Abstract
Pulmonary arterial hypertension (PAH) is a fatal disease with a well-established sexual dimorphism. Activated inflammatory response and altered redox homeostasis, both known to manifest in a sex-specific manner, are implicated in the pathogenic mechanisms involved in PAH development. This study aimed to evaluate the impact of sex and plasma redox status on circulating cytokine profiles. Plasma oxidation-reduction potential (ORP), as a substitute measure of redox status, was analyzed in male and female Group 1 PAH and healthy subjects. The profiles of 27 circulating cytokines were compared in 2 PAH groups exhibiting the highest and lowest quartile for plasma ORP, correlated with clinical parameters, and used to predict patient survival. The analysis of the PAH groups with the highest and lowest ORP revealed a correlation between elevated cytokine levels and increased oxidative stress in females. In contrast, in males, cytokine expressions were increased in the lower oxidative environment (except for IL-1b). Correlations of the increased cytokine expressions with PAH severity were highly sex-dependent and corresponded to the increase in PAH severity in males and less severe PAH in females. Machine learning algorithms trained on the combined cytokine and redox profiles allowed the prediction of PAH mortality with 80% accuracy. We conclude that the profile of circulating cytokines in PAH patients is redox- and sex-dependent, suggesting the vital need to stratify the patient cohort subjected to anti-inflammatory therapies. Combined cytokine and/or redox profiling showed promising value for predicting the patients' survival.
INTRODUCTION
The role of the immune and inflammatory systems in pulmonary arterial hypertension (PAH) has been intensively studied using preclinical models and clinical trials,1-7 and the contribution of various cytokines in the pathogenesis of PAH has been proposed.8-12 The current concept suggests that the initial damage of small pulmonary arteries triggers the recruitment and activation of immune cells, which contribute to vascular remodeling and disease progression, in particular, through an elevated cytokine release. However, the type and intensity of immune system response may vary for each sex.13,14 We have previously reported sexual dimorphism in the inflammatory sub-phenotypes in PAH preclinical models.15-18 Specifically, we identified that male rats developed an inflammatory and/or fibrotic phenotype, while females showed proliferative changes in the absence of inflammatory response.16 Furthermore, males were found to have a more progressive PAH18 that involved the disruption of the endothelial cell barrier in the lungs15 and an over-activation of TLR4-mediated inflammatory signaling.17,18 These data suggest that, at least in preclinical models, vascular remodeling in male rodents exhibits a higher inflammatory component than in females. However, the role of inflammation in patients with PAH remains unclear and, with respect to sex, heterogeneous. For example, the PAH associated with female-dominant autoimmune diseases, such as systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis, etc., displays a woman-to-man ratio ranging from 2:1 to 19:1.19 In contrast, HIV-associated PAH is increased in males,20 with an inverted female-to-male ratio of up to 1:7.7.19 Thus, understanding the role of sex in the inflammatory responses in PAH patients is vital to optimize disease management.
Inflammation and oxidative stress are often considered closely related. Activated by initial damage, inflammatory cells produce high amounts of reactive oxygen species (ROS), exacerbating oxidative stress and tissue damage. This relationship becomes more complicated when the role of sex is taken into account.21 Our recent data indicate that females have a considerably high level of apoptotic cell death in response to cellular stress in the absence of signs of necrotic damage.15,17,22-24 The oxidative environment produced by dysfunctional mitochondria in apoptotic cells limits the immune response and promotes immune tolerance.25,26 Therefore, clearance of apoptotic cells occurs in the absence of pro-inflammatory responses and is associated with a release of anti-inflammatory cytokines.27 In contrast, male cells respond to acute or chronic stress by necrotic cell death.17,18,22-24 Necrotic intracellular content increases the amount of reducing equivalents in the extracellular environment17 and induces a robust immunogenic response.28 This “reductive stress” was described in several inflammation-associated diseases, including PAH.29 Based on this previous research, we propose that sex is a critical regulator of the extracellular redox homeostasis and intensity of inflammatory response. In this study, we analyzed cytokine profiles categorized by sex and oxidative status. The results revealed a significant need for PAH patient cohort stratification based on these 2 parameters.
MATERIALS AND METHODS
Patient cohorts.
PAH and control subjects were prospectively recruited by the University of Arizona (UA). All subjects provided written consent to participate in this study with the approval of the UA institutional human subjects review board. Peripheral venous blood was collected during outpatient clinic visits or right heart catheterization and stored at the University of Arizona Biobank. Care was taken to standardize blood sample collection, preparation, and storage at −80°C, as previously described.17
A total of 140 PAH patients (41 males and 99 females) who met the World Symposium of PH Group 1 criteria30 and 50 healthy subjects (29 males and 21 females) were used in this study for redox and cytokine profiling. Clinical data were extracted from the electronic medical record; 6 minute walk distance (6MWD), brain natriuretic peptide (BNP), and functional class (FC) tests were selected based on the completion of assessment date closest to the date of right heart catheterization. The outcome of time to death was assessed during the 5 year period that followed blood sampling. The cohort characteristics of blood sampling are presented in Table 1.
Table 1.
Demographic data and main clinical parameters for PAH and healthy cohorts
| PAH males | N | Non-PAH males |
N | PAH females |
N | Non-PAH females |
N | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years, median (25-75% IQR) | 58 (52-66) | 41 | 60 (47-69) | 29 | 61 (51-70)* | 99 | 52 (42-58) | 21 | 0.78 |
| Race, n (%) | 36 (88) | 24 (83) | 87 (88) | 18 (86) | |||||
| White | 1 (2) | 1 (3) | 0 (0) | 0 (0) | |||||
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Hispanic | 3 (7) | 1 (3) | 6 (6) | 0 (0) | |||||
| Black | 1 (2) | 3 (10) | 5 (5) | 3 (14) | |||||
| Other | |||||||||
| Non-invasive disease metrics | |||||||||
| NYHA functional class, n (%) | |||||||||
| I | 1 (2) | 3 (3) | |||||||
| II | 8 (20) | 22 (22) | |||||||
| III | 29 (71) | 67 (68) | |||||||
| IV | 3 (7) | 7 (7) | |||||||
| 6-Minute walk distance (m), median (IQR) | 364 (285-414) | 20 | 300 (206-395) | 68 | 0.1 | ||||
| Brain natriuretic peptide (pg/ml), median (IQR) | 99 (41-211) | 39 | 117 (45-298) | 91 | 0.99 | ||||
| Hemodynamics, median (IQR) | |||||||||
| Mean pulmonary arterial pressure (mmHg) | 40 (32.2-53) | 40 | 40 (30-50) | 95 | 0.56 | ||||
| Right atrium pressure (mmHg) | 8 (5-10.5) | 22 | 8 (5-11.5) | 65 | 0.74 | ||||
| Pulmonary vascular resistance (Wood units) | 5.7 (2.9) | 40 | 6 (4) | 93 | |||||
| Pulmonary artery wedge pressure (mmHg) | 10 (7-14) | 40 | 10 (8-13.5) | 93 | 0.64 | ||||
| Cardiac output (L/min) | 5.8 (4.9-6.9) | 40 | 5.7 (4.5-6.6) | 94 | 0.26 | ||||
| Cardiac index (l/min/m2) | 3 (2.3-3.4) | 40 | 2.9 (2.5-3.6) | 94 | 0.64 | ||||
| Cardiac imaging, median (IQR) | 32 (24-40) | 19 | 35 (25.8-42.7) | 38 | 0.17 | ||||
| Cardiac MRI right ventricle ejection fraction (%) | 32 (20.2-40) | 19 | 32 (26-41) | 39 | 0.34 | ||||
| Cardiac MRI right ventricular stroke volume index (mL/m2) | 23 (56.1) | 42 (42.4) | |||||||
| PAH etiology, n (%) | 8 (19.5) | 38 (38.4) | |||||||
| Idiopathic PAH | 2 (4.9) | 8 (8.1) | |||||||
| Connective tissue disease-APAH | 3 (7.3) | 4 (4) | |||||||
| Congenital heart disease-APAH | 2 (4.9) | 3 (3) | |||||||
| Portopulmonary hypertension | 3 (7.3) | 1 (1) | |||||||
| Drugs and toxins-APAH | 0 (0) | 3 (3) | |||||||
| HIV-APAH | 12 (29.3) | 26 (26.3) | |||||||
| Hereditary PAH | 13 (31.7) | 31 (31.3) | |||||||
| Extent of PAH therapy, n (%) | 12 (29.3) | 32 (32.3) | |||||||
| Treatment naïve | 4 (9.8) | 10 (10.1) | |||||||
| Monotherapy | |||||||||
| Dual therapy | |||||||||
| Triple therapy | |||||||||
| Redox status, median (IQR) | |||||||||
| Oxidation-reduction potential | 176.6 (155-201.8)* | 41 | 142 (123-151) | 29 | 179.7 (144.9-201.5)* | 99 | 130 | 21 | 0.64 |
| 5-year survival (%), CI | 48.1 (28.7-65.2) | 30 | 62.7 (50.0-73.0) | 67 | 0.12 | ||||
| Hazard ratio (logrank), CI | 0.60 (0.29-1.24) | 30 | 1.67 (0.81-3.44) | 67 |
Demographic data and clinical parameters depict no significant difference between male and female PAH or healthy control cohorts in age or redox status. Both sexes in the PAH cohort show an equal distribution in age, race, functional class, hemodynamic and cardiac function parameters, and profiles of the PAH-specific therapy. The underlying PAH etiology shows the prevalence of connective tissue disease-associated PAH in females and the higher representation of HIV-associated PAH in male patients. IQR= 25%–75% interquartile range.
P < 0.0001 PAH vs sex-matched healthy subjects in each sex group; P = 0.78 for male PAH vs female PAH patients; P = 0.97 for male vs female healthy subjects; ANOVA Tukey's multiple comparisons test.
Redox parameters evaluation.
Oxidation-reduction potential (ORP) was measured in 30 μL of patient samples electrochemically using RedoxSys Diagnostic System (Aytu BioScience Inc., Englewood, CO), the diagnostic platform that measures ORP in body fluids as described in the manufacturer’s protocol and published.17,31-33
Cytokine multiplex assay.
The Bio-Plex multiplex immunoassay platform permits high throughput identification of proteins in the biological samples using premade or custom-made panels. The Bio-Plex Pro Human Cytokine Group1 Panel 27-Plex (Bio-Rad, M5000KCAF0Y) was used for the analysis of cytokines, chemokines, and growth factors in human plasma of healthy and PAH subjects. Bead-based assay permits the detection of 27 different types of cytokine, chemokine, or growth factor target in a single well of a 96-well microplate. The assay was performed according to the manufacturer’s protocol. Briefly, human plasma was diluted 2-fold with Bio-Plex sample diluent and added to beads covalently coupled to antibodies against 27 targets. After 30 minutes of incubation on a shaker at room temperature, beads were washed, and biotinylated detection antibodies were added for 30 minutes under the same conditions. After a 3 time wash, streptavidin-phycoerythrin (streptavidin-PE) complex was added to bind to the biotinylated detection antibodies for 10 minutes at room temperature. The plate was processed on the Bio-Plex instrument immediately. Data Acquisition at low PMT, RP1 setting, and Analysis Data was performed using the Bio-Plex 200 System (Bio-Rad).
Principal component analysis.
Principal component analysis (PCA) was applied to the controls and PAH patients to visualize high-dimensional data clustering. To analyze and plot the data set, we utilized the Orange software package (version 3.26). Cohorts were disaggregated by sex, and PCA was done on cytokines that showed redox-specific expression profiles. For males, there were ten cytokines (IL-1b, MIP-1a, G-CSF, IL-6, IL-1ra, VEGF, IL-10, Eotaxin, MCP1, IFNg) involved in PCA; for females – thirteen (IL-1b, IL-2, IL-13, IL-7, IL-17, Eotaxin, IL-8, IL-10, MIP1a, IFNg, VEGF, IL-1ra, MCP-1). The built-in Orange software algorithms and intelligent data visualizations techniques (Scatterplot and VizRank) have unbiasedly defined and plotted the coloring class areas with boundaries between healthy controls and high- and low-ORP pulmonary hypertension. Linear projection vectors have identified cytokines involved in the separation of these classes.
Machine learning classification analysis of biomarkers.
For machine learning analysis, we utilized the Orange software package (version 3.26). To identify the best algorithms for classifier learning, we used 6 different algorithms (Random Forest, Support Vector Machine, Neural Network, Naïve Bayes, Logistic Regression, and Stochastic Gradient Descent). The cytokine profile data were randomly split into the train data set (80%) and test data set (20%) repeated 5 times. We have also utilized cross-validation by splitting data into 3-folds. The best algorithms were selected using the performance criteria such as sensitivity, the area under the curve (AUC), and classification accuracy (CA). For the sex-based separation of the patient cohort, the best model based on classification accuracy in a cross-validation study was identified as Stochastic Gradient Descent. For redox-based stratification, the Support Vector Machine model was more accurate. For the prediction of patient mortality, the Neural Network algorithm showed the best accuracy of prediction. The confusion matrix for different classifications was assessed using cross-validation, and the accuracies of predictions reported. ROC curves for each algorithm were plotted, and feature importance for each cytokine was calculated as an information gain value. Student's t-test was used to identify statistical significance between survival and non-survival clinical and cytokines data.
Cytokine analysis by immunoblotting.
Three cytokines representative for each specific group: (1) cytokines that show similar redox sensitivity in males and females (IL-1β); (2) cytokines that precisely elevates in low ORP conditions in males (IL-6), and (3) cytokines that elevate explicitly in the high ORP conditions in females (IL-13) were also analyzed in human plasma by Western Blot analysis as previously described.17 Briefly, 1ul of plasma collected from control subjects or PAH patients with low and high ORP was mixed with reduced 6X Laemmli sample buffer (Boston Bioproducts, Ashland, MA, cat BP-111R). After 5 minute of incubation at 95°C, the samples were loaded on the 4%–20% Mini-PROTEAN TGX Stain-Free gels (Bio-Rad Laboratories, Hercules, CA) and electrophoretically separated using a PowerPac Universal power supply. The samples were transferred using Trans-Blot Turbo transferring system (Bio-Rad Laboratories, Hercules, CA); the membranes were blocked with 5% BSA (Fisher Scientific, Fair Lawn, NJ, cat 9048-46-8) and incubated with anti-IL-1β (Abcam, Cambridge, MA, ab9722), IL-6 (Santa Cruz Biotechnology, Inc., Dallas, Texas, sc-57315) and anti-IL-13 (Abcam, Cambridge, MA, ab201470) antibody diluted to 1:1000 for overnight at 4C. The membranes washed 3 times with Tris-buffered saline (TBST) were incubated with anti-Rabbit IgG, HRP-Linked Antibody (Cell Signaling, Danvers, MA cat 7074S) diluted to 1:5000 for 1 hour at room temperature. The signal was recorded with the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA) using a chemiluminescent protocol and analyzed using Image Laboratory software. The loading was normalized per total sample protein using free stain gels as published.18,34
Statistical analysis.
The normality of the data was assessed by Kolmogorov-Smirnov and Shapiro-Wilk tests. Cytokine expression in groups was reported as mean ± SEM. Stratified analyses based on cytokine profiles were performed, in which differences in continuous variables were assessed using the Student’s t-test for normally distributed data. Correlations were performed utilizing Pearson’s or Spearman's analyses based on the normality of the data. To visualize high-dimensional data clustering, PCA analysis was carried out by the Orange software package (version 3.26). Survival analysis with left-truncated right-censored data was calculated and plotted in R using OIsurv and KMsurv packages. Kaplan-Meier estimates of patient survival and the hazard ratio for the 5 year risk of death were compared between the sexes by a log-rank test. Kaplan-Meier estimates of survival for selected cytokines were based on cut-off values calculated as 95 percentile of the control cohort. Statistical data analyses were carried out using statistical software, GraphPad Prizm version 8.4. P values < 0.05 were considered statistically significant.
RESULTS
PAH and control cohorts.
Table 1 details demographics for both PAH and control cohorts with similar median ages and race distribution. Both sexes in the PAH cohort showed an equal distribution in functional class, with the most prevalent class III (71% and 68% in males and females, correspondingly). There were no sex differences in 6 minute walk distance, brain natriuretic peptide levels, hemodynamic, and cardiac function parameters.
Among underlying PAH etiologies, idiopathic PAH had the highest representation (56.1% and 42.4% in male and female cohorts correspondingly). Both male and female patient cohorts consisted of connective tissue disease (CTD), congenital heart disease, portal hypertension, drugs and toxins, HIV, and hereditary PAH. In accordance with the well-described sex disparities for conditions associated with PAH,19,35 CTD was more prevalent in the female cohort, while HIV had a higher representation in male patients. Both conditions are known to be associated with chronic inflammation.36-38
Approximately 30% of PAH subjects were treatment-naïve, ~30% were placed on PAH monotherapy, another ~30% were on dual PAH therapy, and ~10% of PAH subjects received triple therapy (phosphodiesterase inhibitors, endothelin receptor antagonists, and prostanoids). Notably, the profiles of the PAH-specific therapy were closely matched in male and female PAH subjects, minimizing the effects of therapy on the discovered sex difference in cytokine profiles. We also tested whether PAH therapy affects the plasma redox profile by comparing the Oxidation Reduction Potential (ORP) values in treated and treatment-naïve patients and found no differences between these groups in either sex (Fig. S1).
Kaplan-Meier estimates of patient survival (cohort involved in cytokine analysis) showed a lower survival in males, although this difference didn’t reach statistical significance (Table 1, 5 year survival rates were 62.7%, CI 73%–50% and 48.1%, CI 65.2%–28.7% in female and male patients correspondingly). In contrast, plasma redox status showed significantly greater oxidative stress in PAH patients of both sexes compared to the sex-matched healthy controls; however, there was no significant difference in the redox profile between the sexes inside the PAH group.
The inflammatory response in PAH.
Oxidation-Reduction Potential (ORP) is a measure of the ability of the system to oxidize (lose electrons) or reduce (gain electrons). The redox status of the biological system is complex and depends on the spectrum of short-lived reactive oxygen and/or nitrogen species (RO/NS), RO/NS producing and detoxifying enzymes, and small molecule antioxidants. Therefore, ORP represents a unique integral approach for evaluating the system's final oxidative and/or reductive status. The large body of previously published research confirms the high predictive value of ORP in evaluating the redox status of human plasma and other fluids in health and disease.17,31-33, 39-46 Therefore, we used ORP as the robust and straightforward approach for evaluating patients' plasma redox status. ORP was normally distributed in plasma samples collected from male and female PAH patients (Fig. S2). To investigate whether the inflammatory response depends on the redox status, we selected 2 extreme quartiles, 25% of the most oxidized samples (highest ORP quartile) and 25% of the least oxidized samples (lowest ORP quartile). When both quartiles were combined (plasma redox status is not accounted for), the samples showed a significant elevation of cytokines in the PAH cohort, reproducing data published by other research groups.47,48 Notably, both sexes had comparable changes in the cytokine profile. Thus, we found that IL-1b, IL-1ra, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, G-CSF, IP10, MIP-1a, TNFa, and VEGF were increased in each sex group compared to healthy controls (Table. 2). Eotaxin and FGFb were elevated in females but were unchanged in males. The circulating levels of MIP-1b were low in males with PAH but not in females, and RANTES was below the control level in both sexes. Other cytokines, such as IL-5, IL-9, IL-15, GM-CSF, INFγ, MCP1, and PDGFbb, remained unaltered in each sex compared to healthy subjects.
Table 2.
Cytokine profile in male and female PAH patients
| Cytokines | Males (Control vs PAH) |
Females (Control vs PAH) |
||||
|---|---|---|---|---|---|---|
| P value | Control; PAH (mean±SE), pg/ml |
Fold, Control |
P value | Control; PAH (mean±SE), pg/ml |
Fold, Control |
|
| IL-1β | P = 0.0002 | 0.15±0.017; 0.56±0.098 | 3.7 | P = 0.0005 | 0.13±0.02; 0.60±0.05 | 4.6 |
| IL-1ra | P = 0.0015 | 57.75±5.11; 86.81±6.80 | 1.5 | P < 0.0001 | 87.57±8.05; 161.9±10.88 | 1.8 |
| IL-2 | P < 0.0001 | 1.99±0.35; 5.46±0.55 | 2.7 | P = 0.0036 | 2.31±0.51; 5.12±0.48 | 2.2 |
| IL-4 | P < 0.0001 | 1.05±0.13; 2.96±0.23 | 2.8 | P < 0.0001 | 0.95±0.13; 2.73±0.16 | 2.9 |
| IL-6 | P = 0.0012 | 1.46±0.21; 4.43±0.82 | 3.0 | P < 0.0001 | 1.15±0.19; 6.25±0.74 | 5.4 |
| IL-7 | P < 0.0001 | 7.0±0.81; 27.8±3.18 | 4.0 | P < 0.0001 | 8.49±1.61; 24.03±1.73 | 2.8 |
| IL-8 | P < 0.0001 | 4.16±0.47; 9.55±0.87 | 2.3 | P = 0.0002 | 4.75±0.54; 13.95±1.37 | 2.9 |
| IL-10 | P = 0.0009 | 2.60±0.58; 7.54±1.08 | 2.9 | P = 0.0004 | 1.96±0.35; 7.40±0.82 | 3.8 |
| IL-12 (p70) | P = 0.0006 | 2.81±0.71; 8.53±1.25 | 3 | P = 0.0041 | 2.0±0.37; 7.2±0.89 | 3.6 |
| IL-13 | P < 0.0001 | 0.57±0.10; 2.11±0.22 | 3.7 | P < 0.0001 | 0.54±0.12; 2.00±0.15 | 3.7 |
| IL-17 | P < 0.0001 | 6.30±0.47; 11.17±0.89 | 1.8 | P = 0.0113 | 8.2±1.21; 12.9±0.93 | 1.6 |
| G-CSF | P < 0.0001 | 55.65±5.76; 146.90±9.34 | 2.6 | P < 0.0001 | 65.95±7.88; 147.7±7.23 | 2.2 |
| IP10 | P = 0.0002 | 90.88±8.56; 279.30±46.60 | 3.1 | P < 0.0001 | 118.0±15.16; 353.10±29.28 | 3.0 |
| MIP-1α | P < 0.0001 | 0.48±0.08; 1.87±0.16 | 3.9 | P < 0.0001 | 0.60±0.14; 2.27±0.18 | 3.8 |
| TNFα | P = 0.001 | 28.24±2.93; 43.03±2.88 | 1.5 | P = 0.0034 | 28.18±2.93; 41.53±2.22 | 1.5 |
| VEGF | P = 0.0111 | 75.72±7.02; 114.40±12.61 | 1.5 | P = 0.0014 | 56.67±4.87; 107.90±9.02 | 1.9 |
| Eotaxin | P = 0.1533 | 33.89±4.39; 44.54±5.46 | 1.3 | P = 0.0244 | 27.0±3.37; 45.29±4.93 | 1.7 |
| FGF basic | P = 0.063 | 23.90±2.95; 17.76±1.48 | 0.7 | P = 0.0192 | 30.43±6.52; 20.01±1.0 | 0.7 |
| MIP-1β | P = 0.0265 | 48.09±4.23; 37.73±1.49 | 0.8 | P = 0.6608 | 45.66±2.13; 47.56±2.51 | 1.0 |
| RANTES | P < 0.0001 | 5786±844; 1827±248 | 0.3 | P < 0.0001 | 7296±882; 2376±210 | 0.3 |
| IL-5 | P = 0.0983 | 4.67±1.02; 10.77±2.57 | 2.3 | P = 0.1219 | 5.11±0.90; 9.36±1.25 | 1.8 |
| IL-9 | P = 0.5715 | 53.5±4.70; 56.8±3.19 | 1 | P = 0.3735 | 72.37±5.14; 67.47±2.78 | 0.9 |
| IL-15 | P = 0.775 | 43.2±8.59; 39.8±7.17 | 0.9 | P = 0.2689 | 43.78±10.66; 92.32±18.88 | 2.1 |
| GM-CSF | P = 0.2494 | 0.91±0.25; 1.26±0.18 | 1.4 | P = 0.7414 | 1.59±0.53; 1.38±0.25 | 0.9 |
| IFN γ | P = 0.6449 | 4.42±0.71; 4.00±0.55 | 0.9 | P = 0.084 | 3.53±0.37; 5.35±0.62 | 1.5 |
| MCP1 | P = 0.4719 | 35.4±3.48; 30.2±6.18 | 0.9 | P = 0.845 | 30.76±2.58; 32.21±4.30 | 1.0 |
| PDGF bb | P = 0.446 | 558±68; 440±152 | 0.8 | P = 0.2206 | 570.1±62.23; 444.4±60.38 | 0.8 |
The levels of circulating cytokines, measured in high (most oxidized) and low (least oxidized) ORP quartile, were combined to evaluate the PAH-mediated changes in cytokine profiles without accounting for the redox status. Compared to sex-matched Control subjects, PAH patients of both sexes showed a significant upregulation of the inflammatory response (18 cytokines out of 27 were found to be significantly elevated in males; 19 out of 27 – in females). Without the redox-based stratification, the profile of circulating cytokines was comparable between male and female PAH patients. For each sex, the first number, shown as mean ± SE, represents Control subjects, the second – PAH patients. White fields show the cytokines that were not significantly elevated compared to sex-matched Control; light grey fields represent the cytokines with a P value between 0.05 and 0.001; dark grey fields show cytokine with P < 0.001.
Cytokines profiles with consideration of plasma redox status and patient sex.
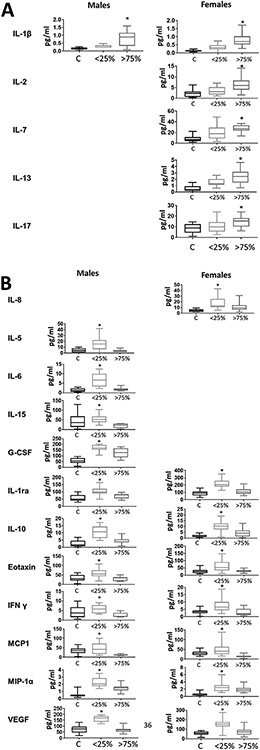
Fig 1 consists of cytokines discovered as ORP-dependent since they were significantly altered in the patients with either the lowest or the highest plasma ORP values. Interestingly, some of these cytokines were not depending on patient sex. Thus, IL-1β was increased only in the most oxidized samples, while IL-1ra, IL-10, Eotaxin, INFy, MCP1, MIP-1a, and VEGF were elevated only in low ORP samples, and these changes were evident in both sexes. In contrast, other cytokines revealed their ORP dependency only in consideration of sex. Thus, IL-2, IL-7, IL-13, and IL-17 were increased in the samples with the highest ORP, specifically in women. IL-8 was increased in females' low ORP group, while IL-5, IL-6, IL-15, and G-CSF were also increased in the low ORP group, but only in males. These results suggest that the redox state of the microenvironment may influence cytokines expression and release. However, despite the commonly accepted interconnection between oxidative stress and inflammation, not all cytokines correlated with ORP. Moreover, sex significantly affected the correlations between cytokine levels and plasma redox state. Thus, female patients have a higher number of cytokines affected by oxidative stress, whereas, in males, all cytokines except IL-1β were upregulated in patients with the least oxidized plasma. The same results were obtained in 3 representative cytokines - IL-1β, IL-6, and IL-13 using an immunoblotting analysis as an alternative method to measure plasma cytokine levels (Fig. S3).
Fig 1.
Redox-based profile of circulating cytokines. The contribution of the redox status was evaluated by comparing the levels of circulating cytokines in the Low-ORP quartile (<25%) vs the High-ORP quartile (>75%) in each sex. Female patients showed more cytokines significantly elevated in High-ORP samples (A). In males, cytokines were predominantly elevated in Low-ORP samples (B). In each graph, Controls subjects are shown in the first boxplot (black contour), the least oxidized samples with the lowest ORP – as a second boxplot (light grey contour), and the most oxidized samples with the highest ORP – as a third boxplot (dark grey contour). Boxplots are presented only for ORP-dependent cytokine (highest or lowest ORP group is significantly different vs controls). *indicate significance (P < 0.05) between highest and lowest ORP by the Students t-test.
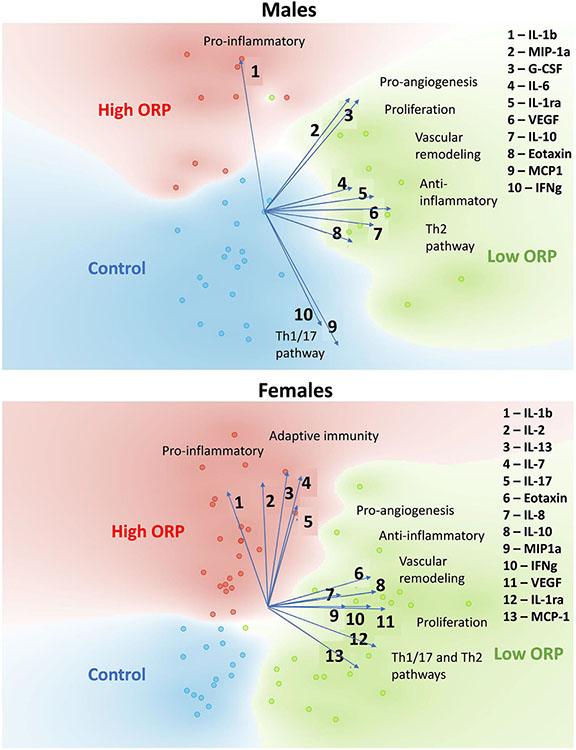
The principal component analysis (PCA) of redox-dependent cytokines showed distinct clustering of control and PAH subjects with low and high ORP status (Fig 2). Importantly, this separation was achieved only when the data were disaggregated by sex, while analysis without sex-based stratification disrupted the clustering (data not shown). This discovery suggests that the contribution of both factors, sex, and redox status, are required to distinguish patients with PAH from healthy controls and could be used for diagnostic purposes. To identify the cytokines that served as the primary determinants of separation of high- or low-ORP PAH patients from the healthy cohort, we plotted the linear projection vectors. In males, IL-1b was the most influential in separating the high-ORP PAH patients from the healthy controls, while MIP-1α, G-CSF, IL-1ra, IL-6, IL-10, VEGF, and Eotaxin all contribute to distinguishing the low-ORP PAH group from controls. In females, cytokines IL-1b, IL-2, IL-7, IL-13, and IL-17 were all involved in the high-ORP group clustering, while Eotaxin, IL-1ra, IL-8, IL-10, VEGF, MIP-1α, IFNγ, and MCP-1 helped to distinguish the low ORP patients.
Fig 2.
Redox-based clustering of control and PAH plasma samples in each sex. Principal component analysis (PCA) of cytokines that differentially express in 2 extreme redox conditions, the most and the least oxidized, revealed the clustering of PAH samples with Low-ORP, High-ORP, and control classes for each sex. The linear projection of individual cytokines showed vectors that are contributed to the separation of the classes. In males, IL-Ib, a pro-inflammatory cytokine, showed the highest involvement in separating patients with High-ORP from controls. MIP-1a, G-CSF, IL-6, IL-1ra, VEGF, IL-10, and Eotaxin exhibited influence on clustering of patients with Low-ORP. In females, not only IL-1b, but also IL-2, IL-13, IL-7, and IL-17 contributed to the clustering of High-ORP samples. The Low-ORP group's separation was driven by Eotaxin, IL-8, IL-10, MIP-1a, IFNg, VEGF, IL-1ra, and MCP-1. Overall, High-ORP clustering is mediated by pro-inflammatory cytokines, and Low-ORP - by proliferative and anti-inflammatory pathways.
In both sexes, the cytokines profiles were categorized as previously described.49 Pro-inflammatory response mediators were the main factors that defined the patients with a high level of oxidative stress in both sexes. This finding corresponds to the well-established interconnection between oxidative stress and inflammation. However, the mediators of angiogenesis, proliferation, vascular remodeling, and anti-inflammatory pathways were found to contribute to the separation of patients with low ORP (or the less oxidized plasma), suggesting that the low oxidation, or increased level of reduced equivalents, could also be involved in the activation of the pathways associated with PAH initiation and progression.
Correlations between the clinical parameters and the cytokine levels.
Although the contribution of the inflammatory component to PAH development is known, most studies do not observe a reproducible correlation between cytokines and disease parameters. We discovered that consideration of sex and/or plasma redox status increases the number of significant correlations. In men, 7 cytokines significantly correlated with the changes in the clinical parameters. Except for G-CSF, the elevated cytokine levels corresponded to increased PAH severity, defined as higher mPAP, PVR, and BNP and lower CO, CI, and 6MWD (Table 3). In women, 14 cytokines significantly correlated with the severity markers, although only 3 (IL-1b, IL-9, and IP10) positively correlated with the PAH severity. The majority of cytokines, such as IL-2, IL-4, IL-5, IL-7, IL-12, IL-13, IL-15, IL-17, and Eotaxin, correlated with a decrease in PAH severity, suggesting that not an elevated production of these cytokines, but rather their decrease corresponds to more severe disease. We conclude that in females, cytokines may simultaneously play a role in the PAH progression and the adaptive responses.
Table 3.
Correlation analysis of PAH severity markers and cytokine expression profile
| Cytokines | Males |
Females |
Interactions | ||||
|---|---|---|---|---|---|---|---|
| clinical parameter | R | P value | clinical parameter | R | P value | P value | |
| IL-1β | CI | 0.24 | P = 0.32 | CI | −0.36 | P = 0.017 | P = 0.045 |
| IL-2 | mPAP | 0.30 | P = 0.19 | mPAP | −0.31 | P = 0.0497 | P = 0.04 |
| IL-4 | BNP | 0.05 | P = 0.84 | BNP | −0.396 | P = 0.039 | P = 0.08 |
| IL-5 | 6MWD | −0.16 | P = 0.68 | 6MWD | 0.75 | P < 0.0001 | P = 0.02 |
| BNP | 0.11 | P = 0.67 | BNP | −0.40 | P = 0.049 | P = 0.16 | |
| IL-7 | 6MWD | −0.005 | P = 0.99 | 6MWD | 0.42 | P = 0.02 | P = 0.4 |
| IL-12p70 | mPAP | 0.20 | P = 0.4 | mPAP | −0.36 | P = 0.03 | P = 0.04 |
| 6MWD | −0.17 | P = 0.63 | |||||
| 6MWD | 0.44 | P = 0.014 | P = 0.01 | ||||
| IL-13 | mPAP | 0.22 | P = 0.36 | mPAP | −0.40 | P = 0.01 | P = 0.048 |
| IL-17 | RVEF | −0.2 | P = 0.52 | RVEF | 0.53 | P = 0.017 | P = 0.031 |
| Eotaxin | CI | −0.02 | P = 0.93 | CI | 0.31 | P = 0.043 | P = 0.65 |
| BNP | 0.12 | P = 0.62 | BNP | −0.33 | P = 0.036 | P = 0.13 | |
| 6MWD | −0.15 | P = 0.68 | 6MWD | 0.37 | P = 0.035 | P = 0.71 | |
| IL-8 | 6MWD | −0.64 | P = 0.046 | 6MWD | 0.1 | P = 0.6 | P = 0.22 |
| BNP | 0.64 | P = 0.002 | BNP | 0.1 | P = 0.52 | P = 0.79 | |
| MIP-1α | mPAP | 0.45 | P = 0.045 | mPAP | −0.07 | P = 0.66 | P = 0.17 |
| FGF basic | 6MWD | −0.25 | P = 0.48 | 6MWD | 0.39 | P = 0.021 | P = 0.047 |
| PVR | 0.51 | P = 0.023 | PVR | −0.02 | P = 0.92 | P = 0.046 | |
| mPAP | 0.52 | P = 0.019 | mPAP | −0.19 | P = 0.23 | P = 0.01 | |
| IFN γ | PVR | 0.33 | P = 0.19 | PVR | −0.38 | P = 0.013 | P = 0.23 |
| mPAP | −0.25 | P = 0.31 | mPAP | −0.33 | P = 0.033 | P = 0.49 | |
| BNP | −0.23 | P = 0.36 | BNP | −0.36 | P = 0.024 | P = 0.21 | |
| CO | −0.49 | P = 0.038 | CO | 0.26 | P = 0.1 | P = 0.07 | |
| IP10 | 6MWD | 0.75 | P = 0.017 | 6MWD | −0.38 | P = 0.027 | P = 0.001 |
| CO | 0.09 | P = 0.71 | CO | −0.45 | P = 0.0027 | P = 0.05 | |
| BNP | 0.49 | P = 0.029 | BNP | 0.42 | P = 0.0064 | P = 0.24 | |
| CI | −0.1 | P = 0.69 | CI | −0.35 | P = 0.021 | P = 0.6 | |
| mPAP | 0.56 | P = 0.01 | mPAP | 0.23 | P = 0.13 | P = 0.53 | |
| PVR | 0.42 | P = 0.069 | PVR | 0.37 | P = 0.014 | P = 0.79 | |
| IL-9 | CO | −0.15 | P = 0.53 | CO | −0.41 | P = 0.008 | P = 0.49 |
| MIP-1β | CO | −0.46 | P = 0.042 | CO | −0.23 | P = 0.13 | P = 0.95 |
| IL-15 | 6MWD | 0.37 | P = 0.5 | 6MWD | 0.75 | P = 0.006 | P = 0.47 |
| G-CSF | CI | 0.47 | P = 0.044 | CI | 0.25 | P = 0.12 | P = 0.34 |
Correlation analysis was done in the PAH cohort disaggregated by sex. A normality test was taken before analysis for each cytokine or clinical parameter. Blue background indicates an increase in PAH severity (defined as higher mPAP, PVR, and BNP; and lower CO, CI, and 6MWD). Beige background indicates a decrease in PAH severity. Bold p-values indicate significant changes.
Only 3 out of twenty-one cytokines significantly correlated with the disease parameters in both sexes; 2 of these, FGFb and INFy, exhibited the opposite effects (a positive correlation with PAH severity in males and a negative correlation in females). Thus, distinct, sex-specific inflammatory profiles differentially contribute to PAH severity.
Cytokine profiling-based predictions.
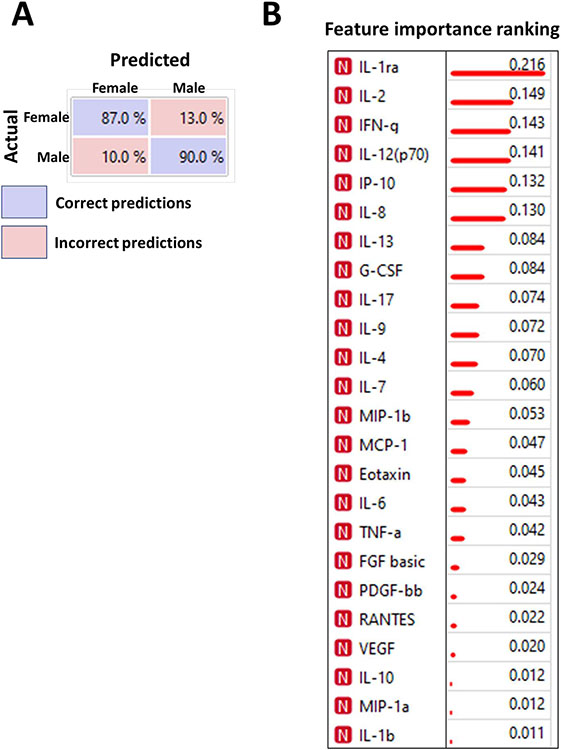
To evaluate the potential contribution of sex in the profile of circulating cytokines, we applied the Machine Learning and/or Deep learning (ML/DL) algorithms. ML models trained to recognize the specific patterns are useful tools to make unbiased predictions of classifications. The confusion matrix shown in Fig 3A indicates the results of ML predictions of patient sex based on the cytokine profiles. We found that ML/DL approach can predict the patient's sex with ~90% accuracy based on the PAH cytokine profile. Although the is no practical use in predicting the sex of the patient, this outcome highlights that the sex-specific profiles of circulating cytokines could be easily identified and separated using ML/DL. The raking of the cytokines shown in Fig 3B represents the level of contribution of each cytokine in the sex-specific separation of the overall profile. These results suggest that IL-1ra, IL-2, INFγ, IL-12(p70), IP10, and IL-8 are the primary influencers that outline the sex difference in the circulating cytokines in PAH.
Fig 3.
Sex-specific separation of PAH patient cohort based on cytokine profiles. Confusion matrix for cross-validation of Stochastic gradient descent machine learning algorithm trained on sex-specific cytokine profiles was able to distinguish males and females with 87%–90% prediction accuracy (A), confirming the presence of distinct sex-based profiles in cytokine expression identifiable by machine learning models. Blue fields indicate correct predictions; red fields – incorrect predictions (algorithm confusions). Cytokines IL-1ra, IL-2, IL-12, IFNg, IP10, and IL-8 were identified as the most potent contributors in the differentiation of male vs female cytokine profiles (B). Information gain values indicate the ranking.
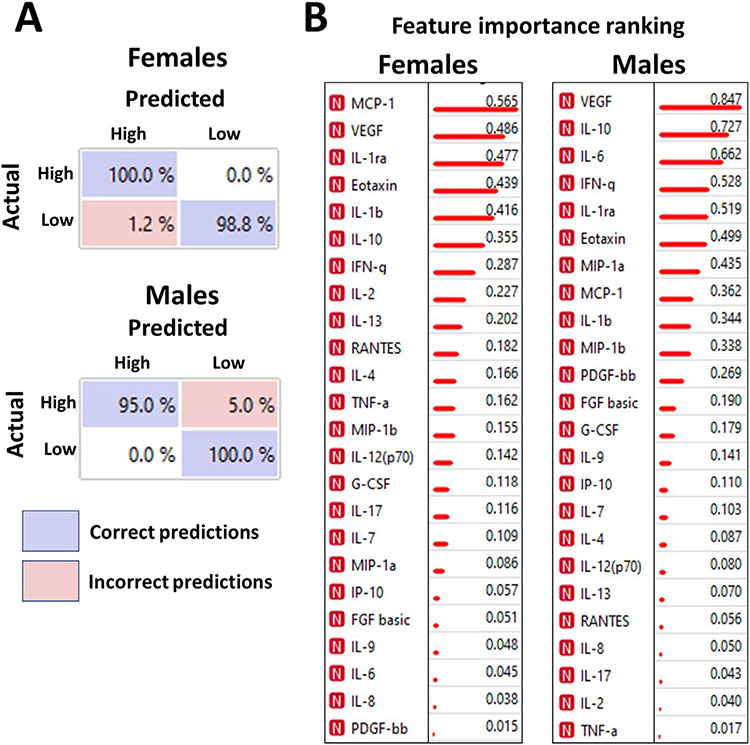
The same ML/DL algorithms were applied to identify the contribution of redox status to the cytokine profile. While no prediction was possible when the analysis was performed on patients of both sexes (data not shown), the sex-specific approach allowed an accurate (95%–100%) prediction of samples with a high or low ORP (Fig 4A). Again, we infer that redox homeostasis significantly contributes to the cytokine profile and/or release, although this contribution is sex-specific. Among the cytokines that determine the redox-specific disaggregation of cytokine profile in females are MCP1, VEGF, IL-1ra, Eotaxin, IL-1β, and IL-10, whereas in males – VEGF, IL-10, IL-6, INFγ, IL-1ra, and Eotaxin (Fig 4B); these are all ORP-dependent cytokines (Fig 2), which explicitly increased in the low-ORP samples, except for IL-1β (Fig 1).
Fig 4.
Redox-specific separation of PAH patient cohort based on cytokine profiles. Confusion matrix for cross-validation of Support Vector Machine trained on redox-specific profiles in each sex group distinguished between High-ORP and Low-ORP plasma samples with 95%–100% prediction accuracy (A). The data confirm that the difference in the redox environment triggers the distinct patterns of cytokine expression that could be accurately recognized by machine learning models. Blue fields indicate correct predictions; red fields – incorrect predictions (algorithm confusions). MCP-1, VEGF, IL-1ra, Eotaxin, IL-1b, and IL-10 were identified as the primary contributors to the redox-based profiling in females, whereas VEGF, IL-10, IL-6, IFNg, IL-1ra were responsible for the redox-based separation in males (B). Information gain values indicate the ranking.
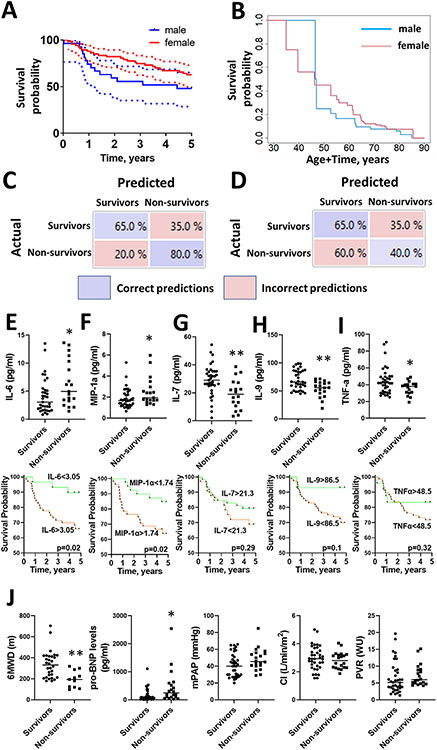
Finally, we applied the ML/DL model to predict patient survival. Compared to the previous analysis done to validate the contribution of sex and redox status in cytokine profiling, this prediction type is highly significant, as there is a demanding need to identify the patients at a high risk of mortality. The 5 year survival in the PAH cohort involved in cytokine profiling was 62.7% (CI 73%–50%) in females and 48.1% (CI 65.2%–28.7%) in male patients (Fig 5A), no significant differences in survival for all patients was found (P = 0.2) between males and females with accounting for left-truncated right-censored data (Fig 5B). The combined cytokine and ORP profiles allowed an accurate statistical classification of survivors vs non-survivors. As shown in the confusion matrix (Fig 5C), the mortality episodes were predicted with 80% accuracy. The same predictive analysis applied for the primary clinical parameters showed a much higher model confusion with accuracy in predicting patient mortality by only 40% (Fig 5D). Although cytokine and clinical markers profiles showed a comparable accuracy for predicting patient survival, the profiling of circulating cytokines could become a useful tool specifically for predicting the episodes of patient mortality. The analysis of cytokine profile in survivals vs non-survivals revealed 5 significantly altered cytokines - IL-6, MIP-1α, IL-7, IL-9, and TNFα (Fig 5E-I). Notably, only IL-6 and MIP-1α were increased in non-survived patients, while rest cytokines were decreased in non-survivals, which corresponds to our results described earlier. The Kaplan-Meier curves compared the survival of patients below and above cut-off values corresponding to the 95% percentile determined in the control subjects. It was discovered that only patients with elevated levels of IL-6 (>3.05pg/ml, P < 0.02, Fig 5E) and MIP-1α (>1.74pg/ml, P < 0.02, Fig 5F) show a significantly lower survival probability. For the rest of cytokines showing the significantly decreased levels in the non-survival group vs survivals, the Kaplan-Meier curves revealed the delayed difference in patient survival that didn’t reach the statistical significance (< 21.3pg/ml for IL-7, P = 0.29, Fig 5G; < 86.5pg/ml for IL-9, P = 0.1, Fig 5H); <48.5pg/ml for TNFα, P = 0.32, Fig 5I). Among the clinical parameters, only 6MWD and pro-BNP levels were significantly changed in survivors vs non-survivors (Fig 5J), while the rest, including mPAP, CI, PVR, were found to be similar in these patient groups.
Fig 5.
Cytokine profile but not clinical parameters predict PAH patient mortality. The Kaplan –Meier estimates of 5 year survival for each sex of patients involved in cytokine study were compared by log-rank test (solid lines; P = 0.12). Dotted lines indicate 0.95% confidence interval (A). Overall survival of patients stratified by sex assuming left truncation indicated no difference between sexes (P = 0.2) (B). Confusion matrix for cross-validation of Neural Network deep learning algorithm trained on the selected cytokine profile+redox status predicted mortality in total (male + female) PAH patient cohort with 80% prediction accuracy (C). Blue fields indicate correct predictions; red fields – incorrect predictions (algorithm confusions). The same machine-learning algorithm applied for the primary clinical parameters predicted patient mortality with 40% accuracy, although it showed a comparable accuracy for predicting patient survival (D). Five cytokines, IL-6, MIP-1α IL-7, IL-9, and TNFa were identified as significantly different between the survivals and non-survivals (E-I). Kaplan-Meier curves confirmed an unfavorable survival for patients with cytokine levels exceeding 95% percentile of the control values for IL-6 and MIP-1α (E, F). IL-7, IL-9, and TNFα showed a non-significant predisposition to the decreased survival in patients with values below the 95% percentile determined in control subjects (G-I). Only 6MWD and pro-BNP levels, but not mPAP, CI, or PVR were significantly different in survivals vs non-survivals (F). *indicate significance P < 0.05, **indicate significance P < 0.01 by the Students t-test.
DISCUSSION
Although inflammation is an essential player in PAH initiation and progression,50 the detailed contribution of inflammatory responses in PAH pathogenesis remains unclear. Prior studies confirmed the increased cytokines expression and secretion in PAH compared to healthy subjects.48,49,51,52 However, the heterogeneity of the PAH population confounds the identification of distinct signatures of the inflammatory profile. The machine learning techniques applied to delineate possible patterns in the inflammatory profiles revealed distinct immune phenotypes of patients with PAH,49 although these phenotypes did not correspond to PAH etiology. These results suggest that an alternative classification of PAH patients is required to understand the factors contributing to inflammatory-based clustering.
In the present study, we applied 2 criteria to stratify the initial PAH cohort. We discretely analyzed male and female samples and further divided patients based on the redox status of plasma. These criteria were selected based on previously published data that indicate a critical role of sexual dimorphism in the manifestation of PAH.15,16,19,53,54 We have also previously shown that the type of cell death is sex-dependent, with female cells more likely to undergo apoptosis while male cells succumb to necrosis.22 The particular type of cell death affects the redox status of the extracellular milieu. Thus, the intracellular content passively released from necrotic cells consists of considerably more reducing equivalents than the extracellular environment17,55 and shifts the overall redox balance to a more reduced and less oxidized state. Apoptosis or ferroptosis, in contrast, executes programmed death with the oxidation of intracellular components to make this process immune-silent.56-58 In this regard, the dependence of plasma redox homeostasis on sex is expected and was indeed confirmed in preclinical animal models and PAH patients.17 Thus, we found that some cytokines known to be produced in response to necrosis but not apoptosis59 were increased only in low-OPR samples and only in males, supporting the proposed interconnection between necrosis, a more reduced/less oxidized environment, and male sex.
Interestingly, in each sex, the samples with high and low ORP were clustered differently, although both exhibited a strong separation from the healthy cohort. Based on these results, we propose that plasma redox homeostasis may represent an essential contributor to the sub-phenotyping of PAH patients and be implemented into underlying pathology. Moreover, in this study, we, for the first time, outlined the cytokines that displayed redox sensitivity, as they were found to be significantly elevated in 1 of the extreme redox conditions – in plasma with the highest or lowest level of oxidation. Although the large body of published literature confirms the increased oxidative stress in the site of inflammation, the dependence of particular cytokines’ secretion on the level of oxidative stress has never been proposed or identified. Our data indicate that IL-1β is a markedly oxidative stress-driven cytokine that achieves the highest expression in an oxidative environment in both male and female patients. However, in males, IL-1β did not significantly correlate with any clinical parameters, suggesting that this oxidative stress-induced cytokine is not the primary contributor to the PAH severity in males. In contrast, in females, IL-1β significantly correlated with a decrease in CI. Other cytokines that showed increased expression in a highly oxidative milieu are IL-2, IL-7, IL-13, and IL-17, all possessing strong proinflammatory characteristics. The remainder of cytokines were increased in the less oxidized milieu, suggesting that the less oxidized environment is more favorable for cytokine production in PAH.
The difference in the redox homeostasis for each sex and the sex-specific correlations between the clinical parameters and circulating cytokines also highlight the importance of sex as a factor separating the PAH cohort into sub-groups. In males, most cytokines positively correlated with the PAH severity, as was defined earlier (higher mPAP, PVR, and BNP, and lower CO, CI, and 6MWD). On the other hand, the increased level of total antioxidant protection in females makes them more protected from oxidative damage associated with inflammation and, thus, more adapted to inflammatory reactions. Therefore, we propose that females are more dependent on the inflammation that cleans and detoxifies the damaged tissue. Indeed, a previously published ML approach revealed that the cluster of patients with the highest percentage of women (80.5%) has the highest level of upregulated cytokines, although this group of patients had the lowest mortality rate among the 4 clusters studied.49 Based on these discoveries, the inflammatory response in females could positively influence the course of PAH, which contributes to the better survival described for the female sex.60
The discovered negative association between cytokine levels and PAH severity in females could also be viewed through the increasingly recognized role of lymphocytes in regulating the course of PAH. PAH patients have a dysfunctional immune system characterized by the decreased relative and absolute number of circulating lymphocytes,61 altered T- and B-cell circulating subsets,62,63 diminished CD8+ T cells,64 functionally defective Natural killer (NK) cells,65 regulatory T cells,66 which predispose to a worse survival.67 The evidence from animal models confirms the critical importance of lymphocyte deficiency or dysfunction in predisposing to PH, as mice with genetic NK deficiency or athymic rats develop PH spontaneously or become more susceptible to pulmonary vascular injury and severe form of PH.68-70 Notably, female athymic rats developed a more severe PH than males.71 It was also noticed that female PAH patients with a significant drop in absolute lymphocytes count have low survival; the effect that was not noticed in males.72 Given that in PAH, males demonstrate higher mortality, the inverted poor prognosis in the immune-deficient females suggests that females are more dependent on an adequate immune function and sensitive to its insufficiency. Indeed, we have previously found that PH female rats had a more pronounced increase in medial thickness in the small pulmonary arteries.16 Since ineffective immune regulation is known to promote a severe angio-obliterative PAH,1 females could benefit from the immune-stimulating properties of some cytokines. Indeed, we discovered that cytokines known to either stimulate differentiation and activation of NK and T cells (IL2, IL12) or produced by already activated NK and T cells (INFγ, IL13) show a significant negative correlation with mPAP in the female patient group (Table 3).
The unbiased ML/DL approach additionally confirmed that sex affects the composition of circulating cytokines, and the resulting profiles could be efficiently separated by sex with an accuracy of around 90%. Among the cytokines identified as the most critical contributors to sex-specific profiling were those that showed a sex-specific effect in their correlations with PAH severity markers. A similar approach confirmed that for each sex, the profiles of cytokines responding to the more or less oxidative environment are also distinct and could be accurately predicted. However, most importantly, cytokines profiles combined with plasma redox status allowed an accurate prediction of patient mortality. IL-6, significantly elevated in non-survival patients and showing a poor survival prognosis in patients with levels exceeding 95% control values cut-off, has recently gained considerable attention in PAH research. Lung-specific overexpression of this inflammatory cytokine produced spontaneous PAH in mice, which was additionally accelerated by hypoxia,73 while smooth muscle-specific deletion of IL-6 receptor protected against experimental PAH.9 Although the circulating levels of IL-6 in PAH patients did not correlate with mPAP, PVR, or 6MWD, they were found to be independently associated with severe RV dysfunction, diminished RV-PA coupling, and low patient survival rate.8,48,74,75 However, treatment with IL-6 receptor antagonist, tocilizumab, was not effective in the unstratified patient cohort,2 supporting the need for a more classified approach.
In addition to tocilizumab, other anti-inflammatory therapeutics were evaluated in clinical trials, highlighting the interest and necessity to control the inflammatory component of PAH. A phase II, randomized, double-blind, placebo-controlled study (NCT0266455876) used ubenimex that inhibits the synthesis of leukotriene B4 (LTB4), responsible for initiating pro-inflammatory signaling in pre-clinical studies.77,78 However, ubenimex also inhibits several other targets not relevant to LTB4. Therefore, the failure of the clinical trial to significantly improve PVR and 6MWD, the primary and secondary study outcomes, could be related to the off-target activity of ubenimex. At the same time, it could be due to using a non-stratified patient cohort and missing the opportunity to target the most susceptible to anti-inflammatory therapy patient groups. Notably, the effects of a combined antioxidant and anti-inflammatory therapy seem more promising. Furthermore, the clinical trial (NCT0203697079) tested bardoxolone methyl that simultaneously possesses antioxidant and anti-inflammatory properties has detected improvements in 6MWD compared to baseline. Notably, 6MWD was also significantly different in our analysis of survival vs non-survival patients, confirming the prognostic value of 6MWD, an independent determinant of patient survival.80-82 Thus, based on the previous studies and our current results, we propose that a combined therapy and the more precise approach that considers patient sex and redox status could improve current and future anti-inflammatory therapies' efficiency and help predict the outcomes.
This study represents a very novel approach to stratifying the patient cohort based on 2 factors known as essential contributors in PAH pathobiology. However, this first-time attempt requires follow-up studies to validate the presented results on larger patient cohorts and patients who reside in different geographical locations. It is also important to implement a further stratification based on PAH etiology or comorbidities, focusing on the sex-dependent conditions. Therapy could alter the cytokine profiles and redox status. Although the therapy profiles of male and female PAH patients were matched in this study, and the ORP values in treated and treatment-naïve patients were similar, the effects of therapy should be investigated more precisely, for example, by comparing the cytokine levels in patients placed on different treatment protocols with treatment-naïve subjects. Future research could also include longitudinal evaluation of circulating cytokine profiles or retrospective assessment of the efficiency of anti-inflammatory therapy based on the patient sex and plasma redox profile. While ORP represents a sum of simultaneously ongoing oxidative-reductive reactions and, therefore, is more suitable to describe a complex and fast-changing balance between oxidants and reductants compared to measuring single redox contributors, future studies may also evaluate the different aspects of redox homeostasis by identifying the key contributors into the final redox balance. Additional research on a better understanding of the mechanisms responsible for the distinct contribution of sex in cytokine release and PAH severity is also required. It could shed light on the new sex-specific targetable mechanisms or the particular type of inflammatory cells responsible for the discovered difference in the cytokine profile. Finally, the discovered connection between the cytokine levels and patient survival would also require further validation and testing. Nevertheless, if confirmed, it could help optimize and personalize the therapeutic options and reduce mortality risk.
Supplementary Material
At A Glance Commentary.
Rafikov R, et al.
Background
Although sex and oxidative stress are known and significant contributors to the pathobiology of PAH, their contribution to the profile of circulating cytokines has never been evaluated.
Translational Significance
In males, circulating cytokines are preferably increased in low oxidative conditions and positively correlate with PAH severity, while in females, cytokine release corresponds to higher oxidative stress but less severe PAH. Thus, the contribution of sex to the inflammatory response and PAH severity highlights the critical importance of the sex-based stratification of patient cohorts subjected to anti-inflammatory therapies. Furthermore, the combined cytokine/redox profiles allow an accurate prediction of PAH mortality.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01HL133085 (OR), R01HL132918 (RR), R01HL 151447(RR), and R01HL136603 (AAD).
Abbreviations:
- AUC
area under the curve
- BNP
brain natriuretic peptide
- CTD
connective tissue disease
- FC
functional class
- NK
Natural killer
- ORP
oxidation-reduction potential
- PAH
pulmonary arterial hypertension
- PCA
Principal component analysis
- ROS
reactive oxygen species
- TBST
Tris-buffered saline
- UA
University of Arizona
Footnotes
Conflicts of interest: All authors have read the Journal’s authorship agreement and policy on disclosing potential conflicts of interest. Two authors, RR and OR, have filed a provisional patent application using the data from this manuscript and have in place an approved plan for managing any potential conflicts arising from this. Other authors have no financial interests that could be perceived as being a conflict of interest.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.trsl.2022.03.013.
DATA AVAILABILITY STATEMENT
The data underlying this study will be shared on request to the corresponding author.
The authors would like to thank Marina Zemskova for her help with the technical aspects of the Bio-Plex instrument.
REFERENCES
- 1.Nicolls MR, Voelkel NF. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am J Respir Crit Care Med 2017;195:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez-Sanchez J, Harlow L, Church C, et al. Clinical trial protocol for TRANSFORM-UK: a therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulm Circ 2018;8:2045893217735820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichimura K, Matoba T, Koga JI, et al. Nanoparticle-mediated targeting of pitavastatin to small pulmonary arteries and leukocytes by intravenous administration attenuates the progression of monocrotaline-induced established pulmonary arterial hypertension in rats. Int Heart J 2018;59:1432–44. [DOI] [PubMed] [Google Scholar]
- 4.Prins KW, Thenappan T, Weir EK, Kalra R, Pritzker M, Archer SL. Repurposing medications for treatment of pulmonary arterial hypertension: what's old is new again. J Am Heart Assoc 2019;8:e011343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seropian IM, Gonzalez GE, Maller SM, Berrocal DH, Abbate A, Rabinovich GA. Galectin-1 as an emerging mediator of cardiovascular inflammation: mechanisms and therapeutic opportunities. Mediators Inflamm 2018;2018:8696543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiekerkoetter E, Kawut SM, de Jesus, Perez VA. New and emerging therapies for pulmonary arterial hypertension. Annu Rev Med 2019;70:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Wang Y, Lei Z. Chrysin ameliorates ANTU-induced pulmonary edema and pulmonary arterial hypertension via modulation of VEGF and eNOs. J Biochem Mol Toxicol 2019;33:e22332. [DOI] [PubMed] [Google Scholar]
- 8.Simpson CE, Chen JY, Damico RL, et al. Cellular sources of interleukin-6 and associations with clinical phenotypes and outcomes in pulmonary arterial hypertension. Eur Respir J 2020;55. https://erj.ersjournals.com/content/erj/55/4/1901761.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura Y, Phan C, Tu L, et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J Clin Invest 2018;128:1956–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savale L, Chaumais MC, O'Connell C, Humbert M, Sitbon O. Interferon-induced pulmonary hypertension: an update. Curr Opin Pulm Med 2016;22:415–20. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zhou Y, Miao L. Pulmonary capillary hemangioma-like pulmonary artery hypertension associated with interferon-alpha therapy. Am J Ther 2020;27(5):e511–4. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1beta and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol 2014;171:5589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva EM, Mariano VS, Pastrez PRA, et al. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS One 2017;12:e0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troy JD, Hill HR, Ewell MG, Frey SE. Sex difference in immune response to vaccination: a participant-level meta-analysis of randomized trials of IMVAMUNE smallpox vaccine. Vaccine 2015;33:5425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafikova O, James J, Eccles CA, et al. Early progression of pulmonary hypertension in the monocrotaline model in males is associated with increased lung permeability. Biology of sex differences 2020;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafikova O, Rafikov R, Meadows ML, Kangath A, Jonigk D, Black SM. The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype. Pulm Circ 2015;5:184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafikov R, Nair V, Sinari S, et al. Gender difference in damage-mediated signaling contributes to pulmonary arterial hypertension. Antioxid Redox Signal 2019;31:917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zemskova M, McClain N, Niihori M, et al. Necrosis-Released HMGB1 (High Mobility Group Box 1) in the progressive pulmonary arterial hypertension associated with male sex. Hypertension 2020;76:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batton KA, Austin CO, Bruno KA, Burger CD, Shapiro BP, Fairweather D. Sex differences in pulmonary arterial hypertension: role of infection and autoimmunity in the pathogenesis of disease. Biol Sex Differ 2018;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butrous G. Human immunodeficiency virus-associated pulmonary arterial hypertension: considerations for pulmonary vascular diseases in the developing world. Circulation 2015;131:1361–70. [DOI] [PubMed] [Google Scholar]
- 21.Rafikov R, James J, McClain N, Tofovic SP, Rafikova O. Role of gender in regulation of redox homeostasis in pulmonary arterial hypertension. Antioxidants (Basel) 2019;8. www.ncbi.nlm.nih.gov/pmc/articles/PMC7145198/pdf/pone.0231267.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zemskova M, Kurdyukov S, James J, McClain N, Rafikov R, Rafikova O. Sex-specific stress response and HMGB1 release in pulmonary endothelial cells. PLoS One 2020;15:e0231267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelbary M, Rafikova O, Gillis EE, et al. Necrosis contributes to the development of hypertension in male, but not female, spontaneously hypertensive rats. Hypertension 2019;74:1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed R, Rafikova O, O'Connor PM, Sullivan JC. box 1 in male compared with female spontaneously hypertensive rats worsens renal ischemia-reperfusion injury. Clin Sci (Lond) 2020;134:1751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008;29:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafikova O, Al Ghouleh I, Rafikov R. Focus on early events: pathogenesis of pulmonary arterial hypertension development. Antioxid Redox Signal 2019;31:933–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou W, Zhang Q, Yan Z, et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 2013;4:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med 2012;18:1360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Torres I, Guarner-Lans V, ME Rubio-Ruiz. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int J Mol Sci 2017;18. www.ncbi.nlm.nih.gov/pmc/articles/PMC5666780/pdf/ijms-18-02098.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53(11):1801913. https://pubmed.ncbi.nlm.nih.gov/30545968/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rael LT, B OR, Kelly MT, Carrick MM, BarOr D. Assessment of oxidative stress in patients with an isolated traumatic brain injury using disposable electrochemical test strips. Electroanalysis 2015;27:2567–73. [Google Scholar]
- 32.Rozemeijer S, Spoelstra-de Man AME, Coenen S, et al. Estimating vitamin C status in critically ill patients with a novel point-of-care oxidation-reduction potential measurement. Nutrients 2019;11(5):1031. https://pubmed.ncbi.nlm.nih.gov/31071996/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldmaier K, Stoppe C, Goetzenich A, et al. Oxidation-reduction potential in patients undergoing transcatheter or surgical aortic valve replacement. Biomed Res Int 2018;2018:8469383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivero-Gutierrez B, Anzola A, Martinez-Augustin O, de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem 2014;467:1–3. [DOI] [PubMed] [Google Scholar]
- 35.Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010;138:1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepp DH. Inflamed response: HIV provokes the immune system into a hyperactive state that can lead to chronic inflammation. Positively aware: the monthly Journal of the Test Positive Aware Network 2011;23:14–7. [PubMed] [Google Scholar]
- 37.Pedersen KK, Pedersen M, Gaardbo JC, et al. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr 2013;63:272–9. [DOI] [PubMed] [Google Scholar]
- 38.Spagnolo P, Cordier JF, Cottin V. Connective tissue diseases, multimorbidity and the ageing lung. Eur Respir J 2016;47:1535–58. [DOI] [PubMed] [Google Scholar]
- 39.Rael LT, Bar-Or R, Mains CW, Slone DS, Levy AS, Bar-Or D. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma 2009;26:1203–11. [DOI] [PubMed] [Google Scholar]
- 40.Spanidis Y, Mpesios A, Stagos D, et al. Assessment of the redox status in patients with metabolic syndrome and type 2 diabetes reveals great variations. Experimental and therapeutic medicine 2016;11:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril 2016;106:566–73, e10. [DOI] [PubMed] [Google Scholar]
- 42.Opacic M, Stevic Z, Bascarevic V, Zivic M, Spasic M, Spasojevic I. Can oxidation-reduction potential of cerebrospinal fluid be a monitoring biomarker in amyotrophic lateral sclerosis? Antioxid Redox Signal 2018;28:1570–5. [DOI] [PubMed] [Google Scholar]
- 43.Spanidis Y, Goutzourelas N, Stagos D, et al. Assessment of oxidative stress in septic and obese patients using markers of oxidation-reduction potential. In vivo 2015;29:595–600. [PubMed] [Google Scholar]
- 44.Stagos D, Goutzourelas N, Ntontou AM, et al. Assessment of eccentric exercise-induced oxidative stress using oxidation-reduction potential markers. Oxidative medicine and cellular longevity 2015;2015:204615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjugstad KB, Rael LT, Levy S, et al. Oxidation-reduction potential as a biomarker for severity and acute outcome in traumatic brain injury. Oxidative medicine and cellular longevity 2016;2016:6974257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rael LT, Bar-Or R, Salottolo K, et al. Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: a retrospective analysis. Scandinavian Journal of trauma, resuscitation and emergency medicine 2009;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groth A, Vrugt B, Brock M, Speich R, Ulrich S, Huber LC. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010;122:920–7. [DOI] [PubMed] [Google Scholar]
- 49.Sweatt AJ, Hedlin HK, Balasubramanian V, et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res 2019;124:904–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gourh P, Arnett FC, Assassi S, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther 2009;11:R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Lin P, Hong C, et al. Serum cytokine profiles in patients with chronic obstructive pulmonary disease associated pulmonary hypertension identified using protein array. Cytokine 2018;111:342–9. [DOI] [PubMed] [Google Scholar]
- 53.Hester J, Ventetuolo C, Lahm T. Sex, Gender, and Sex hormones in pulmonary hypertension and right ventricular failure. Comprehensive Physiology 2019;10:125–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Austin ED, Hamid RY. Not? Sex chromosomes may modify sexual dimorphism in pulmonary hypertension. Am J Respir Crit Care Med. 2018;197:858–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ottaviano FG, Handy DE, Loscalzo J. Redox regulation in the extracellular environment. Circ J 2008;72:1–16. [DOI] [PubMed] [Google Scholar]
- 56.Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell 2019;76:232–42. [DOI] [PubMed] [Google Scholar]
- 57.Kagan VE, Gleiss B, Tyurina YY, et al. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–99. [DOI] [PubMed] [Google Scholar]
- 58.Heckmann BL, Tummers B, Green DR. Crashing the computer: apoptosis vs necroptosis in neuroinflammation. Cell Death Differ 2019;26:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P. Necrosis is associated with IL-6 production but apoptosis is not. Cell Signalling 2006;18:328–35. [DOI] [PubMed] [Google Scholar]
- 60.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–63. [DOI] [PubMed] [Google Scholar]
- 61.Foris V KG, Douschan P, Avian A, Olschewski A, Olschewski H. Neutrophil-to-lymphocyte ratio as a prognostic parameter in pulmonary arterial hypertension. Eur Respir J 2016;48:PA2479. [DOI] [PubMed] [Google Scholar]
- 62.Austin ED, Rock MT, Mosse CA, et al. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med 2010;104:454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;185:311–21. [DOI] [PubMed] [Google Scholar]
- 64.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration 2008;75:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ormiston ML, Chang C, Long LL, et al. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation 2012;126:1099–109. [DOI] [PubMed] [Google Scholar]
- 66.Huertas A, Tu L, Gambaryan N, et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. Eur Respir J 2012;40:895–904. [DOI] [PubMed] [Google Scholar]
- 67.Edwards AL, Gunningham SP, Clare GC, et al. Professional killer cell deficiencies and decreased survival in pulmonary arterial hypertension. respirol 2013;18:1271–7. [DOI] [PubMed] [Google Scholar]
- 68.Ratsep MT, Moore SD, Jafri S, et al. Spontaneous pulmonary hypertension in genetic mouse models of natural killer cell deficiency. American Journal of physiology Lung cellular and molecular physiology 2018;315:L977–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007;175:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicolls MR, Mizuno S, Taraseviciene-Stewart L, et al. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ 2012;2:434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamosiuniene R, Manouvakhova O, Mesange P, et al. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circ Res 2018; 122:1689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jutras-Beaudoin NTV, Lajoie AC, Breuils-Bonnet S, Paulin R, Potus F. Neutrophil-lymphocyte ratio as an independent predictor of survival in pulmonary arterial hypertension: An exploratory study. CJC Open 2022;4:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009;104:236–44, 28p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prins KW, Archer SL, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2018;37:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heresi GA, Aytekin M, Hammel JP, Wang S, Chatterjee S, Dweik RA. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 2014;43:912–4. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez-Cuenca S, Barbarroja N, Vidal-Puig A. Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim Biophys Acta 2015;1851:40–50. [DOI] [PubMed] [Google Scholar]
- 77.Qian J, Tian W, Jiang X, et al. Leukotriene B4 activates pulmonary artery adventitial fibroblasts in pulmonary hypertension. Hypertension 2015;66:1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian W, Jiang X, Tamosiuniene R, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013;5:200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012;98:238–43. [DOI] [PubMed] [Google Scholar]
- 80.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161:487–92. [DOI] [PubMed] [Google Scholar]
- 81.Huang J, Mehta S, Mura M. Early decline in six-minute walk distance from the time of diagnosis predicts clinical worsening in pulmonary arterial hypertension. Respiration 2015;89:365–73. [DOI] [PubMed] [Google Scholar]
- 82.Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2015;34:362–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study will be shared on request to the corresponding author.
The authors would like to thank Marina Zemskova for her help with the technical aspects of the Bio-Plex instrument.