Abstract
Pseudomonas aeruginosa is a major, but opportunistic, respiratory pathogen, which rarely infects healthy individuals, mainly due to the barrier effect of the human airway epithelium (HAE). This review explores the interaction of P. aeruginosa with HAE and the progression of the infection. The basolateral part of the epithelium, which includes the basolateral membrane of the epithelial cells and the basement membrane, is inaccessible in normal tight epithelia with intact junctions. We highlight how P. aeruginosa exploits weaknesses in the HAE barrier to gain access to the basolateral part of the epithelium. This access is crucial to initiate respiratory infection and is mainly observed in the injured epithelium, in repairing or chronically remodeled epithelium, and during extrusion of senescent cells or cell multiplication during normal epithelium renewal. The subsequent adhesion of the bacteria and cytotoxic action of virulence factors, including the toxins delivered by the type 3 secretion system (T3SS), lead to retractions and cell death. Eventually, P. aeruginosa progressively reaches the basement membrane and propagates radially through the basal part of the epithelium to disseminate using twitching and flagellar motility.
Introduction
Pseudomonas aeruginosa is a ubiquitous gram-negative bacterium mainly found in aqueous environments and surfaces [1–4]. This opportunistic pathogen is responsible for various infections, particularly those involving the respiratory tract [5–7]. Owing to intact airway epithelial barrier [8–11] and optimal lung defenses, P. aeruginosa is rarely pathogenic in healthy individuals [12], and the development of infections depends on epithelial injuries [13–16], mucociliary clearance dysfunctions [17], or immune system impairment [18–21]. Acute or chronic pneumonia is limited to immunocompromised patients or those with defective pulmonary function, such as mechanically ventilated patients in intensive care unit [5,22,23], and those with cystic fibrosis (CF) [24,25] or chronic obstructive pulmonary disease (COPD) [7,26–28].
The intact human airway epithelium (HAE) is a physical and functional barrier against pathogens [29,30]. The pseudostratified and differentiated mucociliary bronchial epithelium is usually composed of several different types of cells, supported by the underlying basement membrane [31] (Fig 1). The ciliated and secretory cells, which are tall, functionally differentiated, and highly polarized, reach the apical surface. The basal cells, which are located at the basal pole under the columnar cells, are multipotent progenitors, which generate the other cells during the differentiation process [31–33]. In intact healthy HAE, only apical membranes are exposed to the environment [20,34]. Injuries, varying from junction disruptions to partial shedding of the epithelium, or even to complete denudation of the basement membrane, induce the HAE to initiate a repair process to regenerate and restore its function [35–37]. In chronic inflammatory respiratory diseases such as CF or COPD, the dysregulated inflammation can alter the respiratory epithelial repairing process [14,15,35,38–42].
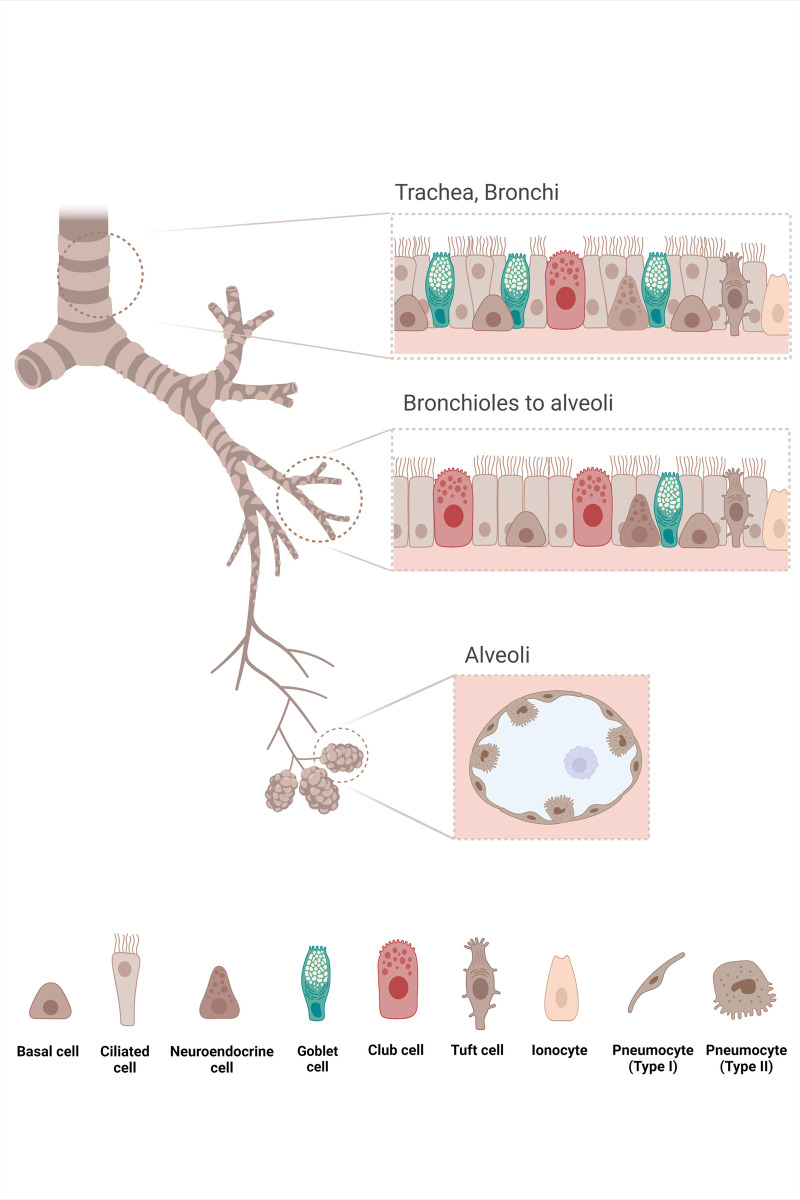
Fig 1. Lung epithelia structure.
The lung is a complex organ composed of conducting airways and gas exchange zones. The conducting airways branch from the trachea to terminal bronchioles that end up in the alveoli. In airways, the lining epithelium provides a physical barrier between the external environment and the underlying parenchyma, whose integrity is maintained by intercellular junctions. The airway epithelium ensures the protection of the lung against inhaled particles, toxins, and pathogens through the mucociliary clearance and secretion of molecules with antibacterial, antioxidant, and antiprotease activity that act in an orchestrated way to protect the epithelium from lesion factors. The diversity of cells constituting the epithelial barrier is adapted to the epithelial functions. In the pseudostratified epithelium lining the trachea and bronchi, different specific cell types such as basal, goblet, and ciliated cells are found. Basal cells are progenitor cells involved in the epithelial renewal and the anchor of the epithelium to the basement membrane through hemidesmosomes. Goblet cells, within subepithelial glands, produce the respiratory mucus that entraps noxious particles and is moved towards the oropharyngeal junction by the coordinated beating of ciliated cells. Some rare secretory Club cells are also described. In the distal bronchioles, the number of basal cells decreases, and the goblet cells are progressively replaced by Club cells, mainly involved in the production of anti-inflammatory factors and surfactant proteins. In addition, other cell types were found more rarely: neuroendocrine cells, which serve as communicators between the immune and nervous system by secreting neuropeptides; Tuft cells, which have chemosensory, neuronal, and immunological functions; and pulmonary ionocytes. The alveoli are lined by type I and type II pneumocytes whose functions are completely different: Type I pneumocytes are involved in the O2/CO2 exchange through their thin cytoplasm, whereas type II pneumocytes secrete surfactant proteins and act as progenitor cells of the alveolar epithelium. Created with BioRender.com.
The airway epithelium protects the lungs from various external assaults (pollutants, pathogens, allergens, etc.) in three ways. First, the airway epithelium is a physical barrier. Its impermeability is ensured by strongly connected cells by tight junctions, adherens junctions, and desmosomes [43,44]. The tight junctions, located near the apical border, delimit the apical plasma membrane from the basolateral plasma membrane of the differentiated cells [45–47]. In healthy epithelium, the basolateral part, which is composed of the basolateral cell membrane and the underlying basement membrane, is not exposed to external agents [29,43]. Second, the airway epithelium can expel inhaled particles from the airways using the mucociliary clearance mechanism. The mucociliary clearance is carried out by the ciliated and secretory cells. It is the main process for removing inhaled foreign particles from the airways [44]. The airway surfaces are lined by epithelial cells and covered with an airway surface layer (ASL) composed of two parts: a mucus layer and a low-viscosity periciliary layer (PCL). Thus, the ASL lubricates airway surfaces and facilitates ciliary beating for efficient mucus clearance [17]. The mucus produced by secretory cells forms a continuous layer on the epithelial surface, and this three-dimensional matrix acts as a physical barrier protecting the underlying epithelia and as a trap for inhaled particles and exogenous microorganisms [48–50]. It is composed of mucins (MUC5ac and MUC5b), which are heavily glycosylated proteins within the mucus to which the pathogens attach [51–53]. Thereby, the coordinated beating of the ciliated cells’ cilia sweeps the trapped bacteria out of the lungs toward the oropharynx where they are swallowed. Third, the airway epithelium is capable of defending itself against infectious agents. The airway epithelium can produce antimicrobial peptides and inflammatory cytokines, which participate in the innate immune response [20,29].
This review discusses the interaction of P. aeruginosa with HAE as it is an integral part of its virulence in the respiratory tract. We will first highlight that the essential step to initiate airway infection is access to the basolateral part of the HAE by P. aeruginosa. Then, we will identify the different steps in the HAE infection by P. aeruginosa and discuss some specific issues, such as how the bacteria adhere to the HAE cells, what is the role of pathogen internalization by HAE cells in infection, and how P. aeruginosa crosses and invades the epithelium.
Access to the basolateral part of HAE is crucial for P. aeruginosa to initiate respiratory infections
1) Breach of the epithelium is a hallmark of P. aeruginosa infections
The HAE plays a key role in defense against P. aeruginosa by being a functional physical barrier thanks to intercellular junctions, mainly tight junctions, leading to epithelial polarity and impermeability. The ability to exploit epithelial breaches to reach the binding receptors in deeper tissues is a hallmark of P. aeruginosa infections. Most, if not all, pathologies caused by this bacterium illustrate this point. Cutaneous infections mostly occur after skin injuries such as extended burns or chronic cutaneous wounds, the latter being quite common among diabetic patients [54,55]. Keratitis or corneal infections usually follow disruption of the corneal epithelium occurring after ocular trauma, surgery, or lesions caused by contact lenses [56–59]. The risk factors of acute otitis externa, which is mainly observed in pools and hot tub users [60,61], include ear canal epithelium disruptions, such as abrasion (scratching, clearing ear canals), maceration, psoriasis, or eczema [60,61]. The likelihood of P. aeruginosa catheter-related urinary tract infections is increased following damage to the epithelium structure during catheter insertion or manipulation [62]. For respiratory infections, the significance of epithelial breaches is also observed, in addition to biofilm formation and impaired mucociliary clearance. HAE lesions are described in CF and COPD patients, but also in those undergoing intubation or mechanical ventilation [13,63–65]. (See below.)
2) HAE breach allows P. aeruginosa access to the basolateral part of the epithelium
Epithelial breaches expose the basolateral part of the epithelium (i.e., the basolateral membrane of HAE cells and/or the basement membrane), which is normally inaccessible in healthy epithelium owing to intact tight junctions [34]. Access to the basolateral part is the main element of P. aeruginosa interaction and is crucial for initiating P. aeruginosa respiratory infections.
Many studies demonstrated that P. aeruginosa poorly interacts with an intact, healthy, and well-polarized HAE. Conversely, numerous reports demonstrated preferential interactions with injured or repairing epithelia, or with nonpolarized cells in the basolateral part of HAE. The first such report showed that in rat tracheal surface, P. aeruginosa interacts in vivo more easily with brush-injured sites than with those that are not injured [66]. Subsequent studies have also shown infrequent adhesion to the normal epithelium but increased adhesion to areas of epithelial damage and the basement membrane in airway culture models [9,67]. Lee and colleagues confirmed the crucial role of epithelial tight junctions in interaction with P. aeruginosa. They showed that bacterial adhesion and toxic effects are more frequently found near the free edges of epithelial wounds, where the basolateral cell plasma membrane is accessible [68]. HAE with intact tight junctions is entirely resistant to P. aeruginosa-induced cell apoptosis, as opposed to nonjunctional epithelia [69]. Although the mucociliary clearance decreases bacterial access to the epithelium, the Puchelle team reported that P. aeruginosa is unable to adhere to normal and uninjured cells because the functional tight junctions play a key role in masking the basolateral main receptors for bacterial attachment (see below). They described the preferential sites of interaction with P. aeruginosa on the exposed basolateral plasma membranes, the denuded basement membrane after injury, as well as the flattened migrating and spreading cells during repair [11,14,15,70,71]. Other studies confirmed that basolateral plasma membranes exposed after injury are more likely to bind P. aeruginosa and that the level of the polarity of the cells is also an important factor, given that nonpolarized migrating cells or incompletely polarized cells are more susceptible to P. aeruginosa binding and intoxication [72,73].
3) Main settings wherein P. aeruginosa could exploit opportunities to gain access to the basolateral part of HAE
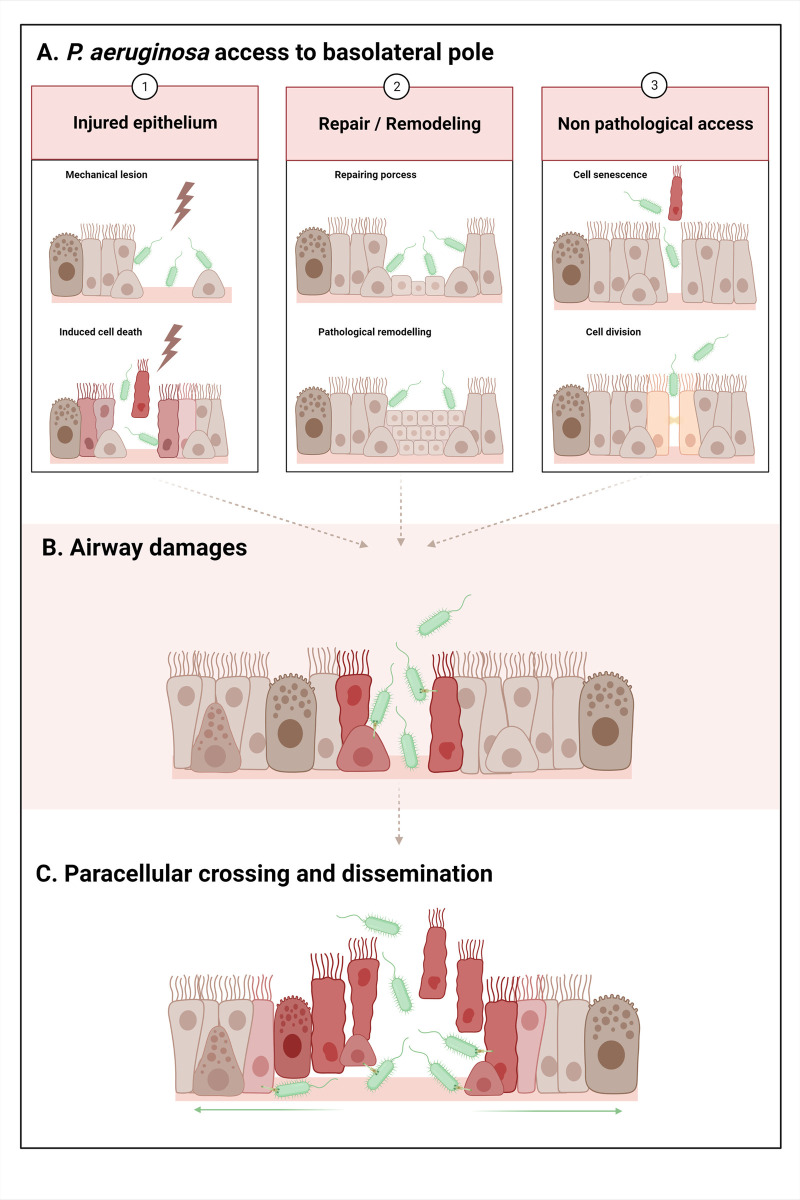
a) Injured epithelium. Following an injury to the epithelium, the basolateral part becomes accessible to P. aeruginosa because the basolateral membrane of adjacent cells and basement membrane are exposed (Fig 2A and left panel).
Fig 2. P. aeruginosa basolateral interaction and progression in the airway epithelium.
A. In the first step of infection, P. aeruginosa gains access to the basolateral part, exploiting various opportunities: 1. Injured epithelium: a. Mechanical lesion: removal of HAE cells and denudation of the basement membrane. Ex: endotracheal tube in VAP. b. Induced epithelial cell death: dying cells undergo retraction, tight junction disruption, and detachment from the adjacent cells and the basement membrane. The injury could be caused by pathogens (viruses, bacteria, etc.), chemical injury (pollutants, toxic compounds, etc.), or excessive inflammatory processes (excess of cytokines, proteases, oxidant stress, etc.). Ex: excess inflammation injuring HAE in CF and COPD. 2. Repair of the epithelium after injury/epithelium remodeling: a. After an injury, HAE undergoes a repair process: basal cell dedifferentiation, spread and migration, transitional squamous metaplasia or basal/mucous hyperplasia, and progressive differentiation. Cells display low differentiation levels, low polarity, and no functional tight junctions. Ex: repair in CF and COPD. b. Chronic and pathologically remodeled epithelium: squamous and goblet cells metaplasia, hyperplasia of surface goblet and basal cells. Cells display low differentiation levels, low polarity, and no functional tight junctions. Ex: remodeled epithelia in CF and COPD. 3. Nonpathological access in differentiated epithelium: transient disruption of tight junctions during: a. Extrusion of a senescent cell. b. Cell division. B. P. aeruginosa virulence factors induce airway damage, notably by T3SS toxin injection on the basolateral membranes of the cells. T3SS effectors (ExoS, ExoT, and ExoU) induce cell retraction and death, facilitating the subsequent access of bacteria to the adjacent or underlying cells and the basement membrane. C. Bacteria cross the epithelium by this paracellular route, gain access to the basal part, and progressively propagate radially through the epithelium to disseminate, using pili and flagella. Created with BioRender.com.
First, this can result from endotracheal tube insertion and manipulation that, not infrequently, induce mechanical damage in ventilator-associated pneumonia (VAP). The endotracheal tube can cause scratching and abrasion of the HAE, removing fragments of the epithelium and leading to denudation of the basement membrane [13,63,74]. In addition, biofilm formation on the plastic surface and mechanical impairment of the mucociliary clearance by the tube facilitate infection [63].
Second, this can result from induced HAE cell death. Dying cells undergo retraction and tight junction disruptions, leading to subsequent detachment from the adjacent cells and the basement membrane. The causes of cell injury are extremely diverse and can be induced by pathogens (viruses, bacteria, etc.), chemical injury (pollutants, toxic substances, allergens, etc.), or hyperactive inflammation (excess of cytokines, proteases, oxidant stress, etc.) [14,35]. Injuries could be part of a potentially vicious circle since HAE injury by itself will attract immune cells. For instance, degranulation of neutrophils contributes to inflammation and tissue damage [19,25,75,76], which could increase the number of breaches and thereby provide an opportunity for P. aeruginosa to reach the basolateral part of HAE. But, a fine-tuned homeostasis has been described in peritoneal serosa, which is likely transposable to other tissues including the lung tissue. Indeed, Uderhardt and colleagues reported that such homeostasis is maintained to prevent the excess of deleterious inflammation in the case of microlesion. Resident tissue macrophages sense rapidly the death of individual cells and sequester the damage owing to extending membrane processes (pseudopods), thus preventing neutrophil activation [77]. In case of too many lesions, such as exemplified below for CF and COPD, the cloaking by resident tissue macrophages is overwhelmed and would not prevent neutrophil-driven inflammation and subsequent tissue damage.
In CF disease, different factors contribute to P. aeruginosa infections. The most common and well-accepted paradigm is the “low volume hypothesis” stating that in the absence of a functional chloride channel CFTR, the airway mucus is dehydrated, hyperviscous, and thickened, thereby compromising the mucociliary clearance [78,79]. The mucus stagnates in the airways, and this aberrant accumulation of mucus provides a nidus for colonization and recurrent infections by opportunistic pathogens, like P. aeruginosa [78]. As CF patients become chronically infected, P. aeruginosa adapts to the specific lung environment by genotypic and phenotypic variations, such as loss of virulence and/or increased resistance to antimicrobials and host immunity. P. aeruginosa usually grows as a biofilm on host tissues/epithelial surfaces during these chronic infections [78,80]. Bacterial multiplication, following their entrapment within the mucus, induces a vigorous inflammatory response. Moreover, the deficit in the immune response due to CFTR dysfunction leads to persistent and hyperactive immunological stimulation, resulting in chronic lung inflammation [18,19,78]. For instance, the decreased CFTR functionality reduces bicarbonate secretion in the airways, then decreases the pH of the mucus in CF patients. It leads to the inactivation of cationic antimicrobial peptides secreted by the host and to mucus tethering and impaired mucus detachment from the lung epithelium [78]. Activated neutrophils and macrophages accumulated in the airways, and these cells release multiples products such as inflammatory cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-8), reactive oxygen species, or protease (like neutrophil elastase), all contributing to the HAE destruction [19,25,75,76]. This excessive and ineffective inflammatory response, probably exacerbated by bacterial toxins, leads to the inadvertent destruction and damage of the HAE [14,18,19,25]. Various injuries to the HAE have been previously described in CF airways, including microlesions, shedding areas (few basal cells are still present or even total denudation of the basement membrane), epithelial sloughing and disorganization of the epithelial tight junctions, etc. [14,39,64,81,82].
Besides the mucociliary clearance elevator, the mucus composition could also play a role in bacterial virulence in the lung. In healthy epithelium, the mucus protects against pathogens’ epithelial adhesion and cytotoxicity and appears to play an important role in suppressing bacterial virulence. It was shown that mucins prevented P. aeruginosa aggregation and adhesion to the underlying surface and that mucins triggered the dispersal of P. aeruginosa biofilm[83,84]. Mucins significantly enhanced twitching motility and decreased biofilm formation [85]. It was also described that mucins attenuated the virulence of P. aeruginosa, decreasing many virulence genes (such as type 3 and 6 secretion systems (T3SS/T6SS), siderophores, and quorum sensing), disintegrating biofilms, and reducing cytotoxicity on human epithelium colorectal cells and burn infections in a porcine model [86]. When mucus structure and/or properties are compromised, the mucus’ protective abilities may be significantly decreased. It is exemplified in CF with a defect of mucin expression and mucus production associated with more susceptibilities to P. aeruginosa infection than in the healthy lung [50].
In COPD patients, the chronic and excessive inflammation due to the causative inhaled irritants (for instance, cigarette smoke) leads to an increased number of neutrophils and macrophages in the lungs, as well as to the activation of airway epithelial cells and mucus hypersecretion. Emphysema and luminal occlusion by aberrant mucus and inflammatory exudate is commonly observed [87]. A reduced mucociliary clearance is also described in COPD [88]. Cells produce pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8, and release elastase. Combined with the products of oxidative stress, it contributes to the HAE breakdown [87,88]. Microbial colonization of the respiratory tract is often observed, which can be associated with acute exacerbation, enhancing the host inflammatory response [7]. This combined pathogen and host actions enhance HAE injuries [7]. Particularly, in COPD, the major alteration of the lung is parenchymal destruction [65]. Coinfections with viruses and bacteria have been described to cause more severe functional lung impairments in COPD patients [89]. Viruses, notably the respiratory syncytial virus, alter the expression of receptor molecules on respiratory epithelial cells and provoke cell death. In turn, an increase in the adhesion and invasion by P. aeruginosa leads to coinfections or superinfections [7,90].
b) Repair of the epithelium after injury/epithelium remodeling. To regenerate and restore its function following injury, the HAE has to repair itself. During this process, the surviving basal cells that are adjacent to the wound edge, spread, and migrate to cover the denuded basement membrane [35,91]. Basal cells then proliferate, forming a transitional squamous metaplasia [37]. Cells undergo a differentiation process, progressively regenerating their polarity and reform junctions, resulting in a complete and functional pseudostratified mucociliary epithelium. During this repairing process, the exposed cells display low or no differentiation levels, low or no polarity, no functional tight junctions, and expressed alternative receptors, favoring interactions with P. aeruginosa [14,15,35,43,70,72] (see “Adhesion” section and Table 1). As for injuries described above, for CF and COPD, P. aeruginosa interactions with repairing HAE are facilitated (Fig 2A and middle panel).
Table 1. P. aeruginosa airway receptors.
| Airway receptors | Type of cells used to evidence airway receptors | Location in healthy airways | Pathological epithelia | PA adhesin | References | |
|---|---|---|---|---|---|---|
| Glycosphingolipids (Lipid rafts components) | Asialo GM1 (GalNAcβ1-4Gal disaccharides) | Primary human nasal epithelial cells (differentiated and not differentiated) | Basal cell membrane | Specific apical membrane expression by regenerating epithelium or CF epithelium | - Type IV pili (C-terminal part) - Flagella |
[16,127–131] |
| Globotriaosylceramide Gb3 (CD77, αGal–βGal–βGlc–Cer saccharides) | Cell lines (H1299 kc, A549) | Unknown | - | LecA | [132–137] | |
| N-glycoproteins | N-glycans | Cell lines (Calu 3) Primary bronchial cell | Apical membrane mainly (expressed on basal cell membrane, but few interactions with PA) | Enhanced expression in damaged epithelium | Type IV pili | [109,138,139]* |
| CFTR | Cell lines (CFT-1, CFBE41o-, A549, WI-38) Primary human bronchial epithelial cells (differentiated) | Apical membrane | Decreased expression in dedifferentiated and remodeled epithelium | LPS | [140–145] | |
| Integrins | α5β1 integrin–Fibronectin | Cell lines (16HBE) Primary nasal cells (differentiated) | Absent in normal airways | Apically exposed and overexpressed in repairing epithelium | OprQa | [70,71,146–148] |
| αvβ5 integrin–Vitronectin | Bronchial tissue | Unknown | Increased expression in repairing epithelium or inflamed epithelium | OprD | [149–152] | |
| Proteoglycans | HSPG (Heparan sulfate proteoglycans) (HS chain) | Cell lines (Calu 3, 16HBE) Primary nasal cell | Basal cell membrane mainly | Apically increased during epithelial injury and dedifferentiation | Flagella | [109,138,153,154]* |
| ECM | Laminin Type I and IV collagens | Not applicable | Basement membrane (ECM beneath the epithelium) | Accessible only in injured epithelium | OprD, OprG, EstA, PA3923b | [14,155,156] |
CFTR, cystic fibrosis transmembrane regulator; ECM, extracellular matrix; LPS, lipopolysaccharides; OMP, outer membrane protein; PA, P. aeruginosa.
aRoger and colleagues described a 50-kDa OMP, probably corresponding to the OprQ porin described later by Arhin and colleagues.
bde Bentzmann and colleagues described 60 kDa OMPs, probably corresponding to the OprD, OprG, EstA, PA3923a porin described later by Paulsson and colleagues.
In CF and COPD, chronic and pathological remodeling, such as squamous metaplasia or hyperplasia of goblet and basal cells, is typically observed [34,38–42,65,82]. As in the repairing process, cells in remodeling epithelia show reduced differentiation, low polarity, absence of functional tight junctions, and expression of alternative receptors, again enhancing their potential interaction with P. aeruginosa [14,15,70] (see “Adhesion” section and Table 1).
c) Access in nonpathological conditions Bacteria can also gain access to the basolateral part of the HAE in differentiated epithelium without any injury or repair process, due to the normal epithelial renewal. Some studies have shown that after a transient disruption of the epithelial junctions, either following extrusion of a senescent cell or cell division during multiplication, the basolateral part becomes exposed and P. aeruginosa can interact with the HAE [92–94]. As previously described for the pathogenic bacteria Listeria monocytogenes in the MDCK cell line [95], Heiniger and colleagues described that P. aeruginosa adhesion to the apical membrane of ciliated cells from primary differentiated human epithelium is very low (see “Adhesion” section). They also showed that the interaction with the cells occurs after a transient disruption of the epithelial barrier during the extrusion of a senescent cell. The basolateral membrane of the ciliated cells is then exposed, and the bacteria can easily interact with it [94]. Disruptions of tight junctions occur during cell divisions or cell senescence and are both natural phenomena ensuring epithelial homeostasis [92,93]. In the MDCK cell line model, Golovkine and colleagues showed that very soon after HAE infection (approximately 3 hours), P. aeruginosa takes advantage of these brief and transient ruptures of the epithelial barrier to gain access to the basolateral part. In this model, cell deaths were not caused yet by P. aeruginosa but were natural/physiological deaths [93] (Fig 2A and right panel).
The clinical impact of the interaction of P. aeruginosa to the basolateral part in vivo in healthy humans might be low and insufficient to develop an infection, as P. aeruginosa is rarely described in community-acquired pneumonia [96]. The functional mucociliary clearance and competent immune defenses are likely to clear most of the P. aeruginosa present in the airways of healthy individuals. Moreover, airway cell divisions/senescence are infrequent, decreasing the probability of encountering P. aeruginosa to initiate infections in healthy individuals. Indeed, (i) the cell turnover rate of the HAE self-renewal is one of the lowest compared to other epithelia, more than 100 days for HAE [97,98] versus 3 to 5 days for intestinal epithelium, 10 days for corneal epithelium, or 20 days for cutaneous epithelium [99–102]; (ii) Golovkine and colleagues described that the opportune interaction of P. aeruginosa is not systematic in their model but is rather a rare event since many sites of cell divisions/deaths were not exploited by the bacteria [93]; (iii) the cloaking by resident tissue macrophages prevents excess tissue damages and preserves tissue homeostasis [77]; and (iv) the few cases of P. aeruginosa community-acquired pneumonia, described in healthy individuals, were often associated with prolonged or repeated exposures to contaminated whirlpools or hot tubs aerosols [61,103–105]. The number of P. aeruginosa observed in these contaminated waters, wherein the bacteria are proliferating, was high (up to 105 colony-forming units (CFU)/mL) [61,105–107]. We can speculate that the high infectious inoculum of P. aeruginosa can counter the strong barrier effect by increasing the probability of encountering the rare physiological breach event, thereby explaining the development of infection in a healthy individual.
Either way, it is noteworthy that the studies describing P. aeruginosa interaction with intact airway epithelium in ex vivo culture models used very high bacterial inocula (from 106 to 108 CFU [72,94,108–112]) or high concentration of purified virulence factors (such as elastase [113–117], LPS [118], rhamnolipids [119,120], and 3OC12-HSL [121–123]). Although both are related to the bacterial load commonly detected in clinical samples [124,125], they correspond to thresholds commonly seen in clinical microbiology laboratories at the time of airway infection diagnosis. Considering the bacterial proliferation in the infected airways, they are likely far higher than the inoculum at the initiation of the epithelial breach. Additional studies are needed to address the relevance of the infectious inoculum in vivo, when P. aeruginosa pulmonary infections occur in CF, COPD, or VAP.
The steps of progression of P. aeruginosa during airway infection
1) Adhesion of P. aeruginosa to the airways
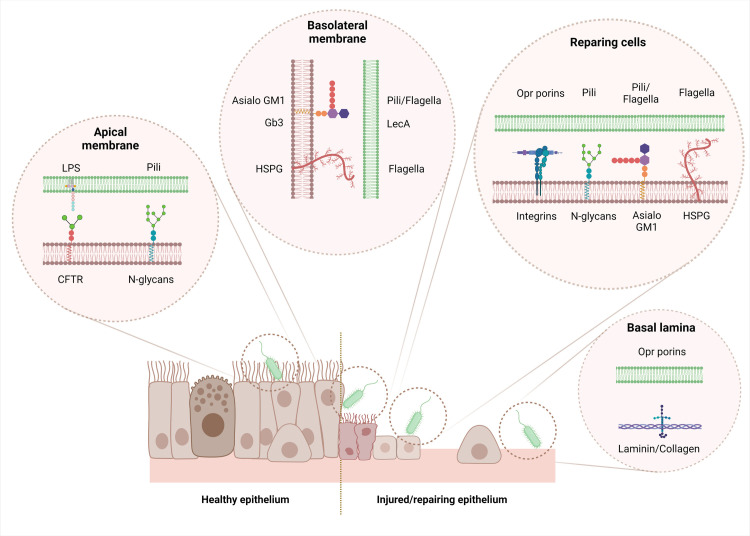
Respiratory infections by P. aeruginosa are usually initiated by the adhesion of the bacteria to the HAE cells or the basement membrane. Appendices of motility and adherence, pili, and flagella are virulence factors present on the bacterial surface, mainly responsible for movement and attachment to the cells [109]. Utilizing different mechanisms of motility (swarming, swimming, and twitching), the bacteria sense the optimal surface for initiating cell surface contact and properly attached to the surface [126]. Several molecules, present on the host epithelial cell and extracellular matrix components and containing long carbon chains, bind the different adhesins of P. aeruginosa (Fig 3 and Table 1).
Fig 3. Airway receptors of P. aeruginosa.
Green membrane: P. aeruginosa; brown membrane: host cells. Created with BioRender.com.
It is noteworthy that some of these receptors, listed in Table 1, are expressed at different locations on the epithelium, depending on specific situations. At the apical side of healthy airway epithelium, very few receptors are present, which strongly limit interaction with P. aeruginosa. For instance, integrins or N-glycans are hardly or not at all present at the apical part of healthy epithelium, but they are overexpressed during the repair process specifically [138]. Asialo GM1 and HSPG are mainly expressed at the basolateral part but are specifically recovered at the apical part in regenerating or CF epithelia [16,128,138]. Migrating cells actively synthesize cellular fibronectin/vitronectin, and their receptors, the integrins, are up-regulated and apically exposed during migration in the repairing process [70,148,151]. As fully detailed in Part I, the adhesion of P. aeruginosa to receptors at the basolateral part of the epithelium can only occur in case of disruption or lack of tight junctions or when cells lose their polarization, which is mainly observed within injured or repairing/remodeled epithelium [9,14–16,67,68,94].
Pili-mediated adhesion, combined with their retractive ability, creates close contact between bacteria and host cells and induces the action of various export systems. In particular, the T3SS is an important contact-dependent virulence factor that injects toxins through the membrane of the host cell [157,158]. Pili mechanical retraction induces the chemosensory system phosphorelay (Chp), leading to stimulation of adenylate cyclase CyaB and up-regulating the signaling molecule adenosine-cyclic monophosphate (cAMP) production, which allosterically activates the virulence factor regulator (Vfr)-dependent virulence system [158,159]. Vfr regulates multiple virulence factors in P. aeruginosa, including the T3SS, but also the T2SS and the quorum sensing [160,161].
2) Airway damage induced by P. aeruginosa
P. aeruginosa virulence factors involved in respiratory infections
P. aeruginosa is an environmental bacterium adapted to the soil and aqueous habitat. It is an opportunistic pathogen found mainly in immunocompromised patients. Human airway pathogenicity of P. aeruginosa is explained by the production of many virulence factors, displaying varied and complementary properties [162,163]. Some factors are involved in motility and adhesion, persistence in the host, iron uptake, or resistance to oxidative stress. The toxins are mostly involved in the destruction of the host cells [163].
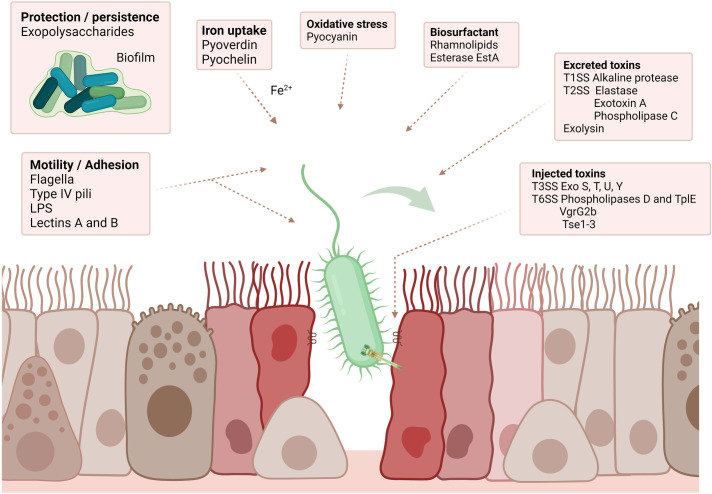
The main virulence factors of P. aeruginosa involved in respiratory infections are illustrated in Fig 4 and their functions are detailed in Table 2.
Fig 4. aeruginosa main virulence factors in respiratory infections.
P. Created with BioRender.com.
Table 2. Virulence factors of P. aeruginosa in respiratory infections.
| Class | Virulence factor | Gene(s) | General function(s) | HAE-specific function(s) | Type of cells used to evidence HAE-specific function(s) |
Molecular host target | SS | Reference |
|---|---|---|---|---|---|---|---|---|
| Motility/Adhesion | Flagella | fliC | Swimming and swarming motility Host cell adhesion and internalization |
Motility and basal adhesion to HAE cells HAE infection progression |
Primary nasal cells, cell lines (Calu 3, 1HAEo-), mice pneumonia | Heparan sulfate, proteoglycans, AsialoGM1 | - | [127,130,138,172] |
| Type IV pili | pilA | Twitching motility Host cell adhesion, biofilm formation |
Motility and apical adhesion to HAE cells Allow T3SS injection. HAE infection progression |
Cell lines (Calu 3) | N-glycans | - | [138,173,174] | |
| LPS | waa, wzy, wzz, wbp, wzx | Resistance to serum killing and phagocytosis, inflammatory response Binding to CFTR Cell junctions’ disruptions and apoptosis |
Apical adhesion to HAE cells Disruption of HAE tight junctions HAE cell apoptosis |
Cell lines (A549, NCL-H292, Beas-2b) | CFTR, Tight junctions (ZO-1) | - | [54,118,175–177] | |
| Lectin A Lectin B |
lecA
lecB |
Host cell adhesion: binding to galactose (LecA) and fucose (LecB) Internalization (LecA) Cytotoxicity (LecA and LecB) |
Apical and basal adhesion to HAE cells HAE cell internalization HAE cell cytotoxicity |
Primary nasal cells, cell lines (H1299, A549), mice pneumonia | Globotriaosylceramide Gb3 (CD77) | - | [132,134,178] | |
| Protection/Persistence | Exopolysaccharides (Alginate, Psl, Pel) |
alg, muc, psl, pel | Biofilm main component: polymeric matrix Persistence, bacteria protection (from IS and antibiotics) |
Biofilm persistence in chronic HAE infection | - | - | - | [167,179–181] |
| Iron uptake | Siderophores: - Pyoverdin - Pyochelin |
pvd
pch |
Iron siderophore, green pigment (pyoverdine) Cytotoxicity (ROS production) |
- | - | DNA, lipid membrane, proteins | - | [182–185] |
| Heme uptake systems: - Pseudomonas heme uptake - Heme assimilation systems |
phu
has |
Extracellular heme acquisition (uptake in the cytoplasm) | - | - | - | - | [185–187] | |
| Oxidative stress | Pyocyanin | phz | Cytotoxicity (ROS production: O2−, H2O2) IS regulation (apoptosis), blue-green pigment |
Inhibition of ciliated HAE function | Primary sheep cells | DNA, lipid membrane, proteins | - | [188–190] |
| Biosurfactant | Rhamnolipid | rhl | Amphiphilic: detergent and solubilizing properties Disruption cell junctions IS regulation (neutrophils lysis) Biofilm formation, motility |
Solubilization of airway surfactant Disruption of HAE tight junctions |
Primary nasal cells | Tight junctions (ZO-1, occludin, JAM-A) | - | [120,191–193] |
| Esterase EstA | estA | Autotransporter enzyme: Hydrolyze glycerol esters Rhamnolipids production |
Disruption of HAE tight junctions | See Rhamnolipid | See Rhamnolipid | - | [194] | |
| Excreted toxins | Alkaline protease | aprA | Protease activity: IS inactivation (antibodies, neutrophils, complement, cytokines) and laminin | Degradation of HAE extracellular matrix: (basement membrane: laminin) | - | Laminin | T1SS | [195,196] |
| Elastase | lasB, (lasA) | Protease activity: elastin, collagen, transferrin, antibodies, complement → Tissue damage Disruption of cell junctions |
Degradation of HAE extracellular matrix (basement membrane and alveolar septum: elastin and collagen) Disruption of HAE tight junctions |
Primary nasal cells, cell lines (Calu 3) | Elastin, Collagen, tight junction (ZO-1, claudin, occludin) | T2SS | [113,114,117,197] | |
| Exotoxin A | toxA | Host cells protein synthesis inhibition: eEF2 inhibition by ADP-ribosylation → Cell death |
HAE cell death | Cell lines (CuFi-1) | eEF2 | T2SS | [198,199] | |
| Phospholipase C | plcH | Hydrolysis of phospholipids (erythrocyte and leukocyte cytolysis), surfactant degradation | Pulmonary surfactant degradation | - | Phosphatidylcholine, Sphingomyelin | T2SS | [200–203] | |
| Exolysin | exlA | Pore-forming toxin: cell membrane disruptions (erythrocytes, leukocytes, epithelial cells) | HAE cell death and retraction | Cell lines (A549), mice pneumonia | Phophoslipid bilayers | - | [108,204,205] | |
| CFTR inhibitory factor | cif | - | TAP-1 mediated-MHC class 1 antigen presentation and CFTR-mediated chloride secretion inhibition | Cell lines (CFBE41o-, A549) | CFTR and TAP-1 | OMV | [206,207] | |
| Injected toxins | Exo S (exoenzyme S) | exoS | GTPase-activating protein activity and ADP ribosyltransferase activity: inhibition of several host cell functions (cell apoptosis, cell division and cell migration inhibitions, junctions and actin cytoskeleton disruptions) | HAE cell death and retraction | Mice pneumonia | Rho family of GTPases, Ras superfamily GTPases | T3SS | [163,208,209] |
| Exo T (exoenzyme T) | exoT | |||||||
| Exo U (exoenzyme U) | exoU | Phospholipase A2 activity: membrane phospholipids hydrolysis (rapid cell necrosis) | Rapid HAE cell death and retraction | Mice pneumonia, cell lines (Beas-2b) | Phospholipids | T3SS | [163,208,210] | |
| Exo Y (exoenzyme Y) | exoY | Adenylate cyclase activity: actin cytoskeleton disruptions | HAE cell death and retraction | Mice pneumonia, cell lines (PMVECR1) | Tau protein (microtubule) | T3SS | [211–214] | |
| Phospholipase D | pldA, pldB | - Host cell internalization - Bacterial competition |
HAE cell internalization | Cell lines (Calu 3) | Akt kinase | H2 (pldA) H3 (pldB) T6SS |
[110,215,216] | |
| VgrG2b | vgrG | - Host cell internalization - Bacterial competition |
HAE cell internalization | Cell lines (Calu 3 | γ-tubulin ring complex (γTuRCn microtubule component) | H2 T6SS | [110,217] | |
| Phospholipase TplE | TplE | - Host cells endoplasmic reticulum disruption - Bacterial competition |
- | - | Endoplasmic reticulum apparatus | H2 T6SS | [218] | |
| Tse1-3 | Tse1-3 | Bacterial competition (peptidoglycan degradation) | - | - | - | H1 T6SS | [215] | |
| Short RNA | sRNA52320 | - | - | Decrease of IL-8 secretion | Bronchial primary cells | Kinases of the LPS-stimulated MAPK pathway | OMV | [219] |
| Global regulatory system | Quorum sensing | las, rhl, pqs, iqs | Autoinducer peptides (for instance, homoserines lactones 3O–C12–HS) detect critical density → activation of 4 regulation systems: impact on virulence factors production and biofilm formation | Disruption of tight junctionsa | - | Tight junctions (ZO-1, ZO-3, JAM-A, occludin) | - | [122,123,164,165,220] |
aDisruption of cells’ tight junction described in the intestinal epithelial model (Caco2 cells), not yet in HAE.
CFTR, cystic fibrosis transmembrane regulator; eEF2, eukaryotic elongation factor 2; HAE, human airway epithelium; H2O2, hydrogen peroxide; LPS, lipopolysaccharide; MHC, major histocompatibility complex; OMV, outer membrane vesicle; O2, superoxide; IS, immune system; ROS, reactive oxygen species; SS, secretion system; TAP, transporter associated with antigen processing; TxSS, type x secretion system.
Most of these virulence factors are not synthesized constitutively but are regulated depending on the needs of the bacteria in different types of settings and collaterally during infection. The production is fine-tuned by quorum sensing, which is a communication system based on the detection of bacterial density in the environment [164]. The communication is mediated by the secretion of small signaling molecules, such as homoserines lactones or quinolones, used as autoinducer messengers. Each bacterium produces these messengers, thereby their concentration depends on the growing bacterial population [164]. When the critical threshold is reached (i.e., quorum), four regulatory systems are activated to coordinate the response of the bacterial population: Las, Rhl, PQS, and IQS systems [164–167]. Around 10% of the P. aeruginosa genome is estimated to be regulated by the quorum sensing system [168].
In some conditions, and notably in CF disease, P. aeruginosa is known to form structured aggregates known as biofilms. After adhesion to a surface, the bacteria congregate in highly organized communities, surrounded by an extracellular matrix composed of exopolysaccharides (alginate, PEL, and PSL), extracellular DNA, lipids, and proteins [169]. This matrix, representing between 50% and 90% of the total volume of the biofilm, strengthens its structure and protects bacteria from effectors of the host’s immune response (such as phagocytosis or antibody actions) and antibiotics, making their eradication very difficult [18,167,169]. In addition, bacteria within the biofilm are exposed to oxygen gradients and nutrient limitations leading to modification of bacterial metabolism. One clinically relevant phenotype that occurred in such a situation is the small colony variant phenotype, which is characterized by slow growth and a loss of cytotoxicity [25]. Biofilm formation is regulated by the quorum sensing system [18,165]. The development of the mucoid phenotype by overproduction of alginate found in biofilms is a strategy for the bacteria to survive in a hostile environment and is mainly observed in P. aeruginosa chronic respiratory infections (such as in CF patients) and biomaterial-associated infections (endotracheal tubes, urinary catheters, etc.) [25,170]. The inert surfaces of the biomaterial are used as a base that facilitates the development of biofilms [18,171].
During the progression of chronic P. aeruginosa infection in CF patients, phenotypic and genotypic changes occur in P. aeruginosa to adapt to the specific mucus-plugging environment. Strains established in this long-term colonization show less inflammation and less cytotoxicity against HAE than the first strains years earlier in the same patient [18]. A switch from acute to chronic phenotype occurred. It has been described to be characterized by down-regulation of some virulence factors (motility with loss of flagellum and pili, protease, rhamnolipids, pigments, …), increased biofilm formation, the apparition of mucoid or small colony variant phenotype, change in LPS, and alteration of quorum sensing system [18,78].
From the first stages of P. aeruginosa interaction with the airway epithelium, the first line of defense of the innate immune system in the lung recognizes P. aeruginosa components and virulence factors, activating an immune reaction to prevent or resolve the infection. Various immunity actors are then recruited, and inflammation processes are observed [19].
First, specific P. aeruginosa patterns are recognized thanks to pattern recognition receptors (PRRs) [19,221]. Three types are described: (i) Secreted PRR, such as Complement C1q, collectins, and surfactant proteins SP-A and D, which bind P. aeruginosa cell wall and mark it for clearance by macrophages and neutrophils. (ii) Transmembrane PRR, the most important actors, represented by the Toll-like receptors (TLRs) present on immune and epithelial cell membranes. They recognize different components of P. aeruginosa call the PAMPs (pathogen-associated molecular patterns). For example, TLR2 recognizes the pili, TLR4 and CFTR the LPS, and TLR5 the flagella. (iii) Cytosolic PRRs, such as TLR9 and NOD-1, can recognize bacterial DNA or cell wall components of the internalized/phagocytosed bacteria.
Various cells coordinate efforts to produce an appropriate innate immune response [18,19,78]. Epithelial cells of the HAE play an important role of alert of P. aeruginosa presence. After TLR-mediated activation, they produce a large panel of pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, Il-6, …) and the chemokine IL-8, which initiate inflammation and recruit other more specialized immune cells such as neutrophils. They also produce antimicrobial peptides (defensin, cathelicidin LL-37) and reactive oxygen species (radical hydroxide, hypothiocyanite), acting directly against P. aeruginosa. Neutrophils play a critical role in P. aeruginosa destruction and the development of the inflammatory response. Not present in the uninfected lung, they are recruited by attractive chemokines released by sentinel cells during infection, such as macrophages or epithelial cells, mediated by PRR signals. They phagocyte and kill the bacteria and they generate a great number of antibacterial molecules, such as reactive oxygen species (superoxide anion, hydrogen peroxide, radical hydroxide, nitric oxide, responsible for an oxidative burst), proteases (elastase), and antimicrobial peptides (defensin, cathelicidin LL-37), lactoferrin and lysozyme. Alveolar macrophages are the resident leukocyte of the lung, acting as the main sentinel for infectious agents’ detection. They recognize P. aeruginosa with their PRR, phagocytose it, and complete the immune cells activation of the HAE cells, through pro-inflammatory cytokines secretion.
After this review of all P. aeruginosa virulence factors involved in respiratory infection, we will focus on the most important damages described in airway infections, caused by the main virulence factors secreted by P. aeruginosa. Two types of degradation will be described: (i) HAE cell damages, wherein the key role of T3SS in host cell retraction and disruption of tight junctions facilitates the subsequent access of bacteria to the adjacent or underlying cells (ii) airway components degradation (basement membrane, surfactant), allowing the radial progression of P. aeruginosa in the epithelium (Fig 2B and 2C).
HAE cells damages: The key role of the T3SS cytotoxic activity
Among all the virulence factors produced by P. aeruginosa, the T3SS and its effectors are commonly considered the major determinant of virulence during infection [157]. This extensively studied secretion system plays a key role in the P. aeruginosa pathogenesis by injecting effectors that include toxins directly into the cytoplasm of the host cells including HAE cells [157]. P. aeruginosa strains that do not express T3SS are less virulent in human clinical infections and animal infection models [157,222,223].
T3SS-mediated delivery of the exoenzymes requires the adhesion of the bacteria to the host cell. As previously described, attachment via pili and their retraction activate signaling pathways, mediated by the Chp system, and cAMP/Vfr circuit, which lead to the transcription of virulence-associated genes, notably T3SS effectors [158,160,161]. Four well-known T3SS effectors have been described (exoenzymes ExoS, ExoT, ExoU, and ExoY), but not all are produced by every P. aeruginosa strain. ExoT and ExoY are found in nearly all strains, but ExoS and ExoU are mutually exclusive [224,225] and rarely found together in the same strain [226,227]. Two types of P. aeruginosa strains are therefore observed, with distinct pathogenesis phenotypes: (i) the “ExoU profile,” expressing ExoT, ExoU, and ExoY, is highly virulent, induces rapid cell death of the host cells, and shows a low rate of cell internalization; and (ii) the “ExoS profile,” expressing the ExoS, ExoT, and ExoY, causes slower death and exhibits a higher rate of internalization. The phenotypes associated with the expression of “ExoU profile” are mainly encountered in acute infections (bacteremia, ocular infections, acute pneumonia, etc.), while the “ExoS profile” is predominant in pulmonary chronic infections (in CF, COPD, etc.) [163,208,228–230].
ExoU, which is considered the most potent of type 3 secreted toxins, has a phospholipase A2 activity that induces host cell membrane disruption by phospholipids hydrolysis. It leads to rapid necrotic death of both epithelial and immune cells. ExoU was mainly described in severe and acute infections, especially respiratory infections [163]. ExoS and ExoT are bifunctional proteins both displaying two distinct enzymatic activities: an N-terminal GTPase-activating protein (GAP) activity and a C-terminal adenosine diphosphate ribosyl-transferase (ADPRT) activity. Inhibiting host cell GTPases, such as Rho-, Rac-, Ras-, Rap-, and Cdc42 GTPases, ExoS, and ExoT cause actin cytoskeleton depolymerization of the HAE cells. It eventually results in the detachment of HAE cells from the basement membrane due to cell retraction (disruption of the cell structure and subsequent tight junctions disruption), cell rounding and apoptosis [157,163,231].
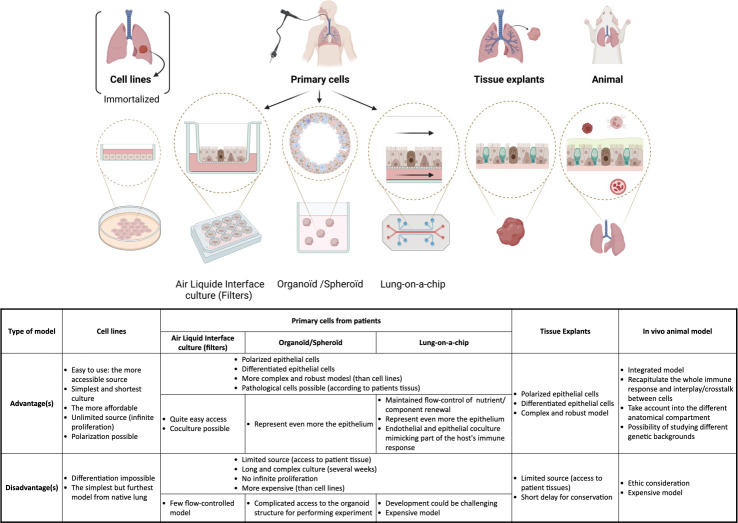
It is worth noting that the studies describing preferential T3SS cytotoxicity towards the basolateral part rather than the apical surface of HAE used mainly differentiated epithelium models, such as human or bovine primary airway epithelial cells cultured at the air–liquid interface (from 7 days to 6 weeks) [68,72,94] (Fig 5 and S1 Table). Such models likely mimic more in vivo conditions than immortalized cell lines [232].
Fig 5. Models of airway epithelia.
It is of the utmost importance to use relevant experimental models to assess the host–pathogen interactions driving P. aeruginosa infection, depending on the physiological relevance needed: Various models exist, from cell lines to primary cells and tissue explants/animal models, each with their advantages and disadvantages. All the models used in the references of the review are listed in S1 Table. Created with BioRender.com.
Altogether, T3SS effectors play a key role in HAE damaging by facilitating the subsequent access of bacteria to the basolateral surface of other HAE cells nearby, allowing P. aeruginosa to break through the epithelium (Fig 2B and 2C).
Other secreted toxins or membrane constituents of P. aeruginosa have been shown to act complementarily to T3SS leading to enhanced cytotoxicity on HAE cells. For instance, the T2SS-secreted Exotoxin A catalyzes the ADP-ribosylation of its host target protein, the eukaryotic elongation factor 2 (eEF2), resulting in the inhibition of protein synthesis and ultimately leading to cell death [198,199]. The LecA and LecB binding proteins have also been shown to participate, at least in part, in a cytotoxic effect on HAE cells and to lung injury in a murine model [134]. The LPS can also induce apoptosis in lung endothelial and epithelial cells [177]. ExlA exolysin, a pore-forming toxin recently discovered in some strains of P. aeruginosa lacking T3SS, forms pores in the host cell membrane, leading to a massive entry of Ca2+ that in turn activates the ADAM10 metalloprotease [233]. The subsequent cleavage of the adherens junctions constituent E cadherin leads to HAE cell membrane disruption and death [108,205].
Some virulence factors affect the cells’ tight junctions and allow P. aeruginosa tissue progression. The proteolytic LasB elastase disrupts the tight junctions by acting on occludin, claudin, or ZO-1 proteins [113,114,116,117]. Rhamnolipids could also play a role thanks to their amphiphilic properties. They are incorporated into membranes and disrupt the architecture of tight junctions after several hours of infection in culture models [119,120,193]. In the same way, the LPS, a membrane component of P. aeruginosa, increases airway epithelium barrier paracellular permeability by disrupting the tight junctions, by affecting ZO-1 and ZO-2 [118,176]. Although studied only in an intestinal epithelium model, the homoserine lactone 3O–C12–HS of the P. aeruginosa quorum sensing has been shown to induce rupture of epithelium integrity [121]. It activates various cellular kinases implicated in tight junction’s functions (p38 or p42/44), leading to a modification of ZO-1, ZO-3, JAM-A and occludin production, as well as a reorganization of the actin cytoskeleton [121–123].
However, the inoculum of P. aeruginosa in the healthy epithelium is likely to be very low and the produced quantity of these factors might probably not be sufficient to break down alone intact tight junctions. Their impact is more likely delayed in P. aeruginosa epithelial invasion, after access to the basolateral part and bacterial proliferation in the infected airways, resulting in an inoculum far higher (commonly detected from 105 to 107 CFU/mL in respiratory clinical samples [124,125]. The role of these components might be then complementary to the action of T3SS-secreted exoenzymes in tissue progression.
Degradation of other components of respiratory barriers
In addition to the factors leading to the breakdown of the cells in respiratory epithelia, P. aeruginosa secretes several factors capable of destroying various components of the extra-epithelial components of the respiratory barriers, such as the basement membrane, the surfactant, and the mucins, which promote bacterial spread in the epithelium (Fig 2B and 2C).
P. aeruginosa produces proteases that play an important role in the degradation of the basement membrane and the mesenchyme. For instance, the LasB elastase, the most important protease of P. aeruginosa, degrades elastin and collagen and participates in tissue invasion and progression into the lung tissue [234]. The alkaline protease cleaves notably laminin, another important and biologically active component of the basement membrane [195].
Several other factors are involved in the degradation of the pulmonary surfactant: phospholipase C, which hydrolyses phospholipids [201,203,235], and the rhamnolipids, amphiphilic glycolipids having detergent and solubilizing properties [192].
It has been shown that P. aeruginosa sdsA1 gene, encoding a secreted sulfatase, plays a central role in the degradation of mucin and that the sdsA1 inhibition decreased the release of sulfate from mucin. sdsA1 mutant showed a decreased mucin gel penetration and an attenuation of P. aeruginosa virulence in leukopenic mice intraperitoneally infected [236].
3) P. aeruginosa internalization in HAE cells
Although P. aeruginosa is primarily an extracellular pathogen, internalization in nonphagocytic cells, such as airway epithelial cells, has been widely described for many years [71,72,217,237–240]. However, these descriptions were based on nonpolarized cells in the majority (S1 Table). Therefore, considering some of the recent findings, this mechanism might be not crucial, and, if observed, only a very small proportion of the bacteria is internalized, depending on bacterial clone and host cell types. The fraction of internalized bacteria in airway epithelium cultured from primary cells cultured at the air–liquid interface was estimated to be very low. From the primary airway cells model, Fleiszig and colleagues found an approximate rate of internalization of approximately 0.0001% within human nasal epithelium or approximately 0.001% in the bovine tracheal epithelium [72]. Conversely, internalization rates in cell lines were higher: approximately 1% to 2% within MDCK or HeLa cells [72,110,138,241] (Fig 5). Furthermore, the host cell membrane composition, the intactness of tight junctions, and the level of cell polarization play a key role in the internalization process. It occurs more frequently on the basolateral cell surface, in cells with disrupted tight junctions or with low polarity levels, the latter being mainly observed on injured or repairing/remodeled epithelium [11,72,73,242–244].
Internalization process
As with many other bacteria, P. aeruginosa internalization involves the formation of membrane lipid rafts [132,245], a signaling platform in the plasma membrane locally enriched in glycosphingolipids and cholesterol, which migrate within the phospholipid bilayer to form lipid aggregates [246]. No lysosomal fusion has been described after vacuole uptake and multiplying bacteria have been observed in the vacuoles [238]. Following bacterial binding, internalization is mediated by the rearrangement of the cellular actin cytoskeleton. The phosphatidylinositol 3-kinase (PI3K) coupled with the Akt pathway is involved in the entry into host cells through both apical and basolateral plasma membranes of epithelial cells [109]. The cytoskeleton microtubules have also been involved in the internalization of the bacteria, via the effectors delivered into cells by the T6SS. This mechanism promotes P. aeruginosa uptake into epithelial cells by interfering with the PI3K/Akt pathway, through the action of several effectors (H3-T6SS-dependent phospholipase D effector (PldB) and H2-T6SS VgrG2b effector), which interact with the γ-tubulin ring complex [110,216,217,247].
P. aeruginosa was shown to interfere with the epithelial polarity to enhance binding to the cells: It can induce a subversion of airway epithelial cell polarity to locally transform the apical into a basal plasma membrane and thus allow bacterial attachment and internalization into cells under. After remodeling, the membrane forms protrusions that internalize bacteria without disrupting the tight junctions [248–250].
Consequences of P. aeruginosa internalization on the pathogenicity
The consequences of P. aeruginosa internalization in airway epithelial cells are still unclear. In an ALI airway epithelium model, which is highly representative of the conditions observed in the in vivo airway epithelium, the extremely low number of internalized bacteria found (around 0.0001%) is likely to indicate a probable accessory role of the internalization in the pathogenesis [72]. Different hypotheses have been proposed.
The invasion of the epithelium. Internalization, as a prerequisite to the invasion of the epithelium, is the most common hypothesis. Some authors argue that internalization, without any killing observed, represents a route for the bacteria to cross the cells, reaches the basal part and the bloodstream system, and allows dissemination to distant organs [109,238]. Thereby, the bacteria could multiply, cross cells to reach the basal part, and invade the surrounding tissues [244,250]. However, all these studies do not define precise mechanisms, and there is no evidence of this transcellular route as the mainstay of its pathogenicity towards the HAE.
Persistence and establishment of chronic infections. As there is no lysosomal killing after internalization, this mechanism has been hypothesized to allow P. aeruginosa to escape from the different effector cells of the host immune response, such as phagocytes, thereby favoring its persistence and establishment of chronic infections. [217,238,242,250]. Internalization could then induce the transition from an acute to a chronic phenotype in P. aeruginosa [110].
P. aeruginosa clearance by airway cell desquamation. This last and contradictory hypothesis proposes that internalization is more detrimental than beneficial for the bacteria. In healthy individuals, P. aeruginosa binding to CFTR channels leads to bacterial internalization and triggers host immune system activation, notably inflammation and neutrophil recruitment, which causes the detachment of the infected cells and their removal by mucociliary clearance [251]. Conversely, in CF patients lacking functional CFTR channels, P. aeruginosa is not internalized, hampering its clearance. The bacteria persist in the dehydrated mucus protected from neutrophils and lead to chronic infections. In this pathology, chronically induced inflammation is ineffective and deleterious [144,237,251].
4) Crossing of the epithelium and dissemination
Recently, a new model has emerged to describe the crossing and the propagation of P. aeruginosa at the HAE. To gain access to the basement membrane, P. aeruginosa uses a paracellular route and then radial propagation through the basal compartment (Fig 2C). This new model excludes the suspected, but never demonstrated, theory of a transcellular route used by P. aeruginosa (by internalization) to cross the epithelium.
Zulianello and colleagues observed the absence of internalization but a paracellular invasion of P. aeruginosa in differentiated airway epithelia when the integrity of cellular junctions was compromised by rhamnolipids [120]. Subsequently, Heiniger and colleagues demonstrated the exact route used by the bacteria between cells to access the basement membrane during infection [94]. After P. aeruginosa adhesion to the basolateral surfaces of ciliated cells, the bacteria inject cytotoxic toxins using T3SS (ExoS and ExoT). The toxins lead to cell retraction and detachment, allowing access to other basolateral membranes of adjacent ciliated cells or the resting basal cells, and the progression of P. aeruginosa through the airway epithelium. Pili-mediated twitching mobility was necessary for this bacterial progression. Radial bacterial dissemination across the entire epithelium was then observed [94]. More recently, Golovkine and colleagues provided a detailed description of this phenomenon. Using a real-time microscopy technique, they described precisely the progression of the paracellular bacterial migration through the epithelium. During the epithelial infection, P. aeruginosa takes advantage of transient ruptures in the tight junctions to access the basolateral part and once the first bacterium has entered into the breach, a cohort of bacteria rapidly follows at the same entry point. A radial propagation from this entry point through the basal compartment followed, involving the injection of the T3SS-secreted toxins (ExoS and ExoT) into the cells. The pili-mediated twitching motility and the flagella were also necessary for this progression. Interestingly, no internalization of the bacteria was observed in this model, making transcellular migration highly unlikely [93].
Conclusions
P. aeruginosa is a major respiratory pathogen, expressing an impressive panel of complexes and complementary virulence factors that, in a well-coordinated manner, promote HAE invasion. However, P. aeruginosa is an opportunistic pathogen that rarely infects healthy individuals, and the barrier effect of the airway epithelium plays a key role in limiting its pathogenicity. P. aeruginosa exploits weaknesses in the HAE barrier to gain access to the basolateral part of the epithelium, which is normally inaccessible in healthy epithelium with intact tight junctions. This access is crucial for initiating infection and represents the common point of all the various clinical pathologies due to these bacteria, ranging from lung infections and corneal infections to catheter-related urinary tract infections.
A new pathogenesis model has emerged, replacing a suspected, but incompletely supported, model, where P. aeruginosa invades through a transcellular route within the epithelium following its internalization. This new paradigm relates to a paracellular crossing of the HAE after access to the basolateral part of the HAE and the radial propagation of P. aeruginosa through the basal compartment. Therapeutic approaches, including the use of compounds that inhibit access of P. aeruginosa to the basolateral membrane, could allow alternative treatment strategies, which have the potential to bypass the classic antibiotics, reducing the emergence of antimicrobial resistance.
Supporting information
ECM, extracellular matrix; ND/NR, not determined/not relevant.
(DOCX)
Acknowledgments
We would like to deeply thank Prof. Stéphane Corvec and Prof. Steve Lory for critically revising the paper, which helped us in keeping with the important scientific content of this review. We are also indebted to Dr. Nicholas Judson for his comments to make this review of interest to a broad scientific community not specialized in microbiology. We thank Dr. Valérian Dormoy for his help in proofreading the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Rutherford V, Yom K, Ozer EA, Pura O, Hughes A, Murphy KR, et al. Environmental reservoirs for exoS+ and exoU+ strains of Pseudomonas aeruginosa. Env Microbiol Rep. 2018;10:485–492. doi: 10.1111/1758-2229.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selezska K, Kazmierczak M, Musken M, Garbe J, Schobert M, Haussler S, et al. Pseudomonas aeruginosa population structure revisited under environmental focus: impact of water quality and phage pressure. Environ Microbiol. 2012;14:1952–1967. doi: 10.1111/j.1462-2920.2012.02719.x [DOI] [PubMed] [Google Scholar]
- 3.Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, Morales G, et al. Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, Ausubel F. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262 [DOI] [PubMed] [Google Scholar]
- 5.Walter J, Haller S, Quinten C, Kärki T, Zacher B, Eckmanns T, et al. Healthcare-associated pneumonia in acute care hospitals in European Union/European Economic Area countries: an analysis of data from a point prevalence survey, 2011 to 2012. Euro Surveill. 2018:23. doi: 10.2807/1560-7917.ES.2018.23.32.1700843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser AR. Pseudomonas aeruginosa virulence and antimicrobial resistance: two sides of the same coin? Crit Care Med. 2014;42:201–202. doi: 10.1097/CCM.0b013e3182a120cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353 [DOI] [PubMed] [Google Scholar]
- 8.Philippon S, Streckert HJ, Morgenroth K. In vitro study of the bronchial mucosa during Pseudomonas aeruginosa infection. Virchows Archiv A Pathol Anat Histopathol. 1993;423:39–43. doi: 10.1007/BF01606430 [DOI] [PubMed] [Google Scholar]
- 9.Tsang KWT, Rutman A, Tanaka E, Lund V, Dewar A, Cole PJ, et al. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur Respir J. 1994;7:1746–1753. doi: 10.1183/09031936.94.07101746 [DOI] [PubMed] [Google Scholar]
- 10.Niederman MS, Rafferty TD, Sasaki CT, Merrill WW, Matthay RA, Reynolds HY. Comparison of Bacterial Adherence to Ciliated and Squamous Epithelial Cells Obtained from the Human Respiratory Tract1–3. Am Rev Respir Dis. 1983;127:85–90. doi: 10.1164/arrd.1983.127.1.85 [DOI] [PubMed] [Google Scholar]
- 11.Plotkowski MC, Chevillard M, Pierrot D, Altemayer D, Zahm JM, Colliot G, et al. Differential adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells in primary culture. J Clin Invest. 1991;87:2018–2028. doi: 10.1172/JCI115231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baltimore RS, Christie CDC, Smith GJW. Immunohistopathologic Localization of Pseudomonas aeruginosa in Lungs from Patients with Cystic Fibrosis: Implications for the Pathogenesis of Progressive Lung Deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650 [DOI] [PubMed] [Google Scholar]
- 13.Ramphal R, Small PM, Shands JW, Fischlschweiger W, Small PA. Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun. 1980;27:614–619. doi: 10.1128/iai.27.2.614-619.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bentzmann S, Plotkowski C, Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am J Resp Crit Care. 1996;154:S155–S162. doi: 10.1164/ajrccm/154.4_Pt_2.S155 [DOI] [PubMed] [Google Scholar]
- 15.de Bentzmann S, Roger P, Puchelle E. Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur Respir J. 1996;9:2145–2150. doi: 10.1183/09031936.96.09102145 [DOI] [PubMed] [Google Scholar]
- 16.de Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski MC, et al. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustamante-Marin XM, Ostrowski LE. Cilia and Mucociliary Clearance. Csh Perspect Biol. 2017;9:a028241. doi: 10.1101/cshperspect.a028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gellatly SL, Hancock REW. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033 [DOI] [PubMed] [Google Scholar]
- 19.Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology. 2010;15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x [DOI] [PubMed] [Google Scholar]
- 20.Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol. 2021;21:347–362. doi: 10.1038/s41577-020-00477-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu-Chen X, Weinstock J, Rastogi D, Koumbourlis A, Nino G. The airway epithelium during infancy and childhood: a complex multicellular immune barrier. basic review for clinicians. Paediatr Respir Rev. 2021;38:9–15. doi: 10.1016/j.prrv.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–919. doi: 10.1378/chest.10-0166 [DOI] [PubMed] [Google Scholar]
- 23.Rello J, Bodi M, Mariscal D, Navarro M, Diaz E, Gallego M, et al. Microbiological testing and outcome of patients with severe community-acquired pneumonia. Chest. 2003;123:174–180. doi: 10.1378/chest.123.1.174 [DOI] [PubMed] [Google Scholar]
- 24.Sousa AM, Pereira MO. Pseudomonas aeruginosa Diversification during Infection Development in Cystic Fibrosis Lungs—A Review. Pathogens. 2014;3:680–703. doi: 10.3390/pathogens3030680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev. 2011;24:29–70. doi: 10.1128/CMR.00036-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy TF. The many faces of Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47:1534–1536. doi: 10.1086/593187 [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47:1526–1533. doi: 10.1086/593186 [DOI] [PubMed] [Google Scholar]
- 28.Parameswaran GI, Sethi S. Pseudomonas infection in chronic obstructive pulmonary disease. Future Microbiol. 2012;7:1129–1132. doi: 10.2217/fmb.12.88 [DOI] [PubMed] [Google Scholar]
- 29.Vareille M, Kieninger E, Edwards MR, Regamey N. The Airway Epithelium: Soldier in the Fight against Respiratory Viruses. Clin Microbiol Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiemstra PS, McCray PB, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45:1150–1162. doi: 10.1183/09031936.00141514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021:1–13. doi: 10.1038/s41385-020-00370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rock JR, Randell SH, Hogan BLM. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajj R, Baranek T, Naour RL, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288 [DOI] [PubMed] [Google Scholar]
- 34.Carlier FM, de Fays C, Pilette C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front Physiol. 2021;12:691227. doi: 10.3389/fphys.2021.691227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coraux C, Hajj R, Lesimple P, Puchelle E. In vivo models of human airway epithelium repair and regeneration. European Respir Rev. 2005;14:131–136. doi: 10.1183/09059180.05.00009702 [DOI] [Google Scholar]
- 36.Coraux C, Hajj R, Lesimple P, Puchelle E. Réparation et régénération de l’épithélium respiratoire. M/S: médecine sciences. 2005;21:1063–1069. [DOI] [PubMed] [Google Scholar]
- 37.Adam D, Perotin J-M, Lebargy F, Birembaut P, Deslée G, Coraux C. Regeneration of airway epithelium. Rev Mal Respir. 2014;31:300–311. doi: 10.1016/j.rmr.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 38.Adam D, Roux-Delrieu J, Luczka E, Bonnomet A, Lesage J, Mérol J-C, et al. Cystic fibrosis airway epithelium remodelling: involvement of inflammation. J Pathol. 2015;235:408–419. doi: 10.1002/path.4471 [DOI] [PubMed] [Google Scholar]
- 39.Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, et al. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery PK. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 2000;117:251S–260S. doi: 10.1378/chest.117.5_suppl_1.251s [DOI] [PubMed] [Google Scholar]
- 41.Cigana C, Lorè NI, Riva C, Fino ID, Spagnuolo L, Sipione B, et al. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep. 2016;6:21465. doi: 10.1038/srep21465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim V, Kelemen SE, Abuel-Haija M, Gaughan JP, Sharafkaneh A, Evans CM, et al. Small Airway Mucous Metaplasia and Inflammation in Chronic Obstructive Pulmonary Disease. Copd J Chronic Obstr Pulm Dis. 2009;5:329–338. doi: 10.1080/15412550802522445 [DOI] [PubMed] [Google Scholar]
- 43.Herard AL, Zahm JM, Pierrot D, Hinnrasky J, Fuchey C, Puchelle E. Epithelial barrier integrity during in vitro wound repair of the airway epithelium. Am J Resp Cell Mol. 2012;15:624–632. doi: 10.1165/ajrcmb.15.5.8918369 [DOI] [PubMed] [Google Scholar]
- 44.Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983;128:S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14 [DOI] [PubMed] [Google Scholar]
- 45.Furuse M, Takai Y. Recent advances in understanding tight junctions. Fac Rev. 2021;10:18. doi: 10.12703/r/10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumbiner B., Structure biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.c749 [DOI] [PubMed] [Google Scholar]
- 47.Stevenson BR, Anderson JM, Bullivant S. The epithelial tight junction: Structure, function and preliminary biochemical characterization. Mol Cell Biochem. 1988;83:129–145. doi: 10.1007/BF00226141 [DOI] [PubMed] [Google Scholar]
- 48.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo J-S, et al. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–L330. doi: 10.1152/ajplung.00440.2002 [DOI] [PubMed] [Google Scholar]
- 49.Webster MJ, Tarran R. Slippery When Wet: Airway Surface Liquid Homeostasis and Mucus Hydration. Curr Top Membr. 2018;81:293–335. doi: 10.1016/bs.ctm.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 50.Wagner CE, Wheeler KM, Ribbeck K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu Rev Cell Dev Bi. 2018;34:189–215. doi: 10.1146/annurev-cellbio-100617-062818 [DOI] [PubMed] [Google Scholar]
- 51.Voynow JA, Rubin BK. Mucins, Mucus, and Sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412 [DOI] [PubMed] [Google Scholar]
- 52.Rose MC, Voynow JA. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005 [DOI] [PubMed] [Google Scholar]
- 53.Girod S, Zahm JM, Plotkowski C, Beck G, Puchelle E. Role of the physiochemical properties of mucus in the protection of the respiratory epithelium. Eur Respir J. 1992;5:477–487. [PubMed] [Google Scholar]
- 54.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4 [DOI] [PubMed] [Google Scholar]
- 55.Mihai MM, Holban AM, Giurcăneanu C, Popa LG, Buzea M, Filipov M, et al. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom J Morphol Embryol. 2014;55:1401–1408. [PubMed] [Google Scholar]
- 56.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2 [DOI] [PubMed] [Google Scholar]
- 57.Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SMJ. Role of the Corneal Epithelial Basement Membrane in Ocular Defense against Pseudomonas aeruginosa. Infect Immun. 2009;77:3264–3271. doi: 10.1128/IAI.00111-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, et al. Microbial Keratitis Predisposing Factors and Morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 59.Kam KW, Yung W, Li GKH, Chen LJ, Young AL. Infectious keratitis and orthokeratology lens use: a systematic review. Infection. 2017;45:727–735. doi: 10.1007/s15010-017-1023-2 [DOI] [PubMed] [Google Scholar]
- 60.Roser DJ, Akker BVD, Boase S, Haas CN, Ashbolt NJ, Rice SA. Pseudomonas aeruginosa dose response and bathing water infection. Epidemiol Infect. 2014;142:449–462. doi: 10.1017/S0950268813002690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rice SA, van den Akker B, Pomati F, Roser D. A risk assessment of Pseudomonas aeruginosa in swimming pools: a review. J Water Health. 2012;10:181–196. doi: 10.2166/wh.2012.020 [DOI] [PubMed] [Google Scholar]
- 62.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J Infect Public Heal. 2009;2:101–111. doi: 10.1016/j.jiph.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 63.Safdar N, Crnich CJ, Maki DG. The Pathogenesis of Ventilator-Associated Pneumonia: Its Relevance to Developing Effective Strategies for Prevention. Respir Care. 2005;50:725–741. [PubMed] [Google Scholar]
- 64.Carson JL, Collier AM, Gambling TM, Knowles MR, Boucher RC. Ultrastructure of airway epithelial cell membranes among patients with cystic fibrosis. Hum Pathol. 1990;21:640–647. doi: 10.1016/s0046-8177(96)90011-8 [DOI] [PubMed] [Google Scholar]
- 65.Jeffery PK. Remodeling and Inflammation of Bronchi in Asthma and Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2004;1:176–183. doi: 10.1513/pats.200402-009MS [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi T, Yamada H. Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am Rev Respir Dis. 1991;144:1147–1152. doi: 10.1164/ajrccm/144.5.1147 [DOI] [PubMed] [Google Scholar]
- 67.Schwarzer C, Fischer H, Machen TE. Chemotaxis and Binding of Pseudomonas aeruginosa to Scratch-Wounded Human Cystic Fibrosis Airway Epithelial Cells. PLos ONE. 2016;11:e0150109. doi: 10.1371/journal.pone.0150109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee A, Chow D, Haus B, Tseng W, Evans D, Fleiszig S, et al. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am J Physiol. 1999;277:L204–L217. doi: 10.1152/ajplung.1999.277.1.L204 [DOI] [PubMed] [Google Scholar]
- 69.Rajan S, Cacalano G, Bryan R, Ratner AJ, Sontich CU, van Heerckeren A, et al. Pseudomonas aeruginosa Induction of Apoptosis in Respiratory Epithelial Cells. Am J Resp Cell Mol. 2000;23:304–312. doi: 10.1165/ajrcmb.23.3.4098 [DOI] [PubMed] [Google Scholar]
- 70.Roger P, Puchelle E, Bajolet-Laudinat O, Tournier JM, Debordeaux C, Plotkowski MC, et al. Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur Respir J. 1999;13:1301–1309. doi: 10.1034/j.1399-3003.1999.13f13.x [DOI] [PubMed] [Google Scholar]
- 71.Plotkowski MC, de Bentzmann S, Pereira SHM, Zahm J-M, Bajolet-Laudinat O, Roger P, et al. Pseudomonas aeruginosa Internalization by Human Epithelial Respiratory Cells Depends on Cell Differentiation, Polarity, and Junctional Complex Integrity. Am J Resp Cell Mol. 1999;20:880–890. doi: 10.1165/ajrcmb.20.5.3408 [DOI] [PubMed] [Google Scholar]
- 72.Fleiszig SM, Evans DJ, Do N, Vallas V, Shin S, Mostov KE. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kazmierczak BI, Mostov K, Engel JN. Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa. Mol Biol Cell. 2004;15:411–419. doi: 10.1091/mbc.e03-08-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klainer AS, Turndorf H, Wu WH, Maewal H, Allender P. Surface alterations due to endotracheal intubation. Am J Medicine. 1975;58:674–683. doi: 10.1016/0002-9343(75)90504-5 [DOI] [PubMed] [Google Scholar]
- 75.Plotkowski MC, Beck G, Tournier JM, Bernardo-Filho M, Marques EA, Puchelle E. Adherence of Pseudomonas aeruginosa to respiratory epithelium and the effect of leucocyte elastase. J Med Microbiol. 1989;30:285–293. doi: 10.1099/00222615-30-4-285 [DOI] [PubMed] [Google Scholar]
- 76.Leroy A-G, Caillon J, Caroff N, Broquet A, Corvec S, Asehnoune K, et al. Could Azithromycin Be Part of Pseudomonas aeruginosa Acute Pneumonia Treatment? Front Microbiol. 2021;12:642541. doi: 10.3389/fmicb.2021.642541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell. 2019;177:541–555.e17. doi: 10.1016/j.cell.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malhotra S, Hayes D, Wozniak DJ. Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface. Clin Microbiol Rev. 2019:32. doi: 10.1128/CMR.00138-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5 [DOI] [PubMed] [Google Scholar]
- 80.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627 [DOI] [PubMed] [Google Scholar]
- 81.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Resp Crit Care. 2005;172:1013–1018. doi: 10.1164/rccm.200410-1398OC [DOI] [PubMed] [Google Scholar]
- 82.Dovey M, Wisseman CL, Roggli VL, Roomans GM, Shelburne JD, Spock A. Ultrastructural morphology of the lung in cystic fibrosis. J Submicr Cytol Path. 1989;21:521–534. [PubMed] [Google Scholar]
- 83.Caldara M, Friedlander RS, Kavanaugh NL, Aizenberg J, Foster KR, Ribbeck K. Mucin Biopolymers Prevent Bacterial Aggregation by Retaining Cells in the Free-Swimming State. Curr Biol. 2012;22:2325–2330. doi: 10.1016/j.cub.2012.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Co JY, Cárcamo-Oyarce G, Billings N, Wheeler KM, Grindy SC, Holten-Andersen N, et al. Mucins trigger dispersal of Pseudomonas aeruginosa biofilms. npj Biofilms Microbiomes. 2018;4:23. doi: 10.1038/s41522-018-0067-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haley CL, Kruczek C, Qaisar U, Colmer-Hamood JA, Hamood AN. Mucin inhibits Pseudomonas aeruginosa biofilm formation by significantly enhancing twitching motility. Can J Microbiol. 2014;60:155–166. doi: 10.1139/cjm-2013-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wheeler KM, Cárcamo-Oyarce G, Turner BS, Dellos-Nolan S, Co JY, Lehoux S, et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat Microbiol. 2019;4:2146–2154. doi: 10.1038/s41564-019-0581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnes PJ, Burney PGJ, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76 [DOI] [PubMed] [Google Scholar]
- 88.Gao W, Li L, Wang Y, Zhang S, Adcock IM, Barnes PJ, et al. Bronchial epithelial cells in COPD. Respirology. 2015;20:722–729. doi: 10.1111/resp.12542 [DOI] [PubMed] [Google Scholar]
- 89.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and Airway Inflammation in Chronic Obstructive Pulmonary Disease Severe Exacerbations. Am J Resp Crit Care. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 90.Jeannoël M, Lina G, Rasigade JP, Lina B, Morfin F, Casalegno JS. Microorganisms associated with respiratory syncytial virus pneumonia in the adult population. Eur J Clin Microbiol. 2019;38:157–160. doi: 10.1007/s10096-018-3407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erjefält JS, Erjefält I, Sundler F, Persson CGA. In vivo restitution of airway epithelium. Cell Tissue Res. 1995;281:305–316. doi: 10.1007/BF00583399 [DOI] [PubMed] [Google Scholar]
- 92.Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke J-D, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071 [DOI] [PubMed] [Google Scholar]
- 93.Golovkine G, Faudry E, Bouillot S, Elsen S, Attrée I, Huber P. Pseudomonas aeruginosa Transmigrates at Epithelial Cell-Cell Junctions, Exploiting Sites of Cell Division and Senescent Cell Extrusion. PLos Pathog. 2016;12:e1005377. doi: 10.1371/journal.ppat.1005377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heiniger RW, Winther-Larsen HC, Pickles RJ, Koomey M, Wolfgang MC. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 2010;12:1158–1173. doi: 10.1111/j.1462-5822.2010.01461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pentecost M, Otto G, Theriot JA, Amieva MR. Listeria monocytogenes Invades the Epithelial Junctions at Sites of Cell Extrusion. PLos Pathog. 2006;2:e3. doi: 10.1371/journal.ppat.0020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T, Hou Y, Wang R. A case report of community-acquired Pseudomonas aeruginosa pneumonia complicated with MODS in a previously healthy patient and related literature review. BMC Infect Dis. 2019;19:130. doi: 10.1186/s12879-019-3765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46:144–154. doi: 10.1016/0014-4827(67)90416-8 [DOI] [PubMed] [Google Scholar]
- 98.Rawlins EL, Hogan BLM. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407 [DOI] [PubMed] [Google Scholar]
- 99.Sharma A, Coles WH. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest Ophth Vis Sci. 1989;30:1962–1971. [PubMed] [Google Scholar]
- 100.Grove GL, Kligman AM. Age-associated changes in human epidermal cell renewal. J Gerontol. 1983;38:137–142. doi: 10.1093/geronj/38.2.137 [DOI] [PubMed] [Google Scholar]
- 101.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Bio. 2014;15:19–33. doi: 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- 102.Darwich AS, Aslam U, Ashcroft DM, Rostami-Hodjegan A. Meta-Analysis of the Turnover of Intestinal Epithelia in Preclinical Animal Species and Humans. Drug Metab Dispos. 2014;42:2016–2022. doi: 10.1124/dmd.114.058404 [DOI] [PubMed] [Google Scholar]
- 103.Christopher JC, Gordon B, Andes D. Hot Tub-Associated Necrotizing Pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 2003;36:e55–e57. doi: 10.1086/345851 [DOI] [PubMed] [Google Scholar]
- 104.Hatchette TF, Gupta R, Marrie TJ. Pseudomonas aeruginosa Community-Acquired Pneumonia in Previously Healthy Adults: Case Report and Review of the Literature. Clin Infect Dis. 2000;31:1349–1356. doi: 10.1086/317486 [DOI] [PubMed] [Google Scholar]
- 105.Rose HD, Franson TR, Sheth NK, Chusid MJ, Macher AM, Zeirdt CH. Pseudomonas Pneumonia Associated With Use of a Home Whirlpool Spa. JAMA. 1983;250:2027–2029. doi: 10.1001/jama.1983.03340150069030 [DOI] [PubMed] [Google Scholar]
- 106.Huhulescu S, Simon M, Lubnow M, Kaase M, Wewalka G, Pietzka AT, et al. Fatal Pseudomonas aeruginosa pneumonia in a previously healthy woman was most likely associated with a contaminated hot tub. Infection. 2011;39:265–269. doi: 10.1007/s15010-011-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mena KD, Gerba CP. Reviews of Environmental Contamination and Toxicology. Rev Environ Contam Toxicol. 2009;201:71–115. doi: 10.1007/978-1-4419-0032-6_3 [DOI] [PubMed] [Google Scholar]
- 108.Basso P, Ragno M, Elsen S, Reboud E, Golovkine G, Bouillot S, et al. Pseudomonas aeruginosa Pore-Forming Exolysin and Type IV Pili Cooperate To Induce Host Cell Lysis. Mbio. 2017;8:e02250–e02216. doi: 10.1128/mBio.02250-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLos Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sana TG, Hachani A, Bucior I, Soscia C, Garvis S, Termine E, et al. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J Biol Chem. 2012;287:27095–27105. doi: 10.1074/jbc.M112.376368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dosunmu EF, Emeh RO, Dixit S, Bakeer MK, Coats MT, Owen DR, et al. The anti-microbial peptide TP359 attenuates inflammation in human lung cells infected with Pseudomonas aeruginosa via TLR5 and MAPK pathways. PLoS ONE. 2017;12:e0176640. doi: 10.1371/journal.pone.0176640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hawdon NA, Aval PS, Barnes RJ, Gravelle SK, Rosengren J, Khan S, et al. Cellular responses of A549 alveolar epithelial cells to serially collected Pseudomonas aeruginosa from cystic fibrosis patients at different stages of pulmonary infection. Fems Immunol Medical Microbiol. 2010;59:207–220. doi: 10.1111/j.1574-695X.2010.00693.x [DOI] [PubMed] [Google Scholar]
- 113.Azghani AO, Gray LD, Johnson AR. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect Immun. 1993;61:2681–2686. doi: 10.1128/iai.61.6.2681-2686.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clark CA, Thomas LK, Azghani AO. Inhibition of Protein Kinase C Attenuates Pseudomonas aeruginosa Elastase–Induced Epithelial Barrier Disruption. Am J Resp Cell Mol. 2011;45:1263–1271. doi: 10.1165/rcmb.2010-0459OC [DOI] [PubMed] [Google Scholar]
- 115.Azghani AO. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am J Resp Cell Mol. 1996;15:132–140. doi: 10.1165/ajrcmb.15.1.8679217 [DOI] [PubMed] [Google Scholar]
- 116.Azghani AO, Miller EJ, Peterson BT. Virulence factors from Pseudomonas aeruginosa increase lung epithelial permeability. Lung. 2000;178:261–269. doi: 10.1007/s004080000031 [DOI] [PubMed] [Google Scholar]
- 117.Nomura K, Obata K, Keira T, Miyata R, Hirakawa S, Takano K, et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res. 2014;15:21. doi: 10.1186/1465-9921-15-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophth Vis Sci. 2000;41:4093–4100. [PubMed] [Google Scholar]
- 119.Wallace CJ, Medina SH, ElSayed MEH. Effect of Rhamnolipids on Permeability Across Caco-2 Cell Monolayers. Pharmaceut Res. 2014;31:887–894. doi: 10.1007/s11095-013-1210-5 [DOI] [PubMed] [Google Scholar]
- 120.Zulianello L, Canard C, Köhler T, Caille D, Lacroix J-S, Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vikström E, Tafazoli F, Magnusson K-E. Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett. 2006;580:6921–6928. doi: 10.1016/j.febslet.2006.11.057 [DOI] [PubMed] [Google Scholar]
- 122.Vikström E, Bui L, Konradsson P, Magnusson K-E. Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur J Cell Biol. 2010;89:584–597. doi: 10.1016/j.ejcb.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 123.Vikström E, Bui L, Konradsson P, Magnusson K-E. The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp Cell Res. 2009;315:313–326. doi: 10.1016/j.yexcr.2008.10.044 [DOI] [PubMed] [Google Scholar]
- 124.Wilson MJB, Martin DE. Quantitative sputum culture as a means of excluding false positive reports in the routine microbiology laboratory. J Clin Pathol. 1972;25:697. doi: 10.1136/jcp.25.8.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalin M, Lindberg AA, Tunevall G. Etiological Diagnosis of Bacterial Pneumonia by Gram Stain and Quantitative Culture of Expectorates: Leukocytes or Alveolar Macrophages as Indicators of Sample Representativity. Scand J Infect Dis. 2015;15:153–160. doi: 10.3109/inf.1983.15.issue-2.05 [DOI] [PubMed] [Google Scholar]
- 126.Khan F, Pham DTN, Oloketuyi SF, Kim Y-M. Regulation and controlling the motility properties of Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2020;104:33–49. doi: 10.1007/s00253-019-10201-w [DOI] [PubMed] [Google Scholar]
- 127.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993;92:1875–1880. doi: 10.1172/JCI116779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davies J, Dewar A, Bush A, Pitt T, Gruenert D, Geddes DM, et al. Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1 antibody and neuraminidase inhibition. Eur Respir J. 1999;13:565–570. doi: 10.1183/09031936.99.13356599 [DOI] [PubMed] [Google Scholar]
- 129.Scharfman A, van Brussel E, Houdret N, Lamblin G, Roussel P. Interactions between Glycoconjugates from Human Respiratory Airways and Pseudomonas aeruginosa. Am J Resp Crit Care. 1996;154:S163–S169. doi: 10.1164/ajrccm/154.4_Pt_2.S163 [DOI] [PubMed] [Google Scholar]
- 130.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa Flagella Activate Airway Epithelial Cells through asialoGM1 and Toll-Like Receptor 2 as well as Toll-Like Receptor 5. Am J Resp Cell Mol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC [DOI] [PubMed] [Google Scholar]
- 131.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR R Domain Leads to Increased Levels of AsialoGM1 and Increased Pseudomonas aeruginosa Binding by Epithelial Cells. Am J Resp Cell Mol. 1998;19:269–277. doi: 10.1165/ajrcmb.19.2.2889 [DOI] [PubMed] [Google Scholar]
- 132.Eierhoff T, Bastian B, Thuenauer R, Madl J, Audfray A, Aigal S, et al. A lipid zipper triggers bacterial invasion. Proc Natl Acad Sci. 2014;111:12895–12900. doi: 10.1073/pnas.1402637111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brandel A, Aigal S, Lagies S, Schlimpert M, Meléndez AV, Xu M, et al. The Gb3-enriched CD59/flotillin plasma membrane domain regulates host cell invasion by Pseudomonas aeruginosa. Cell Mol Life Sci. 2021;78:3637–3656. doi: 10.1007/s00018-021-03766-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chemani C, Imberty A, de Bentzmann S, Pierre M, Wimmerová M, Guery BP, et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun. 2009;77:2065–2075. doi: 10.1128/IAI.01204-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mewe M, Tielker D, Schönberg R, Schachner M, Jaeger K-E, Schumacher U. Pseudomonas aeruginosa lectins I and II and their interaction with human airway cilia. J Laryngol Otol. 2005;119:595–599. doi: 10.1258/0022215054516313 [DOI] [PubMed] [Google Scholar]
- 136.Zheng S, Eierhoff T, Aigal S, Brandel A, Thuenauer R, de Bentzmann S, et al. The Pseudomonas aeruginosa lectin LecA triggers host cell signalling by glycosphingolipid-dependent phosphorylation of the adaptor protein CrkII. Biochim Biophys Acta Mol Cell Res. 2017;1864:1236–1245. doi: 10.1016/j.bbamcr.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 137.Mikolajczyk K, Sikora M, Hanus C, Kaczmarek R, Czerwinski M. One of the two N-glycans on the human Gb3/CD77 synthase is essential for its activity and allosterically regulates its function. Biochem Biophys Res Commun. 2022;617:36–41. doi: 10.1016/j.bbrc.2022.05.085 [DOI] [PubMed] [Google Scholar]
- 138.Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-Mediated Damage Requires Distinct Receptors at the Apical and Basolateral Surfaces of the Polarized Epithelium. Infect Immun. 2010;78:939–953. doi: 10.1128/IAI.01215-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dorscheid DR, Wojcik KR, Yule K, White SR. Role of cell surface glycosylation in mediating repair of human airway epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2001;281:L982–L992. doi: 10.1152/ajplung.2001.281.4.L982 [DOI] [PubMed] [Google Scholar]
- 140.Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerçeker AA, Golan DE, et al. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci. 2002;99:6907–6912. doi: 10.1073/pnas.092160899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brezillon S, Dupuit F, Hinnrasky J, Marchand V, Kälin N, Tümmler B, et al. Decreased expression of the CFTR protein in remodeled human nasal epithelium from non-cystic fibrosis patients. Lab Invest. 1995;72:191–200. [PubMed] [Google Scholar]
- 142.Brézillon S, Hamm H, Heilmann M, Schafers H-J, Hinnrasky J, Wagner TOF, et al. Decreased expression of the cystic fibrosis transmembrane conductance regulator protein in remodeled airway epithelium from lung transplanted patients. Hum Pathol. 1997;28:944–952. doi: 10.1016/s0046-8177(97)90010-1 [DOI] [PubMed] [Google Scholar]
- 143.Ruffin M, Bigot J, Calmel C, Mercier J, Givelet M, Oliva J, et al. Flagellin From Pseudomonas aeruginosa Modulates SARS-CoV-2 Infectivity in Cystic Fibrosis Airway Epithelial Cells by Increasing TMPRSS2 Expression. Front Immunol. 2021;12:714027. doi: 10.3389/fimmu.2021.714027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hampton TH, Ballok AE, Bomberger JM, Rutkowski MR, Barnaby R, Coutermarsh B, et al. Does the ΔF508-CFTR mutation induce a proinflammatory response in human airway epithelial cells? Am J Physiol Lung Cell Mol Physiol. 2012;303:L509–L518. doi: 10.1152/ajplung.00226.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arhin A, Boucher C. The outer membrane protein OprQ and adherence of Pseudomonas aeruginosa to human fibronectin. Microbiology+. 2010;156:1415–1423. doi: 10.1099/mic.0.033472-0 [DOI] [PubMed] [Google Scholar]
- 147.Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Resp Cell Mol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373 [DOI] [PubMed] [Google Scholar]
- 148.Hérard AL, Pierrot D, Hinnrasky J, Kaplan H, Sheppard D, Puchelle E, et al. Fibronectin and its alpha 5 beta 1-integrin receptor are involved in the wound-repair process of airway epithelium. Am J Physiol. 1996;271:L726–L733. doi: 10.1152/ajplung.1996.271.5.L726 [DOI] [PubMed] [Google Scholar]
- 149.Paulsson M, Singh B, Al-Jubair T, Su Y-C, Høiby N, Riesbeck K. Identification of outer membrane Porin D as a vitronectin-binding factor in cystic fibrosis clinical isolates of Pseudomonas aeruginosa. J Cyst Fibros. 2015;14:600–607. doi: 10.1016/j.jcf.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salazar-Peláez LM, Abraham T, Herrera AM, Correa MA, Ortega JE, Paré PD, et al. Vitronectin Expression in the Airways of Subjects with Asthma and Chronic Obstructive Pulmonary Disease. PLoS ONE. 2015;10:e0119717. doi: 10.1371/journal.pone.0119717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pilewski JM, Latoche JD, Arcasoy SM, Albelda SM. Expression of integrin cell adhesion receptors during human airway epithelial repair in vivo. Am J Physiol. 1997;273:L256–L263. doi: 10.1152/ajplung.1997.273.1.L256 [DOI] [PubMed] [Google Scholar]
- 152.Smith JW, Vestal DJ, Irwin SV, Burke TA, Cheresh DA. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- 153.Plotkowski MC, Costa AO, Morandi V, Barbosa HS, Nader HB, Bentzmann SD, et al. Role of heparan sulphate proteoglycans as potential receptors for non-piliated Pseudomonas aeruginosa adherence to non-polarised airway epithelial cells. J Med Microbiol. 2001;50:183–190. doi: 10.1099/0022-1317-50-2-183 [DOI] [PubMed] [Google Scholar]
- 154.Bermejo-Jambrina M, Eder J, Kaptein TM, Hamme JL, Helgers LC, Vlaming KE, et al. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J. 2021;40:e106765. doi: 10.15252/embj.2020106765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paulsson M, Su Y-C, Ringwood T, Uddén F, Riesbeck K. Pseudomonas aeruginosa uses multiple receptors for adherence to laminin during infection of the respiratory tract and skin wounds. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-54622-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Plotkowski MC, Tournier JM, Puchelle E. Pseudomonas aeruginosa strains possess specific adhesins for laminin. Infect Immun. 1996;64:600–605. doi: 10.1128/iai.64.2.600-605.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hauser AR. The Type III Secretion System of Pseudomonas aeruginosa: Infection by Injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Inclan YF, Persat A, Greninger A, Dollen JV, Johnson J, Krogan N, et al. A scaffold protein connects type IV pili with the Chp chemosensory system to mediate activation of virulence signaling in Pseudomonas aeruginosa. Mol Microbiol. 2016;101:590–605. doi: 10.1111/mmi.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci. 2015;112:7563–7568. doi: 10.1073/pnas.1502025112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate Regulation of Bacterial Virulence Genes by a Novel Adenylate Cyclase-Dependent Signaling Pathway. Dev Cell. 2003;4:253–263. doi: 10.1016/s1534-5807(03)00019-4 [DOI] [PubMed] [Google Scholar]
- 161.Fuchs EL, Brutinel ED, Jones AK, Fulcher NB, Urbanowski ML, Yahr TL, et al. The Pseudomonas aeruginosa Vfr Regulator Controls Global Virulence Factor Expression through Cyclic AMP-Dependent and -Independent Mechanisms. J Bacteriol. 2010;192:3553–3564. doi: 10.1128/JB.00363-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klockgether J, Tümmler B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Res. 2017;6:1261. doi: 10.12688/f1000research.10506.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 164.Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6: 56–60. doi: 10.1016/s1369-5274(03)00008-0 [DOI] [PubMed] [Google Scholar]
- 165.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Turkina MV, Vikström E. Bacteria-Host Crosstalk: Sensing of the Quorum in the Context of Pseudomonas aeruginosa Infections. J Innate Immun. 2019;11:263–279. doi: 10.1159/000494069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thi MTT, Wibowo D, Rehm BHA. Pseudomonas aeruginosa Biofilms. Int J Mol Sci. 2020;21:8671. doi: 10.3390/ijms21228671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deep A, Chaudhary U, Gupta V. Quorum sensing and Bacterial Pathogenicity: From Molecules to Disease. J Lab Physicians. 2011;3:4–11. doi: 10.4103/0974-2727.78553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. Fems Microbiol Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moreau-Marquis S, Stanton BA, O’Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther. 2008;21:595–599. doi: 10.1016/j.pupt.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intens Care Med. 1999;25:1072–1076. doi: 10.1007/s001340051014 [DOI] [PubMed] [Google Scholar]
- 172.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/IAI.66.1.43-51.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hahn HP. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9 [DOI] [PubMed] [Google Scholar]
- 174.Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938 [DOI] [PubMed] [Google Scholar]
- 175.Rocchetta HL, Burrows LL, Lam JS. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63:523–553. doi: 10.1128/MMBR.63.3.523-553.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eutamene H, Theodorou V, Schmidlin F, Tondereau V, Garcia-Villar R, Salvador-Cartier C, et al. LPS-induced lung inflammation is linked to increased epithelial permeability: role of MLCK. Eur Respir J. 2005;25:789–796. doi: 10.1183/09031936.05.00064704 [DOI] [PubMed] [Google Scholar]
- 177.Cabrera-Benítez NE, Pérez-Roth E, Ramos-Nuez Á, Sologuren I, Padrón JM, Slutsky AS, et al. Inhibition of endotoxin-induced airway epithelial cell injury by a novel family of pyrrol derivates. Lab Invest. 2016;96:632–640. doi: 10.1038/labinvest.2016.46 [DOI] [PubMed] [Google Scholar]
- 178.Bajolet-Laudinat O, Bentzmann SG, Tournier JM, Madoulet C, Plotkowski MC, Chippaux C, et al. Cytotoxicity of Pseudomonas aeruginosa internal lectin PA-I to respiratory epithelial cells in primary culture. Infect Immun. 1994;62:4481–4487. doi: 10.1128/iai.62.10.4481-4487.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nivens DE, Ohman DE, Williams J, Franklin MJ. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol. 2001;183:1047–1057. doi: 10.1128/JB.183.3.1047-1057.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pier GB, Coleman F, Grout M, Franklin M, Ohman DE. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun. 2001;69:1895–1901. doi: 10.1128/IAI.69.3.1895-1901.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Limoli DH, Jones CJ, Wozniak DJ. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol Spectr. 2015:3. doi: 10.1128/microbiolspec.MB-0011-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xiao R, Kisaalita WS. Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Microbiology+. 1997;143 (Pt 7):2509–2515. doi: 10.1099/00221287-143-7-2509 [DOI] [PubMed] [Google Scholar]
- 184.Ankenbauer RG, Quan HN. FptA, the Fe(III)-pyochelin receptor of Pseudomonas aeruginosa: a phenolate siderophore receptor homologous to hydroxamate siderophore receptors. J Bacteriol. 1994;176:307–319. doi: 10.1128/jb.176.2.307-319.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Minandri F, Imperi F, Frangipani E, Bonchi C, Visaggio D, Facchini M, et al. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect Immun. 2016;84:2324–2335. doi: 10.1128/IAI.00098-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith AD, Wilks A. Differential Contributions of the Outer Membrane Receptors PhuR and HasR to Heme Acquisition in Pseudomonas aeruginosa *. J Biol Chem. 2015;290:7756–7766. doi: 10.1074/jbc.M114.633495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ochsner UA, Johnson Z, Vasil ML. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology+. 2000;146:185–198. doi: 10.1099/00221287-146-1-185 [DOI] [PubMed] [Google Scholar]
- 188.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Husson M-O, Harf-Monteil C, Monteil H. Genre Pseudomonas Pseudomonas aeruginosa La bacille pyocyanique. 2ème édition. Précis de Bactériologie Clinique; 2017. [Google Scholar]
- 190.Jackowski JT, Szepfalusi Z, Wanner DA, Seybold Z, Sielczak MW, Lauredo IT, et al. Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: role of O2 radicals. Am J Physiol. 1991;260:L61–L67. doi: 10.1152/ajplung.1991.260.2.L61 [DOI] [PubMed] [Google Scholar]
- 191.McClure CD, Schiller NL. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr Microbiol. 1996;33:109–117. doi: 10.1007/s002849900084 [DOI] [PubMed] [Google Scholar]
- 192.Abdel-Mawgoud AM, Lépine F, Déziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biot. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Halldorsson S, Gudjonsson T, Gottfredsson M, Singh PK, Gudmundsson GH, Baldursson O. Azithromycin Maintains Airway Epithelial Integrity during Pseudomonas aeruginosa Infection. Am J Resp Cell Mol. 2010;42:62–68. doi: 10.1165/rcmb.2008-0357OC [DOI] [PubMed] [Google Scholar]
- 194.Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger K-E. The Autotransporter Esterase EstA of Pseudomonas aeruginosa Is Required for Rhamnolipid Production, Cell Motility, and Biofilm Formation. J Bacteriol. 2007;189:6695–6703. doi: 10.1128/JB.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iiyama K, Takahashi E, Lee JM, Mon H, Morishita M, Kusakabe T, et al. Alkaline protease contributes to pyocyanin production in Pseudomonas aeruginosa. Fems Microbiol Lett. 2017;364:fnx051. doi: 10.1093/femsle/fnx051 [DOI] [PubMed] [Google Scholar]
- 196.Heck LW, Morihara K, Abrahamson DR. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect Immun. 1986;54:149–153. doi: 10.1128/iai.54.1.149-153.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kessler E, Safrin M, Gustin JK, Ohman DE. Elastase and the LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. J Biol Chem. 1998;273:30225–30231. doi: 10.1074/jbc.273.46.30225 [DOI] [PubMed] [Google Scholar]
- 198.Armstrong S, Yates SP, Merrill AR. Insight into the catalytic mechanism of Pseudomonas aeruginosa exotoxin A. Studies of toxin interaction with eukaryotic elongation factor-2. J Biol Chem. 2002;277:46669–46675. doi: 10.1074/jbc.M206916200 [DOI] [PubMed] [Google Scholar]
- 199.Ferguson TEG, Reihill JA, Walker B, Hamilton RA, Martin SL. A Selective Irreversible Inhibitor of Furin Does Not Prevent Pseudomonas Aeruginosa Exotoxin A-Induced Airway Epithelial Cytotoxicity. PLoS ONE. 2016;11:e0159868. doi: 10.1371/journal.pone.0159868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Songer JG. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6 [DOI] [PubMed] [Google Scholar]
- 201.Terada LS, Johansen KA, Nowbar S, Vasil AI, Vasil ML. Pseudomonas aeruginosa Hemolytic Phospholipase C Suppresses Neutrophil Respiratory Burst Activity. Infect Immun. 1999;67:2371–2376. doi: 10.1128/IAI.67.5.2371-2376.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ostroff RM, Vasil AI, Vasil ML. Molecular comparison of a nonhemolytic and a hemolytic phospholipase C from Pseudomonas aeruginosa. J Bacteriol. 1990;172:5915–5923. doi: 10.1128/jb.172.10.5915-5923.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wargo MJ, Gross MJ, Rajamani S, Allard JL, Lundblad LKA, Allen GB, et al. Hemolytic Phospholipase C Inhibition Protects Lung Function during Pseudomonas aeruginosa Infection. Am J Resp Crit Care. 2011;184:345–354. doi: 10.1164/rccm.201103-0374OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bouillot S, Munro P, Gallet B, Reboud E, Cretin F, Golovkine G, et al. Pseudomonas aeruginosa Exolysin promotes bacterial growth in lungs, alveolar damage and bacterial dissemination. Sci Rep. 2017;7:2120. doi: 10.1038/s41598-017-02349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Reboud E, Elsen S, Bouillot S, Golovkine G, Basso P, Jeannot K, et al. Phenotype and toxicity of the recently discovered exlA-positive Pseudomonas aeruginosa strains collected worldwide. Environ Microbiol. 2016;18:3425–3439. doi: 10.1111/1462-2920.13262 [DOI] [PubMed] [Google Scholar]
- 206.Bomberger JM, Ely KH, Bangia N, Ye S, Green KA, Green WR, et al. Pseudomonas aeruginosa Cif Protein Enhances the Ubiquitination and Proteasomal Degradation of the Transporter Associated with Antigen Processing (TAP) and Reduces Major Histocompatibility Complex (MHC) Class I Antigen Presentation*. J Biol Chem. 2014;289:152–162. doi: 10.1074/jbc.M113.459271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bomberger JM, Ye S, MacEachran DP, Koeppen K, Barnaby RL, O’Toole GA, et al. A Pseudomonas aeruginosa Toxin that Hijacks the Host Ubiquitin Proteolytic System. PLoS Pathog. 2011;7:e1001325. doi: 10.1371/journal.ppat.1001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72:6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ganesan AK, Vincent TS, Olson JC, Barbieri JT. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J Biol Chem. 1999;274:21823–21829. doi: 10.1074/jbc.274.31.21823 [DOI] [PubMed] [Google Scholar]
- 210.Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, et al. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin. ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cowell BA, Evans DJ, Fleiszig SMJ. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol Lett. 2005;250:71–76. doi: 10.1016/j.femsle.2005.06.044 [DOI] [PubMed] [Google Scholar]
- 213.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa Exotoxin Y Is a Promiscuous Cyclase That Increases Endothelial Tau Phosphorylation and Permeability*. J Biol Chem. 2012;287:25407–25418. doi: 10.1074/jbc.M111.301440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kloth C, Schirmer B, Munder A, Stelzer T, Rothschuh J, Seifert R. The Role of Pseudomonas aeruginosa ExoY in an Acute Mouse Lung Infection Model. Toxins. 2018;10:185. doi: 10.3390/toxins10050185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen L, Zou Y, She P, Wu Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol Res. 2015;172:19–25. doi: 10.1016/j.micres.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 216.Bleves S, Sana TG, Voulhoux R. The target cell genus does not matter. Trends Microbiol. 2014;22:304–306. doi: 10.1016/j.tim.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 217.Sana TG, Baumann C, Merdes A, Soscia C, Rattei T, Hachani A, et al. Internalization of Pseudomonas aeruginosa Strain PAO1 into Epithelial Cells Is Promoted by Interaction of a T6SS Effector with the Microtubule Network. Mbio. 2015;6:e00712–e00715. doi: 10.1128/mBio.00712-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang F, Wang X, Wang B, Chen L, Zhao Z, Waterfield NR, et al. The Pseudomonas aeruginosa Type VI Secretion PGAP1-like Effector Induces Host Autophagy by Activating Endoplasmic Reticulum Stress. Cell Rep. 2016;16:1502–1509. doi: 10.1016/j.celrep.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 219.Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, et al. A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016;12:e1005672. doi: 10.1371/journal.ppat.1005672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Smith RS, Harris SG, Phipps R, Iglewski B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol. 2002;184:1132–1139. doi: 10.1128/jb.184.4.1132-1139.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cigana C, Lorè NI, Bernardini ML, Bragonzi A. Dampening Host Sensing and Avoiding Recognition in Pseudomonas aeruginosa Pneumonia. J Biomed Biotechnol. 2011;2011:852513. doi: 10.1155/2011/852513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005 [DOI] [PubMed] [Google Scholar]
- 223.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med. 2012;40:1157–1163. doi: 10.1097/CCM.0b013e3182377906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ozer EA, Nnah E, Didelot X, Whitaker RJ, Hauser AR. The Population Structure of Pseudomonas aeruginosa Is Characterized by Genetic Isolation of exoU+ and exoS+ Lineages. Genome Biol Evol. 2019;11:1780–1796. doi: 10.1093/gbe/evz119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pena C, Cabot G, Gomez-Zorrilla S, Zamorano L, Ocampo-Sosa A, Murillas J, et al. Influence of Virulence Genotype and Resistance Profile in the Mortality of Pseudomonas aeruginosa Bloodstream Infections. Clin Infect Dis. 2015;60:539–548. doi: 10.1093/cid/ciu866 [DOI] [PubMed] [Google Scholar]
- 226.Horna G, Amaro C, Palacios A, Guerra H, Ruiz J. High frequency of the exoU+/exoS+ genotype associated with multidrug-resistant “high-risk clones” of Pseudomonas aeruginosa clinical isolates from Peruvian hospitals. Sci Rep. 2019;9:10874. doi: 10.1038/s41598-019-47303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yousefi-Avarvand A, Khashei R, Ebrahim-Saraie HS, Emami A, Zomorodian K, Motamedifar M. The Frequency of Exotoxin A and Exoenzymes S and U Genes Among Clinical Isolates of Pseudomonas aeruginosa in Shiraz. Iran. Int J Mol Cell Med. 2015;4:167–173. [PMC free article] [PubMed] [Google Scholar]
- 228.Wareham DW, Curtis MA. A genotypic and phenotypic comparison of type III secretion profiles of Pseudomonas aeruginosa cystic fibrosis and bacteremia isolates. Int J Med Microbiol. 2007;297:227–234. doi: 10.1016/j.ijmm.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 229.Hirakata Y, Finlay BB, Simpson DA, Kohno S, Kamihira S, Speert DP. Penetration of Clinical Isolates of Pseudomonas aeruginosa through MDCK Epithelial Cell Monolayers. J Infect Dis. 2000;181:765–769. doi: 10.1086/315276 [DOI] [PubMed] [Google Scholar]
- 230.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology+. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659 [DOI] [PubMed] [Google Scholar]
- 231.Soong G, Parker D, Magargee M, Prince AS. The Type III Toxins of Pseudomonas aeruginosa Disrupt Epithelial Barrier Function. J Bacteriol. 2008;190:2814–2821. doi: 10.1128/JB.01567-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gingerich A, Pang L, Hanson J, Dlugolenski D, Streich R, Lafontaine ER, et al. Hypothiocyanite produced by human and rat respiratory epithelial cells inactivates extracellular H1N2 influenza A virus. Inflamm Res. 2016;65:71–80. doi: 10.1007/s00011-015-0892-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reboud E, Bouillot S, Patot S, Béganton B, Attrée I, Huber P. Pseudomonas aeruginosa ExlA and Serratia marcescens ShlA trigger cadherin cleavage by promoting calcium influx and ADAM10 activation. PLoS Pathog. 2017;13:e1006579. doi: 10.1371/journal.ppat.1006579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bleves S, Viarre V, Salacha R, Michel GPF, Filloux A, Voulhoux R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int J Med Microbiol. 2010;300:534–543. doi: 10.1016/j.ijmm.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 235.Wiener-Kronish JP, Sakuma T, Kudoh I, Pittet JF, Frank D, Dobbs L, et al. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661 [DOI] [PubMed] [Google Scholar]
- 236.Kida Y, Yamamoto T, Kuwano K. SdsA1, a secreted sulfatase, contributes to the in vivo virulence of Pseudomonas aeruginosa in mice. Microbiol Immunol. 2020;64:280–295. doi: 10.1111/1348-0421.12772 [DOI] [PubMed] [Google Scholar]
- 237.Pier GB, Grout M, Zaidi TS, Olsen JC, Johnson LG, Yankaskas JR, et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bucior I, Tran C, Engel J. Assessing Pseudomonas virulence using host cells. Methods Mol Biol. 2014;1149:741–755. doi: 10.1007/978-1-4939-0473-0_57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cappiello F, Grazia AD, Segev-Zarko L, Scali S, Ferrera L, Galietta L, et al. Esculentin-1a-Derived Peptides Promote Clearance of Pseudomonas aeruginosa Internalized in Bronchial Cells of Cystic Fibrosis Patients and Lung Cell Migration: Biochemical Properties and a Plausible Mode of Action. Antimicrob Agents Chemother. 2016;60:7252–7262. doi: 10.1128/AAC.00904-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem. 2005;280:4864–4872. doi: 10.1074/jbc.M411702200 [DOI] [PubMed] [Google Scholar]
- 242.Pereira SH, Cervante MP, Bentzmann S, Plotkowski MC. Pseudomonas aeruginosa entry into Caco-2 cells is enhanced in repairing wounded monolayers. Microb Pathog. 1997;23:249–255. doi: 10.1006/mpat.1997.0153 [DOI] [PubMed] [Google Scholar]
- 243.Kazmierczak BI, Jou TS, Mostov K, Engel JN. Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell Microbiol. 2001;3:85–98. doi: 10.1046/j.1462-5822.2001.00091.x [DOI] [PubMed] [Google Scholar]
- 244.Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yamamoto N, Yamamoto N, Petroll MW, Cavanagh HD, Jester JV. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophth Vis Sci. 2005;46:1348–1355. doi: 10.1167/iovs.04-0542 [DOI] [PubMed] [Google Scholar]
- 246.Lingwood D, Simons K. Lipid Rafts As a Membrane-Organizing Principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- 247.Jiang F, Waterfield NR, Yang J, Yang G, Jin Q. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe. 2014;15:600–610. doi: 10.1016/j.chom.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 248.Engel J, Eran Y. Subversion of mucosal barrier polarity by pseudomonas aeruginosa. Front Microbiol. 2011;2:114. doi: 10.3389/fmicb.2011.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kierbel A, Gassama-Diagne A, Rocha C, Radoshevich L, Olson J, Mostov K, et al. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J Cell Biol. 2007;177:21–27. doi: 10.1083/jcb.200605142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lepanto P, Bryant DM, Rossello J, Datta A, Mostov KE, Kierbel A. Pseudomonas aeruginosa interacts with epithelial cells rapidly forming aggregates that are internalized by a Lyn-dependent mechanism. Cell Microbiol. 2011;13:1212–1222. doi: 10.1111/j.1462-5822.2011.01611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pier GB. The challenges and promises of new therapies for cystic fibrosis. J Exp Med. 2012;209:1235–1239. doi: 10.1084/jem.20121248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ECM, extracellular matrix; ND/NR, not determined/not relevant.
(DOCX)