al.36 The basket-like 64 electrodes would simultaneously contact the endocardium of the atria and record potentials, and the proprietary computer software was used to analyse the electrical signals collected by the basket electrodes to guide the ablation. In the study, rotors were mapped in 97% of the study population, with an average of 2.1 ± 1.0 rotors per patient, and rotor ablation terminated AF or prolonged AF cycle length in 86% of the patients, and 82.4% of the patients were in sinus rhythm during a mean follow-up of 273 days. These favourable outcomes, however, were not reproduced at many other centres37–39. In a meta-analysis, circumferential pulmonary vein isolation, combined with a rotor ablation strategy guided by a basket catheter, was not found superior to pulmonary vein isolation alone.40 The flaws seen with inter-spline bunching resulted in a loss of coverage and contact,41 and only 63.1% of the inter-electrode distances were less than the most stringent spatial resolution required for the identification of rotors in human AF. Moreover, a computer simulation study42 found that several high-density mapping catheters (AFocusII and PentaRay) had inter-electrode spacings below minimum resolution (11.9 mm), suggesting that these catheters have a higher resolution to locate PSs if placed over the rotor core accurately. Although these catheters have satisfactory mapping resolution, they are not panoramic mapping devices that could show overall atrial electrical activity. Therefore, both panoramic mapping catheters and local high-density mapping catheters have advantages and disadvantages, and there are no comparative clinical studies to determine which would provide superior performance.
Recently, electrographic flow (EGF) mapping (Ablamap® software; Ablacon, Wheat Ridge, CO) emerged as a novel method to identify AF driver by estimating atrial cardiac action potential flow. An algorithm that combined Green’s minimal bending energy algorithm and Horn–Schunck Flow algorithm was used to analysed the time-domain information from the unipolar electrograms collected by a basket-like catheter and converted it into the space domain of flow vectors for the identification of singularities where the flow vector angles around a point covered 360°.43 One strength of this method is the potential to distinguish active and passive rotors. Theoretically, it can make up for insufficient local resolution of the basket-like catheter. The ongoing FLOW-AF study (NCT 04473963) will verify the efficacy of driver ablation–guided EGF mapping.
In 2020, the real-time electrogram analysis for the driver (RADAR) system was introduced to guide driver ablation in a prospective, multi-centre study.44 The RADAR system was used in conjunction with a standard mapping system. High-density contact mapping was performed using a coronary sinus catheter as a reference to record electrograms in various anatomic locations. These electrograms were then sorted and stitched together to create a panoramic 3D conduction vector map for each coronary sinus phase. Sites of rotational activity and focal impulses were identified in the individual conduction vector maps. The initial outcome of using the RADAR system was promising, with 55% of patients experiencing AF termination after driver ablation and 82% remaining AF-free during follow-up.
Non-invasive electrocardiographic imaging
Recently, the non-invasive electrocardiographic imaging (ECGi) technique was used to map rotors 45–47. Using an external cardiac 3D mapping system (ECVUE system, Medtronic), patients with AF wore a vest with 252 electrodes and underwent CT scanning to obtain the relationship between the 3D geometry of both atria and the positions of 252 electrodes on the vest.45 Atrial activation during AF was obtained by phase mapping of the recorded ECG. Active driving and passive propagation zones were identified by analysing all accumulated images for each patient. A driver is defined as a focal breakthrough, when the image presents a centrifugal activation from a point or zone, or a spiral wave, when the wave is rotated around a centre on phase progression. In this study, the rate of AF termination during rotor ablation was 70%, and 85% of patients were free from AF recurrence at a 1-year follow-up. The latter AFACART study47 and the TARGET-AF148 trial also observed favourable outcomes of non-invasive ECGi-guided driver ablation for PerAF.
However, the system has some limitations. First, epicardial far-field unipolar signal mapping results in poor stability of atrial signal quality and makes it challenging to distinguish micro-re-entrant from focal breakthrough; second, the activation data are a composite of endocardial, epicardial, and intramural patterns and interactions, and therefore, cannot differentiate the signals from overlapping cardiac anatomy sites, such as coronary sinus and inter-atrial septum. Third, phase mapping tends to introduce false rotors during complex activation patterns.49,50 The conduction of two waves in opposite directions on both sides of a conduction block line may be misinterpreted as a rotor in phase analysis. Hence, although this system is a panoramic high-resolution mapping technology, it has a non-negligible false-positive rate in AF rotor mapping due to the inherent deficiency of external signals.
Non-contact charge density mapping
The charge density (CD) mapping is performed by a combined imaging and multi-electrode mapping system (AcQMap, Acutus Medical, Carlsbad, CA), which has 48 ultrasound transducers to reconstruct atrial chamber anatomy and 48 low-impedance, high-fidelity electrodes for recording biopotential signals. In brief, an inverse algorithm, based on the principles of electrostatic field theory, obtains the global distribution of CD sources across the endocardial surface generated by ultrasonic imaging and then creates global maps of cardiac activation displayed as a spatiotemporal window.51 The UNCOVER AF Trial reported a 72.5% success rate of single ablation for PerAF guided by the AcQMap system.52
The key advantages of CD mapping include (i) global and continuous mapping of AF with a reduction in far-field interference; (ii) enabling the identification of both re-entrant and focal activation; (iii) rapid (<5 min) and non-contact reconstruction of atrial anatomy leading to avoidance of some problems encountered by the impedance-based approach. The major limitations of this mapping method are the inability to study epicardial or transmural mechanisms and the inability to properly and successfully identify the so-called rotor activity.
Local high-density mapping
Dominant frequency mapping
In 2005, Haïssaguerre’s group performed dominant frequency (DF) mapping by using spectral analysis to construct hierarchical gradients of activation frequencies at different atrial regions.53 The DF is calculated from the cycle length of local electrograms, which correlate with the local AF frequency, i.e. a faster local AF waveform frequency is associated with a higher DF. In theory, localized sites with the highest frequency activity are markers of critical AF drivers or rotors. Ablation of these high DF sites achieved AF termination and prolongation of the AF cycle length. However, the latter radiofrequency ablation of drivers of atrial fibrillation study failed to demonstrate the superiority of localized high-frequency source ablation over PVI in either patient with paroxysmal AF or persistent AF.54 The technical limitations of DF mapping may account for these results. The local potentials become more complex as AF progresses; for example, with local fragmented potentials or double potentials, the value obtained from this DF calculation may be faster than that of real drivers or rotors, precluding accurate DF measurement. Basic research has also confirmed that the DF of fragmented potentials generated by the collision of wavefronts around rotors is consistent with that of the rotor core region.55 Therefore, these limitations of DF mapping cannot be ignored, and this method can be used only as a reference for rotor potential analysis.
In 2017, the CARTO-Finder, a novel technique module of the CARTO mapping system (Biosense Webster), enabled a dynamic evaluation of rotational and focal activation during AF and was used to identify areas of rotor domains.56,57 Ablation of rotor domains effectively eliminated frequency gradients, and 70% of patients with long-standing persistent AF were free of AF recurrence at 12 months.56 However, only 13 patients were included in this study, and the efficacy of using CARTO-Finder to target AF drivers will be further confirmed by the ongoing larger multi-centre study (ClinicalTrials.gov Identifier: NCT03064451).
Phase similarity mapping
In 2013, Lin and colleagues demonstrated that regions of AF drivers were characterized by regular, organized, and rapid repetitive activities with a high degree of similarity in bipolar electrogram configuration.58 After that, in their single-centre, randomized study, a novel similarity index (SI) was calculated by using phase analysis of the bipolar signal acquired by the AFocus II catheter to identify potential AF drivers for ablation.59 The obtained electrogram signal underwent phase calculation simulation through a computer system to filter out interference signals and far-field contamination, theoretically improving the accuracy of rotor identification. An SI vector field was then constructed between a pair of the nearest electrodes to analyse the electrical wave propagation around AF drivers based on curvature and divergence forces, and therefore, can distinguish a rotor from a focal source or random fractionated. In this study, an average of 2.6 ± 0.89 high SI regions were mapped per patient, and the rate of AF termination was superior (68% vs. 27%) to that of the control group with a fractionated potential ablation strategy, with a high success rate of 83% in long-term follow-up.
Dispersion mapping
In 2017, Seitz et al. used the PentaRay catheter to identify the region with rotor activity in AF.60 This mapping is based on the theoretical premise that when a PentaRay catheter is placed at the centre of a rotor, its electrode branches will record the sequential excitation phases of rotor wavefronts, i.e. the asynchrony of the bipolar potential or ‘dispersion electrograms.’ However, the study methodology did not exclude fragmented potentials in the dispersion analysis and the area with both fragmented and non-fragmented potentials as an ablation target. Despite the excellent termination rate of AF, extensive ablation is performed on a wide area of atria (15.8–29% of LA surface area), and the unintentional creation of an unnecessary pro-arrhythmic scar cannot be ignored. Recently, the results from our study61,62 showed that HF and dispersion electrogram-guided rotor ablation abruptly terminated AF, substantially improving long-term AF elimination. Notably, it showed a decreased ablation area (4.3–13.3% of LA surface area) and suggested that most fragmented potentials are the by-products of re-entrant rotors that break down at their boundaries. Therefore, local high-density mapping provides more detailed information to distinguish the rotor from complicated AF potentials despite not providing a panoramic imaging of the whole atrium.
Stochastic trajectory analysis of ranked signals mapping
Stochastic trajectory analysis of ranked signals (STAR) is a novel mapping method that compares activation times across electrode pairs to identify atrial regions with earlier activation than neighbouring areas.63 The author defined region as an AF driver if it leads for more than 75% of wavefronts during a recording period. In 2020, Honarbakhsh et al. confirmed the efficacy of STAR-guided ablation in a single-centre, prospective study, and the success rate was 81.5%.64 The STAR mapping method has some potential advantages: it allows both global and sequential mapping and is compatible with different commercially available multi-polar catheters (CARTO, Rhythmia, and EnSite). However, this method cannot distinguish the driver mechanism between rotational and focal. Furthermore, the ablation of early activation areas depended on the operator’s interpretation. The limited coverage and contact of basket catheters and the position and orientation of the catheter may also influence the mapping accuracy.
Focal source and trigger computational algorithm
In 2020, Chauhan et al. reported their proprietary focal source and trigger (FaST) computational algorithm, which allowed automatically identifying sustained periodic bipolar and unipolar QS electrogram morphology during AF.65 Both patients with paroxysmal and persistent AF were enrolled in this validation study. PVI plus FaST site ablation decreased AF recurrence compared with PVI alone, but the result did not reach statistical significance (P = 0.064). One advantage of the FaST mapping is time efficiency: the mean ablation time of FaST sites outside the PV was only 8.5 ± 5.1 min. Notwithstanding, the FaST mapping was not performed comprehensively in this study and may miss some potential focal sources in the right atrium. Furthermore, FaST signals may arise from mechanisms other than focal sources, such as endo-epicardial breakthroughs.
Repetitive-regular activities mapping
Recently, Pappone and colleagues introduced a novel real-time integrated mapping technique to identify potential driver regions characterized by repetitive-regular activities (RRas).66 This mapping method consists of three components: a regular cycle length map, a fragmentation map, and a peak-to-peak voltage measurement. The software integrated the three mapping results and allowed an automated evaluation of multiple mechanisms during AF by identifying regions exhibiting RRas, fragmentation, the conduction velocity of consistent wavefronts, and electrically silent areas. Therefore, it provided a patient-tailored strategy. In this study, a modified PVI, plus RRa site ablation, achieved a higher rate of AF termination (61% vs. 30%) and less AF recurrence (73% vs. 50%) compared with a modified PVI alone.66 One drawback of this approach is that it cannot distinguish the specific electrophysiological characteristic of the repetitive-regular electrograms, such as rotors, focal sources, or micro re-entries.
Driver ablation vs. conventional approach: pooled analysis from clinical trials
At present, the overall AF termination rate of rotor ablation is 44.5 ± 15.5% (Figure 5B), the rate of freedom from AF/AT is 57.7 ± 7.7%, and the rate of freedom from AF is 69.5 ± 4.6% (Figure 5A). Supplementary material online, Table S1 presents clinical studies of different currently available methods for rotor mapping. Although the termination and success rates were different among various mapping methods, the overall mean values were similar. Considerable discrepancy in the rate of successful ablation and AF termination was observed among studies of FIRM-guided approach, which is also the method with the largest number of published studies. A study reported that the AF termination rate and long-term success rate were only 5% and 21% by FIRM-guided ablation.67 The reasons for the difference in results are the defects of the mapping technology itself and the limitations of the study, such as the type of AF included, the follow-up time, and the sample size. Thus, outcomes of single-arm studies were significantly limited by high heterogeneity. However, the number of case-control clinical studies of traditional ablation strategies is relatively small, and we conducted a meta-analysis of 10 clinical trials with published case-control studies (Figure 5C). The significant pooled OR for freedom from AF/AT in these 10 studies using the fixed-effects model was 0.53 [CI, 0.40–0.69 (P = 0.037); I2 = 42% (P < 0.0001)]. Among the ten studies, only Tilz’s findings did not support driver ablation, and the study included paroxysmal AF and performed FIRM-guided ablation alone without PVI. Two other studies also compared AF driver-only ablation to PVI.54,60 Although Seitz’s conclusions favour driver ablation, the incidence of atrial tachycardia is as high as about 30%.56 If these three studies are excluded, freedom from AF/AT produced an OR of 0.45 (CI, 0.32–0.62; P < 0.0001), with minimal heterogeneity between studies (I2 = 0%). It supports the possible benefit of driver ablation as an additional strategy in improving freedom from all arrhythmias compared with conventional ablation alone.
Figure 5.
Comparison of outcomes of rotor ablation guided by currently available clinical mapping methods. (A) Long-term success rate of different mapping methods, (B) rate of AF termination of different mapping methods, and (C) a meta-analysis of 10 clinical trials with published case-control studies.
The controversary of driver studies
Although the overall results of the meta-analysis favoured driver ablation, we cannot ignore the inconsistency of clinical findings. Because there are several factors affecting the outcome of AF ablation, only in terms of mapping methods and ablation strategies, first of all, there are differences in the false-positive rate of different mapping devices, and the success rate is naturally different. Furthermore, the area of ablation lesions and different methods of ablation (linear or patch lesion) can also affect the incidence of iatrogenic arrhythmias. However, for the acute outcome of ablation, driver ablation achieved a higher AF termination rate than conventional ablation,36,59,60,66,68 suggesting an intervention in the maintenance mechanism of AF. Previous studies have also confirmed a favourable long-term outcome in patients with procedural AF termination.69,70
Secondly, the results of studies may also be impacted by sample size and different control groups. Currently, there is still a lack of large-sample randomized controlled studies to directly compared driver ablation and conventional ablation. Previous studies set different ablation strategies as control groups, including PVI,54,66 PVI + linear ablation,36 CAFE ablation,59 or ‘stepwise’ strategy.60,68 It was therefore difficult to compare studies with one another. Moreover, there is also a lack of evidence comparing driver ablation with new strategies that have demonstrated satisfactory clinical outcomes, such as posterior BOX isolation or vein of Marshall ethanol infusion. In addition, most clinical studies have been conducted in ablation-naive patients,47,54,64,66 and a part of these patients may be benefited by PVI alone. In the study of Seitz et al., PVI was not performed, and regions of AF termination were frequently located in the PV antrum.60 Therefore, enrichment of the study population for patients having extra-PV drivers would likely increase the success rate of adjuvant ablation approaches.
Future perspectives
Limitations of mapping technology
AF is neither a purely focal nor a stable re-entry in nature, and sequential local high-density mapping may be limited by global resolution. Although panoramic mapping enables a real-time evaluation of propagation of the entire chamber, it also has many limitations, such as low spatial resolution and suboptimal electrode–tissue contact.71 The current mapping tools are only based on the analysis of unipolar or bipolar electrograms. The unipolar electrograms are vulnerable to far-field potentials, and the morphology of bipolar electrograms is affected by inter-electrode distances and wavefront direction.71 When the electrode resolution is insufficient, the interference is significant, and when the potential complexity is high, it is difficult to identify the false positives and false negatives of the rotor. Before optical mapping can be safely applied to the human body, computer and artificial intelligence technology should be the best way to identify rotors accurately. For example, in the research of RADAR, high-density mapping and computer signal processing technology are integrated to avoid the loss of mapping information and comprehensively analyse the overall electrical activation characteristics of AF, and the signal processing is rapid.
Insufficient understanding of the rotor mechanism
Although the theoretical research on rotors has been far ahead of understanding clinical practice, the clinical AF phenomenon is much more complicated than animal and in vitro models. In addition to the rotor phenomenon, micro re-entry, focal activity, or double-layer activation also participate in the maintenance of AF. However, these theories are not mutually exclusive, and different mechanisms of AF might exist in the same patient.72 This still needs to be revealed by clinical research and basic research.
Conclusion
Studies on AF mechanisms have gradually revealed the complicated properties and diverse 3D forms of rotors that play an essential role in the occurrence and development of the AF substrate. However, limitations in current research methodology and the complexity of the rotor mechanism have rendered it difficult to achieve complementary results between basic science and clinical studies. Although initial studies of rotor ablation have yielded favourable outcomes, there is still a lack of large multi-centre studies to verify the efficacy of rotor ablation. Despite various rotor mapping methods, the current mapping approaches are limited by inadequate resolution. Further theoretical and technological developments are warranted to address the limitations discussed and allow the translation of basic and clinical science results to treat patients with AF.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (Grant No: 81770324 to M.Q. and 82270365 to T.L.) and the Nature Science Foundation of Hubei Province (2018CFB418 to T.L.). The funder has designed this study and decided to publish this manuscript.
Contributor Information
Chang-Hao Xu, Department of Cardiology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, 241 Huaihai West Road, Xuhui District, Shanghai 200030, China.
Feng Xiong, Montreal Heart Institute, Department of Medicine, University of Montreal, 5000, Bélanger street, Montréal, Québec H1T 1C8, Canada.
Wei-Feng Jiang, Department of Cardiology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, 241 Huaihai West Road, Xuhui District, Shanghai 200030, China.
Xu Liu, Department of Cardiology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, 241 Huaihai West Road, Xuhui District, Shanghai 200030, China.
Tao Liu, Montreal Heart Institute, Department of Medicine, University of Montreal, 5000, Bélanger street, Montréal, Québec H1T 1C8, Canada; Department of Cardiology, Renmin Hospital of Wuhan University, No. 238, Jiefang Road, Wuchang District, Wuhan 430061, China; Department of Cardiology, Cardiovascular Research Institute of Wuhan University, No. 238, Jiefang Road, Wuchang District, Wuhan, Hubei 430061, China.
Mu Qin, Department of Cardiology, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, 241 Huaihai West Road, Xuhui District, Shanghai 200030, China.
Supplementary material
Supplementary material is available at Europace online.
References
- 1. Mines GR. On dynamic equilibrium in the heart. J Physiol 1913;46:349–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis T. Oliver-Sharpey Lectures on the nature of flutter and fibrillation of the auricle. Br Med J 1921;1:590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scherf D, Terranova R. Mechanism of auricular flutter and fibrillation. Am J Physiol 1949;159:137–42. [DOI] [PubMed] [Google Scholar]
- 4. Moe GK. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59–70. [DOI] [PubMed] [Google Scholar]
- 5. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–20. [DOI] [PubMed] [Google Scholar]
- 6. Allessie MA. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation. New York: Grune and Stratton Press; 1985. p265. [Google Scholar]
- 7. Wang Z, Pagé P, Nattel S. Mechanism of flecainide's antiarrhythmic action in experimental atrial fibrillation. Circ Res 1992;71. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof CJHJ, Chorro F, Scheffer GJ, Brugada J, Konings K, Zetelaki Zet al. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation 1993;88:736–49. [DOI] [PubMed] [Google Scholar]
- 9. Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone Cet al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:406–26. [PubMed] [Google Scholar]
- 10. Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg 2000;12:2–14. [DOI] [PubMed] [Google Scholar]
- 11. Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of trachycardia. Circ Res 1973;33:54–62. [PubMed] [Google Scholar]
- 12. Comtois P, Kneller J, Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace 2005;7:10–20. [DOI] [PubMed] [Google Scholar]
- 13. Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 1992;355:349–51. [DOI] [PubMed] [Google Scholar]
- 14. Gray RA, Jalife J, Panfilov AV, Baxter WT, Cabo C, Davidenko JMet al. Mechanisms of cardiac fibrillation. Science 1995;270:1222–3; author reply 1224-1225. [PubMed] [Google Scholar]
- 15. Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature 1998;392:75–8. [DOI] [PubMed] [Google Scholar]
- 16. Wiener N, Rosenblueth A. The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex 1946;16:205–65. [PubMed] [Google Scholar]
- 17. Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res 1977;41:9–18. [DOI] [PubMed] [Google Scholar]
- 18. Jalife J. Inward rectifier potassium channels control rotor frequency in ventricular fibrillation. Heart Rhythm 2009;6:S44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamazaki M, Filgueiras-Rama D, Berenfeld O, Kalifa J. Ectopic and reentrant activation patterns in the posterior left atrium during stretch-related atrial fibrillation. Prog Biophys Mol Biol 2012;110:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wellner M, Berenfeld O, Jalife J, Pertsov AM. Minimal principle for rotor filaments. Proc Natl Acad Sci U S A 2002;99:8015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJet al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 2010;122:1674–82. [DOI] [PubMed] [Google Scholar]
- 22. Parameswaran R, Kalman JM, Royse A, Goldblatt J, Larobina M, Watts Tet al. Endocardial-epicardial phase mapping of prolonged persistent atrial fibrillation recordings: high prevalence of dissociated activation patterns. Circ Arrhythm Electrophysiol 2020;13:e008512. [DOI] [PubMed] [Google Scholar]
- 23. de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops Pet al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol 2016;9:e003648. [DOI] [PubMed] [Google Scholar]
- 24. Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation 1998;98:1236–48. [DOI] [PubMed] [Google Scholar]
- 25. Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res 2002;90:E73–87. [DOI] [PubMed] [Google Scholar]
- 26. Zou R, Kneller J, Leon LJ, Nattel S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium. Am J Physiol Heart Circ Physiol 2005;289:H1002–12. [DOI] [PubMed] [Google Scholar]
- 27. Nattel S, Xiong F, Aguilar M. Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms. Nat Rev Cardiol 2017;14:509–20. [DOI] [PubMed] [Google Scholar]
- 28. Calvo CJ, Deo M, Zlochiver S, Millet J, Berenfeld O. Attraction of rotors to the pulmonary veins in paroxysmal atrial fibrillation: a modeling study. Biophys J 2014;106:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LAet al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krinsky VI. Mathematical models of cardiac arrhythmias (spiral waves). Pharmacol Ther B 1978;3:539–55. [DOI] [PubMed] [Google Scholar]
- 31. Cabo C, Pertsov AM, Davidenko JM, Baxter WT, Gray RA, Jalife J. Vortex shedding as a precursor of turbulent electrical activity in cardiac muscle. Biophys J 1996;70:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jalife J. Déjà vu in the theories of atrial fibrillation dynamics. Cardiovasc Res 2011;89:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davila HV, Salzberg BM, Cohen LB, Waggoner AS. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol 1973;241:159–60. [DOI] [PubMed] [Google Scholar]
- 34. Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 1976;191:485–7. [DOI] [PubMed] [Google Scholar]
- 35. Davidenko JM, Kent PF, Chialvo DR, Michaels DC, Jalife J. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A 1990;87:8785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol 2012;60:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gokoglan Yet al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016;13:830–5. [DOI] [PubMed] [Google Scholar]
- 38. Berntsen RF, Haland TF, Skardal R, Holm T. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: a within-patient controlled study with implanted cardiac monitoring. Heart Rhythm 2016;13:1768–74. [DOI] [PubMed] [Google Scholar]
- 39. Tilz RR, Lenz C, Sommer P, Roza MS, Sarver AE, Williams CGet al. Focal impulse and rotor modulation ablation vs. pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: results from the FIRMAP AF study. Europace 2021;23:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohanty S, Mohanty P, Trivedi C, Gianni C, Della Rocca DG, Di Biase Let al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping: insights from a meta-analysis. Circ Arrhythm Electrophysiol 2018;11:e005789. [DOI] [PubMed] [Google Scholar]
- 41. Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar Ket al. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roney CH, Cantwell CD, Bayer JD, Qureshi NA, Lim PB, Tweedy JHet al. Spatial resolution requirements for accurate identification of drivers of atrial fibrillation. Circ Arrhythm Electrophysiol 2017;10:e004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haines DE, Kong MH, Ruppersberg P, Haeusser P, Avitall B, Torok TSet al. Electrographic flow mapping for atrial fibrillation: theoretical basis and preliminary observations. J Interv Card Electrophysiol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choudry S, Mansour M, Sundaram S, Nguyen DT, Dukkipati SR, Whang Wet al. RADAR: a multicenter food and drug administration investigational device exemption clinical trial of persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita Set al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–8. [DOI] [PubMed] [Google Scholar]
- 46. Lim HS, Hocini M, Dubois R, Denis A, Derval N, Zellerhoff Set al. Complexity and distribution of drivers in relation to duration of persistent atrial fibrillation. J Am Coll Cardiol 2017;69:1257–69. [DOI] [PubMed] [Google Scholar]
- 47. Knecht S, Sohal M, Deisenhofer I, Albenque JP, Arentz T, Neumann Tet al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace 2017;19:1302–9. [DOI] [PubMed] [Google Scholar]
- 48. Honarbakhsh S, Dhillon G, Abbass H, Waddingham PH, Dennis A, Ahluwalia Net al. Noninvasive electrocardiographic imaging-guided targeting of drivers of persistent atrial fibrillation: the TARGET-AF1 trial. Heart Rhythm 2022;19:875–84. [DOI] [PubMed] [Google Scholar]
- 49. Podziemski P, Zeemering S, Kuklik P, van Hunnik A, Maesen B, Maessen Jet al. Rotors detected by phase analysis of filtered, epicardial atrial fibrillation electrograms colocalize with regions of conduction block. Circ Arrhythm Electrophysiol 2018;11:e005858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology considerations in phase mapping of human cardiac arrhythmias. Circ Arrhythm Electrophysiol 2016;9:e004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grace A, Willems S, Meyer C, Verma A, Heck P, Zhu Met al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight 2019;4:e126422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Willems S, Verma A, Betts TR, Murray S, Neuzil P, Ince Het al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF Trial. Circ Arrhythm Electrophysiol 2019;12:e007233. [DOI] [PubMed] [Google Scholar]
- 53. Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LFet al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005;112:789–97. [DOI] [PubMed] [Google Scholar]
- 54. Atienza F, Almendral J, Ormaetxe JM, Moya A, Martinez-Alday JD, Hernandez-Madrid Aet al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol 2014;64:2455–67. [DOI] [PubMed] [Google Scholar]
- 55. Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach Det al. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation 2006;113:626–33. [DOI] [PubMed] [Google Scholar]
- 56. Calvo D, Rubin J, Perez D, Moris C. Ablation of rotor domains effectively modulates dynamics of human: long-standing persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2017;10:e005740. [DOI] [PubMed] [Google Scholar]
- 57. Honarbakhsh S, Schilling RJ, Providencia R, Keating E, Chow A, Sporton Set al. Characterization of drivers maintaining atrial fibrillation: correlation with markers of rapidity and organization on spectral analysis. Heart Rhythm 2018;15:1296–303. [DOI] [PubMed] [Google Scholar]
- 58. Lin YJ, Lo MT, Lin C, Chang SL, Lo LW, Hu YFet al. Nonlinear analysis of fibrillatory electrogram similarity to optimize the detection of complex fractionated electrograms during persistent atrial fibrillation. J Cardiovasc Electrophysiol 2013;24:280–9. [DOI] [PubMed] [Google Scholar]
- 59. Lin YJ, Lo MT, Chang SL, Lo LW, Hu YF, Chao TFet al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC Clin Electrophysiol 2016;2:667–78. [DOI] [PubMed] [Google Scholar]
- 60. Seitz J, Bars C, Theodore G, Beurtheret S, Lellouche N, Bremondy Met al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol 2017;69:303–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qin M, Lin RJ, Wu SH, Liu X. Extra pulmonary vein driver mapping and ablation in paroxysmal atrial fibrillation by electrogram dispersion analysis. J Cardiovasc Electrophysiol 2019;30:164–70. [DOI] [PubMed] [Google Scholar]
- 62. Qin M, Jiang WF, Wu SH, Xu K, Liu X. Electrogram dispersion-guided driver ablation adjunctive to high-quality pulmonary vein isolation in atrial fibrillation of varying durations. J Cardiovasc Electrophysiol 2020;31:48–60. [DOI] [PubMed] [Google Scholar]
- 63. Honarbakhsh S, Hunter RJ, Ullah W, Keating E, Finlay M, Schilling RJ. Ablation in persistent atrial fibrillation using stochastic trajectory analysis of ranked signals (STAR) mapping method. JACC Clin Electrophysiol 2019;5:817–29. [DOI] [PubMed] [Google Scholar]
- 64. Honarbakhsh S, Schilling RJ, Finlay M, Keating E, Hunter RJ. Prospective STAR-guided ablation in persistent atrial fibrillation using sequential mapping with multipolar catheters. Circ Arrhythm Electrophysiol 2020;13:e008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chauhan VS, Verma A, Nayyar S, Timmerman N, Tomlinson G, Porta-Sanchez Aet al. Focal source and trigger mapping in atrial fibrillation: randomized controlled trial evaluating a novel adjunctive ablation strategy. Heart Rhythm 2020;17:683–91. [DOI] [PubMed] [Google Scholar]
- 66. Pappone C, Ciconte G, Vicedomini G, Mangual JO, Li W, Conti Met al. Clinical outcome of electrophysiologically guided ablation for nonparoxysmal atrial fibrillation using a novel real-time 3-dimensional mapping technique: results from a prospective randomized trial. Circ Arrhythm Electrophysiol 2018;11:e005904. [DOI] [PubMed] [Google Scholar]
- 67. Steinberg JS, Shah Y, Bhatt A, Sichrovsky T, Arshad A, Hansinger Eet al. Focal impulse and rotor modulation: acute procedural observations and extended clinical follow-up. Heart Rhythm 2017;14:192–7. [DOI] [PubMed] [Google Scholar]
- 68. Lin R, Zeng C, Xu K, Wu S, Qin M, Liu X. Dispersion-guided ablation in conjunction with circumferential pulmonary vein isolation is superior to stepwise ablation approach for persistent atrial fibrillation. Int J Cardiol 2019;278:97–103. [DOI] [PubMed] [Google Scholar]
- 69. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SBet al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 70. O'Neill MD, Wright M, Knecht S, Jais P, Hocini M, Takahashi Yet al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J 2009;30:1105–12. [DOI] [PubMed] [Google Scholar]
- 71. de Groot NMS, Shah D, Boyle PM, Anter E, Clifford GD, Deisenhofer Iet al. Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on Cardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. Europace 2022;24:313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schotten U, Lee S, Zeemering S, Waldo AL. Paradigm shifts in electrophysiological mechanisms of atrial fibrillation. Europace 2021;23:ii9–ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.