Abstract
Aims
The very high-power short-duration (vHP-SD) radiofrequency (RF) ablation concept of atrial fibrillation (AF) treatment by pulmonary vein isolation (PVI) aims for safer, more effective, and faster procedures. Utilizing conventional ablation, the ‘close protocol’ has been verified. Since lesion formation of vHP-SD ablation creates wider but shallower lesions we adapted the close protocol to an individualized and tighter ‘very-close protocol’ of 3–4 mm of inter-lesion distance (ILD) at the anterior and 5–6 mm at the posterior aspect of the left atrium using vHP-SD only. Here, we evaluated the safety and efficacy of vHP-SD ablation for PVI utilizing a very-close protocol in comparison with standard ablation.
Methods and results
A total of 50 consecutive patients with symptomatic AF were treated with a very-close protocol utilizing vHP-SD (vHP-SD group). The data were compared with 50 consecutive patients treated by the ablation-index-guided strategy (control group). The mean RF time was 352 ± 81 s (vHP-SD) and 1657 ± 570 s (control, P < 0.0001), and the mean procedure duration was 59 ± 13 (vHP-SD) and 101 ± 38 (control, P < 0.0001). The first-pass isolation rate was 74% (vHP-SD) and 76% (control, P = 0.817). Severe adverse events were reported in 1 (2%, vHP-SD) and 3 (6%, control) patients (P = 0.307). A 12-month recurrence-free survival was 78% (vHP-SD) and 64% (control, P = 0.142). PVI durability assessed during redo-procedures was 75% (vHP-SD) vs. 33% (control, P < 0.001).
Conclusions
PVI solely utilizing vHP-SD via a very-close protocol provides safe and effective procedures with a high rate of first-pass isolations. The procedure duration and ablation time were remarkably low. A 12-month follow-up and PVI durability are promising.
Keywords: Atrial fibrillation, High-power short-duration, Pulmonary vein isolation, Radiofrequency, Acute efficacy
What’s new?
Here, we evaluated the safety and efficacy of very high-power short-duration ablation for PVI utilizing a very-close protocol in comparison with standard ablation.
The first-pass isolation rate was 74% (vHP-SD) and 76% (control, P = 0.817). Severe adverse events were reported in 1 (2%, vHP-SD) and 3 (6%, control) patients (P = 0.307). A 12-month recurrence-free survival was 78% (vHP-SD) and 64% (control, P = 0.142).
PVI durability assessed during redo-procedures was 75% (vHP-SD) vs. 33% (control, P < 0.001).
PVI solely utilizing vHP-SD via a very-close protocol provides safe and effective procedures with a high rate of first-pass isolations.
Introduction
Catheter ablation-based pulmonary vein isolation (PVI) has shown high procedural success and long-term follow-up rates for the treatment of paroxysmal atrial fibrillation (PAF) and persistent atrial fibrillation (PersAF).1 Recently, novel single-shot systems have shown excellent acute and long-term success rates with decreased procedure time compared with radiofrequency (RF)-based three-dimensional (3D)-mapping and point-by-point PVI.2 Nevertheless, single-shot systems have several limitations because they are mainly designed for PVI only. Furthermore, the adaptability to different PV anatomies is narrowed. 3D-mapping and point-by-point-based PVI received several improvements by implementing contact force (CF) sensing and ablation index (AI)-guided RF ablation shortening procedure time and improving safety and patients outcome.3,4 Recently, high-power short-duration (HP-SD) with a maximum of up to 50 W and very HP-SD (vHP-SD) with a maximum of 90 W have been evaluated and were found to shorten the procedure duration.5,6 The novel QDOT Micro ablation catheter (Biosense Webster, Inc., Diamond Bar, CA, USA) has been developed allowing for real-time assessment of catheter-to-tissue interface temperature and therefore allows temperature-controlled ablation.7 This strategy aims to create shallower but wider lesions in a very short time by reducing conductive heating and increasing resistive heating at the same time. Additionally, collateral tissue damage might be reduced.8 Utilizing conventional RF ablation the ‘close protocol’ with an inter-lesion distance (ILD) of 6 mm has been introduced and verified.9 Since the lesion formation of vHP-SD ablation creates wider but shallower lesions, we adapted the close protocol to an individualized and tighter ‘very-close protocol’ of 3–4 mm ILD at the anterior aspect and 5–6 mm at the posterior aspect of the left atrium using vHP-SD only. Here, we thought to evaluate the safety, efficacy, and follow-up of vHP-SD ablation for PVI utilizing a novel vHP-SD catheter utilizing a very-close protocol in comparison with conventional CF sensing AI-guided RF ablation.
Methods
Inclusion and exclusion criteria
Since September 2020, 50 consecutive patients with symptomatic, drug-refractory PAF, or short-standing PersAF (duration ≤3 months) presented for PVI and were treated with the QDOT Micro catheter (vHP-SD group). A total of 50 consecutive previous patients treated with conventional CF-sensing AI-guided PVI served as the control (control group). The patients were prospectively and consecutively enrolled. Exclusion criteria were prior left atrial (LA) ablation attempts, LA diameter of >60 mm, severe valvular heart disease, or contraindications to post-interventional oral anticoagulation. Transoesophageal echocardiography was performed in all patients prior to PVI to rule out intracardiac thrombi and to assess the LA diameter. No further pre-procedural imaging was performed. In patients on vitamin K antagonists, the procedure was performed under therapeutic INR values of 2–3. In patients on new oral anticoagulants, the morning dose on the day of the procedure was omitted. All patients gave written informed consent and all patient information was anonymized. The study was approved by the local ethics board (Lübeck ablation registry ethical review board number: WF-028/15) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Intraprocedural management
The detailed intraprocedural management for 3D-mapping and AI-guided PVI has been described in previous studies from our group.10 In brief, the procedure was performed under deep sedation using midazolam, fentanyl, and propofol. Three ultrasound-guided right femoral vein punctures were performed and three 8F short sheaths were inserted. Prior to transseptal puncture (TSP), one diagnostic catheter was introduced and positioned inside the coronary sinus. Double TSP was performed under fluoroscopic guidance using a modified Brockenbrough technique with 8.5F transseptal sheaths and puncture needle (SL1 sheath and BRK-1 TSP needle, St. Jude Medical, Inc., St. Paul, MN, USA). Pulmonary vein (PV) angiography was performed to identify the PV ostia. Both sheaths were continuously flushed with heparinized saline (10 mL/h). After TSP heparin, boluses were administered targeting an activated clotting time of >300 s.
Ablation procedure
3D electroanatomic LA reconstruction (CARTO 3 V7, Biosense Webster) was performed via fast anatomical mapping with a multi-electrode mapping catheter (Pentaray or Lasso Nav, Biosense Webster). For the LA voltage map, the bipolar voltage reference interval was set between 0.05 and 0.5 mV. After PV angiography, the ipsilateral PVs were tagged according to 3D-mapping and PV angiography. During PVI, a multi-electrode spiral mapping catheter was positioned inside the ipsilateral PVs. All procedures in both groups have been performed by two highly experienced operators only (R.R.T and C.-H.H.).
The vHP-SD ablation group
In the vHP-SD group, the QDOT Micro catheter was utilized. For all applications, vHP-SD ablation (90 W, 4 s; QMODE+ mode) was performed. The target temperature of the temperature-controlled ablation was 60°C based on the hottest surface thermocouple.7 The irrigation flow rate delays the energy application for a minimum of 2 s before and 4 s after each RF application. A switch to conventional QMODE was always possible by changing the ablation mode. For all cases, a very-close protocol was utilized aiming to perform vHP-SD only. For anterior lesions an ILD of 3–4 mm and for posterior lesions an ILD of 5–6 mm was predefined (Figure 1). The rationale of this proceeding for the anterior aspect was derived from pre-clinical animal studies where a double application of 90 W for 4 s caused a further tissue temperature rise and a 40% deeper lesion formation. The target CF range was 10–25 g. In the case of CF of <10 g, a CF of 5 g was acceptable to start the application. In the case of CF of <5 g, another catheter position was utilized to achieve a stable and continuous contact with CF of >5 g. The final lesion set after vHP-SD-based PVI is shown in Figure 2. An S-shaped temperature probe (CIRCA S-CATH, Circa Scientific, Englewood, CO, USA) was advanced into the oesophagus to monitor the oesophageal temperature (Teso) in all cases of the vHP-SD group. The intraluminal Teso cut-off was set at 38.5°C. During the procedures, special attention was drawn to audible pops and all catheters were checked for charring after removal.
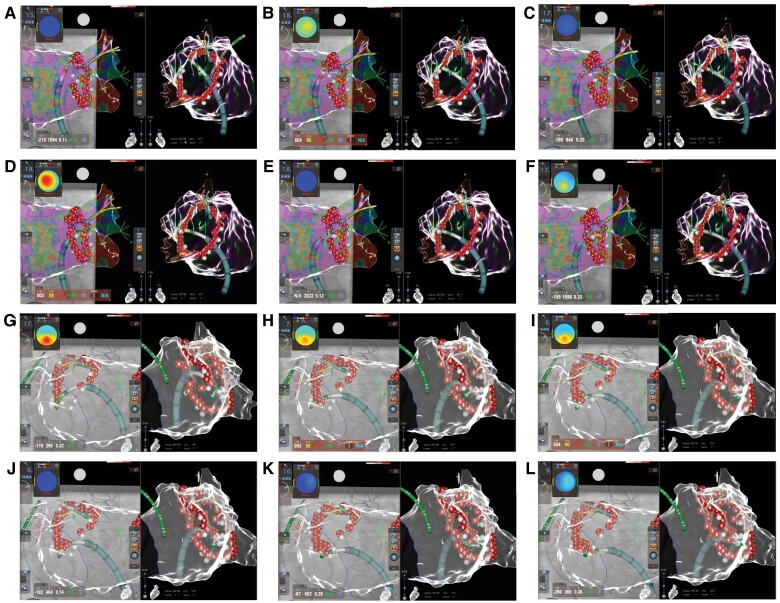
Figure 1.
QDOT micro catheter in QMODE± utilizing the very-close protocol: A–F: posterior aspect: three-dimensional electroanatomic reconstruction (CARTO 3, UNIVIEW module, Biosense Webster) of the left atrium in right anterior oblique (left) and right lateral (right) view. Please note the deployment of very high-power short-duration applications by 90W/4 s. At the posterior area an ILD of 5–6 mm was targeted. G–L: anterior aspect: Three-dimensional electroanatomic reconstruction (CARTO 3, UNIVIEW module, Biosense Webster) of the left atrium in posterior anterior (left) and left lateral (right) view. Please note the deployment of very-high power short duration applications by 90 W/4 s (QMODE+ mode, red–white tags) at the anterior aspect of the left pulmonary veins. At the anterior area an ILD of 3–4 mm was targeted.
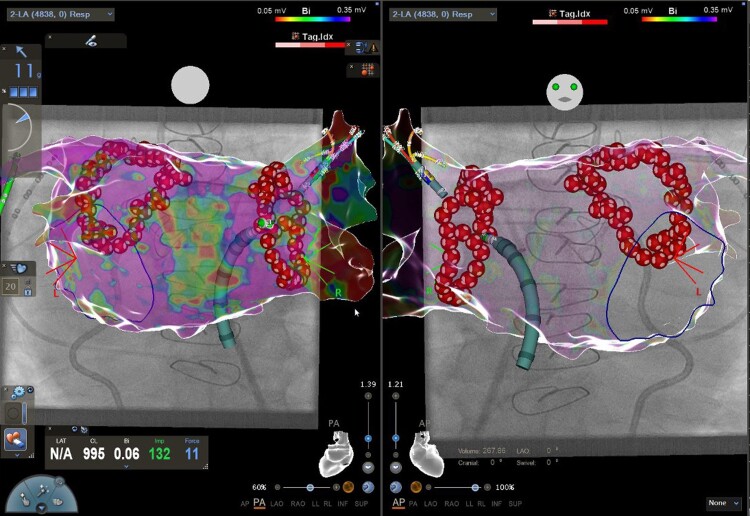
Figure 2.
Final lesions set. Three-dimensional electroanatomic reconstruction (CARTO 3, UNIVIEW module, Biosense Webster) of the left atrium in posterior anterior (left) and anterior posterior (right) view. Please note the two circles of very-high power short duration applications by 90 W/4 s (QMODE+ mode, red–white tags) encircling the right and left pulmonary veins.
Conventional ablation
In the control group, conventional CF-sensing AI-guided ablation was used. Ablation was performed with a Thermocool Smart-touch surround flow catheter (Biosense Webster) in a power-controlled mode. Energy application was limited to 40 W. Target range for CF was 10–40 g. Target AIs were 550, 450, and 380 for the anterior, roof, and posterior segments of the LA, respectively.10 The ILD was set to 5–6 mm. In the case of previously known or periprocedural typical atrial flutter, cavotricuspid isthmus ablation was performed in both groups.
Postprocedural care
A figure-of-eight suture and a pressure bandage were used to prevent femoral bleeding. The pressure bandage was removed after 4 h and the figure-of-eight suture on the next day. Following ablation, all patients underwent transthoracic echocardiography immediately post procedure, after 2 h and at Day 1 after the procedure to rule out a pericardial effusion. New oral anticoagulants were re-initiated 6 h post ablation. Anticoagulation was continued for at least 3 months and continued thereafter based on the patient’s CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs or a new antiarrhythmic drug were prescribed and continued for 3 months post ablation. All patients were treated with proton-pump inhibitors for 6 weeks. Following a 3-month blanking period, patients completed outpatient clinic visits, including ECG and 72 h-Holter ECG at 3, 6, and 12 months. In addition, regular telephone interviews were performed. Additional outpatient clinic visits were immediately initiated in cases of symptoms suggestive of arrhythmia recurrence.
End points
Primary end point
The primary endpoint was defined as freedom from documented AF/atrial tachycardia (AT) recurrence 12 months after PVI, including a 90-day blanking period. Recurrence was defined as any ECG-documented atrial tachyarrhythmia lasting for at least 30 s, including AF, AT, and atrial flutter. Patients completed outpatient clinic visits at 3, 6, and 12 months including ECGs and 24 h-Holter ECGs. In addition, regular telephonic interviews were performed.
Secondary end points
The secondary end points were acute procedural success defined as the ability to confirm electrical isolation with a circular mapping catheter, procedural parameters (e.g. procedure time, LA dwelling time, fluoroscopy time), number and duration of RF applications, number of first-pass isolations as well as periprocedural complications. Periprocedural complications were defined according to the latest guidelines. Only adverse events adjudicated as possible, probable, or definitely related to the ablation procedure were mentioned as safety events. An adverse event was considered serious if it resulted in permanent injury or death, required an intervention for treatment, or required hospitalization for more than 24 h. All other safety events were defined as minor complications.
Second ablation procedure
Patients with AF or atrial tachycardia (AT) recurrence during the follow-up and suitable for a repeat-PVI were scheduled for a second ablation procedure using a 3D-mapping system. The techniques for mapping and RF-based PVI have been previously described. The procedures were performed as per institutional standards. Typically, LA electroanatomic reconstruction was performed using a multipolar mapping catheter. Each individual PV was evaluated for electrical reconnection using the mapping catheter recordings. When non-isolated PVs were identified, an RF-based, point-by-point PVI was performed as per institutional standards. For the treatment of AT high-density mapping utilizing a 3D-mapping system and a multipolar mapping, a catheter was conducted to identify the AT mechanism followed by deployment of standardized ablation lines as previously described.
Statistical analysis
Continuous variables are presented as median with interquartile range [first quartile (Q1), third quartile (Q3)]; they were compared using the Wilcoxon Mann–Whitney test. Categorical variables are presented as absolute and relative frequencies; they were compared using the χ2 test or Fisher’s exact test (in case of small-expected cell frequencies). All P-values are two-sided and a P-value <0.05 was considered significant. Recurrence-free survival was estimated with the Kaplan–Meier method. All calculations were performed with the statistical analysis software SAS (SAS Institute Inc., version 9.3, Cary, NC, USA).
Results
Patient characteristics
One hundred patients with PAF or PersAF were prospectively enrolled in this study. A total of 50 consecutive patients underwent vHP-SD-based PVI utilizing the QMODE+ ablation mode. The data were compared with 50 consecutive previous patients with PVI by conventional CF-sensing AI-guided ablation. Patient baseline characteristics are shown in Table 1. No demographic differences were detected between the groups.
Table 1.
Baseline patient characteristics
| Variable | VHP-SD | Control | P |
|---|---|---|---|
| Patients | 50 | 50 | |
| Age, years | 67 ± 10 | 66 ± 10 | 0.774 |
| LA volume, mL/m2a | 33 ± 10 | 37 ± 7 | 0.142 |
| Duration of AF, months | 28 ± 37 | 19 ± 27 | 0.263 |
| Female gender | 16 (32) | 19 (38) | 0.529 |
| Paroxysmal AF | 26 (52) | 24 (48) | 0.689 |
| Congestive heart failure | 7 (14) | 10 (20) | 0.424 |
| Arterial hypertension | 28 (56) | 29 (58) | 0.840 |
| Diabetes mellitus type 2 | 6 (12) | 2 (4) | 0.140 |
| Coronary artery disease | 12 (24) | 10 (20) | 0.629 |
| Previous TIA/Stroke | 3 (6) | 5 (10) | 0.461 |
| CHA2DS2-VASc score | |||
| ȃ0 | 8 (16) | 9 (18) | 0.790 |
| ȃ1 | 10 (20) | 5 (10) | 0.161 |
| ȃ2 | 9 (18) | 15 (30) | 0.249 |
| ȃ3 | 12 (24) | 10 (20) | 0.629 |
| ȃ≥4 | 11 (12) | 11 (32) | 0.999 |
Values are counts, n (%), or mean (±SD).
AF, atrial fibrillation; LA, left atrium.
Per body surface area.
Procedural characteristics
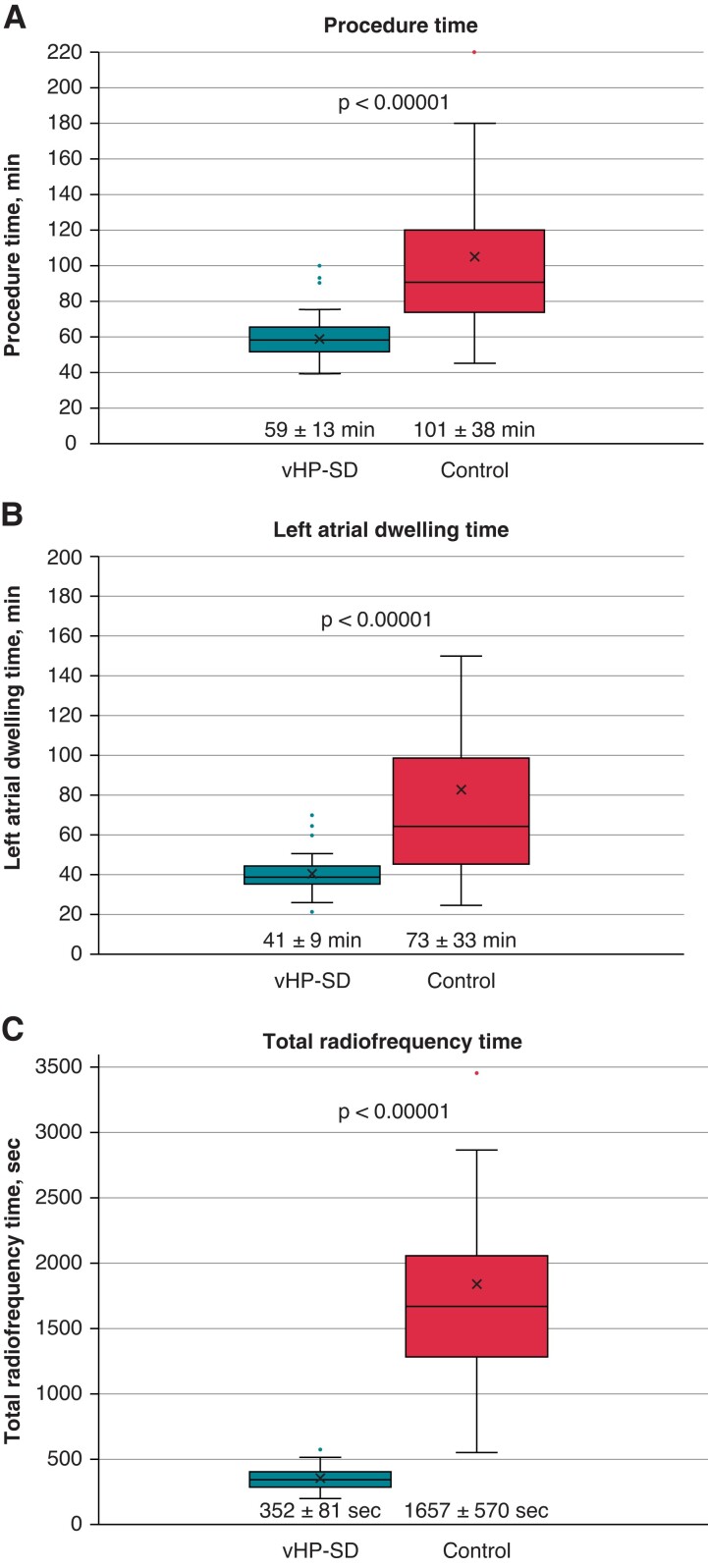
Procedural data are summarized in Tables 2, 3 and Figure 3. All procedures were performed by two experienced operators. Only patients with PVI or PVI plus CTI block were included in this study. All PVs were successfully isolated in either group. With 74% (vHP-SD) and 76% (control), a similar rate (P = 0.817) of first-pass isolations was observed in both groups (first attempt all veins isolated, FAAVI). For right PVs, the rate of first-pass isolation (first-attempt vein isolated, FAVI) was significantly higher in the vHP-SD group (96%) than in control patients (76%), P = 0.004. For left PVs, no difference in FAVI was observed (78% vs. 72%; P = 0.488). Significantly shorter procedure times 59 ± 13 min vs. 101 ± 38 min (P < 0.0001), LA dwelling times 41 ± 9 min vs. 73 ± 33 min (P < 0.0001), and fluoroscopy times 7 ± 3 min vs. 12 ± 6 min (P < 0.0001), were observed for the vHP-SD group. For procedures with PVI only (excluding all patients with additional CTI block, n = 13 in each group), the procedure times were 56 ± 10 min vs. 98 ± 35 min (P < 0.0001). CTI block was achieved by Qmode+ only in all patients (n = 13). In one patient with a repeat procedure, the CTI was checked and was found to be blocked after QMODE+ only.
Table 2.
Procedural details
| Variable | vHP-SD | Control | P |
|---|---|---|---|
| Number of patients | 50 | 50 | |
| Number of PVs | 200 | 200 | |
| Total number of isolated PVs | 200 (100) | 200 (100) | 0.999 |
| FAAVI | 37 (74) | 38 (76) | 0.817 |
| Total procedure time, min | 59 ± 13 | 101 ± 38 | <0.0001 |
| Total procedure time, min (PVI only) | 56 ± 10 | 98 ± 35 | <0.0001 |
| Total LA dwelling time, min | 41 ± 9 | 73 ± 33 | <0.0001 |
| Total fluoroscopy time, min | 7 ± 3 | 12 ± 6 | <0.001 |
| Total amount of contrast agent, mL | 50 ± 13 | 54 ± 28 | 0.364 |
| Total radiofrequency time, s | 352 ± 81 | 1657 ± 570 | <0.0001 |
| Total number of applications | 88 ± 20 | 83 ± 31 | 0.314 |
| Mean application duration, s | 4 ± 0 | 19 ± 6 | <0.0001 |
| Mean contact force, g | 15 ± 3 | 18 ± 3 | 0.212 |
| Mean power/application, Watt | 90 ± 0 | 32 ± 4 | <0.0001 |
| Total delivered power/lesion, Joule | 331 ± 111 | 565 ± 212 | <0.001 |
| Teso Temp. > 38.5°C, n | 18 (36) | — | — |
| Teso Temp. > 38.5°C, n/patient | 0.6 | — | — |
| Max Teso, °C | 42 ± 2 | — | — |
| Cavotricuspid isthmus block, n | 13 (26) | 13 (26) | 0.999 |
| Periprocedural complications | |||
| Severe adverse events | 1 (2) | 3 (6) | 0.307 |
| ȃCardiac tamponade | 0 | 1 (2) | 0.787 |
| ȃSevere bleeding | 1 (2) | 2 (4) | 0.558 |
| ȃPhrenic nerve injury | 0 | 0 | 0.999 |
| ȃStroke or TIA | 0 | 0 | 0.999 |
| Minor complications | 3 (6) | 2 (4) | 0.553 |
| ȃMinor bleeding | 2 (4) | 2 (4) | 0.553 |
| ȃPericardial effusion | 1 (2) | 0 | 0.787 |
| ȃTransient air embolism | 0 | 0 | 0.999 |
| ȃClinical apparent oesophagus injury | 0 | 0 | 0.999 |
| ȃCharring on catheter tip | 0 | 0 | 0.999 |
Values are counts, n (%) or mean (±SD).
PV(s), pulmonary vein(s); PVI, pulmonary vein isolation; FAAVI, first-attempt all veins isolated; LA, left atrium; min, minutes, s, seconds; g, grams.
Table 3.
Procedural details—individual pulmonary vein
| Variable | vHP-SD | Control | P |
|---|---|---|---|
| Right-sided PVs | 50 | 50 | |
| ȃTotal ablation time, s | 172 ± 53 | 802 ± 308 | <0.0001 |
| ȃTotal number of applications | 43 ± 13 | 42 ± 19 | 0.899 |
| ȃMean application duration, s | 4 ± 0 | 19 ± 6 | <0.001 |
| ȃMean contact force, g | 17 ± 4 | 20 ± 5 | <0.001 |
| ȃMean power/application, Watt | 90 ± 0 | 32 ± 4 | <0.0001 |
| ȃTotal delivered power/lesion, Joule | 329 ± 17 | 565 ± 212 | <0.001 |
| ȃFAVI | 48 (96) | 38 (76) | 0.004 |
| Left-sided PVs | 50 | 50 | |
| ȃTotal ablation time, s | 182 ± 51 | 831 ± 375 | <0.0001 |
| ȃTotal number of applications | 46 ± 13 | 44 ± 19 | 0.463 |
| ȃMean application duration, s | 4 ± 0 | 18 ± 14 | 0.001 |
| ȃMean contact force, g | 14 ± 4 | 16 ± 3 | <0.001 |
| ȃMean power/application, Watt | 90 ± 0 | 32 ± 5 | <0.0001 |
| ȃTotal delivered power/lesion, Joule | 334 ± 6 | 543 ± 231 | <0.001 |
| ȃFAVI | 39 (78) | 36 (72) | 0.488 |
Values are counts, n (%), or mean (±SD).
PV(s), pulmonary vein(s); FAVI, first-attempt vein isolated; s, seconds; g, grams.
Figure 3.
Periprocedural data: periprocedural duration: (A) procedure time; (B) left atrial dwelling time; (C) total radiofrequency time, vHP-SD group compared with the control group.
While the total number of applications (P = 0.314) and mean CF (P = 0.212) were similar in both groups, the total ablation time 352 ± 81 min vs. 1657 ± 570 s (P < 0.0001) and mean application duration 4 ± 0 min vs. 19 ± 6 s (P < 0.0001) were significantly shorter in the vHP-SD group. Despite a higher mean power per application in the vHP-SD group 90 ± 0 min vs. 32 ± 4 W (P < 0.0001), the total delivered energy per lesion was significantly lower 331 ± 111 J vs. 565 ± 212 J (P < 0.001). The QMODE+ ablation mode was exclusively used for all procedures in the vHP-SD group. No switch to QMODE was necessary to achieve PVI. No differences were observed between the groups with regard to catheter maneuverability and catheter stability along the targeted PVs. After discharge, all patients received antiarrhythmic drugs post ablation for 3 months.
Safety
No differences in terms of serious adverse events or minor complications were observed. One groin bleeding requiring blood transfusion was observed in the vHP-SD group (2%) and two patients with groin bleeding (one surgical intervention and one blood transfusion) were observed for the control group (4%, P = 0.558). In the control group, one patient suffered from a cardiac tamponade which was detected after finalizing the procedure (2%). The patient was successfully treated via epicardial puncture and aspiration. There were no further severe adverse events such as stroke, phrenic nerve palsy, or atrioesophageal fistula in either group. Concerning minor complications, one patient of the vHP-SD group experienced an asymptomatic pericardial effusion not requiring epicardial puncture or any further intervention (2%). Two patients of each group (4%/4%) experienced minor bleeding of the groin, not requiring intervention or blood transfusion. There were no documented steam pops and no catheter tip charring was detected in either group. An oesophageal temperature probe was utilized only in the vHP-SD group. A Teso >38.5°C was detected in 18 (36%) patients solely at the posterior part of the left PVs. The mean maximum Teso was measured at 42 ± 2°C.
Follow-up and clinical success
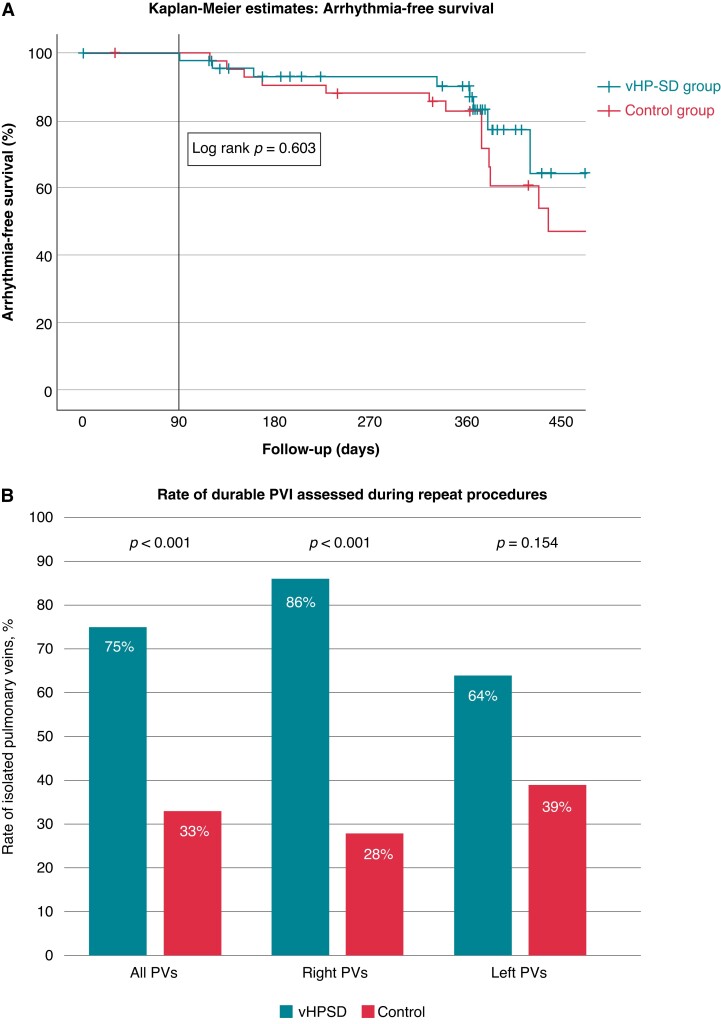
In a total of 89/100 patients (89%), 12-month follow-up was available [rate of loss to follow-up was not different between the groups (vHP-SD: n = 5 vs. control: n = 6, P = 0.749)]. The rate of 12-month AF/AT-free survival after a 90-day blanking period was vHP-SD: 78% vs. control 64% (P = 0.142 Figure 4A). The mean time to recurrence was 344 ± 178 and 359 ± 188 days.
Figure 4.
12-month follow-up and findings of repeat procedures. (A) Kaplan–Meier estimates with 12-month follow-up after the index PVI utilizing very-high power short duration applications by 90 W/4 s (QMODE+) only and the control group. No statistical differences were found concerning 12-month freedom from atrial tachyarrhythmias. (B) Comparison of pulmonary vein durability assessed during repeat procedures of n = 7 (very high-power short-duration group) and n = 9 (control) patients. All four PVs were found to be isolated in 57% of very high-power short-duration group and 0% of control group patients.
Concerning patients with PAF 12-month AF/AT-free survival after a 90-day blanking period was vHP-SD: 83% (20/24) vs. control 67% (15/21), (P = 0.334), and vHP-SD: 71% (14/21) vs. control 61% (14/23), (P = 0.670) or PersAF, respectively.
The findings during repeat procedures are summarized in Table 4 and Figure 4B. A total of 16 patients (vHP-SD group = 7, control group = 9) received a repeat procedure and verification of PVI due to recurrence of AF, atrial tachycardia, typical flutter, or LA appendage closure (Table 4). The median time to reintervention was 12 (9, 16) months for the vHP-SD group and 14 (12, 20) months for the control group (P = 0.516).
Table 4.
Finding during repeat procedures
| Variable | vHP-SD | Control | P |
|---|---|---|---|
| Patients | 7 | 9 | |
| AF recurrence | 3 | 7 | 0.152 |
| Atrial tachycardia/typical flutter | 2/1 | 2/ | 0.377 |
| PV isolation verified during LAA closure | 1 | 0 | 0.565 |
| Time to repeat procedure, months | 12 (9, 16) | 14 (12, 20) | 0.516 |
| PVs | 28 | 36 | |
| Patients with durable complete PVI | 4 (57) | 0 (0) | <0.01 |
| Isolated PVs | 21 (75) | 12 (33) | <0.001 |
| Isolated right PVs | 12 (86) | 5 (28) | <0.001 |
| Isolated left PVs | 9 (64) | 7 (39) | 0.154 |
| CTI block (index procedure) | 1 | 3 | |
| CTI block verified (repeat procedure) | 1 (100) | 2 (67) | 0.572 |
| Ablation strategy during repeat procedure | |||
| Reisolation of not isolated PVs | 7 (100) | 24 (100) | |
| Box-lesion | 2 | 0 | |
| Mitralisthmus line | 1 | 1 | |
| LAA isolation | 1 | 0 | |
| CTI block | 3 | 2 |
Values are counts, n (%), mean (±SD), or median (interquartile range) as appropriate.
AF, atrial fibrillation; CTI, cavotricuspid isthmus block; LAA, left atrial appendage; PV(s), pulmonary vein(s); PVI, pulmonary vein isolation.
Discussion
This study aims to assess efficacy, procedural characteristics, safety, and follow-up during PVI utilizing solely the vHP-SD mode of the QDOT Micro catheter by utilizing a very-close protocol. The data were compared with standard CF-guided ablation. The major findings are
All PVs could be isolated utilizing vHP-SD only, with no necessity to switch the ablation mode to moderate power.
A higher first-pass isolation rate was observed for right-sided PVs compared with control while the overall rate was similar.
RF time, procedure time, and LA dwelling time were significantly reduced utilizing vHP-SD only.
The rate of periprocedural complications were low and no differences were observed between the groups.
During repeat procedures a higher rate of durable PVI was observed for vHP-SD.
The long-term outcome was promising and similar between the groups.
Although the number of single-shot devices for PVI are increasing, the gold standard remains RF-based ablation. The advantages for single-shot devices are shorter procedure times, learning curves, and safety aspects. Recently, the HP-SD concept of RF-based PVI with increased power and shorter duration was introduced and efficacy and safety were shown in previous studies in a power-controlled ablation mode.11
A further improvement of performance was recently shown for the vHP-SD concept utilizing 90Watts for 4 s in a temperature-controlled mode which was recently realized by the QDOT Micro catheter. The six thermocouples of this catheter enable precise temperature measurement and power modulation to avoid tissue overheating, collateral damage, catheter tip charring, and steam pops.12
The concept of RF ablation utilizing the ‘close protocol’ has been verified by different groups and was found to be effective and safe.9 However the lesion formation of vHP-SD ablation creates wider but shallower lesions. Therefore, we suggested an adapted, individualized, and tighter ‘very-close protocol’ of 3–4 mm ILD at the anterior aspect and 5–6 mm at the posterior aspect of the left atrium using vHP-SD only to achieve safe and fast PVI. The present study shows that PVI utilizing the QMODE+ ablation mode provides similar acute success and periprocedural complications rates when compared with the standard CF-sensing AI-guided PVI. The rate of first-pass isolation was relatively high and comparable between the groups. Utilizing the ‘very-close protocol’ PVI was achieved by QMODE+ only. This observation is different from the study by Reddy et al. They reported a necessity of conventional ablation in 26.9% of patients and 5% of PVs.7 The reason for this discrepancy might be the fact that an individualized ‘very-close protocol’ was utilized aiming for a QMODE+ only strategy.
With the QDOT Micro catheter, a switch to conventional ablation mode (QMODE) is always possible, yet it was not necessary in any of our cases to achieve PVI. In our study, no charring, no steam pops, and no clinical apparent oesophageal injuries occurred, suggesting an excellent safety profile of the QMODE+ ablation mode. The fact that the application duration and consequently the total RF ablation time was massively reduced utilizing the QMODE+ translated into significantly reduced median LA dwelling times and a shorter median procedure time.
For PVI only (excluding all patients with additional CTI block, n = 13 in each group), the procedure times were 56 ± 10 vs. 98 ± 35 min (P < 0.0001). With a mean procedure time of <60 min, the vHP-SD strategy offers short procedure times comparable with single-shot devices.13,14,15 Although a comparable procedure time of 55.6 ± 6.6 min for PVI only was reported for the 50 W HP-SD protocol by Chen and colleagues.16 With a total mean RF time of 352 s, this was massively reduced compared with the control group (1657 s). With potentially similar or even faster PVI compared with balloon-based ablation, the ability to set further ablation strategies as well as an excellent safety profile, vHP-SD has the potential for an ideal ablation tool. With a total of 75% durable isolated PVs and 57% of patients showing all four PVs durable isolated this rate was unexpectedly high compared with 33 and 0% for the control group. Data on PV durability for the cryoballoon showed 56–69% durable isolated PVs while all four PVs were shown to be isolated in 21–26% of patients.17,18 Utilizing point-by-point RF ablation via the close protocol, Pooter et al. showed PVI durability of all four PVs in 62% of patients.19 Additionally, prior studies reporting on cryoablation or conventional RF showed durability percentages ranging from 0 to 33%.20 Our observation is strengthening the high efficacy of the QMODE+ only strategy utilizing a very-close protocol. The 12-month follow-up is promising and comparable with recent findings of single-shot devices.
Limitations
This study is the first prospective analysis on 1-year follow-up of vHP-SD only-based PVI in comparison with standard AI-guided PVI. It is a non-randomized analysis resulting in potential biases. Although we are presenting single-center experience with a relatively small number of patients, consecutive patients where prospectively evaluated and all procedures were performed by two highly experienced operators. A Teso probe was provided in all patients of the vHP-SD group. Yet, no post-ablation endoscopy analyses were performed. Therefore, no data on subclinical oesophageal injury are available and especially atrioesophageal fistula typically occur weeks after the procedure. The number of redo-procedures was relatively low; however, we are presenting the first data on PVI durability after vHP-SD-based PVI.
Conclusions
Here, we are reporting on the efficacy and safety of vHP-SD-based PVI utilizing a very-close protocol as compared with standard CF-sensing AI-guided PVI. While demonstrating similar acute and long-term efficacy for PVI, the total ablation time, as well as procedural duration, were impressively low utilizing vHP-SD. The data are promising and is comparable with the data of recent single-shot catheter ablation procedures.
Contributor Information
Christian-H Heeger, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Makoto Sano, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany; Division of Cardiology, Internal Medicine III, Hamamatsu University Hospital, 1-20-1 Handayama, Higashi-ku, Hamamatsu city, Shizuoka 431-3192, Japan.
Sorin Ștefan Popescu, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Behnam Subin, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Marcel Feher, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Huong-Lan Phan, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Bettina Kirstein, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Julia Vogler, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Charlotte Eitel, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Sascha Hatahet, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany.
Karl-Heinz Kuck, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany; LANS Cardio, Stephansplatz 5, Hamburg 20354, Germany.
Roland R Tilz, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, Lübeck D-23538, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany; LANS Cardio, Stephansplatz 5, Hamburg 20354, Germany.
Author contributions
C.-H.H. contributed to concept/design, data collection, data analysis and interpretation, drafting article. M.S., S. Ș.P., B.S., M.F., S.H., B.K., H.-L.P., and K.-H.K. contributed to critical revision and approval. C.E. and J.V. contributed to data collection, critical revision, and approval. R.R.T. contributed to concept/design, data analysis, and interpretation, critical revision, and approval.
Data availability
The data will not be available for other researchers due to ethical reasons.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2020;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJet al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. New Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 3. Hussein A, Das M, Chaturvedi V, Asfour IK, Daryanani N, Morgan Met al. Prospective use of ablation index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2017;28:1037–47. [DOI] [PubMed] [Google Scholar]
- 4. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni Aet al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients. Circ Arrhythm Electrophysiol 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 5. Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler Vet al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Ep Europace 2019;22:388–93. [DOI] [PubMed] [Google Scholar]
- 6. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa Met al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electr 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 7. Reddy VY, Grimaldi M, Potter TD, Vijgen JM, Bulava A, Duytschaever MFet al. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol 2019;7:778–86. [DOI] [PubMed] [Google Scholar]
- 8. Barkagan M, Contreras-Valdes FM, Leshem E, Buxton AE, Nakagawa H, Anter E. High-power and short-duration ablation for pulmonary vein isolation: safety, efficacy, and long-term durability. J Cardiovasc Electr 2018;29:1287–96. [DOI] [PubMed] [Google Scholar]
- 9. Duytschaever M, Pooter JD, Demolder A, Haddad ME, Phlips T, Strisciuglio Tet al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: the CLOSE to CURE study. Heart Rhythm 2020;17:535–43. [DOI] [PubMed] [Google Scholar]
- 10. Münkler P, Kröger S, Liosis S, Abdin A, Lyan E, Eitel Cet al. Ablation index for catheter ablation of atrial fibrillation—clinical applicability and comparison with force-time integral. Circ J 2018;82:CJ-18–0361. [DOI] [PubMed] [Google Scholar]
- 11. Chen S, Schmidt B, Bordignon S, Tohoku S, Urban VC, Schulte-Hahn Bet al. Catheter ablation of atrial fibrillation using ablation index-guided high-power technique: Frankfurt AI high-power 15-month follow-up. J Cardiovasc Electr 2021;32:616–24. [DOI] [PubMed] [Google Scholar]
- 12. Tilz RR, Sano M, Vogler J, Fink T, Saraei R, Sciacca Vet al. Very high-power short-duration temperature-controlled ablation versus conventional power-controlled ablation for pulmonary vein isolation: the fast and furious—AF study. Int J Cardiol Hear Vasc 2021;35:100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heeger CH, Wissner E, Wohlmuth P, Mathew S, Hayashi K, Sohns Cet al. Bonus-freeze: benefit or risk? Two-year outcome and procedural comparison of a “bonus-freeze” and “no bonus-freeze” protocol using the second-generation cryoballoon for pulmonary vein isolation. Clin Res Cardiol 2016;105:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chun KR, Stich M, Furnkranz A, Bordignon S, Perrotta L, Dugo Det al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm 2017;14:495–500. [DOI] [PubMed] [Google Scholar]
- 15. Heeger CH, Wissner E, Mathew S, Hayashi K, Sohns C, Reissmann Bet al. Short tip-big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clin Res Cardiol 2016;105:482–8. [DOI] [PubMed] [Google Scholar]
- 16. Chen S, Schmidt B, Bordignon S, Urbanek L, Tohoku S, Bologna Fet al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: procedural data, lesion analysis, and initial results from the FAFA AI high power study. J Cardiovasc Electr 2019;30:2724–31. [DOI] [PubMed] [Google Scholar]
- 17. Heeger C-H, Rexha E, Maack S, Rottner L, Fink T, Mathew Set al. Reconduction after second-generation cryoballoon-based pulmonary vein isolation—impact of different ablation strategies. Circ J 2020;84:902–10. [DOI] [PubMed] [Google Scholar]
- 18. Heeger C-H, Wissner E, Mathew S, Deiss S, Lemes C, Rillig Aet al. Once isolated, always isolated? Circ Arrhythm Electrophysiol 2018;8:1088–94. [DOI] [PubMed] [Google Scholar]
- 19. Lycke M, O’Neill L, Gillis K, Wielandts J-Y, Waroux J-BLPD, Tavernier Ret al. How close are we toward an optimal balance in safety and efficacy in catheter ablation of atrial fibrillation? Lessons from the CLOSE protocol. J Clin Med 2021;10:4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pooter JD, Strisciuglio T, Haddad ME, Wolf M, Phlips T, Vandekerckhove Yet al. Pulmonary vein reconnection no longer occurs in the majority of patients after a single pulmonary vein isolation procedure. JACC Clin Electrophysiol 2019;5:295–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be available for other researchers due to ethical reasons.