Abstract
Background
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, prone positioning has been widely applied for non-intubated, spontaneously breathing patients. However, the efficacy and safety of prone positioning in non-intubated patients with COVID-19-related acute hypoxemic respiratory failure remain unclear. We aimed to systematically analyze the outcomes associated with awake prone positioning (APP).
Methods
We conducted a systematic literature search of PubMed/MEDLINE, Cochrane Library, Embase, and Web of Science from January 1, 2020, to June 3, 2022. This study included adult patients with acute respiratory failure caused by COVID-19. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed, and the study quality was assessed using the Cochrane risk-of-bias tool. The primary outcome was the reported cumulative intubation risk across randomized controlled trials (RCTs), and the effect estimates were calculated as risk ratios (RRs; 95% confidence interval [CI]).
Results
A total of 495 studies were identified, of which 10 fulfilled the selection criteria, and 2294 patients were included. In comparison to supine positioning, APP significantly reduced the need for intubation in the overall population (RR=0.84, 95% CI: 0.74–0.95). The two groups showed no significant differences in the incidence of adverse events (RR=1.16, 95% CI: 0.48–2.76). The meta-analysis revealed no difference in mortality between the groups (RR=0.93, 95% CI: 0.77–1.11).
Conclusions
APP was safe and reduced the need for intubation in patients with respiratory failure associated with COVID-19. However, it did not significantly reduce mortality in comparison to usual care without prone positioning.
Keywords: Awake prone positioning, COVID-19, Acute hypoxemic respiratory failure, Non-invasive respiratory support
Introduction
Since the 1970s, prone positioning has been proposed as an inexpensive and physiologically justified management strategy for patients with acute respiratory distress syndrome (ARDS). Traditional prone positioning therapy is primarily used for mechanically ventilated patients, and many clinical studies have shown that prone positioning can help improve the prognosis of patients with ARDS.[1,2] The coronavirus disease 2019 (COVID-19) pandemic dramatically increased the number of patients with moderate to severe ARDS treated outside intensive care units (ICUs).[3] The unprecedented shortage of mechanical ventilators and ICU beds led clinicians to extensively use non-invasive respiratory support to treat hypoxemic respiratory failure.[4,5] In this scenario, awake prone positioning (APP) was widely used to reduce the consumption of medical resources. The physiological rationale for prone positioning in ARDS has been well-described.[6,7] Prone positioning can result in an increased lung volume,[8] homogenized pleural pressure,[9] reduced shunting,[10] and improved lung compliance.[11] In patients with COVID-19, prone positioning leads to physiological changes associated with lung-protective ventilation, including increased lung recruitment, decreased collapse, and improved ventilation–perfusion matching.[12] Before the COVID-19 pandemic, no randomized clinical trials had examined the effects of prone positioning in awake and non-intubated patients with hypoxemic respiratory failure. A recent systematic review and meta-analysis showed that APP reduced the need for intubation in COVID-19 patients, particularly among those requiring advanced respiratory support and those in ICU settings.[13] Nevertheless, despite the recommendations in the COVID-19 pneumonia guidelines, the application of APP remains controversial.[14] A recent randomized controlled trial (RCT) including patients with acute hypoxemic respiratory failure (AHRF) due to COVID-19[15] showed that in comparison to usual care without prone positioning, prone positioning did not significantly reduce endotracheal intubation at 30 days. Additionally, no studies have specifically reported the safety of APP. Therefore, we performed a systematic review and meta-analysis of all clinical trials with a control group to assess the effectiveness and safety of APP in COVID-19 patients with AHRF.
Methods
Search strategy
We conducted a systematic literature search of PubMed/MEDLINE, Cochrane Library, Embase, and Web of Science from January 1, 2020 to June 3, 2022. The search strategy involved a combination of the following keywords: APP, high-flow nasal cannula, non-invasive ventilation, COVID-19, and SARS-CoV-2. Only reports published in English were included, and there was no limit to geographical location. Trial registries, including ClinicalTrials.gov, were also used to identify completed and ongoing trials. The search strategy is presented in Supplementary Appendix.
Study inclusion and criteria
We selected all RCTs, and eligible studies only included adult patients (age ≥18 years) with AHRF caused by COVID-19 who received APP alongside any oxygen delivery technique, including simple oxygen therapy, high-flow nasal cannula oxygenation (HFNC), and NIV. We enrolled patients of any sex who were admitted to a ward or ICU. We excluded records of non-randomized trials, such as observational studies, protocols, opinions, editorials, reports, and reviews.
Data extraction and study quality
Data extraction was performed independently and in duplicates to avoid bias. The abstracted data were stored in a customized Excel database, and general and specific characteristics were extracted, including the year of publication, country, study design, patient characteristics, interventions, and outcomes. The RCTs were assessed using the Cochrane risk-of-bias 2 tool (RoB2).[16] Article selection was first performed by two independent reviewers based on titles and abstracts. The reviewers then independently reviewed the full texts of potentially relevant studies to determine their eligibility for inclusion. Data extraction was performed by the two independent reviewers using a data collection form. Disagreements were resolved by a third reviewer, who had a voting decision.
Outcomes
The primary outcome was the need for intubation over the longest follow-up period. Secondary outcomes included mortality, adverse events, and tolerance to prone positioning, described as the duration of prone positioning.
Statistical analysis
We calculated individual and pooled risk ratios (RRs) for dichotomous outcomes with 95% confidence intervals (CIs). Heterogeneity analysis was performed using Cochran's Q statistic and quantified using I2 values.[17] Heterogeneity with I2 values >50% was considered significant. We employed the fixed-effects model or the random-effects model in cases with low or high statistical heterogeneity, respectively. Publication bias for the primary endpoint was assessed by visual inspection of the funnel plot.[18,19] For pooled outcome analyses, a P-value <0.05 was considered significant. The analysis was performed using RevMan 5.4. software (Review Manager, Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). Sensitivity analyses were performed by serially excluding each study to determine the implications of the individual studies for the pooled estimates.[20] Continuous variables were converted into mean and standard deviation (SD) values in accordance with the methodology described by Wan et al.[21,22] We uniformly converted the SpO2/FiO2 ratio at baseline to mean difference (MD) values and also performed subgroup analyses of the factors that may influence the primary outcomes such as the SpO2/FiO2 ratio at baseline.
Results
Study characteristics
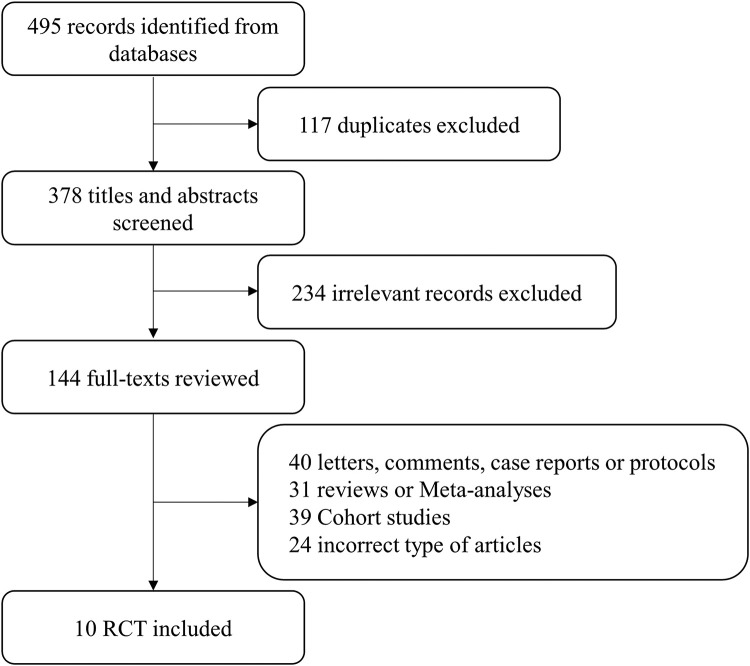
A total of 495 studies were identified using this search strategy. After removing duplicates, 10 studies met the inclusion criteria, as shown in Figure 1. We identified 10 articles for inclusion, of which one was an individual participant-level meta-analysis of six RCTs,[23] and one was included in that meta-analysis.[24] The study characteristics are summarized in Table 1. All studies were published between 2020 and 2022, and a total of 2294 patients, all with COVID-19-related acute respiratory failure, were included in the study. Of these 2294 patients, 1172 (51.09%) underwent APP, and 1122 (48.91%) received usual care treatment. The 10 studies included five multicenter and five single-center studies, and the intubation rate was the primary outcome in all 10 studies. Bias analysis revealed a relatively high risk of bias with good quality in five studies, fair quality in two studies, and poor quality in three studies (Supplementary Figures S1 and S2, Supplementary Table S1). The Cochrane risk-of-bias tool reported potential ambiguity and a high risk of bias, especially loss of accuracy with respect to blinded persons, as shown in the supplementary material (Supplementary Figure S3).
Figure 1.
Flow diagram of study selection. RCT: Randomized controlled trial.
Table 1.
Baseline characteristics of the included studies.
| Study | Country | Study design | Number | Setting | Oxygen therapy method | SpO2/FiO2 of prone position | SpO2/FiO2 of usual care | Prone position duration | Intervention | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| Alhazzani et al.[15] | Canada/Kuwait/Saudi Arabia/USA | RCT | 400 | ICU | High flow, low flow, NIV | 132 (103 -174) | 136 (110-181) | 4.8 (1.8–8.0) h/day | The target duration of prone positioning was 8 h/day to 10 h/day. | Not to prone position |
| Ehrmann et al.[24] | Mexico/France/USA/Spain/Ireland/Canada | Multicenter RCT | 1126 | ICU and general wards | HFNC | 147.9 ± 43.9 | 148.6 ± 43.1 | 5.0 (1.6–8.8) h/day | Lie in the prone position for as long and as frequently as possible each day | Standard care with HFNC |
| Ibarra-Estrada et al.[23] | Mexico | RCT | 430 | COVID-19 unit | HFNC | 134.7 ± 38.7 | 135.5 ± 37.9 | 9.4 (5.6–12.9) h/day | HFNC + APP | HFNC+ standard care |
| Fralick et al.[28] | Canada, USA | Multicenter RCT | 248 | General ward | Nasal cannula, HFNC, face mask | 303 (261-336) | 305 (267-339) | 6 (1.5–12.8) h/72 h | Recommended to adopt a prone position four times a day | Received usual care |
| Jayakumar et al.[32] | India | Multicenter RCT | 60 | ICU | Nasal cannula, face mask, non-rebreather mask, HFNC, or NIV | 185.6 ± 126.1 | 201.4 ± 118.8 | NA | Patients were encouraged to lie prone for a minimum of 6 h in a day | APP was not encouraged |
| Johnson et al.[31] | USA | RCT | 30 | General ward | Room air, nasal cannula | >300 | >300 | 1.6 (0.2–3.1) h/72 h | Patients were instructed to position themselves in prone and lateral position | Usual care |
| Kharat et al.[30] | Switzerland | RCT | 27 | General ward | Nasal cannula | 318 (284–341) | 336 (303–388) | NA | Patients used their mobile phone “timer” function to alternate their body position every 4 h | Nasal cannula+usual care |
| Garcia et al.[33] | USA | Multicenter RCT | 239 | General ward | Room air, nasal cannula, mask, or HFNC | 396 (308-457) | 402 (311-457) | NA | Smartphone-based recommendation to self-prone position during hospitalization | Usual care |
| Rosén et al.[27] | Sweden | Multicenter RCT | 75 | ICU and general ward | HFNC or NIV | 151(131–174) | 157(136–175) | 9.0 (4.4–10.6) h/day | A protocol targeting at least 16 h APP per day was initiated | APP was not encouraged |
| Taylor et al.[29] | USA | RCT | 40 | General ward | Room air, nasal cannula, HFNC, or NIV | >200 | >200 | NA | APP | Usual care |
Data are expressed as mean ± SD or median (interquartile range).APP: Awake prone positioning; COVID-19: Coronavirus disease 2019; HFNC: High-flow nasal cannula oxygenation; ICU: Intensive care unit; NIV: Non-invasive ventilation; RCT: Randomized controlled trial; SD: Standard deviation.
Association of APP with intubation
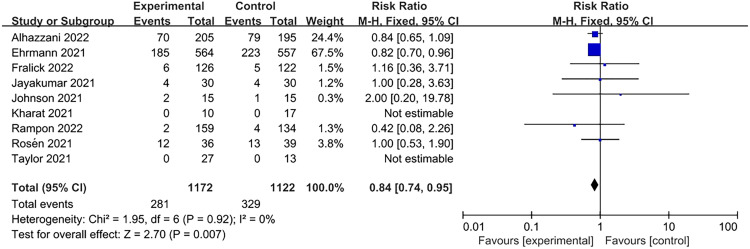
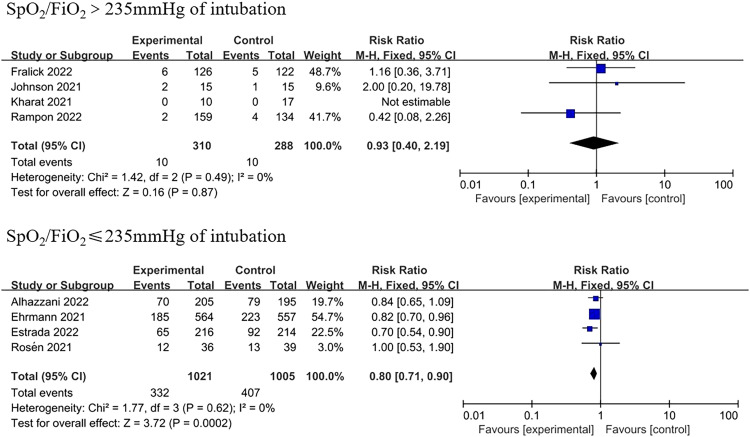
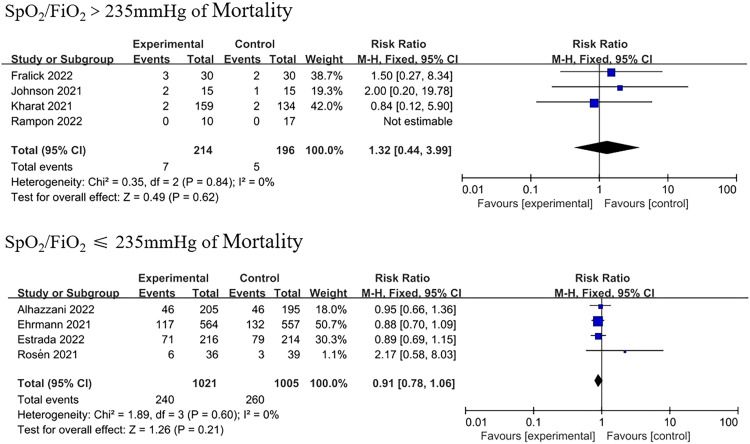
In comparison to the supine position, APP significantly reduced the need for intubation (RR=0.84, 95% CI: 0.74–0.95; Figure 2). Of the 1172 patients in the APP group, 281 were intubated, while 329 of the 1122 patients in the control group were intubated. Only some studies limited the SpO2/FiO2 ratio when recruiting patients, and the subgroup analysis was classified according to the SpO2/FiO2 ratio at baseline (Table 1). An SpO2/FiO2 ratio of 235 corresponded to a PaO2/FiO2 ratio of 200 mmHg.[25] Subgroup analyses showed lower intubation rates for patients with moderate to severe AHRF (four trials; RR=0.80, 95% CI: 0.71–0.90; Figure 3). However, in the studies including patients with an SpO2/FiO2 ratio of >235 mmHg, intubation events were rare and not statistically significant (four trials; RR=0.93, 95% CI: 0.40–2.19; Figure 3).
Figure 2.
Association of APP with intubation. APP: Awake prone positioning; CI: Confidence interval.
Figure 3.
Subgroup analysis of intubation. CI: Confidence interval.
Association of APP with survival
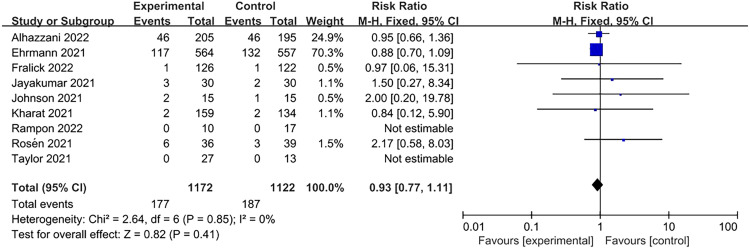
All studies reported that mortality did not significantly improve in patients who underwent prone positioning (RR=0.93, 95% CI: 0.77–1.11; Figure 4) in comparison to those who received usual care. Subgroup analysis on the basis of SpO2/FiO2 levels also did not show statistical significance (Figure 5). Ehrmann et al.[24] provided subgroup results that provided an a priori comparison of severe (SpO2/FiO2 <190; equivalent to PaO2/FiO2 <150 mmHg at enrollment) vs. less severe hypoxemia (SpO2/FiO2 ≥190, equivalent to PaO2/FiO2 ≥150 mmHg at enrollment). They found a significant reduction in survival in patients with an SpO2/FiO2 ratio of ≤190 mmHg patients. Further research is required to investigate the association between mortality and APP.
Figure 4.
Association of APP with intubation mortality. APP: Awake prone positioning; CI: Confidence interval.
Figure 5.
Subgroup analysis of intubation mortality. CI: Confidence interval.
Association between APP and adverse events
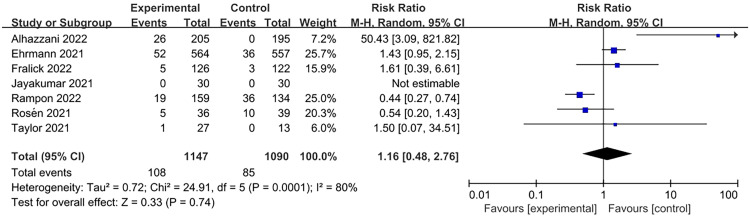
Adverse events such as skin breakage, vomiting, aspiration, musculoskeletal pain, catheter displacement, and cardiac arrest have been reported. A total of eight studies recorded adverse events, and the analysis showed that APP did not increase the incidence of adverse events (RR=1.16, 95% CI: 0.48–2.76; Figure 6). In one of the studies,[26] adverse events occurred only in the intervention group, but not in the control group. In the study by Rosén et al.,[27] adverse effects of cardiac arrest were reported twice in the intervention group and once in the control group; however, the events occurred independently of APP. In the study by Fralick et al.,[28] only significant complications, including aspiration pneumonia and venous thrombosis, occurred in five patients in the prone group and three in the control group.
Figure 6.
Adverse events. CI: Confidence interval.
Duration of APP
Six studies recorded the duration of prone positioning. In the studies that recorded the time to prone positioning, the duration of prone positioning varied from <1 h to 12 h. Therefore, it was not possible to form a basis for the duration of prone positioning (Supplementary Table S2). Four studies specifically recorded the duration of daily prone positioning {4.8 h/day (interquartile range [IQR]: 1.8–8.0 h/day), 5.0 h/day (IQR: 1.6–8.8 h/day), 9.4 h/day (IQR: 5.6–12.9 h/day), and 9.0 h/day (IQR: 4.4–10.6 h/day)}. The intubation rates in these studies were 34.1%, 32.8%, 30.1%, and 33.3%, respectively. No correlation was observed between the duration of prone positioning and the intubation rate.
Discussion
This meta-analysis collected results from 10 RCTs including 2294 patients with acute respiratory failure due to COVID-19. Our study showed that prone positioning significantly reduced the need for intubation but did not significantly increase the incidence of adverse events. Among the 10 studies, three RCTs showed a high risk of bias (Supplementary Figure S2), of which two did not assess intubation events[29,30] and one had a small number of cases.[31] We believe that the bias did not affect the findings for intubation risk. However, some heterogeneity was observed among these studies. Three RCTs described the feasibility of APP; therefore, some patients in the control group also underwent prone positioning.[28,32,33] In our meta-analysis, the primary outcome of the intubation rate was mainly driven by two studies[15,22]: the overall weight of these three studies was 91.9%. Thus, more high-quality RCTs are needed to demonstrate the effects of APP. The subgroup analysis showed that cases with moderate and severe respiratory failure could show more apparent effects, while the intubation rate was not significantly lower in patients with mild respiratory failure in general wards. Alhazzani et al.[15] suggested that severe respiratory failure patients (SpO2/FiO2 ≤150 mmHg) could not benefit from APP on the basis of the subgroup analysis. However, they believed that the false discovery rate was not statistically significant for any pre-planned subgroup. Therefore, these findings should be interpreted cautiously. Although our subgroup analysis showed a significant effect in patients with moderate to moderate and severe respiratory failure, further research is needed to determine whether patients with moderate and severe respiratory failure can benefit from this approach.
One previous study recorded the adverse events in 17 patients with COVID-19 who received APP.[34] They concluded that using prone positioning in non-intubated patients with hypoxemia of COVID-19 was safe; however, many patients could not maintain the prone position for too long. In our study, most RCTs reported adverse events in the APP group; however, few studies have analyzed the statistical differences in adverse events between the prone positioning group and standard care groups. We performed a meta-analysis of eight studies that recorded adverse events and found no significant difference between the prone positioning and control groups, although one of these studies reported no adverse events in the control group. On the basis of these findings, we consider that the application of APP in COVID-19 patients with respiratory failure is a safe option. Among intubated patients, the effect of prone positioning may be related to the duration and initiation of the operation.[26] When analyzing the relationship between the duration of prone positioning and prognosis in spontaneously breathing patients, we found that most patients were less compliant and did not follow prone positioning for the time required for the study. This may be due to intolerance to prone positioning caused by musculoskeletal pain or general discomfort. Although some studies have reported that APP could be performed for 16 h or more (72 h),[35,36] prolonged prone positioning did not reduce intubation rates. Nevertheless, owing to a lack of medical resources, some studies did not accurately record the detailed duration of patients’ daily prone positioning. Only four studies had detailed records,[15,22,23,27] and no significant difference was observed in intubation rates between APP for 5.0 h/day (IQR, 1.6–8.8) and 9.0 h/day (IQR, 4.4–10.6). On the contrary, a recent non-RCT showed that NIV was feasible and safe for early and prolonged (at least 8 h every day) prone positioning treatment, which could reduce 28-day NIV failure, mortality, and intubation rates. APP requires close cooperation among doctors, nurses, and respiratory therapists to ensure success, especially in the initial stages. Patients should understand and cooperate with their healthcare stakeholders, and clinicians should communicate adequately with patients and help improve their comfort. Future larger and well-designed studies (RCTs) are needed to explore the optimal duration of prone positioning.
Strengths and Limitations of the Study
Our meta-analysis included only RCTs; therefore, the quality of evidence was high. Furthermore, we explored safety and efficacy outcomes. Nevertheless, our meta-analysis also had several limitations. First, our primary results were driven by three large-sample studies. Second, in comparison to previous meta-analyses by Li et al.,[13] our meta-analysis included only one more RCT by Alhazzani et al.,[15] and consequently, the main findings remained similar. Third, three studies had a high risk of bias; however, because of the nature of the studies, both doctors and patients knew the group they belonged to, and blinding was not possible. Despite the study bias, the funnel plots for the different outcomes were generally symmetrical (Supplementary Figure S4). Finally, many studies did not record the specific duration of prone positioning and the number of days for which prone positioning was performed, and the heterogeneity of prone positioning time among patients in the same study was also considerable; therefore, it is not possible to accurately understand the different effects of the duration of prone positioning.
Conclusions
APP is an effective treatment for non-intubated patients with hypoxic respiratory failure caused by COVID-19. APP reduced the intubation rate, especially in patients with moderate to severe and severe acute respiratory failure. However, it had no significant effect on mortality. The criteria for optimal duration and performance of APP remain unclear. APP does not increase the incidence of adverse events and can be used safely in unincubated patients with respiratory failure.
Author Contributions
JJW: Conception of the study, data collection, data analysis, data interpretation, manuscript draft, and approval. DNC: conception of the study, data collection, data interpretation, manuscript drafting, and approval. PYD: Data collection, revision, and approval. CCZ: Data collection, revision, and approval. XZ: Data collection, revision, and approval. HL: Data collection, revision, and approval. HX: Conception of the study, data interpretation, revision, and approval. DCC:conception of the work, data analysis, data interpretation, revision, approval; RLW: conception of the work, data analysis, data interpretation, revision, and approval. All the authors have read and approved the final manuscript.
Acknowledgments
We thank all authors for their contributions to this article.
Funding
This study was supported by the Clinical Research Plan of SHDC (grant number: SHDC2020CR2013A) and the Clinical Research Plan of SHDC (grant number: SHDC2020CR5010-003).
Ethics Statement
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Managing Editor: Jingling Bao
Footnotes
Given his role as Editor-in-Chief, Prof. Dechang Chen had no involvement in the peer review of this article and has no access to information regarding its peer review. Prof. Arnaud W. Thille took responsibility for the peer-review progress. Prof. Jean-Louis Teboul who is the co-editor-in-chief made the final decision.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jointm.2023.02.001.
Contributor Information
Hui Xie, Email: xymtm@163.com.
Dechang Chen, Email: 18918520002@189.cn.
Ruilan Wang, Email: wangyusun@hotmail.com.
Appendix. Supplementary materials
References
- 1.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Mancebo J., Fernández R., Blanch L., Rialp G., Gordo F., Ferrer M., et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 3.Coopersmith C.M., Antonelli M., Bauer S.R., Deutschman C.S., Evans L.E., Ferrer R., et al. The surviving sepsis campaign: research priorities for coronavirus disease 2019 in critical illness. Crit Care Med. 2021;49(4):598–622. doi: 10.1097/CCM.0000000000004895. [DOI] [PubMed] [Google Scholar]
- 4.Vitacca M., Nava S., Santus P., Harari S. Early consensus management for non-ICU acute respiratory failure SARS-CoV-2 emergency in Italy: from ward to trenches. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grieco D.L., Maggiore S.M., Roca O., Spinelli E., Patel B.K., Thille A.W., et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47(8):851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholten E.L., Beitler J.R., Prisk G.K., Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touchon F., Trigui Y., Prud'homme E., Lefebvre L., Giraud A., Dols A.M., et al. Awake prone positioning for hypoxaemic respiratory failure: past, COVID-19 and perspectives. Eur Respir Rev. 2021;30(160) doi: 10.1183/16000617.0022-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malbouisson L.M., Busch C.J., Puybasset L., Lu Q., Cluzel P., Rouby J.J. Role of the heart in the loss of aeration characterizing lower lobes in acute respiratory distress syndrome. CT scan ARDS study group. Am J Respir Crit Care Med. 2000;161(6):2005–2012. doi: 10.1164/ajrccm.161.6.9907067. [DOI] [PubMed] [Google Scholar]
- 9.Mutoh T., Guest R.J., Lamm W.J., RK Albert. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis. 1992;146(2):300–306. doi: 10.1164/ajrccm/146.2.300. [DOI] [PubMed] [Google Scholar]
- 10.Gattinoni L., Vagginelli F., Chiumello D., Taccone P., Carlesso E. Physiologic rationale for ventilator setting in acute lung injury/acute respiratory distress syndrome patients. Crit Care Med. 2003;31(4 Suppl):S300–S304. doi: 10.1097/01.CCM.0000057907.46502.7B. [DOI] [PubMed] [Google Scholar]
- 11.Gattinoni L., Taccone P., Carlesso E., Marini J.J. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 12.Fossali T., Pavlovsky B., Ottolina D., Colombo R., Basile M.C., Castelli A., et al. Effects of prone position on lung recruitment and ventilation-perfusion matching in patients with COVID-19 acute respiratory distress syndrome: a combined CT scan/electrical impedance tomography study. Crit Care Med. 2022;50(5):723–732. doi: 10.1097/CCM.0000000000005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Luo J., Pavlov I., Perez Y., Tan W., Roca O., et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(6):573–583. doi: 10.1016/S2213-2600(22)00043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronavirus disease 2019 (COVID-19) treatment guidelines. Available form: https://www.covid19treatmentguidelines.nih.gov [Last accessed on 2023 February 1].
- 15.Alhazzani W., Parhar K.K.S., Weatherald J., Al Duhailib Z., Alshahrani M., Al-Fares A., et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. JAMA. 2022;327(21):2104–2113. doi: 10.1001/jama.2022.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 19.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 20.Patsopoulos N.A., Evangelou E., Ioannidis J.P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 23.Ibarra-Estrada M., Li J., Pavlov I., Perez Y., Roca O., Tavernier E., et al. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial. Crit Care. 2022;26(1):84. doi: 10.1186/s13054-022-03950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrmann S., Li J., Ibarra-Estrada M., Perez Y., Pavlov I., McNicholas B., et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B., et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 26.Reutershan J., Schmitt A., Dietz K., Unertl K., Fretschner R. Alveolar recruitment during prone position: time matters. Clin Sci. 2006;110(6):655–663. doi: 10.1042/CS20050337. [DOI] [PubMed] [Google Scholar]
- 27.Rosén J., von Oelreich E., Fors D., Jonsson Fagerlund M., Taxbro K., Skorup P., et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care. 2021;25(1):209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fralick M., Colacci M., Munshi L., Venus K., Fidler L., Hussein H., et al. Prone positioning of patients with moderate hypoxaemia due to COVID-19: multicentre pragmatic randomised trial (COVID-PRONE) BMJ. 2022;376 doi: 10.1136/bmj-2021-068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor S.P., Bundy H., Smith W.M., Skavroneck S., Taylor B., Kowalkowski M.A. Awake prone positioning strategy for nonintubated hypoxic patients with COVID-19: a pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18(8):1360–1368. doi: 10.1513/AnnalsATS.202009-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharat A., Dupuis-Lozeron E., Cantero C., Marti C., Grosgurin O., Lolachi S., et al. Self-proning in COVID-19 patients on low-flow oxygen therapy: a cluster randomised controlled trial. ERJ Open Res. 2021;7(1):00692–02020. doi: 10.1183/23120541.00692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson S.A., Horton D.J., Fuller M.J., Yee J., Aliyev N., Boltax J.P., et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR) Ann Am Thorac Soc. 2021;18(8):1424–1426. doi: 10.1513/AnnalsATS.202011-1466RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayakumar D., Ramachandran Dnb P., Rabindrarajan Dnb E., Vijayaraghavan Md B.K.T., Ramakrishnan Ab N, Venkataraman Ab R. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection-a multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36(8):918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia M.A., Rampon G.L., Doros G., Jia S., Jagan N., Gillmeyer K., et al. Rationale and design of the awake prone position for early hypoxemia in COVID-19 study protocol: a clinical trial. Ann Am Thorac Soc. 2021;18(9):1560–1566. doi: 10.1513/AnnalsATS.202009-1124SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solverson K., Weatherald J., Parhar K. Tolerability and safety of awake prone positioning COVID-19 patients with severe hypoxemic respiratory failure. Can J Anaesth. 2021;68(1):64–70. doi: 10.1007/s12630-020-01787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musso G., Taliano C., Molinaro F., Fonti C., Veliaj D., Torti D., et al. Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: clinical outcomes and mechanisms for treatment response in the PRO-NIV study. Crit Care. 2022;26(1):118. doi: 10.1186/s13054-022-03937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierucci P., Ambrosino N., Di Lecce V., Dimitri M., Battaglia S., Boniello E., et al. Prolonged active prone positioning in spontaneously breathing non-intubated patients with COVID-19-associated hypoxemic acute respiratory failure with PaO(2)/FiO(2) >150. Front Med. 2021;8 doi: 10.3389/fmed.2021.626321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.