Dear Editor,
We read with great interest the recent article in the Journal of Infection by Gentry et al.,1 which analyzed the clinical outcomes between those who received oral antivirals or not among veterans aging 65 years and older with mild-to-moderate SARS-CoV-2 active infection in the United States (US), using propensity score matching. However, authors did not directly compare the effectiveness of between two drugs, molnupiravir and nirmatrelvir-ritonavir, due to the baseline differences.
In China, people just pulled through the first wave of SARS-CoV-2 caused by omicron subvariants BF.7 and BA.5.2 in the year-end of 2022.2 However, the available antivirals were different between China and US. In China, nirmatrelvir-ritonavir had been granted conditional approval for adults with mild to moderate COVID-19 symptoms and at high risk for progression to severe disease since February 2022.3 Azvudine was initially developed as a broad-spectrum antiviral agent which obtained emergency use to treat adult COVID-19 patients with moderate symptoms in July 2022.4 These two drugs were extensively used to respond the omicron surge in the winter of 2022. By contrast, molnupiravir received emergency use at December 30, 2022, which could be accessed only in a few people during the outbreak.
Unlike the wealth of available data for nirmatrelvir-ritonavir,5 there is limited information published regarding the clinical use of azvudine, as well as the absence of head-to-head trial for antiviral agents, there is a need to understand antivirals effect of available agents in practice, we thus make a comparison of viral load dynamics among hospitalized patients with COVID-19 who received nirmatrelvir-ritonavir or azvudine. The study followed STROBE reporting guideline and was approved by the Ethics Committee of Beijing You’An Hospital and waived the need for informed consent. All patients were Chinese and were determined to be SARS-CoV-2 positive before admitted to COVID-19 wards. Key inclusion criteria were a positive SARS-CoV-2 reverse-transcriptase–polymerase-chain-reaction (RT-PCR) test, defined as cycle threshold (Ct) values < 35 according to Chinese Diagnosis and Treatment Protocol for COVID-19 (Version 10) at admission day or at the next day of admission. The prescription of antivirals and the frequency of nasopharyngeal/oropharyngeal swab collection was at the discretion of the treating physician. RT-PCR tests were performed within 2 h after sample collection. The detection limit of Ct value was set to be 40.
Patients included in the analysis were treated with single antiviral agent. Data were derived from electronic health record and our primary aim was to compare the Ct value dynamic change for different antivirals. Analyses used R software (version 4.1.2), and 2-sided P < 0.05 indicated statistical significance.
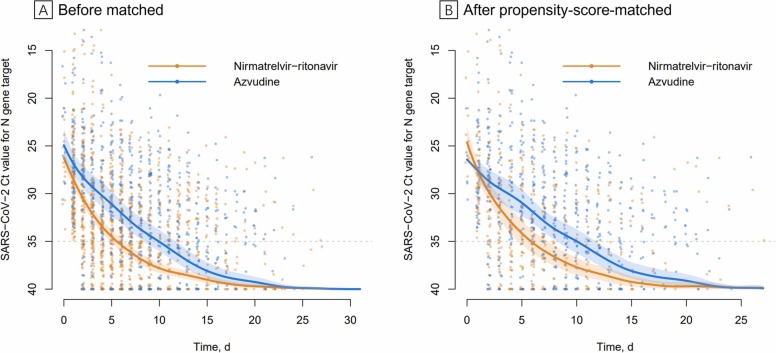
Of 1001 patients with Covid-19 admitted to the hospital, 245 patients were included in the final analysis (Supplementary Fig. 1). Their baseline characteristics according to different antivirals is presented ( Table 1), both in the unmatched and propensity-score-matched analytic cases (Supplementary Fig. 2). The dynamic Ct values were showing in the unmatched ( Fig. 1A) and in propensity-score-matched cohorts (Fig. 1B). In propensity-score-matched analysis, the Ct value was 31.6 (95% CI, 30.4–32.8) on the fitting curve for nirmatrelvir-ritonavir group and 29.5 (95% CI, 28.1–30.9) for azvudine group at day 3, respectively. At day 5, the Ct value on the fitting curve was 34.2 (95 % CI, 32.9–35.4) and 31.0 (95% CI, 29.6–32.3) in the respective group. Patients who received nirmatrelvir-ritonavir tended to have a shorter period to the first RT-PCR negative conversion than those who received azvudine (days, 5.8 [95% CI, 4.7–7.4] vs 10.0 [95% CI, 8.2–11.7]).
Table 1.
Characteristics of patients receiving nirmatrelvir-ritonavir or receiving azvudine, before and after propensity-score matching.
| Before matchinga | After propensity score matchingb | |||
|---|---|---|---|---|
| Patients, No. (%) |
Patients, No. (%) |
|||
| Baseline characteristics | Nirmatrelvir-ritonavir (N = 168) |
Azvudine (N = 77) |
Nirmatrelvir-ritonavir (n = 67) |
Azvudine (n = 67) |
| Age, mean(SD), y | 73.24 (13.58) | 69.09 (16.85) | 70.21 (13.93) | 70.57 (15.42) |
| Female sex | 60 (35.7%) | 30 (39.0%) | 28 (41.8%) | 26 (38.8%) |
| Male sex | 108 (64.3%) | 47 (61.0%) | 39 (58.2%) | 41 (61.2%) |
| Hypertension | 77 (45.8%) | 44 (57.1%) | 35 (52.2%) | 38 (56.7%) |
| Prior myocardial infarction or stroke | 48 (28.6%) | 27 (35.1%) | 23 (34.3%) | 23 (34.3%) |
| Type 2 diabetes | 58 (34.5%) | 18 (23.4%) | 21 (31.3%) | 18 (26.9%) |
| Prior malignancy | 42 (25.0%) | 13 (16.9%) | 16 (23.9%) | 13 (19.4%) |
| Other chronic diseasesc | 84 (50.0%) | 31 (40.3%) | 30 (44.8%) | 30 (44.8%) |
| Mean arterial pressure, mm Hg, median (IQR) | 100.4 (89.8–106.8) | 95.8 (89.5–103.6) | 101.2 (91.1–107.1) | 95.9 (90.3–103.9) |
| Oxygen saturation, %, median (IQR) | 96.30 (94.35–97.80) | 97.00 (96.00–98.00) | 96.90 (94.95–97.98) | 97.00 (95.20–98.00) |
| C-reactive protein, mg/l, median (IQR) | 51.90 (24.30–77.30) | 33.70 (7.18–65.55) | 38.25 (14.95–70.96) | 36.37 (9.50–73.29) |
| Neutrophil count per mm3, median (IQR) | 4.32 (2.82–6.34) | 3.97 (2.55–6.80) | 3.94 (2.69–6.33) | 4.03 (2.55–6.80) |
| Lymphocyte count per mm3, median (IQR) | 0.87 (0.60–1.17) | 0.83 (0.48–1.22) | 0.93 (0.56–1.20) | 0.80 (0.48–1.12) |
| Initial Ct value, median (IQR) | 28.24 (24.97–31.46) | 26.97 (22.98–30.36) | 27.67 (23.79–29.94) | 27.69 (23.88–31.08) |
Before matching, data on the C-reactive protein level were missing for 17 patients, on Neutrophil count for 9, on the Lymphocyte count for 9.
Missing data for patients included in the propensity-score–matched analysis was imputed using multiple imputation.
Other chronic diseases including history of pulmonary diseases, gastrointestinal diseases, liver diseases, kidney diseases or prior organ transplant.
Fig. 1.
Scatter plot of serial cycle threshold values. Cycle threshold (Ct) values from quantitative reverse transcription-polymerase chain reaction targeting N genes from each infected individuals were all presented in the figure. Orange circles indicate patients received nirmatrelvir-ritonavir; blue circles indicate patients received azvudine. The full thick curves represent the mean line of fit derived from a functional principal components analysis method. The shaded areas indicate 95 % credible intervals of the associated curve and their bounds. The dashed line indicates Ct value of 35. The Ct values greater than 40 are denoted by Ct value of 40. 1896 samples collected from all study subjects (n = 245) (A), 1075 samples collected from 67 propensity-score-matched pairs (B).
The inverse-probability-treatment-weighted analysis, and multiple sensitivity analyses, including analyze the patients with Ct value less than 30; and analyze the azvudine treatment within 5 days of admission and uptake azvudine at least 3 days, all showed similar results that nirmatrelvir-ritonavir is superior in viral inhibition (Supplementary Figs. 3–5).
Patients with high viral load of SARS-CoV-2 were prone to severe disease,6, 7 and inhibiting viral replication at early phase could substantially improve the prognosis for patients with COVID-19.8 Although nirmatrelvir-ritonavir and azvudine were both widely used for the treatment of COVID-19, our study suggests that there is a difference in their antiviral effects among hospitalized individuals predominantly elderly. Patients who received nirmatrelvir-ritonavir presented a more rapid virus suppression at the initial stage of hospitalization and an earlier RT-PCR negative conversion than those who received azvudine. To our knowledge, this is the first study that directly compared antiviral effect between different available agents in a clinical setting in the mainland of China. Although we did not make comparison of clinical outcomes, these results push us into rethinking the selection and prioritization of antivirals, for some other medications have shown a comparable efficacy to that of nirmatrelvir-ritonavir.9
Our research is limited with following aspects. First, the study was conducted in a single center. Second, antivirals were not prescribed at the initial stage of COVID-19 in some patients; it thus cannot be determined whether the conclusion of our study would be reproduced in outpatients.
CRediT authorship contribution statement
Concept and design: Y Gao, Z Luo, Y Ma. Drafting of the manuscript: Y Gao, Z Luo, S Ren, Z Duan, Y Han. Data acquisition and management: S Ren, Z Duan, Y Han. Data Entry: S Ren. Statistical analysis: H Liu, Z Gao, X Zhang. Clinical investigators: S Ren, Y Gao. Interpretation of data: all authors. Critical revision of the manuscript for important intellectual: X Zhang, Z Hu, Y Ma. Administrative, technical, or material support: Z Hu, Y Ma. Supervision: Z Hu, Y Ma.
Ethics committee approval
Ethics Committee of Beijing You’An Hospital reviewed the study protocol and waived the need for approval of ethical clearance.
Declaration of Competing Interest
None.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (2019YFC0121704), and R&D Program of Beijing Municipal Education Commission (KM202310025009), and Beijing You'An Hospital, Capital Medical University, Young and Middle-aged Talents Incubation Project (YNKTQN2021003).
Role of the funding source
The sponsors had no function in study design, data collection, analysis, and interpretation of data as well as in writing of the manuscript. All authors had full access to all data. Dr Hu and Dr Ma had the final responsibility for the decision to submit for publication.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.03.023.
Appendix A. Supplementary material
Supplementary material
References
- 1.Gentry C.A., Nguyen P., Thind S.K., Kurdgelashvili G., Williams R.J. Characteristics and outcomes of US Veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J Infect. 2023;86:248–255. doi: 10.1016/j.jinf.2023.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y., Wang L., Feng Z., et al. Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet. 2023;401:664–672. doi: 10.1016/S0140-6736(23)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Medical Products Administration. China grants conditional approval for Pfizer's oral COVID-19 drug; 2022. [cited 2023 March 10]. 〈http://english.nmpa.gov.cn/2022-02/14/c_707085.htm〉.
- 4.National Medical Products Administration. Domestically developed drug joins virus battle; 2022. [cited 2023 March 10]. 〈http://english.nmpa.gov.cn/2022-08/15/c_797867.htm〉.
- 5.Zheng Q., Ma P., Wang M., et al. Efficacy and safety of Paxlovid for COVID-19: a meta-analysis. J Infect. 2023;86:66–117. doi: 10.1016/j.jinf.2022.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. Bmj. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stankiewicz Karita H.C., Dong T.Q., Johnston C., et al. Trajectory of viral RNA load among persons with incident SARS-CoV-2 G614 infection (Wuhan Strain) in association with COVID-19 symptom onset and severity. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z., Gao W., Bao H., et al. VV116 versus nirmatrelvir-ritonavir for oral treatment of covid-19. N Engl J Med. 2023;388:406–417. doi: 10.1056/NEJMoa2208822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material