Abstract
Aims
To evaluate the relationship between spatial heterogeneity of electrocardiographic repolarization and spatial heterogeneity of atrial depolarization with arrhythmic substrate represented by left ventricular fibrosis.
Methods and results
We assessed the associations of T- and P-wave morphology parameters analysed from the standard 12-lead electrocardiograms with left ventricular fibrosis in 378 victims of unexpected sudden cardiac death (SCD) who underwent medico-legal autopsy. Based on autopsy findings, the SCD victims were categorized into four different groups according to different stages of severity of left ventricular fibrosis (substantial fibrosis, moderate patchy fibrosis, scattered mild fibrosis, no fibrosis). T-wave and P-wave area dispersion (TWAd: 0.0841 ± 0.496, 0.170 ± 0.492, 0.302 ± 404, 0.296 ± 0.476, P = 0.008; PWAd: 0.574 ± 0.384, 0.561 ± 0.367, 0.654 ± 0.281, 0.717 ± 0.257, P = 0.011, respectively; low values abnormal), non-dipolar components of T-wave and P-wave morphology (T_NonDipolarABS: 0.0496 ± 0.0377, 0.0571 ± 0.0487, 0.0432 ± 0.0476, 0.0380 ± 0.0377, P = 0.027; P_NonDipolarABS: 0.0132 ± 0.0164, 0.0130 ± 0.0135, 0.0092 ± 0.0117, 0.0069 ± 0.00472, P = 0.005, respectively, high values abnormal), T-wave morphology dispersion (TMD: 45.9 ± 28.3, 40.5 ± 25.8, 35.5 ± 24.9, 33.0 ± 24.6, P = 0.030, respectively, high values abnormal), and P-wave heterogeneity (PWH: 20.0 ± 9.44, 19.7 ± 8.87, 17.9 ± 9.78, 15.4 ± 4.60, P = 0.019, respectively, high values abnormal) differed significantly between the groups with different stages of left ventricular fibrosis. After adjustment with heart weight, T_NonDipolarABS [standardized β (sβ) = 0.131, P = 0.014], PWAd (sβ = −0.161, P = 0.003), P_NonDipolarABS (sβ = 0.174, P = 0.001), and PWH (sβ = 0.128, P = 0.015) retained independent association, and TWAd (sβ = −0.091, P = 0.074) and TMD (sβ = 0.097, P = 0.063) tended to retain their association with the degree of myocardial fibrosis.
Conclusion
Our findings suggest that abnormal values of T- and P-wave morphology are associated with arrhythmic substrate represented by ventricular fibrosis partly explaining the mechanism behind their prognostic significance.
Keywords: Electrocardiography, T-wave, T-wave morphology, P-wave, P-wave morphology, Ventricular repolarization, Atrial depolarization, Myocardial fibrosis, Sudden cardiac death
What’s new.
The present observations suggest that T-wave morphology, representing spatial heterogeneity of ventricular repolarization, and P-wave morphology, representing spatial heterogeneity of atrial depolarization, are associated with ventricular fibrosis, a potential substrate for life-threatening ventricular tachyarrhythmias.
These findings may partly explain the mechanism behind the association of these parameters with the risk of sudden cardiac death.
Introduction
Sudden cardiac death (SCD) is the most common mode of death in western societies. Approximately 70–80% of these deaths are due to coronary artery disease (CAD). During the current treatment era, a vast majority of patients with CAD have well-preserved left ventricular function and numerically most of SCDs occur in these patients. Acute plaque complications may be a less frequent cause of SCD in CAD patients than previously thought suggesting the importance of arrhythmogenic milieu in myocardium as a cause for SCD in CAD patients, particularly during triggering factors, such as ischaemia.1 Substantial previous evidence has linked myocardial fibrosis with the risk of life-threatening ventricular tachyarrhythmias in CAD and dilated cardiomyopathy.2–4 Spatial heterogeneity of electrocardiographic repolarization, represented by T-wave morphology, has been shown to yield prognostic information, e.g. in patients with CAD, and to also be associated with the risk of SCD.5 P-wave abnormalities have been found to predict SCD in general population6 and patients with CAD.7 However, the mechanisms behind the association of spatial heterogeneity of electrocardiographic repolarization and atrial depolarization with the risk of SCD are not fully established. Therefore, we evaluated the association of T-wave morphology and P-wave morphology with the arrhythmic substrate represented by myocardial fibrosis determined in the autopsy of 378 victims of unexpected SCD.
Methods
Study population
The present study population was drawn from the Fingesture database, which consists of consecutive series of 5869 victims of unexpected SCD.8 The database was systemically collected in Northern Finland between the years 1998 and 2017. All of the subjects with SCD underwent a medico-legal autopsy, which was carried out at the Department of Forensic Medicine, University of Oulu, Oulu, Finland, and the National Institute for Health and Welfare, Oulu, Finland. According to Finnish law, a medico-legal autopsy is obligatory if the death occurs without pre-existing known disease or the death is unexpected (Act on the Inquest Into the Cause of Death, 459/1973, 7th paragraph: Finnish law). The used definition for SCD was that the death occurred within 6 h of the onset of the symptoms or if unwitnessed within 24 h when the victim was last seen alive and healthy.9 An experienced forensic pathologist performed the autopsies using contemporary guidelines. The other causes of sudden death than cardiac, such as pulmonary embolism, cerebral haemorrhage, etc., were excluded in autopsy. An evidence of acute coronary complication, defined as an acute intracoronary thrombus, plaque rupture or erosion, intraplaque haemorrhage, or critical stenosis (>75%) in major coronary artery or chronic atherosclerotic lesion with healed scar or fibrosis were used as criteria to classify SCD as ischaemic. Meticulous investigations of myocardium, coronary arteries, and valves were performed, and histological samples were taken from multiple sections of the heart in autopsy. Myocardial fibrosis was classified into four stages of severity: substantial fibrosis, moderate patchy fibrosis, scattered mild fibrosis, and no fibrosis (Figure 1). A very experienced specialist in forensic medicine performed the autopsies and the classification of myocardial fibrosis using standardized protocol. The determination of the degree of fibrosis was based on a comprehensive evaluation of myocardial fibrosis in macroscopic myocardial dissection and histological tissue samples from several myocardial sites. The median time between the last available electrocardiogram (ECG) prior to the event of SCD was 2 years (interquartile range, 0.28, 4.9 years). The autopsy reports, available medical and police reports, and systematic questionnaires for the family members were used to gather information on the SCD victims. A total of 378 SCD victims with good quality digitized 12-lead ECGs in sinus rhythm available from which both spatial heterogeneity of T-morphology and spatial heterogeneity of P-wave morphology could reliably be analysed were included in the present study. Premortem ECGs were recorded approximately on average 2 years prior to the SCD. The study was approved by the Ethics Committee of Northern Ostrobothnia Hospital District and complies with the Declaration of Helsinki.
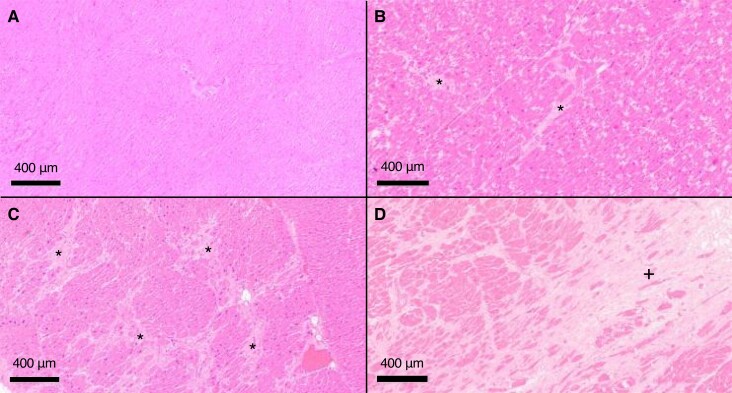
Figure 1.
Example histological myocardial samples representing four stages of fibrosis. No fibrosis (A), scattered mild fibrosis (B), moderate patchy fibrosis (C), and substantial fibrosis (D). *Fibrotic patches; +larger, uniform fibrosis. Haematoxylin-eosin stain.
Electrocardiography
In the present study, we analysed the standard 12-lead resting ECGs of 378 victims of unexpected SCD. The antemortem ECGs of the study SCD victims were previously recorded and available in their health records. The resting 12-lead ECGs with a paper speed of 50 mm/s were analysed in digital mode. All the study subjects were in sinus rhythm and the analysis was made from five consecutive beats. No premature contractions were included in the measured segment.
Analysis of T-wave morphology and P-morphology parameters
Both T-wave morphology and P-wave morphology parameters were analysed automatically from the 12-lead ECGs by using custom-made software. The starting and ending points of the analysed parameters were visually double-checked. T-wave and P-wave area dispersion (TWAd, PWAd, respectively) were obtained from leads I, II, V4–V6. The T-wave area and P-wave area under baseline were considered negative. The average T-wave area and the average P-wave area were divided by the maximum absolute T-wave area and by the maximum absolute P-wave area, respectively, within leads I, II, V4–V6. T-wave area dispersion and PWAd may get values between 1 and −1, and the lower the value the larger the heterogeneity between the leads. T-wave area dispersion and PWAd describe the dispersion of the T-wave area and P-wave area, respectively, between the leads I, II, V4–V6, i.e. spatial heterogeneity of electrocardiographic repolarization and spatial heterogeneity of electrocardiographic atrial depolarization (see Supplementary material online, Figure S1).10 Non-dipolar components of T-wave and P-wave morphology (T_NonDipolarABS and P_NonDipolarABS, respectively) describe the absolute value of the five (from fourth to eighth) singular value components (non-dipolar components) of the T-wave loop and the P-wave loop, respectively.11 Higher values indicate increased spatial heterogeneity of electrocardiographic repolarization and spatial heterogeneity of electrocardiographic atrial depolarization. In T-wave and P-wave morphology dispersion (TMD and PMD, respectively), the average angle between all reconstruction vector pairs in the T-wave loop and the P-wave loop, respectively, is calculated.12 Higher values indicate increased heterogeneity of T-wave morphology and P-wave morphology, respectively (see Supplementary material online, Figure S2). T-wave and P-wave heterogeneity (TWH and PWH, respectively) were determined by measuring the splay of waveforms around the first moment, i.e. around the average waveform of T-wave and P-wave calculated from different leads using second central moment analysis.13 Higher values indicate increased spatial heterogeneity of T-wave morphology and P-wave morphology, respectively.
Statistical analysis
The statistical significance of the differences in continuous variables between the four groups with different stages of myocardial fibrosis was analysed using the one-way analysis of variance. If a parameter differed significantly between the four groups, the statistical significances of the differences between the individual groups were assessed by the least significant difference (LSD) post hoc test. The χ2 test was used to analyse the statistical significance of differences between categorical variables. A regression analysis model was used to evaluate the influence of heart weight on the association of T-morphology and P-morphology parameters with myocardial fibrosis.
Results
The present study population consisted of 378 victims of unexpected SCD. Based on the available data, a total of 96 (25%) of SCD victims of the present analysis had a history of diabetes mellitus, 27 (7%) had a history of acute myocardial infarction, and 26 (7%) had a history of angina pectoris. The mean age of the SCD victims was 65.4 ± 11.4 years. The age did not differ significantly between the four groups of SCD victims with different stages of myocardial fibrosis (Table 1). There were more males in the groups with increasing stages of fibrosis. Based on the autopsy findings, the cause of SCD in 66.5% of the cases was ischaemic heart disease and, in the rest of the cases, the cause was non-ischaemic comprising cardiomyopathies. The proportion of ischaemic heart disease did not differ significantly between the groups with different stages of myocardial fibrosis (Table 1). Heart weight was significantly different between the groups classified according to the severity of myocardial fibrosis. The groups with substantial fibrosis and with moderate patchy myocardial fibrosis had significantly larger heart weight compared with the groups with scattered mild or no myocardial fibrosis (Table 1).
Table 1.
Characteristics of SCD victims in the present study
| Variable | MF | MF | MF | MF | P-value |
|---|---|---|---|---|---|
| Substantial (n = 47) | Moderate patchy (n = 126) | Scattered mild (n = 155) | No (n = 50) | ||
| Age, years | 66.7 ± 11.7 | 64.9 ± 11.6 | 65.8 ± 10.8 | 64.3 ± 13.0 | 0.68 |
| Male gender (%) | 85.1 | 81.0 | 71.0 | 64.0 | 0.023 |
| IHD (%) | 78.7 | 64.3 | 63.2 | 70.0 | 0.22 |
| Heart weighta, g | 534 ± 157 | 490 ± 122 | 435 ± 118 | 435 ± 106 | <0.001 |
| RR_std, ms | 18 ± 24 | 21 ± 38 | 18 ± 29 | 18 ± 27 | 0.86 |
The values are mean ± SD or percentages.
IHD, ischaemic heart disease based on autopsy findings; MF, myocardial fibrosis; RR_std, the RR interval standard deviation measured from the 12-lead ECG; SCD, sudden cardiac death; SD, standard deviation.
In the Bonferroni post hoc test: P < 0.001 between substantial vs. scattered mild stage of fibrosis and between substantial vs. no fibrosis; and P < 0.01 and <0.05 between moderate patchy vs. scattered mild and between moderate patchy vs. no fibrosis, respectively.
Association of T-wave morphology and P-wave morphology parameters with myocardial fibrosis
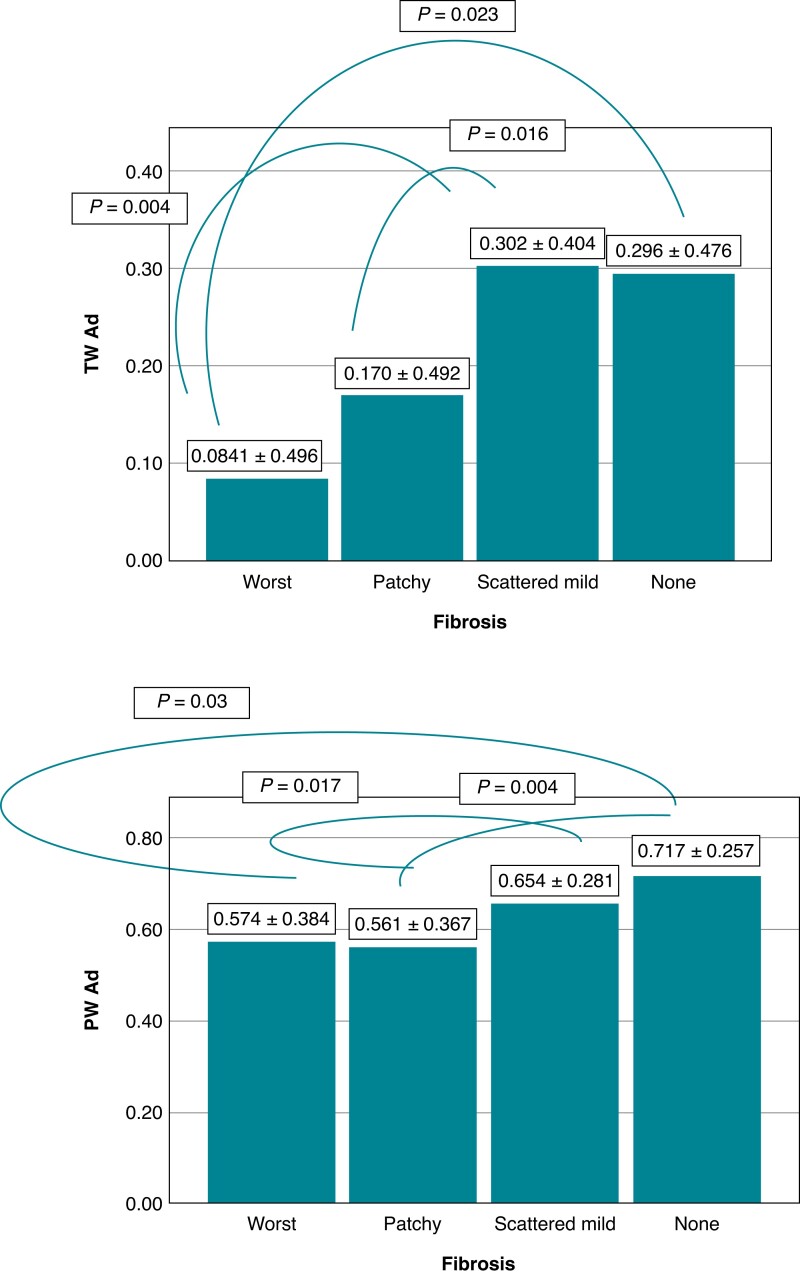
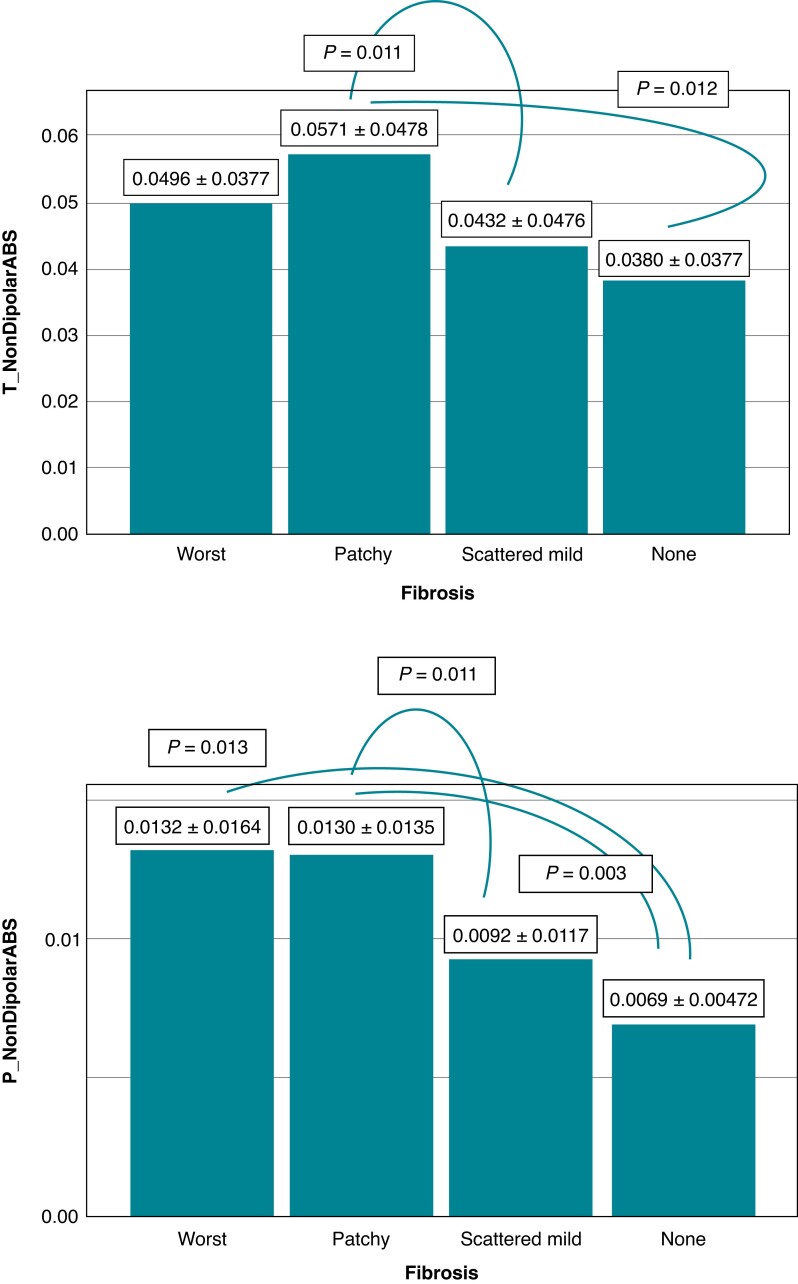
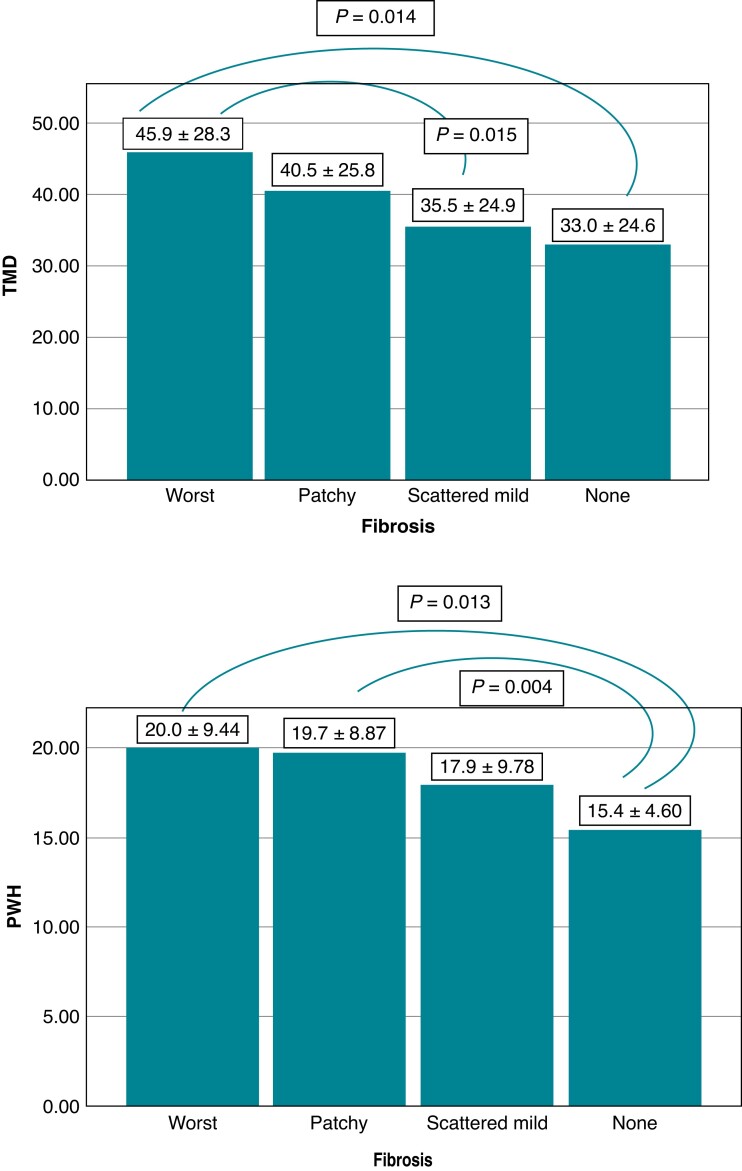
T-wave area dispersion and PWAd differed significantly between the SCD victim groups with different stages of myocardial fibrosis (P = 0.008 and P = 0.011, respectively). Those with substantial myocardial fibrosis had lower values of TWAd and PWAd indicating an association of increased spatial heterogeneity of myocardial repolarization and increased spatial heterogeneity of atrial depolarization, respectively, with myocardial fibrosis (Figure 2). T_NonDipolarABS and P_NonDipolarABS, larger values representing increased spatial heterogeneity of ventricular repolarization and atrial depolarization, respectively, differed significantly between the groups classified based on the severity of myocardial fibrosis (P = 0.027 and P = 0.005, respectively) (Figure 3). There was a significant difference in TMD and PWH between the groups with different stages of myocardial fibrosis (P = 0.030 and P = 0.019, respectively) (Figure 4). Larger values of TMD and PWH indicate increased spatial inhomogeneity of ventricular repolarization and increased spatial inhomogeneity of atrial depolarization, respectively. The values of TWH and PMD (high values abnormal) did not differ significantly between the SCD victim groups with different stages of severity of myocardial fibrosis (worst, patchy, scattered mild, and none) (85.2 ± 58.4, 87.2 ± 68.8, 89.6 ± 81.3, 75.1 ± 46.0, P = 0.66; and 42.5 ± 15.6, 44.2 ± 17.0, 44.2 ± 16.8, 39.4 ± 14.6, P = 0.28, respectively).
Figure 2.
TWAd and PWAd (low values abnormal) differed significantly between the SCD victim groups with different stages of myocardial fibrosis (P = 0.008 and P = 0.011, respectively). (Post hoc LSD P-values are shown in figures.) The values are mean ± SD. Abbreviations: none, no fibrosis; patchy, moderate patchy fibrosis; PWAd, P-wave area dispersion; SCD, sudden cardiac death; scattered mild, scattered mild fibrosis; SD, standard deviation; TWAd, T-wave area dispersion; worst, substantial fibrosis.
Figure 3.
Non-dipolar components of T-wave and P-wave morphology (T_NonDipolarABS, P_NonDipolarABS), larger values representing increased spatial heterogeneity of ventricular repolarization and atrial depolarization, respectively, differed significantly between the groups classified based on the severity of myocardial fibrosis (P = 0.027 and P = 0.005, respectively). (Post hoc LSD P-values are shown in figures.) The values are mean ± SD. Abbreviations: none, no fibrosis; patchy, moderate patchy fibrosis; scattered mild, scattered mild fibrosis; SD, standard deviation; worst, substantial fibrosis.
Figure 4.
TMD (degrees) and PWH (high values abnormal) differed significantly between the SCD victim groups with different stages of myocardial fibrosis (P = 0.030 and P = 0.019, respectively). (Post hoc LSD P-values are shown in figures.) The values are mean ± SD. Abbreviations: none, no fibrosis; patchy, moderate patchy fibrosis; PWH, P-wave heterogeneity; scattered mild, scattered mild fibrosis; SD, standard deviation; TMD, T-wave morphology dispersion; worst, substantial fibrosis.
Heart weight had a direct association with the degree of myocardial fibrosis (β = 0.262, P < 0.001). After adjustment with heart weight, T_NonDipolarABS [standardized β (sβ) = 0.131, P = 0.014], PWAd (sβ = −0.161, P = 0.003), P_NonDipolarABS (sβ = 0.174, P = 0.001), and PWH (sβ = 0.128, P = 0.015) remained independently associated with the degree of myocardial fibrosis, and TWAd (sβ = −0.091, P = 0.074) and TMD (sβ = 0.097, P = 0.063) tended to retain their association with the degree of myocardial fibrosis. Several studied T-wave morphology and P-wave morphology parameters had significant correlations with each other (see Supplementary material online, Table S1). The RR interval (RR) standard deviation (std) (RR_std) measured from the 12-lead ECG did not differ significantly between the groups with different degrees of myocardial fibrosis (Table 1) or did not have any significant logical association with the T-wave morphology or P-wave morphology parameters (see Supplementary material online, Table S1).
The statistical power of the subanalysis of the association of the T-wave morphology and P-wave morphology parameters with myocardial fibrosis in SCD victims with ischaemic (n = 251) and non-ischaemic (n = 127) cause is limited, because of the relatively small number of SCD victims in these subgroups. Nevertheless, the T-wave morphology and P-wave morphology parameters retained a similar association with ventricular fibrosis in these subgroups as in the whole group of SCD victims (see Supplementary material online, Figures S3–S6).
Discussion
In the present study of SCD victims, we found that T-wave morphology, an indicator of spatial heterogeneity of ventricular repolarization, and P-wave morphology, an indicator of spatial heterogeneity of atrial depolarization, were associated with myocardial fibrosis. Ventricular fibrosis generates an arrhythmic substrate and predisposes to life-threatening ventricular tachyarrhythmias.2–4 These observations partly explain the mechanism behind the prognostic significance of T-wave morphology and P-wave morphology.
Increased spatial heterogeneity of T-wave morphology has been shown to yield prognostic information and to predict SCD in general population10 and in patients with CAD.5 Conventional and sophisticated measurements of P-wave abnormalities have been found to predict SCD in general population6,14 and in patients with CAD.7 In the present study, all the P-wave morphology parameters (PWAd, P_NonDipolarABS and PWH) that had a univariate association with myocardial fibrosis retained the independent association after adjustment with heart weight. Of T-wave morphology parameters, only T_NonDipolarABS remained significantly associated with ventricular fibrosis after adjustments. Theoretically, it may be speculated that the spatial heterogeneity of electrocardiographic atrial depolarization may have a somewhat closer independent association with myocardial fibrosis than the spatial heterogeneity of electrocardiographic ventricular repolarization. The association of P-wave morphology with myocardial fibrosis is well in line with recent findings that the variability of P-wave non-dipolar component, particularly when combined with a marker of autonomic cardiac regulation, was relatively accurately, independently, and specifically associated with the risk of SCD in patients with CAD.15 In the present study, several studied T-wave morphology and P-wave morphology parameters were interrelated. The RR_std did not interrelate with the groups with different degrees of myocardial fibrosis or did not have any significant logical interrelation with the T-wave morphology or P-wave morphology parameters supporting the concept that cardiac autonomic regulation and descriptors of ventricular fibrosis, representing arrhythmic substrate, may yield independent prognostic information. Recent data also suggest that acute plaque complications are a less common cause of SCD in CAD patients than previously believed,1 an observation that further underlines the importance of arrhythmic substrate with triggering factors, such as ischaemia and disturbed cardiac autonomic regulation, as a potential cause of SCD without acute coronary syndrome. In the present study, the autopsy-based cause of SCD was ischaemic heart disease with or without plaque complication in the majority of cases. A previous study has shown that some conventional QRS-complex-based parameters and T-inversions were also associated with myocardial fibrosis.16 Our present findings extend these observations to sophisticated measurements of spatial heterogeneity of electrocardiographic ventricular repolarization and spatial heterogeneity of electrocardiographic atrial depolarization.
In a study using cardiac magnetic resonance imaging with late gadolinium enhancement, P-terminal force and P-wave duration were shown to be associated with left ventricular fibrosis.17 Our present findings extend these observations by providing direct evidence that newer P-wave parameters describing spatial heterogeneity of atrial depolarization and myocardial fibrosis are linked. Some other indirect evidence also supports this concept. We have previously observed that the measurements of P-wave spatial heterogeneity have a significant correlation with soluble ST2 and brain natriuretic peptide that are biomarkers that have an association with cardiac fibrosis.7
In ischaemic heart disease, myocardial fibrosis often occurs in the setting of an ischaemic scar, whereas in various types of cardiomyopathies, ventricular fibrosis is usually more diffuse.18 Both in ischaemic heart disease and cardiomyopathies, myocardial fibrosis increases the vulnerability to life-threatening ventricular tachyarrhythmias.2–4 In the present analysis, although the subgroups were relatively small, a similar association was found between T-wave morphology and P-wave morphology and myocardial fibrosis, an observation that suggests that electrocardiographic spatial heterogeneity of repolarization and spatial heterogeneity of atrial depolarization may serve as indicators of arrhythmic substrate represented by ventricular fibrosis, both in ischaemic heart disease and in cardiomyopathies.
There were some limitations in our study. The ECGs suitable for the analysis of the T-wave morphology and P-wave morphology parameters were available only in a minority of the SCD victims and the ECGs were recorded on average 2 years prior to SCD. Myocardial fibrosis was classified into four main categories, but a more detailed subclassification was not available. However, a very experienced specialist in forensic medicine performed the autopsies and the classification of myocardial fibrosis was based on a thorough evaluation as described in the Methods section.
Conclusions
Our present findings suggest that the novel T-wave morphology and P-wave morphology parameters, representing spatial heterogeneity of ventricular repolarization and spatial heterogeneity of atrial depolarization, respectively, are associated with myocardial fibrosis. This may partly explain the mechanism behind their prognostic significance and their relation to the risk of SCD.
Supplementary Material
Contributor Information
Jenni J Hekkanen, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Tuomas V Kenttä, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Lauri Holmström, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Mikko P Tulppo, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Olavi H Ukkola, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Lasse Pakanen, Forensic Medicine Unit, Finnish Institute for Health and Welfare, Hoitajanrinne 1, P.O. Box 310, FI-90101 Oulu, Finland; Department of Forensic Medicine, Medical Research Center Oulu, Research Unit of Internal Medicine, University of Oulu, Aapistie 5B, P.O. Box 5000, FI-90014 Oulu, Finland.
M Juhani Junttila, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Heikki V Huikuri, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Juha S Perkiömäki, Research Unit of Internal Medicine, Division of Cardiology, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, P.O. Box 5000, Kajaanintie 50, 90014 Oulu, Finland.
Supplementary material
Supplementary material is available at Europace online.
Funding
The study was supported by a grant from the Finnish Foundation for Cardiovascular Research, the Yrjö Jahnsson Foundation, the Sigrid Jusélius Foundation, and the Paavo Nurmi Foundation.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Holmström L, Juntunen S, Vähätalo J, Pakanen L, Kaikkonen K, Haukilahti MAEet al. Plaque histology and myocardial disease in victims of sudden cardiac death due to coronary artery disease: the Fingesture study. Eur Heart J 2022:ehac533. (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Disertori M, Rigoni M, Pace N, Casolo G, Mase M, Gonzini Let al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1046–55. [DOI] [PubMed] [Google Scholar]
- 3. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JAet al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 4. Zegard A, Okafor O, de Bono J, Kalla M, Lencioni M, Marshall Het al. Greyzone myocardial fibrosis and ventricular arrhythmias in patients with a left ventricular ejection fraction >35. Europace 2022;24:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirkola JM, Konttinen M, Kenttä TV, Holmström LTA, Junttila MJ, Ukkola OHet al. Prognostic value of T-wave morphology parameters in coronary artery disease in current treatment era. Ann Noninvasive Electrocardiol 2018;23:e12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tereshchenko LG, Henrikson CA, Sotoodehnia N, Arking DE, Agarwal SK, Siscovick DSet al. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc 2014;3:e001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nortamo S, Laitinen I, Passi J, Tulppo M, Ukkola OH, Junttila Jet al. Prognostic significance of P-wave morphology in patients with coronary artery disease. J Cardiovasc Electrophysiol 2019;30:2051–60. [DOI] [PubMed] [Google Scholar]
- 8. Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation 2006;114:1462–7. [DOI] [PubMed] [Google Scholar]
- 9. Hinkle T. Clinical classification of cardiac deaths. Circulation 1982;65:457–64. [DOI] [PubMed] [Google Scholar]
- 10. Kenttä TV, Sinner MF, Nearing BD, Freudling R, Porthan K, Tikkanen JTet al. Repolarization heterogeneity measured with T-wave area dispersion in standard 12-lead ECG predicts sudden cardiac death in general population. Circ Arrhythm Electrophysiol 2018;11:e005762. [DOI] [PubMed] [Google Scholar]
- 11. Zabel M, Malik M, Hnatkova K, Papademetriou V, Pittaras A, Fletcher RDet al. Analysis of T-wave morphology from the 12-lead electrocardiogram for prediction of long-term prognosis in male US veterans. Circulation 2002;105:1066–70. [DOI] [PubMed] [Google Scholar]
- 12. Acar B, Yi G, Hnatkova K, Malik M. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput 1999;37:574–84. [DOI] [PubMed] [Google Scholar]
- 13. Nearing BD, Verrier RL. Tracking cardiac electrical instability by computing interlead heterogeneity of T-wave morphology. J Appl Physiol 2003;95:2265–72. [DOI] [PubMed] [Google Scholar]
- 14. Laitinen I, Kenttä TV, Passi J, Haukilahti MAE, Eranti A, Holkeri Aet al. Prognostic value of P-wave morphology in general population. Europace 2023;25:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hekkanen JJ, Kenttä TV, Tulppo MP, Kiviniemi AM, Ukkola OH, Junttila MJet al. Association of atrial depolarization variability and cardiac autonomic regulation with sudden cardiac death in coronary artery disease. Europace 2022;24:1942–51. [DOI] [PubMed] [Google Scholar]
- 16. Holmström L, Haukilahti A, Vähätalo J, Kenttä T, Appel H, Kiviniemi Aet al. Electrocardiographic associations with myocardial fibrosis among sudden cardiac death victims. Heart 2020;106:1001–6. [DOI] [PubMed] [Google Scholar]
- 17. Win TT, Venkatesh BA, Volpe GJ, Mewton N, Rizzi P, Sharma RKet al. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: the PRIMERI study. Heart Rhythm 2015;12:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jellis C, Martin J, Narula J, Marwick TH. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol 2010;56:89–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.