Abstract
Aims
This study compares clinical outcomes between leadless pacemakers (leadless-VVI) and transvenous ventricular pacemakers (transvenous ventricular permanent-VVI) in subgroups of patients at higher risk of pacemaker complications.
Methods and results
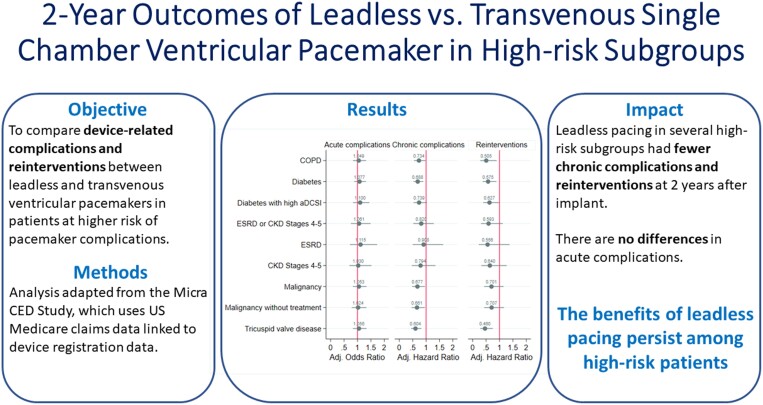
This study is based on the Micra Coverage with Evidence Development (CED) study. Patients from the Micra CED study were considered in a high-risk subgroup if they had a diagnosis of chronic kidney disease Stages 4–5 (CKD45), end-stage renal disease, malignancy, diabetes, tricuspid valve disease (TVD), or chronic obstructive pulmonary disease (COPD) 12 months prior to pacemaker implant. A pre-specified set of complications and reinterventions were identified using diagnosis and procedure codes. Competing risks models were used to compare reinterventions and complications between leadless-VVI and transvenous-VVI patients within each subgroup; results were adjusted for multiple comparisons. A post hoc comparison of a composite outcome of reinterventions and device complications was conducted. Out of 27 991 patients, 9858 leadless-VVI and 12 157 transvenous-VVI patients have at least one high-risk comorbidity. Compared to transvenous-VVI patients, leadless-VVI patients in four subgroups [malignancy, HR 0.68 (0.48–0.95); diabetes, HR 0.69 (0.53–0.89); TVD, HR 0.60 (0.44–0.82); COPD, HR 0.73 (0.55–0.98)] had fewer complications, in three subgroups [diabetes, HR 0.58 (0.37–0.89); TVD, HR 0.46 (0.28–0.76); COPD, HR 0.51 (0.29–0.90)) had fewer reinterventions, and in four subgroups (malignancy, HR 0.52 (0.32–0.83); diabetes, HR 0.52 (0.35–0.77); TVD, HR 0.44 (0.28–0.70); COPD, HR 0.55 (0.34–0.89)] had lower rates of the combined outcome.
Conclusion
In a real-world study, leadless pacemaker patients had lower 2-year complications and reinterventions rates compared with transvenous-VVI pacing in several high-risk subgroups.
Trial Registration
ClinicalTrials.gov ID NCT03039712
Keywords: Leadless pacemakers, Transvenous pacemakers, System reintervention, Complications, High-risk patients
Graphical Abstract
Graphical Abstract.
What’s new?
The Micra leadless pacemaker (leadless-VVI) has been shown to have lower chronic complications and device-related reinterventions compared to transvenous ventricular pacemakers (transvenous-VVI) in a large, real-world population. This study extends the findings of the previous study to demonstrate the benefits of leadless pacing among patients with a higher risk of complications with transvenous pacing.
Leadless-VVI patients with malignancy, diabetes, tricuspid valve disease, and/or chronic obstructive pulmonary disease had fewer chronic complications, and patients with diabetes, tricuspid valve disease, and/or chronic obstructive pulmonary disease had fewer device-related reinterventions than patients receiving transvenous-VVI pacemakers.
Leadless-VVI patients with malignancy, diabetes, tricuspid valve disease, and/or chronic obstructive pulmonary disease had lower rates of the combined outcome of device complications and select device-related reinterventions than transvenous-VVI patients.
Introduction
Leadless pacemakers are an alternative therapeutic device to traditional transvenous pacemakers that obviate the need for transvenous pacing leads. Additionally, they have the potential to reduce pocket related complications since the entire device is implanted within the heart. Evidence from the Micra” Transcatheter Pacing System Investigational Device Exemption (IDE) study showed a 48% reduction in major complications,1 with no dislodgements or procedure-related infections reported. In a post-approval study (PAS), Micra patients had a 63% lower risk of major complications through 12 months post implantation than patients with transvenous pacemakers.2
After its approval for use in USA in 2016, the Center for Medicare and Medicaid Services (CMS) mandated that all leadless (leadless-VVI) pacemaker procedures claimed under CMS coverage be included in a registry as part of its Coverage with Evidence Development (CED) program.3 Linking this registry to claim-based data of patients with traditional transvenous single-chamber (transvenous-VVI) pacemaker procedures from the same implanting sites, the Micra CED study previously demonstrated no differences in aggregate complications and mortality 30 days after implantation (with a statistically higher rate of 30-day pericardial effusions and/or perforations and a lower rate of 30-day device-related complications in the leadless-VVI cohort).4 A follow-up report demonstrated a 31% reduction in chronic complications and a 38% reduction in device-related reinterventions 2 years after implant among leadless-VVI patients compared to transvenous-VVI patients.5 Additionally, a multi-centre study outside of the CED program found that leadless pacing was associated with reductions in device-related complications 1 year after implant compared to transvenous pacing.6
Prior studies have shown that patients with certain co-morbidities may be at increased risk for transvenous pacemaker-related complications.7,8 Whether these observations translate to patients undergoing leadless pacemaker implantation remains poorly understood. The objective of this study is to compare acute and chronic complications, reintervention rates, and mortality in patients implanted with leadless-VVI vs. transvenous-VVI pacemakers in subgroups of patients with a higher risk of transvenous pacemaker complications.
Methods
Study design and population
This study is a retrospective, observational research study based on the longitudinal Micra Coverage with Evidence Development (CED) study (NCT03039712), from which it takes the data source, the initial population, and the baseline characteristics and outcome definitions. The Micra CED study is a continuous-enrolling, longitudinal cohort study that uses administrative claims data to compare complications and reinterventions between patients receiving leadless-VVI pacing systems and patients receiving transvenous-VVI pacemakers in the US Medicare population. Additional details and comparisons of outcomes for the overall population on the Micra CED study have been published previously.4,5,9 The study was approved by the Western Institutional Review Board with a waiver of informed consent and is registered on ClinicalTrials.gov (NCT03039712).
Data
Medicare claims data from 9 March 2017 to 31 December 2019 were used, following the already published protocols on data linkage to the manufacturer device registration data, transvenous-VVI pacemaker patient selection, and general inclusion criteria;4,5,9 this study includes an additional yearly cohort of patients than the 2-year result studies (i.e. those implanted during 2019). Leadless-VVI patients were identified by linking Medicare claims with a registry of patients implanted with Micra leadless pacemaker (Model MC1VR01, Medtronic, Inc.), and identified transvenous-VVI patients using the International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) or Current Procedural Terminology for implants occurring in the inpatient hospital setting or the outpatient hospital setting, respectively (see Supplementary material online, Table S1). Patients with transvenous-VVI pacemakers were excluded if they were implanted by hospitals without any concurrent experience implanting leadless-VVI pacemakers to minimize any selection effects due to transvenous-VVI patients not having the opportunity of receiving a leadless-VVI pacemaker. Patients with less than 12 months of continuous enrolment in Medicare Fee-For-Services prior to implant and patients with a prior cardiovascular implantable electronic device (CIED) were also excluded.
We included in this study as high-risk comorbidities those conditions for whom higher rates of transvenous pacemaker complications have been noted in the literature (higher likelihood of device-related infection,10 bleeding,8 or the desire to preserve vascular access11) and are identifiable in administrative claims within the scope of the Micra CED study. High-risk patients were identified using evidence of a diagnosis present on any encounter during a 12-month lookback period. Patients with end stage renal disease (ESRD) were identified using both diagnosis codes and Medicare enrolment data. Patients with chronic kidney disease (CKD) Stages 4 and 5, malignancy, diabetes, tricuspid valve disease (TVD), and chronic obstructive pulmonary disease (COPD) were identified using diagnosis codes listed in Supplementary material online, Table S2. Diabetes patients were further characterized as to whether they had high-complication diabetes, defined as an adapted Diabetes Complications Severity Index (aDCSI) > 4.12 Other clinical baseline patient characteristics, including coronary artery disease, peripheral vascular disease, atrial fibrillation, left bundle branch block, supraventricular tachycardia, ventricular arrhythmia, steroid use, heart failure, hyperlipidaemia, hypertension, history of any cardiovascular events and procedures (acute myocardial infarction, coronary artery bypass graft, transcatheter aortic valve, and percutaneous coronary intervention), and concomitant transcatheter aortic valve replacement and atrial ablation were identified with diagnosis and procedure codes, listed in Supplementary material online, Table S3, as in previous Micra CED studies.4,5 A Charlson comorbidity index was calculated for each patient.13 Patient demographic characteristics, including age and sex, were identified in the CMS enrolment file.
Outcomes
This study compares acute complications, chronic complications, device reinterventions, and mortality between leadless-VVI and transvenous-VVI patients. Acute complications were defined as embolism and thrombosis, events at the puncture site, cardiac effusion and/or perforation, device-related complications, and other complications, including device-related acute myocardial infarction, postprocedural hematoma or haemorrhage, intraoperative cardiac arrest, pericarditis, vascular complications, haemothorax, and pneumothorax. Chronic complications were defined as those most likely attributable to the device implant or the device itself that may continue to occur outside the acute period and included embolism, thrombosis, device-related complications, including device breakdown, dislodgment, infection, and pocket complications, pericarditis, and haemothorax. Device reinterventions were defined as system revision, lead revision or replacement, system replacement (e.g. replacing a leadless-VVI with a leadless-VVI), system removal, switch to the alternative type of system (switch from leadless-VVI to transvenous-VVI or transvenous-VVI to leadless-VVI), upgrade to a dual-chamber system, or upgrade to a cardiac resynchronization therapy (CRT) device. Mortality was defined as all-cause patient death. Acute complications were identified in any claim in the 30-day period after implant, while chronic complications and device reinterventions were identified in any claim in the 2-year period after implant; all acute and chronic complications and reinterventions were prospectively defined. However, we defined a post hoc composite outcome of selected reinterventions (system revision, lead revision or replacement, system replacement, system removal) and device-related chronic complications (device breakdown, dislodgment, other mechanical complications, infection, device-related pain, stenosis, and pocket complications) in order to capture events more likely to drive health expenditures. Mortality was measured at both 30-day and 2-year periods after implant using Medicare enrolment data; 30-day mortality rates include intra-operative deaths. Codes that identify all outcomes are in Supplementary material online, Table S4. Billing claims were available through 31 December 2020; patients without an event were censored on that date.
Statistical analysis
Overlap weights were used to account for differences in baseline and encounter characteristics between the leadless-VVI and transvenous-VVI cohorts, as in previous studies.4,5 A logistic regression model that included patient baseline and encounter characteristics was used to compute the propensity (i.e. probability) for each patient within each high-risk subgroup to be implanted with a leadless-VVI pacemaker. These scores were used to construct an overlap weight for each patient within each high-risk subgroup and used as weights in the regression models. The overlap weights adjustment strategy uses the probability of receiving therapy with the opposing treatment based on characteristics used to construct the propensity score to place the most weight on patients considered the most exchangeable and the least emphasis on patients who are least likely to receive the opposing therapy.14,15
Logistic regression models were used to estimate unadjusted and overlap-weight adjusted odds ratios of 30-day acute complication rates and 30-day mortality rates and Fine–Gray competing risk models were used to estimate unadjusted and overlap-weight adjusted Hazard ratios of 2-year chronic complication rates, device-related reinterventions rates, mortality rates, and the post hoc composite outcome rates between leadless-VVI patients and transvenous-VVI patients for each high-risk subgroup. Additionally, Fine–Gray competing risk models were estimated by type of chronic complication (device-related complications or other complications) and the components of the composite outcome (device-related complications and selected reinterventions). Standard errors were correlated at the hospital level to account for within-hospital correlation.
A single patient can have more than one comorbidity classified in each high-risk subgroups identified previously. In order to test how leadless pacing performs in patients with multiple high-risk comorbidities, a categorical measure of the number of comorbidities (no high-risk comorbidities, 1 comorbidity, 2 comorbidities, more than 2 comorbidities) was created and, as a post hoc sensitivity analysis, chronic complications, reinterventions, and the composite outcome between leadless-VVI patients and transvenous-VVI patients within each level of this measure.
To correct for the increased probability of false positives due to multiple hypotheses testing, Bonferroni adjustments were used to the standard 5% statistical significance level according to the number of high-risk subgroups and outcomes: by 9 for acute complications, mortality, and reinterventions (9 high-risk subgroups), and by 27 for chronic complications and the composite outcome (9 high-risk subgroups and 3 disaggregated levels of the outcome). With Bonferroni adjustments, comparing P-values to the standard 5% statistical significance level could be misleading. To avoid confusion about the statistical significance of the Odds and Hazard ratios, Bonferroni-adjusted confidence intervals are reported only where statistical significance is achieved when these intervals do not include 1. Events occurring between one and 10 patients were analyzed but not reported to protect beneficiary privacy as required by CMS.16 All statistical analyses were conducted in SAS version 9.4 (SAS Institute).
Results
High-risk subgroups
There are 27 991 de novo implant procedures (12 326 leadless-VVI and 15 665 transvenous-VVI) identified during the study period in the overall cohort, of which 9858 leadless-VVI patients (80.0%) and 12 157 transvenous-VVI patients (77.6%) have at least one high-risk comorbidity; Table 1 shows the number of patients in each of the 9 subgroups defined previously. The largest high-risk subgroup is diabetes (11 936 patients, 42.6%), while the subgroups of patients with ESRD or CKD Stages 4–5 have the fewest patients, accounting for 2032 (7.3%) and 2477 (8.8%) respectively; if these patients are grouped into a single subgroup, they account for 3453 (12.3%) patients. At the same time, the ESRD and CKD Stages 4-5 subgroups have the highest percent of leadless-VVI patients: 74.9% in ESRD patients and 55.2% in CKD Stages 4-5, and 61.1% in the subgroup of ESRD or CKD Stages 4–5 patients. Supplementary material online, Tables S5A–I show patient and implant characteristics of patients in all high-risk subgroups.
Table 1.
High-risk subgroup patient count
| Total N (% of total) |
Leadless-VVI, N |
Transvenous-VVI, N | |
|---|---|---|---|
| Overall population | 27 991 (100%) | 12 326 | 15 665 |
| ȃCOPD | 8372 (29.9%) | 3783 | 4589 |
| ȃDiabetes | 11 936 (42.6%) | 5514 | 6422 |
| ȃȃDiabetes with high aDCSI | 6786 (24.2%) | 3329 | 3457 |
| ȃESRD or CKD Stages 4 and 5 | 3453 (12.3%) | 2110 | 1343 |
| ȃȃESRD | 2032 (7.3%) | 1522 | 510 |
| ȃȃCKD Stages 4 and 5 | 2477 (8.8%) | 1368 | 1109 |
| ȃMalignancy | 7764 (27.7%) | 3499 | 4265 |
| ȃȃMalignancy without treatment | 6602 (23.6%) | 2895 | 3707 |
| ȃTricuspid valve disease | 8162 (29.2%) | 3595 | 4567 |
| No high-risk comorbidities | 5976 (21.3%) | 2468 | 3508 |
| 1 comorbidity | 9928 (35.5%) | 4071 | 5857 |
| 2 comorbidities | 7269 (26.0%) | 3263 | 4006 |
| > 2 comorbidities | 4818 (17.2%) | 2524 | 2294 |
High-risk comorbidities are chronic kidney disease Stages 4 and 5, chronic obstructive pulmonary disease, diabetes with low adapted Diabetes Complication Severity Index, diabetes with high adapted Diabetes Complication Severity Index, end-stage renal disease, malignancy with treatment, malignancy without treatment, and tricuspid valve disease. ESRD and CKD Stages 4 or 5 are not mutually exclusive.
aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease.
Acute complications
Table 2 shows the unadjusted and adjusted odds ratios of the 30-day acute complication rate of leadless-VVI patients compared to transvenous-VVI patients in each of the high-risk subgroups. Acute complication rates of leadless-VVI patients range from 8.6% in the malignancy without treatment subgroup to 14.5% in the ESRD subgroup, and acute complication rates of transvenous-VVI patients range from 7.4% in the diabetes subgroup to 12.0% in the ESRD subgroup. There are no differences in 30-day acute complication rates between leadless-VVI patients and transvenous-VVI patients adjusting for patient characteristics in any of the high-risk subgroups. Supplementary material online, Table S6A and B show the rates of the components of the 30-day acute complications. Leadless-VVI patients have higher unadjusted rates of cardiac effusion and perforations but lower rates of device-related complications than transvenous-VVI patients in all subgroups.
Table 2.
Thirty-day acute complications results
| All acute complications | ||||
|---|---|---|---|---|
| L-VVI rate (%) | TV-VVI rate (%) | Unadjusted OR (confidence interval) |
Adjusted OR (confidence interval) |
|
| COPD | 9.6 | 8.6 | 1.139 (0.916–1.416) |
1.049 (0.833–1.322) |
| Diabetes | 8.9 | 7.4 | 1.227 (1.012–1.487) |
1.077 (0.879–1.319) |
| ȃDiabetes with high aDCSI | 9.8 | 7.9 | 1.279 (0.987–1.656) |
1.100 (0.837–1.447) |
| ESRD or CKD Stages 4 and 5 | 13.2 | 10.6 | 1.279 (0.938–1.745) |
1.061 (0.755–1.490) |
| ȃESRD | 14.5 | 12.0 | 1.254 (0.820–1.915) |
1.115 (0.716–1.737) |
| ȃCKD Stages 4 and 5 | 12.6 | 10.6 | 1.208 (0.838–1.742) |
1.030 (0.697–1.522) |
| Malignancy | 8.8 | 7.8 | 1.146 (0.905–1.451) |
1.053 (0.827–1.340) |
| ȃMalignancy without treatment | 8.6 | 7.9 | 1.102 (0.852–1.427) |
1.024 (0.785–1.335) |
| ȃTricuspid valve disease | 8.8 | 8.1 | 1.105 (0.878–1.391) |
1.056 (0.832–1.339) |
Odds ratios of 30-day acute complication rates between leadless-VVI patients and transvenous-VVI patients for each high-risk subgroup, estimated with logistic regression models. Confidence Intervals adjusted for multiple comparison (number of tests = 9). ESRD and CKD Stages 4 or 5 are not mutually exclusive.
aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; CKD, chronic kidney disease; L-VVI, leadless-VVI; OR, odds ratios; TV-VVI, transvenous-VVI.
Chronic complications
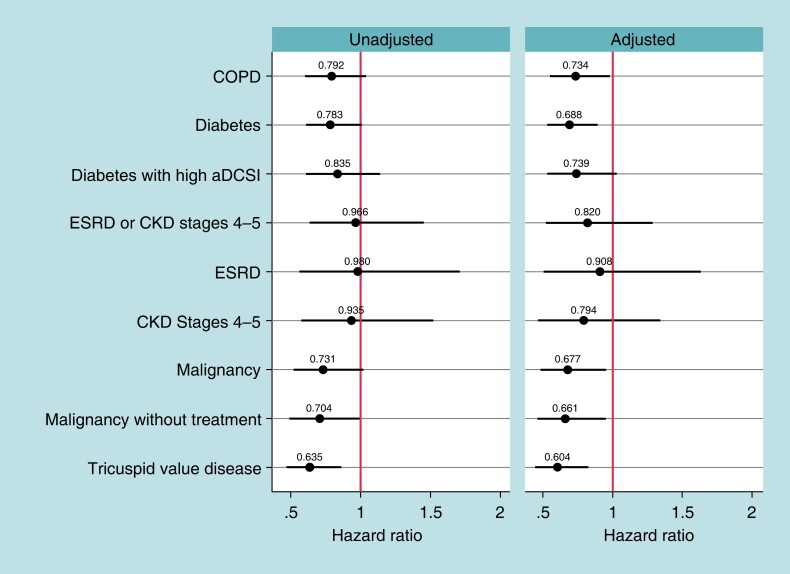
Figure 1 shows the unadjusted and adjusted Hazard ratios of the 2-year chronic complication rates of leadless-VVI patients compared to transvenous-VVI patients for the high-risk subgroups. Prior to adjustment, only the TVD subgroup have a significantly lower chronic complication rate among leadless-VVI compared with transvenous-VVI patients [unadjusted hazard ratio (HR) = 0.64, Bonferroni-adjusted confidence interval (BA-CI) (0.47–0.86)]. Following adjustment for patient characteristics, the COPD, diabetes, malignancy, malignancy without treatment, and TVD subgroups have lower chronic complication rates among the leadless-VVI patients [COPD: adjusted HR = 0.73, BA-CI (0.55–0.98); diabetes: adjusted HR = 0.69, BA-CI (0.53–0.89); malignancy: adjusted HR = 0.68, BA-CI (0.48–0.95); malignancy without treatment: adjusted HR = 0.66, BA-CI (0.46–0.95); TVD: adjusted HR = 0.60, BA-CI (0.44–0.82)]. When evaluating chronic complications by type, leadless-VVI patients have lower device-related chronic complications than transvenous-VVI patients in all high-risk subgroups except ESRD and CKD Stages 4 and 5 (Table 3). Supplementary material online, Table S7A and B show the rates of the components of the 2-year chronic complications.
Figure 1.
Two-year chronic complications results. Notes: Hazard ratios of 2-year chronic complication rates between leadless-VVI patients and transvenous-VVI patients for each high-risk subgroup, estimated with Fine–Gray competing risk models. Spikes represent Bonferroni-adjusted confidence intervals (number of tests = 27). aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease. ESRD and CKD Stages 4 or 5 are not mutually exclusive.
Table 3.
Chronic complications by type
| All chronic complications | Device-related complications | Other complications | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L-VVI rate (%) | TV-VVI rate (%) | Adjusted HR (confidence interval) |
L-VVI rate (%) | TV-VVI rate (%) | Adjusted HR (confidence interval) |
L-VVI rate (%) | TV-VVI rate (%) | Adjusted HR (confidence interval) |
|
| COPD | 5.31 | 6.73 | 0.734 (0.550–0.980) |
2.80 | 5.16 | 0.500 (0.348–0.718) |
2.51 | 1.72 | 1.397 (0.874–2.233) |
| Diabetes | 4.88 | 6.26 | 0.688 (0.530–0.894) |
2.63 | 4.86 | 0.478 (0.352–0.649) |
2.19 | 1.59 | 1.234 (0.810–1.877) |
| ȃDiabetes with high aDCSI | 5.62 | 6.74 | 0.739 (0.531–1.030) |
2.91 | 5.26 | 0.484 (0.322–0.729) |
2.67 | 1.71 | 1.459 (0.854–2.493) |
| ESRD or CKD Stages 4 and 5 | 6.82 | 7.07 | 0.820 (0.522–1.288) |
3.70 | 5.36 | 0.586 (0.341–1.006) |
3.27 | 1.94 | 1.475 (0.689–3.158) |
| ȃESRD | 7.49 | 7.65 | 0.908 (0.503–1.637) |
4.01 | 5.10 | 0.715 (0.352–1.455) |
3.61 | 2.94 | 1.180 (0.467–2.981) |
| ȃCKD Stages 4 and 5 | 6.65 | 7.12 | 0.794 (0.467–1.348) |
3.51 | 5.59 | 0.544 (0.282–1.051) |
3.22 | 1.71 | 1.651 (0.684–3.986) |
| Malignancy | 4.54 | 6.21 | 0.677 (0.481–0.954) |
2.03 | 4.71 | 0.417 (0.269–0.645) |
2.72 | 1.81 | 1.350 (0.826–2.205) |
| ȃMalignancy without treatment | 4.35 | 6.18 | 0.661 (0.459–0.953) |
2.11 | 4.69 | 0.438 (0.278–0.690) |
2.52 | 1.81 | 1.260 (0.745–2.131) |
| Tricuspid valve disease | 4.51 | 7.07 | 0.604 (0.443–0.824) |
2.36 | 5.78 | 0.394 (0.269–0.576) |
2.20 | 1.62 | 1.262 (0.750–2.214) |
Hazard ratios adjusted using overlapping weights. Confidence intervals adjusted for multiple comparisons (number of tests = 27). ESRD and CKD Stages 4 or 5 are not mutually exclusive.
aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR, hazard ratio; L-VVI, leadless-VVI; TV-VVI, transvenous-VVI.
Reinterventions
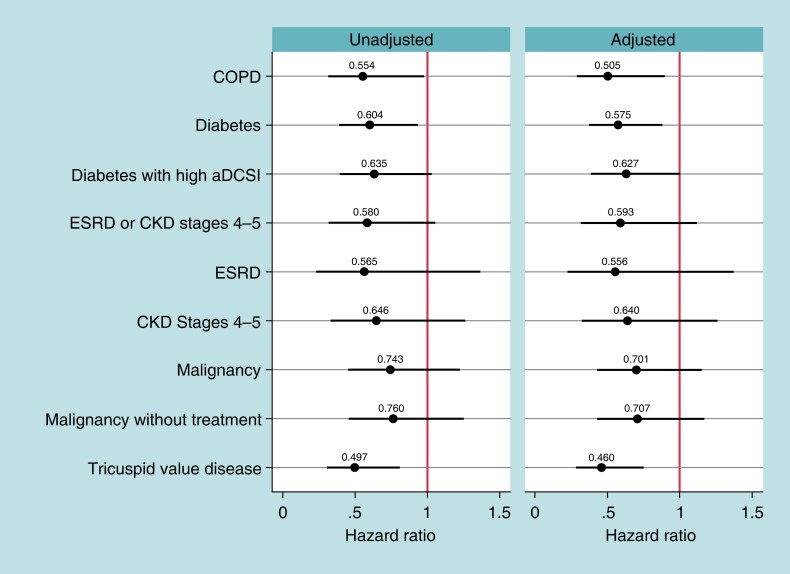
Figure 2 shows the unadjusted and adjusted Hazard ratios comparing 2-year reintervention rates for leadless-VVI patients compared to transvenous-VVI patients among the high-risk subgroups. The COPD, diabetes, and TVD subgroups show lower reintervention rates in leadless-VVI patients [COPD: adjusted HR = 0.51, BA-CI (0.29–0.90); diabetes: adjusted HR = 0.58, BA-CI (0.37–0.89); TVD: adjusted HR = 0.46, BA-CI (0.28–0.76)]. These results are robust to the overlapping weights adjustment. Unadjusted rates are reported in Supplementary material online, Table S8. Supplementary material online, Table S9A and B show the rates of the components of the 2-year reinterventions.
Figure 2.
Two-year device-related reinterventions. Notes: Hazards ratios of 2-year device-related reintervention rates between leadless-VVI patients and transvenous-VVI patients for each high-risk subgroup, estimated with Fine–Gray competing risk models. Spikes represent Bonferroni-adjusted Confidence Intervals (number of tests = 9). aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; ESRD and CKD Stages 4 or 5 are not mutually exclusive.
Composite outcome
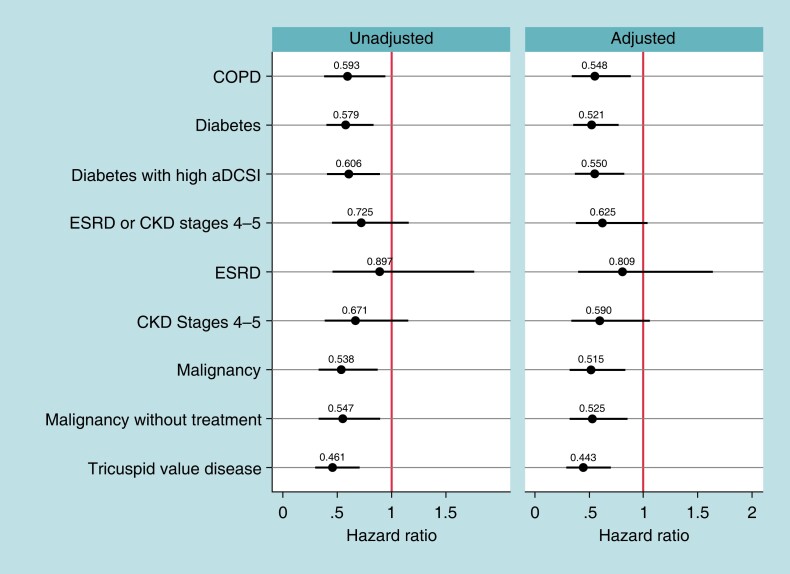
Figure 3 shows the unadjusted and adjusted Hazard ratios comparing the 2-year rates of the composite outcome of device-related complications and select reinterventions for leadless-VVI patients compared to transvenous-VVI patients among the high-risk subgroups. In all high-risk subgroups except those with CKD Stage 4-5 and ESRD, leadless-VVI patients have lower rates of the composite outcome than transvenous-VVI patients [COPD: adjusted HR = 0.55, BA-CI (0.34–0.89); diabetes: adjusted HR = 0.52, BA-CI (0.35–0.77); diabetes with high aDCSI: adjusted HR = 0.55, BA-CI (0.37–0.82); malignancy: adjusted HR = 0.52, BA-CI (0.32–0.83) ; malignancy without treatment: adjusted HR = 0.53, BA-CI (0.32–0.85); TVD: adjusted HR = 0.44, BA-CI (0.28–0.70)]. The difference was driven by device-related chronic complications as there are no statistically significant differences in the selected reintervention rates observed across any subgroup, as seen in the results for the components of this composite outcome in Supplementary material online, Table S10.
Figure 3.
Two-year combined device-related chronic complication and select reinterventions. Notes: hazards ratios of 2-year combined device-related chronic complications (device breakdown, dislodgment, other mechanical complications, infection, device-related pain, device-related stenosis, and pocket complications) and select reinterventions (system revision, lead revision or replacement, system replacement, system removal) rates between leadless-VVI patients and transvenous-VVI patients for each high-risk subgroup, estimated with Fine–Gray competing risk models. Spikes represent Bonferroni-adjusted confidence intervals (number of tests = 27). aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease. ESRD and CKD Stages 4 or 5 are not mutually exclusive.
Mortality
Table 4 shows the unadjusted and adjusted odds ratios and Hazard ratios comparing the 30-day and 2-year all-cause mortality rates between leadless-VVI patients and transvenous-VVI patients per high-risk subgroup. While unadjusted all-cause mortality rates are higher in leadless-VVI patients in all subgroups except ESRD, there is no statistically significant difference in adjusted all-cause mortality rates either at 30-day or 2-year periods in any subgroup.
Table 4.
Mortality rates at 30 days and 2 years
| 30-day mortality | 2-year mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| L-VVI rate (%) | TV-VVI rate (%) | Unadjusted OR (confidence interval) |
Adjusted OR (confidence interval) |
L-VVI rate (%) | TV-VVI rate (%) | Unadjusted HR (confidence interval) |
Adjusted HR (confidence interval) |
|
| COPD | 5.84 | 5.69 | 1.031 (0.795–1.339) |
0.928 (0.710–1.215) |
42.53 | 40.64 | 1.110 (1.003–1.229) |
1.048 (0.944–1.164) |
| Diabetes | 4.72 | 4.50 | 1.053 (0.816–1.358) |
0.871 (0.671–1.131) |
37.05 | 33.57 | 1.175 (1.072–1.288) |
1.041 (0.946–1.146) |
| ȃDiabetes with high aDCSI | 5.92 | 5.84 | 1.013 (0.751–1.365) |
0.901 (0.661–1.228) |
44.46 | 40.93 | 1.147 (1.031–1.277) |
1.049 (0.936–1.175) |
| ESRD or CKD Stages 4 and 5 | 8.48 | 8.34 | 1.016 (0.714–1.447) |
1.003 (0.688–1.462) |
54.41 | 54.73 | 1.018 (0.893–1.161) |
1.004 (0.873–1.155) |
| ȃESRD | 8.48 | 11.37 | 0.722 (0.451–1.156) |
0.802 (0.491–1.309) |
57.56 | 60.98 | 0.913 (0.765–1.090) |
0.978 (0.814–1.175) |
| ȃCKD Stages 4 and 5 | 8.77 | 7.48 | 1.199 (0.813–1.768) |
1.138 (0.754–1.719) |
55.12 | 53.47 | 1.090 (0.934–1.272) |
1.039 (0.882–1.225) |
| Malignancy | 4.97 | 4.22 | 1.205 (0.902–1.608) |
1.033 (0.770–1.386) |
34.95 | 33.15 | 1.116 (1.003–1.242) |
1.023 (0.916–1.143) |
| ȃMalignancy without treatment | 4.32 | 3.97 | 1.098 (0.782–1.543) |
0.975 (0.691–1.375) |
31.85 | 31.16 | 1.072 (0.950–1.209) |
1.010 (0.892–1.142) |
| Tricuspid valve disease | 4.73 | 4.12 | 1.150 (0.841–1.573) |
1.024 (0.745–1.408) |
34.66 | 32.87 | 1.113 (1.000–1.24) |
1.044 (0.935–1.167) |
Hazard ratios and odds ratios adjusted using overlapping weights. Confidence Intervals adjusted for multiple comparisons (number of tests = 9). ESRD and CKD Stages 4 or 5 are not mutually exclusive.
aDCSI, adapted Diabetes Complication Severity Index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR, hazard ratio; L-VVI, leadless-VVI; OR, odds ratio; TV-VVI, transvenous-VVI.
Sensitivity analysis
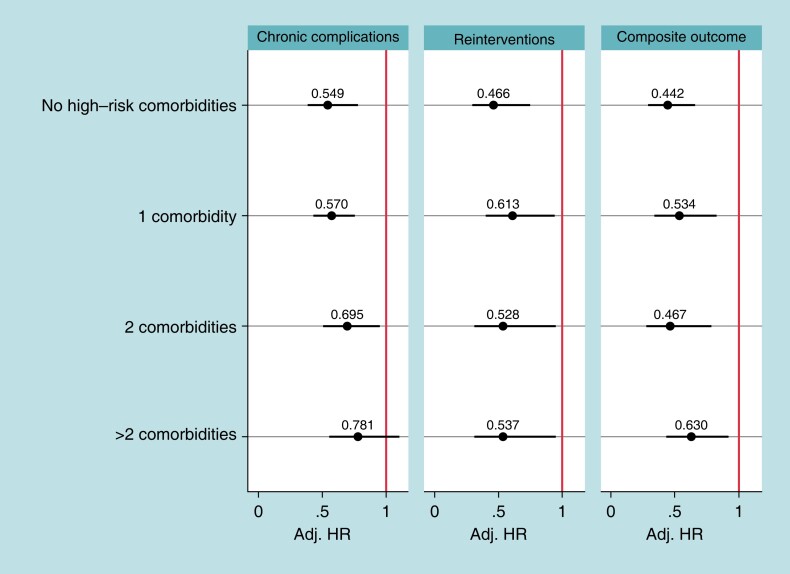
Table 1 includes the patient count by number of high-risk comorbidities. Most patients have only one comorbidity (N = 9928, 35.5%); 21.3% (N = 5976) of all eligible patients do not have any high-risk comorbidities. Patients with more high-risk comorbidities are more likely to have a leadless-VVI pacemaker than a transvenous-VVI pacemaker. Figure 4 shows the Hazard ratios comparing chronic complications, reinterventions, and the composite outcome between leadless-VVI patients and transvenous-VVI patients within each level of the number of high-risk comorbidities measure. Leadless-VVI patients that have 0, 1, and 2 high-risk comorbidities have between 30% and 45% lower chronic complications than transvenous-VVI patients [0 comorbidities: adjusted HR = 0.55, BA-CI (0.39–0.78); 1 comorbidity: adjusted HR = 0.57, BA-CI (0.43–0.76); 2 comorbidities: adjusted HR = 0.70, BA-CI (0.51–0.95)]. For device-related reinterventions and the composite outcome, at all levels of number of comorbidities, leadless-VVI patients have better outcomes than transvenous-VVI patients [reinterventions—0 comorbidities: adjusted HR = 0.47, BA-CI (0.29–0.75); 1 comorbidity: adjusted HR = 0.61, BA-CI (0.40–0.94); 2 comorbidities: adjusted HR = 0.53, BA-CI (0.30–0.93); > 2 comorbidities: adjusted HR = 0.54, BA-CI (0.31–0.95)]. Composite outcome—0 comorbidities: adjusted HR = 0.44, BA-CI (0.30–0.66); 1 comorbidity: adjusted HR = 0.53, BA-CI (0.34–0.84); 2 comorbidities: adjusted HR = 0.47, BA-CI (0.28–0.79); > 2 comorbidities: adjusted HR = 0.63, BA-CI (0.43–0.92).
Figure 4.
Sensitivity analysis results. Notes: Hazard ratios adjusted using overlapping weights. Confidence intervals adjusted for multiple comparisons (number of tests = 12). P-values of interactions are 0.0962 (chronic complications), 0.5408 (reinterventions), and 0.2319 (composite endpoint). High-risk comorbidities are chronic kidney disease Stages 4 and 5, chronic obstructive pulmonary disease, diabetes with low adapted Diabetes Complication Severity Index, diabetes with high adapted Diabetes Complication Severity Index, end-stage renal disease, malignancy with treatment, malignancy without treatment, and tricuspid valve disease. ESRD and CKD Stages 4 or 5 are not mutually exclusive. Conf. Int., confidence internal; HR, hazard ratio; L-VVI, leadless-VVI; TV-VVI, transvenous-VVI.
Discussion
This evaluation of comparative safety of leadless pacemakers shows that leadless-VVI patients with malignancy, diabetes, TVD, and COPD had fewer 2-year chronic complications, and leadless-VVI patients with diabetes, TVD, and COPD had significantly fewer 2-year reinterventions, compared to patients contemporaneously implanted with a transvenous-VVI. Leadless-VVI patients with malignancy, diabetes, TVD, and COPD had lower rates of the combined outcome of device complications and select reinterventions. Transvenous-VVI patients have a higher chronic complication or device-related reintervention rates than leadless-VVI patients in all high-risk subgroups. Adjusted all-cause mortality rates are not statistically different between leadless-VVI and transvenous-VVI patients in any high-risk subgroup. These results confirm the value of leadless-VVI pacemakers in high-risk groups, which is in line with the 2021 ESC guidelines that give a IIa indication in this setting.17 These results are also in line with previous results from other studies on leadless pacing using real world evidence2,4–6 and clinical trial data.1
The main results from this study show that many patients at higher risk of transvenous pacemaker complications have similar reductions in complications and device-related reinterventions associated with leadless pacing than the overall population, including those at higher risk of device infection (COPD, diabetes, malignancy).7 We also find that these reductions in complications that exist for high-comorbidity patients are extended to patients with lower risk of pacemaker complications: results from the sensitivity analysis show that the benefits of leadless pacing are not exclusive to patients with higher risk of complications, as leadless-VVI patients with no high-risk comorbidities also have fewer complications and device-related reinterventions than transvenous-VVI patients with no high-risk comorbidities.
Given the higher likelihood of transvenous pacing complications in patients with end-stage renal disease or receiving haemodialysis, it is expected that leadless pacing would have reduced complications in this population. However, in this subgroup, our study did not find a statistically significant difference in outcomes of leadless-VVI patients vs. transvenous-VVI patients. El-Chami et al.18 found a successful Micra implantation rate in patients in haemodialysis (98.0%), but no statistically significant differences in major complications 12 months after implant. In our study, the lack of statistical evidence of reduction in complications in ESRD patients from leadless pacing contrasts with the volume of ESRD patients that receive a leadless pacemaker: in the ESRD subgroup, three out of four patients receive a leadless-VVI pacemaker, and in the subgroup related to kidney disease, it was the pacemaker of choice for the majority of the patients. This may have been due to the fact that many ESRD patients in the leadless cohort would have been deemed precluded from transvenous pacing, which further contributes to the difficulty in comparing outcomes in this population. Prior research from Garg et al.10 has shown leadless pacing to be a safe and effective alternative for precluded patients, with low and comparable complications rates. Our present analysis, which compares ESRD patients who received a leadless pacemaker with ESRD patients deemed appropriate candidates to receive a transvenous pacemaker, likely underestimates the benefits of leadless pacing in this population. Despite the known benefits of larger sample sizes in observational analyses, the observed sample of ESRD and advanced kidney disease patients is small, particularly patients with transvenous-VVI devices, and our comparison of outcomes in this population is underpowered; more research is warranted for this population. In addition, some of the perceived benefits of leadless-VVI pacemaker in this group could be related to the lower rate of infection. Patients with ESRD on haemodialysis are at risk of recurrent bacteremia with gram positive organisms. Around 70% of patients with Staphylococcus bacteremia and transvenous implantable devices have evidence of endocardial device infection necessitating device removal. Data from the Micra IDE study showed that this is unlikely with leadless-VVI pacemaker.19 It is likely that the 2-year follow-up is not long enough to show this benefit.
Reducing pacemaker complications in high-risk patients is relevant for at least two important reasons: first, high-risk patients are more likely to have a complication; second, the complications in high-risk patients could have more severe outcomes than in the overall population, like pneumothorax in COPD patients or venous complications in diabetic or chronic kidney disease patients. Given the demonstrated benefits of leadless pacing in patients at high risk for pacemaker complications, efforts should be made to expand access to leadless pacing for these patients in places where it is not currently accessible to them, and to increase awareness of these benefits in order to eventually refer high-risk patients to tertiary centres that perform leadless pacing implantations when necessary.
Limitations
This study has several limitations. First, the measured outcomes could be missed, improperly coded, or inadequately documented in administrative claims; prior analyses suggest that this probability is low.9 Second, the overlap weights adjustment are unable to adjust for unobserved confounders that may be correlated with the decision of treating a patient with a leadless-VVI patients, and the study outcomes. However, the overlap weights include several comorbidities related to pacemaker complications, thus reducing the likelihood of bias. Third, there are several conditions that are associated with higher pacemaker complications (e.g. high BMI, previous device infection, cardiac valve replacements) that are not included in this study, as some of these measures are not consistently identifiable in claims data (BMI), not observable given the Micra CED research design (previous device infection), or inadequate sample size (cardiac valve replacements, or a close overlap with the overall sample for hypertension as 93% of the CED population has a history of hypertension). Fourth, a limited sample size of patients with ESRD or advanced chronic kidney disease limits our ability to compare complications in these subgroups. Fifth, our follow-up period is limited to two years, thus we are unable to observe long-term outcomes such as normal device battery depletion. Lastly, the results may not be generalizable to populations outside those eligible for Medicare Fee-For-Services population, particularly younger populations.
Conclusions
In a real-world study of U.S. Medicare patients, the leadless-VVI pacemaker is associated with fewer chronic complications and device-related reinterventions at 2 years compared with transvenous-VVI pacing in several populations with a higher risk of pacemaker complications with transvenous devices. These reductions in complications and reinterventions are also observed in patients without any comorbidity associated with a higher risk of pacemaker complications. These results indicate that patients at higher risk of pacemaker complications also benefit from leadless pacing. Expanded access to this therapy should be considered.
Supplementary Material
Acknowledgements
The authors thank Dedra Fagan, PhD, at Medtronic for assistance in the preparation of this manuscript.
Contributor Information
Serge Boveda, Clinique Pasteur, 45 Avenue de Lombez BP 27617, 31076 Toulouse Cedex 3, France.
Lucas Higuera, Medtronic, Inc., Minneapolis, MN, USA.
Colleen Longacre, Medtronic, Inc., Minneapolis, MN, USA.
Claudia Wolff, Medtronic International Trading Sàrl, Tolochenaz, Switzerland.
Kael Wherry, Medtronic, Inc., Minneapolis, MN, USA.
Kurt Stromberg, Medtronic, Inc., Minneapolis, MN, USA.
Mikhael F El-Chami, Emory University School of Medicine, Atlanta, GA, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported by Medtronic. (ClinicalTrials.gov ID: NCT03039712).
Data availability
The authors are not owners of the data set (data set is owned by the Centers for Medicare and Medicaid Services) and do not have the right to share the data.
References
- 1. Duray GZ, Ritter P, El-Chami M, Narasimhan C, Omar R, Tolosana JMet al. Long-term performance of a transcatheter pacing system: 12-month results from the Micra transcatheter pacing study. Heart Rhythm 2017;14:702–9. [DOI] [PubMed] [Google Scholar]
- 2. El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JPet al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–7. [DOI] [PubMed] [Google Scholar]
- 3. CMS Manual System . Pub 100-03 Medicare National Coverage Determinations. National Coverage Determination (NCD20.8.4): Leadless Pacemakers. Department of Health & Human Services, Centers for Medicare & Medicaid Services. (23 April 2021, date last accessed).
- 4. Piccini JP, El-Chami M, Wherry K, Crossley GH, Kowal RC, Stromberg Ket al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs transvenous single-chamber ventricular pacemaker. JAMA Cardiol 2021;6:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Chami MF, Bockstedt L, Longacre C, Higuera L, Stromberg K, Crossley Get al. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur Heart J 2022;43:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmisano P, Facchin D, Ziacchi M, Nigro G, Nicosia A, Bongiorni MGet al. Rate and nature of complications with leadless transcatheter pacemakers compared with transvenous pacemakers: results from an Italian multicentre large population analysis. Europace 2023;25:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. [DOI] [PubMed] [Google Scholar]
- 8. Tompkins C, McLean R, Cheng A, Brinker JA, Marine JE, Nazarian Set al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011;22:1099–104. [DOI] [PubMed] [Google Scholar]
- 9. Wherry K, Stromberg K, Hinnenthal JA, Wallenfelsz LA, El-Chami MF, Bockstedt L. Using Medicare claims to identify acute clinical events following implantation of leadless pacemakers. Pragmat Obs Res 2020;11:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg A, Koneru JN, Fagan DH, Stromberg K, Padala SK, El-Chami MFet al. Morbidity and mortality in patients precluded for transvenous pacemaker implantation: experience with a leadless pacemaker. Heart Rhythm 2020;17:2056–63. [DOI] [PubMed] [Google Scholar]
- 11. Tan CS, Jie C, Joe J, Irani ZD, Ganguli S, Kalva SPet al. The impact of transvenous cardiac devices on vascular access patency in hemodialysis patients. Semin Dial 2013;26:728–32. [DOI] [PubMed] [Google Scholar]
- 12. Wicke FS, Glushan A, Schubert I, Köster I, Lübeck R, Hammer Met al. Performance of the adapted diabetes complications severity index translated to ICD-10. Am J Manag Care 2019;25:e45–9. [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 14. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Statis Assoc 2018;113:390–400. [Google Scholar]
- 15. Li F, Thomas LE. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 2018;188:250–7. [DOI] [PubMed] [Google Scholar]
- 16. Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LMet al. Identification of heart failure events in Medicare claims: the atherosclerosis risk in communities (ARIC) study. J Card Fail 2016;22:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. [DOI] [PubMed] [Google Scholar]
- 18. El-Chami MF, Clementy N, Garweg C, Omar R, Duray GZ, Gornick CCet al. Leadless pacemaker implantation in hemodialysis patients. JACC: Clin Electrophysiol 2019;5:162–70. [DOI] [PubMed] [Google Scholar]
- 19. El-Chami MF, Soejima K, Piccini JP, Reynolds D, Ritter P, Okabe Tet al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol 2019;42:1105–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are not owners of the data set (data set is owned by the Centers for Medicare and Medicaid Services) and do not have the right to share the data.