Abstract
Chemokines belong to the family of cytokines with chemoattractant properties that regulate chemotaxis and leukocyte migration, as well as the induction of angiogenesis and maintenance of hemostasis. Curcumin, the major component of the Curcuma longa rhizome, has various pharmacological actions, including anti-inflammatory, immune-regulatory, anti-oxidative, and lipid-modifying properties. Chemokines and chemokine receptors are influenced/modulated by curcumin. Thus, the current review focuses on the molecular mechanisms associated with curcumin's effects on chemoattractant cytokines, as well as putting into context the many studies that have reported curcumin-mediated regulatory effects on inflammatory conditions in the organs/systems of the body (e.g., the central nervous system, liver, and cardiovascular system). Curcumin's effects on viral and bacterial infections, cancer, and adverse pregnancy outcomes are also reviewed.
Keywords: Curcumin, Chemokine, Cytokine, Inflammation, Cancer, Infection
Introduction
Chemokines are small (7–13 kDa), signaling proteins that belong to the cytokine family and exert chemoattractant properties. They play an important role in both physiologic and pathological immune responses through the formation of concentration gradients and interactions with their respective receptors on cell surfaces. Almost fifty different chemokines have been identified in humans that induce hemostasis, angiogenesis and regulate chemotaxis and leukocyte adhesion/transmigration. The chemokines are grouped into CC, CXC, CX3C, and XC subfamilies according to the number of amino acids located between the first two structural cysteine residues (Deshmane et al. 2009; Bodnar 2015; Gustavsson 2020). Chemokine receptors are expressed on various cell types, including leukocytes and other non-hematopoietic cells. About twenty types of chemokine receptors have been identified that are 7-transmembrane G protein-coupled receptors (GPCRs). The binding of chemokine to its receptor creates a calcium signaling cascade, activating small GTPases. Activation of integrins and actin polymerization are the downstream events that result in the development of a pseudopod, polarized cell morphology, and ultimately cell movement. The chemokines are then internalized via clathrin-based endocytosis (Cabrero-de las Heras and Martínez-Balibrea 2018; Rajagopal et al. 2010; Proudfoot and Uguccioni 2016). Cascade-activation of downstream signaling proteins is associated with inflammatory processes and leukocyte accumulation in infected or injured tissues. This process is a key step in cell migration and a powerful 'checkpoint' in regulating cell migration. Thus, intentional regulation of cell migration could potentially be accomplished using different therapeutic agents or strategies (Marchese 2014; Maryam Saberi Karimian et al. 2017a; Scholten et al. 2012) (Table 1).
Table 1.
Characteristics of chemokines and chemokine receptors
| Name | Synonyms | Receptor | Target cell | Major function |
|---|---|---|---|---|
| C family | ||||
| XCL1 | Lymphotactin α, SCM-1α, ATAC | XCR1 | T cell, NK, CD8α+ dendritic cell | T cell and NK recruitment |
| XCL2 | Lymphotactin β, SCM-1β | XCR1 | T cell, NK, CD8α+ dendritic cell | Unknown |
| CC family | ||||
| CCL1 | I-309, TCA3, P500, SISe, SCYA1 | CCR8 | Monocyte, neutrophil, T cell (Th2 > Th1) | Monocyte recruitment and endothelial cell migration |
| CCL2 | MCP-1 | CCR2 | T cell (Th2 > Th1), monocyte, basophil, immature dendritic cell, NK | Mixed leukocyte recruitment |
| CCL3 | MIP-1α, LD78 | CCR1, 5 | Monocyte/macrophage, T cell (Th1 > Th2), NK, basophil, immature dendritic cell, eosinophil, neutrophil, astrocyte, fibroblast, osteoclast | Mixed leukocyte recruitment |
| CCL4 | MIP-1 β | CCR1, 5 | Monocyte/macrophage, T cell (Th1 > Th2), NK, basophil, immature dendritic cell, eosinophil, B cell |
T cell, dendritic cell, monocyte and NK recruitment HIV co-receptor |
| CCL5 | RANTES | CCR1, 3, 5 | Monocyte/macrophage, T cell (memory T cell > T cell; Th1 > Th2), NK, basophil, eosinophil, immature dendritic cell | Mixed leukocyte recruitment |
| CCL6 | C10, MRP-1, SCYA6 | CCR1 | Monocyte, B cell, CD4+ T cell, NK | Unknown |
| CCL7 | MARC, MCP-3 | CCR1, 2, 3, 5 | Th2 > Th1 cell, monocyte, eosinophil, basophil, immature dendritic cell, NK | Mixed leukocyte recruitment |
| CCL8 | MCP-2 | CCR1, 2b, 5 | Th2 > Th1 cell, monocyte, eosinophil, basophil, immature dendritic cell, NK | Mixed leukocyte recruitment |
| CCL11 | Eotaxin | CCR3, 5 | Eosinophil, basophil, mast cell, Th2 cell | Eosinophil, basophil and Th2 recruitment |
| CCL12 | MCP-5 | CCR2 | Eosinophil, monocyte, T cell, B cell | Mixed leukocyte recruitment |
| CCL13 | MCP-4, NCC-1, Ckβ6 | CCR2, 3, 5 | Th2 > Th1 cell, monocyte, eosinophil, basophil, dendritic cell | Mixed leukocyte recruitment |
| CCL14a | HCC-1 | CCR1, 3, 5 | Monocyte | Unknown |
| CCL14b | HCC-3 | unknown | Monocyte | Unknown |
| CCL15 | MIP-5, HCC-2 | CCR1, 3 | T cell, monocyte, eosinophil, dendritic cell | Mixed leukocyte recruitment |
| CCL16 | HCC-4, LEC | CCR1, 2, 5, 8 | Monocyte, T cell, NK, immature dendritic cell | Lymphocyte and monocyte recruitment |
| CCL17 | TARC | CCR4, 8 | Th2 > Th1 cell, immature dendritic cell, thymocytes, regulatory T cells | T cell recruitment |
| CCL18 | DC-CK1, PARC | PITPNM3 | Naïve T cell > activated T cell, immature dendritic cell, mantle zone B cell | Lymphocyte and dendritic cell homing |
| CCL19 | MIP-3β, ELC | CCR7 | Naïve T cell, mature dendritic cell, B cell | T cell and dendritic cell migration into para-follicular zones of lymph nodes |
| CCL20 | MIP-3α, LARC | CCR6 | Memory T cells, Th17 cells, blood mononuclear cells, immature dendritic cells, activated B cells, GALT development |
Th17 recruitment Dendritic cell positioning in tissue |
| CCL21 | SLC, 6Ckine, Exodus-2, Ckβ9 | CCR7 | Naïve T cell, B cell, thymocyte, NK, mature dendritic cell | T cell and dendritic cell migration into para-follicular zones of lymph nodes |
| CCL22 | MDC | CCR4 | Immature dendritic cell, NK, T cells (Th2 > Th1), thymocyte, endothelial cell, monocyte, regulatory T cell | NK and T cell recruitment |
| CCL23 | MPIF-1, CK-β | CCR1, FPRL-1 | Monocyte, T cell, resting neutrophil | Monocyte, neutrophil and T cell migration |
| CCL24 | Eotaxin-2, MPIF-2, Ckβ6 | CCR3 | Eosinophil, basophil, T cell | Eosinophil, basophil and Th2 recruitment |
| CCL25 | TECK | CCR9 | Macrophage, thymocyte, dendritic cell, intraepithelial lymphocyte, IgA plasma cell, mucosal memory T cell | Lymphocyte recruitment into intestine |
| CCL26 | Eotaxin-3, MIP-1α, IMAC, TSC-1 | CCR3 | Eosinophil, basophil, fibroblast | Eosinophil, basophil and Th2 recruitment |
| CCL27 | CTACK | CCR10 | Skin homing memory T cell, B cell | T cell recruitment into skin |
| CCL28 | MEC | CCR10 | T cell, eosinophil, IgA+ B cell | T and B cells homing in mucosa |
| CXC family | ||||
| CXCL1 | GRO-α, GRO-1, NAP-3, KC | CXCR2 | Neutrophil, fibroblast | Neutrophil recruitment |
| CXCL2 | GRO-β, GRO-2, NAP-2α | CXCR2 | Neutrophil, fibroblast | Neutrophil recruitment |
| CXCL3 | GRO-γ, GRO-1, NAP-3 | CXCR2 | Neutrophil, fibroblast | Neutrophil recruitment |
| CXCL4 | PF4 | CXCR3b | Fibroblast, endothelial cell | Platelet aggregation |
| CXCL5 | ENA-78 | CXCR2 | Neutrophil, endothelial cell | Neutrophil recruitment |
| CXCL6 | GCP-2 | CXCR2 | Neutrophil, endothelial cell | Neutrophil recruitment |
| CXCL7 | NAP-2 | CXCR1, 2 | Fibroblast, neutrophil, endothelial cell | Neutrophil recruitment |
| CXCL8 | IL-8, NPP-1, MDNCF, GCP-1 | CXCR1, 2 | Neutrophil, basophil, endothelial cell | Neutrophil recruitment |
| CXCL9 | Mig, CRG-10 | CXCR3 | Activated T cell (Th1 > Th2), NK, B cell, endothelial cell, plasmacytoid dendritic cell | Effector T cell recruitment |
| CXCL10 | IP-10, CRG-2 | CXCR3 | Activated T cell (Th1 > Th2), NK, B cell, endothelial cell | Effector T cell recruitment |
| CXCL11 | IP-9, ITAC, β-R1 | CXCR3 | Activated T cell (Th1 > Th2), NK, B cell, endothelial cell | Effector T cell recruitment |
| CXCL12 | SDF-1α/β | CXCR4, 7 | CD34+ bone marrow cells, thymocytes, monocytes/macrophages, naïve activated T cell, B cell, plasma cell, neutrophil, immature and mature dendritic cells, plasmacytoid dendritic cells |
B cell migration into lymph nodes Plasma cell migration into bone marrow |
| CXCL13 | BCA-1, BLC | CXCR5 | Naïve B cell, activated CD4 T cells, immature and mature dendritic cells |
B cell migration into lymph nodes and into follicles T follicular helper cell migration into follicles |
| CXCL14 | BRAK, Bolekine | unknown | T cell, monocyte, B cell | Monocyte and dendritic cell migration |
| CXCL15 | Lungkine, WECHE | unknown | Neutrophil, epithelial cell, endothelial cell | Unknown |
| CXCL16 | Sexckine | CXCR6 | Activated T cell, NKT, endothelial cells | Macrophage scavenger receptor |
| CX3C family | ||||
| CX3CL1 | Fractalkine, Neurotactin, ABCD-3 | CX3CR1 | Activated T cell, monocyte, neutrophil, NK, immature dendritic cell, mast cell, astrocytes, neurons, microglia | T cell, NK and macrophage recruitment |
SCM-1α single C motif-1α, ATAC activation-induced T cell-derived and chemokine-related cytokine, NK natural killer cell, Th helper T cell, MCP-1 Monocyte chemoattractant protein-1, MIP-1α Macrophage inflammatory protein-1α, HIV human immunodeficiency virus, RANTES Regulated on Activation Normal T Cell Expressed and Secreted, MCP-4 Monocyte chemoattractant protein-4, HCC-1 hemofiltrate CC chemokine-1,TARC T cell and activation-related chemokine, ELC Epstein-Barr virus-induced receptor ligand chemokine, GALT gastrointestinal-associated lymphoid tissue, LARC liver and activation-related chemokine, SLC secondary lymphoid tissue chemokine, MDC macrophage-derived chemokine, MPIF-1 myeloid progenitor inhibitory factor-1, MPIF-2 myeloid progenitor inhibitory factor2, TECK thymus-expressed chemokine, NAP-3 neutrophil activation peptide-3, KC keratinocyte chemoattractant, ENA-78 epithelial cell-derived neutrophil-activating factor-78 amino acids, GCP-2 granulocyte chemoattractant protein-2, Mig monokine-inducd by γ-interferon, IP-10 γ-interferon-inducible protein 10, SDF-1α/β stromal cell-derived factor, BLC B-lymphocyte chemokine
Curcumin (diferuloylmethane) is a natural polyphenol from the herb, turmeric. The chemical formula and chemical name are C21H20O6 and 1, 7-bis-(4-hydroxy-3-methoxyphenyl-hepta-1, 6-diene-3, 5-dione, respectively (Shatadal Ghosh et al. 2015; Prasad et al. 2014; Yadollahi and Zargaran 2019), respectively. A molecule of curcumin has a symmetric structure, including two parallel aromatic rings and O-methoxy phenolic groups that are bound to a carbon linker, which has an α, β-unsaturated β-diketone moiety (Yu et al. 2019). Curcumin is the major component of Curcuma longa rhizome (turmeric; Zingiberaceae), a perennial plant, which has been used as a spice in cooking and used in traditional Chinese and Indian natural medicine therapy for centuries. In traditional Asian medicine, curcumin is used to treat various disorders, including rheumatism, liver disorders, insect bites, cough, sinusitis, and anorexia (Bahrami et al. 2019; Mohammad Mohajeri et al. 2018). Curcumin is a safe phytochemical that elicits a wide range of biological actions in the human body. It shows anti-inflammatory, antioxidant, lipid-modulating, anti-thrombotic, immunomodulatory, hepatoprotective, anti-diabetic, anti-tumor, and neuroprotective actions (Qadir et al. 2016; Bavarsad et al. 2019; Ghasemi et al. 2019; Iranshahi et al. 2009; Panahi et al. 2017b; Parsamanesh et al. 2018; Sahebkar and Henrotin 2016; Alidadi et al. 2020; Heidari et al. 2022; Vahedian-Azimi et al. 2022; Mohammed et al. 2021).
In addition, curcumin influences various cells and molecular targets like growth factors, cytokines/chemokines, hormones, transcription factors, cell adhesion molecules, protein kinases, redox state enzymes, and receptors (Esatbeyoglu et al. 2012; H. Zhou et al. 2011; Mashayekhi-Sardoo et al. 2021; Mohajeri et al. 2020; Soltani et al. 2021; Ganjali et al. 2017b; Momtazi-Borojeni et al. 2018). The anti-inflammatory effects of curcumin are mediated by downregulation in the activity of cyclooxygenase-2 (COX-2), lipoxygenase (LOX), inflammasome, and inducible nitric oxide synthase (iNOS) (Goel et al. 2008b, 2008a; Hassanzadeh et al. 2020). Moreover, curcumin also inhibits IL-2, IL-6, IL-8, IL-12, TNF-α, macrophage inhibitory protein (MIP), and the production of monocyte chemoattractant protein-1 (MCP-1) pro-inflammatory cytokines and chemokines (Abe et al. 1999), as well as down-regulates mitogen-activated and janus kinases (Natarajan and Bright 2002; Siwak et al. 2005). These biological effects appear to be achieved by curcumin-mediated inhibition of NF-κB (Surh et al. 2001; Zhong et al. 2012; Lee et al. 2005; Ji et al. 2009; Zhao et al. 2014).
The current review discusses curcumin's anti-inflammatory and regulatory effects on chemokines and chemokine receptors in various in vitro and in vivo (human and experimental animal models) studies in different medical conditions and diseases.
Inhibitory effects of curcumin on chemokines
CCL2 (MCP-1) (Fig. 1)
Fig. 1.
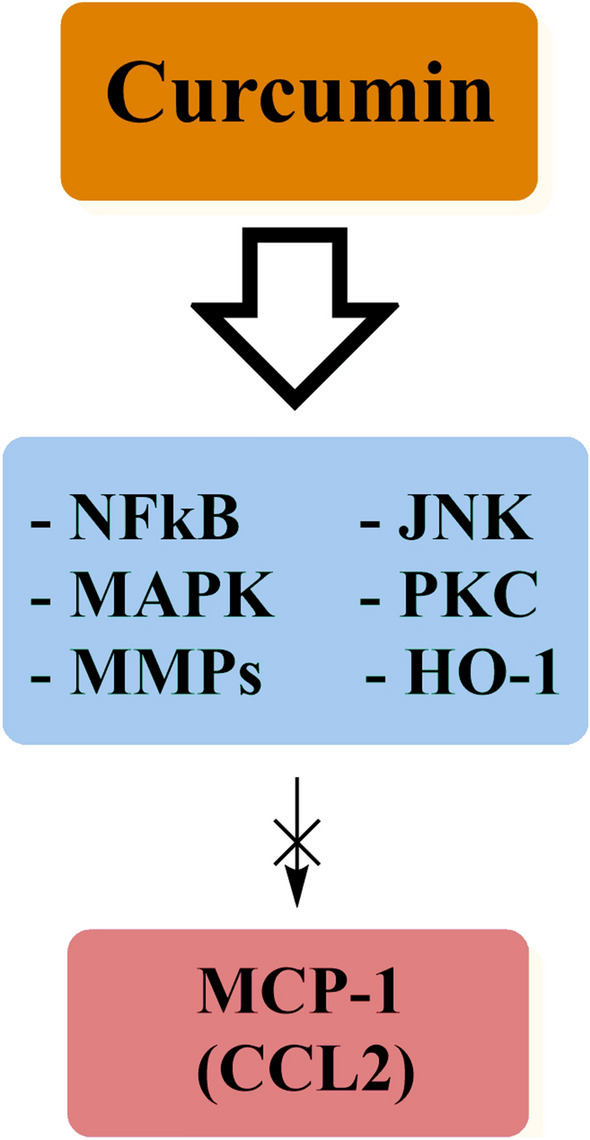
Various molecular targets through which curcumin inhibits MCP-1
CCL2 or monocyte chemoattractant/chemotactic protein-1 (MCP-1) is the major chemotactic protein for monocytes (Mohammadi et al. 2019). Inhibition of CCL2 function may reduce immune cell attraction to inflammation sites and slow the inflammatory response's progression. Curcumin was shown to inhibit MCP-1 production in different cell types and attenuate monocyte recruitment (Abe et al. 1999; Young et al. 2014; Karimian et al. 2017b; Liu et al. 2014; Huang et al. 2016; Jain et al. 2009; Tu et al. 2012; Pan et al. 2013a). In addition, studies with animal models have shown that curcumin reduces MCP-1 expression and alleviates inflammatory disorders, although human studies are limited (Maryam Saberi Karimian et al. 2017a).
Most preclinical studies have reported that curcumin exerts its effects by regulating the MAPK and NF-κB signaling pathways (Panahi et al. 2017a; Antoine et al. 2013; Nagaraju et al. 2015; Zhao et al. 2015; Kim et al. 2005; Cao et al. 2015; Bukhari et al. 2014; Mimche et al. 2012; Chung et al. 2012). Zhang et al. reported that curcumin could reduce the mRNA expression of LPS-induced CCL2 in the rat C6 astrocytoma cell line in a dose-dependent manner through inhibition of the JNK signaling pathway (Zhang et al. 2012). However, Herman and colleagues suggested that CCL2 activity could be inhibited via protein kinase C (PKC) and matrix metalloproteinases (MMP) (Herman et al. 2009). Liu et al. reported that curcumin inhibited ox-LDL-induced MCP-1 expression in macrophages via the NF-κB and JNK pathways (Liu et al. 2014). Zhong et al. evaluated the effects of curcumin on LPS-induced MCP-1 production in the RAW264.7 macrophage cell line and demonstrated that curcumin reduced MCP-1 expression in a concentration-dependent manner. These authors showed that this effect occurred due to upregulation in the expression of heme oxygenase-1 (HO-1), which inhibits the production of LPS-induced reactive oxygen species (ROS) (Zhong et al. 2013). Bao et al. reported that curcumin reduced LPS-induced MCP-1 expression in the fetus-derived glomerular mesangial cells (Bao et al. 2003).. In addition, Tham and colleagues showed that curcumin could downregulate CCL2 expression in human umbilical vein endothelial cells (HUVEC) at concentrations lower than 12.5 μM after 24 h of exposure to curcumin (Tham et al. 2015). Moreover, Ziaei et al. reported that concentrations of curcumin lower than 12.5 μM were able to significantly inhibit TNF-α-induced expression of MCP-1 in HUVECs after 3 h of exposure (Ziaei et al. 2015). Meng et al. also showed that curcumin pre-treatment (3–50 μM/L) could attenuate the LPS-induced expression of MCP-1 in a dose-dependent manner in vascular smooth muscle cells (VSMCs) (Meng et al. 2013). These studies demonstrate the universal CCL2-inhibitory effects conferred by curcumin.
CXCR4/CXCL12
CXCR4 and its ligand, CXCL12, or stromal cell-derived factor-1 (SDF-1), are involved in the development of hematopoietic, endothelial, and nervous system tissues and regulate the migration, homing, and survival of progenitor cells during embryogenesis. In addition, the CXCR4/CXCL12 axis plays a key role in HIV infection, stem cell mobilization, autoimmune disorders, cancers, and tissue regeneration (Shishodia 2013; Peled et al. 2012). Several studies have shown that curcumin induces CXCR4 expression in the follicular lymphoma cell line (Skommer et al. 2007) and mediates the migration of retinal endothelial cells in humans (Sameermahmood et al. 2008). In addition, curcumin has been shown to impair the expression of CXCL12 and decrease select proteins in human tumor cell lines. Interestingly, using an orthotropic mouse model, it has been demonstrated that curcumin-mediated inhibition of CXCL12 expression serves to sensitize colorectal cancer cells to capecitabine by modulating the expression of CXCR4 (Kunnumakkara et al. 2009).
CCR7/CCL21
Fu et al. conducted a study to evaluate the effects of curcumin on healthy human circulatory leukocyte-derived fibrocytes and reported that curcumin treatment (20 μM for 72 h) significantly reduced CCR7 expression (Fu et al. 2015). It appears that the differentiation and migration of human circulatory fibrocytes occurs via regulation of the CCR7/CCL21 axis, in particular, reduction in the expression of CCR7 (Sun et al. 2017).
Chemokine-based therapeutic effects of curcumin (Table 2)
Table 2.
Chemokine-based therapeutic effects of curcumin in experimental models
| Curcumin dose/concentration | Type of model | Findings | Ref | |||
|---|---|---|---|---|---|---|
| Inflammatory diseases | Nervous system | In vitro models | 0, 2.5 and 5 μM | LPS-induced U373-MG human astrocyte cell line as a model of MS | The MCP-1 expression ↓ | (Seyedzadeh et al. 2014) |
| 1, 5, 10, 15 and 20 μM for 24 h | Mouse N9 microglial cells | HIV-1 gp120-induced expression of MCP-1 ↓ | (Guo et al. 2013) | |||
| 10 and 25 μM curcumin 30 min prior to LPS treatment | LPS-treated microglial cells and astrocytes | The LPS-induced expression of MCP-1 ↓ | (Chen et al. 2015) | |||
| 40 mg/kg |
Rat model of acute SCI LPS-challenged astrocytes |
RANTES expression ↓ | (M. S. Lin et al. 2011) | |||
| 100 mg/kg for 14 days | Rat model of sciatic nerve CCI |
NF-κB p65 and CX3CR1 in the spinal cord and DRG ↓ CCI-induced neuropathic pain ↓ |
(H. Cao et al. 2014) | |||
| In vivo models | 100 mg/kg | PTZ-induced rat model of chronic epilepsy |
Glial cell activation ↓ MCP-1 expression in the hippocampus and cortex ↓ Cognitive deficits ↓ |
(Kaur et al. 2015) | ||
|
20 mg/kg PLGA-encapsulated Cur-NPs |
Mouse model of EBI after experimentally-induced SAH | The expression of MCP-1, MIP-2, and CINC-1 ↓ | (Z. Y. Zhang et al. 2017) | |||
| 100 mg/kg | Both in vitro experiments and an in vivo rat model |
The expression of MCP-1, CXCL10, and RANTES ↓ Infiltration of T cells and macrophages ↓ Glial scar formation ↓ Inflammation-induced fibrosis ↓ |
(Yuan et al. 2017) | |||
| 50, 100 and 200 mg/kg for 10 days | Arthritic pain in a rat model of spinal cord inflammation |
Activation of glial cells ↓ Production of MCP-1 and MIP-1α inflammatory mediators in the spinal cord ↓ |
(Chen et al. 2015) | |||
| 0.8 g/kg for 12 weeks | p25Tg transgenic mice as experimental animal model for AD |
Neuro-inflammation and neurodegeneration ↓ Production of MIP-1α ↓ |
(Sundaram et al. 2017) | |||
| Liver | In vivo models | 100 mg/kg | Rat model of metabolic and chemical-induced NASH |
CX3CL1 ↑ RANTES ↓ |
(Pickich et al. 2019) | |
| 200 mg/kg for 4 weeks |
Mouse model of CCl4-induced liver fibrosis (in vivo) |
MCP-1 and CCL7 ↓ Infiltration of Ly6Chigh monocytes in the liver ↓ |
(X. A. Zhao et al. 2018) | |||
|
RAW264.7 cells (in vitro) | ||||||
| 200 mg/kg for 6 weeks | Rat model of CCl4-induced liver fibrosis |
MCP-1 expression ↓ Liver injuries ↓ |
(Tu et al. 2012) | |||
| 50 mg/kg for 8 weeks | Rat model of CCl4-induced liver fibrosis |
CXCL12/CXCR4 biological axis ↓ Hepatic stellate cell activation and migration ↓ |
(Qin et al. 2018) | |||
| 200 mg/kg | Murine model of ConA-induced hepatitis |
CXCL10 ↓ Disease severity ↓ |
(Tu et al. 2011) | |||
| Cardiovascular disorders | In vitro models | 0.1, 1, 5 and 10 μM curcumin + 0.1, 0.5, 1 and 5 μM luteolin | TNF-α-induced vascular inflammation in EA.hy926 human endothelial cell line | MCP-1 expression ↓ | (L. Zhang et al. 2019) | |
| combined curcumin (500 mg/kg) and luteolin (500 mg/kg) therapy for 1 week | Mouse aortic endothelial cells | |||||
| Not mentioned | oxLDL-treated macrophages | MCP-1 expression ↓ | (T. Liu et al. 2014) | |||
| Kidney diseases | In vivo models | 1 and 5 mg/kg for 3 days |
Murine renal cells (in vivo) Human HK-2 renal tubular epithelial cell line (in vitro) |
MCP-1 and CXCL8 expression ↓ | (F. Zhong et al. 2011) | |
| 30 mg/kg over a 5-week period | Factor-H-deficient mice | MCP-1 expression ↓ | (Jacob et al. 2013) | |||
| 100 mg/kg/day for 8 weeks | STZ-induced diabetic nephropathy rat model |
Levels of inflammatory mediators (i.e., MCP-1) ↓ Macrophage recruitment into the renal tissue ↓ |
(Soetikno et al. 2011) | |||
| 120 mg/kg for 5 days both as pre-treatment and post-treatment | Rat model of cisplatin-induced nephrotoxicity |
Pre-treatment; the levels of the CXCL8 chemokine ↓ and inflammation and toxicity ↓ Post-treatment; no positive effects |
(Kumar et al. 2017) | |||
| 100 mg/kg with/without cisplatin | Mice with cisplatin-induced nephrotoxicity | The expression of MCP-1 ↓ | (Ueki et al. 2013) | |||
| Lungs | In vitro models | Not mentioned | Hydrogen peroxide-treated human A549 alveolar epithelial cells | IL-8 and ROS production ↓ | (Biswas et al. 2005) | |
| 100 μM for 24 h | AEC II isolated from the rat model of COPD | IL-8, MCP-1, and MIP-2α inflammatory mediators ↓ | (Gan et al. 2016) | |||
| Not mentioned | BEAS-2B human bronchial epithelial cell line | Ovalbumin and IL-4-induced MCP-1 overexpression ↓ | (Zhu et al. 2019) | |||
| In vivo models | 0.2, 0.5, 1 and 2% w/w for 7 days as pre-treatment | NTHi lysate exposured mice |
KC expression in BALF ↓ Neutrophil recruitment to the lungs ↓ |
(Moghaddam et al. 2009) | ||
| 75, 150 and 300 mg/kg | Murine model of bleomycin-induced ALI | CXCL1, CXCL5 and CXCR12 in the lungs ↓ | (Gouda and Bhandary 2018) | |||
| Not mentioned | Mouse model of acute LPS-induced lung injury | MIP-2 in the BALF ↓ | (J. Kim et al. 2016) | |||
| 50 mg/kg | Murine model of staphylococcus aureus-induced ALI |
MCP-2 and KC levels ↓ Infiltration of neutrophils into lung tissue ↓ |
(Xu et al. 2015) | |||
| 50 mg/ kg for 5 days as prophylaxis | mice with viral-induced ARDS | MCP-1 expression in both in inflammatory infiltrates and lung tissue ↓ | (Avasarala et al. 2013) | |||
| Not mentioned | Murine model of chronic asthma | Ovalbumin and IL-4-induced MCP-1 overexpression ↓ | (Zhu et al. 2019) | |||
| 5 mg/kg | Mouse model of ovalbumin-induced chronic asthma |
CCL11 expression ↓ Fibrosis ↓ |
(Chauhan et al. 2017) | |||
| 20 and 100 mg/kg | Mouse model of ovalbumin-induced allergic asthma | Eotaxin expression ↓ | (Shahid et al. 2019) | |||
| Bowel diseases | In vitro models | 50 μg/mL | CEC |
Mucosal infiltration of neutrophils ↓ MIP-2, KC, and MIP-1α expression and secretion ↓ |
(Larmonier et al. 2011) | |
| 50 μM | T-84 human colorectal carcinoma cells and young adult mouse colonocytes (YAMC) | The colonic expression of CXCL9, CXCL10, and CXCL11 ↓ | (Midura-Kiela et al. 2012) | |||
| In vivo models | 0.2% w/w nanoparticle curcumin 7 days before model induction | DSS-induced murine model of experimental colitis | Mucosal expression of CXCR1 and CXCR2 ↓ | (Ohno et al. 2017) | ||
| Joints | In vitro models | 1–20 μM liposomal curcumin (Lipocurc™) | SW982 human synovial fibroblast and RAW264 murine macrophage cell line | The expression of inflammatory chemokines ↓ | (Kloesch et al. 2016) | |
| 3 μM; pre-treatment for 30 min | human OASFs | The MCP-1-induced VCAM-1 expression ↓ | (Y. M. Lin et al. 2012) | |||
| Others | In vivo models | 0.3 and 1 μM curcumin and its analog, CUR-Br before induction | TPA-induced ear edema murine mode (in vivo) | KC/CXCL1 ↓ | (Rakariyatham et al. 2019) | |
| 10 μM | Murine model of RP (in vivo) |
Progression of retinal degeneration ↓ Activation of microglia cells ↓ following the CCL2 expression inhibition |
(Y. Wang et al. 2017b) | |||
| 6, 30 and 60 μg/kg | New Zealand white rabbits (in vivo) | Improved the wound healing process via the significant ↓ in IL-1, IL-6, and IL-8 | (Jia et al. 2014) | |||
| 50 μM | LPS-stimulated neutrophils (in vitro) |
Neutrophil infiltration ↓ MIP-1α, MIP-1β, IL-8, and GRO-α expression ↓ |
(Antoine et al. 2013) | |||
| Murine model of air pouch inflammation (in vivo) | ||||||
| Infectious diseases | Bacterial infections | In vitro models | Not mentioned | HMEECs | CXCL5 expression ↓ | (Konduru et al. 2016) |
| 200 μM for 5 to 60 min | Live Moraxella catarrahalis bacteria-exposed Detroit 562 pharyngeal cell line as in vitro model of mucositis | The release of IL-8 and MCP-1 ↓ | (Lüer et al. 2012) | |||
| In vivo models | 500 mg/kg for 6 and 18 weeks | Mouse model of H. pylori-induced infection | CCL20, CCL5, CXCL1, CXCL10, CXCL11, and CCL25 expression ↓ | (Santos et al. 2015) | ||
| 50 mg/kg for 1 h before and 1 h after induction | Murine model of NTHi-induced OM | CXCL5 expression ↓ | (Konduru et al. 2016) | |||
| Viral infections | In vivo models | PLGA-encapsulated curcumin | HSV-2-infected mice |
Severity of HSV-2 infection and risk for HIV ↓ The production of MCP-1 and other inflammatory mediators ↓ |
(Vitali et al. 2020) | |
| In vitro models | Pre-treatment with 5 or 50 μM | gp-120- exposed GECs | CXCL8, RANTES, and IP-10 expression ↓ | (Ferreira et al. 2015) | ||
| Cancer | In vitro models | 30 μM | Human PC3 prostate cancer cell line |
Adhesion, invasion and motility ↓ CCL2 activity ↓ |
(Herman et al. 2009) | |
| 25 μM | MDA-MB-231 human metastatic breast cancer cell line and human primary mammary cancer-derived cells | CXCL1 and CXCL2 expression ↓ | (Kronski et al. 2014) | |||
| 15 μM | Human PC3 prostate cancer cell line | CXCL1 and CXCL2 expression ↓ | (Killian et al. 2012) | |||
| Oxaliplatin and curcumin | Primary colorectal cancer cells with liver metastasis-derived cells | CXCL1 expression ↓ | (Ruiz de Porras et al. 2016) | |||
| 5–40 μM for 24 h | SW620 colorectal cancer cell line | NKD2-Wnt-CXCR4 signaling pathway ↓ | (Z. Zhang et al. 2016) | |||
| 10, 25 and 50 μM for 24, 48 and 72 h | SKOV3 human ovarian cancer cell line | CXCL12 and CXCR4 expression ↓ | (Xiaoling et al. 2010) | |||
| In vivo models | Curcuminoids formulated for 8 weeks | Patients with solid tumors | Serum levels of MCP-1 ↓ | (Panahi et al. 2014a) | ||
| 500 mg, three times a day for 4 weeks | Male patients with pulmonary complications arising from sulfur mustard intoxication | MCP-1 ↓ | (Panahi et al. 2015b) | |||
| FOLFOX + 2 g/day curcumin | Colorectal cancer patients | No significant changes in the serum levels of CXCL1 following treatment | (Howells et al. 2019) | |||
| 50 mg/kg for 4 days | Murine model of colon cancer | CXCL1 and CXCL2 expression ↓ | (Sakai et al. 2016) | |||
| Topical curcumin (2 μM) and urosilic acid combined therapy | Murine model of skin tumor | CXCL2 expression ↓ | (Tremmel et al. 2019) | |||
| Curcumin gum formulation (30 min chewing and packing) | Human healthy volunteers | Serum CXCL1 levels ↓ | (Boven et al. 2019) | |||
| Curcumae radix extract (CRE) | Murine model of breast cancer metastasis (MMTV-PyMT transgenic mice) (in vivo) |
CCR7 expression ↓ Motility and cellular migration ↓ |
(Kaya et al. 2019) | |||
| MCF7 cells (in vitro) | ||||||
| 500 mg capsule form of phytosomal curcumin (CCP) | Murine model of GBM |
MCP-1 expression in TAM ↑ Destruction of GBM cells ↑ |
(Mukherjee et al. 2018) | |||
| Pregnancy | In vivo models | 100 μg/kg | LPS-induced adverse pregnancy outcomes in a mouse model | MIF, MCP-1, and MIP-1 expression ↓ | (J. Zhou et al. 2017) | |
| 0.36 mg/kg | LPS-induced pre-eclampsia in a rat model |
MCP-1 expression ↓ Blood pressure ↓ Concentration of urinary proteins ↓ |
(Gong et al. 2016) | |||
| In viro models | 1, 5, 10, 20 and 40 μg/ml for 24, 48 and 72 h | EESC cells | MCP-1 and RANTES expression ↓ | (Chowdhury et al. 2019) | ||
LPS lipopolysaccharide, MS multiple sclerosis, PTZ pentylenetetrazole, PLGA poly lactide-co-glycolide, Cur-NPs curcumin nanoparticles, EBI early brain injury, SAH subarachnoid hemorrhage, CINC-1 chemokine-induced neutrophil chemoattractant-1, CCI chronic constrictive injury, DRG dorsal root ganglion, SCI spinal cord injury, AD Alzheimer's disease, NASH non-alcoholic steatohepatitis, CCl4 carbon tetrachloride, ConA Concavalin A, oxLDL Oxidized low-density lipoprotein, STZ streptozotocin, ROS reactive oxygen species, KC keratinocyte chemoattractant, BALF broncho-alveolar lavage fluid, COPD Chronic obstructive pulmonary disease, NTHi typeable Heamophilus influenza, AEC II alveolar epithelial cell type II, ALI acute lung injury, ARDS acute respiratory distress syndrome, CEC colonic epithelial cells, DSS dextran sodium sulfate, YAMC young adult mouse colonocytes, OASF osteoarthritis synovial fibroblasts, CUR-Br 7 7’-bromo-curcumin, TPA 12-O-tetradecanoylphorbol-13-acetate, RP retinitis pigmentosa, H. pylori Helicobacter pylori, OM otitis media, HMEEC human median ear epithelial cell, HSV-2 herpes simplex virus-2, HIV human immunodeficiency virus, GEC gp-120- exposed genital epithelial cell, FOLFOX folinic acid/5-fluorouracil/oxaliplatin chemotherapy, HNSCC head and neck squamous cell carcinoma, CRE curcumae radix extract, CCP phytosomal curcumin, GBM glioblastoma, TAM tumor-associated macrophage and microglia, EESC eutopic endometrium-derived stromal cells
Inflammatory diseases
Nervous system (Fig. 2)
Fig. 2.
Chemokine-based therapeutic effects of curcumin on nervous system
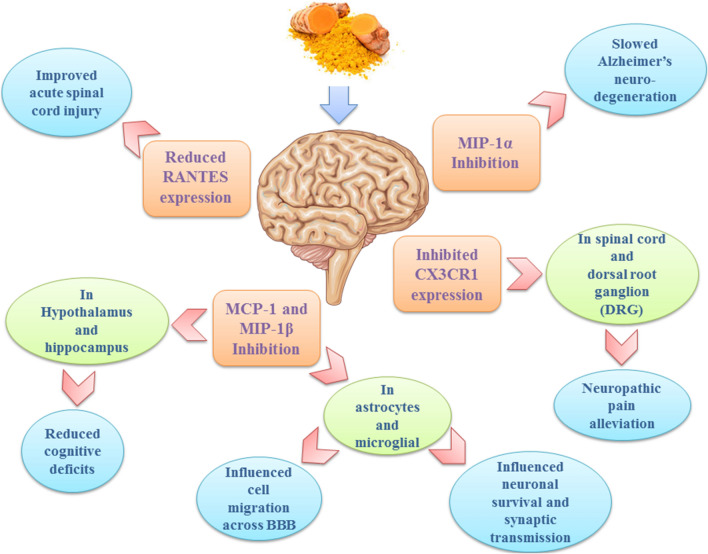
Astrocytes are the most abundant glial cells in the central nervous system (CNS) and are involved with maintaining hemostasis and neuronal function (Röhl et al. 2007; Farina et al. 2007). Astrocytes influence the migration of immune cells across the blood–brain barrier (BBB) via MCP-1 expression (Ransohoff et al. 2003). Curcumin has been documented to significantly reduce the production of pro-inflammatory cytokines and chemokines, including MCP-1 and MIP-1β, in astrocytes and microglial cells through the inhibition of JNK phosphorylation (Qureshi et al. 2018; Chen et al. 2015; Zhang et al. 2012). Seyedzadeh and colleagues evaluated the immunomodulatory effects of curcumin on the LPS-induced U373-MG human astrocyte cell line as an in vitro model of multiple sclerosis and demonstrated that curcumin could attenuate the expression of the MCP-1, which serves as the key chemoattractant for recruitment of immune cells into the CNS (Seyedzadeh et al. 2014). In addition, Guo et al. reported that curcumin pre-treatment could attenuate the production of MCP-1 in the mouse N9 microglial cell line (Guo et al. 2013).
Following brain injury, microglial cells and astrocytes release inflammatory cytokines and chemokines that influence neuron survival and synaptic transmission, as well as increase brain excitability in epilepsy (Solito and Sastre 2012; Walker and Sills 2012; Vezzani et al. 2012). Kaur et al. evaluated the effects of curcumin on glial cell activation and the reduction in cognitive deficits in the pentylenetetrazole (PTZ)-induced rat model of chronic epilepsy and showed that 100 mg/kg of curcumin significantly inhibited glial cell activation. In addition, the expression of MCP-1 was reduced in the hippocampus and cortex with improved cognitive (Kaur et al. 2015). Furthermore, Zhang et al. evaluated the anti-inflammatory effects of poly (lactide-co-glycolide) (PLGA)-encapsulated curcumin nanoparticles (Cur-NPs) in subarachnoid hemorrhage-induced BBB disruption. Using a mouse model of early brain injury (EBI) after experimentally-induced subarachnoid hemorrhage (SAH), these authors demonstrated that the expression of inflammatory chemokines, including MCP-1, MIP-2, and CINC-1 (chemokine-induced neutrophil chemoattractant-1), were significantly reduced following treatment with 20 mg/kg nano-curcumin suggesting that curcumin conferred protective effects in SAH (Zhang et al. 2017).
Spinal cord injury leads to glial scar formation by astrocytes, which severely hinders neuron regeneration. Using both in vitro experiments and an in vivo rat model, Yuan et al. reported that curcumin inhibits glial scar formation and inflammation-induced fibrosis. The authors demonstrated that curcumin downregulated the expression of MCP-1, CXCL10, and RANTES chemokines, as well as reduced the infiltration of T cells and macrophages by inhibiting NF-κB signaling in astrocytes (Yuan et al. 2017).
Chen et al. evaluated the anti-inflammatory effects of curcumin on arthritic pain in a rat model of spinal cord inflammation and demonstrated that oral treatment with curcumin attenuated both the activation of glial cells and the production of MCP-1 and MIP-1α inflammatory mediators in the spinal cord. Additionally, it was shown by these authors that the LPS-induced expression of MCP-1 was significantly reduced in cultured microglial cells and astrocytes (Chen et al. 2015). In another study using an experimental rat model, Cao and colleagues evaluated the effects of curcumin on pain threshold and NF-κB and CX3CR1 expression following sciatic nerve chronic constrictive injury (CCI). Cao et al. reported that curcumin significantly reduced the elevated expression of NF-κB p65 and CX3CR1 in the spinal cord and dorsal root ganglion (DRG) and alleviated CCI-induced neuropathic pain (Cao et al. 2014). In another study, Lin et al. showed that curcumin reduced the expression of RANTES both in a rat model of acute spinal cord injury (SCI) and LPS-challenged astrocytes in vitro (Lin et al. 2011).
To evaluate the therapeutic effects of curcumin on neuroinflammation and neurodegeneration in Alzheimer's disease (AD), Sundaram et al. treated p25Tg transgenic mice (which overexpress p25 and act as an experimental animal model for AD) and demonstrated that curcumin efficiently counteracts glial cell activation and the production of inflammatory cytokines and chemokines such as MIP-1α. These authors convincingly demonstrated that curcumin inhibited p25-mediated neuroinflammation and the progression of neurodegeneration in this experimental animal model of AD (Sundaram et al. 2017).
Th17 lymphocytes can migrate across the BBB and induce the recruitment of immune cells, which leads to inflammation in the CNS (Xie et al. 2011; Kebir et al. 2007). IL-17, the major cytokine secreted by Th17 cells, induces the production of CXCL1, CXCL2, and CXCL8/IL-8 chemokines and their receptors (i.e., CXCR1 and CXCR2). These chemokines and their receptors are involved in the recruitment of lymphocytes and monocytes from the circulation into the CNS and, therefore, have been suggested to play a critical role in the pathogenesis of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). Additionally, CXCL8 is involved with the infiltration of neutrophils (Carlson et al. 2008). Using the well-established EAE animal model, curcumin treatment was shown to decrease the recruitment and differentiation of inflammatory cells, especially Th17, in the CNS (Xie et al. 2009; Natarajan and Bright 2002). It would appear that these inhibitory functions result from the downregulation of NFκB and disruption of CXCL1 and CXCL2 signaling pathways (Bachmeier et al. 2008; Choi et al. 2010).
Liver
Potential antioxidant, anti-inflammatory, and antifibrogenic effects of curcumin have been suggested to account for its beneficial hepatoprotective properties (Ghosh et al. 2011). All of these effects result from the pleiotropic and multi-target activity of curcumin. Accordingly, the numerous effects of curcumin on multiple organs and signaling pathways serve to regulate a wide variety of biochemical processes and influence the expression of different genes involved in liver health and homeostasis (Vera‐Ramirez et al. 2013). We now present multiple lines of evidence that highlight the hepatoprotective effects of curcumin via chemokine-dependent processes.
Pickich et al. evaluated the effects of curcumin on the serum levels of cytokines and chemokines in a rat model of metabolic (i.e., Western diet (WD)) and chemical (i.e., carbon tetrachloride (CCl4))-induced non-alcoholic steatohepatitis (NASH). They reported that curcumin treatment resulted in a 121% increase in Fractalkine (or CX3CL1, which may have an anti-inflammatory and anti-fibrotic role in the liver) (The levels of RANTES or CCL5 that has increased expression in hepatocytes in both toxic and diet-induced liver injury and is associated with the pathogenesis and progression of non-alcoholic fatty liver disease was decreased by 22% Kirovski et al. 2010; Seki et al. 2009).
Ly6Chigh monocytes facilitate liver fibrosis by inducing the production of pro-inflammatory and pro-fibrotic cytokines and chemokines (Pellicoro et al. 2014). MCP-1 and CCL7 are major players in the recruitment and migration of Ly6Chigh monocytes (Brempelis and Crispe 2016). Zhao et al. reported that the mRNA expression of both MCP-1 and CCL7 was dramatically increased in a mouse model of CCl4-induced liver fibrosis and that treatment with curcumin significantly reduced the expression of these inflammatory chemokines and decreased the infiltration of Ly6Chigh monocytes in the liver. Moreover, these same authors noted that RAW264.7 cells co-cultured with curcumin resulted in a significant decrease in the expression of MCP-1 and CCL7. Interestingly, macrophages were induced to the M1 phenotype in a dose-dependent manner (Zhao et al. 2018). In another study, Tu et al. demonstrated that curcumin treatment (200 mg/kg for 6 weeks) in a rat model of CCl4-induced liver fibrosis significantly reduced liver injuries due to MCP-1 and the inhibition of other mediators of inflammation (Tu et al. 2012).
It has been reported that curcumin influences various chemokines and exerts protective effects in the liver. Qin et al. reported the anti-fibrotic effects of curcumin in liver fibrosis via a dose-dependent inhibition of the CXCL12/CXCR4 biological axis, which leads to blockage of hepatic stellate cell activation and migration (Qin et al. 2018). Furthermore, using a murine model of Concavalin A (ConA)-induced hepatitis, Tu et al. demonstrated that pre-treatment with 200 mg/kg curcumin reduced the levels of inflammatory mediators, including the CXCL10 chemokine, and alleviated disease severity (Tu et al. 2011).
Cardiovascular disorders
The cardiovascular protective effects of curcumin have been demonstrated in recent studies. Curcumin has previously been shown to ameliorate cardiac fibrosis, atherosclerosis, and myocardial ischemia and infarction, as well as provide anti-inflammatory, antioxidant, and anti-apoptotic properties in various cardiovascular-related disorders (Pourbagher-Shahri et al. 2021; Li et al. 2020). There is an association between cardiovascular disease (CVD) and secreted cytokines, which serve as biomarkers of primary-phase inflammation (Karimian et al. 2017b). In other words, elevated chronic inflammation plays a key role in the development and progression of CVD (Ruparelia et al. 2017). Increased levels of inflammatory mediators lead to the simultaneous activation and adhesion of monocytes to the endothelium and the uptake of oxidized low-density lipoproteins (oxLDL). These processes facilitate the migration of monocytes to the sub-endothelial space and induce the proliferation of smooth muscle and foam cells and, subsequently, the formation of plaque (Ganjali et al. 2017a). MCP-1 and CXCL8/IL-8 chemokines are considered essential mediators in these events. Zhang et al. evaluated the effects of combined curcumin and luteolin therapy on the synergistic inhibition of TNF-α-induced vascular inflammation in human and mouse vascular cells. They reported that MCP-1 expression was significantly reduced in human endothelial cells with umbilical vein endothelial cell properties and mouse aortic endothelial cells. They showed that this effect resulted from the inhibition of NF-κB translocation to the nucleus (Zhang et al. 2019). Additionally, Liu et al. reported that curcumin significantly reduced MCP-1 expression in oxLDL-treated macrophages by inhibiting the JNK and NF-κB pathways, which suggests that the vascular protective effects of curcumin are related to anti-inflammation and anti-atherosclerosis (Liu et al. 2014). Hence, it would appear that preparing drug formulations of curcumin that overcome obstacles such as low oral absorption and rapid metabolism might suggest its use for the treatment of CVD and CVD-associated disorders via a chemokine-dependent mechanism of action.
Kidney diseases
Different kidney disorders are often characterized by significant metabolic and nutritional disruptions that lead to oxidative stress, as well as uncontrolled and chronic inflammatory responses (de Almeida Alvarenga et al. 2018). Hence, curcumin has been proposed as an important nutritional adjuvant and therapeutic agent for various kidney-associated disorders, since it targets several signaling pathways important to kidney function.
As previously discussed, MCP-1 is essential for monocyte/macrophage infiltration and may play an important role in the development of tubulointerstitial fibrosis (TIF). Blocking the MCP-1/CCR2 pathway can inhibit the progression of fibrosis via the reduction in the recruitment of M1 macrophages (Wada et al. 2004; Kitagawa et al. 2004). In the rat model of unilateral ureteral obstruction (UUO), subcutaneous administration of curcumin significantly attenuated the overexpression of MCP-1 mRNA in the obstructed kidney (Jones et al. 2000). In a model of LPS-induced nephritis, the therapeutic effects of curcumin were mediated by the inhibition of MCP-1 expression and a decrease in monocyte recruitment. Zhong et al. reported that curcumin could efficiently reduce LPS-induced mRNA overexpression of MCP-1 in both murine renal cells and the HK-2 renal tubular epithelial cell line. These same authors further reported that curcumin partially controlled the secretion of MCP-1 and CXCL8 chemokines (Zhong et al. 2011). Similar downregulation of MCP-1 levels has also been reported in factor-H-deficient mice resulting from the intraperitoneal administration of curcumin (30 mg/kg) over a 5-week period (Jacob et al. 2013).
Soetinko and colleagues evaluated the effects of 100 mg/kg/day of curcumin for 8 weeks in the streptozotocin (STZ)-induced diabetic nephropathy rat model. These authors demonstrated that curcumin dramatically reduced the levels of inflammatory mediators (i.e., MCP-1) and macrophage recruitment into the renal tissue of diabetic mice. Macrophage infiltration into glomeruli leads to progressive glomerular injury and ultimately results in tubular and glomerular destruction (Soetikno et al. 2011). Similar results have been reported for a couple of curcumin derivatives (B06 and C66), which effectively reduced plasma levels of TNF-α and MCP-1 and improved renal fibrosis, histological abnormalities, and dysfunction in STZ-induced diabetic mice (Adhikary et al. 2004; Pan et al. 2013b).
Cisplatin is a chemotherapy agent for various types of cancers, and several reports have shown that high doses lead to nephrotoxicity in about 20% of patients (Yao et al. 2007). Kumar et al. evaluated the protective effects of curcumin both as pre-treatment and post-treatment in a rat model of cisplatin-induced nephrotoxicity. They reported that curcumin pre-treatment significantly reduced levels of the CXCL8 chemokine, as well as other inflammatory mediators, and attenuated inflammation and toxicity, while curcumin post-treatment showed no positive effects (Kumar et al. 2017).. In another study using the same mouse model of cisplatin-induced nephrotoxicity, Ueki and colleagues reported that curcumin treatment dramatically reduced the expression of MCP-1 and other mediators in the kidney (Ueki et al. 2013).
Lungs
Chronic obstructive pulmonary disease (COPD) is associated with bronchial damage due to injury to both the epithelial and endothelial cell layers. Elevated levels of CXCL2 and IL-8 chemokines have been reported with COPD, which induce neutrophil recruitment to the lung parenchyma and the production of both proteinases and elastases (Lelli et al. 2017; Overbeek et al. 2013; Kobayashi and DeLeo 2009). It has been shown that in hydrogen peroxide-treated human A549 alveolar epithelial cells, curcumin decreased IL-8 and the production of reactive oxygen species (ROS) (Biswas et al. 2005). Additionally, curcumin significantly reduced the levels of the keratinocyte chemoattractant (KC) chemokine (which is a murine homologuehomolog for human IL-8) in broncho-alveolar lavage fluid (BALF), and also led to the inhibition of neutrophil recruitment to the lungs (Moghaddam et al. 2009).
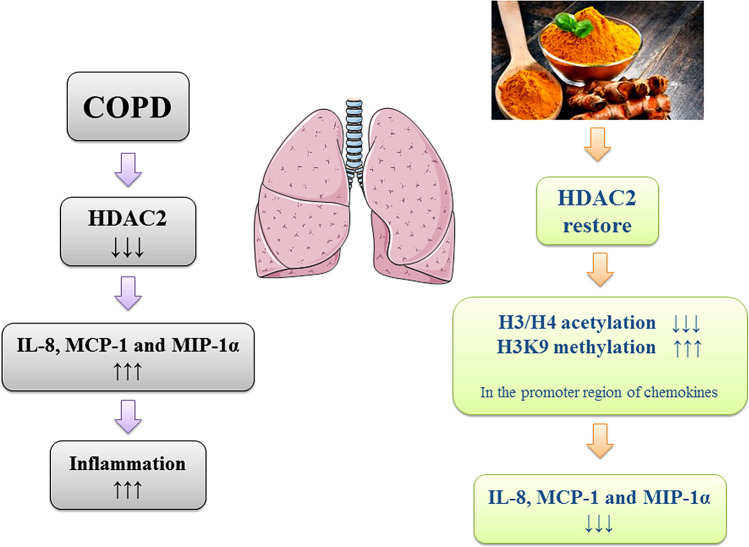
Using an experimental rat model of COPD, Gan et al. reported that the expression of inflammatory mediators, including IL-8, MCP-1, and MIP-2α, were upregulated in alveolar epithelial cells type-II (AEC-II), and that the expression of histone deacetylase-2 (HDAC2) protein was significantly reduced. Following curcumin supplementation in this model, HDAC2 expression was restored, and H3/H4 acetylation and H3K9 methylation in the promoter region of the chemokines was decreased and increased, respectively, which led to a downregulation in the expression of the aforementioned inflammatory chemokines (Gan et al. 2016) (Fig. 3).
Fig. 3.
Chemokine-based anti-inflammatory effects of curcumin in COPD
In another study, Gouda et al. investigated inflammatory pathways that are regulated by curcumin in a murine model of bleomycin-induced acute lung injury (ALI) and reported that the mRNA expression of CXCL1, CXCL5, and CXCR12 were significantly reduced in lung homogenates of curcumin-treated mice (Gouda and Bhandary 2018). Additionally, Kim and colleagues showed that curcumin dramatically reduced the expression of MIP-2 and other inflammatory mediators in the BAL fluid of mice with acute LPS-induced lung injury (Kim et al. 2016).
Xu et al. previously demonstrated that curcumin improved the respiratory condition in mice with staphylococcus aureus-induced ALI and reduced the levels of inflammatory cytokines and chemokines (e.g., MCP-2 and KC) but also decreased the infiltration of neutrophils into lung tissue (Xu et al. 2015). Additionally, Avasarala et al. have evaluated the anti-inflammatory and immunomodulatory effects of curcumin in mice with viral-induced acute respiratory distress syndrome (ARDS) and determined that curcumin prophylaxis (treatment) before the induction of inflammation significantly reduced the expression of inflammatory cytokines and chemokines (including MCP-1) in both inflammatory infiltrates and lung tissue (Avasarala et al. 2013).
Asthma is an inflammatory disorder that is characterized by pulmonary infiltration of eosinophils, neutrophils, and lymphocytes, as well as mucosal hypersecretion and airway hyper-responsiveness (AHR) (Elias et al. 2003). Importantly, MCP-1 (CCL2) is a critical inflammatory mediator in asthmatic airway epithelial cells (Hwang et al. 2017). Zhu et al. reported that curcumin significantly attenuated ovalbumin and IL-4-induced MCP-1 overexpression both in lung tissue of mice in a murine model of chronic asthma, as well as in the BEAS-2B cell line (human bronchial epithelial cells), and it appears that the inhibitory effects of curcumin were mediated by inactivation of the PPARγ-dependent NFκB signaling pathway (Zhu et al. 2019). Chauhan et al. evaluated the impact of intranasal curcumin on the inhibition of pulmonary fibrosis in a mouse model of ovalbumin-induced chronic asthma and reported that curcumin dramatically reduced the expression of eotaxin (CCL11). CCL11 binds to its specific receptor, CCR3, on eosinophils, basophils, and mast cells and leads to the recruitment of these inflammatory cells to the airways. Moreover, CCL11/CCR3 interaction plays a vital role in the pathogenesis of asthma and airway remodeling and has pro-fibrotic effects on lungs and bronchial fibroblasts (Chauhan et al. 2017). Lastly, using the mouse model of ovalbumin-induced allergic asthma, Shahid et al. reported that curcumin significantly reduced the mRNA expression of inflammatory mediators such as eotaxin (Shahid et al. 2019).
Bowel diseases
In a very interesting in vitro study using colonic epithelial cells (CECs), it was shown that curcumin (50 μg/mL) decreased mucosal infiltration of neutrophils and significantly reduced the expression and secretion of MIP-2, KC, and MIP-1α chemokines by neutrophils and CECs (Larmonier et al. 2011). Along these lines, Ohno et al. reported that curcumin nanoparticles significantly reduced the mucosal mRNA expression of inflammatory cytokines and chemokines, including CXCR1 and CXCR2, in the dextran sodium sulfate (DSS)-induced murine model of experimental colitis. These same authors also reported that there was a dramatic decrease in the infiltration of Gr-1 neutrophils into the colon mucosa in the aforementioned model of experimental colitis (Ohno et al. 2017). Finally, Midura-Kiela and colleagues reported that curcumin decreased the colonic expression of CXCL9 (Mig), CXCL10 (IP-10), and CXCL11 (I-TAC), which are all CXCR3 ligands. All three chemokines are involved in epithelial dysfunction and the pathogenesis of inflammatory bowel disease (IBD). Specifically, these chemokines are secreted by colonic epithelial cells in an IFN-γ-induced manner and are a chemoattractant for activated T-lymphocytes and NK cells, although it should be noted that curcumin inhibits IFN-γ signaling (Midura-Kiela et al. 2012).
Osteoarthritis joints
Kloesch et al. reported that a liposomal curcumin formulation could significantly reduce the expression of inflammatory cytokines and chemokines both in SW982 human synovial fibroblast and RAW264 murine macrophage cell lines (Kloesch et al. 2016). Interestingly, Lin et al. have previously shown that in the synovial fluid of osteoarthritis (OA) patients, MCP-1 production is increased and that its interaction with CCR2 leads to enhanced VCAM-1 expression in osteoarthritis synovial fibroblasts (OASFs). This overall process of increased MCP-1 and elevated VCAM-1 expression is involved in the inflammatory process in synovial fibroblasts. This same group of authors also reported that a 30-min period of curcumin pre-treatment (3 μM) could inhibit the MCP-1-induced increase in VCAM-1 expression (Lin et al. 2012).
Other inflammatory diseases
Rakariyatham et al. have demonstrated that the curcumin analog, 7, 7'-Bromo-curcumin (CUR-Br), exhibits higher chemical stability and greater anti-inflammatory effects when compared to curcumin. Using the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema murine model, these same authors also reported that CUR-Br could more efficiently reduce the expression of inflammatory cytokines and chemokines, including KC/GRO (CXCL1), which is a key factor for the recruitment of neutrophils (Rakariyatham et al. 2019).
Wang et al. evaluated the therapeutic effects of curcumin in a murine model of retinitis pigmentosa (RP) in which retinal degeneration (rd1) occurs. Using this model, they showed that curcumin could effectively inhibit the expression of inflammatory mediators such as CCL2, as well as inhibit the activation of microglia cells and, as a result, slow the progression of retinal degeneration (Wang et al. 2017b).
In a study by Jia and colleagues, the effect of intravenously-administered curcumin was assessed for its role in wound healing in New Zealand white rabbits. They demonstrated that curcumin could improve the wound healing process by significantly reducing inflammatory cytokines and chemokines, including IL-1, IL-6, and IL-8 (Jia et al. 2014).
Lastly, Antoine et al. evaluated the anti-inflammatory effects of curcumin on neutrophil activation and infiltration and showed that the expression of MIP-1α, MIP-1β, IL-8, and GRO-α chemokines were reduced in LPS-stimulated neutrophils in vitro, whereas, in the well-established murine model of air pouch inflammation, curcumin significantly decreased the production of multiple inflammatory cytokines and chemokines, including MIP-1α and MIP-1β, as well as dramatically reduced neutrophil infiltration (Antoine et al. 2013).
Infectious diseases
Bacterial infection
Recently, a number of studies have investigated various antibacterial effects of curcumin. Suppressing bacterial DNA replication, inhibiting pathogen motility, and influencing the integrity of the microbial cell membrane are among the most important antibacterial mechanisms of action (Teow et al. 2016). In addition, curcumin administration has been shown to affect the expression of specific microbial genes, such as mecA (Rai et al. 2008), as well as toxin binding activity (Na et al. 2011). In this review, we have focused on how curcumin affects both chemokine-related, as well as host-related protective mechanisms in the context of bacterial infections.
Curcumin has been suggested as a dietary supplement for preventing mucosal destruction associated with Helicobacter pylori (H. pylori) infection. Santos and colleagues evaluated the anti-inflammatory effects of curcumin on H. pylori-induced infection in an experimental mouse model and reported that curcumin significantly reduced the expression of pro-inflammatory mediators including CCL20, CCL5, CXCL1, CXCL10, CXCL11, and CCL25 in the infected mice (Santos et al. 2015).
In another study, Konduru et al. evaluated the therapeutic effects of curcumin on CXCL5 expression in both an in vivo murine model (non-typeable Haemophilus influenza (NTHi)-induced otitis media (OM)) and an in vitro model using human median ear epithelial cells (HMEECs). These authors reported that NTHi-induced CXCL5 expression was significantly reduced via direct inhibition of IκK-β phosphorylation, together with inhibition of p38 MAPK (Konduru et al. 2016).
Lastly, Luer et al. evaluated the anti-inflammatory effects of curcumin on mucositis in an in vitro model. The Detroit 562 pharyngeal cell line was exposed to live Moraxella catarrahalis bacteria. These authors showed that treatment with curcumin (200 μM) for 5 to 60 min completely inhibited the release of inflammatory cytokines and chemokines, including IL-8 and MCP-1. Moreover, they determined that repetitive exposure to curcumin led to repetitive inhibition of these mediators for 4 to 6 h (Lüer et al. 2012).
Viral infection
In addition to the antifungal and antimicrobial properties of curcumin, recent studies have shown that curcumin also possesses antiviral activity against several infections caused by a virus (Jennings and Parks 2020). Therefore, below we present the chemokine-related antiviral effects of curcumin.
Herpes simplex virus-2 (HSV-2) is a common sexually transmitted virus that induces the recruitment of HIV-targeted immune cells and is considered a risk factor for human immunodeficiency virus (HIV) infection in female genital tracts. Vitali et al. reported that intravaginal (but not oral or intraperitoneal) delivery of curcumin nanoparticles to the genital tract of mice could attenuate tissue inflammation and diminish the production of pro-inflammatory mediators, including TNF-α, IL-6, and MCP-1, and potentially lead to both a reduction in the severity of HSV-2 infection and decreased risk for HIV (Vitali et al. 2020).
In a different study, Ferreira and colleagues evaluated the anti-inflammatory potential of curcumin in genital epithelial cells (GECs) as a means to protect them from HIV-1 and HSV-2 viruses. These authors demonstrated that curcumin pre-treatment could restore the integrity of the mucosal barrier, along with inhibition of gp120-mediated upregulation of inflammatory cytokines and chemokines, which included CXCL8, RANTES, and IP-10. These are critical chemoattractants for recruiting HIV target cells to the female genital tract (FGT). Thus, it would appear that curcumin can inhibit and/or control virus proliferation in the FGT (Ferreira et al. 2015).
Cancer (Fig. 4)
Fig. 4.
Chemokine-based anti-tumor effects of curcumin
Many different studies have documented the efficacy of curcumin as an adjunct chemotherapeutic agent for a variety of cancers. Some of these cancers include breast cancer, prostate cancer, squamous cell carcinoma of the head and neck, lung cancer, and brain tumors. The proposed mechanism of action underlying the beneficial effects of curcumin in various cancers is related to the induction of apoptosis and curcumin-mediated suppression of tumor cell proliferation and invasion. The therapeutic effects of curcumin in cancer occur through a variety of cell signaling pathways (Tomeh et al. 2019). Below, we describe and summarize different studies that have been conducted to evaluate the anticancer potential of curcumin as it pertains to the regulation of chemokines.
It has been previously reported that curcumin can inhibit lung metastasis in mice (Bachmeier et al. 2007). Another study reported that curcumin supplementation for 5 days following the removal of a primary mammary tumor in mice leads to a reduced incidence of metastasis to the lungs (Aggarwal et al. 2005). Interestingly, curcumin decreases both the expression of MCP-1 and IL-1β inflammatory mediators, which are critical for tumorigenesis (Abe et al. 1999). Panahi et al. reported that curcuminoids formulated to have an increased bioavailability (since curcumin alone is poorly absorbed after oral administration) reduced the serum levels of MCP-1 in patients with solid tumors after 8 weeks of treatment (Panahi et al. 2014b). These same authors also determined that 500 mg curcumin, taken three times a day for 4 weeks, modulated the expression of MCP-1 in male patients with pulmonary complications arising from sulfur mustard intoxication (Panahi et al. 2015a).
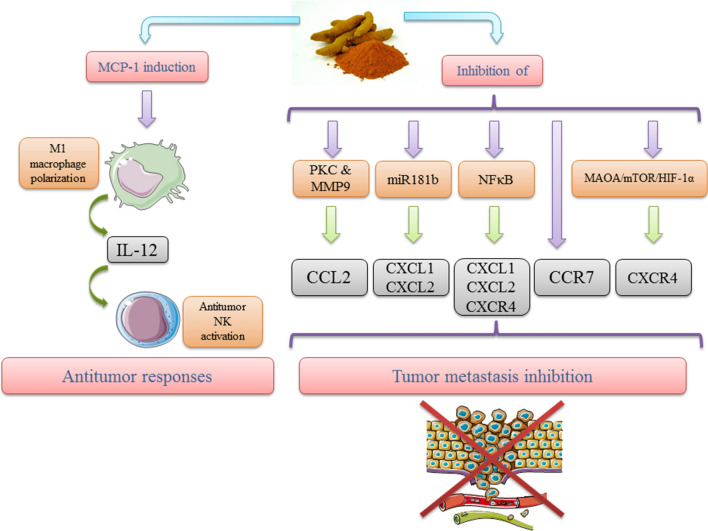
In addition, in prostate cancer, CCL2 has an important role in the development of metastasis to the bone (Bandyopadhyay 2014). Herman et al. reported that 30 μM curcumin could downregulate CCL2 activity by inhibiting protein kinase C (PKC) and matrix metalloproteinase-9 (MMP-9) in human PC-3 and decreasing adhesion, invasion, and motility in a PC3 cell line in vitro (Herman et al. 2009).
Notably, curcumin can significantly regulate the gene expression of 62 genes in MDA-MB-231 breast cancer cells, and the genes for the pro-inflammatory chemokines CXCL1 and CXCL2 are among the genes that are most strongly downregulated (Bachmeier et al. 2008). Both chemokines have pivotal roles in the migration, proliferation, metastasis, and angiogenesis of tumor cells of various organs (Youngs et al. 1997; Loukinova et al. 2000). Kronski et al. reported that curcumin treatment of both MDA-MB-231 (human metastatic breast cancer cell line) and human primary mammary cancer-derived cells could downregulate the expression of CXCL1 and CXCL2 chemokines via downregulation of miR181b (Kronski et al. 2014). It was shown that miR181b modulation could influence tumor progression and metastasis (Bachmeier et al. 2018). Moreover, studies conducted with the prostate cancer metastasis model showed that curcumin could influence the CXCL1 and CXCL2 chemokines, induce apoptosis, inhibit proliferation, and modulate several metastasis-accelerating factors. In an independent study, Killian and colleagues reported that curcumin could inhibit NFκB activation and reduce the expression of CXCL1 and CXCL2, which leads to the abolishment of the autocrine/paracrine loop between these two chemokines and NFκB, as well as reduces the development of metastasis (Killian et al. 2012).
In breast cancer cells, it appears that after curcumin inhibits NF-κB and the chemokines CXCL1 and CXCL2, the downregulation of CXCL1 leads to reduced expression of CXCR4 as a CXCL12/SDF-1 receptor. This overall process represents a metastasis-accelerating axis that could potentially serve as an avenue for therapeutic intervention (Bachmeier et al. 2008; Burger and Peled 2009). However, CXCL1 expression is limited to only a few types of breast cancer cells; however, it exists in the signature of metastasis to the lung (Albini et al. 2008; Minn et al. 2005). In addition, there is evidence that CXCL1 is associated with metastatic development of colorectal cancer, and tumors that highly express this chemokine have poor prognosis and survival (Wang et al. 2017a; Zhuo et al. 2018). Ruiz de Porras and colleagues have demonstrated that a cell culture of primary colorectal cancer cells with liver metastasis-derived cells had a high baseline expression of CXCL1 that was responsive to combination therapy employing oxaliplatin and curcumin, and these authors suggested that curcumin regulates the expression of CXCL1 via downregulation of NF-κB (Ruiz de Porras et al. 2016). However, in a study by Howells et al., comparing the effects of FOLFOX (folinic acid/5-fluorouracil/oxaliplatin chemotherapy) and CUFOX (FOLFOX + 2 g/day of oral curcumin) as therapeutic approaches in colorectal cancer patients, no significant differences were seen between plasma levels of CXCL1 before and after the intervention (Howells et al. 2019). 5-fluorouracil (5-FU) is a cancer chemotherapeutic agent that upregulates the expression of CXCL1 and CXCL2 in the bowel and also triggers neutrophil recruitment. Unfortunately, 5-FU treatment for colon cancer typically results in diarrhea. To this end, Sakai et al., using a murine model of colon cancer, reported that curcumin could inhibit 5-FU-mediated diarrhea by downregulating the expression of CXCL1 and CXCL2 in an NFκB-dependent manner (Sakai et al. 2016).
Tremmel and colleagues evaluated the inhibitory effects of topical curcumin and ursolic acid on skin tumor progression and reported that this combination therapy could significantly reduce the expression of inflammatory cytokines and chemokines such as CXCL2. The combination therapy more efficiently abrogates tumor development compared to either agent used alone as monotherapy (Tremmel et al. 2019).
In head and neck squamous cell carcinoma associated with the oral cavity (HNSCC), Boven et al. evaluated the effects of a curcumin gum formulation as a means to prevent HNSCC associated with the oral cavity and showed that chewing and packing the gum against the buccal mucosa for 30 min in healthy volunteers could significantly reduce the serum levels of inflammatory mediators. Among the chemokines measured in this pilot study, which included CXCL1, IL-8, IP-10, and MIP-1α, a significant reduction in serum CXCL1 levels was observed at 30 min and 4 h after chewing the curcumin gum (Boven et al. 2019).
Notably, CCR7 also plays a crucial role in metastasis and has emerged as a novel biomarker for metastasis to lymph nodes in breast cancer. Additionally, this chemokine receptor has been proposed as a prognostic indicator of metastasis in esophageal carcinoma (Legler et al. 2014; Cabioglu et al. 2005; Liu et al. 2013). Kaya et al. reported that curcumae radix extract (CRE) has anti-metastatic effects on MCF7 cells and in the murine model of breast cancer metastasis (MMTV-PyMT transgenic mice) and dramatically inhibits motility and cellular migration, as well as regulation in the gene expression of metastatic markers, including CCR7, metalloproteinase-9, and c-fus and c-jun proto-oncogenes (Kaya et al. 2019).
Downregulation of CXCR4 as a ‘metastasis-accelerating’ chemokine is another effect of curcumin on chemokines. It has been shown that CXCR4 is associated with tumor motility and invasion, and curcumin has been suggested for CXCR4-mediated inhibition of colorectal cancer progression (Zhang et al. 2016). Zhang et al. previously reported that curcumin inhibits the NKD2-Wnt-CXCR4 signaling pathway in the SW620 colorectal cancer cell line and decreases tumor development (Zhang et al. 2016). In another study, Du et al. demonstrated that cancer-associated fibroblasts (CAFs) could induce epithelial-to-mesenchymal transition (EMT) in prostate cancer cells, as well as accelerate CXCR4 expression and invasion via the monoamine oxidase A/mammalian target of rapamycin (mTOR)/hypoxia-inducible factor-1α (MAOA/mTOR/HIF-1α) signaling pathway. Moreover, these same authors showed that curcumin abrogates invasion and CAF-induced EMT, as well as decreases ROS production and the expression of CXCR4 and IL-6R (Du et al. 2015). Finally, Xiaoling et al. reported that curcumin could dramatically inhibit the expression of CXCL12 and CXCR4, as well as invasion and motility potential, in the SKOV3 human ovarian cancer cell line in a dose-dependent manner (Xiaoling et al. 2010).
It has also been previously suggested that MCP-1 activates and induces M1 macrophages to produce IL-12, a natural killer (NK) cell activator. Thus, the release of MCP-1 in the brain could potentially recruit anti-tumor cells and activate anti-tumor responses. Mukherjee et al. reported that phytosomal curcumin (CCP) could significantly induce the expression of MCP-1 in tumor-associated macrophage and microglia (TAM) in a murine model of glioblastoma (GBM; a primary cerebral tumor) and facilitate the destruction of GBM cells (Mukherjee et al. 2018).
Adverse pregnancy outcomes
Zhou et al. reported that curcumin could ameliorate LPS-induced adverse pregnancy outcomes in a mouse pregnancy model by (1) upregulating Akt phosphorylation in the placenta, (2) reducing the expression of chemokines, such as macrophage migration inhibitory factor (MIF), MCP-1, and MIP-1, and (3) inhibiting the recruitment of CD86+ macrophages to the placenta (Zhou et al. 2017). In another independent study, pregnant rats received LPS to induce pre-eclampsia and then received treatment with curcumin. This study showed that curcumin could reduce blood pressure and the concentration of urinary proteins, as well as reduce the expression of inflammatory mediators such as TLR4, NFκB, IL-6, and MCP-1 (Gong et al. 2016).
Lastly, Chowdhury and colleagues evaluated the effects of curcumin on the secretion of pro-inflammatory and pro-angiogenic cytokines and chemokines in cell culture of eutopic endometrium-derived stromal cells (EESC) in comparison to normal endometrial stromal cells (NESC). They demonstrated that curcumin significantly reduced the expression of inflammatory cytokines and chemokines, such as MCP-1 and RANTES, in a dose- and duration-dependent manner (Chowdhury et al. 2019).
Conclusion
The regulatory and anti-inflammatory effects of curcumin are well established, and it exerts its pharmacological effects through various molecular targets. In general, the biological effects of curcumin are broadly thought to be achieved by inhibition of NF-κB, although, more specifically, the actual anti-inflammatory effects of curcumin are primarily mediated by downregulation in the activity COX-2, LOX, and iNOS. The present review article discussed the effects of curcumin on chemokines and chemokine receptors both in in vitro studies and in vivo experimental animal models of different pathologies that included inflammation in various organs, autoimmune diseases, cancer, and bacterial and viral infections. The findings of these studies have shown that curcumin can exert its inhibitory effects on chemokines, which generally function as pro-inflammatory mediators and are responsible for the recruitment of immune cells to sites of inflammation. As it relates to the neuroprotective effects provided to neuroglia and neurons, curcumin modulates the expression of different chemokines and reduces degeneration, injuries, and deficits associated with these cells' neuroinflammation.
Moreover, the inhibitory effect of curcumin on hepatic, cardiovascular, pulmonary, and renal inflammatory conditions results in decreased fibrosis and other inflammation-associated histological complications. Lastly, in the case of cancer, curcumin can inhibit chemokine expression, leading to inhibition of tumor metastasis and activating specific chemokines that recruit anti-tumor immune cells to the tumor microenvironment. However, clinical trials to treat these various disease states/pathologic disorders with curcumin in humans are limited. Thus, we would suggest that additional clinical studies evaluating curcumin use in humans are needed to reach unequivocal conclusions about the therapeutic effects of curcumin on these various disorders/disease states.
Acknowledgements
None
Authors' contributions
Not applicable.
Funding
None.
Availability of data and material
The data are available upon request.
Declarations
Competing interests
Muhammed Majeed is the founder of Sami-Sabinsa group of companies.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39(1):41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Adhikary L, Chow F, Nikolic-Paterson DJ, Stambe C, Dowling J, Atkins RC, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47(7):1210–1222. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11(20):7490–7498. doi: 10.1158/1078-0432.ccr-05-1192. [DOI] [PubMed] [Google Scholar]
- Albini A, Mirisola V, Pfeffer U. Metastasis signatures: genes regulating tumor-microenvironment interactions predict metastatic behavior. Cancer Metastasis Rev. 2008;27(1):75–83. doi: 10.1007/s10555-007-9111-x. [DOI] [PubMed] [Google Scholar]
- Alidadi M, Jamialahmadi T, Cicero AFG, Bianconi V, Pirro M, Banach M, et al. The potential role of plant-derived natural products in improving arterial stiffness: a review of dietary intervention studies (Review) Trends Food Sci Technol. 2020;99:426–440. doi: 10.1016/j.tifs.2020.03.026. [DOI] [Google Scholar]
- Antoine F, Simard JC, Girard D. Curcumin inhibits agent-induced human neutrophil functions in vitro and lipopolysaccharide-induced neutrophilic infiltration in vivo. Int Immunopharmacol. 2013;17(4):1101–1107. doi: 10.1016/j.intimp.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Avasarala S, Zhang F, Liu G, Wang R, London SD, London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS ONE. 2013;8(2):e57285. doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier B, Nerlich AG, Iancu CM, Cilli M, Schleicher E, Vené R, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19(1–4):137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Höhneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29(4):779–789. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Killian PH, Melchart D. The role of curcumin in prevention and management of metastatic disease. Int J Mol Sci. 2018 doi: 10.3390/ijms19061716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A, Majeed M, Sahebkar A. Curcumin: a potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol. 2019;2:1–17. doi: 10.1007/s13402-019-00442-2. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D. Farmer to pharmacist: curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front Chem. 2014;2:113. doi: 10.3389/fchem.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao HY, Chen RH, Huang SM, Pan XQ, Fei L. Curcumin inhibited the proliferation and extracellular matrix production of human mesangial cells. Zhonghua Er Ke Za Zhi. 2003;41(11):822–826. [PubMed] [Google Scholar]
- Bavarsad K, Barreto GE, Hadjzadeh MAR, Sahebkar A. Protective effects of curcumin against ischemia-reperfusion injury in the nervous system (review) Mol Neurobiol. 2019;56(2):1391–1404. doi: 10.1007/s12035-018-1169-7. [DOI] [PubMed] [Google Scholar]
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7(1–2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Chemokine regulation of angiogenesis during wound healing. Adv Wound Care. 2015;4(11):641–650. doi: 10.1089/wound.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven L, Holmes SP, Latimer B, McMartin K, Ma X, Moore-Medlin T, et al. Curcumin gum formulation for prevention of oral cavity head and neck squamous cell carcinoma. Laryngoscope. 2019;129(7):1597–1603. doi: 10.1002/lary.27542. [DOI] [PubMed] [Google Scholar]
- Brempelis KJ, Crispe IN. Infiltrating monocytes in liver injury and repair. Clin Transl Immunol. 2016;5(11):e113. doi: 10.1038/cti.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari SN, Lauro G, Jantan I, Bifulco G, Amjad MW. Pharmacological evaluation and docking studies of α, β-unsaturated carbonyl based synthetic compounds as inhibitors of secretory phospholipase A2, cyclooxygenases, lipoxygenase and proinflammatory cytokines. Bioorg Med Chem. 2014;22(15):4151–4161. doi: 10.1016/j.bmc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11(16):5686–5693. doi: 10.1158/1078-0432.ccr-05-0014. [DOI] [PubMed] [Google Scholar]
- Cabrero Heras S, Martínez-Balibrea E. CXC family of chemokines as prognostic or predictive biomarkers and possible drug targets in colorectal cancer. World J Gastroenterol. 2018;24(42):4738. doi: 10.3748/wjg.v24.i42.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zheng JW, Li JJ, Meng B, Li J, Ge RS. Effects of curcumin on pain threshold and on the expression of nuclear factor κ B and CX3C receptor 1 after sciatic nerve chronic constrictive injury in rats. Chin J Integr Med. 2014;20(11):850–856. doi: 10.1007/s11655-013-1549-9. [DOI] [PubMed] [Google Scholar]
- Cao F, Liu T, Xu Y, Xu D, Feng S. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int J Clin Exp Pathol. 2015;8(6):6037–6045. [PMC free article] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205(4):811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan PS, Dash D, Singh R. Intranasal curcumin inhibits pulmonary fibrosis by modulating matrix metalloproteinase-9 (MMP-9) in ovalbumin-induced chronic asthma. Inflammation. 2017;40(1):248–258. doi: 10.1007/s10753-016-0475-3. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Dai L, Zhao LX, Zhu X, Cao S, Gao YJ. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci Rep. 2015;5:10278. doi: 10.1038/srep10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Park JW, Kim HY, Kim YH, Kim SM, Son YH, et al. Cellular factors involved in CXCL8 expression induced by glycated serum albumin in vascular smooth muscle cells. Atherosclerosis. 2010;209(1):58–65. doi: 10.1016/j.atherosclerosis.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Chowdhury I, Banerjee S, Driss A, Xu W, Mehrabi S, Nezhat C, et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J Cell Physiol. 2019;234(5):6298–6312. doi: 10.1002/jcp.27360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Choi SH, Choi JA, Chuck RS, Joo CK. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol vis. 2012;18:1966–1972. [PMC free article] [PubMed] [Google Scholar]
- de Almeida Alvarenga L, de Oliveira Leal V, Borges NA, de Aguiar AS, Faxén-Irving G, Stenvinkel P, et al. Curcumin-A promising nutritional strategy for chronic kidney disease patients. J Funct Foods. 2018;40:715–721. doi: 10.1016/j.jff.2017.12.015. [DOI] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X, et al. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int J Oncol. 2015;47(6):2064–2072. doi: 10.3892/ijo.2015.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111(3):291–297. doi: 10.1172/jci17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin–from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ferreira VH, Nazli A, Dizzell SE, Mueller K, Kaushic C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE. 2015;10(4):e0124903. doi: 10.1371/journal.pone.0124903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XY, Zhang DW, Li YD, Zhao PW, Tang YQ, Niu JZ, et al. Curcumin treatment suppresses CCR7 expression and the differentiation and migration of human circulating fibrocytes. Cell Physiol Biochem. 2015;35(2):489–498. doi: 10.1159/000369714. [DOI] [PubMed] [Google Scholar]
- Gan L, Li C, Wang J, Guo X. Curcumin modulates the effect of histone modification on the expression of chemokines by type II alveolar epithelial cells in a rat COPD model. Int J Chron Obstruct Pulmon Dis. 2016;11:2765–2773. doi: 10.2147/copd.s113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjali S, Blesso CN, Banach M, Pirro M, Majeed M, Sahebkar A. Effects of curcumin on HDL functionality. Pharmacol Res. 2017;119:208–218. doi: 10.1016/j.phrs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Ganjali S, Blesso CN, Banach M, Pirro M, Majeed M, Sahebkar A. Effects of curcumin on HDL functionality (review) Pharmacol Res. 2017;119:208–218. doi: 10.1016/j.phrs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Ghasemi F, Bagheri H, Barreto GE, Read MI, Sahebkar A. Effects of curcumin on microglial cells (review) Neurotox Res. 2019;36(1):12–26. doi: 10.1007/s12640-019-00030-0. [DOI] [PubMed] [Google Scholar]
- Ghosh N, Ghosh R, Mandal V, Mandal SC. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011;49(9):970–988. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52(9):1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gong P, Liu M, Hong G, Li Y, Xue P, Zheng M, et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta. 2016;41:45–52. doi: 10.1016/j.placenta.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Gouda MM, Bhandary YP. Curcumin down-regulates IL-17A mediated p53-fibrinolytic system in bleomycin induced acute lung injury in vivo. J Cell Biochem. 2018;119(9):7285–7299. doi: 10.1002/jcb.27026. [DOI] [PubMed] [Google Scholar]
- Guo L, Xing Y, Pan R, Jiang M, Gong Z, Lin L, et al. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS ONE. 2013;8(8):e70565. doi: 10.1371/journal.pone.0070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M. New insights into the structure and function of chemokine receptor: chemokine complexes from an experimental perspective. J Leukocyte Biol. 2020;2:2. doi: 10.1002/JLB.2MR1219-288R. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A. Curcumin: an inflammasome silencer (review) Pharmacol Res. 2020;159:2. doi: 10.1016/j.phrs.2020.104921. [DOI] [PubMed] [Google Scholar]
- Heidari Z, Daei M, Boozari M, Jamialahmadi T, Sahebkar A. Curcumin supplementation in pediatric patients: a systematic review of current clinical evidence (review) Phytother Res. 2022;36(4):1442–1458. doi: 10.1002/ptr.7350. [DOI] [PubMed] [Google Scholar]
- Herman JG, Stadelman HL, Roselli CE. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int J Oncol. 2009;34(5):1319–1327. [PMC free article] [PubMed] [Google Scholar]
- Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–1139. doi: 10.1093/jn/nxz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Liu Y, Xiong Y, Wu H, Wang G, Sun Z, et al. Curcumin protects against liver fibrosis by attenuating infiltration of Gr1hi monocytes through inhibition of monocyte chemoattractant protein-1. Discov Med. 2016;21(118):447–457. [PubMed] [Google Scholar]
- Hwang YP, Jin SW, Choi JH, Choi CY, Kim HG, Kim SJ, et al. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem Toxicol. 2017;99:162–169. doi: 10.1016/j.fct.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo (Article) Eur J Cancer Prev. 2009;18(5):412–415. doi: 10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- Jacob A, Chaves L, Eadon MT, Chang A, Quigg RJ, Alexander JJ. Curcumin alleviates immune-complex-mediated glomerulonephritis in factor-H-deficient mice. Immunology. 2013;139(3):328–337. doi: 10.1111/imm.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11(2):241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MR, Parks RJ. Curcumin as an antiviral agent. Viruses. 2020;12(11):1242. doi: 10.3390/v12111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60(1):135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Xie P, Hong SJ, Galiano R, Singer A, Clark RA, et al. Intravenous curcumin efficacy on healing and scar formation in rabbit ear wounds under nonischemic, ischemic, and ischemia-reperfusion conditions. Wound Repair Regen. 2014;22(6):730–739. doi: 10.1111/wrr.12231. [DOI] [PubMed] [Google Scholar]
- Jones EA, Shahed A, Shoskes DA. Modulation of apoptotic and inflammatory genes by bioflavonoids and angiotensin II inhibition in ureteral obstruction. Urology. 2000;56(2):346–351. doi: 10.1016/s0090-4295(00)00608-7. [DOI] [PubMed] [Google Scholar]
- Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017;33:55–63. doi: 10.1016/j.cytogfr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Kaya P, Lee SR, Lee YH, Kwon SW, Yang H, Lee HW, et al. Curcumae radix extract decreases mammary tumor-derived lung metastasis via suppression of C–C chemokine receptor type 7 expression. Nutrients. 2019 doi: 10.3390/nu11020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian PH, Kronski E, Michalik KM, Barbieri O, Astigiano S, Sommerhoff CP, et al. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33(12):2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174(12):8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- Kim J, Jeong SW, Quan H, Jeong CW, Choi JI, Bae HB. Effect of curcumin (Curcuma longa extract) on LPS-induced acute lung injury is mediated by the activation of AMPK. J Anesth. 2016;30(1):100–108. doi: 10.1007/s00540-015-2073-1. [DOI] [PubMed] [Google Scholar]
- Kirovski G, Gäbele E, Dorn C, Moleda L, Niessen C, Weiss TS, et al. Hepatic steatosis causes induction of the chemokine RANTES in the absence of significant hepatic inflammation. Int J Clin Exp Pathol. 2010;3(7):675–680. [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165(1):237–246. doi: 10.1016/s0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloesch B, Gober L, Loebsch S, Vcelar B, Helson L, Steiner G. In vitro study of a liposomal curcumin formulation (Lipocurc™): toxicity and biological activity in synovial fibroblasts and macrophages. In Vivo. 2016;30(4):413–419. [PubMed] [Google Scholar]
- Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med. 2009;1(3):309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konduru AS, Lee BC, Li JD. Curcumin suppresses NTHi-induced CXCL5 expression via inhibition of positive IKKβ pathway and up-regulation of negative MKP-1 pathway. Sci Rep. 2016;6:31695. doi: 10.1038/srep31695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronski E, Fiori ME, Barbieri O, Astigiano S, Mirisola V, Killian PH, et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol Oncol. 2014;8(3):581–595. doi: 10.1016/j.molonc.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sulakhiya K, Barua CC, Mundhe N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol Cell Biochem. 2017;431(1–2):113–122. doi: 10.1007/s11010-017-2981-5. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, et al. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer. 2009;125(9):2187–2197. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- Larmonier CB, Midura-Kiela MT, Ramalingam R, Laubitz D, Janikashvili N, Larmonier N, et al. Modulation of neutrophil motility by curcumin: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(2):503–515. doi: 10.1002/ibd.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid Redox Signal. 2005;7(11–12):1612–1620. doi: 10.1089/ars.2005.7.1612. [DOI] [PubMed] [Google Scholar]
- Legler DF, Uetz-von Allmen E, Hauser MA. CCR7: roles in cancer cell dissemination, migration and metastasis formation. Int J Biochem Cell Biol. 2014;54:78–82. doi: 10.1016/j.biocel.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Lelli D, Sahebkar A, Johnston TP, Pedone C. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol Res. 2017;115:133–148. doi: 10.1016/j.phrs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Li H, Sureda A, Devkota HP, Pittalà V, Barreca D, Silva AS, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343. doi: 10.1016/j.biotechadv.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Lin MS, Sun YY, Chiu WT, Hung CC, Chang CY, Shie FS, et al. Curcumin attenuates the expression and secretion of RANTES after spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J Neurotrauma. 2011;28(7):1259–1269. doi: 10.1089/neu.2011.1768. [DOI] [PubMed] [Google Scholar]
- Lin YM, Hsu CJ, Liao YY, Chou MC, Tang CH. The CCL2/CCR2 axis enhances vascular cell adhesion molecule-1 expression in human synovial fibroblasts. PLoS ONE. 2012;7(11):e49999. doi: 10.1371/journal.pone.0049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Song L, Wang Z. CCR7: A metastasis and prognosis indicator of postoperative patients with esophageal carcinoma. Hepatogastroenterology. 2013;60(124):747–750. doi: 10.5754/hge12846. [DOI] [PubMed] [Google Scholar]
- Liu T, Li C, Sun H, Luo T, Tan Y, Tian D, et al. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm Res. 2014;63(10):841–850. doi: 10.1007/s00011-014-0758-9. [DOI] [PubMed] [Google Scholar]
- Loukinova E, Dong G, Enamorado-Ayalya I, Thomas GR, Chen Z, Schreiber H, et al. Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. Oncogene. 2000;19(31):3477–3486. doi: 10.1038/sj.onc.1203687. [DOI] [PubMed] [Google Scholar]
- Lüer S, Troller R, Aebi C. Antibacterial and antiinflammatory kinetics of curcumin as a potential antimucositis agent in cancer patients. Nutr Cancer. 2012;64(7):975–981. doi: 10.1080/01635581.2012.713161. [DOI] [PubMed] [Google Scholar]
- Marchese A. Endocytic trafficking of chemokine receptors. Curr Opin Cell Biol. 2014;27:72–77. doi: 10.1016/j.ceb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi-Sardoo H, Mashayekhi-Sardoo A, Roufogalis BD, Jamialahmadi T, Sahebkar A. Impact of curcumin on microsomal enzyme activities: drug interaction and chemopreventive studies (Article) Curr Med Chem. 2021;28(34):7122–7140. doi: 10.2174/0929867328666210329123449. [DOI] [PubMed] [Google Scholar]
- Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol Sin. 2013;34(7):901–911. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, Laubitz D, Ghishan FK, Kiela PR. Curcumin inhibits interferon-γ signaling in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G85–96. doi: 10.1152/ajpgi.00275.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimche PN, Thompson E, Taramelli D, Vivas L. Curcumin enhances non-opsonic phagocytosis of Plasmodium falciparum through up-regulation of CD36 surface expression on monocytes/macrophages. J Antimicrob Chemother. 2012;67(8):1895–1904. doi: 10.1093/jac/dks132. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam SJ, Barta P, Mirabolfathinejad SG, Ammar-Aouchiche Z, Garza NT, Vo TT, et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30(11):1949–1956. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri M, Behnam B, Cicero AF, Sahebkar A. Protective effects of curcumin against aflatoxicosis: a comprehensive review. J Cell Physiol. 2018;233(4):3552–3577. doi: 10.1002/jcp.26212. [DOI] [PubMed] [Google Scholar]
- Mohajeri M, Bianconi V, Ávila-Rodriguez MF, Barreto GE, Jamialahmadi T, Pirro M, et al. Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system (Review) Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104765. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019;66:1–16. doi: 10.1016/j.jnutbio.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Mohammed ES, El-Beih NM, El-Hussieny EA, El-Ahwany E, Hassan M, Zoheiry M. Effects of free and nanoparticulate curcumin on chemically induced liver carcinoma in an animal model (Article) Arch Med Sci. 2021;17(1):218–227. doi: 10.5114/aoms.2020.93739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, Johnston TP, Abdollahi E, Sahebkar A. Curcumin: a natural modulator of immune cells in systemic lupus erythematosus (Review) Autoimmun Rev. 2018;17(2):125–135. doi: 10.1016/j.autrev.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Fried A, Hussaini R, White R, Baidoo J, Yalamanchi S, et al. Phytosomal curcumin causes natural killer cell-dependent repolarization of glioblastoma (GBM) tumor-associated microglia/macrophages and elimination of GBM and GBM stem cells. J Exp Clin Cancer Res. 2018;37(1):168. doi: 10.1186/s13046-018-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HS, Cha MH, Oh D-R, Cho C-W, Rhee JH, Kim YR. Protective mechanism of curcumin against Vibrio vulnificus infection. FEMS Immunol Med Microbiol. 2011;63(3):355–362. doi: 10.1111/j.1574-695X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- Nagaraju GP, Zhu S, Ko JE, Ashritha N, Kandimalla R, Snyder JP, et al. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015;357(2):557–565. doi: 10.1016/j.canlet.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168(12):6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, Sugimoto M, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE. 2017;12(10):e0185999. doi: 10.1371/journal.pone.0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS ONE. 2013;8(1):e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang X, Wang Y, Cai L, Ren L, Tang L, et al. Targeting JNK by a new curcumin analog to inhibit NF-kB-mediated expression of cell adhesion molecules attenuates renal macrophage infiltration and injury in diabetic mice. PLoS ONE. 2013;8(11):e79084. doi: 10.1371/journal.pone.0079084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhu G, Wang Y, Cai L, Cai Y, Hu J, et al. Attenuation of high-glucose-induced inflammatory response by a novel curcumin derivative B06 contributes to its protection from diabetic pathogenic changes in rat kidney and heart. J Nutr Biochem. 2013;24(1):146–155. doi: 10.1016/j.jnutbio.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A. Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med. 2014;22(5):851–857. doi: 10.1016/j.ctim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28(10):1461–1467. doi: 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Ghanei M, Bashiri S, Hajihashemi A, Sahebkar A. Short-term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: positive results of a randomized double-blind placebo-controlled trial. Drug Res (stuttg) 2015;65(11):567–573. doi: 10.1055/s-0034-1389986. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015;34(6):1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, et al. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. 2017;25(1):25–31. doi: 10.1007/s10787-016-0301-4. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Khalili N, Sahebi E, Namazi S, Reiner Ž, Majeed M, et al. Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial (Article) Complement Ther Med. 2017;33:1–5. doi: 10.1016/j.ctim.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Parsamanesh N, Moossavi M, Bahrami A, Butler AE, Sahebkar A. Therapeutic potential of curcumin in diabetic complications (review) Pharmacol Res. 2018;136:181–193. doi: 10.1016/j.phrs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Peled A, Wald O, Burger J. Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs. 2012;21(3):341–353. doi: 10.1517/13543784.2012.656197. [DOI] [PubMed] [Google Scholar]
- Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Pickich MB, Hargrove MW, Phillips CN, Healy JC, Moore AN, Roberts MD, et al. Effect of curcumin supplementation on serum expression of select cytokines and chemokines in a female rat model of nonalcoholic steatohepatitis. BMC Res Notes. 2019;12(1):496. doi: 10.1186/s13104-019-4540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbagher-Shahri AM, Farkhondeh T, Ashrafizadeh M, Talebi M, Samargahndian S. Curcumin and cardiovascular diseases: focus on cellular targets and cascades. Biomed Pharmacother. 2021;136:111214. doi: 10.1016/j.biopha.2020.111214. [DOI] [PubMed] [Google Scholar]
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Proudfoot AE, Uguccioni M. Modulation of chemokine responses: synergy and cooperativity. Front Immunol. 2016;7:183. doi: 10.3389/fimmu.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir MI, Naqvi ST, Muhammad SA. Curcumin: a polyphenol with molecular targets for cancer control. Asian Pac J Cancer Prev. 2016;17(6):2735–2739. [PubMed] [Google Scholar]
- Qin L, Qin J, Zhen X, Yang Q, Huang L. Curcumin protects against hepatic stellate cells activation and migration by inhibiting the CXCL12/CXCR4 biological axis in liver fibrosis: a study in vitro and in vivo. Biomed Pharmacother. 2018;101:599–607. doi: 10.1016/j.biopha.2018.02.091. [DOI] [PubMed] [Google Scholar]
- Qureshi M, Al-Suhaimi EA, Wahid F, Shehzad O, Shehzad A. Therapeutic potential of curcumin for multiple sclerosis. Neurol Sci. 2018;39(2):207–214. doi: 10.1007/s10072-017-3149-5. [DOI] [PubMed] [Google Scholar]
- Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochemical Journal. 2008;410(1):147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discovery. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakariyatham K, Du Z, Yuan B, Gao Z, Song M, Pan C, et al. Inhibitory effects of 7,7'-bromo-curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation. Eur J Pharmacol. 2019;858:172479. doi: 10.1016/j.ejphar.2019.172479. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Röhl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129(1):43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep. 2016;6:24675. doi: 10.1038/srep24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14(3):133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebkar A, Henrotin Y. Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials (Review) Pain Medicine (united States) 2016;17(6):1192–1202. doi: 10.1093/pm/pnv024. [DOI] [PubMed] [Google Scholar]
- Sakai H, Kai Y, Oguchi A, Kimura M, Tabata S, Yaegashi M, et al. Curcumin inhibits 5-fluorouracil-induced up-regulation of CXCL1 and CXCL2 of the colon associated with attenuation of diarrhoea development. Basic Clin Pharmacol Toxicol. 2016;119(6):540–547. doi: 10.1111/bcpt.12619. [DOI] [PubMed] [Google Scholar]
- Sameermahmood Z, Balasubramanyam M, Saravanan T, Rema M. Curcumin modulates SDF-1alpha/CXCR4-induced migration of human retinal endothelial cells (HRECs) Invest Ophthalmol vis Sci. 2008;49(8):3305–3311. doi: 10.1167/iovs.07-0456. [DOI] [PubMed] [Google Scholar]
- Santos AM, Lopes T, Oleastro M, Gato IV, Floch P, Benejat L, et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7(1):306–320. doi: 10.3390/nu7010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten DJ, Canals M, Maussang D, Roumen L, Smit MJ, Wijtmans M, et al. Pharmacological modulation of chemokine receptor function. Br J Pharmacol. 2012;165(6):1617–1643. doi: 10.1111/j.1476-5381.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119(7):1858–1870. doi: 10.1172/jci37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedzadeh MH, Safari Z, Zare A, Gholizadeh Navashenaq J, Razavi SA, Kardar GA, et al. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int Immunopharmacol. 2014;22(1):230–235. doi: 10.1016/j.intimp.2014.06.035. [DOI] [PubMed] [Google Scholar]
- Shahid H, Shahzad M, Shabbir A, Saghir G. Immunomodulatory and anti-inflammatory potential of curcumin for the treatment of allergic asthma: effects on expression levels of pro-inflammatory cytokines and aquaporins. Inflammation. 2019;42(6):2037–2047. doi: 10.1007/s10753-019-01066-2. [DOI] [PubMed] [Google Scholar]
- Shishodia S. Molecular mechanisms of curcumin action: gene expression. BioFactors. 2013;39(1):37–55. doi: 10.1002/biof.1041. [DOI] [PubMed] [Google Scholar]
- Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104(4):879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- Skommer J, Wlodkowic D, Pelkonen J. Gene-expression profiling during curcumin-induced apoptosis reveals downregulation of CXCR4. Exp Hematol. 2007;35(1):84–95. doi: 10.1016/j.exphem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (lond) 2011;8(1):35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E, Sastre M. Microglia function in Alzheimer's disease. Front Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani S, Boozari M, Cicero AFG, Jamialahmadi T, Sahebkar A. Effects of phytochemicals on macrophage cholesterol efflux capacity: impact on atherosclerosis (review) Phytother Res. 2021;35(6):2854–2878. doi: 10.1002/ptr.6991. [DOI] [PubMed] [Google Scholar]
- Sun X, Liu Y, Li C, Wang X, Zhu R, Liu C, et al. Recent advances of curcumin in the prevention and treatment of renal fibrosis. Biomed Res Int. 2017;2017:2418671. doi: 10.1155/2017/2418671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Cheong WF, Wenk MR, et al. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer's disease. J Alzheimers Dis. 2017;60(4):1429–1442. doi: 10.3233/jad-170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Teow S-Y, Liew K, Ali SA, Khoo AS-B, Peh S-C. Antibacterial action of curcumin against Staphylococcus aureus: a brief review. J Trop Med. 2016;2016(2):1. doi: 10.1155/2016/2853045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham CL, Hazeera Harith H, Wai Lam K, Joong Chong Y, Singh Cheema M, Roslan Sulaiman M, et al. The synthetic curcuminoid BHMC restores endotoxin-stimulated HUVEC dysfunction: specific disruption on enzymatic activity of p38 MAPK. Eur J Pharmacol. 2015;749:1–11. doi: 10.1016/j.ejphar.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci. 2019;20(5):1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremmel L, Rho O, Slaga TJ, DiGiovanni J. Inhibition of skin tumor promotion by TPA using a combination of topically applied ursolic acid and curcumin. Mol Carcinog. 2019;58(2):185–195. doi: 10.1002/mc.22918. [DOI] [PubMed] [Google Scholar]
- Tu CT, Han B, Liu HC, Zhang SC. Curcumin protects mice against concanavalin A-induced hepatitis by inhibiting intrahepatic intercellular adhesion molecule-1 (ICAM-1) and CXCL10 expression. Mol Cell Biochem. 2011;358(1–2):53–60. doi: 10.1007/s11010-011-0920-4. [DOI] [PubMed] [Google Scholar]
- Tu CT, Yao QY, Xu BL, Wang JY, Zhou CH, Zhang SC. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression. Food Chem Toxicol. 2012;50(9):3343–3351. doi: 10.1016/j.fct.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng. 2013;115(5):547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Vahedian-Azimi A, Abbasifard M, Rahimi-Bashar F, Guest PC, Majeed M, Mohammadi A, et al. Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials (review) Nutrients. 2022 doi: 10.3390/nu14020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Ramirez L, Pérez-Lopez P, Varela-Lopez A, Ramirez-Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. BioFactors. 2013;39(1):88–100. doi: 10.1002/biof.1057. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Auvin S, Ravizza T, Aronica E (2012) Glia-neuronal interactions in ictogenesis and epileptogenesis: role of inflammatory mediators. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper's Basic Mechanisms of the Epilepsies Bethesda MD: © 2012, Michael A Rogawski, Antonio V Delgado-Escueta, Jeffrey L Noebels, Massimo Avoli and Richard W Olsen [PubMed]
- Vitali D, Bagri P, Wessels JM, Arora M, Ganugula R, Parikh A, et al. Curcumin can decrease tissue inflammation and the severity of hsv-2 infection in the female reproductive mucosa. Int J Mol Sci. 2020 doi: 10.3390/ijms21010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, et al. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15(4):940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- Walker L, Sills GJ. Inflammation and epilepsy: the foundations for a new therapeutic approach in epilepsy? Epilepsy Curr. 2012;12(1):8–12. doi: 10.5698/1535-7511-12.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77(13):3655–3665. doi: 10.1158/0008-5472.can-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yin Z, Gao L, Sun D, Hu X, Xue L, et al. Curcumin delays retinal degeneration by regulating microglia activation in the retina of rd1 mice. Cell Physiol Biochem. 2017;44(2):479–493. doi: 10.1159/000485085. [DOI] [PubMed] [Google Scholar]
- Xiaoling MU, Jing Z, Fang X, Liangdan T (2010) Curcumin inhibits invasion and metastasis in the human ovarian cancer cells SKOV3 by CXCL12-CXCR4 axis (Article). Afr J Biotechnol 9(48):8230–8234. https://www.scopus.com/inward/record.uri?eid=2-s2.0-78649836793&partnerID=40&md5=b306054ad8b73fee808ad8cd6d86ccb0.
- Xie L, Li XK, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, et al. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol. 2009;9(5):575–581. doi: 10.1016/j.intimp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Xie L, Li XK, Takahara S. Curcumin has bright prospects for the treatment of multiple sclerosis. Int Immunopharmacol. 2011;11(3):323–330. doi: 10.1016/j.intimp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Xu F, Diao R, Liu J, Kang Y, Wang X, Shi L. Curcumin attenuates staphylococcus aureus-induced acute lung injury. Clin Respir J. 2015;9(1):87–97. doi: 10.1111/crj.12113. [DOI] [PubMed] [Google Scholar]
- Yadollahi A, Zargaran B. The beneficial effects of curcumin on cardiovascular diseases and their risk factors. Rev Clin Med. 2019;6(1):12–19. [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- Young NA, Bruss MS, Gardner M, Willis WL, Mo X, Valiente GR, et al. Oral administration of nano-emulsion curcumin in mice suppresses inflammatory-induced NFκB signaling and macrophage migration. PLoS ONE. 2014;9(11):e111559. doi: 10.1371/journal.pone.0111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs SJ, Ali SA, Taub DD, Rees RC. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71(2):257–266. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sun J, Wang R, Liu J, Wang P, Wang C. Curcumin management of myocardial fibrosis and its mechanisms of action: a review. Am J Chin Med. 2019;47(08):1675–1710. doi: 10.1142/S0192415X19500861. [DOI] [PubMed] [Google Scholar]
- Yuan J, Liu W, Zhu H, Chen Y, Zhang X, Li L, et al. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017;1655:90–103. doi: 10.1016/j.brainres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Zhao LX, Cao DL, Zhang X, Gao YJ, Xia C. Curcumin inhibits LPS-induced CCL2 expression via JNK pathway in C6 rat astrocytoma cells. Cell Mol Neurobiol. 2012;32(6):1003–1010. doi: 10.1007/s10571-012-9816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen H, Xu C, Song L, Huang L, Lai Y, et al. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016;35(5):2615–2623. doi: 10.3892/or.2016.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Jiang M, Fang J, Yang MF, Zhang S, Yin YX, et al. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol Neurobiol. 2017;54(1):1–14. doi: 10.1007/s12035-015-9635-y. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang X, Zhang L, Virgous C, Si H. Combination of curcumin and luteolin synergistically inhibits TNF-α-induced vascular inflammation in human vascular cells and mice. J Nutr Biochem. 2019;73:108222. doi: 10.1016/j.jnutbio.2019.108222. [DOI] [PubMed] [Google Scholar]
- Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J Neurosci. 2014;39(8):1391–1402. doi: 10.1111/ejn.12502. [DOI] [PubMed] [Google Scholar]
- Zhao F, Gong Y, Hu Y, Lu M, Wang J, Dong J, et al. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: translocation of nuclear factor-κB as potential target. Mol Med Rep. 2015;11(4):3087–3093. doi: 10.3892/mmr.2014.3079. [DOI] [PubMed] [Google Scholar]
- Zhao XA, Chen G, Liu Y, Chen Y, Wu H, Xiong Y, et al. Curcumin reduces Ly6C(hi) monocyte infiltration to protect against liver fibrosis by inhibiting Kupffer cells activation to reduce chemokines secretion. Biomed Pharmacother. 2018;106:868–878. doi: 10.1016/j.biopha.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Zhong F, Chen H, Han L, Jin Y, Wang W. Curcumin attenuates lipopolysaccharide-induced renal inflammation. Biol Pharm Bull. 2011;34(2):226–232. doi: 10.1248/bpb.34.226. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Liu T, Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-κB pathways in rat vascular smooth muscle cells. Inflamm Res. 2012;61(1):61–67. doi: 10.1007/s00011-011-0389-3. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Liu T, Lai W, Tan Y, Tian D, Guo Z. Heme oxygenase-1-mediated reactive oxygen species reduction is involved in the inhibitory effect of curcumin on lipopolysaccharide-induced monocyte chemoattractant protein-1 production in RAW264.7 macrophages. Mol Med Rep. 2013;7(1):242–246. doi: 10.3892/mmr.2012.1138. [DOI] [PubMed] [Google Scholar]
- Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Miao H, Li X, Hu Y, Sun H, Hou Y. Curcumin inhibits placental inflammation to ameliorate LPS-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated Akt. Inflamm Res. 2017;66(2):177–185. doi: 10.1007/s00011-016-1004-4. [DOI] [PubMed] [Google Scholar]
- Zhu T, Chen Z, Chen G, Wang D, Tang S, Deng H, et al. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPARγ-dependent NF-κB signaling pathway in vivo and in vitro. Mediators Inflamm. 2019;2019:4927430. doi: 10.1155/2019/4927430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo C, Wu X, Li J, Hu D, Jian J, Chen C, 2018. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep. [DOI] [PMC free article] [PubMed]
- Ziaei A, Hoppstädter J, Kiemer AK, Ramezani M, Amirghofran Z, Diesel B. Inhibitory effects of teuclatriol, a sesquiterpene from salvia mirzayanii, on nuclear factor-κB activation and expression of inflammatory mediators. J Ethnopharmacol. 2015;160:94–100. doi: 10.1016/j.jep.2014.10.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhuo C, Wu X, Li J, Hu D, Jian J, Chen C, 2018. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are available upon request.