Abstract
Tisotumab vedotin-tftv, an antibody-drug conjugate indicated for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy, demonstrated clinically meaningful and durable responses with a manageable safety profile in the pivotal phase II innovaTV 204 clinical trial. Based on the proposed mechanism of action of tisotumab vedotin, experience from clinical trials, and the US prescribing information, certain adverse events (AEs) including ocular AEs, peripheral neuropathy, and bleeding have been identified as AEs of interest. This article highlights practical considerations and provides recommendations to support the management of selected AEs associated with tisotumab vedotin. Central to monitoring of patients on tisotumab vedotin is a comprehensive care team comprised of oncologists, advanced practice providers (including nurse practitioners, physician assistants, and pharmacists), and other specialists such as ophthalmologists. As ocular AEs may be less familiar to gynecologic oncology practitioners, adherence to the “Premedication and Required Eye Care” section outlined in the US prescribing information, as well as the incorporation of ophthalmologists into the oncology care team, can help provide timely and appropriate eye care for patients receiving tisotumab vedotin.
Cervical cancer is the fourth most frequently diagnosed and fourth most deadly cancer in women worldwide (Arbyn et al., 2020; Bray et al., 2018). Patients with recurrent or metastatic cervical cancer (r/mCC) experience a significant burden from disease-related symptoms, such as vaginal bleeding and abdominal pain, as well as adverse events (AEs) associated with anti-cancer treatments (Issah et al., 2011; Kim et al., 2015). Treatment-related AEs (TRAEs) can have a significant impact on patient quality of life (Davidson, 2011), which may result in patients discontinuing or declining treatment. Therefore, it is critical that the comprehensive care team understands how to prevent and manage AEs associated with treatments.
OVERVIEW OF TISOTUMAB VEDOTIN
Tisotumab vedotin (Tivdak) is a tissue factor-directed antibody-drug conjugate (ADC) comprising a fully human monoclonal antibody specific for tissue factor conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease-cleavable linker, which enables preferential release of MMAE within target cells and concomitant cell death (Breij et al., 2014; Hong et al., 2020). Tissue factor is highly prevalent in a number of solid tumors, including cervical cancer, with expression in cancer cells elevated compared with normal cells (Cocco et al., 2011a, 2011b). The physiological function of tissue factor (initiating the coagulation cascade after vascular injury) can be co-opted by tumor cells to promote tumor growth, angiogenesis, and metastasis (Förster et al., 2006; van den Berg et al., 2012). In September 2021, tisotumab vedotin received accelerated approval from the US Food and Drug Administration for the treatment of adult patients with r/mCC with disease progression on or after chemotherapy (Seagen Inc. & Genmab US Inc., 2022). This indication was granted based on tumor response rate and durability of response. Continued approval is contingent upon confirmation of clinical benefit.
In the pivotal phase II innovaTV 204 study (NCT03438396), tisotumab vedotin was evaluated in patients with r/mCC previously treated with doublet chemotherapy plus bevacizumab (if eligible; Coleman et al., 2021). A total of 101 patients (median age 50 years) received tisotumab vedotin 2 mg/kg (up to a maximum of 200 mg) as an intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. Patients had received either one (71 patients, 70%) or two (30 patients, 30%) prior lines of systemic therapy for r/mCC, and 64 patients (63%) had received previous bevacizumab plus doublet chemotherapy as first-line therapy.
Tisotumab vedotin demonstrated clinically meaningful and durable antitumor activity, with an objective response rate (as assessed by an independent review committee) of 24% (95% confidence interval [CI] = 16%–33%) and a median duration of response of 8.3 months; antitumor activity was observed regardless of histology, prior treatment, or tissue factor expression. Median time to response was 1.4 months, indicating rapid antitumor responses with potential antitumor activity within the first two treatment cycles. Median progression-free survival (PFS), 6-month PFS rate, and median overall survival were 4.2 months, 30% (95% CI = 21%–40%), and 12.1 months, respectively. Most AEs were mild to moderate in severity, with 93 patients (92%) experiencing a TRAE, while 28 patients (28%) experienced at least one grade 3 or higher TRAE. Thirteen subjects (13%) had serious TRAEs, one of which was fatal (Coleman et al., 2021). Prespecified AEs of interest included ocular AEs (which occurred in the first-in-human innovaTV 201 study), peripheral neuropathy (a known MMAE-related AE), and bleeding (Hong et al., 2020; Masters et al., 2018; Yamamoto et al., 1992).
Given the limited treatment options for patients with r/mCC, it is essential to understand how to safely and effectively use newly approved therapies to optimize their clinical benefit. This article highlights select AEs observed with tisotumab vedotin in clinical trials and provides practical recommendations to support the comprehensive care team when managing patients receiving tisotumab vedotin.
WARNINGS AND PRECAUTIONS WITH TISOTUMAB VEDOTIN
Health-care professionals treating patients with tisotumab vedotin should be aware of certain warnings and precautions, as outlined in the US prescribing information (Seagen Inc. & Genmab US Inc., 2022). There is a boxed warning for ocular toxicity, as tisotumab vedotin caused changes in the corneal epithelium and conjunctiva, resulting in changes in vision, including severe vision loss and corneal ulceration. Additionally, the US prescribing information contains warnings for peripheral neuropathy, hemorrhage, pneumonitis, and embryo-fetal toxicity. Providers should refer to the current tisotumab vedotin prescribing information for the most up-to-date dose modification and AE management guidance as additional data become available.
The following section discusses select common AEs observed with tisotumab vedotin from clinical trials and provides measures to mitigate their risk and manage them if they occur.
CLINICAL MANAGEMENT OF OCULAR ADVERSE EVENTS
Ocular AEs occurred in 60% of patients with cervical cancer treated with tisotumab vedotin across clinical trials (Seagen Inc. & Genmab US Inc., 2022). These AEs were initially observed in the phase I/II first-in-human study of tisotumab vedotin (innovaTV 201; NCT02001623); they were most commonly conjunctivitis, dry eye, ulcerative keratitis, blepharitis, and keratitis (Seagen Inc. & Genmab US Inc., 2022). Ocular AEs observed with tisotumab vedotin could be related to tissue factor expression in the eye (Ando et al., 2011; Cho et al., 2011).
To help mitigate risk and manage ocular AEs, an eye care plan based on clinical trial experience was developed (Figure 1) and outlined in the “Premedication and Required Eye Care” section of the US prescribing information. This includes an oncology care team in partnership with an eye care provider and incorporates: 1) ophthalmic exams at baseline, prior to each dose, and as clinically indicated; 2) eye drops; 3) cold packs; 4) avoidance of contact lens use; and 5) prompt referral to an eye care provider for symptom management and dose modifications as appropriate. In clinical practice, communication between the care team usually begins on the initial visit, with ophthalmology helping to determine if tisotumab vedotin is a suitable treatment by assessing the risk of baseline ocular AEs that may worsen with treatment. The oncology care team's referral specifies which ocular assessments are needed; post assessment, the eye care provider shares documentation of findings and recommended actions. The assessment is important in order to mitigate the risk of ocular AEs prior to starting treatment, while the documentation allows the care team to compare previous assessments and potentially detect subtle ocular changes as they may occur. In the first-in-human phase I trial innovaTV 201, ocular event incidence was reduced from 80% in patients enrolled prior to implementation (n = 15) of mitigation measures to 60% in patients enrolled after implementation of such measures (n = 40; Hong et al., 2020).
Figure 1.
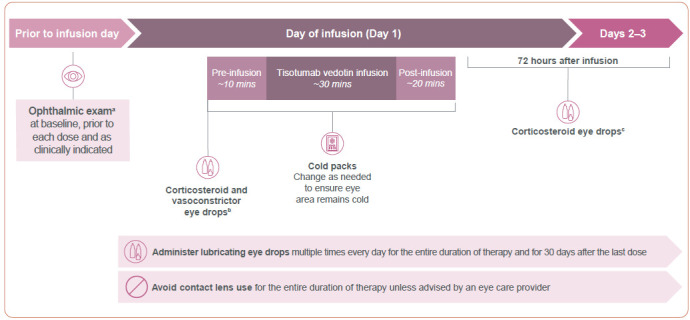
Required eye care to mitigate risk of ocular AEs in patients treated with tisotumab vedotin. AE = adverse event. Examples are based on the innovaTV 204 clinical trial. Information from Seagen Inc. & Genmab US Inc. (2022).
aOphthalmic exam includes visual acuity and slit-lamp exam by an eye care provider.
bPrior to infusion, administer 1 drop of dexamethasone 0.1% per eye and 3 drops of brimonidine tartrate 0.2% per eye.
cFor 72 hours after each infusion, 1 drop of dexamethasone 0.1% administered per eye as prescribed.
In innovaTV 204, patients with active ocular surface disease, Stevens-Johnson syndrome, or a history of cicatricial conjunctivitis were excluded (Coleman et al., 2021). A total of 47 patients (47%) had a medical history of an ocular condition or pretreatment ocular AE that had ended or was ongoing at cycle 1. Considering the median age of patients in innovaTV 204 (50 years), and that aging is associated with increased susceptibility to ocular conditions (Akpek & Smith, 2013), such baseline findings were expected and did not preclude patients from starting tisotumab vedotin. All investigators and enrolled patients were required to adhere to the eye care plan. In total, 54 patients (53%) in innovaTV 204 reported at least one ocular TRAE. Most events were mild to moderate in nature: 25 patients (25%) had grade 1 events, 27 patients (27%) had grade 2 events, and 2 patients (2%) had grade 3 events (both ulcerative keratitis; Coleman et al., 2021). The most common ocular TRAEs were conjunctivitis (26%), dry eye (23%), and keratitis (11%). Regardless of relatedness, the occurrence of treatment-emergent ocular AEs was generally comparable between patients with a medical history of or pretreatment ocular AE vs. patients without (57% vs. 52%, respectively). There was a slightly higher incidence of corneal disorders in patients with prior or ongoing ocular conditions vs. patients without (28% vs. 15%, respectively).
A total of 138 ocular events of any grade were observed among the 54 patients (Coleman et al., 2021). Eighty-six percent of these events resolved by the time of the last follow-up. The median time to onset of the first event was 1.4 months, and the median time to resolution was 0.7 months from documented onset.
Symptoms
Ocular AEs associated with tisotumab vedotin are typically symptomatic and detectable, although the oncology care team may be unfamiliar with their variable clinical presentation (Figure 2). Conjunctivitis can be associated with itching, pain, burning, and redness or scaling around the eyelids (Azari & Barney, 2013; Varu et al., 2019). Keratitis refers to corneal inflammation and can be associated with redness, mucopurulent secretions, discharge, foreign body sensation, pain, reduced visual acuity, blurred vision, and light sensitivity (Cronau et al., 2010; Shah et al., 2017; Sharma, 2001). Diagnosis of keratitis may require a slit-lamp exam (Upadhyay et al., 2015). Blepharitis refers to inflammation of the eyelids, and symptoms can include redness, irritation, loss of eyelashes, blurred or fluctuating vision, and eyelid swelling or sticking (Amescua et al., 2019; Duncan & Jeng, 2015). Dry eye is a condition involving dryness of the ocular surface and has been observed in patients receiving chemotherapy (Clayton, 2018; Maino et al., 2000). Dry eye typically involves ocular pain accompanied by light sensitivity, foreign body sensation, dryness, and irritation (Clayton, 2018). Attention to symptoms through proactive monitoring is critical for facilitating early intervention and management.
Figure 2.
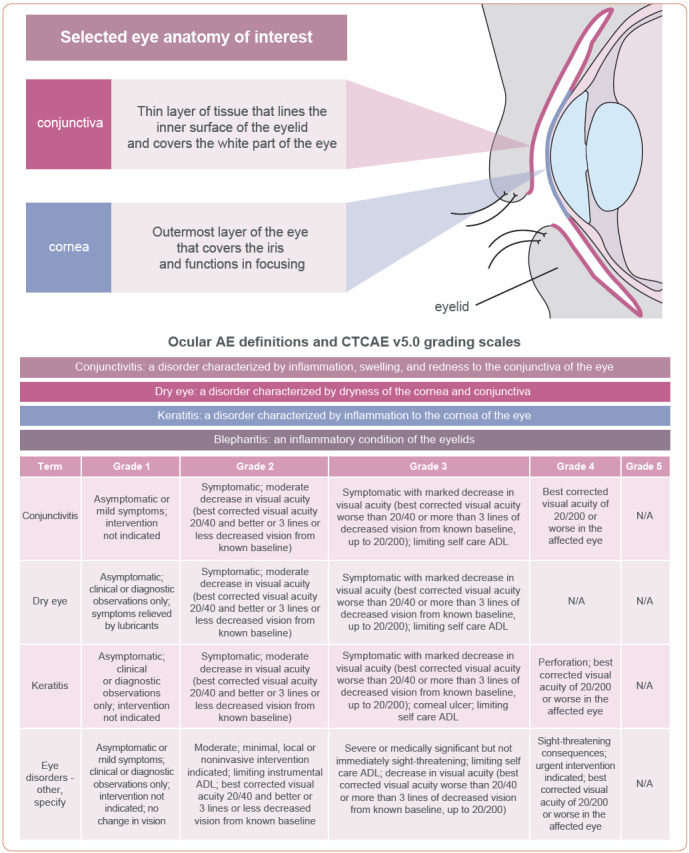
Anatomy of the eye and grading criteria for ocular AEs. ADL = activities of daily living; AE = adverse event; CTCAE = Common Terminology Criteria for Adverse Events. Information from Duncan & Jeng (2015); US Department of Health and Human Services (2017).
Risk Mitigation
Clinicians should adhere to the recommendations described in the “Premedication and Required Eye Care” section of the US prescribing information (section 2.2) to reduce the risk of ocular AEs in patients receiving tisotumab vedotin (Figure 1). The baseline eye exam by an eye care provider should include a visual acuity test and slit-lamp exam at baseline, prior to each dose, and as clinically indicated. Premedication includes several types of eye drops. Topical corticosteroid eye drops may reduce the risk of epithelial proliferation in the eye. Initial prescription and all renewals of any corticosteroid medication should be made only after a slit-lamp exam. The vasoconstrictive properties of topical ocular vasoconstrictor drops and cold packs may reduce ocular blood flow and the delivery of tisotumab vedotin. Cold packs should be placed prior to the start of infusion after eye drop administration and kept over the eye area throughout the infusion and for approximately 20 minutes after the infusion (Figure 1). Cold packs should be changed as needed to ensure the eye area remains cold. A scarf or headband may be used to secure the cold pack over the eyes. Use of topical lubricating eye drops for the duration of therapy and for 30 days after the last dose may reduce dryness and irritation. It is advised that patients avoid contact lenses for the entire duration of therapy unless advised by their eye care provider; patients may be at an increased risk of irritation or infection in the eyes when wearing and inserting or removing contact lenses.
Attention to patient symptoms and proactive monitoring throughout treatment is important for early recognition of ocular AEs and rapid intervention. If not managed early, conditions such as conjunctivitis and keratitis can progress to ulcers and perforation (Azari & Barney, 2013; Titiyal et al., 2006; US Department of Health and Human Services, 2017; Varu et al., 2019). Patients should be evaluated by an eye care provider regularly to help ensure the appropriate use of steroid eye drops, and timely diagnosis and intervention of ocular symptoms to inform potential dose modifications (Seagen Inc. & Genmab US Inc., 2022). To streamline this process, the ophthalmologist should be incorporated into the comprehensive care team by establishing referral pathways between departments or between centers that may not have ophthalmology departments. Open communication between ophthalmologists, oncologists, and advanced practice providers will allow for rapid referral of patients to ensure that they receive appropriate management as soon as possible if ocular AEs occur.
Additionally, patients and caregivers should receive guidance on mitigating the risk of ocular AEs. Patients should be educated on ocular AE symptoms and encouraged to promptly report any symptoms. Moreover, patients should be advised to self-administer preservative-free lubricating eye drops frequently, and bring all eye drops to each infusion appointment. Other important counselling points include maintaining good hygiene around the eyes and being cautious about using irritants (e.g., cosmetics such as mascara or eyeliner) that may aggravate ocular symptoms.
Management
Based on ocular AE diagnosis by the ophthalmologist, the oncology care team should respond by implementing the appropriate dose modifications (Table 1) and reductions (Table 2) to allow for improvement or resolution of ocular symptoms. Patients may experience multiple ocular events, potentially resulting in multiple rounds of dose modification.
Table 1. Tisotumab Vedotin Dose Modification Guidelines for Ocular AEs, Peripheral Neuropathy, Hemorrhage, and Pneumonitis.
| Adverse reaction | Severity | Occurrence | Tisotumab vedotin dose modification |
|---|---|---|---|
| Keratitisa | SPK | Any | Monitor. |
| Confluent superficial keratitis | First occurrence | Withhold dose until SPK or normal, then resume treatment at the next lower dose level. | |
| Second occurrence | Permanently discontinue. | ||
| Ulcerative keratitis or perforation | Any | Permanently discontinue. | |
| Conjunctival ulcerationa | Any ulceration | First occurrence | Withhold dose until complete conjunctival re-epithelialization, then resume treatment at the next lower dose level. |
| Second occurrence | Permanently discontinue. | ||
| Conjunctival or corneal scarring or symblepharona | Any scarring or symblepharon | Any | Permanently discontinue. |
| Conjunctivitis and other ocular adverse reactionsa | Grade 1 | Any | Monitor. |
| Grade 2 | First occurrence | Withhold dose until grade ≤ 1, then resume treatment at the same dose. | |
| Second occurrence | Withhold dose until grade ≤ 1, then resume treatment at the next lower dose level. If no resolution to grade ≤ 1, permanently discontinue. | ||
| Third occurrence | Permanently discontinue. | ||
| Grade 3 or 4 | Any | Permanently discontinue. | |
| Peripheral neuropathy | Grade 2 | Any (initial or worsening of pre-existing condition) | Withhold dose until grade ≤ 1, then resume treatment at the next lower dose level. |
| Grade 3 or 4 | Any | Permanently discontinue. | |
| Hemorrhage | Any-grade pulmonary or CNS | Any | Permanently discontinue. |
| Grade 2 in any other location | Any | Withhold until resolved, then resume treatment at the same dose. | |
| Grade 3 in any other location | First occurrence | Withhold dose until resolved, then resume treatment at the same dose. | |
| Second occurrence | Permanently discontinue. | ||
| Grade 4 in any other location | Any | Permanently discontinue. | |
| Pneumonitis | Grade 2 | Any | Withhold dose until grade ≤ 1 for persistent or recurrent pneumonitis, consider resuming treatment at next lower dose level. |
| Grade 3 or 4 | Any | Permanently discontinue. |
Note. AE = adverse event; CNS = central nervous system; SPK = superficial punctate keratitis. Information from Seagen Inc. & Genmab US Inc. (2022).
Refer patients to an eye care provider promptly for an assessment of new or worsening ocular symptoms.
Table 2. Tisotumab Vedotin Dose Reduction Schedule.
| Dose level | |
|---|---|
| Starting dose | 2 mg/kg |
| First dose reduction | 1.3 mg/kg |
| Second dose reduction | 0.9 mg/kga |
Note. Information from Seagen Inc. & Genmab US Inc. (2022).
Permanently discontinue in patients who cannot tolerate 0.9 mg/kg.
Oncology practitioners who are accustomed to using the Common Terminology Criteria for Adverse Events (CTCAE) grading scale should be aware that ocular symptom grading using CTCAE is not common practice for ophthalmologists. Thus, clear communication between oncology practitioners and ophthalmologists on AE grading criteria is important to inform potential dose modifications.
Case Report of a Patient With Ocular AEs
A 48-year-old female presented with cervical cancer with metastases to the lung, pelvic lymph nodes, and locoregional lymph nodes, and was administered tisotumab vedotin at 2.0 mg/kg every 3 weeks. The patient had a normal ophthalmological evaluation at baseline and adhered to the eye care plan.
Six days after cycle 3, the patient reported red eyes. She was referred to an ophthalmologist, who diagnosed grade 1 conjunctivitis and prescribed steroid eye drops (dexamethasone monofree eye drops 0.2%); the event resolved after a total duration of 12 days from onset of symptoms, and the eye drops were stopped.
Six days after cycle 4, the patient reported tired, watery eyes and a red right eye. The ophthalmologist diagnosed a second occurrence of grade 1 conjunctivitis and prescribed steroid drops again (dexamethasone monofree eye drops 0.1%). The event resolved after a total duration of 11 days from symptom onset.
Six days after cycle 5, the patient reported heavy eyes and experienced a new occurrence of grade 2 conjunctivitis. The following day, despite treatment with steroid eye drops, the patient reported heavy and red eyes and subsequently consulted an ophthalmologist. A slit-lamp exam showed abnormal findings in both eyes. The patient was diagnosed with grade 2 conjunctivitis and mild blepharitis, and was prescribed topical antibiotics and topical steroid drops (single-dose ofloxacin and dexamethasone monofree eye drops) for 1 week. The conjunctivitis resolved following treatment with topical antibiotics and continuation of steroid drops, and no dose modifications were required. The total event duration was 7 days from onset of symptoms.
Six days after cycle 6, the patient reported red eyes. The ophthalmologist was again consulted and diagnosed the patient with a second occurrence of grade 2 conjunctivitis, evidenced by moderate bulbar hyperemia in both eyes. A slit-lamp exam showed abnormal right and left eyelids, and grade 2 blepharitis. The patient's grade 2 conjunctivitis was treated with ofloxacin and dexamethasone monofree eye drops, and resolved after a total duration of 10 days from onset of symptoms. The tisotumab vedotin dose was subsequently reduced to 1.3 mg/kg, in accordance with the mitigation plan in the study protocol.
Twenty-two days after cycle 7, the patient's ophthalmological exam showed blepharitis (slit-lamp test abnormal). The patient underwent additional examination 13 days later; all ophthalmological examinations indicated mild punctate keratitis. Three days later, the patient received tisotumab vedotin at a lower dose (0.9 mg/kg). Five days later, the patient's scan showed progressive disease and no further treatment was given.
Key Takeaways
This case report demonstrates that ocular AEs can be recurrent and may require multiple visits to the ophthalmologist. It is important to note that ocular AE profiles can vary considerably for individual patients over time and between different patients. The establishment of referral pathways facilitates timely access to ophthalmologists, and clinicians should utilize the expertise of ophthalmologists through early referral of patients for prompt diagnosis and rapid management.
CLINICAL MANAGEMENT OF PERIPHERAL NEUROPATHY
Peripheral neuropathy occurred in 42% of patients with cervical cancer treated with tisotumab vedotin across clinical trials (Seagen Inc. & Genmab US Inc., 2022). Peripheral neuropathy is a clinically significant AE that has been observed with several anticancer therapies, including MMAE-containing ADCs and combination therapy with platinum and taxanes (Coleman et al., 2021; Kitagawa et al., 2015; Masters et al., 2018; Zajączkowska et al., 2019). There is currently limited data on resolution of peripheral neuropathy associated with tisotumab vedotin with long-term follow-up; however, based on data from other vedotin ADCs (e.g., brentuximab vedotin), most observed peripheral neuropathy events resolved or improved over time (Ansell et al., 2022).
In innovaTV 204, patients with grade ≥ 2 peripheral neuropathy were excluded from enrollment. At baseline, 20 patients (20%) had a medical history of or ongoing pretreatment peripheral neuropathy at cycle 1 (Coleman et al., 2021). Peripheral neuropathy TRAEs (by individual rather than preferred term) occurred in 33 patients (33%), with 17 patients (17%) having grade 1 events, 9 patients (9%) having grade 2 events, and 7 patients (7%) having grade 3 events (Coleman et al., 2021). The most common peripheral neuropathy TRAEs were neuropathy peripheral (all grade, 10%; grade 3, 2%), peripheral sensory neuropathy (all grade, 9%; grade 3, 2%), and peripheral sensorimotor neuropathy (all grade, 5%; grade 3, 2%).
There was a total of 47 peripheral neuropathy events among the 33 patients, of which 10 (21%) resolved within 30 days after the last dose. The median time to onset was 3.1 months, and the median time to resolution after initial onset was 0.6 months (Coleman et al., 2021). Among the 5 patients who experienced TRAEs leading to treatment discontinuation or dose reduction, there were a total of 6 treatment-related peripheral neuropathy events.
Symptoms
Peripheral neuropathy may be associated with impaired deep tendon reflexes (Murillo, Jr. et al., 2008). Different subtypes of peripheral neuropathy can vary in presentation. Peripheral sensory neuropathy can be associated with numbness, paresthesia, dysesthesia, burning sensations, aching, and neuropathic pain, and peripheral motor neuropathy is typically associated with muscle weakness. Peripheral sensorimotor neuropathy often involves distal paresthesia (Murillo, Jr et al., 2008).
Management
As peripheral neuropathy is often underreported, it is important for clinicians to educate their patients so that they can identify and report symptoms of peripheral neuropathy as early as possible. Early recognition and intervention are critical for facilitating effective management and prevention of permanent treatment discontinuation. All patients receiving tisotumab vedotin should be closely monitored for new or worsening symptoms of peripheral neuropathy throughout treatment, and for resolution of symptoms prior to any continuation of therapy. Advanced practice providers in oncology are generally familiar with assessing peripheral neuropathy. Helpful practices include thorough and well-documented baseline assessments of preexisting neuropathy and questions aimed at determining if symptoms impact or interfere with a patient's day-to-day activities.
Tisotumab vedotin–related peripheral neuropathy can be managed using dose modifications (Table 1) and the dose reduction schedule (Table 2). Clinicians should provide patients with information on how to alleviate peripheral neuropathy symptoms (Tanay & Armes, 2019). Nonpharmacologic interventions include neurostimulation (e.g., transcutaneous electrical nerve stimulation), acupuncture, massage, meditation, dietary supplements, and rehabilitation for functional deficits (e.g., physical or occupational therapy; Stubblefield et al., 2009). Pharmacologic treatments for pain associated with peripheral neuropathy include duloxetine, gabapentin, or pregabalin (Loprinzi et al., 2020).
Case Report of a Patient With Peripheral Neuropathy
A 69-year-old female presented with cervical cancer and metastases to the right abdominal oblique and pelvic lymph nodes, and was administered tisotumab vedotin at 2.0 mg/kg every 3 weeks. The patient had a past medical history of grade 1 neuropathy, diabetes, and recurrent deep vein thrombosis; prior therapy included carboplatin and paclitaxel.
Twenty-two days after cycle 8, the patient reported increased balance problems and difficulty walking around her home due to fear of falling. An examination found that the patient had bilateral numbness in the legs, localized below the knees, and small signs of sensory neuropathy in her fingertips; she was diagnosed with grade 3 peripheral neuropathy and asked for a walker or wheelchair to facilitate mobility.
Treatment was withheld, and the patient's peripheral neuropathy improved from grade 3 to grade 2 by her clinic visit 15 days later. However, the patient still required a walker.
Subsequent radiographical examination of the patient revealed disease progression, and she did not resume treatment. At the time of treatment discontinuation, the patient's peripheral neuropathy was ongoing at grade 2 and considered unlikely to fully resolve in the near future.
Key Takeaways
This case emphasizes the need for a detailed baseline assessment of patients and documentation of preexisting neuropathy to help determine the cause of neuropathy if it occurs. It is important to note that peripheral neuropathy may present differently between individuals or for the same individual over time. To optimize management, it is important to consider the severity of each event at its worst during the treatment cycle and how quickly the event recovers to a lower grade. This case demonstrates the importance of holding the treatment dose according to the dose reduction schedule. Patients should be encouraged to report changes in event severity throughout their treatment cycle.
CLINICAL MANAGEMENT OF BLEEDING EVENTS
As tisotumab vedotin is directed to cells expressing tissue factor, and the primary role of tissue factor is to initiate the coagulation cascade after vascular injury, bleeding events were prespecified as AEs of interest (Alley et al., 2019; Coleman et al., 2021; de Goeij et al., 2015; Yamamoto et al., 1992). Bleeding events occurred in 62% of patients with cervical cancer treated with tisotumab vedotin across clinical trials (Seagen Inc. & Genmab US Inc., 2022). Patients with known coagulation defects or ongoing major bleeding were excluded from enrollment (Coleman et al., 2021). Eligibility criteria relating to platelet count have been previously reported; patients on anticoagulation therapy were included subject to protocol-defined criteria (Coleman et al., 2021). The management of bleeding events and use of anticoagulation or antiplatelet therapy should be personalized to patient needs and per clinician judgement and standard of care.
In innovaTV 204, TRAEs of bleeding occurred in 39 patients (39%) and were mostly grades 1 or 2; 34 patients (34%) had grade 1 events, 3 patients (3%) had grade 2 events, and 2 patients (2%) had grade 3 events (rectal hemorrhage and cystitis hemorrhagic; Coleman et al., 2021). At the time of first dosing of tisotumab vedotin (cycle 1), there were 11 patients (11%) with a medical history of or pretreatment bleeding condition; they were most commonly hematuria and vaginal hemorrhage. In 7 patients (7%), their bleeding condition was ongoing at cycle 1. The most common bleeding TRAEs were epistaxis in 30 patients (30%), vaginal hemorrhage in 7 patients (7%), and hematuria in 3 patients (3%). The occurrence of vaginal hemorrhage and hematuria may be associated with underlying conditions, such as local tumor growth or previous pelvic radiotherapy. Of the 30 patients with epistaxis, 28 (28%) had grade 1 events and 2 (2%) had grade 2 events. The elevated incidence of epistaxis compared with other forms of bleeding observed with tisotumab vedotin may be due to tissue factor expression in the nasal epithelium (Shimizu et al., 2015) and the significant vascularization of the nose (Kucik & Clenney, 2005; Manfredini et al., 2000). Rates of treatment-emergent epistaxis were similar for patients with and without a history of bleeding (36% vs. 39%, respectively).
Of the 57 total bleeding events, most (90%) resolved by the last safety follow-up visit 30 days after the last dose (Coleman et al., 2021). The median time to onset of the first event was 0.3 months, and the median time to resolution was 0.5 months. Consistent with the findings of the innovaTV 201 study, there were no clinically meaningful changes in prothrombin time, international normalized ratio, or activated partial thromboplastin time (de Bono et al., 2019). Moreover, in innovaTV 204, bleeding events did not result in any substantial changes in clinical management for patients receiving tisotumab vedotin.
Management
Standard interventions should be employed as needed to manage any bleeding in tisotumab vedotin–treated patients; refer to Table 1 for dose modification guidelines for hemorrhage.
OTHER ADVERSE EVENTS
Pneumonitis can occur in patients treated with vedotin-based ADCs, including tisotumab vedotin. Patients should be monitored, and appropriate dose modifications should be followed, as described in Table 1.
Gastrointestinal AEs are often experienced by patients receiving anticancer therapies and have the potential to significantly impact their quality of life (O'Reilly et al., 2020). It is important to consider that it may be challenging to differentiate between treatment-related symptoms, and disease-related and ongoing symptoms (Andreyev et al., 2012). In innovaTV 204, the most common gastrointestinal TRAEs were nausea in 27 patients (27%; 17 with grade 1, 10 with grade 2) and decreased appetite in 11 patients (11%; 6 with grade 1, 5 with grade 2). Patients undergoing treatment with tisotumab vedotin may receive prophylactic antiemetics and other management strategies (e.g., hydration or steroids, per institutional guidelines; Coleman et al., 2021; Dielenseger et al., 2019; Miller & Kearney, 2004; Vacirca et al., 2018).
Chemotherapies, including microtubule-disrupting agents, are associated with high rates of alopecia (Rossi et al., 2017). For example, 87% of patients treated with the microtubule-disrupting agent paclitaxel have been reported to experience alopecia (Hospira Inc., 2011). For patients with r/mCC, alopecia may negatively impact a patient's quality of life, especially given the relatively younger age of this patient population (Ancuţa et al., 2012). Alopecia was observed in innovaTV 201 (treatment emergent, 22 patients, 40%) and innovaTV 204 (treatment related, grade 1, 13 patients [13%]; grade 2, 25 patients [25%]; Coleman et al., 2021; Hong et al., 2020). Based on clinical trial experience, alopecia typically occurs within the first 2 treatment cycles. Scalp cooling or other risk-mitigation measures may be applied per local guidelines to reduce the risk of alopecia for patients, with appropriate considerations of potential risks (Coleman et al., 2021; Rugo et al., 2017). Moreover, it is recommended that the comprehensive care team counsel patients that madarosis (loss of eyebrows or eyelashes) may occur, although this was not observed in innovaTV 204. Madarosis may result in patients involuntarily rubbing their eyes, potentially leading to an increased risk of other ocular AEs through infection or ocular surface inflammation.
Other common TRAEs observed in innovaTV 204 are summarized in Table 3.
Table 3. Other TRAEs Reported in innovaTV 204.
| Grades 1–2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Patients with ≥ 1 TRAE, n (%) | 65 (65) | 25 (25) | 2 (2) | 1 (1) |
| TRAEs with an incidence ≥ 10%, or any grade ≥ 3 event, n (%) | ||||
| Nausea | 27 (27) | 0 | 0 | 0 |
| Fatigue | 24 (24) | 2 (2) | 0 | 0 |
| Myalgia | 15 (15) | 0 | 0 | 0 |
| Anemia | 12 (12) | 1 (1) | 0 | 0 |
| Asthenia | 12 (12) | 1 (1) | 0 | 0 |
| Arthralgia | 12 (12) | 0 | 0 | 0 |
| Decreased appetite | 11 (11) | 0 | 0 | 0 |
| Pruritus | 10 (10) | 1 (1) | 0 | 0 |
| Constipation | 8 (8) | 1 (1) | 0 | 0 |
| Neutropenia | 1 (1) | 3 (3) | 0 | 0 |
| Hypomagnesemia | 2 (2) | 0 | 1 (1) | 0 |
| Dehydration | 1 (1) | 1 (1) | 0 | 0 |
| Hypertension | 1 (1) | 1 (1) | 0 | 0 |
| Hypokalemia | 1 (1) | 1 (1) | 0 | 0 |
| Stomatitis | 1 (1) | 1 (1) | 0 | 0 |
| Colitis | 0 | 1 (1) | 0 | 0 |
| Creatinine renal clearance decreased | 0 | 1 (1) | 0 | 0 |
| Cystitis hemorrhagic | 0 | 1 (1) | 0 | 0 |
| Hypocalcemia | 0 | 1 (1) | 0 | 0 |
| Infection | 0 | 1 (1) | 0 | 0 |
| Infusion site extravasation | 0 | 1 (1) | 0 | 0 |
| Lymphocyte count decreased | 0 | 1 (1) | 0 | 0 |
| Neutropenic sepsis | 0 | 0 | 1 (1) | 0 |
| Platelet count decreased | 0 | 0 | 1 (1) | 0 |
| Pneumonia | 0 | 1 (1) | 0 | 0 |
| Pulmonary embolism | 0 | 1 (1) | 0 | 0 |
| Septic shock | 0 | 0 | 0 | 1 (1) |
Note. Study population (n = 101). TRAE = treatment-related adverse event. Information from Coleman et al. (2021).
DISCUSSION
In innovaTV 204, tisotumab vedotin demonstrated clinically meaningful, durable, and rapid responses in patients with r/mCC, as well as a manageable safety profile (Coleman et al., 2021). Many cancer treatments are associated with AEs that can have a significant impact on patient quality of life (Davidson, 2011). Therefore, it is critical that both patients and the comprehensive care team are aware of potential AEs associated with novel therapies to enable effective management.
Clinicians treating patients with cancer may be unfamiliar with ocular AEs, and such events may be quite distressing for patients to experience. Diligently monitoring patients and adhering to premedication and required eye care can effectively mitigate the risk and help manage symptoms of ocular AEs, as demonstrated in clinical trials of tisotumab vedotin conducted in various practice settings (Coleman et al., 2021; Hong et al., 2020). Patients should be counseled on the symptoms of ocular AEs and encouraged to report any symptoms as soon as they occur. The management of ocular AEs can be optimized through a comprehensive approach to patient care, including active surveillance for symptoms by oncology professionals as well as by integrating eye care providers into the oncology care team for regular ophthalmic exams, diagnosis, and AE management.
Peripheral neuropathy is commonly observed across a broad range of MMAE-containing ADCs and chemotherapies, and can be expected with the use of tisotumab vedotin (Coleman et al., 2021; Kitagawa et al., 2015; Masters et al., 2018; Zajączkowska et al., 2019). Key components of optimal management for peripheral neuropathy include close monitoring of patients by clinicians and patient education around signs and symptoms, both to facilitate early intervention with dose modifications and prevent treatment discontinuation. Nonpharmacologic and pharmacologic interventions may be considered for alleviating symptoms and improving patient quality of life (Loprinzi et al., 2020; Stubblefield et al., 2009).
Many patients with cancer experience bleeding events either as a result of underlying disease pathophysiology (e.g., local tumor invasion) or effects related to treatment (Johnstone & Rich, 2017). Based on data from innovaTV 204, most treatment-related bleeding events associated with the use of tisotumab vedotin (most commonly epistaxis) were low grade and resolved without clinical intervention, and the use of tisotumab vedotin was not associated with any clinically relevant changes in key coagulation parameters (Coleman et al., 2021). Clinicians should continually monitor patients for bleeding events and treat with standard interventions as necessary.
Future studies of tisotumab vedotin will continue to provide data on the safety profile and help guide AE management. A confirmatory phase III study of tisotumab vedotin vs. investigator's choice of chemotherapy for the second- or third-line treatment of patients with r/mCC is ongoing (NCT04697628). Tisotumab vedotin is also being evaluated in combination with other therapies for r/mCC, and as a monotherapy in other solid tumors (NCT03786081; NCT03913741; NCT03485209; NCT03657043).
CONCLUSIONS
Overall, tisotumab vedotin has a manageable safety profile. In innovaTV 204, AEs of interest included ocular AEs, peripheral neuropathy, and bleeding events, all of which can be managed with patient education and proactive monitoring, in addition to prompt intervention with dose modifications and supportive care. Moreover, ocular AEs can be managed by following required eye care and using dose modifications as necessary. Our recommendations are intended to help the comprehensive care team manage treatment with tisotumab vedotin, limit treatment-related toxicity, and maximize treatment adherence.
Acknowledgment
The authors would like to thank all patients who participated in innovaTV 201 and innovaTV 204 and their families. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Michael Q. Steinman, PhD, and Francesca Murphy, BA; editorial support, including figure preparation, formatting, proofreading, and submission, was provided by Travis Taylor, BA, all of Scion, London, UK, supported by Seagen Inc. according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The sponsor was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Akpek, E. K., & Smith, R. A. (2013). Overview of age-related ocular conditions. American Journal of Managed Care, 19(5 Suppl), S67–S75. https://www.ajmc.com/view/ace011_13may_agingeye_akpek [PubMed] [Google Scholar]
- Alley, S. C., Harris, J. R., Cao, A., Heuvel, E. G.-v. d., Velayudhan, J., Satijn,…Breij, E. C. (2019). Abstract 221: Tisotumab vedotin induces anti-tumor activity through MMAE-mediated, Fc-mediated, and Fab-mediated effector functions in vitro. Cancer Research, 79(13 Supplement), 221–221. 10.1158/1538-7445.AM2019-221 [DOI] [Google Scholar]
- Amescua, G., Akpek, E. K., Farid, M., Garcia-Ferrer, F. J., Lin, A., Rhee, M. K.,…American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. (2019). Blepharitis preferred practice pattern®. Ophthalmology, 126(1), P56–P93. 10.1016/j.ophtha.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Ancuţa, B., Nemes, R., Scurtu, S., Shencker, M., & Iliescu, D. (2012). Quality of life in cervical cancer survivors. Acta Medica Marisiensis, 58(5), 275–277. [Google Scholar]
- Ando, R., Kase, S., Ohashi, T., Dong, Z., Fukuhara, J., Kanda, A.,…Ishida, S. (2011). Tissue factor expression in human pterygium. Molecular Vision, 17, 63–69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3021580/ [PMC free article] [PubMed] [Google Scholar]
- Andreyev, H. J., Davidson, S. E., Gillespie, C., Allum, W. H., Swarbrick, E., British Society of Gastroenterology,…Faculty of Clinical Oncology Section of the Royal College of Radiologists. (2012). Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut, 61(2), 179–192. 10.1136/gutjnl-2011-300563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell, S. M., Radford, J., Connors, J. M., Długosz-Danecka, M., Kim, W. S., Gallamini, A.,…ECHELON-1 Study Group. (2022). Overall survival with brentuximab vedotin in stage III or IV Hodgkin's lymphoma. The New England Journal of Medicine, 387(4), 310–320. 10.1056/nejmoa2206125 [DOI] [PubMed] [Google Scholar]
- Arbyn, M., Weiderpass, E., Bruni, L., de Sanjosé, S., Saraiya, M., Ferlay, J., & Bray, F. (2020). Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health, 8(2), e191–e203. 10.1016/S2214-109X(19)30482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari, A. A., & Barney, N. P. (2013). Conjunctivitis: A systematic review of diagnosis and treatment. JAMA, 310(16), 1721–1729. 10.1001/jama.2013.280318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Breij, E. C., de Goeij, B. E., Verploegen, S., Schuurhuis, D. H., Amirkhosravi, A., Francis, J.,…Parren, P. W. (2014). An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Research, 74(4), 1214–1226. 10.1158/0008-5472.CAN-13-2440 [DOI] [PubMed] [Google Scholar]
- Cho, Y., Cao, X., Shen, D., Tuo, J., Parver, L. M., Rickles, F. R., & Chan, C.-C. (2011). Evidence for enhanced tissue factor expression in age-related macular degeneration. Laboratory Investigation, 91(4), 519–526. 10.1038/labinvest.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, J. A. (2018). Dry eye. New England Journal of Medicine, 378(23), 2212–2223. 10.1056/NEJMra1407936 [DOI] [PubMed] [Google Scholar]
- Cocco, E., Varughese, J., Buza, N., Bellone, S., Glasgow, M., Bellone, M.,…Santin, A. D. (2011a). Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: Implications for immunotherapy with hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor. BMC Cancer, 11, 263. 10.1186/1471-2407-11-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco, E., Varughese, J., Buza, N., Bellone, S., Lin, K. Y., Bellone, M.,…Santin, A. D. (2011b). Tissue factor expression in ovarian cancer: implications for immunotherapy with hI-con1, a factor VII-IgGF(c) chimeric protein targeting tissue factor. Clinical & Experimental Metastasis, 28(7), 689–700. 10.1007/s10585-011-9401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, R. L., Lorusso, D., Gennigens, C., González-Martin, A., Randall, L., Cibula, D.,…innovaTV 204/GOG-3023/ENGOT-cx6 Collaborators. (2021). Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncology, 22(5), 609–619. 10.1016/S1470-2045(21)00056-5 [DOI] [PubMed] [Google Scholar]
- Cronau, H., Kankanala, R. R., & Mauger, T. (2010). Diagnosis and management of red eye in primary care. American Family Physician, 81(2), 137–144. https://www.ncbi.nlm.nih.gov/pubmed/20082509 [PubMed] [Google Scholar]
- Davidson, S. (2011). Treatment for advanced cervical cancer: Impact on quality of life. Critical Reviews in Oncology/Hematology, 79(1), 24–30. 10.1016/j.critrevonc.2010.07.002 [DOI] [PubMed] [Google Scholar]
- de Bono, J. S., Concin, N., Hong, D. S., Thistlethwaite, F. C., Machiels, J. P., Arkenau, H. T.,…Lassen, U. (2019). Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1-2 trial. Lancet Oncology, 20(3), 383–393. 10.1016/s1470-2045(18)30859-3 [DOI] [PubMed] [Google Scholar]
- de Goeij, B. E. C. G., Satijn, D., Freitag, C. M., Wubbolts, R., Bleeker, W. K., Khasanov, A.,…Parren, P. W. H. I. (2015). High turnover of tissue factor enables efficient intracellular delivery of antibody–drug conjugates. Molecular Cancer Therapeutics, 14(5), 1130–1140. 10.1158/1535-7163.MCT-14-0798 [DOI] [PubMed] [Google Scholar]
- Dielenseger, P., Börjeson, S., Vidall, C., Young, A., & Jahn, P. (2019). Evaluation of antiemetic practices for prevention of chemotherapy-induced nausea and vomiting (CINV): Results of a European oncology nurse survey. Supportive Care in Cancer, 27(11), 4099–4106. 10.1007/s00520-019-04697-1 [DOI] [PubMed] [Google Scholar]
- Duncan, K., & Jeng, B. H. (2015). Medical management of blepharitis. Current Opinion in Ophthalmology, 26(4), 289–294. 10.1097/ICU.0000000000000164 [DOI] [PubMed] [Google Scholar]
- Förster, Y., Meye, A., Albrecht, S., & Schwenzer, B. (2006). Tissue factor and tumor: Clinical and laboratory aspects. Clinica Chimica Acta, 364(1-2), 12–21. 10.1016/j.cca.2005.05.018 [DOI] [PubMed] [Google Scholar]
- Hong, D. S., Concin, N., Vergote, I., de Bono, J. S., Slomovitz, B. M., Drew, Y.,…Coleman, R. L. (2020). Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clinical Cancer Research, 26(6), 1220–1228. 10.1158/1078-0432.CCR-19-2962 [DOI] [PubMed] [Google Scholar]
- Hospira Inc. (2011). Taxol (paclitaxel) package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Issah, F., Maree, J. E., & Mwinituo, P. P. (2011). Expressions of cervical cancer-related signs and symptoms. European Journal of Oncology Nursing, 15(1), 67–72. 10.1016/j.ejon.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Johnstone, C., & Rich, S. E. (2017). Bleeding in cancer patients and its treatment: A review. Annals of Palliative Medicine, 7(2), 265–273. https://apm.amegroups.com/article/view/17761 [DOI] [PubMed] [Google Scholar]
- Kim, Y. J., Munsell, M. F., Park, J. C., Meyer, L. A., Sun, C. C., Brown, A. J.,…Ramondetta, L. M. (2015). Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecologic Oncology, 139(3), 553–558. 10.1016/j.ygyno.2015.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, R., Katsumata, N., Shibata, T., Kamura, T., Kasamatsu, T., Nakanishi, T.,…Yoshikawa, H. (2015). Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: The open-label randomized phase III trial JCOG0505. Journal of Clinical Oncology, 33(19), 2129–2135. 10.1200/jco.2014.58.4391 [DOI] [PubMed] [Google Scholar]
- Kucik, C. J., & Clenney, T. (2005). Management of epistaxis. American Family Physician, 71(2), 305–311. https://www.ncbi.nlm.nih.gov/pubmed/15686301 [PubMed] [Google Scholar]
- Loprinzi, C. L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D. L.,…Hershman, D. L. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology, 38(28), 3325–3348. 10.1200/jco.20.01399 [DOI] [PubMed] [Google Scholar]
- Maino, D. M., Tran, S., & Mehta, F. (2000). Side effects of chemotherapeutic oculo-toxic agents: A review. Clinical Eye and Vision Care, 12(3–4), 113–117. 10.1016/s0953-4431(00)00053-9 [DOI] [PubMed] [Google Scholar]
- Manfredini, R., Gallerani, M., & Portaluppi, F. (2000). Seasonal variation in the occurrence of epistaxis. American Journal of Medicine, 108(9), 759–760. 10.1016/s0002-9343(00)00437-x [DOI] [PubMed] [Google Scholar]
- Masters, J. C., Nickens, D. J., Xuan, D., Shazer, R. L., & Amantea, M. (2018). Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investigational New Drugs, 36(1), 121–135. 10.1007/s10637-017-0520-6 [DOI] [PubMed] [Google Scholar]
- Miller, M., & Kearney, N. (2004). Chemotherapy-related nausea and vomiting - past reflections, present practice and future management. European Journal of Cancer Care, 13(1), 71–81. 10.1111/j.1365-2354.2004.00446.x [DOI] [PubMed] [Google Scholar]
- Murillo, Jr., J. R., Cox, J. E., & Oholendt, M. S. (2008). An overview of chemotherapy-induced peripheral neuropathy. Journal of Pharmacy Practice, 21(2), 138–145. 10.1177/0897190008315056 [DOI] [Google Scholar]
- O'Reilly, M., Mellotte, G., Ryan, B., & O'Connor, A. (2020). Gastrointestinal side effects of cancer treatments. Therapeutic Advances in Chronic Disease, 11, 2040622320970354. 10.1177/2040622320970354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, A., Fortuna, M. C., Caro, G., Pranteda, G., Garelli, V., Pompili, U., & Carlesimo, M. (2017). Chemotherapy-induced alopecia management: Clinical experience and practical advice. Journal of Cosmetic Dermatology, 16(4), 537–541. 10.1111/jocd.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo, H. S., Klein, P., Melin, S. A., Hurvitz, S. A., Melisko, M. E., Moore, A.,…Cigler, T. (2017). Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA, 317(6), 606–614. 10.1001/jama.2016.21038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagen Inc., & Genmab US Inc. (2022). Tivdak (tisotumab vedotin) package insert. seagendocs.com/Tivdak_Full_Ltr_Master.pdf
- Shah, A., Talati, M., & Mauger, T. (2017). Medical and surgical management of Pasteurella canis infectious keratitis. IDCases, 9, 42–44. 10.1016/j.idcr.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. (2001). Keratitis. Bioscience Reports, 21(4), 419–444. 10.1023/a:1017939725776 [DOI] [PubMed] [Google Scholar]
- Shimizu, S., Ogawa, T., Takezawa, K., Tojima, I., Kouzaki, H., & Shimizu, T. (2015). Tissue factor and tissue factor pathway inhibitor in nasal mucosa and nasal secretions of chronic rhinosinusitis with nasal polyp. American Journal of Rhinology & Allergy, 29(4), 235–242. 10.2500/ajra.2015.29.4183 [DOI] [PubMed] [Google Scholar]
- Stubblefield, M. D., Burstein, H. J., Burton, A. W., Custodio, C. M., Deng, G. E., Ho, M.,…Von Roenn, J. H. (2009). NCCN task force report: Management of neuropathy in cancer. Journal of National Comprehensive Cancer Network, 7(Suppl 5), S1–S28. 10.6004/jnccn.2009.0078 [DOI] [PubMed] [Google Scholar]
- Tanay, M. A., & Armes, J. (2019). Lived experiences and support needs of women who developed chemotherapy-induced peripheral neuropathy following treatment for breast and ovarian cancer. European Journal of Cancer Care, 28(3), e13011. 10.1111/ecc.13011 [DOI] [PubMed] [Google Scholar]
- Titiyal, J. S., Negi, S., Anand, A., Tandon, R., Sharma, N., & Vajpayee, R. B. (2006). Risk factors for perforation in microbial corneal ulcers in north India. British Journal of Ophthalmology, 90(6), 686–689. 10.1136/bjo.2005.079533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay, M. P., Srinivasan, M., & Whitcher, J. P. (2015). Diagnosing and managing microbial keratitis. Community Eye Health, 28(89), 3–6. https://pubmed.ncbi.nlm.nih.gov/26435583 [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2017). Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Vacirca, J., Caruana, D., Calcanes, G., Mosier, M., Boccia, R., & McBride, A. (2018). Hydration requirements with emetogenic chemotherapy: Granisetron extended-release subcutaneous versus palonosetron. Future Oncology, 14(14), 1387–1396. 10.2217/fon-2017-0720 [DOI] [PubMed] [Google Scholar]
- van den Berg, Y. W., Osanto, S., Reitsma, P. H., & Versteeg, H. H. (2012). The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood, 119(4), 924–932. [DOI] [PubMed] [Google Scholar]
- Varu, D. M., Rhee, M. K., Akpek, E. K., Amescua, G., Farid, M., Garcia-Ferrer, F. J.,…American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. (2019). Conjunctivitis Preferred Practice Pattern®. Ophthalmology, 126(1), P94–P169. 10.1016/j.ophtha.2018.10.020 [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., Nakagaki, T., & Kisiel, W. (1992). Tissue factor-dependent autoactivation of human blood coagulation factor VII. Journal of Biological Chemistry, 267(27), 19089–19094. https://www.ncbi.nlm.nih.gov/pubmed/1527033 [PubMed] [Google Scholar]
- Zajączkowska, R., Kocot-Kępska, M., Leppert, W., Wrzosek, A., Mika, J., & Wordliczek, J. (2019). Mechanisms of chemotherapy-induced peripheral neuropathy. International Journal of Molecular Sciences, 20(6), 1451. 10.3390/ijms20061451 [DOI] [PMC free article] [PubMed] [Google Scholar]


