Abstract
Aims
Late gadolinium enhancement cardiac magnetic resonance (CMR) permits characterization of left ventricular ischaemic scars. We aimed to evaluate if scar core mass, border zone (BZ) mass, and BZ channels are risk markers for subsequent ventricular arrhythmia (VA) in ST-segment elevation myocardial infarction (STEMI).
Methods and results
A sub-study of the DANish Acute Myocardial Infarction-3 multi-centre trial and Danegaptide phase II proof-of-concept clinical trial in which a total of 843 STEMI patients had a 3-month follow-up CMR. Of these, 21 patients subsequently experienced VA during 100 months of follow-up and were randomly matched 1:5 with 105 controls. A VA event was defined as: ventricular tachycardia, ventricular fibrillation, or sudden cardiac death. Ischaemic scar characteristics were automatically detected by specialized software. We included 126 patients with a median left ventricular ejection fraction of 51.0 ± 11.6% in cases with VA vs. 55.5 ± 8.5% in controls (P = 0.10). Cases had a larger mean BZ mass and more often BZ channels compared to controls [BZ mass: 17.2 ± 10.3 g vs. 10.3 ± 6.0 g; P = 0.0002; BZ channels: 17 (80%) vs. 44 (42%); P = 0.001]. A combination of ≥17.2 g BZ mass and the presence of BZ channels was five times more prevalent in cases vs. controls (P ≤ 0.00001) with an odds ratio of 9.40 (95% confidence interval 3.26–27.13; P ≤ 0.0001) for VA. This identified cases with 52% sensitivity and 90% specificity.
Conclusion(s)
Scar characterization with CMR indicates that a combination of ≥17.2 g BZ mass and the presence of BZ channels had the strongest association with subsequent VA in STEMI patients.
ClinicalTrials.gov
Unique identifier: NCT01435408 (DANAMI 3-iPOST and DANAMI 3-DEFER), NCT01960933 (DANAMI 3-PRIMULTI), and NCT01977755 (Danegaptide).
Keywords: Cardiac magnetic resonance, Late gadolinium enhancement, Scar, ST-segment elevation myocardial infarction, Ventricular arrhythmia
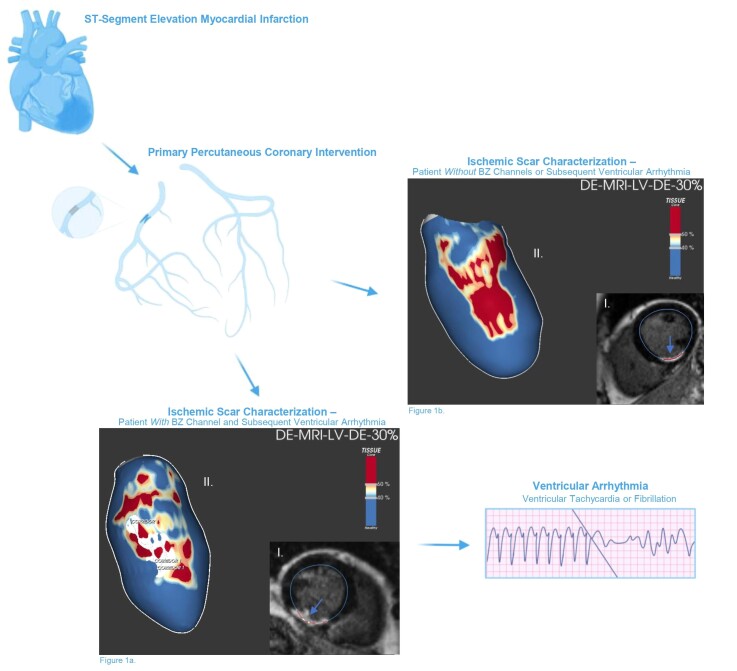
Graphical Abstract
Graphical Abstract.
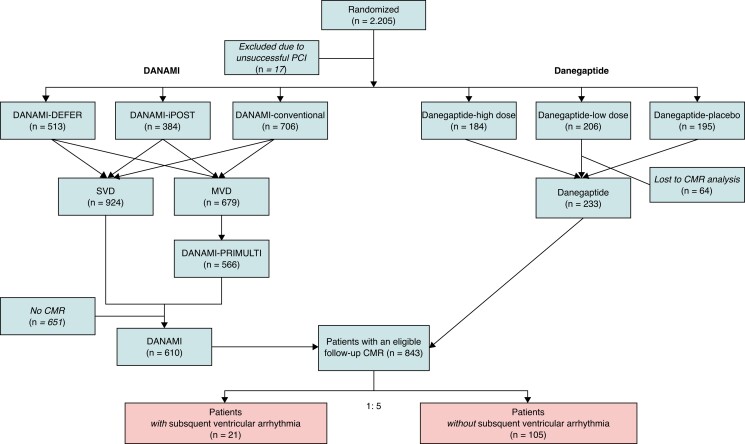
Flowchart in the study population from ST-segment myocardial infarction to arrhythmic endpoint.
What’s new?
Scar characterization with late gadolinium enhancement cardiac magnetic resonance indicates that a combination of ≥17.2 g border zone mass and the presence of border zone channels has the strongest association with subsequent ventricular arrhythmia in ST-segment elevation myocardial infarction.
Individual scar characteristics, including total scar mass, border zone mass, and the presence of border zone channels themselves, were also associated with subsequent ventricular arrhythmia.
Introduction
Advances in both medical and interventional strategies have improved outcomes in patients presenting with ST-segment elevation myocardial infarction (STEMI). However, morbidity and mortality remain substantial.1 Risk stratification of scar-related ventricular arrhythmia (VA) after MI is critical, but currently difficult. Reduced left ventricular ejection fraction (LVEF) is considered the gold standard marker for identifying patients at arrhythmogenic risk post-STEMI.2 It is the main indication for prophylactic implantable cardioverter defibrillator (ICD).2 However, a universal measure of cardiac function does not give direct information about the underlying arrhythmic substrate, and the accuracy in predicting VA in STEMI remains suboptimal.3 Consequently, a substantial number of patients with significant VA post-STEMI, including sudden cardiac death (SCD), do not receive an ICD, and a significant proportion of patients with a prophylactic ICD may never experience VA or relevant ICD therapy.
A detailed characterization of the ischaemic scar and its components, core mass, border zone (BZ) mass, and BZ channels (corridors of BZ that cross core scar and connect two areas of healthy tissue), can be assessed after STEMI using a novel late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) technique, and individual components have been shown to be potential risk markers of future arrhythmic events.4 BZ channels may correlate well with the electrical substrate of slow conductivity or VA isthmuses identified with voltage mapping during scar-related VA ablation procedures and consequently correspond with the arrhythmogenic substrate of the scar.5 However, definite BZ characteristics and their association with VA are currently unclear.
In this retrospective LGE-CMR sub-study of two STEMI populations, we hypothesized that detailed arrhythmogenic BZ substrate characterization 3 months after STEMI could identify risk markers for subsequent VA and may therefore contribute to risk stratification in patients surviving a STEMI.
Methods
Study design
This study is a retrospective, nested, case-control sub-study of the DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction (DANAMI-3) multicentre trial6 and Danegaptide phase II proof-of-concept clinical trial.7
The DANAMI-3 trial investigated the effect of three treatment modalities namely deferred stenting (DANAMI 3-Deferred Stenting vs. Conventional Primary Percutaneous Coronary Intervention), ischaemic post-conditioning (DANAMI 3-Ischaemic Post-Conditioning vs. Conventional Primary Percutaneous Intervention), and complete revascularization of multi vessel disease in patients with STEMI (DANAMI 3-Complete vs. Culprit-Only Revascularization to Treat Multivessel Disease After Early PCI for STEMI). A total of 1.620 patients were admitted for primary percutaneous coronary intervention (PCI) treatment at Rigshospitalet, Copenhagen University Hospital, from 2011 to 2014, and randomized in the trial. A subset had CMR performed acutely and after 3 months.
The Danegaptide trial evaluated the effect of a suggested cardio protective compound, Danegaptide, on myocardial salvage in patients with STEMI. From 2013 to 2015, a total of 585 patients were admitted for primary PCI and randomly enrolled in the study at Rigshospitalet, Copenhagen University Hospital.
The trials were approved by the Central Danish Ethics Committee and were performed in accordance with the Declaration of Helsinki.
Thus, a total of 2.205 patients were randomized (DANAMI n = 1.620, Danegaptide n = 585), of which 17 patients were excluded due to unsuccessful PCI (Figure 1). Of 843 patients with a 3-month follow-up CMR, a total of 22 patients subsequently experienced VA. One patient was excluded due to poor image quality. Thus, 21 patients were randomly matched 1:5 with 105 controls without subsequent VA, who constituted the study population. Matching was performed using following variables: LVEF, infarct location, and revascularization status in patients with multi-vessel disease. We did matching to reduce the risk of confounding and based matching variables on their pre-established prognostic value in terms of hard end points.8,9 The revascularization status was defined at the time of STEMI. Non-revascularized lesions were considered angiographically significant if at least 70% of the vessel diameter on visual estimation was stenotic or showed 50–69% stenosis together with a fractional flow reserve (FFR) measurement of 0.80 or less.
Figure 1.
Flowchart of the study population (n = 126).
Study population
Patients presented with chest pain lasting ≤12 h, acute electrocardiographic ST elevation, and were treated guided by contemporary international guidelines,1 in addition to the experimental treatment.
Detailed description of the DANAMI-3 studies has been previously published.6,7,10–12 Briefly, three different treatment strategies in STEMI were tested [(i) immediate stenting vs. deferred strategy of stent implantation, (ii) mechanical post-conditioning vs. conventional primary PCI, and (iii) FFR-guided complete revascularization or culprit only revascularization]. Patients included in Danegaptide had thrombolysis in myocardial infarction (TIMI) flow 0–1, had single vessel disease, and were tested in a clinical proof-of-concept study with the therapeutic potential of Danegaptide at two-dose levels administered at the time of primary PCI.
Cardiovascular magnetic resonance
The CMR protocols for DANAMI-3 and Danegaptide have previously been described.13 In brief, a 1.5 T scanner was used (Avanto or Espree scanner; Siemens, Erlangen, Germany), and infarcted myocardium was directly quantified via LGE. As earlier studies have shown that the infarction reaches its final size after approximately 30 days, this sub-study only includes patients with a 3-month follow-up CMR scan.
Detection of arrhythmic substrate
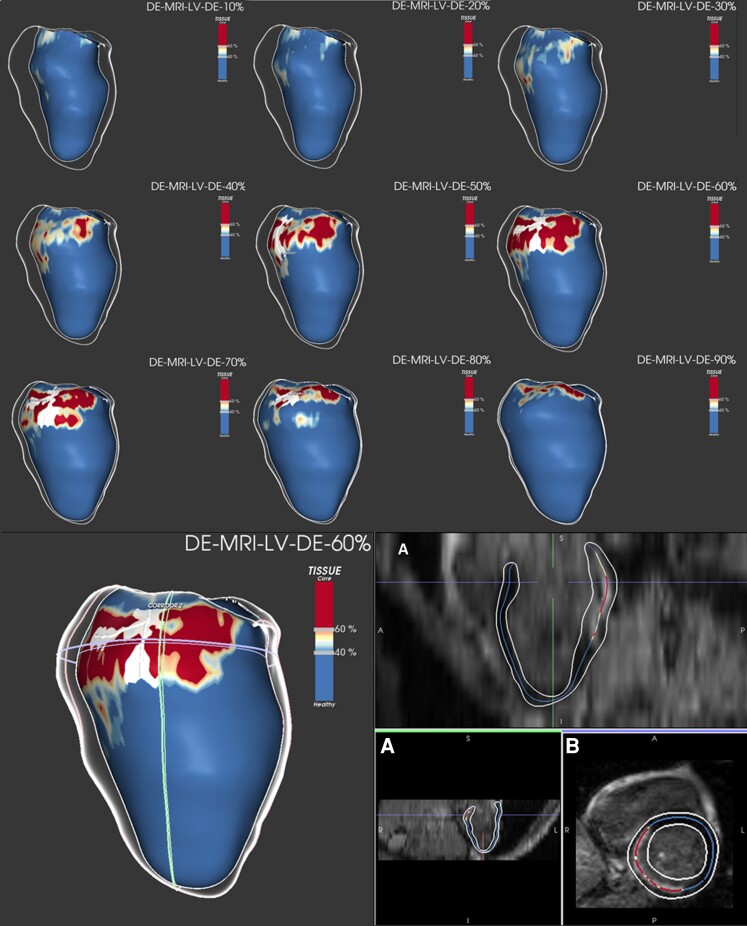
All image data analysis was performed at the Cardiac MRI Research Unit, Rigshospitalet, using the ADAS 3D investigational software tool (ADAS 3D, Galgo Medical SL, Barcelona, Spain), as previously described.14 The LGE-CMR imaging post-processing is illustrated in Figure 2. In brief, myocardial-fibrotic tissue, identified by gadolinium enhancement, was divided into core and BZ using an algorithm based on maximum pixel signal intensity (MPSI). The term BZ was used since only grey zone scar around the core scar was quantified. The core was defined as a region with signal intensity >60% of MPSI in the scar area, whereas BZ was defined as the region with signal intensity <60% and >40% of MPSI. A BZ channel was automatically defined as a corridor of BZ connecting two areas of normal myocardium flowing between two core areas or between a core area and a valve annulus.15
Figure 2.
LGE-CMR imaging post-processing with ADAS 3D software in a STEMI patient with BZ channel identification. Upper panel: LGE-CMR reconstruction of the LV showing an inferobasal scar of a STEMI patient (core in red, BZ in yellow, and healthy myocardium in blue). A white line is drawn over the surface, representing the BZ channel. The substrate progress is seen through different layers, from the endocardium (10–40%) to the epicardium (50–90%), with a defined BZ channel in different layers (50–80%). Lower left panel: LGE-CMR reconstruction of the same patient with the substrate seen from the epicardium (layer 60%). Lower right panel: LGE-CMR raw images in long (A) and short axis (B) used by the software to detect scar, core, BZ, and BZ channels (core in red, BZ in yellow, and healthy myocardium in blue). BZ, border zone; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance; LV, left ventricle; STEMI, ST-segment elevation myocardial infarction;
The mean time of one image analysis (including image import and segmentation) was 20 min (range 15–40 min).
Study end points
The primary endpoint was the presence of BZ tissue and BZ channels and the association with subsequent VA 3 month after STEMI. A VA event was defined as composite arrhythmic event endpoint that included: sustained ventricular tachycardia (VT) lasting more than 30 s, ventricular fibrillation (VF), or SCD defined as death occurring unexpectedly in an otherwise stable subject last seen alive ≤24 h previously, with circumstances suggestive of sudden death.16 The secondary endpoints were cardiovascular and all-cause mortality. All events were validated through medical record review, and the exact clinical presentation of each event is specified in the results section. All patients in the control group went through medical record review to make sure none of them had a VA event and to verify the type of death. Patients were followed from the time of the 3-month follow-up CMR scan until the end point of interest, death, or end of the study, whichever came first.
Statistics
Demographic, procedural, and end point characteristics are presented as mean ± standard deviation for continuous variables and frequencies and percentages for categorical variables. The Wilcoxon rank-sum test was used to evaluate continuous variables and the χ2 test or Fisher’s exact test (when appropriate) for categorical values. Missing values are reported. The odds ratios (ORs) of VA were analysed in five separate uni- and multivariable logistic regression models for scar (model 1), core (model 2), and BZ mass (model 3), the presence of BZ channels (model 4), and the combination of a cutpoint of ≥17.2 g BZ mass [evaluated with receiver operating curves (ROCs)] and the presence of BZ channels (model 5). Only scar characteristics were significant in the univariate analyses (P-value ≤ 0.05); thus, we adjusted each of the five models for LVEF ≤ 35% and left ventricular (LV) mass because of their established prognostic value. In order to analyse the effect of scar mass and the presence of BZ channels on VA, we did a two-way analysis of variance (ANOVA) and three multivariable logistic regression models for the presence of BZ channels adjusted for scar mass, LVEF ≤ 35%, and LV mass (model 1); core mass, LVEF ≤ 35%, and LV mass (model 2); and BZ mass, LVEF ≤ 35%, and LV mass (model 3). We did separate models since core and BZ mass depend on scar mass. ROCs were performed to estimate the predictive value of scar variables and to identify cutpoints of interest. There was no identifiable optimal cutpoint for LVEF. A sensitivity analysis on VA and all-cause mortality was performed in a randomly selected subset of excluded patients without a 3-month follow-up CMR (n = 345).
All analyses were performed with R Core Team (2022) (R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; URL https://www.R-project.org/). A two-sided P-value ≤0.05 was considered statistically significant.
Results
Patient sample
A total of 21 STEMI patients underwent a 3-month LGE-CMR and subsequently experienced VA during 100.3 months follow-up. After randomly matching 1:5 with 105 STEMI patients also undergoing a 3-month LGE-CMR, but without known VA, a total of 126 patients were enrolled in this sub-study. The time of inclusion was balanced in the two groups with year 2013 as median inclusion time (P = 0.5). Baseline clinical characteristics, angiographic, and procedural parameters are summarized in Table 1, without statistically significant differences between the two groups. The medications at discharge, including beta-blocker and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, were balanced between cases and controls, and we found no associations between the medications at discharge and VA during follow-up (see Supplementary material online, Table S1).
Table 1.
Baseline clinical characteristics, angiographic, and procedural parameters in the study population (n = 126)
| STEMI-patients with subsequent ventricular arrhythmia, n = 21 | STEMI-patients without subsequent ventricular arrhythmia, n = 105 | P-value | |
|---|---|---|---|
| Age at STEMI, year | 58.0 ± 10.5 | 57.9 ± 8.5 | 0.78 |
| Male sex, n (%) | 19 (90) | 90 (86) | 0.54 |
| BSA, m2 (IQR) | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.00 |
| Randomization, DANAMI, n (%): | |||
| ȃDEFER, deferred stenting | 4 (19) | 19 (18) | 0.92 |
| ȃiPOST, ischaemic post-conditioning | 4 (19) | 20 (19) | 1.00 |
| ȃPRIMULTI, complete | 6 (29) | 30 (29) | 1.00 |
| Randomization, Danegaptide, high, n (%) | 2 (10) | 11 (10) | 1.00 |
| Hypertension, n (%) | 7 (35) | 35 (33) | 0.84 |
| Diabetes, n (%) | 3 (14) | 6 (6) | 0.17 |
| Dyslipidaemia, n (%) | 7 (35) | 33 (31) | 0.71 |
| CKD, n (%) | 0 | 0 | |
| Smoking: | 0.42 | ||
| ȃFormer, n (%) | 5 (25) | 41 (39) | |
| ȃActive, n (%) | 13 (60) | 47 (45) | |
| Familiar IHD, n (%) | 12 (57) | 45 (43) | 0.24 |
| Known IHD, n (%) | 1 (5) | 2 (2) | 0.43 |
| Previous ischaemic stroke, n (%) | 1 (5) | 4 (4) | 0.85 |
| Known CHF, n (%) | 4 (21) | 23 (22) | 0.92 |
| Angiographic and procedural parameters, n (%): | |||
| ȃAnterior located infarction, | 10 (47) | 49 (47) | 0.92 |
| ȃLocation of culprit lesion: | 0.92 | ||
| ȃȃȃLAD | 10 (48) | 49 (47) | |
| ȃȃȃCx | 4 (19) | 17 (16) | |
| ȃȃȃRCA | 7 (33) | 39 (37) | |
| ȃCTO | 1 (5) | 7 (7) | 0.74 |
| ȃMVD | 6 (29) | 28 (27) | 0.86 |
| ȃComplete revascularization | 15 (71) | 77 (73) | 0.86 |
| Medications at discharge, n (%): | |||
| ȃAspirin | 20 (95) | 104 (99) | 0.20 |
| ȃTicagrelor | 5 (24) | 15 (14) | 0.28 |
| ȃPrasugrel | 15 (71) | 87 (83) | 0.22 |
| ȃClopidogrel | 0 | 2 (2) | 0.52 |
| ȃBeta-blocker | 16 (76) | 95 (90) | 0.10 |
| ȃACE or ARB | 11 (52) | 43 (41) | 0.33 |
| ȃStatin | 21 (100) | 105 (97) | 0.43 |
Data are presented as mean ± standard deviation for continuous variables and number and percentages for categorical variables. Missing: smoking (2%); diabetes (1%), dyslipidaemia (2%); CKD (23%); familiar IHD (2%); known IHD (10%); previous ischaemic stroke (1%); echocardiographic LVEF at follow-up (8%); CHF (10%); statin (1%).
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BSA, body surface area; CKD, chronic kidney disease; CHF, chronic heart failure; CTO, chronic total occlusion; Cx, circumflex artery; DANAMI, DANish Acute Myocardial Infarction; DEFER, deferred stenting vs. conventional primary percutaneous coronary intervention; IHD, ischaemic heart disease; iPOST, ischaemic post-conditioning vs. conventional primary percutaneous intervention; IQR, interquartile range; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; MVD, multi-vessel disease; PRIMULTI, complete vs. culprit lesion only revascularization; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction.
Ventricular arrhythmic events
A total of 21 patients experienced a VA event with a median follow-up of 30.6 months after STEMI. The clinical characteristics of the arrhythmic events are listed in Supplementary material online, Table S2. The VA events were distributed as follows: 10 of 21 had a VF or VT out-/in-hospital cardiac arrest; 7/21 patients had VT, of which 4 of 7 had hospital episodes terminated by antiarrhythmics or cardioversion, and 3 of 7 had episodes terminated by ICD shocks; and the last 4 of 21 patients had SCD. A total of 3 of 21 had a primary prevention ICD implanted before their VA event, and 8 of 21 of the patients with a subsequent VA received a secondary prevention ICD. The remaining 10 of 21 patients did not receive an ICD due to reversible causes (5/10) or death (5/10). A total of 4 of 21 patients were admitted with acute MI in relation to the VA event.
Delayed enhanced cardiac magnetic resonance results
The LGE-CMR substrate derived parameters are shown in Table 2. The LV function was preserved; however, a trend towards a lower LVEF and number of patients with LVEF ≤35% were seen in cases with vs. controls. Cases presented significantly larger median total scar mass (consisting of core- and BZ mass), core mass, and BZ mass compared with controls. In total, 134 BZ channels were identified in 61 (48%) patients, with a median of 2 BZ channels per patient in both cases and controls. A significantly higher proportion of cases had BZ channels compared to controls. The distribution of scar mass plotted against BZ mass, core mass, the presence of BZ channels, and number of BZ channels in the study population are shown in Supplementary material online, Figure S1A–D. Two-way ANOVA revealed no statistically significant interaction between the effects of scar mass and the presence of BZ channels (P = 0.08).
Table 2.
Late gadolinium enhancement cardiac magnetic resonance derived substrate parameters in the study population (n = 126)
| STEMI-patients with subsequent ventricular arrhythmia, n = 21 | STEMI-patients without subsequent ventricular arrhythmia, n = 105 | P-value | |
|---|---|---|---|
| LV mass, mL | 131.0 ± 25.1 | 124.0 ± 28.7 | 0.65 |
| LVEDV, mL | 192.0 ± 50.0 | 179.0 ± 38.9 | 0.78 |
| LVESV, mL | 94.0 ± 40.4 | 76.0 ± 28.6 | 0.12 |
| LVEF, % | 51.0 ± 11.6 | 55.5 ± 8.5 | 0.10 |
| ȃLVEF ≤35%, n (%) | 2 (10) | 2 (2) | 0.07 |
| Location LGE tissue, n (%): | 0.79 | ||
| ȃInferior | 10 (47) | 56 (53) | |
| ȃAnterior | 7 (33) | 26 (25) | |
| ȃLateral | 2 (10) | 8 (8) | |
| ȃSeptal | 2 (10) | 10 (9) | |
| ȃApical | 5 (5) | ||
| Scar mass, g | 23.6 ± 17.4 | 16.0 ± 9.9 | 0.001 |
| Core mass, g | 7.1 ± 7.7 | 4.6 ± 4.8 | 0.01 |
| BZ mass, g | 17.2 ± 10.3 | 10.3 ± 6.0 | 0.0002 |
| Presence BZc, n (%) | 17 (80) | 44 (42) | 0.001 |
| Number of BZc | 2.0 ± 1.4 | 2.0 ± 1.1 | 0.26 |
| Layer location BZc, n (%): | 0.53 | ||
| ȃEndocardial | 10 (65) | 24 (55) | |
| ȃEpicardial | 2 (12) | 3 (7) | |
| ȃBoth | 4 (23) | 17 (38) | |
| Location BZc, n (%): | 0.84 | ||
| ȃInferior | 8 (50) | 26 (59) | |
| ȃAnterior | 6 (38) | 13 (30) | |
| ȃLateral | 2 (13) | 3 (7) | |
| ȃSeptal | 1 (6) | 2 (5) |
Data are presented as mean ± standard deviation for continuous variables and number and percentages for categorical variables. Missing: LVEF (1%); LVEDV (1%); LVESV (1%).
BZc, border zone channels; IQR, interquartile range; LGE, late gadolinium enhancement; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction.
We found a trend towards a larger LV mass, end-diastolic volume, and LV end-systolic volume in cases vs. controls.
The univariate ORs for VA revealed that none of the clinical baseline characteristics were associated with recurrent VA follow-up (see Supplementary material online, Table S1), whereas all scar parameters were significantly associated with the primary endpoint (Table 3). Four multivariable logistic regression models were created for scar, core, and BZ mass and the presence of BZ channels, adjusted by LVEF and LV mass (Table 3). We found scar mass, BZ mass, and the presence of BZ channels to be independent predictors of VA, whereas core mass, LVEF, and LV mass did not predict VA in any of the models. Likewise, we did three multivariable logistic regression models for BZ channels, adjusted by: scar mass, LVEF, and LV mass (model 1); core mass, LVEF, and LV mass (model 2); and BZ mass, LVEF, and LV mass (model 3) and found the presence of BZ channels to be an independent predictor of VA in model 1 + 2 (Table 4). In model 3, the presence of BZ channels and BZ mass were both predictors of VA.
Table 3.
Univariable and multivariable odds ratios for ventricular arrhythmia of each scar determinant
| Multivariable | Univariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Model 1 | ||||||
| ȃScar mass | 1.06 | 1.01–1.11 | 0.01 | 1.06 | 1.02–1.10 | 0.002 |
| ȃLVEF ≤35% | 1.06 | 0.09–12.37 | 0.96 | 5.37 | 0.71–40.48 | 0.10 |
| ȃLV mass | 0.99 | 0.97–1.01 | 0.49 | 1.00 | 0.99–1-02 | 0.64 |
| Model 2 | ||||||
| ȃCore mass | 1.07 | 0.97–1.18 | 0.19 | 1.09 | 1.02–1.18 | 0.02 |
| ȃLVEF ≤35% | 1.90 | 0.15–24.64 | 0.62 | |||
| ȃLV mass | 1.00 | 0.98–1.02 | 0.85 | |||
| Model 3 | ||||||
| ȃBZ mass | 1.11 | 1.03–1.20 | <0.01 | 1.11 | 1.04–1.18 | 0.001 |
| ȃLVEF ≤35% | 1.38 | 0.14–13.47 | 0.78 | |||
| ȃLV mass | 0.99 | 0.97–1.01 | 0.46 | |||
| Model 4 | ||||||
| ȃPresence of BZc | 5.40 | 1.62–18.01 | <0.01 | 5.89 | 1.85–18.72 | 0.003 |
| ȃLVEF ≤35% | 2.70 | 1.62–21.24 | 0.35 | |||
| ȃLV mass | 1.00 | 0.98–1.02 | 0.97 | |||
| Model 5 | ||||||
| ȃBZ mass ≥17.2 g and BZc | 9.68 | 2.70–34.76 | <0.001 | 9.40 | 3.26–27.13 | <0.0001 |
| ȃLVEF ≤35% | 1.08 | 0.12–9.60 | 0.95 | |||
| ȃLV mass | 0.99 | 0.97–1.01 | 0.50 | |||
BZ, border zone; BZc, BZ channels; LV, left ventricular; LVEF, LV ejection fraction; OR, odds ratio.
Table 4.
Multivariable odds ratios for ventricular arrhythmia of the presence of border zone channels
| OR | 95% CI | P-value | |
|---|---|---|---|
| Model 1 | |||
| ȃPresence of BZc | 3.87 | 1.02–14.73 | 0.04 |
| ȃScar mass | 1.03 | 0.98–1.09 | 0.24 |
| ȃLVEF ≤35% | 1.29 | 0.12–14.41 | 0.83 |
| ȃLV mass | 0.99 | 0.98–1.09 | 0.24 |
| Model 2 | |||
| ȃPresence of BZc | 5.55 | 1.48–20.77 | 0.01 |
| ȃCore mass | 0.99 | 0.88–1.12 | 0.92 |
| ȃLVEF ≤35% | 1.03 | 0.22–38.42 | 0.42 |
| ȃLV mass | 1.00 | 0.88–1.12 | 0.92 |
| Model 3 | |||
| ȃPresence of BZc | 3.52 | 0.96–12.94 | 0.04 |
| ȃBZ mass | 1.08 | 0.99–1.17 | 0.07 |
| ȃLVEF ≤35% | 1.26 | 0.13–11.88 | 0.84 |
| ȃLV mass | 0.99 | 0.97–1.01 | 0.49 |
BZ, border zone; BZc, border zone channels; LV, left ventricle; LVEF, LV ejection fraction; OR, odds ratio.
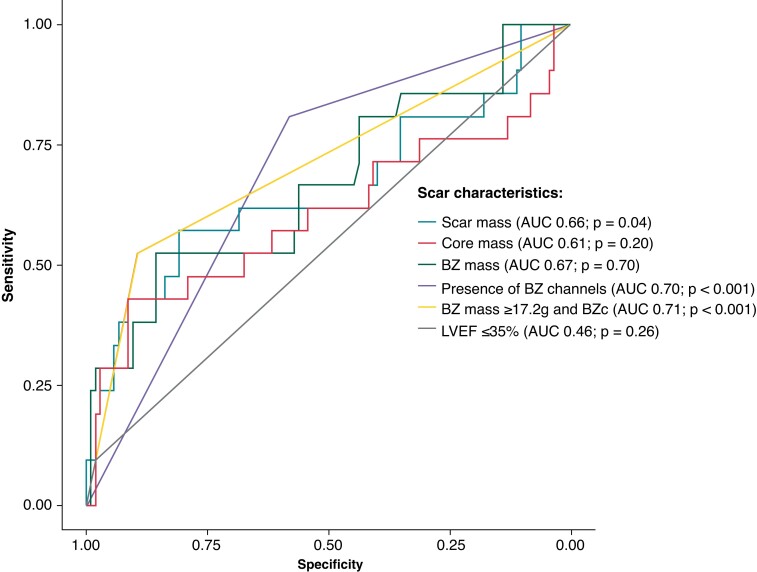
The overall diagnostic performance of the different scar characteristics to differentiate cases and controls is shown in Table 5. The AUC for BZ mass was of 0.67 [95% confidence interval (CI) 0.51–0.81; P = 0.04] and for the presence of BZ channels of 0.70 (95% CI 0.60–0.79; P = 0.0001). The ROC curves for all the scar parameters analysed are shown in Figure 3. The cutpoints for the identification of patients with documented VA are listed in Table 5. The cutpoint for BZ mass was of ≥17.2 g (52% sensitivity, 86% specificity).
Table 5.
The diagnostic performance of the different scar characteristics; and the prevalence of patients above the optimal cutpoint of the different scar characteristics or LVEF ≤35% in cases (n = 21) vs. controls (n = 105)
| AUC | 95% CI | P-value | Optimal cutpoint | Sensitivity | Specificity | No. patients with the optimal cutpoint | P-value | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 21) | Controls (n = 105) | ||||||||||
| Scar mass | 0.66 | 0.51–0.81 | 0.04 | 22.9 | 0.57 | 0.81 | 12 (57) | 20 (19) | 0.0003 | 0.38 | 0.90 |
| Core mass | 0.61 | 0.44–0.77 | 0.20 | 11.5 | 0.43 | 0.91 | 9 (43) | 19 (18) | 0.01 | 0.83 | 0.17 |
| BZ mass | 0.67 | 0.51–0.81 | 0.04 | 17.2 | 0.52 | 0.86 | 11 (52) | 16 (15) | 0.0002 | 0.41 | 0.90 |
| BZc | 0.70 | 0.60–0.79 | 0.0001 | 0.56 | 0.81 | 17 (81) | 44 (42) | 0.001 | 0.28 | 0.94 | |
| BZ mass ≥17.2 g and BZc | 0.71 | 0.60–0.82 | 0.0003 | 0.52 | 0.90 | 11 (52) | 11 (10) | <0.00001 | 0.50 | 0.90 | |
| LVEF ≤35% | 0.46 | 0.77–1.06 | 0.26 | — | — | — | — | — | — | 0.83 | 0.17 |
BZ, border zone; BZc, BZ channels; LVEF, left ventricular ejection fraction; NPV, negative predictive value; PPV, positive predictive value. No optimal cutpoint was identifiable for LVEF ≤35%.
Figure 3.
ROC curves for the different scar determinants. BZc, border zone channel; ROC, receiver operating curve.
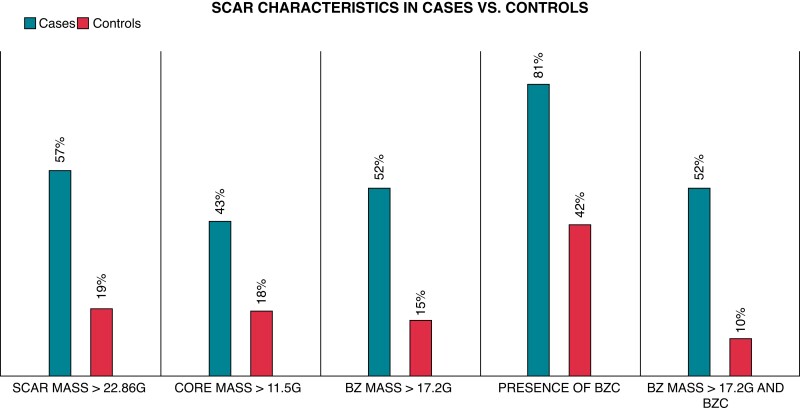
In Table 5 and Figure 4, the distribution of the optimal cutpoints for each scar parameter in cases vs. controls is shown. We found that a BZ mass ≥17.2 g was 4.3 times more prevalent in cases vs. controls. To identify the best model to predict VA events, we performed a sub-analysis on patients with a combination of a cutpoint of BZ mass ≥17.2 g and the presence of BZ channels. This combination proved five times more prevalent in cases vs. controls (Table 5 and Figure 4) and was an independent predictor of subsequent VA (Table 3). The AUC for this combination was 0.71 (95% CI 0.60–0.82; P =0.0003) and showed a 52% sensitivity and 90% specificity for the identification of patients with documented VA (Table 5 and Figure 3). Finally, we found a high negative predictive value for: scar mass <22.9 g, BZ mass <17.2 g, non-presence of BZ channels and a combined endpoint of BZ mass <17.2 g and non-presence of BZ channels. Whereas core mass ≥11.5 g and LVEF ≤35% had a high positive predictive value. However, the number of patients with LVEF ≤35% was low (cases, n = 2; controls, n = 2).
Figure 4.
Bar chart depicting the prevalence of the optimal cutpoint of the diffent scar characteristics in cases (n = 21) vs. controls (n = 105). BZ, border zone; BZc, BZ channels.
Cardiovascular death
The clinical characteristics of cardiovascular vs. all-cause mortality in the study population are shown in Supplementary material online, Table S3. Among patients in this cohort, 13 patients died; of which 6 of 13 died of cardiovascular causes with a median time from STEMI to death of 68.0 months. All patients with cardiovascular death had a previous or immediate VA event compared to one patient in the group of all-cause mortality (6/6 vs. 1/7; P = 0.004). The scar determinants were larger in patients with vs. without cardiovascular death.
Sensitivity analysis
Due to the low frequency of VA and mortality, we performed a sensitivity analysis on the frequency of VA events and all-cause mortality in a randomly selected subset of excluded patients without a 3-month follow-up CMR (n = 345). In this subset, we found a total of 26 (8%) VA events and 47 (14%) events of all-cause mortality. Compared with our study, a total of 22 of 843 (3%) of the patients with a 3-month CMR had subsequent VA and 13 of 126 (10%) of the study population died during follow-up.
Discussion
This nested case-control study describes myocardial scar characteristics visualized by LGE-CMR in a post-STEMI population and the association with subsequent VA at a long-term follow-up.
Our sub-study contributed with two novel findings. Firstly, it revealed that the combination of ≥17.2 g BZ mass and the presence of BZ channels was strongly associated with subsequent VA with a five-time higher prevalence in cases vs. controls. Secondly, individual scar characteristics, including total scar mass, BZ mass, and the presence of BZ channels themselves, were associated with subsequent VA. However, the sample size was small why conclusions must be taken with caution.
Although LVEF is the current key clinical parameter in post-STEMI arrhythmic risk stratification and ICD indication, the need for better predictors of VA is evident. The value of prophylactic ICDs has primarily been established in patients with severely reduced LVEF, though it has been suggested that these patients may represent a minority of the total SCD and only 25–30% of patients with an ICD for primary prevention will get appropriate therapy.17 Two recent studies by Zegard et al.18,19 have demonstrated that greyzone myocardial fibrosis is strongly associated with VA and SCD, also in patients with LVEF >35%. This confirmed in other studies in which scar size and its heterogeneity, assessed with cardiac LGE-CMR, have been proved to be independent predictors of arrhythmic events and cardiovascular death in patients with a healed MI.4,20 However, the combination of a specific cutpoint of BZ mass and the presence of BZ channels has not been investigated elsewhere in a post-STEMI population with mostly preserved LVEF using this unique LGE-CMR post-processing imaging technique.
Recent studies have demonstrated that both the BZ channels detection technique and the presence of BZ channels are feasible and predictive factors for subsequent VT recurrence after VT ablation21,22 and correlate well with voltage channels identified under endocardial voltage mapping during scar-related VA ablation procedures.15,23 Few studies have also investigated the BZ channel characterization as risk stratification for VA and SCD using the same imaging method as ours. Acosta et al.24 found the presence, extension, heterogeneity, and qualitative distribution of BZ tissue of myocardial scar to independently predict appropriate ICD therapies and SCD. Similarly, Jáuregui et al.25 demonstrated that the BZ channels mass was the strongest independent variable associated with the occurrence of VT in post-MI patients. Results are comparable to the current. Sánchez-Somonte et al.26 showed that the absence of BZ channels and a scar mass <10 g identified patients with a low risk of VA in patients with ICD in primary prevention. Correspondingly, we found high negative predictive values for scar mass, BZ mass, the non-presence of BZ channels, and a combined endpoint of <17.2 g BZ mass and the non-presence of BZ channels, stressing the potential usefulness of scar characteristics to rule out patients at risk of VA. However, in contrast to our study, the studies addressed above exclusively included patients with impaired LV function, and the impact of an optimal cutpoint of BZ mass in combination with the presence of BZ channels has not been explored elsewhere. We show that not only were scar characteristics independently associated with VA events in post-STEMI patients, even with mostly preserved LVEF, but also that a combination of a specific cutpoint of BZ mass and the presence of BZ channels had a strong association with future VA events and highly separated cases from controls. We did not find any significant difference in the number of BZ channels between cases and controls. One could speculate that an increased number of BZ channels may be related to an increased risk of VA. However, to our knowledge, this has not been demonstrated in earlier studies using the same method as ours.5,14,15,21,24,25 Also, our study population was small and might not reach statistical significance due to lack of power.
The baseline demographics including medications at discharge were balanced between the two groups. The relevance of primary prevention ICD in patients with medical optimization is debated. Beta-blocker and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker treatment have been suggested to prevent of VA.27 However, we found no associations between the medications at discharge and VA during follow-up. Larger clinical trials are required to confirm this.
An accurate risk stratification tool is crucial for effective treatment strategy to avoid SCD after STEMI. This sub-study allows a better understanding of the arrhythmogenic BZ substrate among the ischaemic population with mostly preserved LVEF, though the sample size and the number of events were small. Based on the current evidence and our results, LGE-CMR post-processing imaging of LV scar in post-STEMI patients, including a well-defined cutpoint of BZ mass and BZ channels determination, may be considered as part of a multi-parametric approach to arrhythmic risk stratification and guide the need for ICD implantation, also in patients with preserved LV function. Randomized, controlled trials investigating this strategy are warranted.
Conclusion(s)
Scar characterization with CMR indicated that a combination of ≥17.2 g BZ mass and the presence of BZ channels had the strongest association with subsequent VA in STEMI patients. Additional studies to confirm the present findings are needed to explore this potential new risk factor.
Limitations
The primary limitation of this subs-study is the low rate of arrhythmic events, though the incidence is similar to larger studies previously published.9,15 Correspondingly, mortality was low. Furthermore, a relatively large number of patients did not undergo CMR, introducing the risk of selection bias. We performed a sensitivity analysis in a subset of patients not undergoing a 3-month follow-up CMR. In this, the mortality rate was comparable; however, the incidence of VA events was higher. Excluded patients and patients lost to CMR likely represent some of the most critically ill patients whose clinical condition likely correlate with the size of LV fibrosis, low LVEF, and subsequent VA. Additionally, it is well described that the risk of arrhythmia is highest the first months post-STEMI,28 and the limited number of patients included in this sub-study with only 21 clinical VA events indicates that the results should be interpreted with caution due to the risk of overfitting the analyses. Finally, data could be biased due to over-time events for example heart failure or new MI that may be associated with the risk of a VA event. Still, our results show a strong association between scar characteristics and subsequent VA in a randomly matched 1:5 nested case-control in a consecutive population of STEMI and supporting earlier studies. Further studies with larger sample sizes are needed to confirm the current findings.
Supplementary Material
Contributor Information
Anna F Thomsen, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Litten Bertelsen, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Christian Jøns, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Reza Jabbari, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Jacob Lønborg, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Kasper Kyhl, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Christoffer Göransson, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Lars Nepper-Christensen, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Kiril Atharovski, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Kathrine Ekström, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Hans-Henrik Tilsted, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Frants Pedersen, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Lars Køber, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Thomas Engstrøm, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Niels Vejlstrup, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Peter Karl Jacobsen, Department of Cardiology, Rigshospitalet University Hospital, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The DANAMI-3 trial was funded by the Research Council of Rigshospitalet, the Danish Agency for Science, Technology and Innovation, and the Danish Council for Strategic Research (grant 09-066994). The Danegaptide phase II proof-of-concept clinical trial was funded by Zealand Pharma A/S, Denmark.
Data availability
Data are not available due to privacy/ethical restrictions.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno Het al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 2. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl Get al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005;352:2581–8. [DOI] [PubMed] [Google Scholar]
- 4. Penela D, Acosta J, Andreu D, Ortiz-Perez JT, Bosch X, Perea RJet al. Identification of the potentially arrhythmogenic substrate in the acute phase of ST-segment elevation myocardial infarction. Heart Rhythm 2017;14:592–8. [DOI] [PubMed] [Google Scholar]
- 5. Andreu D, Ortiz-Pérez JT, Fernández-Armenta J, Guiu E, Acosta J, Prat-González Set al. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace 2015;17:938–45. [DOI] [PubMed] [Google Scholar]
- 6. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang Let al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet 2015;386:665–71. [DOI] [PubMed] [Google Scholar]
- 7. Engstrøm T, Nepper-Christensen L, Helqvist S, Kløvgaard L, Holmvang L, Jørgensen Eet al. Danegaptide for primary percutaneous coronary intervention in acute myocardial infarction patients: a phase 2 randomised clinical trial. Heart 2018;104:1593–9. [DOI] [PubMed] [Google Scholar]
- 8. Moss AJ, Brown MW, Cannom DS, Daubert JP, Estes M, Foster Eet al. Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol. Ann Noninvasive Electrocardiol 2005;10:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mark DB, Nelson CL, Anstrom KJ, Al-Khatib SM, Tsiatis AA, Cowper PAet al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the sudden cardiac death in heart failure trial (SCD-HeFT). Circulation 2006;114:135–42. [DOI] [PubMed] [Google Scholar]
- 10. Kelbæk H, Høfsten DE, Køber L, Helqvist S, Kløvgaard L, Holmvang Let al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet 2016;387:2199–206. [DOI] [PubMed] [Google Scholar]
- 11. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Clemmensen Pet al. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol 2017;2:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Høfsten DE, Kelbæk H, Helqvist S, Kløvgaard L, Holmvang L, Clemmensen Pet al. The third DANish study of optimal acute treatment of patients with ST-segment elevation myocardial infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only. Am Heart J 2015;169:613–21. [DOI] [PubMed] [Google Scholar]
- 13. Kyhl K, Ahtarovski KA, Nepper-Christensen L, Ekström K, Ghotbi AA, Schoos Met al. Complete revascularization versus culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease. JACC Cardiovasc Interv 2019;12:721–30. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva Eet al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 2012;14:1578–86. [DOI] [PubMed] [Google Scholar]
- 15. Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias Det al. Cardiac magnetic resonance–aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm 2017;14:1121–8. [DOI] [PubMed] [Google Scholar]
- 16. Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–64. [DOI] [PubMed] [Google Scholar]
- 17. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau Ret al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 18. Zegard A, Okafor O, de Bono J, Kalla M, Lencioni M, Marshall Het al. Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 2021;77:29–41. [DOI] [PubMed] [Google Scholar]
- 19. Zegard A, Okafor O, de Bono J, Kalla M, Lencioni M, Marshall Het al. Greyzone myocardial fibrosis and ventricular arrhythmias in patients with a left ventricular ejection fraction >35%. Europace 2022;24:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TKet al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinto L, Sanchez P, Alarcón F, Garre P, Zaraket F, Prat-Gonzalez Set al. Cardiac magnetic resonance to predict recurrences after ventricular tachycardia ablation: septal involvement, transmural channels, and left ventricular mass. Europace 2021;23:1437–45. [DOI] [PubMed] [Google Scholar]
- 22. Arbustini E, Kramer CM, Narula J. Arrhythmogenic potential of border zone after myocardial infarction. JACC Cardiovasc Imaging 2018;11:573–6. [DOI] [PubMed] [Google Scholar]
- 23. Roca-Luque I, Van Breukelen A, Alarcon F, Garre P, Tolosana JM, Borras Ret al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace 2020;22:598–606. [DOI] [PubMed] [Google Scholar]
- 24. Acosta J, Fernández-Armenta J, Borràs R, Anguera I, Bisbal F, Martí-Almor Jet al. Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy. JACC Cardiovasc Imaging 2018;11:561–72. [DOI] [PubMed] [Google Scholar]
- 25. Jáuregui B, Soto-Iglesias D, Penela D, Acosta J, Fernández-Armenta J, Linhart Met al. Cardiovascular magnetic resonance determinants of ventricular arrhythmic events after myocardial infarction. Europace 2022;24:938–47. [DOI] [PubMed] [Google Scholar]
- 26. Sánchez-Somonte P, Quinto L, Garre P, Zaraket F, Alarcón F, Borràs Ret al. Scar channels in cardiac magnetic resonance to predict appropriate therapies in primary prevention. Heart Rhythm 2021;18:1336–43. [DOI] [PubMed] [Google Scholar]
- 27. Ruwald MH, Ruwald ACH, Jons C, Alexis J, McNitt S, Zareba Wet al. Effect of metoprolol versus carvedilol on outcomes in MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Am Coll Cardiol 2013;61:1518–26. [DOI] [PubMed] [Google Scholar]
- 28. Gorenek B, Lundqvist CB, Terradellas JB, Camm AJ, Hindricks G, Huber Ket al. Cardiac arrhythmias in acute coronary syndromes: position paper from the joint EHRA, ACCA, and EAPCI task force. Eur Heart J Acute Cardiovasc Care 2015;4:386–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available due to privacy/ethical restrictions.