Abstract
Preeclampsia (PE) is a disorder that affects approximately 5% to 10% of pregnant women. Timely and accurate identification of PE and assessment of its severity are crucial. Therefore, it is necessary to develop predictive indicators which are easily measured in routine antenatal examinations to enable the early detection of PE and assess its severity. We designed a single-center retrospective study in our daily work to assess whether the serum levels of fibrinogen to albumin ratio (FAR), fibrinogen (Fib), albumin (ALB), prothrombin time, calcium (Ca), activated partial thrombin time, creatinine (Cr), D-dimer(D-D), platelet, white blood cell, neutrophil, and lymphocyte counts could help in assessing PE and evaluating its severity. Our findings showed that the serum levels of FAR, Cr, Fib, and D-D were significantly higher in the severe preeclampsia group (sPE) compared with the control and mild preeclampsia groups, whereas the levels of ALB and Ca were significantly lower in sPE patients. In addition, no differences were found between the control and PE groups in terms of prothrombin time, activated partial thrombin time, platelet, white blood cell, neutrophils, and lymphocytes counts. Furthermore, FAR is a novel and better indicator for evaluating the severity of PE, which has not been reported before. And it is an independent risk factor for the development of sPE. In conclusion, the serum levels of FAR, Cr, D-D and Fib were positively correlated with PE, whereas ALB and Ca were negatively correlated with PE severity, which might be valuable in evaluating the severity of PE. FAR proved to be a feasible diagnostic marker for sPE with sensitivity and specificity comparable to those of ALB and Fib.
Keywords: ALB, biomarker, Ca, FAR, inflammation, severe preeclampsia
1. Introduction
Preeclampsia (PE) is a pregnancy disorder that causes hypertension and proteinuria after 20 weeks of gestation. It affects approximately 5% to 10% of pregnant women.[1] In addition to maternal multiple organ dysfunction and intrauterine growth restriction, PE is associated with several adverse outcomes including fetal, neonatal, and maternal mortality.[2] Although the etiology of PE remains controversial, clinical and pathological studies suggest that systemic inflammatory responses,[3,4] immune dysfunction,[5] coagulation/fibrinolytic system,[6] and abnormal placentation[7] play central roles in the pathogenesis of this syndrome. Prenatal care and timely diagnosis are still necessary for the treatment of preeclampsia, as well as for proper management and timely delivery. The diagnosis of PE remains a challenge and is based on a combination of clinical presentation and nonspecific biomarkers. Therefore, it is necessary to develop specific predictive biomarkers which are easily measured in routine antenatal examinations to enable the early detection of PE and assess its severity.
Previous studies have reported that angiogenic factors such as soluble fms-related receptor tyrosine kinase 1, and placental growth factor are essential biomarkers in PE.[8,9] Guo et al[10] have shown that Epstein-Barr virus induced 3 is upregulated in the early onset preeclampsia, indicating that the detection of excessive Epstein-Barr virus induced 3 from maternal plasma is an excellent biomarker of early onset preeclampsia. Other studies have reported that the expression of inflammatory factors such as interleukin 6 [11] and higher neutrophil-to-lymphocyte ratio [12] are valuable predictive markers of PE. Although these studies have been conducted on the predictive value of biochemical tests for PE outcomes, these markers are single studies and are difficult to measure in clinical laboratories. In addition to above-mentioned biomarkers, albumin-based markers are used inflammatory conditions since it is a negative acute phase marker. For example C-reactive protein to albumin ratio has been introduced as a marker of inflammation.[13] Other parameters, such as white blood cell (WBC) and platelet were also suggested as markers of inflammatory conditions.[14,15] Since preeclampsia is also associated with some degree of inflammatory burden,[4] it is reasonable to study other inflammatory markers in preeclampsia. In addition, fibrinogen to albumin ratio (FAR) is a new inflammatory marker and has been proven to have excellent predictive value for abortus imminent,[16] systemic lupus erythematosus,[17] and ascending aortic aneurysm.[18] However, there has been no reliable research on whether FAR can predict PE or evaluate its severity.
PE is a complex and systemic vascular disorder, therefore, in the present study, we combined blood tests including automated full blood count (platelet [PLT], WBC, neutrophil and lymphocyte counts), biochemistry [albumin (ALB), calcium (Ca), creatinine (Cr)], coagulation function tests [D-dimer (D-D), prothrombin time (PT), fibrinogen (Fib), activated partial thrombin time (APTT)], and a new inflammatory marker FAR, which are related to PE and easy to measure. We then investigated whether the twelve serum parameters mentioned above differed significantly between the mild preeclampsia (mPE) and severe preeclampsia (sPE) groups. Collectively, our data suggest that FAR is a novel and better indicator for evaluating preeclampsia severity, which has never been reported before. Besides, this is an independent risk factor for the development of sPE.
2. Methods
2.1. Study participants
A retrospective observational study was conducted at the Second Hospital of Shandong University from January 2017 to August 2021, and written informed consent was obtained from all participants. This study was approved by the Ethics Committee of the Second Hospital of Shandong University (KYll-2023-182). In the present study, 237 pregnant women were included in the final analysis, including 73 normotensive healthy pregnant controls, 61 mPE, and 103 sPE patients. The subjects with normal pregnancies were matched in terms of gestational age and pregnancy body weight to the PE patients. During pregnancy, all control pregnant women had normal blood pressure and immune system profiles and had no history of chronic diseases, such as chronic hypertension, preexisting renal disease, or immunological diseases. The diagnostic criteria for PE were based on those recommended by the American College of Obstetrics and Gynecology in 2019.[19] sPE was considered if it was characterized by a sustained systolic blood pressure of > 160 mm Hg or sustained diastolic blood pressure of > 110 mm Hg or symptoms that included at least one of the following: severe proteinuria (>3 + on dipstick or proteinuria > 5 g/24-hour urine collection), visual impairment and headache, impaired liver function, thrombocytopenia, pulmonary edema, heart failure, oligohydramnios, renal insufficiency, or fetal growth restriction. mPE was defined as the diagnostic criterion for PE, but not for sPE. The medical records provided details of the patients medical history and treatment before admission. Patients with any of the following items at the time of screening were excluded: Comorbidities including chronic hypertension, chronic nephropathy, liver disease, heart disease, blood system disease, recurrent spontaneous abortion, immunological diseases, or other comorbidities; The patient failed to follow up or the data were incomplete.
2.2. Data collection
Blood specimens were harvested immediately after the diagnosis of PE was confirmed, or after a routine antenatal visit. Specimens of blood were centrifuged at 4000 revolutions per minute for 10 minutes and analyzed within 2 hours. Briefly, a Sysmex XN9000 hematology analyzer (Sysmex Corporation, Japan) was used for blood routine analysis. Fib, APTT, PT, and D-D levels were analyzed using a Werfen ACLTOP700 hematology analyzer (Werfen Corporation, Spain). In addition, we collected 5 mL of venous blood in a gold tube with a clot activator and gel, and the serum was collected to measure ALB, Cr, and Ca levels using a Cobas C702 automatic analyzer (Roche Corporation, Switzerland). FAR was determined by dividing the plasma Fib concentration by the serum ALB levels. In our clinical laboratory, all tests were performed in accordance with ISO15189.
2.3. Statistical analysis
Statistical analyses were performed using soft of SPSS version 25.0 (IBM Co., Armonk, NY). Clinical information was obtained from the medical records of pregnant women. The variances between groups were compared using Levene homogeneity test or the F test. For continuous variables, descriptive statistics were calculated as the mean (X) + standard deviation or median and interquartile range. The student t test and Mann-Whitney U test were used to evaluate continuous variables. Multiple group comparisons were conducted using 1-way analysis of variance. Factors associated with sPE were evaluated using a logistic regression analysis. A receiver operating characteristic (ROC) curve was used to estimate biomarkers that were positively correlated with sPE. The area under the curve (AUC) was calculated to evaluate predictive power. P < .05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
A total of 237 pregnant women were divided into 3 groups: healthy normotensive pregnant controls (control, n = 73), mPE (n = 61), and sPE (n = 103). Table 1 compared the clinical characteristics of the control, mPE, and sPE groups, including age, gestational age, gravidity, blood pressure, body mass index (BMI), and urine ALB. The data revealed that the mean age of the women who developed sPE (32.77 ± 5.77) was higher than that of the control (29.95 ± 3.9) and mPE (30.69 ± 5.48) groups (P = .002). We also discovered that the mean BMI for the control, mPE, and sPE groups was 28.25 ± 3.05 kg/m2, 31.91 ± 4.72 kg/m2, and 30.81 ± 4.33 kg/m2, respectively. The BMI of the PE group was higher than that of the control group (P < .001). The 3 groups of pregnant women indicated no significant differences in gravidity (P = .785). A significant difference was found between the sPE and the control group in terms of gestational age at delivery (P < .001). Furthermore, the blood pressure and urine ALB of both the mPE and sPE groups were significantly higher than that of the control group (P < .001). As expected, some indicators were positively correlated with sPE, such as age, BMI, systolic blood pressure, diastolic blood pressure and urine ALB, whereas gestational age had a negative correlation.
Table 1.
Summary of maternal characteristics of the control, mild preeclampsia and severe preeclampsia groups.
| Characteristics | Control | mPE | sPE | P value |
|---|---|---|---|---|
| Age (yr) | 29.95 ± 3.90 | 30.69 ± 5.48 | 32.77 ± 5.77 | .002 |
| BMI (kg/m2) | 28.25 ± 3.05 | 31.91 ± 4.72 | 30.81 ± 4.33 | <.001 |
| Gestational age at delivery (wk) | 39.74 ± 0.99 | 37.66 ± 2.76 | 34.33 ± 3.29 | <.001 |
| Parity (n) | 1.21 ± 0.44 | 1.26 ± 0.54 | 1.22 ± 0.46 | .785 |
| Systolic blood pressure (mm Hg) | 118.52 ± 10.30 | 147.78 ± 10.15 | 167.96 ± 18.59 | <.001 |
| Diastolic blood pressure (mm Hg) | 78.42 ± 7.70 | 96.23 ± 8.21 | 106.69 ± 14.61 | <.001 |
| Urine albumin (g/24 h) | - | 1.11 ± 1.07 | 5.74 ± 5.15 | <.001 |
P: 1-way sANOVA.
BMI = Body mass index, mPE = mild preeclampsia, sPE = severe preeclampsia.
3.2. FAR is positively correlated with PE severity
These aforementioned markers in Table 1 are recognized indicators of PE status; however, some indicators remain controversial or even unknown. To further investigate the relationship between PE and other clinical indicators, student t test or Mann-Whitney U test analysis was conducted. Serum parameters were listed in Table 2. As reported in other studies, the lower the ALB level, the more severe the disease of PE.[20] In addition, we found a significant difference in FAR and Cr levels between the control and mPE or sPE groups. Cr, Fib, D-D, and FAR levels were significantly higher in the sPE group than in the control and mPE groups, while the levels of ALB and Ca were significantly lower in sPE patients. Moreover, the PT in the PE group was lower than that in the control group, while the APTT of the sPE group was higher than that of the control group. However, neither PT nor APTT was statistical different among the 3 groups. In addition, there were no differences in PLT, WBC, neutrophil or lymphocyte counts between the control and PE groups. In conclusion, serum levels of FAR, Cr, D-D and Fib were positively associated while ALB and Ca were negatively correlated with PE severity. Additionally, PT, APTT, PLT, WBC, neutrophil and lymphocyte counts were unrelated to the severity of PE. More importantly, our findings indicate that FAR is positively correlated with PE severity for the first time.
Table 2.
The tested markers values in different groups.
| Variables | Control | mPE | sPE | Control vs mPE | Control vs sPE | mPE vs sPE |
|---|---|---|---|---|---|---|
| Ca | 2.18 ± 0.06 | 2.19 ± 0.15 | 2.10 ± 0.17 | 0.799 | 0.001 | 0.001 |
| PLT | 222.46 ± 61.22 | 212.48 ± 61.40 | 212.83 ± 76.44 | 0.189 | 0.370 | 0.680 |
| Cr | 45.31 ± 9.71 | 51.65 ± 9.54 | 58.69 ± 16.39 | 0.015 | < 0.001 | 0.012 |
| ALB | 38.66 ± 4.78 | 34.46 ± 4.34 | 28.68 ± 3.98 | < 0.001 | < 0.001 | < 0.001 |
| Fib | 4.02 ± 0.50 | 4.04 ± 0.65 | 4.34 ± 0.77 | 0.878 | 0.001 | 0.001 |
| APTT | 28.74 ± 3.80 | 28.52 ± 3.19 | 29.31 ± 5.08 | 0.833 | 0.398 | 0.496 |
| PT | 11.85 ± 3.49 | 11.04 ± 0.97 | 11.23 ± 1.01 | 0.082 | 0.153 | 0.352 |
| D-D | 1.341 (1.073–1.766) | 1.252 (0.998–1.795) | 1.500 (1.180–3.020) | 0.611 | 0.002 | 0.006 |
| FAR | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.15 ± 0.03 | < 0.001 | < 0.001 | <0.001 |
| WBCs | 10.14 ± 5.34 | 9.53 ± 2.94 | 9.73 ± 3.00 | 0.417 | 0.567 | 0.690 |
| neutrophils | 7.49 ± 4.42 | 7.32 ± 3.05 | 7.35 ± 2.75 | 0.796 | 0.814 | 0.949 |
| lymphocytes | 1.88 ± 0.79 | 1.65 ± 0.54 | 1.81 ± 0.60 | 0.058 | 0.538 | 0.113 |
Data are presented as mean ± standard deviation (SD) when the parameters were normally distributed or median and interquartile ranges for non-normally distributed variables.
ALB = albumin, APTT = activated partial thrombin time, Ca = calcium, Cr = creatinine, D-D = D-dimer, FAR = fibrinogen to albumin ratio, Fib = fibrinogen, mPE = mild preeclampsia, PLT = platelet, PT = prothrombin time, sPE = severe preeclampsia, WBC = white blood cell.
3.3. FAR is an independent risk factor for the development of sPE
In order to identify independent risk factors for sPE, univariate logistic regression analysis of the above 6 indicators with differences was performed. Table 3 suggests that FAR (OR: 2.679 [95% CI: 1.106–6.490]; P = .029) was an independent risk factor for the development of sPE.
Table 3.
Univariate conditional logistic regression model analysis of severe preeclampsia.
| Variables | B | SE | Wald | P value | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| ALB | −0.227 | 0.174 | 1.698 | .193 | 0.797 | 0.567 | 1.121 | |
| Ca | −1.874 | 2.369 | 0.626 | .429 | 0.154 | 0.001 | 15.950 | |
| Cr | 0.039 | 0.034 | 1.301 | .254 | 1.040 | 0.972 | 1.111 | |
| Fib | −0.920 | 1.485 | 0.384 | .536 | 0.399 | 0.022 | 7.323 | |
| D-D | 0.000 | 0.023 | 0.000 | .983 | 1.000 | 0.956 | 1.045 | |
| FAR*100 | 0.985 | 0.451 | 4.764 | .029 | 2.679 | 1.106 | 6.490 | |
ALB = albumin, Ca = calcium, CI = confidence intervals, Cr = creatinine, D-D = D-dimer, FAR = fibrinogen to albumin ratio, Fib = fibrinogen.
3.4. ROC curve of FAR in blood serum predicted sPE
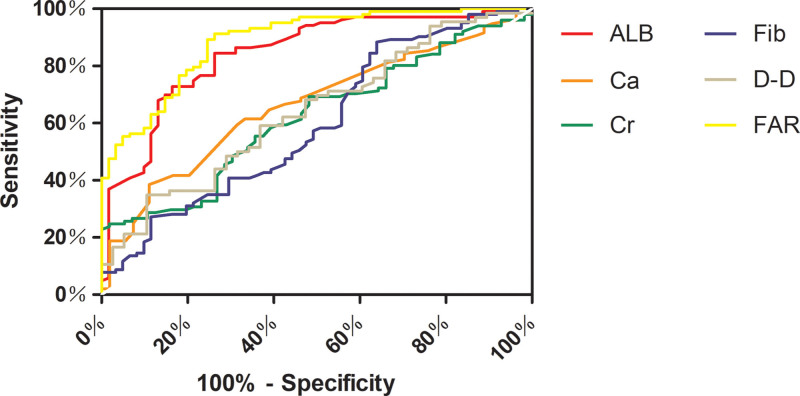
ROC curve was used to further clarify the effect of these indicators (FAR, ALB, Ca, Cr, D-D, and Fib) with statistical significance in distinguishing between mPE and sPE. (Fig. 1). Table 4 proved the AUC of each marker for the prediction of sPE. Specifically, ROC analysis revealed that the AUC for FAR was 0.888 (95% CI: 0.838–0.938; P < .001; sensitivity, 91.26%; specificity, 73.77%) and the AUC for ALB was 0.844 (95% CI: 0.781–0.907; P < .001, sensitivity, 84.47%; specificity, 73.77%). Among these indicators, FAR presented the highest predictive value for sPE. A combination of indicators may improve the accuracy of evaluating the severity of PE.
Figure 1.
The ROC curve analysis of different variables predicting severe preeclampsia. ALB = albumin, Ca = calcium, Cr = creatinine, Fib = fibrinogen, D-D = D-dimer, FAR = fibrinogen to albumin ratio, ROC = receiver operating characteristic.
Table 4.
Predicted values of different variables.
| Variables | Cutoff value | Sensitivity | Specificity | AUC | Sig. | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| ALB | 32.25 | 84.47 | 73.77 | 0.844 | <0.001 | 0.781 | 0.907 |
| Ca | 2.115 | 61.46 | 66.67 | 0.663 | <0.001 | 0.575 | 0.751 |
| Cr | 70.45 | 24.75 | 98.21 | 0.621 | 0.012 | 0.532 | 0.709 |
| Fib | 3.685 | 88.35 | 36.07 | 0.598 | 0.035 | 0.507 | 0.690 |
| D-D | 2.162 | 34.85 | 89.47 | 0.641 | 0.017 | 0.532 | 0.749 |
| FAR | 0.123 | 91.26 | 73.77 | 0.888 | <0.001 | 0.838 | 0.938 |
ALB = albumin, AUC = area under the curve, Ca = calcium, CI = confidence intervals, Cr = creatinine, D-D = D-dimer, FAR = fibrinogen to albumin ratio, Fib = fibrinogen.
4. Discussion
PE is a systemic vascular disorder with signs of metabolic disease or a combination of different types of organ dysfunction. Many biomarkers have been studied for PE diagnosis and prediction, such as placenta-derived biomarkers (sENG, soluble fms-related receptor tyrosine kinase 1 and placental growth factor [8,9]), serum metabolomics (proline betaine and proline [21]), and inflammatory factors (interleukin 6,[11] neutrophil-to-lymphocyte ratio [12]). However, owing to the complexity and testing limitations of the above-mentioned biomarkers, clinical manifestations, hypertension, and proteinuria are still required to diagnose PE and evaluate its severity. Meanwhile, clinical performance is very complex, while inflammatory indicators and proteinuria may be easily affected by infection, kidney and blood disorders. Therefore, there is an urgent need to develop practical laboratory markers that can boost the early detection of PE and prognosticate its severity.
In addition, the routine screening indicators of the coagulation system for PE are Fib and D-D. However, studies on Fib have been controversial. One study identified the level of Fib was significantly decreased in sPE, and Fib ≤ 2.87g/L was a potential cutoff value for screening PE.[22] A previous study indicated that the Fib level was significantly higher in patients with PE, and patients with sPE had significantly higher levels of Fib than pregnant women with mPE.[23] In our research, we found the level of Fib increased with the severity of PE. We believed that the reason for the differences in the results of Fib is that the level of Fib is a dynamic process. Consequently, we investigated the FAR indicator.
FAR is a ratio of fibrinogen and albumin, which is generated at no extra cost when biochemistry and coagulation function tests are performed in routine antenatal examinations. Multiple studies have suggested that elevated levels of FAR are associated with many diseases, such as abortus imminent,[16] systemic lupus erythematosus,[17] acute coronary syndrome,[24] rheumatoid arthritis[25] and ankylosing spondylitis,[26] which are associated with an inflammatory state. Hence, it has been concluded that FAR may be a better indicator for many systemic inflammation diseases. In the present study, we discovered that FAR was elevated in the sPE group compared with that in the control or mPE group. In contrast, unlike FAR, we did not detect differences in leukocyte, neutrophil, or lymphocyte counts among the groups. These results might be related to the small study population, early pregnancy age, and mild systemic inflammation in the participants. However, FAR appeared to be more sensitive than the other indicators.
We also found that the value of FAR was significantly higher than those of Fib and ALB when predicting the severity of PE. The reason for this may be that there was a positive correlation between Fib and sPE but a negative correlation between ALB and sPE. The indicator of FAR included positive and negative factors; as a result, it enlarged the variation of different stages of pregnancy. Thus, the predicted value of FAR was more valuable than that of either Fib or ALB alone. Additionally, ALB was also assessed and reported as a marker of poor disease control along with traditional markers in diabetes mellitus,[27] hypertension,[28,29] metabolic syndrome,[30,31] and thyroiditis.[32,33] Hence, a combination of multiple indicators can considerably increase the diagnostic and predictive efficiency of PE compared with a single indicator. The markers we discovered, particularly FAR, have considerable potential in the prediction of severity for preeclampsia with no additional cost. Meanwhile, these would both improve the efficiency of diagnosis and reduce the financial burden of patients on therapies.
The limitation of the present study was that it was a single-center retrospective study, and evidence of its effectiveness in clinical practice remains to be confirmed in further clinical studies. Therefore, a multi-center randomized prospective study with a large sample size is necessary to explore the roles of these markers in diagnosing PE and assessing the severity and onset of PE.
5. Conclusion
We believe that alterations in FAR, Fib, D-D, ALB, Ca, and Cr might play critical roles in the pathogenesis of PE. Our findings indicate that these indicators may have clinical significance in evaluating PE severity. Furthermore, FAR had a higher predictive value for predicting the severity of PE than the other indicators. More importantly, the routine markers tested in this study were readily derived in daily clinical practice. Further prospective large-scale trials will help elucidate the roles of these biomarkers in predicting the risk and severity of PE.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020QH278).
Author contributions
Conceptualization: Hanxiao Ren, Xiaoqing Zhao.
Data curation: Hanxiao Ren, Wei Liu, Aijun Niu, Xiaoqing Zhao.
Funding acquisition: Hanxiao Ren.
Methodology: Hanxiao Ren, Aijun Niu, Xiaoqing Zhao.
Project administration: Hanxiao Ren, Xiaoqing Zhao.
Writing – original draft: Hanxiao Ren, Wei Liu, Aijun Niu, Xiaoqing Zhao.
Writing – review & editing: Hanxiao Ren, Wei Liu, Aijun Niu, Xiaoqing Zhao.
Abbreviations:
- ALB
- albumin
- APTT
- activated partial thrombin time
- AUC
- area under the curve
- BMI
- body mass index
- Ca
- calcium
- Cr
- creatinine
- D-D
- D-dimer
- FAR
- fibrinogen to albumin ratio
- Fib
- fibrinogen
- mPE
- mild preeclampsia
- PE
- preeclampsia
- PLT
- platelet
- PT
- prothrombin time
- ROC
- receiver operating characteristic
- sPE
- severe preeclampsia
- WBC
- white blood cell
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Ren H, Liu W, Niu A, Zhao X. Fibrinogen to albumin ratio, a novel serum indicator for evaluating the severity of preeclampsia: A single-center retrospective study. Medicine 2023;102:13(e33419).
Contributor Information
Hanxiao Ren, Email: hanxiao_ren@163.com.
Wei Liu, Email: lw403427813@163.com.
Aijun Niu, Email: 1015570577@qq.com.
References
- [1].Agrawal A, Wenger NK. Hypertension during pregnancy. Curr Hypertens Rep. 2020;22:64. [DOI] [PubMed] [Google Scholar]
- [2].Yang Y, Le Ray I, Zhu J, et al. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw Open. 2021;4:e218401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kametas NA, Nzelu D, Nicolaides KH. Chronic hypertension and superimposed preeclampsia: screening and diagnosis. Am J Obstet Gynecol. 2022;226(2s):S1182–95. [DOI] [PubMed] [Google Scholar]
- [4].Mercnik MH, Schliefsteiner C, Fluhr H, et al. Placental macrophages present distinct polarization pattern and effector functions depending on clinical onset of preeclampsia. Front Immunol. 2022;13:1095879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miller D, Motomura K, Galaz J, et al. Cellular immune responses in the pathophysiology of preeclampsia. J Leukoc Biol. 2022;111:237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thakur S, Sharma V, Kaur D, et al. Angiotensin-converting enzyme (ACE) insertion/deletion (I/D) polymorphism as a conjoint regulator of coagulation, fibrinolytic, and RAAS pathway in infertility and associated pregnancy complications. J Renin Angiotensin Aldosterone Syst. 2022;2022:1695769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsubara K, Matsubara Y, Uchikura Y, et al. Pathophysiology of preeclampsia: the role of exosomes. Int J Mol Sci. 2021;22:2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42–50. [DOI] [PubMed] [Google Scholar]
- [9].Kikas T, Inno R, Ratnik K, et al. C-allele of rs4769613 near FLT1 represents a high-confidence placental risk factor for preeclampsia. Hypertension. 2020;76:884–91. [DOI] [PubMed] [Google Scholar]
- [10].Guo F, Zhang B, Yang H, et al. Systemic transcriptome comparison between early-and late-onset pre-eclampsia shows distinct pathology and novel biomarkers. Cell Prolif. 2021;54:e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barron A, Manna S, McElwain CJ, et al. Maternal pre-eclampsia serum increases neurite growth and mitochondrial function through a potential IL-6-dependent mechanism in differentiated SH-SY5Y cells. Front Physiol. 2022;13:1043481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yakiştiran B, Tanaçan A, Altinboğa O, et al. Role of derived neutrophil-to-lymphocyte ratio, uric acid-to-creatinine ratio and delta neutrophil index for predicting neonatal outcomes in pregnancies with preeclampsia. J Obstet Gynaecol. 2022;42:1835–40. [DOI] [PubMed] [Google Scholar]
- [13].Bilgin S, Kurtkulagi O, Atak Tel BM, et al. Does C-reactive protein to serum albumin ratio correlate with diabEtic nephropathy in patients with type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021;15:1071–4. [DOI] [PubMed] [Google Scholar]
- [14].Demirkol ME, Aktas G, Bilgin S, et al. C-reactive protein to lymphocyte count ratio is a promising novel marker in hepatitis C infection: the clear hep-c study. Rev Assoc Med Bras (1992). 2022;68:838–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang X, Xu Q, Yang S, et al. Toll-like receptors and thrombopoiesis. Int J Mol Sci. 2023;24:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Usta CS, Atik TK, Ozcaglayan R, et al. Does the fibrinogen/albumin ratio predict the prognosis of pregnancies with abortus imminens? Saudi Med J. 2021;42:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dai LL, Chen C, Wu J, et al. The predictive value of fibrinogen-to-albumin ratio in the active, severe active, and poor prognosis of systemic lupus erythematosus: a single-center retrospective study. J Clin Lab Anal. 2022;36:e24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuyumcu MS, Aydin O. Fibrinogen-to-albumin ratio may be a predictor for ascending aortic aneurysm. Rev Assoc Med Bras (1992). 2021;67:868–72. [DOI] [PubMed] [Google Scholar]
- [19].ACOG practice bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1. [DOI] [PubMed] [Google Scholar]
- [20].Vázquez-Arredondo JG, Vázquez-Rodríguez JG. Plasma colloid osmotic pressure in preeclampsia. Review of the Mexican literature 1997-2018. Cir Cir. 2021;89:547–52. [DOI] [PubMed] [Google Scholar]
- [21].Chen T, He P, Tan Y, et al. Biomarker identification and pathway analysis of preeclampsia based on serum metabolomics. Biochem Biophys Res Commun. 2017;485:119–25. [DOI] [PubMed] [Google Scholar]
- [22].Chen Y, Lin L. Potential value of coagulation parameters for suggesting preeclampsia during the third trimester of pregnancy. Am J Med Sci. 2017;354:39–43. [DOI] [PubMed] [Google Scholar]
- [23].Duan Z, Li C, Leung WT, et al. Alterations of several serum parameters are associated with preeclampsia and may be potential markers for the assessment of PE severity. Dis Markers. 2020;2020:7815214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [24].Li M, Tang C, Luo E, et al. Relation of fibrinogen-to-albumin ratio to severity of coronary artery disease and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Biomed Res Int. 2020;2020:1860268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Afifi N, B MM, Abdel Ghani AM, et al. Value of albumin-fibrinogen ratio and CRP-albumin ratio as predictor marker of disease activity in Egyptian RA patients, correlated with musculoskeletal sonography. Open Access Rheumatol. 2020;12:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu M, Huang Y, Huang Z, et al. The role of fibrinogen to albumin ratio in ankylosing spondylitis: correlation with disease activity. Clin Chim Acta. 2020;505:136–40. [DOI] [PubMed] [Google Scholar]
- [27].Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23:1098–102. [DOI] [PubMed] [Google Scholar]
- [28].Manolis AA, Manolis TA, Melita H, et al. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med. 2022;102:24–39. [DOI] [PubMed] [Google Scholar]
- [29].Aktas G, Khalid A, Kurtkulagi O, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. 2022;134:297–302. [DOI] [PubMed] [Google Scholar]
- [30].Kabasawa K, Hosojima M, Ito Y, et al. Association of metabolic syndrome traits with urinary biomarkers in Japanese adults. Diabetol Metab Syndr. 2022;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kocak MZ, Aktas G, Erkus E, et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras (1992). 2019;65:9–15. [DOI] [PubMed] [Google Scholar]
- [32].Zhang Y, Xie R, Ou J. A U-shaped association between serum albumin with total triiodothyronine in adults. J Clin Lab Anal. 2022;36:e24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto’s thyroiditis is associated with elevated serum uric acid to high density lipoprotein-cholesterol ratio. Rom J Intern Med. 2021;59:403–8. [DOI] [PubMed] [Google Scholar]