Background:
Newer navigational bronchoscopy technologies render peripheral lung lesions accessible for biopsy and potential treatment. We investigated whether photodynamic therapy (PDT) delivered via navigational bronchoscopy is feasible and safe for ablation of peripheral lung tumors.
Methods:
Two studies evaluated PDT in patients with solid peripheral lung tumors followed by clinical follow-up (nonresection study, N=5) or lobectomy (resection study, N=10). Porfimer sodium injection was administered 40 to 50 hours before navigational bronchoscopy. Lesion location was confirmed by radial probe endobronchial ultrasonography. An optical fiber diffuser was placed within or adjacent to the tumor under fluoroscopic guidance; laser light (630 nm wavelength) was applied at 200 J/cm of diffuser length for 500 seconds. Tumor response was assessed by modified Response Evaluation Criteria in Solid Tumors at 3 and 6 months postprocedure (nonresection study) and pathologically (resection study).
Results:
There were no deaths, discontinuations for adverse events, or serious or grade ≥3 adverse events related to study treatments. Photosensitivity reactions occurred in 8 of 15 patients: 6 mild, 1 moderate, 1 severe (elevated porphyrins noted in blood after treatment). Among 5 patients with clinical follow-up, 1 had complete response, 3 had stable disease, and 1 had progressive disease at 6 months follow-up. Among 10 patients who underwent lobectomy, 1 had no evidence of tumor at resection (complete response), 3 had 40% to 50% tumor cell necrosis, 2 had 20% to 35%, and 4 had 5% to 10%.
Conclusion:
PDT for nonthermal ablation of peripheral lung tumors was feasible and safe in this small study. Further study is warranted to evaluate efficacy and corroborate the safety profile.
Key Words: photodynamic therapy, porfimer sodium, peripheral lung tumors, non–small cell lung cancer
Detection of pulmonary nodules has increased due in part to wide-spread use of chest computerized tomography (CT)1 to screen for early-stage, peripherally located lung cancers.2 Non–small cell lung cancer (NSCLC) diagnosed at stage 1 has a 70% to 90% 5-year survival,3 whereas the overall 5-year survival for lung cancer is 19%.4 With novel bronchoscopic technologies, such as electromagnetic navigation bronchoscopy and radial probe endobronchial ultrasound (REBUS), smaller peripherally located lung tumors can be safely reached and biopsied.5–8 It seems likely that these technologies could be utilized to deliver antitumor therapy to peripheral lung lesions.
First-line treatment for peripheral lung lesions is surgical resection by lobectomy.9 For patients who decline surgery or are not surgical candidates due to pulmonary or cardiovascular comorbidities, secondary options include stereotactic body radiotherapy (SBRT) or percutaneous ablation methods.9 Both therapies have important limitations—radiation pneumonitis with SBRT and complications due to percutaneous ablation methods, including radiofrequency ablation (RFA) and limited efficacy in larger tumors.9,10 Thus, additional therapeutic options for inoperable, early-stage peripheral lung lesions are needed.
Photodynamic therapy (PDT) has been used for the ablation of central airway tumors for more than 3 decades.11 PDT is approved for the treatment of microinvasive endobronchial NSCLC in patients for whom surgery is not indicated, and for reducing obstruction and palliation of symptoms in patients with obstructing endobronchial NSCLC.12 Complete response (CR) rates of 85% have been achieved in nonsurgical candidates with early-stage central airway lung cancer.13,14 In the last decade, transthoracic needle PDT and navigational bronchoscopy PDT have produced partial responses and successful ablation in small studies of peripheral lung tumors.15,16 In PDT, a photosensitizing agent (typically porfimer sodium) is injected intravenously (IV) and retained primarily in the tumor and to a lesser degree in normal tissue. After the administration of the photosensitizing agent, laser light is delivered to the target tumor tissue and margins with a fiberoptic diffuser placed adjacent to or within the tumor.17,18 The light activates the photosensitizer to produce reactive oxygen species leading to tumor cell death through direct cellular and secondary vascular effects,17 which result in less injury to normal tissue than thermal ablative techniques.19
We evaluated the safety and feasibility of PDT for ablation of peripheral lung tumors in 2 phase 1 studies.
METHODS
Study Design and Patients
Two phase 1, single-arm studies at 6 sites in the United States evaluated porfimer sodium administration followed by PDT via navigational bronchoscopy in patients with peripheral lung tumors. The first study enrolled patients with primary lung cancer or solid tumor metastases in the peripheral lung (including oligometastasis), who were not candidates for surgery or unwilling to undergo surgical resection. The second study enrolled patients with solid peripheral lung tumors before surgical resection. Both studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines for good clinical practice. The study protocols were approved by the Institutional Review Boards at each participating site. Eligible patients signed an informed consent form before enrollment.
In both studies, eligible patients were ≥18 years of age with peripheral lung tumors of any malignant histology type, who had not undergone radiation therapy and were deemed likely to survive for at least 3 months. Nonmenopausal or nonsterile females of childbearing potential had to be negative for serum β-human chorionic gonadotropin at study entry and willing to use a medically acceptable form of birth control. Tumors had to be ≤3 cm (nonresection tumor study) or <5 cm (resection tumor study), located in the peripheral lung as defined by Radiation Therapy Oncology Group protocols—that is, the primary tumor not touching a 2-cm volume in all directions around the proximal bronchial tree (distal 2 cm of the trachea, main stem bronchi, and lobar bronchi),20,21 clearly observable on CT without contrast and accessible for unrestricted illumination with PDT. The following tumors were excluded: small cell lung cancers; solid tumors in a central location defined by Radiation Therapy Oncology Group protocols as within 2 cm of the proximal bronchial tree or within 2 cm of a major structure (eg, aorta, heart, trachea, pericardium, superior vena cava, pulmonary artery, esophagus, vertebral body, or spinal canal);20,21 concurrent nonsolid malignancy; and tumors invading a major blood vessel. Key patient exclusion criteria were known porphyria or hypersensitivity to porfimer sodium, porphyrin-like compounds, or its excipients; planned surgical procedure in the next 90 days (except surgical resection of the treated tumor); female patients with intention to breast feed; coexisting ophthalmic disease likely to require slit-lamp examination in the next 90 days; PDT within 3 months or chemotherapy/immunotherapy within 4 weeks before informed consent; and blood parameters of grade 3 or higher on the Common Terminology Criteria for Adverse Events 5-point scale or grade 2, if judged clinically significant by the investigator.
Procedures
Porfimer sodium (Photofrin; Pinnacle Biologics Inc., Bannockburn, IL), 2 mg/kg of body weight, was administered as a single slow IV injection. Afterward, patients were considered photosensitive and had to avoid exposure of eyes and skin to direct sunlight or bright indoor light for at least 30 days. Patients were trained to perform a light challenge at home on follow-up day 44 by exposing a small patch of skin to sunlight for 10 minutes to test for residual phototoxicity before having any exposure to direct sunlight or bright indoor light. If no phototoxicity reaction (eg, erythema, edema, and blistering) occurred within 24 hours, the patient could gradually increase exposure to sunlight and direct indoor light.
Approximately 40 to 50 hours after porfimer sodium administration, navigational bronchoscopy with REBUS was performed to confirm lesion location. A fiber optic diffuser (Optiguide DCYL700 series flexible cylindrical diffuser; Concordia Laboratories Inc.) matching the tumor length was placed within or adjacent to the tumor under fluoroscopic guidance, and laser light (630 nm wavelength) was applied at 200 J/cm of diffuser length for 500 seconds. In the tumor resection study, surgical resection was performed 12 to 18 days after PDT application, with the exception of 1 patient whose resection was delayed until 32 days post PDT because of a deep vein thrombosis that required referral to a thoracic surgeon.
Assessments
Safety evaluation included adverse events classified by Common Terminology Criteria for Adverse Events severity category, seriousness, and relatedness to study treatment, photosensitivity reactions, laboratory assessments, physical exams, vital signs, and concomitant medications.
Tumor response in the nonresection study was assessed using CT scan mass size criteria of the Modified Response Evaluation Criteria in Solid Tumors (Modified RECIST)22 at 3 and 6 months after PDT application. In the resection study, macroscopic and microscopic examinations of the tumor and surrounding healthy lung tissue evaluated the effect of PDT on the tumor and healthy/nontumor lung tissue (0.75 cm radius for eccentrically placed fiber or 1.5 cm diameter for centrally placed fiber), as defined by cytological and pathological evaluation. A CR was no evidence of tumor on pathology testing. Percent tumor necrosis was used to measure partial response.
Tumor specimens from the resection study were assessed by an independent expert pathologist (ARUP Laboratories, Salt Lake City, UT). Residual tumor size was measured using the longest axis of tumor length (note: interceding zones of necrosis may have been present within the span of the axis). We defined abnormal surrounding lung alveolar tissue as areas having 1 or more of the following reactions more than 1.0 cm from the tumor border or “kill zone”: cavitation, pneumonitis, interstitial fibrosis, necrosis, large vessel damage, or acute alveolar damage.23 Other parameters assessed included reactive type 2 pneumocyte hyperplasia, localized parenchymal hemorrhage, mucus plugging/bronchitis in the surrounding lung tissue, and organizing pneumonia pattern. These latter features were not classified as abnormal because they are somewhat expected as either peritumoral tissue reactions or procedure-related changes.
Statistical Analysis
Statistical analyses were performed using SAS Version 9.4. For the calculation of tumor response, change in tumor size from baseline was derived for each subject as postbaseline evaluation size minus baseline evaluation size defined as the closest nonmissing measurement from a scheduled or unscheduled visit before porfimer sodium administration.
RESULTS
Five patients (mean age: 59.8 y) were enrolled in the nonresection tumor study and 10 patients (mean age: 61.9 y) were enrolled in the resection tumor study. All 15 patients received porfimer sodium 2 mg/kg by IV push and PDT light application and completed the study. There were no instances of PDT laser or optical fiber failure. Patient baseline characteristics are shown in Table 1. Men and women were equally represented. Most tumors were adenocarcinomas (67%) or squamous cell carcinomas (27%), with a mean size of 1.7 cm (range: 1.1 to 2.8) for nonresected tumors and 2.28 cm (range: 1.3 to 3.3) for resected tumors. Most patients (n=10) had extremely fair or fair skin types.
TABLE 1.
Baseline Patient and Tumor Characteristics
| Nonresection Study (N=5) | Resection Study (N=10) | |
|---|---|---|
| Age (y), mean (SD) (range) | 59.8 (6.99) | 61.9 (4.72) |
| (51, 68) | (54, 70) | |
| Sex, n (%) | ||
| Male | 1 (20) | 6 (60) |
| Female | 4 (80) | 4 (40) |
| Childbearing potential | 4 (100) | 4 (100) |
| Race, n (%) | ||
| White | 4 (80) | 10 (100) |
| Black | 1 (20) | 0 |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 0 | 0 |
| Not Hispanic/Latino | 5 (100) | 10 (100) |
| Body mass index (kg/m2), mean (SD) | 29.5 (9.71) | 25.4 (3.84) |
| ECOG performance status, n (%) | ||
| 0 | 1 (20) | 7 (70) |
| 1 | 4 (80) | 3 (30) |
| Skin phototype, n (%) | ||
| I—extremely fair skin | 2 (40) | 1 (10) |
| II—fair skin | 1 (20) | 6 (60) |
| III—medium skin | 1 (20) | 3 (30) |
| IV—olive skin | 0 | 0 |
| V—moderately pigmented dark skin | 0 | 0 |
| VI—markedly pigmented dark skin | 1 (20) | 0 |
| Tumor size (cm), mean (SD) (range) | 1.70 (0.76) | 2.28 (0.63) |
| (1.1, 2.8) | (1.3, 3.3) | |
| Tumor type, n (%) | ||
| Adenocarcinoma | 2 (40) | 8 (80) |
| Squamous cell carcinoma | 2 (40) | 2 (20) |
| Carcinoid | 1 (20) | 0 |
ECOG indicates Eastern Cooperative Oncology Group.
Nonresection Tumor Study
Tumor responses to PDT for the 5 nonresection patients are shown in Table 2. Four patients each received 1 porfimer sodium injection, 3 of whom had a single PDT laser light application with fiber placement adjacent to the tumor. The other patient had a single laser light application with intratumor fiber placement in 3 separate tumor positions and the 200 J/cm divided among these 3 positions. One patient received a porfimer sodium injection followed by 2 PDT laser light applications 2 days apart with intratumor fiber placement. This patient had a second porfimer sodium injection 63 days later followed by 2 more PDT laser light applications 3 days apart with adjacent fiber placement.
TABLE 2.
Tumor Response After PDT Light Applications
| Patient # | Diagnosis | Tumor Size (cm) | PDT Light Applications (Day 3) | Tumor Response | Days From PDT to Surgery | Tumor Cell Necrosis (%) | Brisk Inflammatory Reaction |
|---|---|---|---|---|---|---|---|
| Nonresection study | |||||||
| 1 | Squamous cell carcinoma | 2.8 | 1 | 3 mo: SD 6 mo: PD | — | — | — |
| 2 | Carcinoid | 1.6 | 1 | 3 mo: SD 6 mo: SD | — | — | — |
| 3 | Adenocarcinoma metastasis from colon | 1.5 | 3 | 3 mo: SD 6 mo: SD | — | — | — |
| 4 | Squamous cell carcinoma | 1.1 | 2, 2*;2*, 1* | 3 mo: CR 6 mo: CR | — | — | — |
| 5 | Adenocarcinoma | 1.3 | 1 | 3 mo: SD 6 mo: SD | — | — | — |
| Resection study | |||||||
| 1 | Adenocarcinoma | 3.00 | 1 | No | 12 | 20 | Yes |
| 2 | Squamous cell | 1.30 | 1 | No | 13 | 40 | Yes |
| 3 | Adenocarcinoma | 2.60 | 1 | No | 14 | 40 | Yes |
| 4 | Adenocarcinoma | 2.77 | 3 | No | 15 | 35 | Yes |
| 5 | Squamous cell | 1.80 | 1 | Yes† | 32 | No residual tumor | Not applicable |
| 6 | Adenocarcinoma | 3.30 | 2 | No | 15 | 10 | Yes |
| 7 | Adenocarcinoma | 2.10 | 3 | No | 12 | 5 | Yes |
| 8 | Adenocarcinoma | 1.70 | 2 | No | 12 | 10 | Yes |
| 9 | Adenocarcinoma | 2.10 | 1 | No | 13 | 50 | Yes |
| 10 | Adenocarcinoma | 2.10 | 4‡ | No | 18 | 10 | Yes |
PDT applications after first porfimer sodium injection: 2 on day 3, 2 on day 5; after second porfimer sodium injection: 2 on day 3, 2 on day 6.
No nonviable/necrotic tumor at resection.
4 placements for total dose of 200 J.
—indicates not evaluated, no tumor resection in this study; PDT, photodynamic therapy.
At 3 months follow-up, 1 patient had a CR and 4 had stable disease (SD); at 6 months follow-up, 1 patient had continued CR, 3 had continued SD, and 1 had progressive disease (PD) (Table 2). The CR occurred in the patient who received 4 PDT laser light applications—2 at the initial treatment and 2 more 63 days later.
Resection Tumor Study
Tumor responses to PDT for the 10 resection patients are shown in Table 2. Five patients received 1 PDT laser light application, 4 with intratumor fiber placement, and 1 with adjacent fiber placement (Table 2). Two patients received 2 PDT laser light applications, both with intratumor fiber placement. Two patients received 3 PDT laser light applications: 1 patient had intratumor fiber placement and 1 had adjacent fiber placement. One patient received 1 laser light application with 4 separate positions (2 intratumor and 2 adjacent), with a total 200 J/cm divided among those 4 placements.
Nine of 10 patients underwent lobectomy 12 to 18 days after PDT application, of which 3 patients had 40% to 50% tumor cell necrosis, 2 had 20% to 35% tumor cell necrosis, and 4 had 5% to 10% tumor cell necrosis (Table 2). One patient underwent lobectomy 32 days after PDT application and was considered a CR based on no evidence of residual tumor. Figure 1 shows an organizing pneumonia pattern with no residual tissue necrosis in the “kill zone” in this patient.
FIGURE 1.
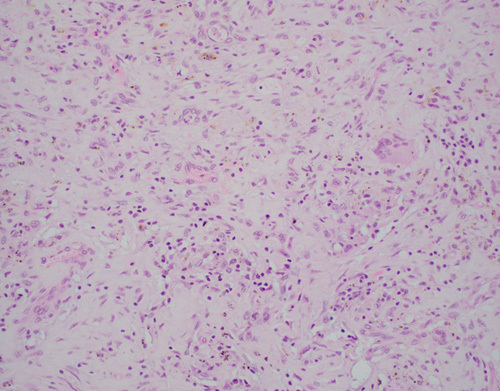
High-power (×20) photomicrograph of tissue response to photodynamic therapy consistent with an organizing pneumonia pattern in the patient with no residual tumor at resection.
The mean (SD) percent tumor cell necrosis was 22% (17.67). A positive brisk inflammatory reaction was observed in the 9 (90%) patients with residual tumor. Surrounding tissue was normal in 6 (60%) patients and abnormal in 4 (40%) patients. Abnormal findings noted in surrounding tissue were cavitation and large caliber vessel (≥0.5 mm) damage indicated by fibrinoid necrosis, thrombus, and vasculitis in 1 (10%) patient; moderate interstitial fibrosis in 1 (10%) patient; and pneumonitis in 2 (20%) patients. Histopathologic findings secondary to surgery included increased alveolar macrophages with atypical/reactive type 2 pneumocytes in all 10 (100%) patients, an organizing pneumonia pattern in 7 (70%) patients and hemorrhage in 6 (60%) patients. In 3 patients with procedure-related hemorrhage, embedded necrosis within the hemorrhage zones was not considered an abnormal histologic reaction in the normal lung fields.
Safety
There were no deaths, and no patients discontinued either study due to adverse events. Among the adverse events reported in ≥20% of patients, erythema (40%) is known to be associated with porfimer sodium exposure, and complications associated with surgical resection—pleural effusion (53%), atelectasis (40%), pneumothorax (33%), and creatinine increase (40%)—occurred primarily in patients who underwent lobectomy and were not deemed related to porfimer sodium or PDT (Table 3).
TABLE 3.
Adverse Events Occurring in ≥20% of Patients
| Preferred Term | All Treated Patients (N=15) n (%) |
|---|---|
| Porfimer sodium photosensitivity and PDT light application | |
| Erythema | 6 (40.0) |
| Surgical complications | |
| Pleural effusion* | 8 (53.3) |
| Atelectasis* | 6 (40.0) |
| Blood creatinine increased | 6 (40.0) |
| Pneumothorax* | 5 (33.3) |
| Blood glucose increased | 5 (33.3) |
| Haemoglobin decreased | 4 (26.7) |
| Blood albumin decreased | 4 (26.7) |
| Blood pressure increased | 4 (26.7) |
| Blood pressure systolic increased | 3 (20.0) |
| Haematocrit decreased | 3 (20.0) |
| Heart rate increased | 3 (20.0) |
| Pulmonary function test abnormal | 3 (20.0) |
| Red blood cell count decreased | 3 (20.0) |
| Respiratory rate increased | 3 (20.0) |
Most of these events occurred in patient who underwent lobectomy: 7 of 8 pleural effusion, 4 of 6 atelectasis, and all 5 pneumothorax.
PDT indicates photodynamic therapy.
Ten of 15 patients (67%) experienced 26 adverse events considered possibly related to porfimer sodium or PDT by the investigator. These included single grade 1 events of rash, flushing, skin exfoliation, swelling face, blisters, and asymptomatic pleural effusion identified on chest x-ray; single grade 2 events of photosensitivity reaction and procedural pain; and 18 events (grade 1=17, Grade 2=1) of erythema due to photosensitivity reactions (9 events reported in a single patient who received 2 porfimer sodium injections). Overall, 8 of 15 patients (53%) experienced photosensitivity events (Fig. 2), including 6 mild reactions, 1 moderate reaction, and 1 severe reaction in a patient with elevated porphyrins in the blood associated with porphyria noted after the procedure. There were no clinical hemorrhagic complications directly related to PDT light application or porfimer sodium injection.
FIGURE 2.
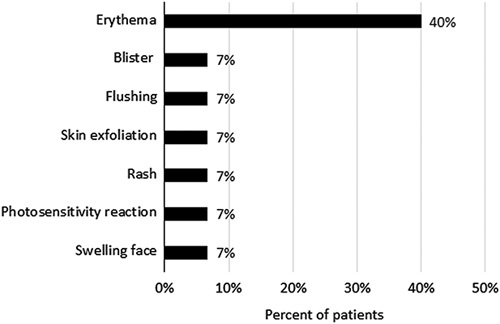
Summary of photosensitivity events. The percent of all patients (N=15) who experienced each type of photosensitivity event is shown. Overall, 8 patients (53%) experienced photosensitivity events; some experienced more than 1 event.
Three of 15 patients (20%), all of whom underwent lobectomy, experienced grade 3 adverse events of anemia, hemothorax, deep vein thrombosis, vomiting, and colon cancer. One patient experienced grade 4 events of hypoxia and hemorrhagic shock, all of which were deemed unrelated to PDT or porfimer sodium. The 14 serious adverse events (Table 4) reported in 4 patients (27%), all occurred in patients who underwent lobectomy and were deemed unrelated to PDT or porfimer sodium.
TABLE 4.
Serious Adverse Events, None of Which Were Deemed Treatment Related*
| Preferred Term | All Treated Patients (N=15) n (%) |
|---|---|
| Anemia | 1 (6.7) |
| Atrial fibrillation | 1 (6.7) |
| Gastrointestinal hemorrhage | 1 (6.7) |
| Gastroesophageal reflux disease | 1 (6.7) |
| Sepsis | 1 (6.7) |
| Colon cancer | 1 (6.7) |
| Dyspnea | 1 (6.7) |
| Hemothorax | 1 (6.7) |
| Hypoxia | 1 (6.7) |
| Pleural effusion | 1 (6.7) |
| Pneumonia aspiration | 1 (6.7) |
| Deep vein thrombosis | 1 (6.7) |
| Shock hemorrhagic | 1 (6.7) |
All occurred in the resection tumor study (ie, patients who underwent lobectomy).
DISCUSSION
In 2 small phase 1 studies, navigational bronchoscopy with REBUS to deliver PDT to peripheral lung tumors was feasible and seemed to be safe and well tolerated. Furthermore, unexpected treatment-emergent adverse events reported with percutaneous RFA and microwave ablation were not observed in these pilot studies. The initial tumor response to a single application of PDT in these studies was encouraging, and the majority of photosensitivity reactions were mild (ie, grade 1: painless erythema and erythema covering <10% body surface area). Among the 5 unresected tumors at 6 months follow-up, there was 1 CR and 3 SD. Residual tumor assessment of the 10 resected tumors showed 1 complete tumor response in a patient who underwent lobectomy 32 days post PDT application and 5% to 50% tumor cell necrosis in the remaining 9 patients who underwent lobectomy 12 to 18 days post PDT application. We hypothesize that the organizing pneumonia pattern and absence of necrosis observed in the CR patient may reflect the longer time between PDT application and tumor resection.
The current treatments for early-stage NSCLC patients who decline surgery or are not surgical candidates have limitations. Although SBRT provides local or regional control of up to 98% of inoperable tumors and overall survival ranges from 43% to 95% out to 3 years, it carries a risk of radiation pneumonitis, which can be severe and, rarely, fatal.24,25 In a multicenter, prospective, single-arm study of SBRT for inoperable stage 1 NSCLC, the rate of grade 3 or 4 pulmonary-specific or respiratory tract-specific toxicity was 16% and the rate of protocol-specified hypoxia or pneumonitis was 8%.20 This risk was even higher in patients with interstitial lung disease and it can be fatal.26,27 Percutaneous RFA has been utilized for decades in medically inoperable lung cancer, but its use is limited to tumors smaller than 3 cm in diameter because of its inability to ablate larger areas.10,28–30 RFA has shown lower local control rates than SBRT or sublobar resection. More recently, percutaneous microwave ablation has emerged as an alternative to RFA to treat larger tumors with similar or slightly better local control rates and survival.31–33 Like any percutaneous ablative technique, however, the pleura is invariably violated, and the rate of pneumothorax can be as high as 62%.9,10 As most patients with medically inoperable lung cancer have very poor lung reserve, this high rate of pneumothorax can be a prohibitive risk. An analysis of 1000 lung RFA sessions reported major complications in ~10% of cases, including lung abscess, bleeding requiring transfusion, pneumothorax, and bronchopleural fistula.34
PDT-mediated cell death is associated with both tumor necrosis and antitumor immune responses.35 Light activation of the photosensitizing agent that accumulates in the target sites generates singlet oxygen and reactive oxygen species in cancer cells. These cytotoxic reactive oxygen species lead to tumor cell death via apoptosis or necrosis and damage the target sites resulting in tumor destruction.35 In our patient with no residual tumor on resection, we hypothesize that necrosis after tumor cell ablation transitioned to the observed organizing pneumonia pattern over the 32 days between PDT application and resection. Because the biological effect of PDT is photochemical rather than thermal, injury to normal surrounding tissue is less than with thermal ablative techniques. There is remarkably little effect on connective tissues like collagen, thus the basic mechanical integrity of organs is maintained and PDT-necrotized areas heal well with less fibrosis and scarring than most other forms of localized necrosis (ie, thermal ablation) and without cumulative toxicity.19,36 For example, the fibrosis observed in about 80% of patients treated with high-dose SBRT makes differentiating between benign radiologic changes and local recurrence challenging.36,37 This phenomenon was not observed after PDT in our study. The impact that thermal ablation therapies (eg, microwave or steam) have on adjacent organs, vessels, and tissue was not seen with PDT in our study. This is expected as the biological effect of PDT is photochemical rather than thermal and, thus, normal tissue injury is less than with thermal ablative techniques.19
In addition to directly affecting tumor cells, PDT can impact vasculature both within the tumoral region and surrounding lung tissue, and the inflammatory reaction can either be localized to the tumor area or potentially impact the adjacent parenchyma.23 A brisk inflammatory reaction, observed in 9 of 10 patients with residual tumor after PDT, was included as a parameter of residual tumor assessment because it has been found to be a favorable prognostic indicator in other tumors (such as colorectal cancer) and such a reaction can indicate a host lymphoid reaction to tumor cells.38 Brisk inflammatory reaction is especially relevant to NSCLC, as it can be included as part of the anti-PD-1-axis immunotherapy evaluation.39 In addition, studies suggest that local delivery of PDT can result in systemic neutrophilia, induction of acute phase proteins, increased circulating levels of complement proteins, and systemic release of proinflammatory cytokines, all of which indicate the presence of a systemic inflammatory response with T-cell–mediated antitumor immune response.40 In studies in laboratory and animal models, tumor ablation by PDT reduced the tumor mass and induced the release of tumor antigen and proinflammatory mediators, leading to regression in distant, antigen-positive tumors outside the illumination field, an effect mediated by tumor antigen-specific cytotoxic T cells.41–43
PDT applied via navigational bronchoscopy could be a reasonable alternative to SBRT or percutaneous ablation for medically inoperable peripheral solid lung tumors. Bronchoscopic ablation is bound to have a more favorable safety profile, thanks to the absence of high pneumothorax risk, and alveolo-pleural fistula associated with percutaneous ablations. The potential for photosensitivity reactions (mostly mild) associated with porfimer sodium can be mitigated through enhanced patient education regarding the avoidance of sunlight and bright indoor light.44 Furthermore, as PDT is not radiation, adjuvant radiation therapy after PDT or radiation therapy for the rescue of local recurrence after PDT could be considered in selected patients.
Although the initial tumor response to a single application of PDT was encouraging, these small feasibility studies were not designed to optimize or assess efficacy and did not include treated or untreated comparators. It is possible that more time may be required after PDT application to achieve maximal tumor necrosis and antitumor immune responses than the 12 to 18 days in our study. Larger controlled studies are needed to evaluate safety and efficacy, including multiple fiber placements in a single light session and the need for multiple light sessions.
CONCLUSION
In 2 small studies, PDT delivered via navigational bronchoscopy for nonthermal ablation of peripheral lung tumors was feasible and safe, warranting further studies to evaluate its efficacy and corroborate its safety profile in larger populations.
ACKNOWLEDGMENTS
The authors thank Laurie LaRusso, MS, ELS for medical writing support, paid for by Concordia Laboratories Inc.
Footnotes
Disclosure: The studies were sponsored and conducted by Concordia Laboratories Inc. S.B. received a research grant from Pinnacle Biologics during this study; support for attending meetings and travel from Pulmonx and Circulogene; honoraria for speaking engagements from GlaxoSmithKline, Boehringer Ingelheim, Sanofi Genzyme, and Regeneron; and consulting fees from Johnson & Johnson, Circulogene and Veracyte; he also holds equity and stocks in Veracyte and Circulogene. R.I.B. has no current relationships to disclose; in the past, he has received educational and research grants from Medtronic, Circulogene, Olympus, and Pinnacle Biologics. Jiten D. Patel, Hiren J. Mehta, and Benjamin L. Witt have no relationships to disclose. J.S.F. has received consulting fees from Noah Medical and Cook Medical and payments for DSMB membership from Covance; and holds stock options from Noah Medical and VIDA Diagnostics. S.D.M. is an educational consultant for Pinnacle Biologics and previously participated in their PDT registry. K.Y. is a consultant for Concordia Healthcare. R.F.C. has received research grants to his institution from Concordia Healthcare and consulting fees from Siemens and Intuitive Surgical.
Contributor Information
Sandeep Bansal, Email: bansalmd@gmail.com.
Rabih I. Bechara, Email: rbechara@augusta.edu.
Jiten D. Patel, Email: Jiten.Patel2@providence.org.
Hiren J. Mehta, Email: Hiren.Mehta@medicine.ufl.edu.
J. Scott Ferguson, Email: jsferguson@medicine.wisc.edu.
Benjamin L. Witt, Email: benjamin.l.witt@aruplab.com.
Septimiu D. Murgu, Email: smurgu@medicine.bsd.uchicago.edu.
Kazuhiro Yasufuku, Email: tghyasufuku@gmail.com.
Roberto F. Casal, Email: RFCasal@mdanderson.org.
REFERENCES
- 1. Sabath BF, Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis. 2019;11:2628–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e78S–e92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7:170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 5. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belanger AR, Akulian JA. An update on the role of advanced diagnostic bronchoscopy in the evaluation and staging of lung cancer. Ther Adv Respir Dis. 2017;11:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchol Interv Pulmonol. 2012;19:91–97. [DOI] [PubMed] [Google Scholar]
- 8. Jensen KW, Hsia DW, Seijo LM, et al. Multicenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodules. J Bronchology Interv Pulmonol. 2012;19:195–199. [DOI] [PubMed] [Google Scholar]
- 9. Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest.. 2012;142:1620–1635. [DOI] [PubMed] [Google Scholar]
- 10. Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 11. Wisnivesky JP, Yung RC, Mathur PN, et al. Diagnosis and treatment of bronchial intraepithelial neoplasia and early lung cancer of the central airways: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest . 2013;143(suppl 5):e263S–e277S. [DOI] [PubMed] [Google Scholar]
- 12. PHOTOFRIN (porfimer sodium) INJECTION, prescribing information. Concordia Laboratories Inc.: St. Michael, Barbados; 2015.
- 13. Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol. 1993;11:1852–1857. [DOI] [PubMed] [Google Scholar]
- 14. Kato H, Furukawa K, Sato M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer. 2003;42:103–111. [DOI] [PubMed] [Google Scholar]
- 15. Chen KC, Lee JM. Photodynamic therapeutic ablation for peripheral pulmonary malignancy via electromagnetic navigation bronchoscopy localization in a hybrid operating room (OR): a pioneering study. J Thorac Dis. 2018;10(suppl 6):S725–S730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato H. Our experience with photodynamic diagnosis and photodynamic therapy for lung cancer. J Natl Compr Canc Netw. 2012;10(suppl 2):S3–S8. [DOI] [PubMed] [Google Scholar]
- 17. Shafirstein G, Battoo A, Harris K, et al. Photodynamic therapy of non-small cell lung cancer. Narrative Review and Future Directions. Ann Am Thorac Soc. 2016;13:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris K, Oakley E, Bellnier D, et al. Endobronchial ultrasound-guidance for interstitial photodynamic therapy of locally advanced lung cancer-a new interventional concept. J Thorac Dis. 2017;9:2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fielding DI, Buonaccorsi G, Cowley G, et al. Interstitial laser photocoagulation and interstitial photodynamic therapy of normal lung parenchyma in the pig. Lasers Med Sci. 2001;16:26–33. [DOI] [PubMed] [Google Scholar]
- 20. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA.. 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 23. Musani AI, Veir JK, Huang Z, et al. Photodynamic therapy via navigational bronchoscopy for peripheral lung cancer in dogs. Lasers Surg Med. 2018;50:483–490. [DOI] [PubMed] [Google Scholar]
- 24. Yamashita H, Takahashi W, Haga A, et al. Radiation pneumonitis after stereotactic radiation therapy for lung cancer. World J Radiol. 2014;6:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prezzano KM, Ma SJ, Hermann GM, et al. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol. 2019;10:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takeda A, Sanuki N, Enomoto T, et al. Subclinical interstitial lung disease: is it a risk factor for fatal radiation pneumonitis following stereotactic body radiotherapy? Lung Cancer. 2014;83:112. [DOI] [PubMed] [Google Scholar]
- 27. Onishi H, Marino K, Yamashita H, et al. Case series of 23 patients who developed fatal radiation pneumonitis after stereotactic body radiotherapy for lung cancer. Technol Cancer Res Treat. 2018;17:1533033818801323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol. 2011;6:2044–2051. [DOI] [PubMed] [Google Scholar]
- 29. Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. [DOI] [PubMed] [Google Scholar]
- 30. Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:160–166. [DOI] [PubMed] [Google Scholar]
- 31. Lu Q, Cao W, Huang L, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol. 2012;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng A, Ye X, Yang X, et al. Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol. 2016;27:1806–1814. [DOI] [PubMed] [Google Scholar]
- 33. Healey TT, March BT, Baird G, et al. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28:206–211. [DOI] [PubMed] [Google Scholar]
- 34. Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. AJR Am J Roentgenol. 2011;197:W576–W580. [DOI] [PubMed] [Google Scholar]
- 35. Hwang HS, Shin H, Han J, et al. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharm Investig. 2018;48:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dahele M, Palma D, Lagerwaard F, et al. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol. 2011;6:1221–1228. [DOI] [PubMed] [Google Scholar]
- 37. Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR) -- can we distinguish fibrosis from recurrence? A systematic review of the literature. Pract Radiat Oncol. 2013;3(2 suppl 1):S11–S12. [DOI] [PubMed] [Google Scholar]
- 38. Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res. 2010;46:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mroz P, Szokalska A, Wu MX, et al. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PLoS One. 2010;5:e15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Shaughnessy MJ, Murray KS, La Rosa SP, et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin Cancer Res. 2018;24:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kleinovink JW, van Driel PB, Snoeks TJ, et al. Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors. Clin Cancer Res. 2016;22:1459–1468. [DOI] [PubMed] [Google Scholar]
- 44. Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg. 2010;89:1744–1748. [DOI] [PubMed] [Google Scholar]


