Abstract
The accumulation of advanced glycation end-products (AGEs) in the organic matrix of bone with aging and chronic disease such as diabetes is thought to increase fracture risk independently of bone mass. However, to date, there has not been a clinical trial to determine whether inhibiting the accumulation of AGEs is effective in preventing low-energy, fragility fractures. Moreover, unlike with cardiovascular or kidney disease, there are also no pre-clinical studies demonstrating that AGE inhibitors or breakers can prevent the age- or diabetes-related decrease in the ability of bone to resist fracture. In this review, we critically examine the case for a long-standing hypothesis that AGE accumulation in bone tissue degrades the toughening mechanisms by which bone resists fracture. Prior research into the role of AGEs in bone has primarily measured pentosidine, an AGE crosslink, or bulk fluorescence of hydrolysates of bone. While significant correlations exist between these measurements and mechanical properties of bone, multiple AGEs are both non-fluorescent and non-crosslinking. Since clinical studies are equivocal on whether circulating pentosidine is an indicator of elevated fracture risk, there needs to be a more complete understanding of the different types of AGEs including non-crosslinking adducts and multiple non-enzymatic crosslinks in bone extracellular matrix and their specific contributions to hindering fracture resistance (biophysical and biological). By doing so, effective strategies to target AGE accumulation in bone with minimal side effects could be investigated in pre-clinical and clinical studies that aim to prevent fragility fractures in conditions that bone mass is not the underlying culprit.
Keywords: Advanced glycation end-product, bone quality, collagen type I, crosslinking, toughness, biomarkers, aging, diabetes, mass spectrometry
1. Introduction
The organic matrix primarily influences the inelastic deformation of bone, while the hard, inorganic phase composed of calcium (Ca2+)-phosphate (PO43−) mineral influences elastic deformation [1]. Plasticity refers to the ability of a material to dissipate energy through the generation of permanent damage during inelastic deformation, and post-yield toughness is one mechanical property characterizing this ability. Characterized by an apparent elastic modulus, elasticity pertains to the reversible response of a material to loading. Marking the transition between elastic and inelastic (plastic) deformation, yield strength characterizes the ability of a material to resist permanent deformation. Determination of these and other mechanical properties from a stress vs. strain curve can be found in Section 2.5. In cases of minimal organic matrix content (by mass or by volume fraction), bone is brittle (i.e., it will fracture with little to no deformation after yielding). With the typical amounts of organic matrix (approximately 45% by volume) in human cortical bone that is properly organized into inter-connected collagen fibrils and infused with mineral as well as arranged into multi-scale interfaces to absorb energy and divert cracks, healthy bone can sustain occasional loads that exceed its yield strength. Being a living tissue, the damage generated during an overloading event is repaired by remodeling. Also in homeostasis, it is repaired faster than it can accumulate to cause frank fracture of a bone. When the distribution of microdamage overwhelms the ability of this targeted remodeling to repair bone tissue, as occurs with sudden increases in the intensity of loading (e.g., shift from not running to daily running), stress fractures can occur in otherwise healthy tissue.
From a clinical perspective, osteoporosis ensues from a loss of bone mass or bone mineral density, thereby increasing fracture risk. Higher fracture risk however is not always attributable to lower bone mass. From another perspective then, the end-result of osteoporosis (i.e., a low-energy, fragility fracture) occurs because of an inability of unhealthy bone to repair microdamage via remodeling [2], limit the accumulation of microdamage throughout the tissue, and hinder, if not arrest, the subsequent initiation and propagation of cracks [2]. Why the latter occurs is complex due to the hierarchical arrangement of collagen type I (col I) and other collagens into mineralized collagen fibrils (MCFs), which i) bind to non-collagenous proteins (NCPs), bound water, and extrafibrillar mineral and ii) are arranged in shifting orientations between lamellae [3]. Undoubtedly though, age- and disease-related changes in the organic matrix – whether cell-mediated, biochemical, or biophysical in nature – contribute to the occurrence of fragility fractures.
At present, widely prescribed medications to treat osteoporosis were developed to either inhibit bone resorption, thereby impeding a loss in bone mass (e.g., bisphosphonates, selective estrogen receptor modulators, anti-RANKL antibody) or promote bone formation (recombinant human parathyroid hormone (1-34), parathyroid hormone-related protein (1-34), and anti-sclerostin antibody), thereby countering bone mass loss. Intermittent injections of rhPTH(1-34) or rhPTHrP(1-34) also stimulate remodeling or bone turnover, whereas the affinity of nitrogen-containing bisphosphonates for mineral suppresses turnover. Arguably then, these therapies indirectly affect the organic matrix, but whether there is sufficient turnover stimulation to restore the inherent quality of the organic matrix or sufficient turnover suppression to degrade the organic matrix is clinically unknown. Unknown too is whether the quality of newly formed bone tissue is degraded in age and disease. As such, targeting specific chemical moieties in the extracellular matrix (ECM) of bone requires a better understanding of the biology underlying aging, disease, and drug effects on the organic matrix. Regardless, all current osteoporosis medications primarily decrease fracture risk by acting on osteoblasts including osteocytes (anabolic) or osteoclasts (anti-catabolic), not on correcting deleterious changes that occur within the organic phase with aging and disease progression (Table 1).
Table 1.
A summary of age- and disease-related changes in the organic matrix that are thought to contribute to bone fragility. Relationships derived from studies that tested correlations in human bone, augmented with some evidence from animal studies.
| Change In Organic Matrix | Change in Apparent-level Mechanical Properties of Cortical Bone | Also Correlates with | Notes |
|---|---|---|---|
| Increase in AGE crosslinking (PEN) and in fluorescent AGEs | Loss of strength, ductility, toughness, and fracture toughness [7,8,12,34] No correlation: [162] |
Loss of LOX crosslinking and increase in AGE adducts [32] | Studies reporting negative correlations between PEN and mechanical properties of bone also report significant correlations with other characteristics. |
| Increase in AGE adduct content (specifically CML) [12, 34] | Loss of fracture toughness [87,89] | Increase in PEN and loss of LOX crosslinking [32] | This is the latest area of interest for correlation studies between AGEs and bone mechanical properties. |
| Loss of LOX crosslinking (especially immature crosslinks) or decrease in ratio of immature to mature enzymatic crosslinks [162] | Weak relationship with loss of cortical bone fracture toughness in adult humans [162] Supported by a) the comparisons between young and adult human bone [163,164] and b) by loss of strength and toughness in lathyrism models [128,129] |
Increase in PEN and CML [32] | This area was explored early-on and new results redirect attention to immature LOX crosslinks again. Immature LOX crosslinks are more difficult to quantify. Mature enzymatic crosslink content tend to be relatively constant in adults. |
| Denatured collagen content | Loss of strength and work-to-fracture [130,165] | Increase in PEN [130,165] | It is very difficult to measure denatured collagen without creating more denaturation during extraction |
| Degradation of network integrity of demineralized bone | Loss of work-to-fracture and fracture toughness [13,86] | Willett et al. [13] were unable to confirm the correlation between thermal stability and J-integral reported by Zioupos et al. [86] | |
| Degradation of collagen in terms of the strength of the demineralized bone | Loss of work-to-fracture [7] | Increase in PEN [7] | Consistent with the loss of nativity and integrity of the bone collagen, the strength of the bone collagen is also degraded. |
Over the lifespan of humans, post-yield toughness of cortical bone decreases to a greater extent than the strength (yield or ultimate) of cortical bone at the apparent level (i.e., independent of macrostructure but not microstructure), an observation initially made by Burstein et al. in the 1970’s [4] and subsequently confirmed in the 90’s [5,6] and 2000’s [7,8]. Increase in tissue mineralization combined with an increase in intra-cortical porosity with aging could be a possible explanation for this observation as there is an inverse relationship between the mineral volume fraction and bone toughness across multiple species encompassing a large range in mineral content relative to dry mass of bone [9]. Also, mineral density and porosity are positively and negatively correlated with strength, respectively. However, the mean degree of mineralization does not appear to correlate with age between 20 years and 99 years of age [5,10], presumably because bone remodeling continues throughout the lifespan of humans. In the largest sample of donors to date (99 females and 94 males), analysis of sections of the femur mid-shaft by contact micro-radiography revealed a weak correlation between degree of tissue mineralization and donor age (female only) in which the correlation was negative with mineralization peaking during the 5th decade when menopause occurs [11]. Thus, hypermineralization is likely not the primary cause of the age-related decline in bone toughness.
The age-related decrease in bone strength at the apparent level is a manifestation of the decrease in apparent bone density, a product of porosity and mineralization. On the other hand, the age-related decrease in toughness is independent of porosity [5,12] and apparent volumetric bone mineral density (vBMD) [13]. Given the importance of col I and other collagens to the toughness of bone, a reasonable supposition then is that the age-related decline in bone toughness, namely post-yield toughness, is due to a loss in the integrity of the organic matrix.
Of the possibilities explaining the age-related decrease in the inherent quality of the organic matrix of bone, the accumulation of advanced glycation end-products (AGEs) has garnered considerable interest with multiple review articles published over the years [14–19]. For one, they accumulate in cortical bone [7,8,20,21] and trabecular bone with age [22], and the presence of AGEs in proteins has been implicated in the pathogenesis of multiple complications associated with diabetes [23,24]. Despite decades of research into the role of AGEs in mechanical properties of bone, inhibition of AGEs with compounds such as pyridoxamine, a B6 vitamer, or alagebrium chloride (ALT-711), an analog of phenacylthiazolium (PTC), is not actively being investigated in clinical trials to prevent fractures (Table 2). As such, we critically examined the evidence that AGE accumulation contributes to bone fragility with the goal of working toward a comprehensive understanding of their role in the mechanical behavior of bone.
Table 2.
AGE inhibitor drug candidates that to Phase 3 clinical trials
| Drug candidate | Clinical trial and outcome | Current status | Refs. |
|---|---|---|---|
| Aminoguanidine | Phase 3 trial in patients with Type 2 diabetic nephropathy; terminated due to toxic side effects | No longer in development | [166] |
| Pyridoxamine (Pyridorin) | Phase 3 trial in patients with diabetic nephropathy; showed efficacy in patients with early disease stages | Other trials are ongoing1 | [167] |
| Thiamine | Pilot clinical trials in patients with Type 2 diabetes and micro-albuminuria; demonstrated efficacy | Phase 3 trial is not planned | [56,168] |
| ALT-711 | Phase 3 trial in sedentary adults with arterial stiffness; demonstrated no efficacy. | No longer in development2 | [169] |
| TRC4186 | Phases 1 and 2 trials in diabetic patients with heart failure; demonstrated efficacy | Phase 3 trial is planned3 | [170] |
A pilot clinical trial “AGEs and bone material strength in Type 2 diabetes” is underway (ClinicalTrials.gov: NCT03778580);
Thirteen trials involving Alagebrium (ALT-711) (ClinicalTrials.gov: NCT00739687, NCT00516646, NCT00557518, NCT00662116, NCT00089713, NCT00043836, NCT01417663, NCT01913301, NCT01014572, NCT00045981, NCT00045994, NCT00302250, NCT00277875)
a Phase 3 clinical trial in patients with heart failure, diastolic dysfunction and Type 2 diabetes has been announced (ClinicalTrials.gov: NCT04507347).
2. The interest in AGEs as a potential contributor to bone fragility
Dictated by the mechanical loading environment of the anatomical site and requirements of mineral homeostasis, bone tissue can exist for years to decades before being replaced by the coupled actions of osteoclasts and osteoblasts. Since the formation of AGEs is a result of time-dependent, non-enzymatic reactions, AGE accumulation was an early candidate for the cause of the decline in bone toughness with advanced aging. Then, when the disproportionate increase in fracture risk among patients with type 2 diabetes (T2D) for a given areal bone mineral density (aBMD) was established in epidemiology studies [25,26], AGE accumulation was postulated to contribute to the elevated fracture risk in diabetes [16]. Here, we describe why AGEs, in particular the crosslinking type, are commonly thought to be an important contributor to bone fragility (i.e., osteoporosis).
2.1. AGE formation
AGEs form via enzyme-independent pathways. Since AGE formation involves multiple pathways with many intermediates [27,28], the study of their functional role in pathophysiology is challenging. Broadly, their formation involves reactions between amino groups in protein or lipids, on one hand, and glucose and/or products of glucose degradation and metabolism, on the other [27]. As a result, modifications are made to lysine and arginine residues and to amino groups in certain lipids such as phosphatidylethanolamine [29]. In bone, AGEs accumulate at multiple Lys and Arg sites of col I, other collagens, and various non-collagenous proteins (NCPs) found in the ECM.
Multiple AGEs have been identified over the years and are either crosslinks (i.e., covalent bonds between neighboring proteins or lipids) or adducts (i.e., a modification to the sidechain of a protein or lipid) [30]. While crosslinks bind or interconnect proteins and lipids together, adducts change the structure of the amino acid, which can alter the microenvironment of the modified protein sites and subsequently alter protein function [31]. For example, the formation of Nε-(carboxymethyl)lysine (CML) adds a carboxyl group to the residual ε-amine of a lysine resulting in a switch from positive ionic charge to negative charge under physiologic pH. Furthermore, there is growing evidence that the formation of AGEs interferes with normal enzymatic post-translational modifications (PTMs). In 2020, Arakawa et al. [32] demonstrated negative correlations between AGE content and the levels of the immature, lysyl-oxidase-derived collagen crosslinks in human trabecular bone supporting the idea by Hudson et al. [33], who analyzed mouse tendons, that glycation hinders enzymatic crosslinking.
AGE crosslinks have received much of the attention among the bone community studying bone quality over the last two decades because of the putative idea that such crosslinks would over-stabilize the collagen and other proteins and result in reduced “plasticity” (stable irreversible deformation) of the collagen fibrils, thereby degrading the inherent toughness of the tissue [34,35]. However, recent work by Arakawa et al. [32], using mass spectroscopy methods, has demonstrated that, of the measurable AGEs, adducts are much more abundant than crosslinks by at least one or two orders of magnitude. Currently, only the non-enzymatic crosslink pentosidine (PEN) is easily quantified as it survives acid hydrolysis and naturally fluoresces [36]. Another AGE crosslink glucosepane (GSPN) is not fluorescent nor easily isolated, but may be more abundant than PEN in bone since it is much more abundant than PEN in skin (Fig. 1) and increases in col I with age (GSPN = 301 x e(0.016 x age in years)) and diabetes [37]. Unlike PEN, GSPN has not been quantified in bone even though increasing levels of GSPN have been found to be more strongly correlated with microvascular diseases than PEN [38].
Figure 1: Relative amount of non-enzymatic modifications to skin col I from the Diabetes Control and Intervention Trial.
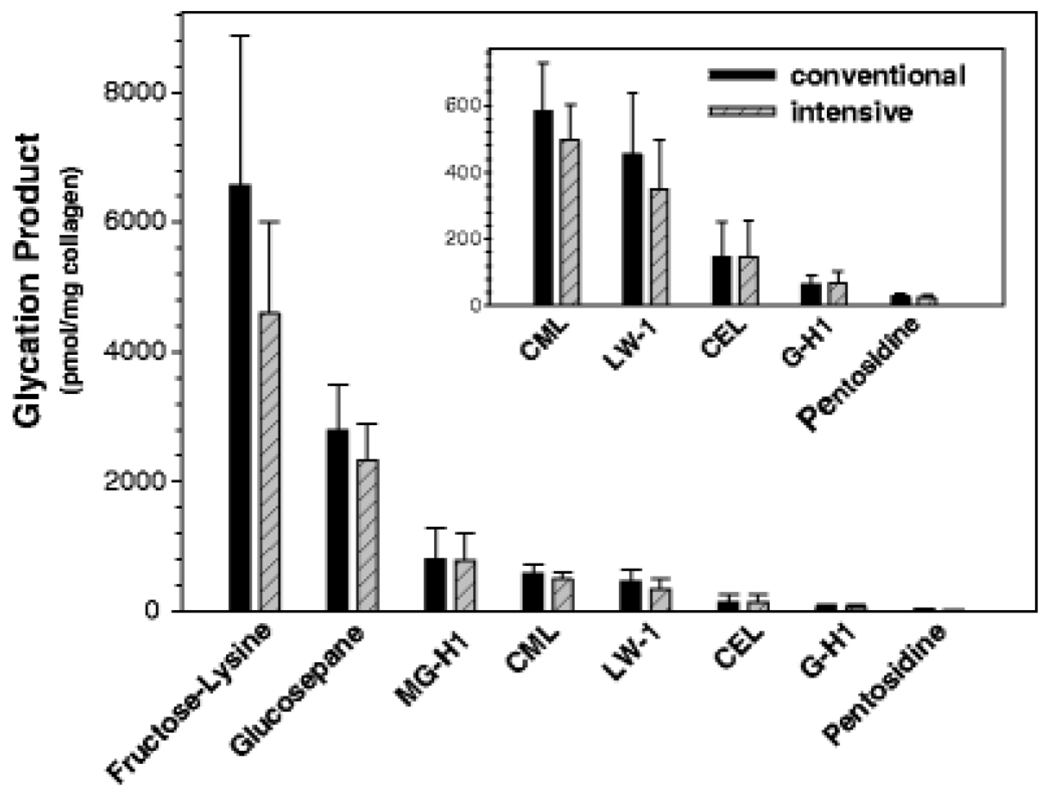
AGEs were quantified in skin biopsies of subjects with type 1 diabetes with either conventional or intensive control of glycemia. The amount of glucosepane in collagen I is 70 times greater than the amount of pentosidine in collagen I (Reproduced from Monnier et al. Glycoconjugate J. 2016).
2.2. Different types of non-enzymatic post-translational modifications
PTM is a final step in biosynthesis and conditioning of protein macromolecules. Following translation, the individual amino acid residues within the protein are structurally modified by specific enzymes to optimize protein functionality and/or stability. Involving specific hydroxylases and lysyl oxidases, two examples of enzymatic PTMs are hydroxylation of lysine and proline residues and the formation of lysine and hydroxylysine crosslinks in col I, respectively [48,49]. In addition to enzymatic modifications, non-enzymatic (NE) environmental conditioning of protein structure – particularly via oxidation, nitration, deamidation, halogenation and glycation of amino acid side chains – causes numerous NE-PTMs (Table 3).
Table 3.
Major physiologically relevant non-enzymatic post-translation modifications a
| Modification | Modifying agent | Targeted residues/groups | Comment | Ref |
|---|---|---|---|---|
| Fructosyllysine | glucose | Lys, N-terminal and PE amino groups | AGE | [145] |
| CML | glucose, glyoxal, glycolaldehyde, PUFA | Lys, N-terminal amino groups | oxidative AGE, ALE | [171] |
| CEL | methylglyoxal, PUFA | Lys, N-terminal amino groups | AGE, ALE | [172] |
| G-H1 | glyoxal | Arg | AGE | [73] |
| MG-H1 | methylglyoxal | Arg | AGE | [59] |
| Argpyrimidine | methylglyoxal | Arg | AGE | [173] |
| Oxidation | hydroxyl and superoxide radicals | Cys, Met, Trp | NE-PTM | [174–176] |
| Halogenation | HOCI, HOBr | Tyr, Trp, Lys | NE-PTM | [177,178] |
| Nitration | peroxynitrite | Tyr | NE-PTM | [176] |
| Deamidation | n/a | Asn and Gln | NE-PTM | [53] |
| MOLD/GOLD b | methylglyoxal, glyoxal | Lys | AGE | [179,180] |
| Pentosidine b | glucose | Lys and Arg | oxidative AGE | [181] |
| Glucosepane b | glucose | Lys and Arg | AGE | [182] |
This table includes only major physiologically relevant NE-PTMs and is not a comprehensive list of known NE-PTMs. CML, carboxymethyllysine; CEL, carboxyethyllysine; G-H1, 5-hydroimidazolone; MG-H1, 5-hydromethyl imidazolone; MOLD/GOLD, methylglyoxal/glyoxal lysine dimer; PUFA, polyunsaturated fatty acids; HOCI, hypochlorous acid; HOBr, hypobromous acid; PE, phosphatidylethanolamine; AGE, advanced glycation end-products; ALE, advanced lipoxidation end-products.
AGE crosslinks
Even though NE-PTMs are formed spontaneously, they preferentially target sites within a given protein structure as dictated by the microenvironment (e.g., pH, oxidative stress, glucose concentration) [50–53]. The presence of low levels of NE-PTMs is indicative of a normal and healthy condition, but further accumulation of NE-PTMs may affect protein functionality and lead to pathology. Long-lived ECM proteins such as col I and other collagens are especially vulnerable to functional damage as they can accumulate more NE-PTMs than proteins with a high turnover rate and are not protected by robust cellular antioxidant defenses.
AGEs constitute a subclass of NE-PTMs that derive from glycation (indirect) and glycoxidation (direct) pathways where glucose and/or its degradation products react with lysine, arginine, and N-terminal residues in proteins as well as with amino groups in lipids (Table 3). Accumulation of AGEs has been implicated in pathogenesis of diabetic complications, including nephropathy, retinopathy, and neurodegenerative disorders [54–56], mobility [57], and in aging [37]. Because formation of many AGEs involves oxidative reactions (Table 3), their accumulation often accompanies inflammatory diseases that are not directly associated with hyperglycemia (e.g., cancers) [58].
2.3. Detecting and quantifying AGEs
Analysis of AGEs under physiological conditions can be challenging because of the relatively low levels of these modifications in vivo. Nevertheless, several analytical approaches have been successfully applied to AGE quantitation in human and animal tissues. Immunodetection techniques (immunohistochemistry, Western blotting, and ELISA) allow for a highly sensitive analysis of AGE localization within tissues and semi-quantitative measurements of AGE levels [59,60]. The variety of commercially available anti-AGE antibodies however is limited. These antibodies are either nonspecific (i.e., recognize multiple AGE structures) or specific to CML, MG-H1 or PEN. More recently, a polyclonal anti-glucosepane antibody has been developed and approbated in aging mouse retinae [61]. The immunodetection techniques do not allow for discovery research as the antibodies are developed against known AGEs.
High-performance liquid chromatography (HPLC) with absorbance and/or fluorescence detection allows for analyses of individual AGEs following tissue hydrolysis. The chromatographic step prior to analysis enables AGE measurements in complex mixtures and improves sensitivity. The technique is usually limited to fluorescent AGEs such as PEN and argpyrimidine [62]. In the bone field, the HPLC assessment of pyridinoline crosslinks (PYD and deoxy-PYD or DPD) and PEN is a variation of the method by Bank et al. [63]. Pre-column derivatization can expand the range of detectable structures [62], but with the advances of mass spectrometry applications, derivatization is now rarely used in AGE analysis.
A more general approach to quantifying total fluorescent AGEs (fAGEs) involves the use of a spectrofluorometer or similar instruction to measure the fluorescence (370-nm Ex, 440-nm Em) of tissue hydrolysates [64]. This technique is often used in conjunction with the more specific HPLC methods because it lacks specificity [65]. It of course cannot detect and quantify non-fluorescent AGEs.
Fourier transform infrared and Raman spectroscopy (FTIR and RS) can detect molecular vibrations of specific chemical groups and are thus sensitive to modifications of proteins due to glycation [66]. In particular, RS has been applied to glycated human bone [67] and quantification of glycation-mediated changes in diabetic mouse bone [68–71]. The technique has the potential to provide non-invasive assessments of AGEs in patients. However, direct detection of individual AGEs in the complex in vivo environment can be challenging due to relatively low sensitivity and specificity [72].
Mass spectrometry (MS) technology has been applied to the analysis of AGEs in tissues using several different approaches: 1) liquid-chromatography tandem mass-spectrometry (LC-MSMS) after exhaustive tissue digestion or hydrolysis, 2) LC-MSMS following digestion with specific proteases, and 3) imaging mass spectrometry (IMS). In the first approach, the total tissue levels of multiple AGEs can be analyzed simultaneously and with very high sensitivity using isotopically labeled standards [73]. In the second approach, the levels of the AGE-modified residues at specific positions within the protein primary structure can be determined [53]. IMS allows for simultaneous determination of tissue localization of multiple AGEs without the use of antibodies [74]. Gas chromatography coupled with mass spectrometry (GC-MS) has also been used in tissue AGE analyses [75]. The MS approaches are amenable to discovery research as the MS data can be interrogated to search for mass-charge shifts corresponding to unknown AGEs.
2.4. Correlations between AGE content and mechanical properties of human bone
In support of the possibility that AGE accumulation contributes to bone fragility, measurements of AGE levels, mainly PEN and fAGEs, in bone matrix negatively correlate with measures of bone plasticity, toughness, and fracture toughness (Table 1). Upon extracting uniform, ‘engineering’ specimens from cadaveric bone, bone fragility can be assessed in terms of the relevant mechanical properties measured using a variety of mechanical tests.
When probing the effects of PTMs in the organic phase, such as AGEs, on the mechanical properties of cortical bone, tests that involve tensile stresses such as bending and some fracture tests are more sensitive because they engage mechanisms provided and controlled by the organic matrix and water [76]. In common mechanical tests of uniform specimens, material properties of bone are derived from engineering stress vs. engineering strain curves (Fig. 2A). The elastic or proportional limit normally marks the onset of permanent deformation that starts once the applied stress is sufficient to cause microdamage and can be detected as the onset of non-linearity by a loss in secant modulus. This is more commonly quantified using the concept of the yield strength (YS) of the material, following the conventional engineering use of the 0.2% offset rule (Fig. 2A). However, bone does not exhibit a clear transition from elastic to inelastic behavior because it is not a purely elastic material. Rather, before the onset of permanent damage, the mechanical behavior is viscoelastic meaning the modulus and indeed the yield strength depend on loading rate, the initial stress vs. strain response is not perfectly linear, and hysteresis, albeit small, occurs during cyclic loading. Post-yield strain and secant modulus characterize the ability of the tissue to deform beyond the yield point and before fracturing when damage irreversibly but stably forms (Fig. 2A). The area under the curve captures the ability of the bone tissue to absorb mechanical work and is most often referred to as toughness (T) or the work-to-fracture (Wf). The maximum stress achieved during the test is the ultimate stress also known as the ultimate strength (σu). The strengths of cortical bone are lower in tension than compression (Fig. 2B).
Figure 2. Mechanical testing of cortical bone in tension and compression.
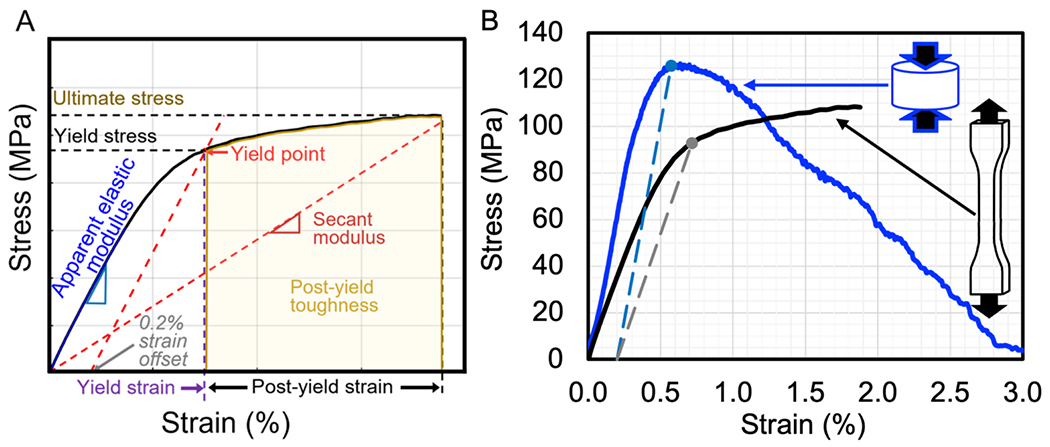
A typical mechanical test (monotonic load-to-failure in tension) generates force vs. displacement data that is converted to an engineering stress vs. strain curve from which mechanical properties are determined. Toughness is the area under the entire curve up to the point of fracture (ultimate stress), while post-yield toughness is the area under the curve after a yield point that is conventionally identified by the 0.2% offset method (intersection of the curve and a linear line with a slope equal to apparent modulus and extending from 0.2% strain) (A). In load-to-failure tests of human cortical bone at 5 mm/min, bone typically has a lower ultimate stress in tension than in compression. To mark the yield point on each curve, the long dashed lines are parallel to the apparent modulus of each test and start from 0.2% strain (B).
Fracture toughness is the resistance of a material to the growth of a pre-existing crack or flaw driven by mechanical stresses [77]. Fracture toughness in materials like cortical bone is best measured in terms of R-curves from which meaningful single point fracture toughness values can be extracted [78–80]. In bending tests of single-edge notched beams (Fig. 3A), KIC represents the intensity of the stress field around a crack tip required to initiate crack growth [77] and is often calculated using the load at the onset of non-linearity in force vs. displacement curves (Fig. 3B) or the load at the onset of detectable crack growth [81]. JIc represents the incremental energetic cost of crack growth at the point of crack growth initiation (Fig. 3C). J- integral (J-int) represents the incremental energetic cost of crack growth at the point of instability or catastrophic fracture after a non-negligible about of stable crack growth [12]. Unlike KIC which originates from linear elastic fracture mechanics, JIc and J-int are derived from non-linear fracture mechanics [77] and have been shown to better represent the fracture behavior of cortical bone because J captures the non-negligible effects of post-yield behavior [82]. As noted above, organic phase modifications, such as PTMs and AGEs, affect the post-yield behavior more than the pre-yield behavior of cortical bone. Therefore, J-based parameters are more sensitive to these changes than are K-based parameters.
Figure 3. Fracture toughness testing of cortical bone.
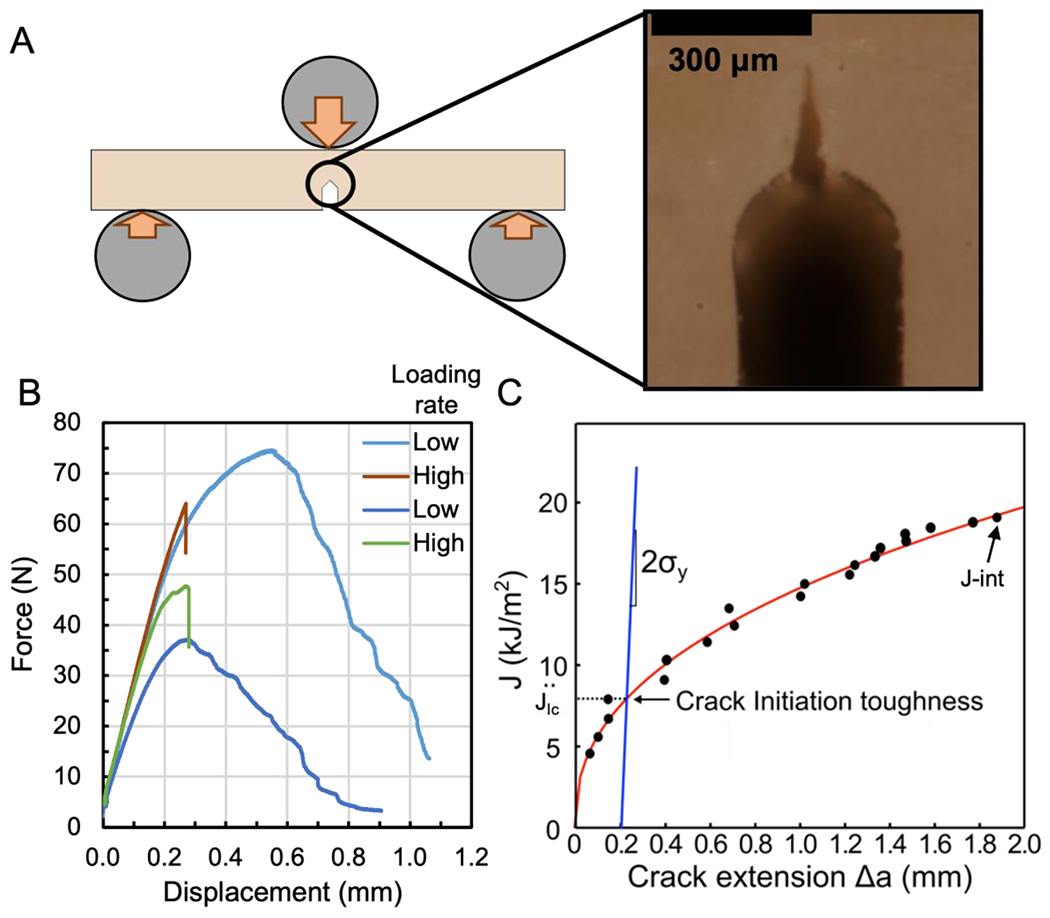
The single-edge notched beam (SENB) is typically loaded in three-point bending until a crack emanating from a micro-notch propagates through the specimen (A). In the magnified photo, the sharpened notch creates a stress concentration to start crack growth. As the loading rate of human cortical SENBs increases from low and high, the final fracture displacement decreases (B). In the schematic of an R-curve, the determination of JIc is based on a construction line (e.g., a slope = yield strength, σy, of cortical bone) and characterizes fracture toughness at crack growth initiation while J-int is fracture toughness at fracture instability (C).
Analyzing fresh cortical bone from 30 unique cadaveric femurs, Wang et al. reported that ultimate bending stress (σu,B), total work-to-fracture (Wf), and the critical stress intensity factor (KIc) to propagate a crack through a uniform beam of bone were all lower in the elderly group (>70 years; 4 males & 6 females) than in the young adult group (19-49 years; 8 males & 2 females) while the concentration of PEN (mmol/mol of col I) was higher in the elderly than the young group [7]. From tensile tests of demineralized bone, the investigators also observed that the failure strength of the organic matrix was also lower with age. Among the 30 donors, PEN negatively correlated with σu,B (r=−0.55), Wf (r=−0.62), and KIc (r=−0.48); whereas the tensile strength of collagen only correlated with Wf (r=+0.54) [7]. In subsequent tensile testing of cortical bone specimens from cadaveric tibia (8 females 53-90 years and 9 males 49-87 years) by the same lab, the overall tensile toughness and post-yield toughness (i.e., energy dissipated during failure and after permanent deformation occurs) were negatively correlated with PEN (r=−0.602 & r=−0.671, respectively) [8].
As for trabecular bone, Karim and Vashishth analyzed cores from the proximal tibia of 23 donors between 18 and 97 years (11 males and 12 females) [22]. Determining mechanical properties from unconfined compression tests to an apparent strain of 1.1%, PEN positively correlated with yield strain (Spearman r = +0.46) and ultimate strain (Spearman r = +0.44) as did fAGEs content. Correlations between toughness and either PEN or total fAGEs were negative but not significant. In these prior studies, mature enzymatic collagen crosslinks (PYD and DPD) did not correlate with the mechanical properties of bone.
With respect to femoral neck strength, Abraham et al. [83] analyzed cadaveric tibiae and femurs from 28 elderly donors by multiple techniques. Using backwards, stepwise regression, they reported that the combination of areal BMD of the femoral neck (macrostructural level), indentation distance increase from cyclic reference point indentation of the tibia (material level), cortical porosity of the tibia (microstructural level), and fluorescent AGEs of tibia cortical bone (ultrastructural level) were independent contributors to the prediction of the maximum force that the proximal femur could withstand in single stance configuration. Characterizing bone at a similar length scale as the force to break the proximal femur, aBMD had the strongest association with femoral neck strength (r=+0.755). Despite characterizing bone at the nanometer length scale, bulk fAGEs (tibia) was also significantly associated femoral neck strength (r=−0.336).
We conducted a comprehensive study of femoral cortical bone from a general population of 54 human donors [13]. We applied hydrothermal isometric tension (HIT) testing to evaluate the connectivity of the collagen network. HIT testing, while mechanistically relevant, involves decalcification of the tissue and super-physiological temperatures to melt the collagen and generate the measured tension. The connectivity parameter, Max Slope, positively correlates with crosslinking [65,84] and other factors that increase connections within the collagen and negatively correlates with fragmentation of the collagen [81,84,85]. In this study [13], there was a relatively strong, positive correlation between the bone collagen network connectivity measure Max Slope and the fracture toughness of the cortical bone indicating that the degree of connectivity within the collagenous matrix is related to the tissue’s ability to resist crack growth – a finding similar to those of a study by Zioupos et al. approximately twenty years earlier [86]. Notably, in our study, PEN content was not a significant factor in the statistical models when attempting to explain variation in the fracture toughness properties and did not correlate with the network connectivity of the demineralized bone either. This suggests that loss of fracture resistance of cortical bone is at least partially affected by a loss in the connectivity or integrity of the collagenous matrix which is independent of amount of AGE crosslink PEN present in the tissue.
Recently, the hypothesis of a negative relationship between AGE adducts and cortical bone mechanical properties has been tested. Matrix-bound CML correlated with crack propagation toughness of cortical bone as determined for 2 female donors (23 and 97 yo) and 4 male donors (25, 50, 61, 79 yo) [87] in which the mechanical properties were determined in a previous study [88]. More recently, the authors of this review recently found a negative, weak correlation between CML content and fracture toughness in a set of femoral cortical bone specimens from 53 donors [89]. CML increased with donor age (r = 0.560, p < 0.0001), and weakly correlated with transverse crack growth initiation fracture toughness (KIc; r = −0.284, p = 0.0293) and transverse J- at crack instability (J-int; r = −0.281, p = 0.0313). In multivariate general linear models (GLMs) with the fracture toughness as the dependent variable, CML content and our bone collagen network connectivity parameter, Max Slope, suggested a small negative contribution for CML and stronger positive contribution for Max Slope to the prediction of the fracture toughness measures.
While PEN content is often found to negatively correlate with the mechanical properties of bone, bone collagen network connectivity appears to be more predictive of cortical bone resistance to fracture, possibly because of generally very low PEN levels and mechanistic contributions from other crosslinks. Also, the non-crosslink CML adduct may contribute to these correlative effects by accumulating in tandem with other AGEs but disrupting normal enzymatic crosslinking (See Section 4).
One key challenge in conducting these types of studies using human cadaveric tissues is the large inter-donor variability. This therefore requires large sets of specimens to provide sufficient statistical power to detect strong correlations between parameters. This may explain differences in findings between studies; some with 10 to 20 donors versus others with 50 or more.
2.5. Age-related decrease in toughness and increase in PEN of rodent bone
Analysis of bones in pre-clinical models of aging support the negative association between mechanical properties and AGEs, namely PEN. Between 6-mo. (or 12-mo.) and 20-mo. of age, the cortical bone of the radius diaphysis lost toughness in male Fischer F344 rats, while PEN and the enzymatic PYD in cortical bone increased between 6-mo. and 20-mo. [90]. Between 6- mo. and 20-mo. of age, the cortical bone of the femur diaphysis lost toughness in male and female BALB/c mice, while PEN and PYD in the same bone increased between these two age groups [91]. In both pre-clinical studies, other changes to bone such as an increase in tissue mineral density and a decrease in bound water were observed. Also, in an early study of demineralized femora from male Wistar rats, the thermal stability, as determined by shrinkage temperature and melting temperature, decreased with age (2-, 5-, 15-, and 25-mo.) [92]. Lastly, we found that the relative abundance of deamidation, a spontaneous, non-enzymatic modification of Asn or Gln, at select sites of col I triple helix negatively correlated with toughness in BALB/c mice and that the resulting age-related increase in negative charge altered hydrogen bonding between col I and water [53]. This is consistent with the accumulation of CML observed in studies mentioned above. Nonetheless, the cause of the apparent decline in the molecular stability and connectivity of collagen I in human bone with age remains unknown.
2.6. Inconsistencies in whether PEN or AGE content increases as toughness of bone decreases in pre-clinical rodent models of type 1 diabetes and type 2 diabetes
The injection of streptozotocin (STZ) to kill islet cells, reduce the production of insulin, and increase circulating blood glucose in growing, male F344 (130 ± 25 mg/dl vs. 436 ± 72 mg/dl) and Sprague-Dawley rats (126 ± 38 mg/dl vs. 410 ± 69 mg/dl) caused an increase in PEN as determined for cortical bone of the femur but did not affect the energy-to-fracture (Uf) of the femur diaphysis subjected to three-point bending [93]. In this study, Uf was the area under the moment (Force x Span / 4) vs. span-adjusted displacement (12 x Displacement / Span2) curve meaning bone structure contributed to its determination, but post-yield displacement of the femur, an indicator of brittleness, was also not lower in the diabetic rats compared to their controls. In fact, the primary effect of type 1 diabetes (T1D) in this pre-clinical model on mechanical behavior of cortical bone was a reduction in the ultimate moment endured by the femur mid-shaft during the load-to-failure bending test. This measurement of bone strength is dependent on the structure and material strength of the mid-diaphysis. The loss in bone strength occurred in both strains of rats and was likely due to the STZ-induced loss of circulating insulin reducing periosteal expansion, thereby resulting in a reduced cross-sectional moment of inertia of the diaphysis [93].
In a rat model of spontaneous T2D (male WBN/Kob rat), fasting blood glucose increased from 100 ± 10 mg/dl to 450 ± 50 mg/dl between 10-mo. and 16-mo. of age as did PEN in the cortical bone of the femur diaphysis [94]. These levels were significantly higher in the diabetic WBN/Kob rats than in the non-diabetic, age-/sex-matched Wistar rats. Coincident with onset of hyperglycemia, the work-to-fracture (Wf) and the ultimate load of the femur diaphysis during three-point bending were lower in diabetic than in non-diabetic animals. However, the decline in the Wf and ultimate load as the duration of diabetes increased (10- to 18-mo.) in the WBN/Kob rats was modest unlike the rise in PEN over the same age range. Moreover, pooling all the WBN/Kob rats from the 10 age groups (1-mo. and 2-mo. to 18-mo. in 2-mo. intervals), PEN was positively correlated with Wf (Pearson r = +0.312) and ultimate load (Pearson r = +0.460). In another rat model of spontaneous T2D in which a high fight diet (HFD) was used to time the onset of hyperglycemia (ZDSD rat), toughness and fracture toughness (KIc), but not ultimate bending stress, progressively decreased between 16-wk., when the male rats were switched to the HFD for 7 weeks, and 29-wk. of age, while PEN and the enzymatic PYD in cortical bone increased [95]. These mechanical properties and PEN did not change between 16-wk and 29- wk in the non-diabetic, male CD(SD) rats (PYD similarly increased). Compared to CD(SD) rats with glucose levels of 86 ± 8 mg/dl, the ZDSD rats with glucose levels of 320 ± 51 mg/dl lost weight as they aged suggesting other organs were also being deleteriously affected by the unchecked diabetes.
There are multiple mouse models of T2D, each with advantages and disadvantages in their ability to phenocopy human complications of the disease [96], but few mouse studies have reported differences in PEN or fAGEs. Devlin et al. [97] found a trend toward higher fAGE in the cortical bone of diabetic TallyHO femurs compared to the non-diabetic SWR/J femurs (p=0.074). Blood glucose was variable among the TallyHO mice with some having fasting levels below 250 mg/dl, but on average, the levels were 2.5 times higher than those of SWR/J mice. Three-point bending tests revealed that the femur diaphysis of the TallyHO mice was clearly more brittle than the femur diaphysis of the SWR/J mice with 59% and 48% lower post-yield displacement in the diabetic animals at 8-wk. and 17-wk. of age. In a follow-up study of the brittle bone phenotype in the TallyHO model of juvenile-onset T2D with similar variance in glucose levels (non-fasting: 126 to 205 mg/dl for SWR/J and 206 to 578 mg/dl for TallyHO), Creecy et al. [69] found that the low bone toughness (femur diaphysis) at 16-wk. did not progressively worsen at 34-wk. of age (18 more weeks of hyperglycemia). Moreover, fAGE significantly increased with age but the difference between SWR/J and TallyHO only trended toward being higher in the diabetic animals (p=0.095). PEN, on the other hand, did not depend on the age or strain of the animal. As for the KK-Ay mouse model of spontaneous T2D, PEN in de-embedded proximal femurs was not significantly different between the diabetic Ay/a mice and the non-diabetic a/a littermates at 20 weeks of age, which is approximately 12 weeks of hyperglycemia at euthanasia (Ay/a: 426.5 ± 49.2 mg/dL vs. a/a: 234.5 ± 5.1 mg/dL) [98]. Interestingly, an infrared spectroscopy marker of mean collagen maturity or mature-to-immature crosslinking ratio was 12% higher in the proximal femur from the diabetic mice compared to the non-diabetic mice. Unfortunately, the study did not include mechanical testing of long bones. Lastly, in a diet-induced obesity (DIO) model of T2D, male C57BL/6 mice fed a HFD between 10-weeks and 32-weeks of age developed significantly higher blood glucose levels after 23- weeks of age reaching levels between 260 mg/dl and 340 mg/dl compared to the mice fed a low fat diet with levels between 190 mg/dl and 240 mg/dl [71]. In the femurs, there was an increase and decrease in fAGEs and crack initiation toughness (KIc) that negatively correlated (Pearson r = −0.80, p=0.0017) in addition to a negative correlation with interfibrillar spacing of mineral crystals (r = − 0.62, p = 0.032).
In summary, rodent models of diabetes partially support the idea that bone toughness decreases and AGE content in the matrix increases as the duration of hyperglycemia advances over time. Of note, the relationships between mechanical properties and AGE levels of bone depend on i) the degree to which the animals are hyperglycemic relative to control animals, ii) the age of the animal at which glucose levels increase and the duration of hyperglycemia before euthanasia, and iii) whether animals in each group are littermates, whether the animal prone to diabetes carries a mutation, or whether the control animals are similar genetically but a different strain nonetheless.
2.7. Clinical associations between urinary or serum AGEs and fracture risk
There is evidence that patients with osteoporosis have elevated levels of AGEs in their skeleton or in their circulation. In two publications by Saito and colleagues [99,100], discarded proximal femurs were acquired from hemiarthroplasty cases involving a fragility fracture of the femoral neck (females between 72 and 86 years of age) and compared to cadaveric proximal femurs from age-matched donors (females between 69 and 89 years of age). Cortical bone or trabecular bone, depending on the study, was extracted from the femoral neck region, fractionated into low- (1.7-2.0 g/ml) and high-density (>2.0 g/ml) mineral, and then hydrolyzed for collagen crosslink analysis. PEN in the low-density fraction of cortical bone was higher in the fracture case compared to the cadaver control [99], whereas PEN in both the low- and high-density fractions of trabecular bone was significantly higher in the fracture group than in the cadaver, non-fracture group [100].
In a follow-up study from the same group of investigators but involving 432 post-menopausal Japanese women (45-85 years) who were selected from the prospective Nagano Cohort Study after confirming they were not being treated for osteoporosis, baseline urinary pentosidine levels – determined by the same HPLC assay as bone PEN but normalized to mg of Cr instead of mol of collagen and log transformed – was a significant predictor of incident vertebral fractures over 5-year follow up period (5.2±3.3 years) [101]. Moreover, the highest quartile of PEN was an independent risk factor for a vertebral (VB) fracture (fx) after adjusting the Cox’s proportional hazard model for baseline age, lumbar aBMD, and number of previous vertebral fractures (Table 4). In a similar study involving 396 post-menopausal Caucasian women living in France, baseline urinary PEN was significantly higher by ~8% in women who experienced a fracture (vertebral and non-vertebral) than women who did not experience a fracture over the 10-year follow-up (10.1±2.6 years) [102]. However, the association between urinary PEN and increased fracture risk was not significant after adjusting for age, previous fracture, and hip T-score (Table 4). When the Cox proportional hazards model with multiple covariables was limited to 5 years of follow-up to better match the study in Japan, urinary PE was still not an independent predictor of fracture risk (for possible reasons for the discrepancy between the studies, see [102].)
Table 4.
Associations between fracture risk and AGE measurements as indicated by hazard ratio (HR), relative risk (RR), or odds ratio (OR). Red font indicates a non-significant association.
| Population | Sample size | Follow up (years) | Predictor | Unadjusted risk ratio (95% Cl) | Adjusted risk ratio (95% Cl) | Fx site | P-value | Ref |
|---|---|---|---|---|---|---|---|---|
| Post-menopausal Japanese women | 432 | 5.2±3.3 | A log increase in urinary PE | HR: 5.16 (2.95-8.78) | Not reported | VB | <0.01 | [101] |
| highest quartile of urinary PE over other PE quartiles | Not reported | 1.33 (1.01-1.76)a | VB | 0.04 | ||||
| Post-menopausal French women | 396 | 10.1±2.6 | A log increase in urinary PE | HR: 2.65 (1.23-5.76) | HR: 1.23 (0.54-2.82) b | Any | 0.01; 0.62 | [102] |
| highest quartile of urinary PE over other PE quartiles | HR: 1.55 (0.98-2.48) | HR: 1.16 (0.72-1.90) b | Any | 0.06; 0.56 | ||||
| Post-menopausal Japanese women | 765 | 5.1 c | 1 SD increase in urinary PE | Not reported | HR: 1.18 (1.05-1.33)d | VB | 0.005 | [103] |
| HR: 1.11 (0.88-1.41)d | Long bone | 0.381 | ||||||
| Elderly Caucasian and African American men and women (≥65 yo) | 3373 | 9.22 (5.12, 11.42) c | 1 SD increase in serum CML | HR: 1.27 (1.16-1.40 | HR: 1.25 (1.13-1.38) e | Hip | <0.001;<0.001 | [105] |
| HR: 1.18 (1.06-1.31)f | 0.003 | |||||||
| Post-menopausal Japanese women | 517 | No (−) prevalent fx vs. (+) prevalent fx | 1 SD increase in urinary log-PE | Not reported | OR: 1.93 (1.09-3.41)g | Any | 0.024 | [104] |
| Elderly females and males (70-79 yo) in the US with and without diabetes | 427 (non- DM) | 7.5±2.7 | 1 SD increase in urinary log-PE | RR: 0.97 (0.72-1.30)h | RR: 1.08 (0.79-1.49) i | Any | 0.817; 0.630 | [106] |
| 501 (T2D) | RR: 1.50 (1.22-1.85)h | RR: 1.42 (1.10-1.83) i | <0.00; 0.007 | |||||
| Japanese men and women (50-85 yo) with T2D | 77 (M) | No (−) VB fx vs. (+) VB fx | 1 SD increase in serum PE | Not reported | OR: 0.79 (0.41-1.52)j | VB | 0.475 | [107] |
| 76 (F) | Not reported | OR: 2.50 (1.09-5.73)j | 0.030 | |||||
| Age-matched females and males in the US with and without diabetes | 2332 (non-DM) | Clinical Fx: 10.9±5.2 | 1 SD increase in log CML | 1.07 (0.98–1.16)k | 1.03 (0.94–1.13)l | Any | 0.16; 0.50 | [109] |
| 712 (T2D) | Clinical Fx: 9.6±5.1 | 1 SD increase in log CML | 1.45 (1.22–1.73) | 1.49 (1.24– 1.79) | < 0.001<0.001 |
covariates in model were baseline age, lumbar aBMD, and number of prevalent vertebral fractures
covariates in model were baseline age, T-score of hip aBMD, and number of prevalent fractures
median follow up with or without interquartile range being reported (otherwise, mean±SD)
covariates in model were baseline age, body weight, diabetes mellitus, lumbar aBMD, prior fracture, and presence of back pain
covariates in model were age, gender, race/ethnicity, and clinic site
covariates in model were age, gender, race/ethnicity, and clinic site plus prevalent coronary heart disease, smoking, body mass index (BMI), alcohol use, level of physical activity, and baseline eGFR
covariates in model were age, gender, body weight and height, aBMD, smoking, alcohol drinking, urine NTx, serum hCRP, diabetes mellitus, and hypertension
covariates in model were age, gender, and race/ethnicity
covariates in model were age, gender, race/ethnicity, current smoker, baseline aBMD of hip, baseline weight, weight loss, cystatin-C, HbA1C, and use of vitamin D supplements, calcium supplements, and relevant medications.
covariates in model were age, body weight and height, HbA1C, eGFR, duration of diabetes, duration of postmenopausal state (F), presence of retinopathy or neuropathy, alcohol consumption, smoking, prevalent nonvertebral fractures, lumbar aBMD, and use of insulin/pioglitazone.
covariates in “unadjusted” model were age, race, sex, and clinic site.
covariates in “adjusted” model were age, race, sex, clinic site, current smoking status, total hip BMD, weight, weight loss of 5+ pounds in year before baseline, cystatin-C, A1c, and medication use.
Collecting urine from post-menopausal women in the Nagano cohort (N=765), urinary PEN was associated with an elevated VB fx risk but not an elevated long bone fx risk (Table 4). Based on receiver operating characteristic (ROC) analysis, there was a moderate improvement in risk classification when urinary PEN was added to 10-year probabilities of a fragility fracture: C statistic = 0.690 (95% CI: 0.639-0.741) using FRAX alone to classify vertebral body fracture vs. C statistic = 0.732 (95% CI: 0.686-0.778) combining FRAX and PEN [103]. In the most recent analysis involving the cohort of post-menopausal women in Japan, urinary PEN, as determined by ELISA instead of HPLC, was significantly associated with the prevalence of fracture before and after adjusting for known risk factors (Table 4) [104].
Circulating PEN is an imprecise marker of AGE content in bone because the urine and serum levels depend on resorption activity. To the best of our knowledge, there isn’t a published study testing the ability of combining markers of AGEs, bone resorption (CTX), and/or bone formation (P1NP) to predict fracture risk.
Pentosidine is not the only AGE to be associated with fracture risk. Applying an ELISA approach to the measurement of CML, a non-crosslinking AGE, in serum from participants (N=3373) in the US-based, prospective Cardiovascular Health Study, CML was significantly associated with increased risk of a hip fracture with and without adjustment for known risk factors (Table 4) [105]. Like urinary PEN (Fig. 4), the proportion of subjects who remained fracture free decreased as the quartile of CML at baseline increased (i.e., going from the lowest CML levels in the <25th percentile to the highest CML levels in the >75th percentile).
Figure 4. Kaplan-Meier curves of fracture occurrence for different quartiles of AGEs.
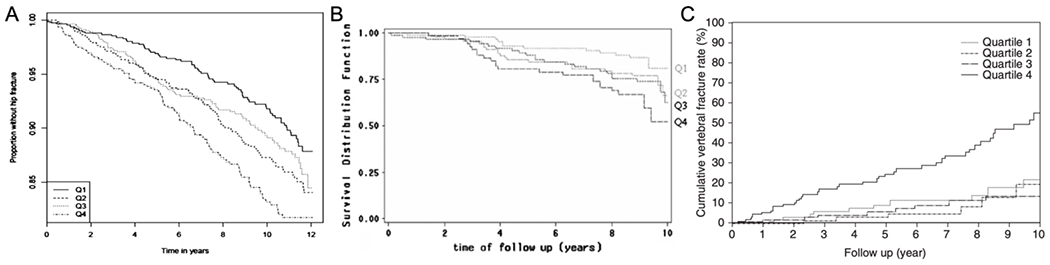
In three independent studies, those individuals with a circulating AGE level in the highest quartile (>75th percentile) were much more likely to experience a fracture over a 10-year period than individuals in the lowest quartile (<25th percentile): (A) Proportion of subjects without a hip fracture vs. time to follow-up for serum CML (Reproduced from Barzilay et al. J Bone Miner Res. 2014;), (B) Fraction of subjects without vertebral or peripheral fracture vs. time to follow-up for urinary PE (Reproduced from Gineyts et al. Osteoporos Int. 2010), and (C) Cumulative incidence of a vertebral fracture vs. time to follow-up for urinary PE (Reproduced from Shiraki et al. J Bone Miner Metab. 2008).
An association between pentosidine and elevated fracture risk has been observed in the elderly with T2D. Using the same HPLC analysis as Gineyts et al. [102], Schwartz et al. [106] compared urinary PEN between 427 subjects without diabetes (73.4±2.9 years of age) and 501 subjects with T2D (73.6±2.9 years of age). There was no difference in urinary PEN between the 2 groups with or without adjusting for age, gender, kidney function (eGFR), and previous weight loss. However, in logistical regression models, the log of PEN was significantly associated with a higher fracture risk but only for those with diabetes (Table 4). The significant relative risk (RR) in the T2D group and the non-significant RR in the non-diabetes group remained nearly the same after adjusting for additional factors such as glucose-lower medications and osteoporosis medications.
When serum PEN was measured in 77 Japanese men (50-80 years of age) and 76 Japanese post-menopausal women (50-85 years of age) with type 2 diabetes (HbA1c = 9.1±2.5% and 9.1±1.8%, respectively), there was not a significant difference in PEN between those men without vertebral fractures and those with a VB fx (Table 4). However, serum PEN was significantly higher in women with a VB fx than in women without a fracture [107]. The 28 men with vertebral fracture(s) were older than the men without a fracture, but the duration of diabetes was not significant between the two groups. The women with a VB fx, on the other hand, were not only older but had diabetes for a longer time (18.0±11.4 years) than women without a fracture. Upon adjusting the logistic regression for risk factors, serum PEN was significantly associated with the presence of vertebral fractures in women but not in the men (Table 4). In a similar study with a small sample size (7 males and 33 females), serum PEN was 72% higher in the VB fx group than in the non-fx group (p=0.04) [108]. In a recent study of fracture risk in T2D with the largest cohort to date [109], 1 SD increase in log CML (serum) was associated with ~1.5 times higher clinical fracture incidence for adults with T2D but not adults without diabetes (Table 4). Baseline CML was significantly higher in T2D than in non-diabetes. Once confined to prevalent VB fx, there was no significant association between log CML and fracture risk [109]. For those interested in how AGEs may cause bone fragility in chronic kidney disease, see the review article by Damrath et al. [110].
For the most part, clinical measurements of PEN in the previously cited studies either did not correlate or weakly correlated with aBMD (hip or spine), resorption marker (CTX or NTX), and duration of diabetes. PEN correlated with the age of the subjects, but there was considerable scatter in the relationship, which was not always linear. The fracture risk marginally increases with an increase in circulating PEN (Table 4), but this increase does not appear at the present time to be clinically meaningful since PEN is currently not part of the diagnosis of osteoporosis. A high PEN or CML value though is concerning as the likelihood of remaining fracture free is significantly reduced when the level is higher than 75th percentile (Fig. 4). Prospective studies likely need to demonstrate that an AGE marker adds value to the prediction of fracture risk by FRAX before serum or urinary measurements of PEN or CML become the standard of care in diagnosing osteoporosis regardless of whether a patient has diabetes.
2.8. Diabetes-related changes in bone that coincide with AGE accumulation
There are multiple diabetes-related changes to bone that possibly portend a loss of fracture resistance. For example, five different groups reported that the ability of the tibia mid-diaphysis to resist impact micro-indentation using an instrument called the OsteoProbe was lower in adults with type 2 diabetes than those without T2D [111–115]. While the determinants of Bone Material Strength Index (BMSi), the primary measurement of the OsteoProbe, are debatable [116,117], Samakkarnthai et al. [118] observed negative correlations between BMSi and skin AGEs (via autofluorescence) in 171 subjects with T2D (r=−0.30, p<0.0001) and 108 age-matched subjects without diabetes (r=−0.23, p=0.020). In a previous study of postmenopausal women with (N=16) and without T2D (N=19), BMSi was only correlated with skin autofluorescence in the T2D group (r=−0.65, p=0.006 compared to control: r=−0.24, p=0.92) [112]. As another example, two studies acquired discarded proximal femurs from patients who elected to undergo total hip arthroplasty, due to osteoarthritis of the hip, so that differences in cortical and trabecular bone between non-T2D (HbA1c < 6.5%; n≥19) and T2D (HbA1c > 6.5%; nࣙ12) could be ascertained [119,120]. In both studies, fluorescent AGEs (fAGEs) in the trabecular bone were not significantly different between the 2 groups. However, fAGEs in cortical bone [119] and PEN in trabecular bone [120] was significantly higher in T2D than in non-T2D subjects. In one study, trabecular fAGE was positively and negatively correlated with yield stress and post-yield displacement of trabecular bone (r=+0.56 and r=−0.66, respectively) [119], while in the other study, trabecular fAGE was not a significant predictor of ultimate stress and post-yield strain of trabecular bone [120]. The latter study involved stepwise linear regression in which trabecular BV/TV, mineral-to-matrix ratio, and age were positive contributors to the prediction of ultimate stress (adj-R2=0.694) and age, matrix maturity ratio, and PEN were negative contributors to the prediction of post-yield strain (adj-R2=0.116). The challenge in quantifying the relative contribution of AGEs to bone fragility is the multiple, hierarchical characteristics that all simultaneously contribute to the toughness and strength of bone.
3. Effect of in vitro glycation/ribation models on the mechanical properties of bone
One approach to the challenge of quantifying the relative contribution of AGEs to bone fragility has been to conduct in vitro experiments with controlled variables and conditions. The most common approach involves the incubation of bone specimens in physiological solutions (phosphate buffered saline, PBS, Hanks’ balanced salt solution, and HBSS, simulated body fluid, SBF) with and without reducing sugars, such as glucose and ribose. See Table 5 for a comprehensive listing of studies. These reducing sugars diffuse into the matrix of the bone and form a multitude of reaction products (i.e., adducts, crosslinks) through the process of non-enzymatic glycation (NEG; aka ribation or glycation). Commonly used ribose as well as the less reactive glucose leverage the Maillard reaction pathway [121]. Incubating bone in ribose at neutral pH readily forms a robust accumulation of PEN and fAGE relative to controls [64,65]. After the incubation process, glycated/ribated specimens are mechanically tested along with their incubated controls, and sometimes with non-incubated ‘native’ controls [65,122] to elucidate the effect of NEG on the mechanical behavior of bone. Widely used over the last two decades (Table 5), this approach continues today.
Table 5.
The effect of in vitro glycation on the mechanical properties of bone. Studies reporting no effect of glycation on a mechanical property shaded in blue grey.
| Species (age) | Incubation protocol (buffer, pH, anti-microbial) | Effect on AGEs | Effect on mechanical properties a | Ref |
|---|---|---|---|---|
| Cortical | bone | |||
| Human tibia (34-to 85-yr.) | 0.6 M ribose (HBSS with 30 mM HEPES, pH 7.2-7.4, PI) for 7 days at 37 °C | 104% higher fAGEs | No significant difference in longitudinal crack initiation toughness | [88] |
| 35% lower longitudinal crack growth toughness (compact tension) | ||||
| 42% reduction in failure strain, 72% lower creep rate, and 40% lower residual strain for stress levels > 100 MPa in multicyclic creep tests | ||||
| Human tibia (57-to 97-yr.) | 100 mM glucose (HBSS with 10 mM HEPES, pH 7.2-7.6, PI & 100 μg/mL streptomycin) for 3 and 7 days at 50 °C | 175% higher fAGEs after 7 days of incubation | ~40% higher indentation distance increase (IDI) after 3 days | [151] |
| ~18% higher IDI after 7 days | ||||
| No other cyclic reference point indentation (cRPI) properties reported | ||||
| Human tibia (57-to 86-yr.) | 0.6 M ribose (HBSS with 30 mM HEPES, pH 7.2-7.6, PI); 10 days at 37 °C | 136% higher fAGEs | 56% lower transverse crack initiation toughness (single edge notched beam) | [183] |
| 55% lower microindentation modulus | ||||
| No differences in cRPI properties including IDI | ||||
| Bovine tibia (20-mo.) | 0.67 M ribose (HBSS pH 7.3-7.6, 100 mL/L of toluene & chloroform & 0.5 mg/mL of gentamicin) for 15 days at 37 °C | Whitish compared to brownish color | No significant differences in elastic modulus and yield stress, but higher secant stiffness | [184] |
| Lower post-yield strain and damage fraction | ||||
| No significant difference in J-integral but lower plastic component of J-int with quasi-static loading rate | ||||
| No significant differences in the elastic and plastic component of the J-int under fall-like loading rate | ||||
| Bovine tibia (1.5- to 2-yr.) irradiated at 33 kGy | 1.8 M ribose (PBS, pH 7.4, NS) for 24 h at 60 °C | No effect due to irradiation 100-fold increase in PEN | Relative to irradiated control, no significant difference in modulus | [185] |
| 10% and 20% higher yield and ultimate stress | ||||
| 4% and 39% higher yield and failure strain | ||||
| 73% higher work-to-fracture (three-point bending tests of machined specimens) | ||||
| Bovine femur /tibia (18-mo.) | 0.67 M ribose (HBSS b NS c, 10 mL/L of toluene & chloroform & 0.5 mg/mL of gentamicin) for 3, 8, 11, 17, 29, 38 days at 37 °C | Whitish compared to dark brownish color fAGE = 1416 × [1– e−time/12] | No significant difference in compressive & tensile modulus, regardless of time | [64] |
| Tensile properties after 29-days | ||||
| 4.2% higher yield strain | ||||
| 9.3% higher yield stress | ||||
| 12.3% lower DF d | ||||
| no difference in post-yield strain | ||||
| Bovine femur (3-mo.) | 0.2 M ribose (PBS, pH neutral, 1% penicillin-streptomycin & 1% fungizone & PIf) for 15 days at 37 °C * | PEN not detected in control and ~250 mmol/mol in ribose | No significant differences in yield stress, yield strain, ultimate stress, ultimate strain, post-yield strain, energy to yield, and post-yield energy to fracture (three-point bending of machined specimens), and micro-hardness. | [186] |
| Bovine femur (18-mo.) | 0.67 M ribose (HBSS, NS, 10 mL/L of toluene & chloroform & 0.5 mg/mL of gentamicin) for 21 days at 37 °C | NS | No significant difference in thermal stability of mineralized bone. (Significantly higher melting temperature in ribated bone after demineralization in 45% formic acid) | [187] |
| Bovine metatarsal (18-to 24-mo.) | 0.6 M ribose (SBF g with HEPES h, pH 7.4, 10 mL/L of toluene & chloroform & 0.5 mg/mL gentamycin) for 14 days at 37 °C |
88% higher fAGEs PEN not detected in control and ~16 mmol/mol in ribose | No significant differences in modulus, yield stress, ultimate stress, and yield strain. | [65] |
| 26% and 27% lower post-yield strain and failure strain | ||||
| 21% and 29% lower toughness and post-yield toughness | ||||
| 11.5% and 11% DF and post-yield strain/failure strain (3pt bending of machined specimens) | ||||
| Rat femur/ tibia (4-mo.) | 0.2 M glucose (PBS e, pH 7.4, 0.1% sodium azide) for 60 days at 29 °C * | Whitish compared to brownish color | No significant differences in ultimate force, stiffness, displacement, energy-to-yield, and energy-to-fracture (three-point bending of diaphysis). | [188] |
| Trabecular | bone | |||
| Human proximal femurs (42-to 97-yr.) | 0.6 M ribose (HBSS with 30 mM HEPES, pH 7.3-7.6, PI) for 7 days at 37 °C | Whitish compared to brownish color; 90% higher fAGEs | No significant differences in apparent modulus, yield strain, yield stress, ultimate strain, and ultimate stress 92% lower post-yield strain energy and 38% lower DF (compression tests of cores) | [189] |
| Human tibial plateau (64-yr.) | 0.6 M ribose (HBSS with 30 mM HEPES, pH 7.3-7.6, PI) for 7 days at 37 °C | NS | No significant differences in apparent modulus at either 0.6% strain or 1.1% strain. | [190] |
| No difference in toughness at 0.6% strain | ||||
| 30% lower toughness at 1.1% strain (compression tests of trabecular cores). | ||||
| Porcine mandibular condyles | 0.2 M ribose (PBS; pH 7.4; 0.1% sodium azide & PI) for 15 days at 37 °C * | 200% higher PEN | No significant difference in tissue-level modulus (nanoindentation on dry trabeculae). | [191] |
Higher or lower change with glycation reported as 100 × (sugar – control) / control
Hanks’ balanced salt solution (HBSS) is typically Hanks buffer and 1.3 mM CaCI2
Not specified in the paper (NS)
Damage fraction (DF) is 1 – secant modulus at failure / initial modulus
Phosphate buffered saline (PBS)
Protease inhibitor (PI) cocktail is typically either a tablet (e.g., Roche Diagnostics) or 25 mM ε-amino-η-caproic acid, 5 mM benzamide, and 10 mM N-ethylmalemide
Simulated body fluid (SBF)
HEPES is a zwitterionic buffer
changed incubation media every day, every 3 days, every 4 days (volume not always specified), 5 times per week
3.1. Findings from in vitro AGE accumulation studies involving bone
In the first study to use in vitro ribation, Vashishth et al. [64] incubated bovine cortical bone specimens in 0.67 M ribose solution and found that the treatment increased fAGE, stiffened the bone col I (measured after decalcification) and led to a reduction in the ability of the tissue to plastically deform before fracture (detected as a ‘damage fraction’ parameter based on the secant modulus, Fig. 2A). Over the two decades since this publication, variations of this approach have been applied to both human and animal bone specimens leading to mixed and inconsistent results (Table 5). Confounded by differences in buffered solutions, concentration of sugar, incubation time and temperature, species of bone, antimicrobial agents, and in mechanical testing, studies either report no effect of AGE accumulation on the mechanical properties of bone or report a significant decrease in post-yield (plastic) properties of bone (Table 5).
Despite discrepancies in the in vitro glycation/ribation studies (Table 5), the consensus includes suppressed plasticity (Fig. 5A), suppressed ability to sustain damage stably (damage fraction based on the secant modulus at fracture), and suppressed fracture toughness (Fig. 5B). Interestingly, these effects seem to depend on the development of extensive browning of the ribated specimens.
Figure 5. The mechanical effects of the non-enzymatic glycation/ribation model on bone.
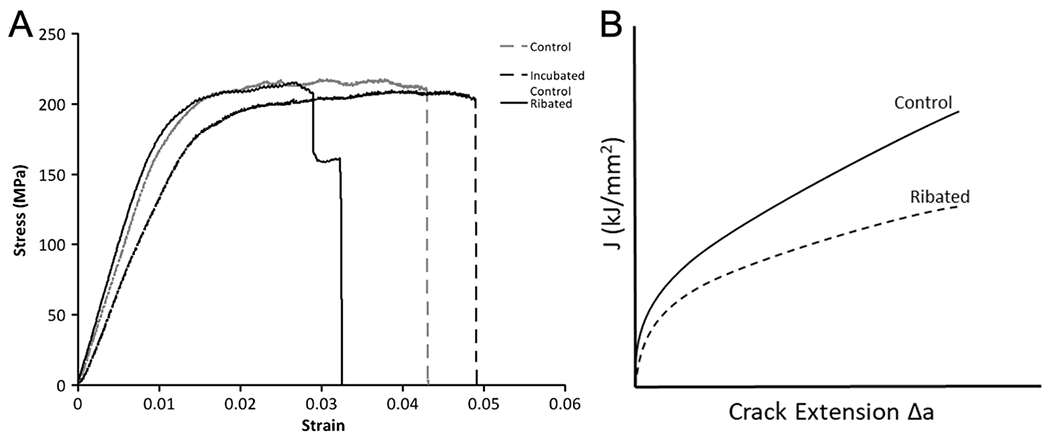
The effects of the non-enzymatic ribation model on mechanical behavior of bovine cortical bone tissue tested in three-point bending (A). Note the loss of post-yield strain in the Ribated group. (Reproduced from Willett et al. Bone. 2013). Schematic of the effects of the non-enzymatic ribation model on the R-curve of cortical bone (B), based on measurements reported in Jia et al. JMBBM 2021.
3.2. Mechanism by which in vitro glycation/ribation affects bone tissue
In the Willett et al. study (Table 5), ribation of bovine cortical bone significantly decreased post-yield strain (Table 5) while it increased PEN content and thermomechanical measures of collagen network connectivity, which are dependent on crosslinking and measured by hydrothermal isometric tension testing (HIT). Interestingly, within the ribated group, PEN and half-life of stress decay during HIT testing were positively correlated with measures of strain accommodation (post-yield strain) and energy absorption (work-to-fracture) before failure [65]. Clearly, the non-enzymatic ribation model of AGE accumulation reduced the cortical bone tissue’s toughness through a reduced capacity for post-yield strain accommodation. However, the positive correlations suggest that increased crosslinking may not provide a complete explanation for this ‘embrittlement’.
The AGE-related reduction in post-yield strain of cortical bone is often attributed to increased pathological inter-helical (helix-to-helix) collagen crosslinking that suppresses bone tissue plasticity [16,123]. Similar conclusions have previously been reached for soft tissues in which tendon viscoelasticity [124] and fibril-level sliding and plasticity mechanisms [125] are inhibited. Interestingly, work on individual collagen fibrils from tendons treated with methylglyoxal, a potent reactive metabolite that produces AGEs, demonstrated that treated fibrils were stiffer, stronger and less viscoelastic, but not necessarily more brittle [126]. Unfortunately, it is not clear how this translates to bone tissue, which is composed of mineralized collagen fibrils with presumably different mechanics due to their different nanocomposite structure and hierarchical organization.
Unlike normal collagen crosslinking catalyzed by the lysyl oxidase (LOX) enzyme, which forms crosslinks from a telopeptide to an helical site [16], it was thought that AGE crosslinks form between helical sites and prevent intra-fibrillar sliding. Computational models have been developed to explain this mechanism in which enzymatic crosslinks are at the terminal end of each collagen triple helix and non-enzymatic crosslinks are at random sites between collagen interfaces conferring and reducing inelastic deformation, respectively [123]. However, Chiue et al. [127] found that incubating rat tail tendons in ribose (<30 mM) mainly forms crosslinks at the same sites as the LOX crosslinks (i.e., telopeptide region to helical region), supporting the idea that AGEs can hinder enzymatic collagen crosslinking [32,33] and strengthening concerns regarding the role of NEG/AGE crosslinks in the age- and diabetes-related decline in fracture resistance. Greater certainty regarding the amount and location of AGE crosslinks and adducts in the organic matrix of bone is needed to inform such computational models of bone’s mechanical behavior. This calls for standardization of protocols to modify AGEs in bone and to detect different AGEs with respect to their location.
3.2.1. Insufficient control precludes accumulation of known AGEs
In addition to the questions regarding the location and consequence of the crosslinks formed during incubation of bone in ribose, this model of AGE accumulation is not sufficiently controlled to isolate the effects of specific crosslinks or adducts or other products alone. The spontaneous NEG reactions form a multitude of products that are difficult to measure. It is unclear which, if any, adducts are formed with ribation and what their effects are on the mechanical behavior of the tissue. In vivo, the formation of adducts, such as CML, may result in meaningful alterations in the profile of enzymatic and non-enzymatic collagen crosslinks [38] and in the charge distribution along the surface of a collagen molecule [59], thereby disrupting critical ionic and hydrogen bonding interactions among col I, mineral, water, and NCPs [53]. Certainly, the in vitro model does not recapitulate a loss of total crosslinking. In fact, total crosslinking increases. It is unknown if the surface charge of the col I and other collagens is modified by in vitro ribation.
Furthermore, Garnero et al. [122] noted that col I in ‘younger’ bovine bone tissue will mature when incubated in vitro in similar conditions to the controls in the ribation model. They used fetal bovine cortical bone and detected increased mature enzymatic collagen crosslink levels along with increased PEN levels. Willett et al. [65] also noted that, in bovine specimens from older steers than those used by Garnero et al. [122], the mature pyridinoline crosslinks (PYD and DPD) increased in both incubated controls and ribated specimens relative to non-incubated controls. In both studies, the bone tissue gained toughness due to incubation alone. Notably, this is consistent with the results of lathyrism studies that demonstrate the importance of the mature enzymatic collagen crosslinks towards bone tissue strength and toughness [128,129].
3.2.2. In vitro models are not consistent with the pathology
The in vitro model of AGE accumulation increases crosslinking, stabilization, stiffening, and strengthening of col I and other collagens in bone. Using differential scanning calorimetry and hydrothermal isometric tension (HIT) testing, incubating bovine cortical bone in 0.6 M ribose for 7 days at 37 °C (Table 5) increased thermal stability and connectivity of the organic matrix respectively [65]. This increase in stability and connectivity is not consistent with studies of human and primate cortical bone over a wide range of ages. Early studies of cadaveric bone found that the collagen network degrades. For example, Wang et al. observed that an age-related loss of tensile strength coincided with increased susceptibility to proteolysis by α- chymotrypsin, which is indicative of collagen network degradation and denaturation [7,130]. In the analysis of baboon femurs (5-26 years of age) and human femurs (19-89 years of age), the fracture toughness of the bone tissue negatively correlated with the percent of denatured collagen [130,131]. In their analysis of cadaveric femurs (35-92 years of age), Zioupos et al. found a negative correlation between network connectivity of demineralized bone (measured using a technique similar to HIT) and age and a positive correlation between the network connectivity and work-to-fracture as determined by three-point bending tests of uniform samples [86]. We found similar results in our recent study [13] (mentioned in Section 2.4) in which there was a relatively strong, positive correlation between bone collagen network connectivity measures and the fracture toughness of human cortical bone.
Based on this correlation, increased collagen network connectivity following AGE accumulation by non-enzymatic ribation could be expected to improve strength, plasticity and fracture toughness, but it does not. The non-specific accumulation of AGEs in in vitro glycation studies may promote and degrade the toughness of bone such that the observed difference, or lack thereof, in the mechanical behavior of bone depends on incubation conditions. Therefore, the findings from in vitro ribose-based, non-enzymatic glycation models should be treated with caution. They have led to a putative but possibly incorrect understanding of the role of AGEs in the pathology of bone fragility. There are important limitations and concerns that must be considered and addressed. There is a gap between the in vitro models and the known in vivo pathology. This represents an opportunity for the development of new in vitro models that are more pathophysiology relevant and mimetic.
4. The relative sparsity of AGE crosslinks and abundance of non-crosslink AGE adducts
Largely due to the in vitro ribation model and the elevated levels of PEN in aging and diseases, such as diabetes, the field has been focused on the concept of extraneous crosslinking-related suppression of bone tissue plasticity. Unfortunately, there is little empirical evidence that the effect observed in the model is consistent with the in vivo mechanism, as discussed above. Furthermore, and until recently, there has been a challenging issue with the quantity of the AGEs measured, specifically PEN [132].
The amount of LOX crosslinks in mature human bone is on the order of 1 to 1.3 moles per mol of collagen [32,133]. Of this, 1 mol per mol of collagen are the immature, divalent, LOX-derived crosslinks, and 0.1 to 0.3 mol per mol are the mature, trivalent enzymatic crosslinks. Strikingly, PEN content in bone is two to three orders of magnitude lower, in the 0.001 to 0.010 mol per mol range, than these enzymatic crosslinks. This low amount and the evidence that PEN may primarily form at the same sites as LOX crosslinks, rather than interconnecting helical regions of two neighboring col I molecules [127], challenges the idea that PEN plays a clinically meaningful role in bone fragility and suggests that it may simply be indicative of other AGEs.
Recently, a comprehensive mass spectrometry study by Arakawa et al. [32] demonstrated again that PEN content in bone is very small compared to the LOX crosslinks. Furthermore, PEN was orders of magnitude less abundant than four AGE adducts (Fig. 6). These AGE adducts were found to strongly correlate with PEN in a positive manner and occur at levels comparable to the LOX crosslinks. Therefore, while PEN, and perhaps GSPN, likely play a minor mechanistic role in bone fragility, PEN may be indicative of other AGEs that are not necessarily crosslinks but can affect bone mechanics. This alternative mechanism which does not involve crosslinks has yet to be tested.
Figure 6. AGEs and enzymatic crosslinks in human cancellous bone from lateral tibia plateau.
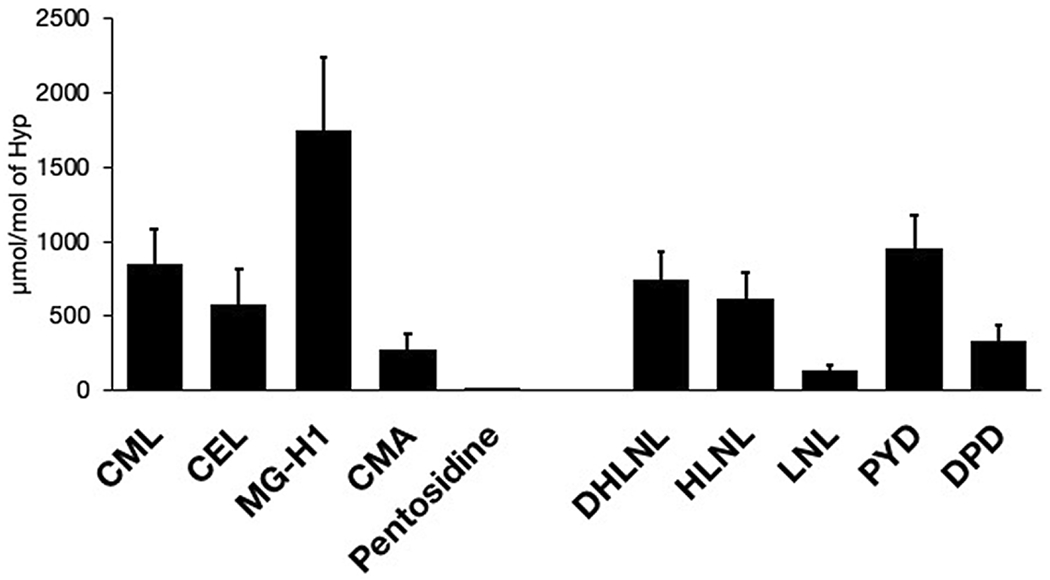
The filled bar and error bar reflect mean value and standard deviation. Abbreviations: Hyp, hydroxyproline; CML, Nε-(carboxymethyl)lysine; CEL, Nε-(carboxyethyl)lysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine 1; CMA, Nω-(carboxymethyl)arginine; DHLNL, dihydroxylysinonorleucine; HLNL, hydroxylysinonorleucine; LNL, lysinonorleucine; PYD, pyridinoline; DPD, deoxypyridinoline. (Reproduced from Arakara et al., Scientific Reports, 2020;)
Interestingly, Arakawa et al. demonstrated negative correlations between AGE adduct content and LOX crosslink content [32]. If AGE adducts compete for sites of enzymatic crosslinking on col I and other collagens (lysine, arginine), they could cause a net loss of enzymatic crosslinking (or maturation) and an overall decline in the stability, connectivity and force-carrying capacity of bone’s organic matrix, similar to those observed in human bone (Table 1) and to the effects measured in lathyrism models in which LOX crosslinking is inhibited [128,129]. One challenge to this hypothesis is that these AGE adducts would need to form early in the synthesis of new osteiod (i.e., bone collagenous matrix before mineralization) in order to compete with LOX for these reactive sites. At this time, the reaction kinetics of the formation of AGE adducts are poorly understood. Furthermore, it might require many years of deposition of compromised osteoid via osteonal remodeling before the mechanical properties of the bone tissue would be compromised enough to lead to bone fragility and perhaps fracture. Bone turnover in diabetes is thought to be slower than normal [134–136]. This may partially explain the gradual time course of bone fragility in diabetic patients and other disease groups.
In summary, measurable AGE crosslinks are not sufficiently abundant to affect bone fragility while non-crosslink AGE adducts may be. (Note: the levels of the most abundant physiological crosslink glucosepane have not yet been measured in bone.) The mechanisms of bone collagen stability and connectivity degradation do not appear to be directly related to PEN or CML but may be linked to a) loss of total LOX crosslinking due to competition with AGE adduct formation and b) other forms of collagen network degradation.
5. Mechanisms of AGE inhibition and challenges in clinical application of AGE inhibitors
Extensive AGE research over the past several decades resulted in the following proposed mechanisms of AGE inhibition:
Blocking adduction of glucose to lysine residue either via scavenging glucose or through protection of protein amino groups (e.g., polyamines [137] and aspirin [138], respectively).
Blocking oxidation of Amadori intermediate to AGEs (e.g., pyridoxamine [139]).
Scavenging of reactive dicarbonyl compounds derived from glucose autoxidation or oxidation of glucose protein adducts (e.g., aminoguanidine [140], pyridoxamine [141]).
Scavenging of reactive oxygen species (e.g., pyridoxamine [142]).
Breaking the existing AGE crosslinks (e.g., N-phenacylthiazolium bromide [143]).
However, since practically all the mechanistic studies have been conducted in vitro with or without cultured cells, it remains to be seen whether these mechanisms are also present in vivo. For example, it has been suggested that most AGE inhibitors in vivo act primarily via chelation of redox-active transition metal ions, mainly iron and copper [144]. Also targeting an intermediate of glycation reactions is a potential strategy to inhibit AGE accumulation in vivo. When glucose reacts with lysine, the resulting adduct undergoes intramolecular rearrangement forming the Amadori product, a key intermediate in AGE pathways [145]. An enzyme like amadoriase can de-glycate lysine residues and thus could be an in vivo treatment to inhibit AGE accumulation [146].
Dozens of AGE inhibitors have been proposed as drug candidates based on in vitro and animal studies, including synthetic compounds, phytochemicals, and plant extracts (for recent reviews, see [24,147]). In preclinical studies, inhibition of AGEs by pyridoxamine has been shown to inhibit AGE levels in skin and kidney while limiting progression of renal disease in diabetic rats [148]. Similarly, benfotiamine treatment inhibited retinal AGE levels and ameliorated diabetic retinopathy in the rat model [149]. Yet, despite significant research and clinical efforts in the past 35 years, only a few AGE inhibitors progressed to Phase 3 clinical trials (Table 2) and none of these potential therapeutic treatments received FDA approval. A challenge in translating the inhibition of AGE formation is understanding the biological effects of these inhibitors in vivo.
To date, there have been in vitro studies of AGE inhibitors to prevent bone fragility. With respect to human trabecular bone, inhibition of AGEs was investigated using phenacyl thiazolium bromide (PTB), a crosslink breaker [150]. Adding 2 different concentrations of PTB during ribation (0.6 M ribose at 37 °C for 3 days or 7 days) reduced fAGEs in trabecular bone from young donors (19-49 years) but less so in bone from old donors (50-80 years). In a similar study involving glycation (100 mM glucose at 50 °C for 7 days) of cortical human bone from female donors (57-97 years), the addition of either aminoguanidine or pyridoxamine prevented the accumulation of fAGEs [151]. Finally, upon collecting femurs from male C57BL/6J mice fed a high fat diet from 10-weeks to 32-weeks of age, femur mid-shafts were incubated for 7 days at 37 °C in phenacyl thiazolium chloride (PTC). Compared to non-PTC-treated femurs, this AGE breaker reduced fAGEs by 41.2% and increased maximum fracture toughness (KIc,max) by 35% [71]. The in vitro treatment with PTC however did not affect crack initiation toughness (KIc,init). While these results are encouraging, there are no published in vivo studies demonstrating effects of AGE inhibitors on AGE levels or pathophysiology of bone. Research is needed to understand the relative effects of AGE inhibitors on the material behavior of bone and on bone metabolism (e.g., resorption).
6. Limitations and Future Directions
One key limitation is that most human cadaveric tissue and in vivo animal model studies report negative correlations between AGEs and toughness rather than demonstrate causation or mechanistic insight. A second pertains to the lack of validated protocols to detect and quantify the different types of AGEs. Microplate readers and liquid chromatography can only quantify the bulk levels of fluorescent AGEs in a piece of bone tissue and so miss numerous non-enzymatic modifications of the organic matrix. These traditional techniques also do not indicate where in proteins the AGEs occur. These limitations may be overcome by the standardization of protocols between labs and the adoption of mass spectroscopy and proteomics techniques. Unfortunately, this requires greater labor and expense.
As another important limitation, in vitro glycation and ribation models meant to reveal mechanism are inconsistent in results between studies and with the established hallmarks of the pathology observed in human bone specimens. This includes the type of reactant and the AGEs formed, and their relative quantity, location and specific effects on the organic matrix. Again, these models and their protocols could be standardized and modified to include other factors involved in the pathology such as alternative reactive sugars and metabolites and established inflammatory factors such as oxidative stress. One possible avenue of research to elucidate mechanisms is the incubation of bone in glucose or ribose with different types of AGE inhibitors such that specific AGEs (e.g., CML over glucosepane) form [152]. This strategy can be couple with mechanical testing of human bone or rodent bone to determine which AGEs affect which mechanical properties.
The idea of competition between AGE adducts and LOX-derived crosslinks is a compelling hypothesis that to be tested requires interdisciplinary studies spanning multi-scale mechanics and biochemistry. For example, if overall collagen crosslinking is decreased due to adduct formation instead of enzymatic crosslink formation, how does this affect strength and toughening mechanisms such as collagen unravelling [153]? Rodent models may be useful addressing such questions. The maturation of enzymatic collagen crosslinking occurs with age in rodents and can be disrupted with a toxin β-aminopropionitrile [129], while AGE formation can be manipulated with high sugar or high fat diets and/or AGE inhibitors. The challenge again with in vivo studies is separating biological effects of any exogeneous compound and biophysical effects of PTMs.
Since non-collagenous proteins (NCPs) contribute to toughening mechanisms of bone at the molecular scale [154–156], there is the possibility NE-PTMs of NCPs (i.e., AGE accumulation on other proteins in bone) affect fibril-level deformation and sliding in a manner that is detrimental to the ability of bone to resist fracture. NCPs, such as osteopontin and osteocalcin, are small proteins that may be modified by AGE formation [157]. Though not as abundant as the collagens, NCPs are present in bone tissue and are thought to facilitate mineralization and adhesion between collagen and mineral [155]. The effects of AGEs on NCP structure and function, and subsequent effects on bone mechanics, have not been thoroughly investigated to date.
Since clinical trials of AGE inhibitors would require a large sample size to capture a difference in fracture incidence between a placebo group and a treatment group, a surrogate would be beneficial if it tracked treatment-related changes in bone. The standard surrogate, DXA-derived aBMD, is not expected to show efficacy unless the biological effects of AGE inhibitors prove to include a reduction in bone resorption and/or a promotion of bone formation. Thus, research is also needed to validate new biomarkers or surrogates of bone matrix quality. Two possible candidates are the OsteoProbe that assesses the ability of a person’s tibia to resist impact microindentation [158] and ultrashort-time-to-echo magnetic resonance imaging (UTE-MRI) that assesses bound and pore water concentrations of a person’s tibia as positive and negative correlates, respectively, of mechanical properties of human cortical bone [159]. In a study of cadaveric tibiae from female donors, BMSi correlated with fAGEs (r=−0.613, p<0.001) [160]. When cadaveric cortical bone samples were incubated in 0.1 M or 0.5 M ribose for 4 weeks, a large accumulation of AGEs decreased bound water, as determined by 1H nuclear magnetic resonance relaxometry, the basis of UTE-MRI [161]. Studies are needed to establish whether BMSi or bound water concentration are related to fracture incidence in the context of elevated AGEs.
7. Conclusions
Given the importance of collagen type I to the fracture resistance of bone, it is widely accepted that the age- and disease-related declines in bone toughness, namely post-yield toughness, are due to degradation of the organic matrix. Because glycation-mediated, non-enzymatic post-translation modifications (AGEs) accumulate in the organic matrix of bone with aging and chronic diseases such as diabetes, AGE accumulation is thought to be one reason why an individual’s fracture risk is higher than expected for a given X-ray-based measurement of bone mass at the hip or spine (T-score). While there is plenty of in vitro evidence that AGEs lower bone toughness, though not all glycation studies are supportive, in vivo evidence of AGE accumulation contributing to bone fragility is lacking. For example, the role of AGE adducts in the mechanical behavior of bone is unknown despite being far more abundant than AGE crosslinks. This gap in knowledge is problematic because the accumulation of AGE adducts has the potential to hinder enzymatic crosslinking and to negatively alter stabilizing interactions among collagens, water, non-collagenous proteins, and mineral.
Before collagen degradation can be translated to the clinic, several key gaps in knowledge must be addressed: i) causal relationships between AGEs and degradation in bone toughness and ii) use of methods to locate and quantify various AGEs in collagens and non-collagenous proteins. Otherwise, targeting AGE accumulation in bone to prevent or treat bone fragility is a difficult proposition. In addition, adopting serum or urinary measurements of AGEs as a clinical tool to diagnose osteoporosis or bone fragility likely requires a prospective study showing such AGE markers add value to the prediction of fracture risk by FRAX. Lastly, the opportunity is there for pre-clinical studies to assess the efficacy of AGE inhibitors or breakers in their ability to promote the ability of bone to resist fracture.
Highlights.
Accumulation of AGEs may contribute to bone fragility with aging and disease.
Whether crosslinking AGEs or AGE adducts are detrimental is unknown.
There is a need to quantify various AGEs and their location on proteins in bone.
Acknowledgements
Without support from National Institute of Arthritis and Musculoskeletal, and Skin Diseases, the Canadian Institutes of Health Research, and the VA Office of Research and Development, our research would not be possible. The content herein is of course the responsibility of the authors and does not necessarily represent the official views of these funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Burr DB, The contribution of the organic matrix to bone’s material properties, Bone. 31 (2002) 8–11. 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- [2].Burr DB, Martin RB, Schaffler MB, Radin EL, Bone remodeling in response to in vivo fatigue microdamage, J Biomech. 18 (1985) 189–200. 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- [3].Unal M, Creecy A, Nyman JS, The Role of Matrix Composition in the Mechanical Behavior of Bone, Curr Osteoporos Rep. 16 (2018) 205–215. 10.1007/s11914-018-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burstein AH, Reilly DT, Martens M, Aging of bone tissue: mechanical properties, J Bone Joint Surg Am. 58 (1976) 82–6. [PubMed] [Google Scholar]
- [5].McCalden RW, McGeough JA, Barker MB, Court-Brown CM, Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure., The Journal of Bone and Joint Surgery American Volume. 75 (1993) 1193–1205. [DOI] [PubMed] [Google Scholar]
- [6].Zioupos P, Currey JD, Changes in the Stiffness, Strength, and Toughness of Human Cortical Bone With Age, Bone. 22 (1998) 57–66. 10.1016/s8756-3282(97)00228-7. [DOI] [PubMed] [Google Scholar]
- [7].Wang X, Shen X, Li X, Agrawal CM, Age-related changes in the collagen network and toughness of bone., 31 (2002) 1–7. [DOI] [PubMed] [Google Scholar]
- [8].Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X, Age- related factors affecting the postyield energy dissipation of human cortical bone, J Orthopaed Res. 25 (2007) 646–655. 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Currey JD, Physical characteristics affecting the tensile failure properties of compact bone, J Biomech. 23(1990) 837–844. 10.1016/0021-9290(90)90030-7. [DOI] [PubMed] [Google Scholar]
- [10].Bala Y, Farlay D, Boivin G, Bone mineralization: from tissue to crystal in normal and pathological contexts, Osteoporosis Int. 24 (2013) 2153–2166. 10.1007/s00198-012-2228-y. [DOI] [PubMed] [Google Scholar]
- [11].Bergot C, Wu Y, Jolivet E, Zhou LQ, Laredo JD, Bousson V, The degree and distribution of cortical bone mineralization in the human femoral shaft change with age and sex in a microradiographic study, Bone. 45 (2009) 435–442. 10.1016/j.bone.2009.05.025. [DOI] [PubMed] [Google Scholar]
- [12].Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS, Identifying Novel Clinical Surrogates to Assess Human Bone Fracture Toughness, J Bone Miner Res. 30 (2015) 1290–1300. 10.1002/jbmr.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Willett TL, Dapaah DY, Uppuganti S, Granke M, Nyman JS, Bone collagen network integrity and transverse fracture toughness of human cortical bone, Bone. 120 (2019) 187–193. 10.1016/j.bone.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hein GE, Glycation endproducts in osteoporosis--is there a pathophysiologic importance?, Clinica Chimica Acta. 371 (2006) 32–36. 10.1016/j.cca.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [15].Vashishth D, The role of the collagen matrix in skeletal fragility., Curr Osteoporos Rep. 5 (2007) 62–66. 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- [16].Saito M, Marumo K, Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus., Osteoporosis International. 21 (2010) 195–214. 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- [17].Sroga GE, Vashishth D, Effects of bone matrix proteins on fracture and fragility in osteoporosis., Current Osteoporosis Reports. 10 (2012) 141–150. 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nyman JS, Makowski AJ, The Contribution of the Extracellular Matrix to the Fracture Resistance of Bone, Curr Osteoporos Rep. 10 (2012) 169–177. 10.1007/s11914-012-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burr DB, Changes in bone matrix properties with aging., Bone. 120 (2019) 85–93. 10.1016/j.bone.2018.10.010. [DOI] [PubMed] [Google Scholar]
- [20].Saito M, Age-related changes in biochemical characteristics of collagen from human weight-bearing and non-weight-bearing bone, Tokyo Jikeikai Medical Journal. 114 (1999) 327–337. [Google Scholar]
- [21].Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A, Advanced glycation end products and bone loss during aging, Annals of New York Academy of Science. 1043 (2005) 710–717. 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- [22].Karim L, Vashishth D, Heterogeneous Glycation of Cancellous Bone and Its Association with Bone Quality and Fragility, Plos One. 7 (2012) e35047. 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Monnier VM, Sell DR, Nagaraj RH, Miyata S, Grandhee S, Odetti P, Ibrahim SA, Maillard Reaction-Mediated Molecular Damage to Extracellular Matrix and Other Tissue Proteins in Diabetes, Aging, and Uremia, Diabetes. 41 (1992) 36–41. 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- [24].Freund MA, Chen B, Decker EA, The Inhibition of Advanced Glycation End Products by Carnosine and Other Natural Dipeptides to Reduce Diabetic and Age- Related Complications, Compr Rev Food Sci F. 17 (2018) 1367–1378. 10.1111/1541-4337.12376. [DOI] [PubMed] [Google Scholar]
- [25].Vestergaard P, Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis, Osteoporosis Int. 18 (2007) 427–444. 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- [26].Janghorbani M, Dam RMV, Willett WC, Hu FB, Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture., American Journal of Epidemiology. 166 (2007) 495–505. 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- [27].Thorpe SR, Baynes JW, Maillard reaction products in tissue proteins: New products and new perspectives, Amino Acids. 25 (2003) 275–281. 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- [28].Avery NC, Bailey AJ, The effects of the Maillard reaction on the physical properties and cell interactions of collagen, Pathol Biol. 54 (2006) 387–395. 10.1016/j.patbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [29].Sookwong P, Nakagawa K, Fujita I, Shoji N, Miyazawa T, Amadori-Glycated Phosphatidylethanolamine, a Potential Marker for Hyperglycemia, in Streptozotocin-Induced Diabetic Rats, Lipids. 46 (2011) 943. 10.1007/s11745-011-3588-3. [DOI] [PubMed] [Google Scholar]
- [30].Suzuki A, Yabu A, Nakamura H, Advanced glycation end products in musculoskeletal system and disorders., Methods. 2020 (2020) 4889. 10.1016/j.ymeth.2020.09.012. [DOI] [PubMed] [Google Scholar]
- [31].Nagai R, Shirakawa J, Fujiwara Y, Ohno R, Moroishi N, Sakata N, Nagai M, Detection of AGEs as markers for carbohydrate metabolism and protein denaturation, J Clin Biochem Nutr. 55 (2014) 1–6. 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Arakawa S, Suzuki R, Kurosaka D, Ikeda R, Hayashi H, Kayama T, Ohno R, Nagai R, Marumo K, Saito M, Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone, Sci Rep-Uk. 10 (2020) 18774. 10.1038/s41598-020-75923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hudson DM, Archer M, King KB, Eyre DR, Glycation of type I collagen selectively targets the same helical domain lysine sites as lysyl oxidase–mediated cross-linking, The Journal of Biological Chemistry. 293 (2018) 15620–15627. 10.1074/jbc.ra118.004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, Busse B, Alliston T, Ager JW, Ritchie RO, Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales., Proceedings of the National Academy of Sciences. 108 (2011) 14416–14421. 10.1073/pnas.1107966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fessel G, Li Y, Diederich V, Guizar-Sicairos M, Schneider P, Sell DR, Monnier VM, Snedeker JG, Advanced Glycation End-Products Reduce Collagen Molecular Sliding to Affect Collagen Fibril Damage Mechanisms but Not Stiffness, Plos One. 9 (2014) e110948. 10.1371/journal.pone.0110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, Monnier VM, Pentosidine: A molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia, Diabetes Metabolism Rev. 7 (1991) 239–251. 10.1002/dmr.5610070404. [DOI] [PubMed] [Google Scholar]
- [37].Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM, Glucosepane Is a Major Protein Cross-link of the Senescent Human Extracellular Matrix RELATIONSHIP WITH DIABETES*, J Biol Chem. 280 (2005) 12310–12315. 10.1074/jbe.m500733200. [DOI] [PubMed] [Google Scholar]
- [38].Monnier VM, Genuth S, Sell DR, The pecking order of skin Advanced Glycation Endproducts (AGEs) as long-term markers of glycemic damage and risk factors for micro- and subclinical macrovascular disease progression in Type 1 diabetes, Glycoconjugate J. 33 (2016) 569–579. 10.1007/s10719-016-9702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH, N- Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth, J Neurochem. 80 (2002) 998–1008. 10.1046/j.0022-3042.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- [40].Dattilo BM, Fritz G, Leclerc E, Kooi CWV, Heizmann CW, Chazin WJ, The Extracellular Region of the Receptor for Advanced Glycation End Products Is Composed of Two Independent Structural Units †, Biochemistry-Us. 46 (2007) 6957–6970. 10.1021/bi7003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz DS, Schmidt AM, Hoffmann R, Shekhtman A, Advanced Glycation End Product Recognition by the Receptor for AGEs, Structure. 19 (2011) 722–732. 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Yan SD, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM, N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression., J Biological Chem. 274 (1999) 31740–9. [DOI] [PubMed] [Google Scholar]
- [43].Buetler TM, Leclerc E, Baumeyer A, Latado H, Newell J, Adolfsson O, Parisod V, Richoz J, Maurer S, Foata F, Piguet D, Junod S, Heizmann CW, Delatour T, N(epsilon)-carboxymethyllysine-modified proteins are unable to bind to RAGE and activate an inflammatory response., Mol Nutr Food Res. 52 (2008) 370–8. 10.1002/mnfr.200700101. [DOI] [PubMed] [Google Scholar]
- [44].THORNALLEY PJ, BATTAH S, AHMED N, KARACHALIAS N, AGALOU S, BABAEI-JADIDI R, DAWNAY A, Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry, Biochem J. 375 (2003) 581–592. 10.1042/bj20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M, Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs, Cells. 11 (2022) 1312. 10.3390/cells11081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO, Advanced glycation endproducts in food and their effects on health, Food Chem Toxicol. 60 (2013) 10–37. 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- [47].Delgado-Andrade C, Roncero-Ramos I, Carballo J, Rufián-Henares J, Seiquer I, Navarro MP, Composition and functionality of bone affected by dietary glycated compounds., Food Funct. 4 (2013) 549–56. 10.1039/c2fo30187c. [DOI] [PubMed] [Google Scholar]
- [48].Knott L, Bailey AJ, Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance, Bone. 22 (1998) 181–187. 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- [49].Hudson DM, Eyre DR, Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification?, Connect Tissue Res. 54 (2013) 245–251. 10.3109/03008207.2013.800867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Thornalley PJ, Rabbani N, Protein damage in diabetes and uremia—identifying hotspots of proteome damage where minimal modification is amplified to marked pathophysiological effect, Free Radical Res. 45 (2010) 89–100. 10.3109/10715762.2010.534162. [DOI] [PubMed] [Google Scholar]
- [51].Chetyrkin S, Mathis M, Pedchenko V, Sanchez OA, McDonald WH, Hachey DL, Madu H, Stec D, Hudson B, Voziyan P, Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues., Biochemistry. 50 (2011) 6102–6112. 10.1021/bi200757d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jahouh F, Hou S, Kováč P, Banoub JH, Determination of glycation sites by tandem mass spectrometry in a synthetic lactose- bovine serum albumin conjugate, a vaccine model prepared by dialkyl squarate chemistry, Rapid Commun Mass Sp. 26 (2012) 749–758. 10.1002/rcm.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Creecy A, Brown KL, Rose KL, Voziyan P, Nyman JS, Post-translational modifications in collagen type I of bone in a mouse model of aging, Bone. 143 (2021) 115763. 10.1016/j.bone.2020.115763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stitt A, Frizzell N, Thorpe S, Advanced Glycation and Advanced Lipoxidation: Possible Role in Initiation and Progression of Diabetic Retinopathy, Curr Pharm Design. 10 (2004) 3349–3360. 10.2174/1381612043383124. [DOI] [PubMed] [Google Scholar]
- [55].Gooch C, Podwall D, The diabetic neuropathies., Neurologist. 10 (2004) 311–22. 10.1097/01.nrl.0000144733.61110.25. [DOI] [PubMed] [Google Scholar]
- [56].Rabbani N, Thornalley PJ, Emerging role of thiamine therapy for prevention and treatment of early- stage diabetic nephropathy, Diabetes Obes Metabolism. 13 (2011) 577–583. 10.1111/j.1463-1326.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- [57].Chen J-H, Lin X, Bu C, Zhang X, Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies, Nutr Metabolism. 15 (2018) 72. 10.1186/s12986-018-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Muthyalaiah YS, Jonnalagadda B, John CM, Arockiasamy S, Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression, Glycoconjugate J. 38 (2021) 717–734. 10.1007/s10719-021-10031-x. [DOI] [PubMed] [Google Scholar]
- [59].Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF, Increased serum levels of the specific AGE-compound methylglyoxal- derived hydroimidazolone in patients with type 2 diabetes, Metabolis. 52 (2003) 163–167. 10.1053/meta.2003.50035. [DOI] [PubMed] [Google Scholar]
- [60].Pozzi A, Zent R, Chetyrkin S, Borza C, Bulus N, Chuang P, Chen D, Hudson B, Voziyan P, Modification of collagen IV by glucose or methylglyoxal alters distinct mesangial cell functions., Journal of the American Society of Nephrology : JASN. 20 (2009) 2119–2125. 10.1681/asn.2008080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Streeter MD, Rowan S, Ray J, McDonald DM, Volkin J, Clark J, Taylor A, Spiegel DA, Generation and Characterization of Anti-Glucosepane Antibodies Enabling Direct Detection of Glucosepane in Retinal Tissue, Acs Chem Biol. 15 (2020) 2655–2661. 10.1021/acschembio.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ahmed N, Thornalley PJ, Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence, Biochem J. 364 (2002) 15–24. 10.1042/bj3640015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM, Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run., Journal of Chromatography. B, Biomedical Sciences and Applications. 703 (1997) 37–44. [DOI] [PubMed] [Google Scholar]
- [64].Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP, Influence of nonenzymatic glycation on biomechanical properties of cortical bone, Bone. 28 (2001) 195–201. 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- [65].Willett TL, Sutty S, Gaspar A, Avery N, Grynpas M, In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone., Bone. 52 (2013) 611–622. 10.1016/j.bone.2012.11.014. [DOI] [PubMed] [Google Scholar]
- [66].McAvan BS, France AP, Beilina B, Barran PE, Goodacre R, Doig AJ, Quantification of protein glycation using vibrational spectroscopy, Analyst. 145 (2020) 3686–3696. 10.1039/c9an02318f. [DOI] [PubMed] [Google Scholar]
- [67].Unal M, Uppuganti S, Leverant CJ, Creecy A, Granke M, Voziyan P, Nyman JS, Assessing glycation- mediated changes in human cortical bone with Raman spectroscopy, J Biophotonics. 11 (2018) e201700352. 10.1002/jbio.201700352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rubin MR, Paschalis EP, Poundarik A, Sroga GE, McMahon DJ, Gamsjaeger S, Klaushofer K, Vashishth D, Advanced Glycation Endproducts and Bone Material Properties in Type 1 Diabetic Mice, Plos One. 11 (2016) e0154700. 10.1371/journal.pone.0154700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Creecy A, Uppuganti S, Unal M, Bunn RC, Voziyan P, Nyman JS, Low bone toughness in the TallyHO model of juvenile type 2 diabetes does not worsen with age, Bone. 110 (2018) 204–214. 10.1016/j.bone.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shitole P, Choubey A, Mondal P, Ghosh R, Influence of Low Dose Naltrexone on Raman Assisted Bone Quality, Skeletal Advanced Glycation End-products and Nano-mechanical Properties in Type 2 Diabetic Mice Bone, Mater Sci Eng C. 123 (2021) 112011. 10.1016/j.msec.2021.112011. [DOI] [PubMed] [Google Scholar]
- [71].LLabre JE, Sroga GE, Tice MJL, Vashishth D, Induction and rescue of skeletal fragility in a high-fat diet mouse model of type 2 diabetes: An in vivo and in vitro approach, Bone. 156 (2022) 116302. 10.1016/j.bone.2021.116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Alsamad F, Brunel B, Vuiblet V, Gillery P, Jaisson S, Piot O, In depth investigation of collagen non-enzymatic glycation by Raman spectroscopy, Spectrochimica Acta Part Mol Biomol Spectrosc. 251 (2021) 119382. 10.1016/j.saa.2020.119382. [DOI] [PubMed] [Google Scholar]
- [73].Thornalley PJ, Rabbani N, Detection of oxidized and glycated proteins in clinical samples using mass spectrometry — A user’s perspective, Biochimica Et Biophysica Acta Bba - Gen Subj. 1840 (2014) 818–829. 10.1016/j.bbagen.2013.03.025. [DOI] [PubMed] [Google Scholar]
- [74].Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, Hudson BG, Caprioli RM, Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles., Journal of Lipid Research. 55 (2014) 1375–1385. 10.1194/jlr.m049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW, Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat., Kidney International. 61 (2002) 939–950. 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- [76].Samuel J, Park J-S, Almer J, Wang X, Effect of water on nanomechanics of bone is different between tension and compression, J Mech Behav Biomed. 57 (2016) 128–138. 10.1016/j.jmbbm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Anderson TL, Fracture Mechanics, (2017). 10.1201/9781315370293. [DOI] [Google Scholar]
- [78].Vashishth D, Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements, J Biomech. 37 (2004) 943–946. 10.1016/j.jbiomech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [79].Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO, Mechanistic aspects of fracture and R-curve behavior in human cortical bone, Biomaterials. 26 (2005) 217–231. 10.1016/j.biomaterials.2004.02.017. [DOI] [PubMed] [Google Scholar]
- [80].Yang QD, Cox BN, Nalla RK, Ritchie RO, Fracture length scales in human cortical bone: The necessity of nonlinear fracture models, Biomaterials. 27 (2006) 2095–2113. 10.1016/j.biomaterials.2005.09.040. [DOI] [PubMed] [Google Scholar]
- [81].Woodside M, Willett TL, Elastic–plastic fracture toughness and rising JR-curve behavior of cortical bone is partially protected from irradiation–sterilization-induced degradation by ribose protectant, J Mech Behav Biomed. 64 (2016) 53–64. 10.1016/j.jmbbm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- [82].Yan J, Mecholsky JJ, Clifton KB, How tough is bone? Application of elastic–plastic fracture mechanics to bone, Bone. 40 (2007) 479–484. 10.1016/j.bone.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [83].Abraham AC, Agarwalla A, Yadavalli A, McAndrew C, Liu JY, Tang SY, Multiscale Predictors of Femoral Neck In Situ Strength in Aging Women: Contributions of BMD, Cortical Porosity, Reference Point Indentation, and Nonenzymatic Glycation, J Bone Miner Res. 30 (2015) 2207–2214. 10.1002/jbmr.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Iranmanesh F, Willett TL, A linear systems model of the hydrothermal isometric tension test for assessing collagenous tissue quality, J Mech Behav Biomed. 125 (2022) 104916. 10.1016/j.jmbbm.2021.104916. [DOI] [PubMed] [Google Scholar]
- [85].Burton B, Gaspar A, Josey D, Tupy J, Grynpas MD, Willett TL, Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization, Bone. 61 (2014) 71–81. 10.1016/j.bone.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [86].Zioupos P, Currey JD, Hamer AJ, The role of collagen in the declining mechanical properties of aging human cortical bone, J Biomed Mater Res. 45 (1999) 108–116. . [DOI] [PubMed] [Google Scholar]
- [87].Thomas CJ, Cleland TP, Sroga GE, Vashishth D, Accumulation of carboxymethyllysine (CML) in human cortical bone., Bone. 110 (2018) 128–133. 10.1016/j.bone.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Poundarik AA, Wu P-C, Evis Z, Sroga GE, Ural A, Rubin M, Vashishth D, A direct role of collagen glycation in bone fracture., Journal of the Mechanical Behavior of Biomedical Materials. 52 (2015) 120–130. 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Willett TL, Dapaah DY, Tupy J, Uppuganti S, Nyman JS, N-ε-(Carboxymethyl)Lysine Correlates With The Degradation of Human Cortical Bone Fracture Resistance, in: ORS 2022 Annual Meeting, Tampa, Florida, 2022: p. 435. https://www.ors.org/abstract-search/. [Google Scholar]
- [90].Uppuganti S, Granke M, Makowski AJ, Does MD, Nyman JS, Age-related changes in the fracture resistance of male Fischer F344 rat bone, Bone. 83 (2016) 220–232. 10.1016/j.bone.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Creecy A, Uppuganti S, Girard MR, Schlunk SG, Amah C, Granke M, Unal M, Does MD, Nyman JS, The age-related decrease in material properties of BALB/c mouse long bones involves alterations to the extracellular matrix, Bone. 130 (2020) 115126. 10.1016/j.bone.2019.115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Danielsen CC, Age-related thermal stability and susceptibility to proteolysis of rat bone collagen., The Biochemical Journal. 272 (1990) 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, Wang X, Type 1 Diabetes in Young Rats Leads to Progressive Trabecular Bone Loss, Cessation of Cortical Bone Growth, and Diminished Whole Bone Strength and Fatigue Life, J Bone Miner Res. 24 (2009) 1618–1627. 10.1359/jbmr.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Saito M, Fujii K, Mori Y, Marumo K, Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats., Osteoporosis International. 17 (2006) 1514–1523. 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- [95].Creecy A, Uppuganti S, Merkel AR, O’Neal D, Makowski AJ, Granke M, Voziyan P, Nyman JS, Changes in the Fracture Resistance of Bone with the Progression of Type 2 Diabetes in the ZDSD Rat, Calcified Tissue Int. 99 (2016) 289–301. 10.1007/s00223-016-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fajardo RJ, Karim L, Calley VI, Bouxsein ML, A Review of Rodent Models of Type 2 Diabetic Skeletal Fragility, J Bone Miner Res. 29 (2014) 1025–1040. 10.1002/jbmr.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Devlin MJ, Vliet MV, Motyl K, Karim L, Brooks DJ, Louis L, Conlon C, Rosen CJ, Bouxsein ML, Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse., Endocrinology. 155 (2014) 3806–3816. 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hunt HB, Pearl JC, Diaz DR, King KB, Donnelly E, Bone Tissue Collagen Maturity and Mineral Content Increase With Sustained Hyperglycemia in the KK-Ay Murine Model of Type 2 Diabetes., Journal of Bone and Mineral Research. 33 (2018) 921–929. 10.1002/jbmr.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Saito M, Fujii K, Soshi S, Tanaka T, Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture., Osteoporosis International. 17 (2006) 986–995. 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- [100].Saito M, Fujii K, Marumo K, Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls., Calcified Tissue International. 79 (2006) 160–168. 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- [101].Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T, Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures, J Bone Miner Metab. 26 (2008) 93–100. 10.1007/s00774-007-0784-6. [DOI] [PubMed] [Google Scholar]
- [102].Gineyts E, Munoz F, Bertholon C, Sornay-Rendu E, Chapurlat R, Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study, Osteoporosis Int. 21 (2010) 243–250. 10.1007/s00198-009-0939-5. [DOI] [PubMed] [Google Scholar]
- [103].Tanaka S, Kuroda T, Saito M, Shiraki M, Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women, J Bone Miner Res. 26 (2011) 2778–2784. 10.1002/jbmr.467. [DOI] [PubMed] [Google Scholar]
- [104].Shiraki M, Kashiwabara S, Imai T, Tanaka S, Saito M, The association of urinary pentosidine levels with the prevalence of osteoporotic fractures in postmenopausal women, J Bone Miner Metab. 37 (2019) 1067–1074. 10.1007/s00774-019-01017-9. [DOI] [PubMed] [Google Scholar]
- [105].Barzilay JI, Bůžková P, Zieman SJ, Kizer JR, Djoussé L, Ix JH, Tracy RP, Siscovick DS, Cauley JA, Mukamal KJ, Circulating Levels of Carboxy- Methyl- Lysine (CML) Are Associated With Hip Fracture Risk: The Cardiovascular Health Study, J Bone Miner Res. 29 (2014) 1061–1066. 10.1002/jbmr.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, H. Study Aging, and Body Composition, Pentosidine and Increased Fracture Risk in Older Adults with Type 2 Diabetes, J Clin Endocrinol Metabolism. 94 (2009) 2380–2386. 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T, Serum Pentosidine Levels Are Positively Associated with the Presence of Vertebral Fractures in Postmenopausal Women with Type 2 Diabetes, Journal of Clinical Endocrinology & Metabolism. 93 (2008) 1013–1019. 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- [108].Choi D-H, Lee S-M, Lim S-A, Choi Y-S, Feasibility of Serum Pentosidine Level as a Potential Risk Factor for Osteoporotic Vertebral Compression Fracture., Asian Spine Journal. 12 (2018) 992–997. 10.31616/asj.2018.12.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Dhaliwal R, Ewing SK, Vashishth D, Semba RD, Schwartz AV, Greater Carboxy-Methyl-Lysine Is Associated With Increased Fracture Risk in Type 2 Diabetes, J Bone Miner Res. 37 (2022) 265–272. 10.1002/jbmr.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Damrath JG, Creecy A, Wallace JM, Moe SM, The impact of advanced glycation end products on bone properties in chronic kidney disease, Curr Opin Nephrol Hypertens. Publish Ahead of Print (2021) 411–417. 10.1097/mnh.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Farr JN, Drake MT, Amin S, Melton LJ, McCready LK, Khosla S, In Vivo Assessment of Bone Quality in Postmenopausal Women With Type 2 Diabetes, J Bone Miner Res. 29 (2014) 787–795. 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Furst JR, Bandeira LC, Fan W-W, Agarwal S, Nishiyama KK, McMahon DJ, Dworakowski E, Jiang H, Silverberg SJ, Rubin MR, Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes, J Clin Endocrinol Metabolism. 101 (2016) 2502–2510. 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M, Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population- Based Study, J Bone Miner Res. 32 (2017) 1062–1071. 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- [114].Holloway-Kew KL, Betson A, Rufus-Membere PG, Gaston J, Diez-Perez A, Kotowicz MA, Pasco JA, Impact microindentation in men with impaired fasting glucose and type 2 diabetes, Bone. 142 (2021) 115685. 10.1016/j.bone.2020.115685. [DOI] [PubMed] [Google Scholar]
- [115].Syversen U, Mosti MP, Mynarek IM, Vedal TSJ, Aasarød K, Basso T, Reseland JE, Thorsby PM, Asvold BO, Eriksen EF, Stunes AK, Evidence of impaired bone quality in men with type 1 diabetes: a cross-sectional study, Endocr Connect. 10 (2021) 955–964. 10.1530/ec-21-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Buckley K, Kerns JG, Vinton J, Gikas PD, Smith C, Parker AW, Matousek P, Goodship AE, Towards the in vivo prediction of fragility fractures with Raman spectroscopy, J Raman Spectrosc. 46 (2015) 610–618. 10.1002/jrs.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Diez-Perez A, Bouxsein ML, Eriksen EF, Khosla S, Nyman JS, Papapoulos S, Tang SY, Technical note: Recommendations for a standard procedure to assess cortical bone at the tissue-level in vivo using impact microindentation, Bone Reports. 5 (2016) 181–185. 10.1016/j.bonr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Samakkarnthai P, Sfeir JG, Atkinson EJ, Achenbach SJ, Wennberg PW, Dyck PJ, Tweed AJ, Volkman TL, Amin S, Farr JN, Vella A, Drake MT, Khosla S, Determinants of Bone Material Strength and Cortical Porosity in Patients with Type 2 Diabetes Mellitus, J Clin Endocrinol Metabolism. 105 (2020) dgaa388. 10.1210/clinem/dgaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Karim L, Moulton J, Vliet MV, Velie K, Robbins A, Malekipour F, Abdeen A, Ayres D, Bouxsein ML, Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes, Bone. 114 (2018) 32–39. 10.1016/j.bone.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hunt HB, Torres AM, Palomino PM, Marty E, Saiyed R, Cohn M, Jo J, Warner S, Sroga GE, King KB, Lane JM, Vashishth D, Hernandez CJ, Donnelly E, Altered Tissue Composition, Microarchitecture, and Mechanical Performance in Cancellous Bone From Men With Type 2 Diabetes Mellitus., Journal of Bone and Mineral Research. 34 (2019) 1191–1206. 10.1002/jbmr.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sroga GE, Siddula A, Vashishth D, Glycation of Human Cortical and Cancellous Bone Captures Differences in the Formation of Maillard Reaction Products between Glucose and Ribose, Plos One. 10 (2015) e0117240. 10.1371/journal.pone.0117240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD, Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone, Bone. 38 (2006) 300–309. 10.1016/j.bone.2005.09.014. [DOI] [PubMed] [Google Scholar]
- [123].Siegmund T, Allen MR, Burr DB, Failure of mineralized collagen fibrils: Modeling the role of collagen cross-linking, J Biomech. 41 (2008) 1427–1435. 10.1016/j.jbiomech.2008.02.017. [DOI] [PubMed] [Google Scholar]
- [124].Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG, Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue, Matrix Biology : Journal of the International Society for Matrix Biology. 59 (2017) 95–108. 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- [125].Lee JM, Veres SP, Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon, J Appl Physiol. 126 (2019) 832–841. 10.1152/japplphysiol.00430.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Svensson RB, Smith ST, Moyer PJ, Magnusson SP, Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons, Acta Biomaterialia. 70 (2018) 270–280. 10.1016/j.actbio.2018.02.005. [DOI] [PubMed] [Google Scholar]
- [127].Chiue H, Yamazoye T, Matsumura S, Localization of the dominant non-enzymatic intermolecular cross-linking sites on fibrous collagen, Biochemical and Biophysical Research Communications. 461 (2015) 445–449. 10.1016/j.bbrc.2015.04.011. [DOI] [PubMed] [Google Scholar]
- [128].Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R, Gamsjaeger S, Buchinger B, Roschger A, Phipps R, Boskey AL, Dall’Ara E, Varga P, Zysset P, Klaushofer K, Roschger P, Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral., Bone. 49 (2011) 1232–1241. 10.1016/j.bone.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].McNerny EM, Gong B, Morris MD, Kohn DH, Bone Fracture Toughness and Strength Correlate With Collagen Cross- Link Maturity in a Dose- Controlled Lathyrism Mouse Model, J Bone Miner Res. 30 (2015) 455–464. 10.1002/jbmr.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang X, Li X, Shen X, Agrawal CM, Age-Related Changes of Noncalcified Collagen in Human Cortical Bone, Ann Biomed Eng. 31 (2003) 1365–1371. 10.1114/1.1623488. [DOI] [PubMed] [Google Scholar]
- [131].Wang X, Bank R, Tekoppele J, Athanasiou K, Agrawal C, Effect of collagen denaturation on bone biomechanical integrity, in: 45th Annual Meeting of the Orthopaedic Research Society, 1999: p. 782. [Google Scholar]
- [132].Pritchard JM, Willett TL, Pentosidine as a Biomarker for Poor Bone Quality and Elevated Fracture Risk, in: Patel VB, Preedy VR (Eds.), Springer, 2017: pp. 355–392. 10.1007/978-94-007-7693-7_32. [DOI] [Google Scholar]
- [133].Saito M, Marumo K, Fujii K, Ishioka N, Single-Column High-Performance Liquid Chromatographic-Fluorescence Detection of Immature, Mature, and Senescent Cross-Links of Collagen, Anal Biochem. 253 (1997) 26–32. 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- [134].Gerdhem P, Isaksson A, Åkesson K, Obrant KJ, Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus, Osteoporosis Int. 16 (2005) 1506–1512. 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- [135].Starup-Linde J, Lykkeboe S, Handberg A, Vestergaard P, Høyem P, Fleischer J, Hansen TK, Poulsen PL, Laugesen E, Glucose variability and low bone turnover in people with type 2 diabetes, Bone. 153 (2021) 116159. 10.1016/j.bone.2021.116159. [DOI] [PubMed] [Google Scholar]
- [136].Rubin MR, de Boer IH, Backlund J-YC, Arends V, Gubitosi-Klug R, Wallia A, Gregory NS, Barnie A, Burghardt AJ, Lachin JM, Braffett BH, Schwartz AV, Biochemical Markers of Bone Turnover in Older Adults with Type 1 Diabetes, J Clin Endocrinol Metabolism. (2022). 10.1210/clinem/dgac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Méndez JD, Leal LI, Inhibition of in vitro pyrraline formation by l-arginine and polyamines, Biomed Pharmacother. 58 (2004) 598–604. 10.1016/j.biopha.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [138].Rao GN, Lardis MP, Cotlier E, Acetylation of lens crystalline: A possible mechanism by which aspirin could prevent cataract formation, Biochem Bioph Res Co. 128 (1985) 1125–1132. 10.1016/0006-291x(85)91057-5. [DOI] [PubMed] [Google Scholar]
- [139].Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG, Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions., The Journal of Biological Chemistry. 278 (2003) 46616–46624. 10.1074/jbc.m307155200. [DOI] [PubMed] [Google Scholar]
- [140].Webster J, Urban C, Berbaum K, Loske C, Alpar A, GÄrtner U, Arriba SGD, Arendt T, MÜnch G, The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal, Neurotox Res. 7 (2005) 95–101. 10.1007/bf03033780. [DOI] [PubMed] [Google Scholar]
- [141].Voziyan PA, Metz TO, Baynes JW, Hudson BG, A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation., The Journal of Biological Chemistry. 277 (2002) 3397–3403. 10.1074/jbc.m109935200. [DOI] [PubMed] [Google Scholar]
- [142].Chetyrkin SV, Mathis ME, Ham A-JL, Hachey DL, Hudson BG, Voziyan PA, Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: inhibition by pyridoxamine., Free Radical Biology & Medicine. 44 (2008) 1276–1285. 10.1016/j.freeradbiomed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [143].Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P, An agent cleaving glucose-derived protein crosslinks in vitro and in vivo, Nature. 382 (1996) 275–278. 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- [144].Nagai R, Murray DB, Metz TO, Baynes JW, Chelation: A Fundamental Mechanism of Action of AGE Inhibitors, AGE Breakers, and Other Inhibitors of Diabetes Complications, Diabetes. 61 (2012) 549–559. 10.2337/db11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Monnier VM, Sell DR, Dai Z, Nemet I, Collard F, Zhang J, The Role of the Amadori Product in the Complications of Diabetes, Ann Ny Acad Sci. 1126 (2008) 81–88. 10.1196/annals.1433.052. [DOI] [PubMed] [Google Scholar]
- [146].Monnier VM, Sell DR, Prevention and Repair of Protein Damage by the Maillard Reaction In Vivo, Rejuv Res. 9 (2006) 264–273. 10.1089/rej.2006.9.264. [DOI] [PubMed] [Google Scholar]
- [147].Sarmah S, Roy AS, A review on prevention of glycation of proteins: Potential therapeutic substances to mitigate the severity of diabetes complications, Int J Biol Macromol. 195 (2022) 565–588. 10.1016/j.ijbiomac.2021.12.041. [DOI] [PubMed] [Google Scholar]
- [148].Alderson NL, Chachich ME, Frizzell N, Canning P, Metz TO, Januszewski AS, Youssef NN, Stitt AW, Baynes JW, Thorpe SR, Effect of antioxidants and ACE inhibition on chemical modification of proteins and progression of nephropathy in the streptozotocin diabetic rat, Diabetologia. 47 (2004) 1385–1395. 10.1007/s00125-004-1474-8. [DOI] [PubMed] [Google Scholar]
- [149].Hammes H-P, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M, Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy, Nat Med. 9 (2003) 294–299. 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- [150].Bradke BS, Vashishth D, N-Phenacylthiazolium Bromide Reduces Bone Fragility Induced by Nonenzymatic Glycation, Plos One. 9 (2014) e103199. 10.1371/journal.pone.0103199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Abar O, Dharmar S, Tang SY, The effect of aminoguanidine (AG) and pyridoxamine (PM) on ageing human cortical bone, Bone Jt Res. 7 (2018) 105–110. 10.1302/2046-3758.71.bjr-2017-0135.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sroga GE, Vashishth D, Controlled Formation of Carboxymethyllysine in Bone Matrix through Designed Glycation Reaction., Jbmr Plus. 5 (2021) e10548. 10.1002/jbm4.10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Seelemann CA, Willett TL, Empirical evidence that bone collagen molecules denature as a result of bone fracture., J Mech Behav Biomed. 131 (2022) 105220. 10.1016/j.jmbbm.2022.105220. [DOI] [PubMed] [Google Scholar]
- [154].Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D, Dilatational band formation in bone., Proceedings of the National Academy of Sciences. 109 (2012) 19178–19183. 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Morgan S, Poundarik AA, Vashishth D, Do Non-collagenous Proteins Affect Skeletal Mechanical Properties?, Calcified Tissue Int. 97 (2015) 281–291. 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Cavelier S, Dastjerdi AK, McKee MD, Barthelat F, Bone toughness at the molecular scale: A model for fracture toughness using crosslinked osteopontin on synthetic and biogenic mineral substrates, Bone. 110 (2018) 304–311. 10.1016/j.bone.2018.02.022. [DOI] [PubMed] [Google Scholar]
- [157].Thomas CJ, Cleland TP, Zhang S, Gundberg CM, Vashishth D, Identification and characterization of glycation adducts on osteocalcin., Anal Biochem. 525 (2017) 46–53. 10.1016/j.ab.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Schoeb M, Hamdy NAT, Malgo F, Winter EM, Appelman-Dijkstra NM, Added Value of Impact Microindentation in the Evaluation of Bone Fragility: A Systematic Review of the Literature, Front Endocrinol. 11 (2020) 15. 10.3389/fendo.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Manhard MK, Nyman JS, Does MD, Advances in imaging approaches to fracture risk evaluation, Transl Res. 181 (2017) 1–14. 10.1016/j.trsl.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Abraham AC, Agarwalla A, Yadavalli A, Liu JY, Tang SY, Microstructural and compositional contributions towards the mechanical behavior of aging human bone measured by cyclic and impact reference point indentation, Bone. 87 (2016) 37–43. 10.1016/j.bone.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Nyman JS, Uppuganti S, Unal M, Leverant CJ, Adabala S, Granke M, Voziyan P, Does MD, Manipulating the Amount and Structure of the Organic Matrix Affects the Water Compartments of Human Cortical Bone, JBMR Plus. 3 (2019) e10135. 10.1002/jbm4.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gauthier R, Follet H, Langer M, Gineyts E, Rongiéras F, Peyrin F, Mitton D, Relationships between human cortical bone toughness and collagen cross-links on paired anatomical locations., Bone. 112 (2018) 202–211. 10.1016/j.bone.2018.04.024. [DOI] [PubMed] [Google Scholar]
- [163].Berteau J-P, Gineyts E, Pithioux M, Baron C, Boivin G, Lasaygues P, Chabrand P, Follet H, Ratio between mature and immature enzymatic cross-links correlates with post-yield cortical bone behavior: An insight into greenstick fractures of the child fibula, Bone. 79 (2015) 190–195. 10.1016/j.bone.2015.05.045. [DOI] [PubMed] [Google Scholar]
- [164].Depalle B, Duarte AG, Fiedler IAK, Pujo-Menjouet L, Buehler MJ, Berteau J-P, The different distribution of enzymatic collagen cross-links found in adult and children bone result in different mechanical behavior of collagen., Bone. 110 (2018) 107–114. 10.1016/j.bone.2018.01.024. [DOI] [PubMed] [Google Scholar]
- [165].Wang X, Bank RA, TeKoppele JM, Hubbard GB, Althanasiou KA, Agrawal CM, Effect of Collagen Denaturation on the Toughness of Bone, Clin Orthop Relat R. 371 (2000) 228–239. 10.1097/00003086-200002000-00027. [DOI] [PubMed] [Google Scholar]
- [166].Freedman BI, Wuerth J-P, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS, for the A.I.S. Investigators, Design and Baseline Characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II), Control Clin Trials. 20 (1999) 493–510. 10.1016/s0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- [167].Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB, Group CS, Pyridorin in type 2 diabetic nephropathy., Journal of the American Society of Nephrology : JASN. 23 (2012) 131–136. 10.1681/asn.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Beltramo E, Mazzeo A, Porta M, Thiamine and diabetes: back to the future?, Acta Diabetol. 58 (2021) 1433–1439. 10.1007/s00592-021-01752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Oudegeest-Sander MH, Rikkert MGMO, Smits P, Thijssen DHJ, van Dijk APJ, Levine BD, Hopman MTE, The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: A randomized factorial design trial, Exp Gerontol. 48 (2013) 1509–1517. 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Chandra KP, Shiwalkar A, Kotecha J, Thakkar P, Srivastava A, Chauthaiwale V, Sharma SK, Cross MR, Dutt C, Phase I clinical studies of the advanced glycation end-product (AGE)-breaker TRC4186: safety, tolerability and pharmacokinetics in healthy subjects., Clin Drug Invest. 29 (2009) 559–75. 10.2165/11315260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [171].Ahmed MU, Thorpe SR, Baynes JW, Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein., J Biol Chem. 261 (1986) 4889–4894. 10.1016/S0021-9258(19)89188-3. [DOI] [PubMed] [Google Scholar]
- [172].Ahmed MU, Frye EB, Degenhardt TP, Thorpe SR, Baynes JW, N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins., Biochem J. 324(Pt 2) (1997) 565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wilker SC, Chellan P, Arnold BM, Nagaraj RH, Chromatographic Quantification of Argpyrimidine, a Methylglyoxal-Derived Product in Tissue Proteins: Comparison with Pentosidine, Anal Biochem. 290 (2001) 353–358. 10.1006/abio.2001.4992. [DOI] [PubMed] [Google Scholar]
- [174].Finley EL, Dillon J, Crouch RK, Schey KL, Identification of tryptophan oxidation products in bovine α- crystallin, Protein Sci. 7 (1998) 2391–2397. 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Taylor SW, Fahy E, Murray J, Capaldi RA, Ghosh SS, Oxidative Post-translational Modification of Tryptophan Residues in Cardiac Mitochondrial Proteins*, J Biol Chem. 278 (2003) 19587–19590. 10.1074/jbc.c300135200. [DOI] [PubMed] [Google Scholar]
- [176].Perkins BA, Rabbani N, Weston A, Ficociello LH, Adaikalakoteswari A, Niewczas M, Warram J, Krolewski AS, Thornalley P, Serum Levels of Advanced Glycation Endproducts and Other Markers of Protein Damage in Early Diabetic Nephropathy in Type 1 Diabetes, Plos One. 7 (2012) e35655. 10.1371/journal.pone.0035655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Brown KL, Darris C, Rose KL, Sanchez OA, Madu H, Avance J, Brooks N, Zhang M-Z, Fogo A, Harris R, Hudson BG, Voziyan P, Hypohalous Acids Contribute to Renal Extracellular Matrix Damage in Experimental Diabetes, Diabetes. 64 (2015) 2242–2253. 10.2337/db14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].He C, Song W, Weston TA, Tran C, Kurtz I, Zuckerman JE, Guagliardo P, Miner JH, Ivanov SV, Bougoure J, Hudson BG, Colon S, Voziyan PA, Bhave G, Fong LG, Young SG, Jiang H, Peroxidasin-mediated bromine enrichment of basement membranes, Proc National Acad Sci. 117 (2020) 15827–15836. 10.1073/pnas.2007749117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Degenhardt TP, Thorpe SR, Baynes JW, Chemical modification of proteins by methylglyoxal., Cell Mol Biology Noisy-Le-Grand France. 44 (1998) 1139–45. [PubMed] [Google Scholar]
- [180].Odani H, Shinzato T, Usami J, Matsumoto Y, Frye EB, Baynes JW, Maeda K, Imidazolium crosslinks derived from reaction of lysine with glyoxal and methylglyoxal are increased in serum proteins of uremic patients: evidence for increased oxidative stress in uremia, Febs Lett. 427 (1998) 381–385. 10.1016/s0014-5793(98)00416-5. [DOI] [PubMed] [Google Scholar]
- [181].Grandhee SK, Monnier VM, Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors, J Biol Chem. 266 (1991) 11649–11653. 10.1016/s0021-9258(18)99006-x. [DOI] [PubMed] [Google Scholar]
- [182].Monnier VM, Sun W, Sell DR, Fan X, Nemet I, Genuth S, Glucosepane: a poorly understood advanced glycation end product of growing importance for diabetes and its complications, Clin Chem Lab Med. 52 (2019) 21–32. 10.1515/cclm-2013-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Merlo K, Aaronson J, Vaidya R, Rezaee T, Chalivendra V, Karim L, In Vitro-Induced High Sugar Environments Deteriorate Human Cortical Bone Elastic Modulus and Fracture Toughness., Journal of Orthopaedic Research. 38 (2020) 972–983. 10.1002/jor.24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Jia S, Gong H, Cen H, Shi P, Zhang R, Li Z, Bi X, Influence of non-enzymatic glycation on the mechanical properties of cortical bone, J Mech Behav Biomed. 119 (2021) 104553. 10.1016/jjmbbm.2021.104553. [DOI] [PubMed] [Google Scholar]
- [185].Willett TL, Burton B, Woodside M, Wang Z, Gaspar A, Attia T, γ-Irradiation sterilized bone strengthened and toughened by ribose pre-treatment., Journal of the Mechanical Behavior of Biomedical Materials. 44 (2015) 147–155. 10.1016/j.jmbbm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- [186].Viguet-Carrin S, Farlay D, Bala Y, Munoz F, Bouxsein ML, Delmas PD, An in vitro model to test the contribution of advanced glycation end products to bone biomechanical properties, Bone. 42 (2008) 139–149. 10.1016/j.bone.2007.08.046. [DOI] [PubMed] [Google Scholar]
- [187].Tręacz H, Wójtowicz K, Wlizło-Dyś E, Dyś W, Effect of “in vitro” induced glycation on thermostability of bone tissue., International Journal of Biological Macromolecules. 51 (2012) 561–565. 10.1016/j.ijbiomac.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [188].Reddy GK, Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues., 21 (2003) 738–743. 10.1016/s0736-0266(03)00006-8. [DOI] [PubMed] [Google Scholar]
- [189].Tang SY, Zeenath U, Vashishth D, Effects of non-enzymatic glycation on cancellous bone fragility, Bone. 40 (2007) 1144–1151. 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Tang SY, Vashishth D, Non-enzymatic glycation alters microdamage formation in human cancellous bone., Bone. 46 (2010) 148–154. 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Willems NMBK, Langenbach GEJ, Stoop R, den Toonder JMJ, Mulder L, Zentner A, Everts V, Higher number of pentosidine cross-links induced by ribose does not alter tissue stiffness of cancellous bone., Materials Science and Engineering C. 42 (2014) 15–21. 10.1016/j.msec.2014.05.006. [DOI] [PubMed] [Google Scholar]


