Abstract
Aims
The aim of this study was to investigate the electrophysiological characteristics and long-term outcome of patients undergoing substrate-based ablation of left posterior fascicular ventricular tachycardia (LPF-VT) guided by targeting of fragmented antegrade Purkinje potentials (FAPs) during sinus rhythm.
Methods and results
This study retrospectively analysed 50 consecutive patients referred for ablation. Substrate mapping during sinus rhythm was performed to identify the FAP that was targeted by ablation. FAPs were recorded in 48 of 50 (96%) patients during sinus rhythm. The distribution of FAPs was located at the proximal segment of posterior septal left ventricle (LV) in two (4.2%) patients, middle segment in 33 (68.8%) patients, and distal segment in 13 (27.1%) patients. In 32 of 48 (66.7%) patients, the FAP displayed a continuous multicomponent fragmented electrogram, while a fragmented, split, and uncoupled electrogram was recorded in 16 (33.3%) patients. Entrainment attempts at FAP region were performed successfully in seven patients, demonstrating concealed fusion and the critical isthmus of LPF-VT. Catheter ablation targeting at the FAPs successfully terminated the LPF-VT in all 48 patients in whom they were seen. Left posterior fascicular (LPF) block occurred in four (8%) patients after ablation. During a median follow-up period of 61.2 ± 16.8 months, 47 of 50 (94%) patients remained free from recurrent LPF-VT.
Conclusion
Ablation of LPF-VT targeting FAP during sinus rhythm results in excellent long-term clinical outcome. FAPs were commonly located at the middle segment of posterior septal LV. Region with FAPs during sinus rhythm was predictive of critical site for re-entry.
Keywords: Fragmented antegrade Purkinje potential, Catheter ablation, Fascicular ventricular tachycardia, Long-term outcome
What’s new?
Substrate-based mapping and targeting of the fragmented antegrade Purkinje potentials (FAPs) during sinus rhythm is a highly successful strategy resulting in excellent long-term clinical outcome with relatively low risk of inadvertent iatrogenic left posterior fascicular (LPF) block during ablation.
FAPs identified during sinus rhythm are strongly predictive of critical sites for re-entry.
FAPs were located at the middle segment of posterior septal LV in majority of patients with LPF-ventricular tachycardia.
Introduction
Left posterior fascicular ventricular tachycardia (LPF-VT) is the most common type of fascicular ventricular tachycardia (FVT).1 Re-entry involving various branches of the Purkinje network emanating from the left fascicles with differential conduction properties has been considered to be the underlying mechanism.2–4 Application of radiofrequency energy during ventricular tachycardia (VT) has become mainstay ablation strategy and proved feasible and effective.4,5 However, not infrequently (10–40% of the time), VT may be non-inducible or non-sustained at the time of planned catheter ablation though intensive electrophysiological stimulation and isoproterenol infusion.6,7 We have previously described an approach targeting sites that demonstrate abnormal wide, low-frequency potentials during sinus rhythm suggestive of antegrade activation of the Purkinje network along the posterior septal left ventricle (LV) (so-called fragmented antegrade Purkinje potential, FAP).8 In this study, we further investigated the detailed electrophysiological characteristics and long-term outcome of patients undergoing catheter ablation of LPF-VT guided by FAPs during sinus rhythm.
Methods
Study population
From October 2012 to January 2015, consecutive patients with symptomatic, electrocardiographically documented LPF-VT referred for electrophysiology study and index ablation were included for present analysis (including 25 patients from our previous study.8) All patients had cardiac imaging with transthoracic echocardiography to confirm absence of structural heart diseases. All patients provided written informed consent prior to the catheter ablation. This study was approved by the institutional ethics committee.
Electrophysiological study
After discontinuation of all antiarrhythmic drugs for at least five half-lives, all patients underwent electrophysiological evaluation. An electrophysiological study was performed with the patient in the fasting state under conscious sedation. Two quadripolar catheters were positioned at the His bundle region and right ventricular apex, respectively, and a decapolar catheter was deployed to coronary sinus (CS) under fluoroscopy via the femoral veins. Twelve-lead surface electrocardiogram (ECG) and intracardiac electrograms were recorded simultaneously by a digital multichannel system (LabSystem PRO, Bard Electrophysiology, Lowell, MA, USA). Bipolar signals were filtered at 30–500 Hz, and unipolar signals were filtered at 0.05–500 Hz.
Stimulation protocol was performed from the right ventricular apex, right ventricular outflow tract and CS at two drive trains with up to three extrastimuli and incremental burst pacing at a cycle length up to 250 ms. If tachycardia could not be induced at the baseline condition, burst pacing and programmed ventricular stimulation with ventricular extrastimulus testing were repeated with the administration of isoproterenol (1–3 µg/min).
Substrate mapping during sinus rhythm
A 3.5 mm irrigated catheter (NaviStar, Biosense Webster Inc., Diamond Bar, CA, USA) was introduced into the LV via retrograde aortic approach. Three-dimensional (3D) electroanatomic mapping was performed using the CARTO mapping system (Biosense Webster, Inc., Diamond Bar, CA, USA). Intravenous heparin was administered to maintain an activated clotting time of 250 s. Detailed point-by-point LV mapping was initially performed during sinus rhythm, and the left His-Purkinje system [including left posterior fascicle (LPF) and left anterior fascicle] was reconstructed by mapping the antegrade Purkinje potentials (PPs). Then, more detailed mapping of the Purkinje fibre network was performed in the vicinity of the LPF during sinus rhythm. We have previously described ‘FAP’ which was defined as an abnormal wide, fragmented, and low-frequency potential documented preceding ventricular activation during sinus rhythm.8 The following electrophysiological features of FAPs were analysed: peak-to-peak amplitude, the area of the FAP, and the distribution of FAP.
Activation and entrainment mapping during ventricular tachycardia
After mapping in sinus rhythm, VT was re-induced, and activation mapping was performed via the remap process based on the previous LV geometry within the posterior septal sites to record the earliest PP. Whenever possible, entrainment at the FAP region with decreasing pacing output (from 20 mA/1 ms to pacing threshold) was performed to confirm participation of the targeted site in VT. The relation between the FAP during sinus rhythm and the earliest PP during VT was analysed.
Catheter ablation
Radiofrequency catheter ablation was delivered at the FAP region during sinus rhythm with temperature-controlled mode at 43°C and irrigation rate at 17 mL/min. Energy output was started at 30 W and increased up to 40 W, and power delivery was maintained for 30–60 s at each ablation site. The endpoint of ablation was defined as non-inducibility of VT after ablation despite programmed stimulation and isoproterenol infusion and elimination of the FAPs during sinus rhythm.
Follow-up
Continuous telemetric monitoring was performed for 24 h after the procedure. After hospital discharge, patients were seen in follow-up in outpatient clinic or virtually (via telephone) every 3 months for the first year post-ablation and every 6 months thereafter. All patients underwent a 12-lead ECG or 24-hour Holter monitoring during follow-up. If the patients had any rhythm-related symptoms, a 12-lead ECG or 24-hour 12-lead Holter monitoring was performed to document the cause of the symptoms.
Statistical analysis
All continuous variables are presented as mean ± standard deviations. The categorical variables are expressed as numbers and percentages. Categorical variables were compared using χ2 analysis. Continuous variables were compared using the Student’s t-test or Mann–Whitney U test, depending on data distribution. A two-tailed value of P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 21.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
Between October 2012 and January 2015, a total of 50 consecutive patients underwent catheter ablation for LPF-VT at the Guangdong Cardiovascular Institute. The median age was 27.5 ± 9.6 years, and 76% were male. All patients had structurally normal hearts, with a mean LV ejection fraction of 63.6 ± 9.7% and LV end-diastolic diameter of 46.4 ± 4.7 mm. The patients on average failed 1.8 ± 1.1 antiarrhythmic drugs prior to ablation. Of note, there were 26 patients in whom intravenous verapamil given during sustained arrhythmia episodes was able to successfully terminate LPF-VT prior to ablation (Table 1).
Table 1.
Baseline clinical characteristics
| Patients | n = 50 |
|---|---|
| Age (years) | 27.5 ± 9.6 |
| Male gender (%) | 38(76) |
| Hypertension, n (%) | 7(14) |
| No. of failed AADs | 1.8 ± 1.1 |
| LVDd (mm) | 46.4 ± 4.7 |
| LVEF (%) | 63.6 ± 9.7 |
Values are expressed as mean ± SD or as n (%).
AAD, antiarrhythmic drug; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction.
Electrophysiological study
At the time of the electrophysiological study, clinical VT was induced in all patients. LPF-VT could be induced in the baseline state with programmed extrastimulus testing or burst ventricular stimulation in 26 patients, while in 2 patients programmed atrial stimulation was able to induce LPF-VT. In remaining 22 patients, LPF-VT induction required isoproterenol infusion. The mean cycle length of inducible VT was 350 ± 38 ms. All inducible VTs demonstrated right bundle branch block morphology and superior axis. VA dissociation during VT was observed in 40 patients, and the remaining 10 patients presented 1:1 VA conduction. The His bundle potential was activated retrogradely during VT in all patients.
Electrophysiological features of FAPs during sinus rhythm
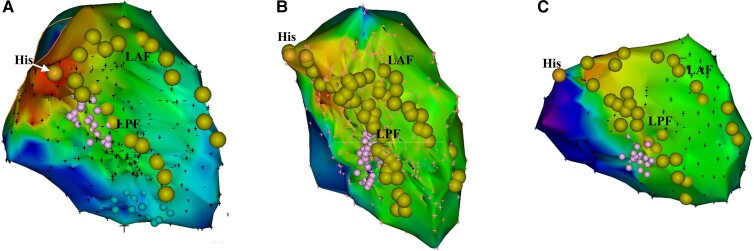
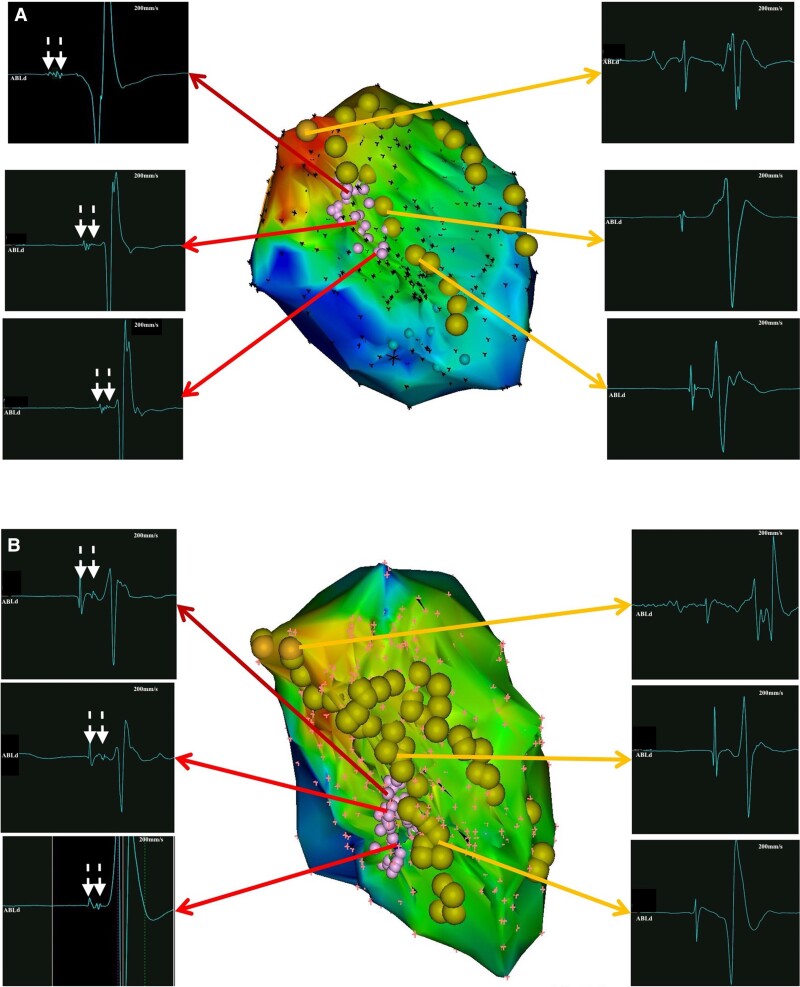
Detailed LV mapping was completed during sinus rhythm in all patients. FAPs were recorded in 48 of 50 (96%) patients during sinus rhythm. The distribution of FAPs included the proximal segment of the posterior septal LV in 2 (4.2%) patients, middle segment in 33 (68.8%) patients, and distal segment in 13 (27.1%) patients (Figure 1). The mean area of FAPs was 1.47 ± 0.92 cm2. In 32 of 48 (66.7%) patients, the FAP displayed a continuous multicomponent fragmented electrogram, while a fragmented, split, and uncoupled electrogram was recorded in 16 (33.3%) patients (Figure 2). Moreover, bipolar voltage of FAP was noted to be significantly lower than that of adjacent PPs (0.27 ± 0.01 mV vs. 1.42 ± 0.65 mV, P < 0.001) (Table 2). No voltage transition between FAP and adjacent PPs was observed.
Figure 1.
Distribution of FAPs. The examples of distribution of FAPs including the proximal segment of posterior septal LV (A), middle segment (B), and distal segment (C). FAP, fragmented antegrade Purkinje potential; LAF, left anterior fascicle; LPF, left posterior fascicle; LV, left ventricle.
Figure 2.
Morphology of single FAP. The FAP displayed a continuous multicomponent fragmented electrogram (A) and a fragmented, split, and uncoupled electrogram (B). FAP, fragmented antegrade Purkinje potential.
Table 2.
Electrophysiological characteristics of FAPs
| Electrophysiological characteristics | Overall (n = 50) |
|---|---|
| FAP recordings, n (%) | 48 (96) |
| Distribution of FAPs, n (%) | |
| ȃProximal segment of the posterior septal LV | 2 (4.2) |
| ȃMiddle segment of the posterior septal LV | 33 (68.8) |
| ȃDistal segment of the posterior septal LV | 13 (27.1) |
| Area of FAPs (cm2) | 1.47 ± 0.92 |
| Morphology of single FAP, n (%) | |
| ȃContinuous multicomponent fragmented electrogram | 32(66.7) |
| ȃFragmented, split, and uncoupled electrogram | 16 (33.3) |
| Bipolar voltage of FAP (mV) | 0.27 ± 0.01 |
Values are expressed as mean ± SD or as n (%).
FAP, fragmented antegrade Purkinje potential; LV, left ventricle.
Mapping during ventricular tachycardia
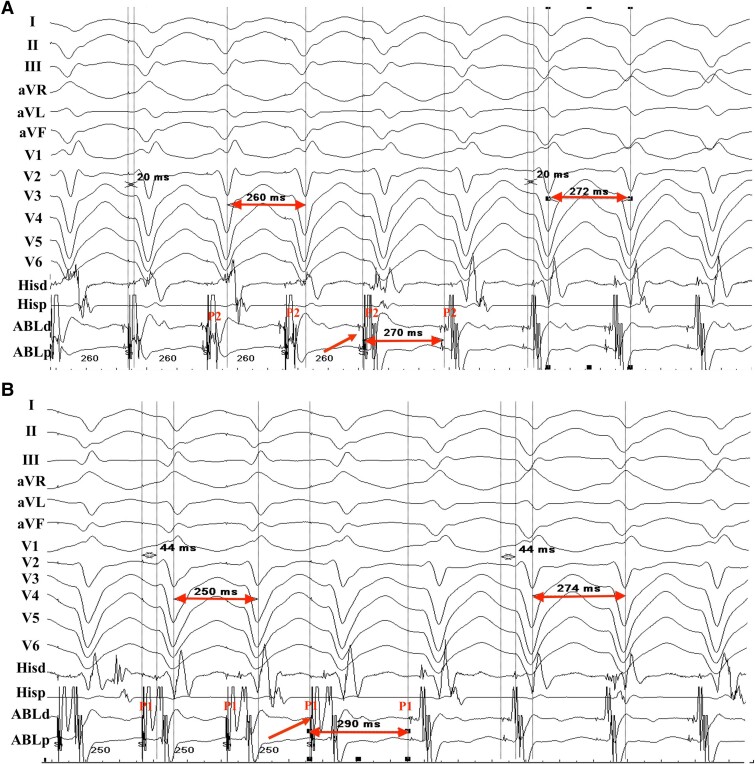
LPF-VT was sustained in 11 patients. The earliest retrograde PP was located at the distal segment of the FAP region recorded during sinus rhythm in these 11 patients. Entrainment attempts at the FAP region were performed successfully in 7 of 11 cases, demonstrating concealed fusion and the critical isthmus of LPF-VT (Figure 3).
Figure 3.
Example of entrainment from the FAP region during the LPF-VT in patient 12 with a tachycardia cycle length of 272 ms. (A) Selective capture of P2 was achieved during entrainment from the FAP region at pacing cycle length of 260 ms, and surface ECG demonstrated a concealed fusion of QRS morphology. The postpacing interval of P2 was almost equal to cycle length of LPF-VT. (B) P1 was selectively captured during entrainment from the FAP region at pacing cycle length of 250 ms. The surface ECG represented a concealed fusion of QRS morphology. The post-pacing interval of P1 was slightly longer than cycle length of LPF-VT. ECG, electrocardiogram; FAP, fragmented antegrade Purkinje potential; LPF-VT, left posterior fascicular ventricular tachycardia.
Catheter ablation and follow-up
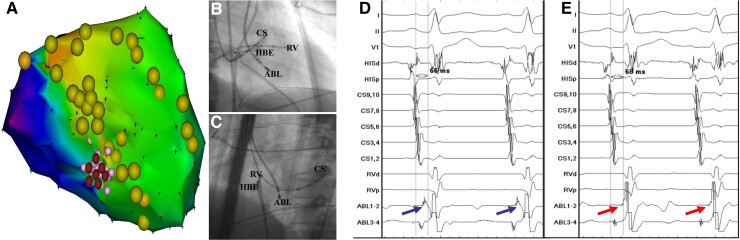
Radiofrequency catheter ablation targeting at the FAP region successfully terminated the LPF-VT in 48 patients, while in the 2 patients in whom FAP was not identified, ablation targeting the earliest PP was successful, rendering all patients completely non-inducible for clinical or non-clinical VT with programmed stimulation and isoproterenol challenge. FAPs during sinus rhythm after ablation were eliminated in these 48 patients (Figure 4). Of note, LPF block occurred in four (8%) patients after ablation, which had no clinical significance or adverse sequelae over long-term follow-up. No procedural-related complications were observed during the ablation. During a follow-up period of 61.2 ± 16.8 months, 47 of 50 (94%) patients remained asymptomatic without documented recurrence of VT. Of these three recurrent patients, two patients received repeat ablation. FAP was still recorded in the two patients, and successful ablation was achieved with elimination of the FAP.
Figure 4.
Successful ablation targeting FAPs during sinus rhythm in patient 5. (A) Note that an area with FAP was located within the posterior Purkinje fibre network and successful ablation was achieved by targeting FAPs. (B and C). The radiographic images of RAO 30°and LAO 45° showed the catheter positions. The ablation catheter was located at the posterior septal LV. (D and E). The FAPs were recorded before ablation and FAPs were eliminated after ablation. ABL, ablation catheter; CS, coronary sinus; FAP, fragmented antegrade Purkinje potential; HBE, His bundle electrogram; LAO, left anterior oblique; LV, left ventricle; RAO, right anterior oblique; RV, right ventricle.
Discussion
Major findings
In the present study, we found that: (i) substrate-based mapping and targeting of the FAPs during sinus rhythm is a highly successful strategy resulting in excellent long-term clinical outcome with relatively low risk of inadvertent iatrogenic LPF block during ablation; (ii) FAPs identified during sinus rhythm are strongly predictive of critical sites for re-entry; and (iii) FAPs were located at the middle segment of posterior septal LV in majority of patients with LPF-VT.
Presence of FAPs
Various ablation approaches with successful outcomes have been previously described including targeting the earliest PP or diastolic potential during LPF-VT5,9,10 or linear ablation at the middle segment of the LPF during sinus rhythm.7,11 However, the limitations of these approaches are obvious, such as non-inducibility or non-sustained nature of LPF-VT and development of inadvertent LPF block. We previously described a novel substrate-based approach to ablation of LPV-VT during sinus rhythm.8 Utilizing a 3D electroananomical mapping system, FAPs were found along posterior septal LV during sinus rhythm in patients with LPF-VT, while not observed in healthy subjects. In the present study, the FAPs were able to be recorded in the vast majority (96%) of patients. The presence of abnormal antegrade PP during sinus rhythm suggests that the region with FAPs is the electroanatomical substrate for LPF-VT and may be indicative of a specific disorder involving the posterior Purkinje network.
Electrophysiological characteristics of FAPs
In this study, we found that the FAPs were clustered at middle segment of posterior septal LV in more than two-thirds of patients. This finding additionally supports previous studies that the approach of targeting at the middle segment of the LPF during sinus rhythm can be an effective treatment strategy.6,7 Furthermore, FAPs were frequently noted to display continuous multicomponent fragmented electrograms. The FAP region was associated with lower bipolar voltage than the adjacent PP. Another interesting finding in the present study was that the earliest retrograde PP during LPF-VT coincided with the distal segment of the FAP region recorded during sinus rhythm. Most importantly, successful entrainment attempts at the FAP region were achieved in 7 of 11 cases during VT. The elimination of the FAPs with ablation during sinus rhythm resulted in successful and durable elimination of VT. Therefore, the FAPs are likely to represent the slow conduction region and may be critical sites for re-entry.
Catheter ablation
Although catheter ablation of LPF-VT can be performed successfully, LPF-VT recurrence is reported, especially in patients with non-inducible LPF-VT.7 A successful ablation of non-inducible LPF-VT may pose a difficult challenge in a clinical scenario. The ablation approach utilized in this study may provide several advantages. First, using non-inducibility as the procedural endpoint may be confounded by the fact that the mechanical termination is frequently encountered during activation mapping. Elimination of the FAPs during sinus rhythm may be a simpler and more objective endpoint to confirm ablation success. Second, this approach may minimize the risk of inadvertently causing iatrogenic LPF block in otherwise a cohort of patients who are typically young otherwise healthy, which in previous studies was used as an acceptable procedural endpoint during delivery of linear ablation.6,7,12 In our study, LPF block was only observed in a small minority (8%) of patients after ablation. Liu et al.5 reported that 20% of patients developed new onset LPF block by targeting the earliest PP. Third, procedure duration may be shortened obviating the need for aggressive and lengthy stimulation protocols to induce VT.
Long-term clinical outcome
Our previous study has demonstrated the favourable outcome guided by FAPs ablation. However, mean follow-up was limited to 16 ± 7 months.8 This study reports excellent long-term outcome targeting the FAPs in sinus rhythm. Freedom from recurrent LPF-VT was reported in 47 of 50 (94%) patients during a median follow-up period of more than 5 years. The data presented in this study provide new evidence that abolishing the critical area during sinus rhythm resulting in excellent long-term outcome.
Study limitations
This study has several limitations. This was a retrospective single-center study with limited sample size. Furthermore, only patients with LPF-VT underwent ablation, and therefore, our findings might not be applicable for patients with other types of FVT. In addition, the accurate re-entry circuit was not well defined in this study because of the complex of the Purkinje system. Finally, multielectrode catheters (i.e. HD Grid, PentaRay) were not used in our study, which may provide more high-fidelity mapping and visualization of FAPs and PPs. Future studies with a high resolution mapping catheter will provide a better understanding of the characterization of FAP.
Conclusion
Ablation of LPF-VT targeting FAPs during sinus rhythm is associated with excellent long-term clinical outcome and can minimize iatrogenic LPF block during ablation. FAPs were commonly localized to the middle segment of posterior septal LV. Regions with FAPs during sinus rhythm likely represent critical sites for re-entry.
Contributor Information
Hui-Qiang Wei, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Zili Liao, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Yuanhong Liang, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Xianhong Fang, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Hongtao Liao, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Hai Deng, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Wei Wei, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Yingjie Huang, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Yang Liu, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Fangzhou Liu, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Weidong Lin, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Jackson J Liang, Division of Cardiovascular Medicine, Cardiac Arrhythmia Service, University of Michigan Health System, Ann Arbor, MI, USA.
Yumei Xue, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Shulin Wu, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Xianzhang Zhan, Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, No.106 Zhongshan 2nd Road, Yuexiu District, 510000 Guangzhou, China.
Funding
This work was supported by the Science and Technology Planning Program of Guangdong Province (grant number 2019B020230004).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Kapa S, Gaba P, DeSimone CV, Asirvatham SJ. Fascicular ventricular arrhythmias: pathophysiologic mechanisms, anatomical constructs, and advances in approaches to management. Circ Arrhythm Electrophysiol 2017;10:e002476. [DOI] [PubMed] [Google Scholar]
- 2. Nogami A, Naito S, Tada H, Taniguchi K, Okamoto Y, Nishimura Set al. . Demonstration of diastolic and presystolic Purkinje potentials as critical potentials in a macroreentry circuit of verapamil-sensitive idiopathic left ventricular tachycardia. J Am Coll Cardiol 2000;36:811–23. [DOI] [PubMed] [Google Scholar]
- 3. Ouyang F, Cappato R, Ernst S, Goya M, Volkmer M, Hebe Jet al. . Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macroreentry within the Purkinje network. Circulation 2002;105:462–9. [DOI] [PubMed] [Google Scholar]
- 4. Liu Q, Shehata M, Jiang R, Yu L, Chen S, Zhu Jet al. . Macroreentrant loop in ventricular tachycardia from the left posterior fascicle: new implications for mapping and ablation. Circ Arrhythm Electrophysiol 2016;9:e004272. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Fang Z, Yang B, Kojodjojo P, Chen H, Ju Wet al. . Catheter ablation of fascicular ventricular tachycardia: long-term clinical outcomes and mechanisms of recurrence. Circ Arrhythm Electrophysiol 2015;8:1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin D, Hsia HH, Gerstenfeld EP, Dixit S, Callans DJ, Nayak Het al. . Idiopathic fascicular left ventricular tachycardia: linear ablation lesion strategy for noninducible or nonsustained tachycardia. Heart Rhythm 2005;2:934–9. [DOI] [PubMed] [Google Scholar]
- 7. Luo B, Zhou G, Guo X, Liu X, Yang J, Sun Qet al. . Long-term outcome of catheter ablation for left posterior fascicular ventricular tachycardia with the development of left posterior fascicular block and characteristics of repeat procedures. Int J Cardiol 2017;236:203–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhan XZ, Liang YH, Xue YM, Shehata M, Liao HT, Fang XHet al. . A new electrophysiologic observation in patients with idiopathic left ventricular tachycardia. Heart Rhythm 2016;13:1460–7. [DOI] [PubMed] [Google Scholar]
- 9. Long DY, Dong JZ, Sang CH, Jiang CX, Tang RB, Yan Qet al. . Isolated conduction within the left His-Purkenje system during sinus rhythm and idiopathic left ventricle tachycardia: findings from mapping the whole conduction system. Circ Arrhythm Electrophysiol 2013;6:522–7. [DOI] [PubMed] [Google Scholar]
- 10. Zhou G, Lu X, Nie Z, Chen S, Wei Y, Cai Let al. . QRS complex axis deviation changing in catheter ablation of left fascicular ventricular tachycardia. Europace 2020;22:1688–96. [DOI] [PubMed] [Google Scholar]
- 11. Chen M, Yang B, Zou J, Shan Q, Chen C, Xu Det al. . Non-contact mapping and linear ablation of the left posterior fascicle during sinus rhythm in the treatment of idiopathic left ventricular tachycardia. Europace 2005;7:138–44. [DOI] [PubMed] [Google Scholar]
- 12. Fishberger SB, Olen MM, Rollinson NL, Rossi AF. Creation of partial fascicular block: an approach to ablation of idiopathic left ventricular tachycardia in the pediatric population. Pacing Clin Electrophysiol 2015;38:209–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.