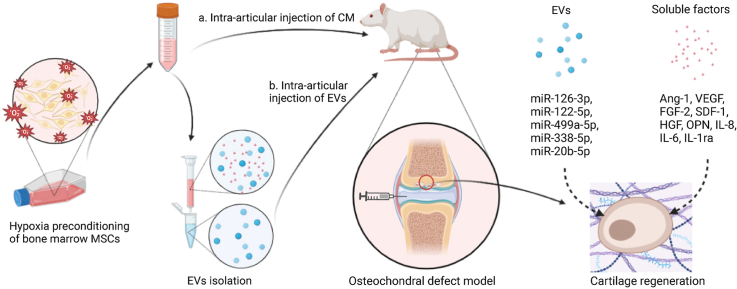
Abstract
Secretome derived from mesenchymal stem cells (MSCs) have profound effects on tissue regeneration, which could become the basis of future MSCs therapies. Hypoxia, as the physiologic environment of MSCs, has great potential to enhance MSCs paracrine therapeutic effect. In our study, the paracrine effects of secretome derived from MSCs preconditioned in normoxia and hypoxia was compared through both in vitro functional assays and an in vivo rat osteochondral defect model. Specifically, the paracrine effect of total EVs were compared to that of soluble factors to characterize the predominant active components in the hypoxic secretome. We demonstrated that hypoxia conditioned medium, as well as the corresponding EVs, at a relatively low dosage, were efficient in promoting the repair of critical-sized osteochondral defects and mitigated the joint inflammation in a rat osteochondral defect model, relative to their normoxia counterpart. In vitro functional test shows enhancement through chondrocyte proliferation, migration, and matrix deposition, while inhibit IL-1β-induced chondrocytes senescence, inflammation, matrix degradation, and pro-inflammatory macrophage activity. Multiple functional proteins, as well as a change in EVs’ size profile, with enrichment of specific EV-miRNAs were detected with hypoxia preconditioning, implicating complex molecular pathways involved in hypoxia pre-conditioned MSCs secretome generated cartilage regeneration.
Keywords: Hypoxia preconditioned MSCs, Secretome, Extracellular vesicles, Cartilage regeneration, Joint inflammation
Graphical abstract
Highlights
-
•
Hypoxia preconditioning enhanced MSCs secretome for a better therapeutic effect on articular cartilage repair.
-
•
Most beneficial effects of hypoxia preconditioned MSCs secretome were associated with EVs instead of soluble factors.
-
•
Hypoxia preconditioning to MSCs altered the size profile of EVs subpopulation as well as miRNAs contents.
1. Introduction
Articular cartilage is a smooth connective tissue covering the surface of long bones in synovial joints. Due to the avascular structure of articular cartilage, injured cartilage has limited self-repair capacity. After an injury to synovial joints, robust and active cellular and molecular responses occur in the joint, including cell necrosis, apoptosis, cartilage matrix degradation, as well as synovial inflammation [1]. If these acute responses are not mitigated or left unchecked, the joint injury can lead to post-traumatic arthritis (PTA), which is responsible for approximately 12% of the overall prevalence of symptomatic OA in the United States [2]. In the clinic, Microfracture, Mosaicplasty, and Autologous Chondrocyte Implantation (ACI) are currently the most common surgical treatments for well-defined cartilage defects [3]. However, issues such as formation of fibrocartilage, donor site morbidity, and poor long term outcomes are serious challenges for these traditional treatments [[4], [5], [6]]. Therefore, therapeutic treatments with improved outcomes, that are less invasive, would be of important clinical value for cartilage repair.
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be harvested from various tissues [7]. The capability of self-regeneration, differentiation and immunomodulation makes MSCs an attractive cell source in regenerative medicine. Despite their therapeutic effect, studies have however reported limited survival of MSCs [8] and few differentiated cells [9] after transplantation, suggesting that the therapeutic effect of MSCs are predominantly through their paracrine function. MSC secretome refers to a plethora of biologically active factors including cytokines, chemokines, growth factors, and bioactive factors packaged in the extracellular vesicles (EVs) secreted from MSCs into surrounding extracellular space [10,11]. As the composition of MSC secretome is extremely sensitive to the surrounding microenvironment [12,13], alteration to culture conditions could be strategically employed to engineer MSC secretome.
To date, most of the in vitro expansion condition of MSCs is at normoxia (20% oxygen), which is dramatically higher than the oxygen concentration in MSC's tissue niches and was reported to induce early cell senescence and genetic instability in MSCs [14]. Physiologically, the oxygen tension of bone marrow, adipose tissue and umbilical cord is at 1–9% [15,16], 5–9% [17], and 1–6% [18]), respectively. Subjecting MSC to hypoxia condition of 1–5% was found to increase MSCs stemness and differential property [[19], [20], [21], [22]] and has thus been a promising cell manufacturing process in tissue regeneration. Moreover, hypoxia preconditioning has been widely reported to enhance the paracrine activity of stem cells [23,24]. Conditioned medium (CM) from hypoxia primed MSCs were reported to have beneficial effects on various tissue injury and disease models [[25], [26], [27], [28]]. The therapeutic effect of EVs from hypoxia preconditioned MSCs has also been validated in many pre-concept studies, including myocardial infarction [29,30], bone fracture [31], spinal cord injury [32], skin wound [33], and osteoarthritis [34,35]. These findings indicated that hypoxia preconditioning could be an engineering strategy to improve MSC secretome for a better therapeutic effect.
In this study we investigated the paracrine function of secretome from hypoxia preconditioned MSCs in articular cartilage injury, in comparison to normoxia secretome. In particular, total EVs were separated from the soluble factors to characterize the predominant active components in the hypoxia secretome. Furthermore, we screened differentially expressed soluble factors as well as EV-miRNAs in MSC secretome to explore the potential molecular mechanisms. In vitro functional studies were performed on MSCs, chondrocytes, and macrophages to assess the paracrine effect, followed by in vivo validation on rat osteochondral injury model.
2. Materials and methods
2.1. Cell culture
2.1.1. Human bone marrow MSCs
Primary human bone marrow MSCs were purchased from Lonza Inc. (Walkersville, MD) and RoosterBio Inc. (Frederick, MD), supplied at passage 2. Human BM-MSCs were cultured in 1 g/L d-Glucose Dulbecco's Modified Eagle's Medium (LG DMEM) supplemented with 10% fetal bovine serum (FBS) (HyClone), 1% GlutaMAX (Thermo Fisher Scientific), and 1% penicillin-streptomycin (P/S, Life Technologies, USA). Cells at passage 5 were used for all experiments.
2.1.2. Rat bone marrow MSCs
Sprague-Dawley (SD) rat bone marrow mesenchymal stem cells were purchased from Cyagen Inc. (USA), supplied at passage 2 (Cat. # RAWMX-01001). Rat BM-MSCs were cultured in complete MSC growth media (Cat. # GUXMX-90011, Cyagen, USA). Cells at passage 4 were used for all experiments.
2.1.3. Swine articular cartilage chondrocytes
Chondrocytes were isolated from the articular cartilage of the pigs as described in previous study [36]. Chondrocytes were cultured in LG DMEM supplemented with 10% FBS and 1% GlutaMAX, and 1% P/S. Cells at passage 2 were used for all experiments.
2.1.4. Human monocytic THP-1 cells
The human monocytic THP-1 cells (TIB-202, ATCC) were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS. Cells at passage 11 were used for all experiments.
2.2. Hypoxia preconditioning and CM collection
When cultured MSCs reached 70%–80% confluency, they were rinsed with PBS three times and replaced with blank LG-DMEM and cultured under different oxygen tension (20%, 5%, and 1%) for 24 h. CM was collected as normoxia (20% oxygen tension) conditioned medium (NCM), hypoxia-5% oxygen tension conditioned medium (HCM-5%), and hypoxia-1% oxygen tension conditioned medium (HCM-1%). The collected CM was centrifuged at 500 g for 5 min, followed with 4000 g for 10 min to remove cell debris. Cell numbers were counted after CM collection and were used to normalize the CM before use. The CM was concentrated 10 times using protein concentrators with a molecular weight cut-off of 3 kDa (Thermo Fisher Scientific, USA) and stored in −20 °C. Concentrated human MSC-derived CM was diluted to niche (1x) concentration and used in all the in vitro functional assays and secretome and EV characterization. Rat MSC-derived CM was used for in vivo studies.
2.3. Functional assays
2.3.1. Cell proliferation assay
Human MSCs or swine chondrocytes were seeded in 96 wells plates at 2000 cells/well or 5000 cells/well, respectively. After cell attachment, the expansion medium was replaced with CM diluted in low serum culture medium (LG DMEM supplemented with 0.5% FBS). Low serum culture medium without CM served as negative control (NC). Cell amounts were quantified by Quant-iT PicoGreen dsDNA assay kit (Life Technologies, USA) at different timepoint.
2.3.2. Cell migration assay
The effect of CM on migration ability of MSCs and chondrocytes was assessed using a Transwell culture (Millipore, Germany). MSCs (3 x 104) or chondrocytes (5 x 104) were suspended in 300 μl of low serum culture medium and placed into the upper chamber. The CM was diluted in low serum culture medium and added in 700 μl to the lower chamber of each Transwell culture. Low serum culture medium without CM served as negative control (NC). After 16 h incubation, the migrated cells, were fixed in 4% (v/v) paraformaldehyde and stained with haematoxylin and eosin (Sigma Aldrich). The cells in five randomly selected fields at 100X magnification were counted to indicate migration cells.
2.3.3. Chondrocyte senescence assay
Chondrocytes were seeded at 2 x 104 cells/well in a 24-well plate in DMEM containing 10% FBS. 10 ng/ml IL-1β (RnD systems) was added to the expansion media after cell attachment to induce cell senescence for 24 h. CM diluted in low serum culture medium was added to the cells after the induction, in the presence of 10 ng/ml IL-1β. Chondrocytes induced with IL-1β but without any CM treatment served as positive control (PC), whereas negative control (NC) consisted of chondrocytes without the addition of IL-1β and CM treatments. After 48 h of CM treatment, chondrocytes were stained with Senescent Cells Staining Kit (Merck). The cells in five randomly selected fields at 100X magnification were counted, and the ratio of senescent cells to total cells was calculated.
2.3.4. Chondrocyte re-differentiation
1.5 x 105 chondrocytes suspended in chondrogenic media were centrifuged at 200 g for 5 min to form pellets. After overnight culture, the media was replaced with 500 μL of chondrogenic media in the presence of 2 ng/mL TGF-β3 (RnD Systems, Canada), and supplemented with 50 μL of 10x concentrated CM or blank LG DMEM (NC). The chondrogenic medium was changed every 2 days. At 7 days, chondrocyte pellets were harvested for RT-PCR, histological staining, and quantification of ECM.
Chondrocyte redifferentiation was also examined under IL-1β induced inflammation. Chondrocyte pellets was cultured in 500 μL of chondrogenic media in the presence of 5 ng/mL TGF-β3 and 10 ng/ml IL-1β, supplemented with 50 μL of 10x concentrated CM. Chondrocyte pellets cultured with 5 ng/mL TGF-β3 and 10 ng/ml IL-1β but without any CM treatment served as positive control (PC), whereas negative control (NC) consisted of 5 ng/mL TGF-β3 treated chondrocytes without the addition of IL-1β and CM treatments.
2.3.5. Macrophage polarization assay
2 x 105/mL THP-1 cells were differentiated into M0 macrophages by incubation with 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma) for 24 h. This was followed by 24 h rest in complete RPMI 1640 medium (Gibco). M0 macrophages were polarized into M1 macrophages by incubation with 10 ng/ml LPS (Sigma) and 20 ng/ml IFN-γ (RnD, USA). CM was included during M1 macrophage differentiation and incubated for 48 h, then cells were harvested for RT-qPCR. The protein amount of IL-1β, TNF-α, and IL-6 released from the macrophage was measured using ELISA kits accordingly (BMS224-2, BMS213-2, and BMS223-4, Life Technologies, USA).
2.3.6. MSC osteogenic differentiation assay
MSCs were seeded at 4 x 104 cells/well on a 24-well plate. After overnight culture, the media was replaced with 500 μL of osteogenic media and supplemented with 50 μL of 10x concentrated CM, EV, EV depleted (CMdEV) component, or blank LG DMEM (NC). The osteogenic medium was changed every 2 days. At 7 days, cells were harvested for mRNA expression analysis of osteogenic gene ALP by RT-qPCR. Osteogenic medium was composed of low glucose DMEM supplemented with 0.5% FBS, 50 μg/ml ascorbic acid, 1 mM sodium pyruvate, 10 μM dexamethasone and 10 mM β-glycerophosphate.
2.4. Quantitative real-time polymerase chain reaction (RT-qPCR)
The total RNAs of cells were extracted with RNeasy® Mini Kit (Qiagen, Germany). The reverse transcription was conducted with 100 ng of total RNA using iScript™ cDNA synthesis kit (Bio-Rad, USA). Real-time PCR was performed using the Power SYBR® green PCR master mix on ABI Step One Plus Real-time PCR System (Applied Biosystems, Life Technologies, USA). The expression levels of targeted genes were normalized to the reference gene β-actin and were then calculated using the 2-ΔΔCt formula with reference to the day 0 cells. Primer sequences of targeted genes were listed in Supplemental Table 1.
2.5. Luminex assay
The concentrated CM was normalized to cell numbers before analyzed by Magnetic Luminex Assay (R & D Systems, USA). The fluorescent intensities of selected proteins (IL-6, IL-8, PDGF-AA, PDGF-BB, MMP-1, MCP-1, VEGF, OPN, IL-1ra, LIF, FGF2, Angiopoietin-1, GDF-15, HGF, BMP-2, BMP-4, TIMP-1, CXCL12/SDF-1, MMP-13, TSP2, TGF-β1, TGF-β3) were determined with Luminex MAGPIX fluorescent imager, and protein concentrations were automatically calculated by Luminex xPONENT software.
2.6. EVs and soluble factors isolation
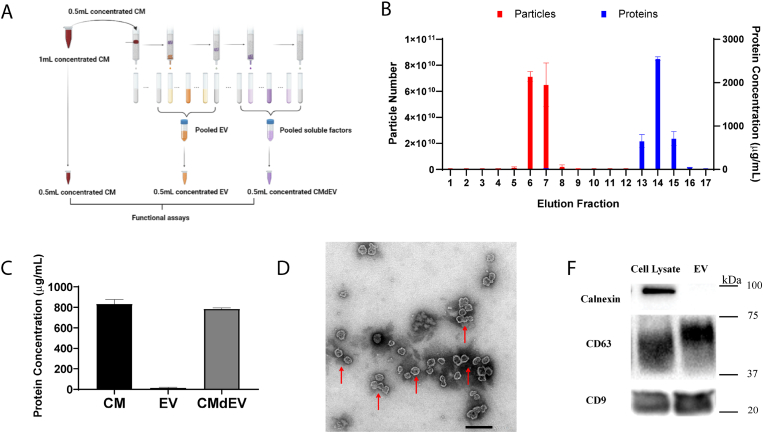
EVs and soluble factors were segregated using size-exclusion chromatography on a q-EV column (Izon, New Zealand). Elution was collected at 0.5 mL per fraction (Fig. 3A). Protein concentrations of EVs and soluble factors in the fractions were determined by a Micro BCA Protein Assay Kit (Thermofisher, USA) according to the manufacturer's instructions. Nanoparticle tracking analysis (NTA) performed on a NanoSight NS500 instrument (NanoSight Ltd, UK) analyzed the particle size and numbers of EVs. The morphology of EVs was verified by transmission electron microscopy (TEM; JEOL JEM-1220). Expression of CD9 (ab263019, Abcam), CD63 (ab134045, Abcam), and Calnexin (ab133615, Abcam) in EVs and cell lysate were assayed by western blotting.
Fig. 3.
Efficient separation of EVs and soluble factors from CM. (A) Illustration of EVs and soluble factors isolation using SEC. (B) Particle number and protein concentration of individual fractions eluted from q-EV column. (C) Protein concentration of original CM and isolated CMdEV samples. (D) Transmission electron microscopy (TEM) of isolated EVs. Scale bar = 200 nm. (E) Western blotting assay of CD63 and CD9, and Calnexin in MSC cell lysate and EVs.
2.7. miRNA sequencing
RNAs were extracted from EVs using q-EV RNA extraction kit column (Izon, New Zealand). The sRNA library preparation and sequencing analysis was performed by Novogene (Singapore) with Illumina NextSeq 500 system. MiRNA expression levels were estimated by TPM (transcript per million): Normalized expression = mapped reads*1000000. P-value of 0.05 was set as the threshold for significantly differential expression by default. Prediction of the target gene of miRNA was performed by miRanda (http://www.microrna.org). Subsequently, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway for the target genes were analyzed with Tarbase v7.0.
2.8. In vivo repair of cartilage defects via intra-articular injection of CM or EV
All animal procedures were performed according to the guidelines of the Institutional Animal Care and Use Committee at National University of Singapore (IACUC protocol: R20-0258). 12-weeks old female SD rats with a weight range of 300–400 g were used. The osteochondral defect (1.5 mm diameter, 1 mm depth) was created manually on the trochlear grooves of rat distal femurs with a drill. The animals were randomly allocated to each group.
Rat bone marrow MSCs were used to generate CM and EV for in vivo study, following the same protocol as indicated in Section 2, 2.2.6. For CM study, there were three groups (n = 6 in each group): defects treated with serum-free media (SFM), defects treated with NCM (50X), and defect treated with HCM (50X). For the EV study, there were five groups (n = 6 in each group): defects treated with serum free media (SFM), defects treated with NEV (50X) or NEV2 (100X), and defects treated with HEV (50X) or HEV2 (100X). Intra-articular injections of SFM, CM or EV (100 μL/knee) were administered immediately after the surgery and subsequently with weekly injection. At 6 weeks, rats were euthanized by CO2 inhalation, and distal femora and synovial membrane were harvested for analysis.
The distal femora were fixed in 10% buffered formalin for a week before micro-CT imaging by microCT scanner (Bruker micro-CT, Kontich, Belgium). Then samples were decalcified by 30% formic acid and were cut into 5 μm sections. For cartilage regeneration analysis, sections of defect tissues were subjected to haematoxylin and eosin (Sigma-Aldrich, USA), Safranin O (Sigma-Aldrich, USA), Masson's trichrome staining (Sigma-Aldrich, USA), and type I collagen (C3867, Sigma-Aldrich, USA) and type II collagen (Clone 6B3, Chemicon, USA) immunostsining. The quality of cartilage regeneration was assessed by a modified O'Driscoll scoring system (Supplemental Table 2). For analysis of synovitis, sections of synovial membrane were subjected to haematoxylin and eosin (Sigma-Aldrich, USA) staining and CD206 (ab64693, Abcam) immunostaining, and scored according to synovitis scoring system (Supplemental Table 3). All the stained sections were scanned by Nikon Eclipse Ti2-E Motorized Inverted Research Microscope and the scoring was conducted by three independent and blinded researchers.
2.9. Statistical analysis
All analyses were conducted using GraphPad Prism 8.4.3. A p-value <0.05 was considered to indicate a significant difference. The statistical significance between two groups was evaluated by two-sample t-test. For multiple groups, the statistical significance was evaluated by one-way analysis of variance (ANOVA) followed with a post hoc Tukey test. All data are presented as the mean ± SD for at least three independent biological replicates.
3. Results
3.1. Hypoxia pre-conditioning enhanced the cartilage regenerative and anti-inflammatory paracrine functions of MSC secretome
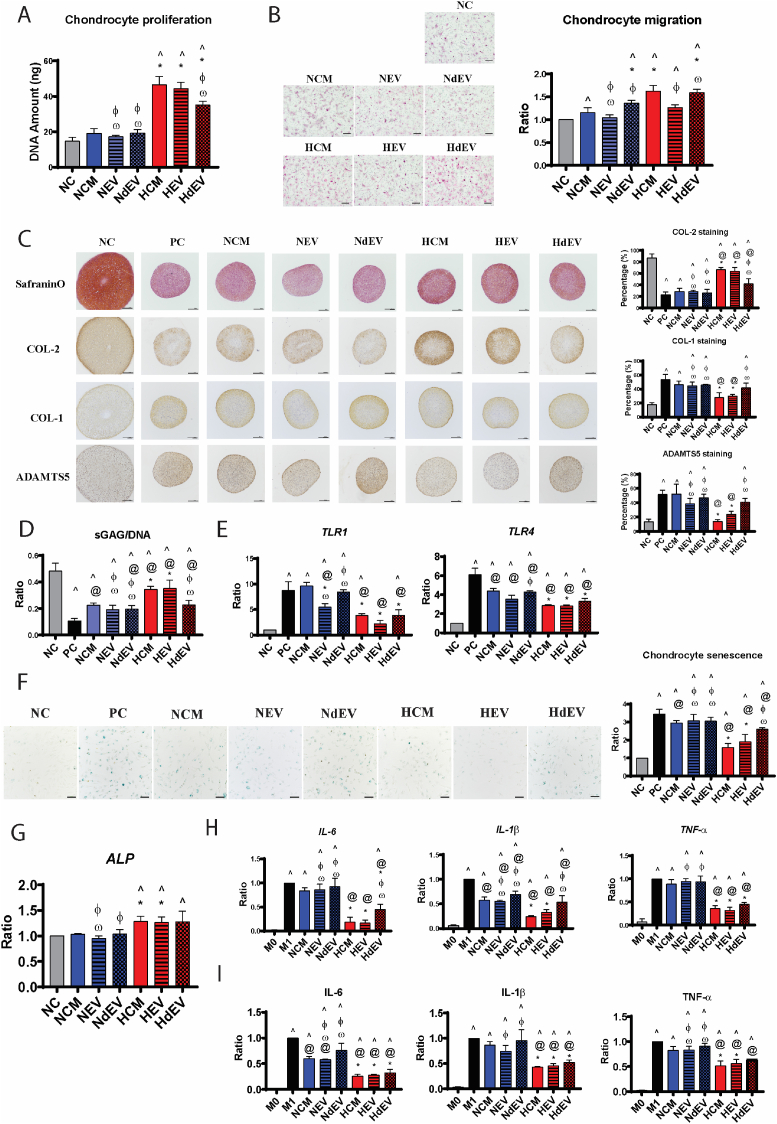
We first determine whether hypoxia preconditioning enhanced the paracrine functions of MSC secretome. The effects of HCM were compared with NCM on different cell types in the joint, including chondrocytes, MSC and macrophages, through a series of in vitro functional assays (Fig. 1).
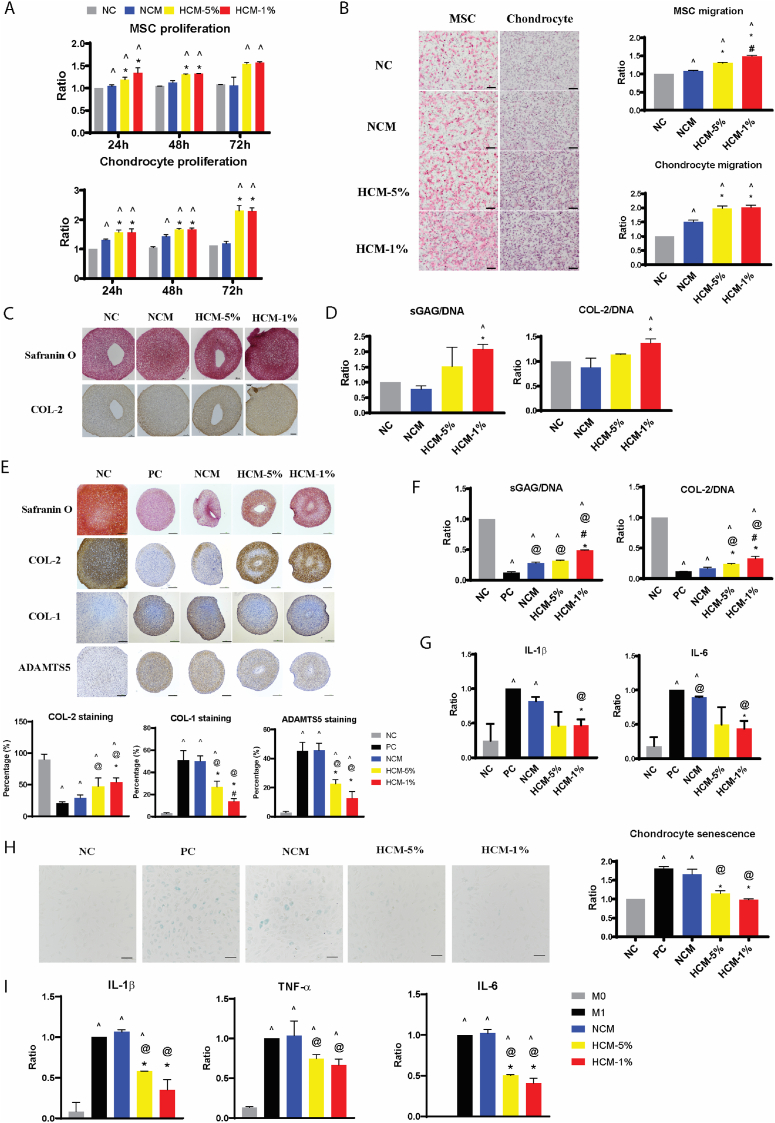
Fig. 1.
Hypoxia preconditioning enhanced the paracrine functions of human BM-MSC in vitro. (A) The proliferation of MSCs and chondrocytes were analyzed by quantitation of DNA amounts after 24, 48, and 72 h treated with NCM, HCM-5%, and HCM-1%, presented as ratio to 24 h NC group. (B) H&E staining of migrated MSCs and chondrocytes, and quantitative analysis of cell migration, presented as ratio to NC group. Scale bar = 200 μm. (C) Safranin O and COL-2 immunohistochemical staining, and (D) quantitative analysis of sGAG and COL-2 deposition, in chondrocyte pellets after 7 days in non-inflammation condition. Scale bar = 200 μm. (E) Safranin O staining and immunohistochemical staining, and quantitative analysis of COL-2, COL-1, ADAMTS5 content, of chondrocyte pellets after 7 days in IL-1β induced inflammation. Scale bar = 200 μm. (F) Quantitative analysis of sGAG and COL-2 deposition, and (G) transcription of IL-1β and IL-6 genes in chondrocyte pellets after 7 days in IL-1β induced inflammation. Data is presented as ratio to NC group. (H) SA-beta-Gal staining of senescent chondrocytes (blue colour) and quantitative analysis of senescent cells, presented as ratio to NC group. Scale bar = 100 μm. All data represent the mean ± standard deviation, n = 4. ^ denotes p < 0.05 compared to the NC; @ denotes p < 0.05 compared to the PC; * denotes p < 0.05 compared to NCM. # denotes p < 0.05 compared to HCM-5%. (I) Transcription of IL-1β, TNF-α, and IL-6 genes in macrophages, presented as ratio to M1 macrophage. Data represent the mean ± standard deviation, n = 4. ^ denotes p < 0.05 compared to the M0; @ denotes p < 0.05 compared to the M1; * denotes p < 0.05 compared to NCM. # denotes p < 0.05 compared to HCM-5%.
DNA quantification showed a time dependent effect of HCM on MSC and chondrocyte proliferation (Fig. 1A). HCM-1% and HCM-5% induced similar enhancement on MSC proliferation over a period of 3 days, while NCM has no effect. The enhanced mitogenic effect of HCM was also observed on chondrocytes, at a significantly higher levels compared to NCM, especially at 72 h.
The chemotactive activity, as evaluated by Transwell assay, showed that HCM-5% and HCM-1% induced more migration of both chondrocytes and MSCs, relative to NCM (Fig. 1B). In addition, HCM-1% showed a greater pro-migration function than HCM-5% on MSC.
The chondrogenic potential of MSC secretome was examined using chondrocyte 3D pellet culture. Low concentration of TGF-β3 (2 ng/ml) included in chondrogenic differentiation media induced ECM deposition demonstrated by Safranin O and COL-2 immunohistochemical staining (NC group, Fig. 1C). Pellets treated with NCM did not enhance ECM deposition, while treatment with HCM-1% consistently generated better chondrocyte anabolic activity, indicated by increased quantification of sGAG and type II collagen deposition (Fig. 1D).
The effect of CM on chondrocyte cartilage formation was also investigated under inflammatory conditions, with a basal media containing 5 ng/ml TGF-β3 and 10 ng/ml IL-1β. In inflammation Positive Control group (PC; + IL-1β), the deposition of proteoglycan and type II collagen were dramatically decreased, with concomitant increased expression of extracellular matrix degrading enzyme, ADAMTS5, and fibrocartilage marker, type I collagen, compared with the non-inflammatory Negative Control (NC; no addition of IL-1β), indicated by Safranin O and immunohistochemical staining (Fig. 1E). In the presence of HCM treatment, but not NCM, an obvious increase of proteoglycan and type II collagen while significant suppression of type I collagen and ADAMTS5 was observed (Fig. 1E). The superior beneficial effect of HCM on cartilage matrix deposition compared to NCM was also validated through quantification of type II collagen and sGAG, with HCM-1% having significantly better pro-anabolic effect than HCM-5% (Fig. 1F). The expression of inflammatory cytokine genes IL-1β and IL-6 were considerably reduced in inflammatory chondrocytes treated with HCM-1% compared to NCM and PC group (Fig. 1G). IL-1β treatment also markedly increased senescence of chondrocytes (PC; Fig. 1H). Treatment with HCM-5% and HCM-1% drastically decreased IL-1β-triggered senescence to levels equivalent to the negative control (NC). NCM, on the other hand, has no effect on attenuating chondrocytes senescence.
To mimic the in vivo inflammation-induced macrophage activity, human monocytic THP-1 cells were used to generate undifferentiated macrophage (M0), then differentiated into pro-inflammatory macrophage (M1). HCM and NCM were added during the M1 differentiation period in the presence of LPS and IFN-γ. Gene expression of pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 were significantly increased in M1 macrophage (Fig. 1I). Treatment with HCM-5% and HCM-1%, but not NCM, significantly suppressed transcription of these cytokines (Fig. 1I).
Taken together, these finding showed that hypoxia preconditioning was able to significantly potentiate paracrine functions of MSC secretome with enhanced migration, proliferation and chondrogenic capability, as well as providing better chondro-protective and anti-inflammatory activities. In comparison to HCM-5%, HCM-1% exhibited better effect on MSC migration and chondrocyte cartilage formation in both non-inflammatory and inflammatory conditions. Thus 1% oxygen tension was selected as the hypoxic condition in the following in vitro and in vivo experiments.
3.2. HCM promoted tissue regeneration in osteochondral defect
The therapeutic effects of HCM and NCM were evaluated in rat osteochondral defect model, and serum free medium (SFM) was set as the control group.
Macroscopic images at 6 weeks showed that HCM treatment improved neo tissue formation and integration to adjacent cartilage, while SFM and NCM treatment only partially filled the defects with distinct boundary remaining between neo and host tissue (Fig. 2A). Osteochondral bone regeneration was evaluated by micro-CT scan of the defects (Fig. 2B). Micro-CT quantitative analysis showed that HCM-treated defects had the highest bone volume, which was significantly higher than NCM-treated defects and SFM-treated defects (Fig. 2C). HCM-treated defects also showed increased trabecular thickness (Tb.th) while decreased trabecular separation (Tb.sp) compared to SFM group, although no significant difference compared to NCM-treated defects (Fig. 2C). No difference in trabecular number (Tb.N) of the regenerated subchondral bone was observed among all the groups. In all measured parameters, NCM treatment did not registered improvement relative to SFM control.
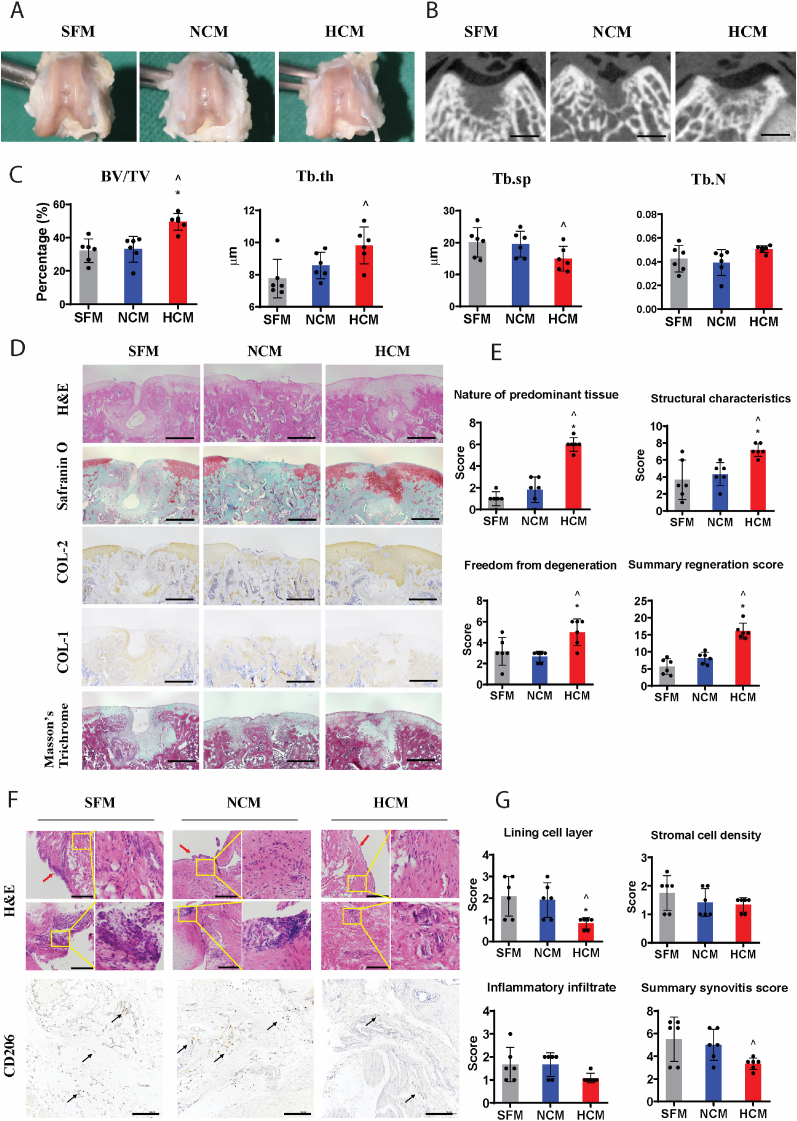
Fig. 2.
Therapeutic effect of HCM in rat osteochondral defect model. (A) Macroscopic appearance of osteochondral defects at 6 weeks post-surgery. (B) The cross-section of micro-CT scan at the middle of defects Scale bar = 1 mm. (C) Quantitative analysis of bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) of the regenerated subchondral bone. (D) Histological and immunohistochemical staining of the repaired cartilage. Scale bar = 500 μm. (E) The modified O'Driscoll score for cartilage regeneration with three parameters, including nature of predominant tissue, structural charactertisitics, and freedom from degeneration. (F) Histological and immunohistochemical staining of synovial membrane. Red arrows indicate lining cell layers. Yellow boxes indicate synovial stromal with and without infiltrated leukocytes (follicle-like aggregates). Black arrows indicate CD206 positive cells. Scale bar = 200 μm. (G) The synovitis score of synovial membrane with three parameters, including lining cell layer, stromal cell density, and inflammatory infiltrate. (I) Data are presented as mean ± standard deviation, n = 6 per group. ^ denotes p < 0.05 compared to the SFM group; * denotes p < 0.05 compared to NCM group.
Histologically, HCM-treated defects showed predominantly hyaline cartilage formation characterised by the presence of spherical chondrocytes (H&E staining) and high expression of proteoglycan (Safranin O staining) and type II collagen, and low expression of type I collagen within the blue zone (total collagen fibres area) of Masson's trichrome staining (Fig. 2D). In contrast, NCM and SFM-treated defects showed mostly fibroblast cells and predominantly stained with type I collagen, but not for s-GAG and type II collagen (Fig. 2D). Statistically, defects evaluated by the modified O'Driscoll scoring system showed that HCM-treated defects had a significantly higher score in the nature of predominant tissue, structural characteristics, freedom from degeneration, and the total cartilage regeneration than NCM-treated defects and SFM-treated defects, while NCM treatment showed no difference with SFM (Fig. 2E).
Synovitis is indicated by enlargement of lining cell layer, increased stromal cell density and inflammatory infiltrate in synovial membrane, which can be scored by the synovitis scoring system (Supplemental Table 3) based on H&E staining and CD206 (a marker of macrophages) staining (Fig. 2F). HCM treatment significantly reduced lining cell layer thickness compared to SFM and NCM (Fig. 2G). Although there is not a statistically difference in the score of stromal cell density and inflammatory infiltrate among groups, HCM treatment resulted in significantly lower total synovitis score than SFM group, while NCM treatment showed no difference with SFM (Fig. 2G).
From these results, we concluded that HCM could promote the regeneration of osteochondral defects and mitigate synovial inflammation at 6 weeks post-surgery, whereas NCM is unable to generate the same therapeutic effect. The in vivo outcome is consistent with the in vitro functional studies.
3.3. Efficient separation of EVs and soluble factors from CM
To characterize the paracrine contribution of soluble factors and EVs in the MSC secretome, EVs were separated from the soluble factors using size exclusion chromatography (SEC) (Fig. 3A). NTA and Micro BCA analysis indicate that EVs were well separated from the soluble protein component (Fig. 3B). EV was eluted from fraction 4 to fraction 9, as evidenced by high concentration of particles that peaked at fraction 6–7, and insignificant protein concentration. The protein concentration was dramatically increased from fraction 12 onwards and peaked in fraction 14, while EV particles were undetectable in fraction 13–17 (Fig. 3B). EV-elution fractions (Fraction 1–9) were pooled as EV component, and the soluble protein-rich but EV-poor fractions (Fraction 13–17) were pooled as EV depleted (CMdEV) component. 0.22 μm filtering was not used during the proceeding to ensure that total EVs was isolated. The total protein amounts of CMdEV sample were similar compared to the original CM (Fig. 3C), indicative of good recovery of soluble factors through SEC. Visualization of the pooled EV component by TEM with negative staining showed that particles possess a circular morphology with intact membrane (Fig. 3D). Western blot analysis showed that the EV sample was positive with surface markers CD63 and CD9 but negative with endoplasmic reticulum (ER) integral protein Calnexin (Fig. 3E). These results validated the efficient separation of EVs from the soluble factors.
3.4. EVs play predominant roles in the paracrine function of HCM
We then determined the paracrine contribution of soluble factors and EVs in MSC secretome. Hypoxia preconditioned EV (HEV) and normoxic EV (NEV), soluble factors from hypoxia preconditioned MSC (HdEV) and normoxic MSC (NdEV), as well as original HCM and NCM, were prepared according to Fig. 3A and compared through a series of functional assays.
The proliferation assay of chondrocytes after 48 h treatment was used to compare the mitogenic function of EVs and soluble factors (Fig. 4A). NCM, NEV, and NdEV treatments all showed no beneficial effect on chondrocytes proliferation when compared to NC group. HCM and HEV treatments induced similar enhancement effect, while the mitogenic effect of HdEV was less prominent when compared to HCM and HEV. This result suggested that HEV played a more predominant role in promoting chondrocyte proliferation than soluble factors in HCM.
Fig. 4.
Comparison of EVs and soluble factors' paracrine functions in vitro. (A) The proliferation of chondrocytes was analyzed by quantitation of DNA amounts after 48 h treatment, presented as ratio to NC group. (B) H&E staining of migrated chondrocytes and quantitative analysis of cell migration. Scale bar = 200 μm. (C) Histological and immunohistochemical staining of chondrocyte pellets after 7 days with IL-1β induced inflammation and quantitative analysis of COL-2, COL-1, ADAMTS5 content. Scale bar = 200 μm. (D) Quantitative analysis of sGAG deposition in chondrocyte pellets after 7 days with IL-1β induced inflammation. (E) Transcription of TLR1 and TLR4 genes in chondrocyte pellets after 7 days with IL-1β induced inflammation. (F) SA-beta-Gal staining of senescent chondrocytes (blue colour) and quantitative analysis of senescence, presented as ratio to NC group. Scale bar = 100 μm. (G) Transcription of ALP genes in MSCs after 7 days osteogenic differentiation, presented as ratio to NC group. All data represent the mean ± standard deviation, n = 4. ^ denotes p < 0.05 compared to the NC; @ denotes p < 0.05 compared to the PC; * denotes p < 0.05 compared to NCM; φ denotes p < 0.05 compared to HCM; ω denotes p < 0.05 compared to HEV. (H) Transcription of IL-1β, TNF-α, and IL-6 genes in macrophages, presented as ratio to M1 macrophages. (I) Quantification of cytokine proteins (IL-1β, TNF-α, and IL-6) released from macrophages, presented as ratio to M1 macrophages. Data represent the mean ± standard deviation, n = 4. ^ denotes p < 0.05 compared to the M0; @ denotes p < 0.05 compared to the M1; * denotes p < 0.05 compared to NCM; φ denotes p < 0.05 compared to HCM; ω denotes p < 0.05 compared to HEV.
Next, the chemotactive effect of EVs and soluble factors was compared with chondrocyte migration assay (Fig. 4B). In normoxia groups, soluble factors (NdEV) showed a greater chemotactive effect than NCM and NEV, while NEV has no effect compared to NC group. In hypoxia groups, HdEV showed a similar chemotactive effect as HCM and was significantly greater than HEV. Although the pro-migrating effect of HEV showed no difference with NCM, HEV was more chemotactive than NEV. This result implicated that soluble factors, instead of EVs, contained the predominant component contributing to the chemotactive effect of hypoxic secretome.
The chondrocyte protective effect of EVs and soluble factors were compared in 7-days inflammatory chondrocyte pellets. The increased beneficial effect on cartilage matrix deposition compared to inflammatory control group (PC) were only observed in HCM, HEV, and HdEV treated chondrocyte pellets, but not in NCM, NEV, NdEV groups (Fig. 4C&D). HEV showed a similar pro-anabolic effect as HCM with a significantly better effect than HdEV, validated by Safranin O staining, type II collagen staining (Fig. 4C), and quantification of sGAG (Fig. 4D). HEV also showed a similar suppression effect as HCM on type I collagen and ADAMTS5 expression, while HdEV treatment was insufficient to reduce expression of these markers when compared to PC group (Fig. 4C).
TLR1 and TLR4 pathways are two major inflammatory signaling pathways in chondrocytes, which promote the expression of catabolic factors while suppress cellular anabolic activities [48]. The transcription of TLR1 gene was considerably reduced in inflammatory chondrocytes treated with HCM, HEV, HdEV as well as NEV when compared to PC, NCM and NdEV (Fig. 4E). HCM, HEV, HdEV also exhibited inhibition effect on TLR4 gene expression compared to NCM and NdEV (Fig. 4E). In addition, HEV showed a greater inhibition effect on TLR1 gene expression compared to NEV, but no difference on TLR4 gene expression. HEV also exhibited no difference on TLR1 and TLR4 genes expression compared to HdEV. This result implicated that only EVs in HCM was able to reverse the imbalance cartilage homeostasis induced by IL-1β. Although soluble factors in HCM showed similar inhibition effect on TLR1 and TLR4 inflammatory signaling as HEV, they were not sufficient to promote chondrocyte anabolic activities and decrease catabolic activities.
HEV showed a similar anti-senescence effect as HCM, significantly better than HdEV, while HdEV showed no difference with NCM, NEV, and NdEV. Although NCM treatment reduced chondrocyte senescence compared to PC group, both NEV and NdEV treatment alone showed no inhibition effect (Fig. 4F). This result implicated that although both EVs and soluble factors contributed to the hypoxic MSC anti-senescence paracrine signaling, hypoxia treatment potentiated the anti-senescence paracrine signaling in the EV components in HCM.
The osteogenic potential of CM, EV, or CMdEV was analyzed by MSCs osteogenesis assay (Fig. 4G). NCM, NEV, and NdEV treatments all showed no effect on MSC osteogenesis when compared to NC group, while HCM, HEV, and HCM-EV treated MSCs all showed enhanced osteogenesis compared to NC group. Only HCM and HEV treatments induced significantly higher expression of osteogenic marker ALP than NCM treatment (Fig. 4G).
The anti-inflammatory effect EVs and soluble factors was also compared in pro-inflammatory macrophage differentiation. According to the gene transcription (Fig. 4H) and protein quantification (Fig. 4I) results, treatment with HCM and HEV significantly supressed the gene and protein expression of inflammatory cytokines IL-6, IL-1β and TNF-α when compared to M1 and NCM groups. Treatment with NCM, NEV, or NdEV did not affect the protein level of IL-1β and TNF-α when compared to M1 (Fig. 4I), although the decreased transcription of IL-1β was observed (Fig. 4H). In addition, significantly more decreased TNF-α protein level was detected in HCM and HEV treated pro-inflammatory macrophages, but not in HdEV, when compared to NCM, NEV and NdEV treatments (Fig. 4I).
Taken together, most of the beneficial effects of HCM were predominantly associated with the EV component, while the soluble factors contributed a smaller but significant effect. These beneficial effects include the induction of chondrocyte proliferation and anabolic activity during inflammation while suppressing catabolic activity and senescence. The exception was the chemotaxis of chondrocytes in which soluble factors has more prominent effect.
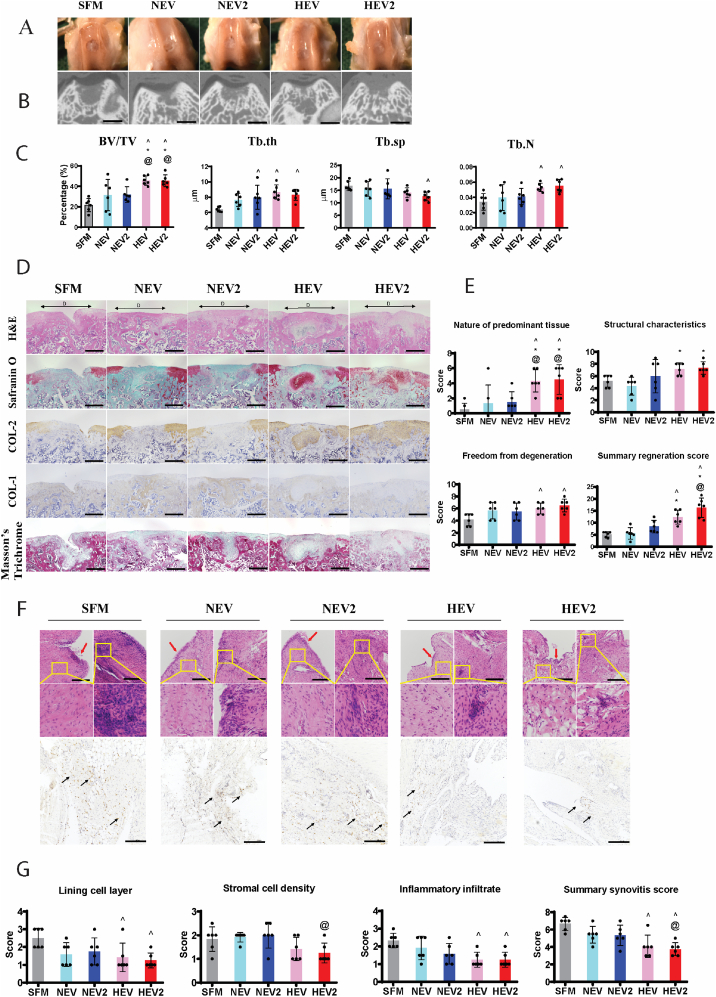
3.5. HEV promoted tissue regeneration in osteochondral defect
The therapeutic effects of EVs in rat osteochondral repair were tested at 2 dosages, EV (50X) and EV2 (100X). Delivery of serum free medium (SFM) was set as the control group.
Macroscopic images at 6 weeks showed that all the defects treated with HEVs exhibited good neo tissue filling and surface regularity (Fig. 5A). Conversely, NEVs treatment only partially filled the defects and showed distinct boundary of the defects (Fig. 5A).
Fig. 5.
Therapeutic effect of HEVs in rat osteochondral defect model. (A) Macroscopic appearance of osteochondral defects at 6 weeks post-surgery. (B) The cross-section of micro-CT scan at the middle of defects. Scale bar = 1 mm. (C) Quantitative analysis of bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) of the regenerated subchondral bone. (D) Histological and immunohistochemical staining of the repaired cartilage. D with black arrows indicate defect areas. 150X magnification. Scale bar = 500 μm. (E) The modified O'Driscoll score for cartilage regeneration with three parameters, including nature of predominant tissue, structural characteristics, and freedom from degeneration. (F) Histological and immunohistochemical staining of synovial membrane. Red arrows indicate lining cell layers. Yellow boxes indicate synovial stromal with and without leukocytic infiltration (follicle-like aggregates). Black arrows indicate CD206 positive cells. Scale bar = 200 μm. (G) The synovitis score of synovial membrane with three parameters, including lining cell layer, stromal cell density, and inflammatory infiltrate. Data are presented as mean ± standard deviation, n = 6 per group. ^ denotes p < 0.05 compared to the SFM group; * denotes p < 0.05 compared to NEV group. @ denotes p < 0.05 compared to the NEV2 group.
Micro-CT scan of the defects treated with HEV and HEV2 showed better subchondral bone regeneration compared to NEV and NEV2 (Fig. 5B). Quantification of regenerated subchondral bone volume in Fig. 5C showed that the regenerated bone volume in HEV treated defects and HEV2 treated defects was significantly higher than NEV, NEV2 and SFM-treated defects. NEV2, HEV, and HEV2 treated defects all showed increased trabecular thickness (Tb.th) compared to SFM treated defects. Both HEV and HEV2 treated defects showed increased trabecular number (Tb.N) compared to SFM group, while only HEV2 treated defects showed decreased trabecular separation (Tb.sp) compared to SFM group (Fig. 5C).
Histologically, HEV and HEV2 treatment resulted in hyaline cartilage regeneration, composed mainly of sGAG, type II collagen and minimal type I collagen (Fig. 5D). Both NEV and NEV2 treatment formed fibrous tissue that stained positive for type I collagen while negative for type II collagen and sGAG. Consistently, HEV and HEV2 treated defects showed significantly higher score in the nature of predominant tissue than NEV and NEV2 (Fig. 5E). Moreover, both HEV and HEV2 treatments showed significantly higher score in the structural characteristics and freedom from degeneration compared to SFM group, while NEV and NEV2 treatments showed no statistic difference with SFM group (Fig. 5E). In the summary score, HEV and HEV2 treatments showed significantly higher cartilage regeneration score than SFM and NEV treated defect, and HEV2 treatment showed significantly higher score than NEV2 (Fig. 5E).
Only HEV and HEV2 treated synovial membrane showed significantly decreased lining cell layer thickness and leukocyte infiltration compared to SFM group, while NEV and NEV2 treatments showed no difference (Fig. 5F&G). In the summary synovitis score, HEV and HEV2 showed the best anti-inflammation effect to synovial membrane compared to SFM group, and HEV2 treatment resulted in significantly decreased synovitis compared to NEV2 treatment (Fig. 5G).
When comparing to the regenerative outcome with HCM (Fig. 2D, E, F, G), we found that HEV exhibited less cartilage regeneration and inflammation modulation, implicating that the soluble factors in HCM indeed contributed to the therapeutic effect on cartilage repair. However, administration of higher concentration of HEVs, i.e., HEV2, yield comparable cartilage regeneration as well as anti-inflammatory effect with HCM, implicating that the beneficial effect of soluble factors could be compensated by increasing the dosage of EVs.
3.6. Hypoxia preconditioning altered the profiles of soluble factors and EVs
We next investigated the influence of hypoxia preconditioning on the profiles of secreted soluble factors. Luminex protein array was conducted to compare the expression of 22 proteins in hypoxia and normoxia preconditioned MSCs secretome (Fig. 6A). The up-regulated soluble factors in HCM include Ang-1, VEGF, FGF-2, SDF-1, HGF, OPN, IL-8, IL-6, and IL-1ra. The expression of other soluble factors such as PDGF-AA, PDGF-BB, MMP-1, MCP-1, LIF, GDF-15, BMP-2, BMP-4, TIMP-1, MMP-13, TSP-2, TGF-β1, TGF-β3 did not show significant difference between normoxic and hypoxic secretome.
Fig. 6.
Influence of hypoxia preconditioning on MSCs secretome profile. (A) Up-regulated soluble factors in hypoxia preconditioned MSC secretome. Data represent the mean ± standard deviation. * denotes p < 0.05 compared normoxia. (B) Particle number and size analysis of HEV and NEV. All the samples were normalized to cell number before measured by NTA. (C) Heatmap of eight differentially expressed miRNAs in HEV compared to NEV (p < 0.05). The color from red to blue represents the log10 (TPM+1) value from large to small. (E) Gene Ontology (GO) analysis of miR-126-3p and miR-122-5p target genes. (E, F) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of miR-126-3p and miR-122-5p target genes.
The influence of hypoxia preconditioning on the size profiles of secreted EVs were investigated using NTA. Although the total EV amounts (size within 50–500 nm) were similar between HEV and NEV, there was a significant increase in the size range of larger EV subpopulation (200–500 nm) in HEV relative to NEV (Fig. 6B). In addition, a secondary peak size of around 270 nm was detected in HEV compared to that in NEV (320–350 nm) (Fig. 6B). This result implicated that although hypoxia preconditioning did not alter the total quantity of EVs, it might affect the size and yield of the larger EVs.
As it is widely acknowledged that miRNAs in EVs exert predominant biological functions, we profiled the miRNA expression in HEV and NEV through high-throughput miRNA sequencing. Eight miRNAs (miR-126-3p, miR-122b-3p, miR-122-5p, miR-31-3p, miR-499a-5p, miR-499b-3p, miR-338-5p, and miR-20b-5p) were screened out with a different expression between HEV and NEV (Fig. 6C). Among these differentially expressed miRNAs, the top three most abundant miRNAs, miR-126-3p, miR-122b-3p, and miR-122-5p, were selected for functional enrichment analysis. A total of 1687 target genes of miR-126-3p and miR-122-5p were acquired after the prediction with Tarbase, and we did not find target genes for has-miR-122b-3p. The known functions of these predicted target genes were identified by GO and KEGG pathway analysis. Based on GO analysis, most of the miR-126-3p and miR-122-5p target genes were enriched in pathways regulating cellular nitrogen compound metabolic process, organelle, and ion binding in the biological process (BP), cellular component (CC), and molecular function (MF), respectively (Fig. 6D). KEGG pathway analysis revealed that most of the miR-126-3p and miR-122-5p target genes were closely related with proteoglycans in cancer pathway (Fig. 6E). Specifically, miR-122-5p target genes were significantly involved in ECM-receptor interaction pathway (Fig. 6F).
4. Discussion
To study the molecular mechanisms of hypoxia preconditioned MSCs secretome induced regenerative effect, it is necessary to compare the contribution of EVs and soluble factors. Most studies either focused on the MSC-CM or the isolated EV, while few studies investigated the effect of EV-depleted CM. In this study, SEC [37] was used to separate EV from the soluble factors efficiently. We demonstrated for the first time that CM, as well as the corresponding EVs, derived from hypoxia preconditioned human BM-MSC efficiently promoted the cartilage repair and subchondral bone regeneration of critical-sized cartilage injury as well as mitigated joint inflammation in a rat osteochondral defect model. In vitro functional assays showed that that most of the beneficial effects of HCM were predominantly associated with EVs, while the soluble factors contributed less but significant effect.
Contrary to the previous report by Zhang et al. [38,39], we found that normoxic CM and EVs, at the amount we used (50x), failed to promote cartilage and subchondral bone regeneration, and showed no inhibiting effect on synovial inflammation. 50x CM has an estimated dosage of 20 μg EV, which is one-fifth of the EV dosage used in Zhang et al.‘s study at 100 μg EV [11,12]. Hypoxia preconditioning thus represents an effective and easy to implement treatment method that could substantially enhance the osteochondral regeneration and anti-inflammatory paracrine activities of MSC. On the other hand, we found that isolated HEV (50X), at equivalent concentration as HCM (50X), exhibited less cartilage regeneration and anti-inflammation outcome, implicating that the soluble factors in HCM also contributed to the therapeutic effect on cartilage repair.
Several proteins were found to have expression up-regulated in the hypoxic MSCs secretome that could be related to the enhanced HdEV therapeutic effect. The elevated chondrocyte proliferation by HdEV (relative to NCM or NdEV) could be attributed to the heightened levels of VEGF and FGF2, given that VEGF [40] and FGF-2 [41] were reported to increase chondrocyte mitotic ability. FGF-2 was also reported to maintain chondrocyte phenotype during expansion [42], which could be associated with the decreased senescent chondrocyte treated with HdEV. Chemokines with cell recruiting function such as HGF [43], SDF-1 [44], OPN [45], IL-8 [46] that were significantly increased in hypoxia MSC secretome could contribute to the significantly enhanced chondrocyte migration treated with HdEV. An increased level of anti-inflammatory factors such as HGF, IL-1ra, and OPN found in hypoxic MSC secretome could have countered the IL-1β induced inflammatory effect on chondrocytes. IL-1ra, as a receptor antagonist of IL-1β, was shown to reduce chondrocyte inflammation [47]. The anti-inflammatory role of HGF has been demonstrated in many studies, mainly through inhibiting GSK3β-mediated activation of NF-κB and downstream inflammatory cytokines expression [48]. OPN was also reported to generate an anti-inflammatory effect on chondrocytes, as treatment with OPN inhibited IL-1β triggered production of NO and PGE2 in human OA cartilage [49]. IL-1β activates TLRs inflammatory signalling in chondrocyte [50]. Correspondingly, the increased anti-inflammatory effect of hypoxic soluble factors compare to normoxic soluble factors was validated with the decreased TLR1 and TLR4 expression in inflamed chondrocytes, as well as reduced expression of IL-1β, IL-6, and TNF-α in pro-inflammatory macrophages. In addition, HGF [51,52], VEGF [52], and IL-6 [53] have been reported to have anti-apoptotic ability and could be important for cell survival after injury. The angiogenic ability associated with Ang-1 [54], VEGF [55], and HGF [56] could also play important roles in angiogenesis that is a prerequisite for subchondral bone regeneration [57] in osteochondral lesion regeneration. Collectively, these up-regulated trophic factors in hypoxia preconditioned MSC secretome could contribute to cartilage regeneration, even though the beneficial effect of hypoxic soluble factors is in general not as strong as HEV, except for chondrocyte migration.
Although the mechanism of how hypoxic EVs generated beneficial effects is still under exploration, most studies focused on the nucleic acid contents in EVs with next generation of RNA sequencing techniques. To date, several miRNAs have been validated to enrich in hypoxic EV and contribute to the beneficial effects on chondrocytes, including miR-26a-5p [58], miR-216a-5p [34], miR-181c-5p [35], miR-18a-3p [35], and miR-205-5p [59]. Interestingly, none of these miRNAs previously associated with hypoxia-enhanced MSC-EV therapeutic effect to cartilage repair and OA was upregulated in HEV in this study. Among the most upregulated were miR-126-3p and miR-122-5p. In previous cartilage-related study, miR-126-3p has been reported to promote chondrocyte migration and proliferation while inhibit apoptosis and inflammation [60]. MiR-122-5p was found to decrease IL-1β and TNF-α-induced inflammation in chondrocytes [61,62]. In addition, over expression of miR-122-5p in MSC-EV was reported to enhance bone regeneration in osteonecrosis of the femoral head (ONFH) rabbit model [63]. GO and KEGG enrichment analysis of their target genes indicated association with cellular metabolism and ECM receptor interaction pathways, which provides the potential targets for further mechanism study.
The disparity in the upregulated miRNAs by hypoxia preconditioning found in our study compared to others could be caused by many experimental factors, such as MSC sources, hypoxia conditions, and EV isolation methods. Unlike previous studies that used only small EVs (<150 nm) [34,35,58,59], we used total EVs without excluding large EVs. Notably, within large EVs of 200–500 nm, a distinct subgroup of around 270 nm was detected in HEV, which was absent in the NEV. One possible explanation for this shift is that decreasing the size of secreted large particles could be a strategy to save energy due to the limitation of energy production under hypoxia [64]. Generally, large EVs are made up of micro-vesicles (MVs) and apoptotic bodies, which are both derived from plasma membrane [65]. The study on large EVs has been limited due to low abundancy, lack of specific surface markers to distinguish them from small EVs, and the difficulty in separating the subpopulations of EVs according to size with conventional isolation techniques [66]. With differential ultracentrifuge to separate large EVs and small EVs, it was reported that different size EVs contained different proteins, mRNAs, and miRNAs [67]. Gorgun et al. found that miRNA profile in small EVs was more homogeneous than large EVs, suggesting that small EVs were less affected by environmental stimulation than large EVs [68]. Given the distinct size shift in larger EV populations in our hypoxia-condition MSCs, further studies should investigate the specific content of MSC large EVs in the future.
5. Conclusion
Hypoxia preconditioning was demonstrated to enhance MSCs secretome for a better therapeutic effect on articular cartilage repair, compared to traditional normoxia cultured MSC secretome. Moreover, most of the beneficial effects of hypoxia preconditioned MSCs secretome such as promoting proliferation, anabolism, anti-inflammation was predominantly associated with EVs, while the soluble factors contributing a smaller but significant effect. Interestingly, hypoxia preconditioning to MSCs showed less effect on total EVs production but altered the profile of EVs subpopulation. Administration of increased amount of EVs could compensate the effect of soluble factors, resulted in comparable cartilage regeneration and anti-inflammatory effect with complete MSCs secretome. In conclusion, delivery of hypoxic MSCs EVs could provide an augmented cell-free clinical application to inhibit joint inflammation and promote cartilage regeneration.
CRediT authorship contribution statement
Yanmeng Yang: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Writing – original draft. Yingnan Wu: Resources, Investigation. Dahou Yang: Resources, Investigation. Shu Hui Neo: Investigation. Nurul Dinah Kadir: Validation. Doreen Goh: Validation. Jian Xiong Tan: Validation. Vinitha Denslin: Investigation. Eng Hin Lee: Funding acquisition, Writing – review & editing. Zheng Yang: Conceptualization, Supervision, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Prof. Herbert Schwarz, Department of Physiology, NUS, for providing THP-1 cells. This work was supported by National Medical Research Council of Singapore (MOH-000371-00) and the National Research Foundation, Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program, through Singapore-MIT Alliance for Research and Technology (SMART): Critical Analytics for Manufacturing Personalized-Medicine (CAMP) Inter-Disciplinary Research Group. YY was supported by NUS Research Scholarship.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.03.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Punzi L., Galozzi P., Luisetto R., Favero M., Ramonda R., Oliviero F., Scanu A. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2(2) doi: 10.1136/rmdopen-2016-000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown T.D., Johnston R.C., Saltzman C.L., Marsh J.L., Buckwalter J.A. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 3.Marcacci M., Filardo G., Kon E. Treatment of cartilage lesions: what works and why? Injury. 2013;44:S11–S15. doi: 10.1016/S0020-1383(13)70004-4. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y.Z., Zhang S.F., Qi Y.Y., Wang L.L., Ouyang H.W. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell Transplant. 2011;20(5):593–607. doi: 10.3727/096368910X532738. [DOI] [PubMed] [Google Scholar]
- 5.Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grøntvedt T., Solheim E., Strand T., Roberts S., Isaksen V., Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. JBJS. 2004;86(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Vasiliadis H.S., Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst. Rev. 2010;10 doi: 10.1002/14651858.CD003323.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36(4):568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Eggenhofer E., Benseler V., Kroemer A., Popp F.C., Geissler E.K., Schlitt H.J., Baan C.C., Dahlke M.H., Hoogduijn M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noiseux N., Gnecchi M., Lopez-Ilasaca M., Zhang L., Solomon S.D., Deb A., Dzau V.J., Pratt R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Gnecchi M., Zhang Z., Ni A., Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusuma G.D., Carthew J., Lim R., Frith J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cell. Dev. 2017;26(9):617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada J., Albo C., Benguria A., Dopazo A., Lopez-Romero P., Carrera-Quintanar L., Roche E., Clemente E., Enriquez J., Bernad A. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19(5):743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer P., Meyer L., Eckert A.W., Berginski M., Schubert J. Measurement of oxygen partial pressure in the mandibular bone using a polarographic fine needle probe. Int. J. Oral Maxillofac. Surg. 2006;35(3):231–236. doi: 10.1016/j.ijom.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., Yusuf R., Côté D., Vinogradov S.A., Scadden D.T., Lin C.P. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodson L. Adipose tissue oxygenation: effects on metabolic function. Adipocyte. 2014;3(1):75–80. doi: 10.4161/adip.27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjöstedt S., Rooth G., Caligara F. The oxygen tension of the blood in the umbilical cord and the intervillous space. Arch. Dis. Child. 1960;35(184):529–533. doi: 10.1136/adc.35.184.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Rocca Y., Fonticoli L., Rajan T.S., Trubiani O., Caputi S., Diomede F., Pizzicannella J., Marconi G.D. Hypoxia: molecular pathophysiological mechanisms in human diseases. J. Physiol. Biochem. 2022;78(4):739–752. doi: 10.1007/s13105-022-00912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang O.K., Noh Y.W., Hong J.T., Lee J.-W. Hypoxia pretreatment promotes chondrocyte differentiation of human adipose-derived stem cells via vascular endothelial growth factor. Tissue Engineering and Regenerative Medicine. 2020;17:335–350. doi: 10.1007/s13770-020-00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F., Zachar V., Pennisi C.P., Fink T., Maeda Y., Emmersen J. Hypoxia enhances differentiation of adipose tissue-derived stem cells toward the smooth muscle phenotype. Int. J. Mol. Sci. 2018;19(2):517. doi: 10.3390/ijms19020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J.P., Liao Y.T., Wu S.H., Chiang E.R., Hsu S.h., Tseng T.C., Hung S.C. Mesenchymal stem cells from a hypoxic culture improve nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2020;14(12):1804–1814. doi: 10.1002/term.3136. [DOI] [PubMed] [Google Scholar]
- 23.Hu X., Xu Y., Zhong Z., Wu Y., Zhao J., Wang Y., Cheng H., Kong M., Zhang F., Chen Q. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ. Res. 2016;118(6):970–983. doi: 10.1161/CIRCRESAHA.115.307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin H.-S., Lee S., Kim Y.-M., Lim J.-Y. Hypoxia-activated adipose mesenchymal stem cells prevents irradiation-induced salivary hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem Cell. 2018;36(7):1020–1032. doi: 10.1002/stem.2818. [DOI] [PubMed] [Google Scholar]
- 25.Chen L., Xu Y., Zhao J., Zhang Z., Yang R., Xie J., Liu X., Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C.-P., Chio C.-C., Cheong C.-U., Chao C.-M., Cheng B.-C., Lin M.-T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013;124(3):165–176. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 27.Chang W., Kim R., Park S.I., Jung Y.J., Ham O., Lee J., Kim J.H., Oh S., Lee M.Y., Kim J. Enhanced healing of rat calvarial bone defects with hypoxic conditioned medium from mesenchymal stem cells through increased endogenous stem cell migration via regulation of ICAM-1 targeted-microRNA-221. Mol. Cell. 2015;38(7):643. doi: 10.14348/molcells.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiz A.M., Jr., Gionet-Gonzales M.A., Lee M.A., Leach J.K. Conditioning of myoblast secretome using mesenchymal stem/stromal cell spheroids improves bone repair. Bone. 2019;125:151–159. doi: 10.1016/j.bone.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L.-P., Tian T., Wang J.-Y., He J.-N., Chen T., Pan M., Xu L., Zhang H.-x., Qiu X.-T., Li C.-C. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Shao K., Liu C., Li C., Yu B. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur. Rev. Med. Pharmacol. Sci. 2019;23(15):6691–6699. doi: 10.26355/eurrev_201908_18560. [DOI] [PubMed] [Google Scholar]
- 31.Liu W., Li L., Rong Y., Qian D., Chen J., Zhou Z., Luo Y., Jiang D., Cheng L., Zhao S. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Liu W., Rong Y., Wang J., Zhou Z., Ge X., Ji C., Jiang D., Gong F., Li L., Chen J. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflammation. 2020;17(1):1–22. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X.-F., Wang T., Wang Z.-X., Huang K.-P., Zhang Y.-W., Wang G.-L., Zhang H.-J., Chen Z.-H., Wang C.-Y., Zhang J.-X. Hypoxic ucMSC-secreted exosomal miR-125b promotes endothelial cell survival and migration during wound healing by targeting TP53INP1. Mol. Ther. Nucleic Acids. 2021;26:347–359. doi: 10.1016/j.omtn.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong Y., Zhang J., Jiang D., Ji C., Wang J., Ge X., Tang P., Yu S., Cui W., Cai W. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325–342. doi: 10.1016/j.actbio.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Zhang B., Tian X., Qu Z., Hao J., Zhang W. Hypoxia-preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes. 2022;12(2):225. doi: 10.3390/membranes12020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tee C.A., Yang Z., Yin L., Wu Y., Han J., Lee E.H. Improved zonal chondrocyte production protocol integrating size-based inertial spiral microchannel separation and dynamic microcarrier culture for clinical application. Biomaterials. 2019;220 doi: 10.1016/j.biomaterials.2019.119409. [DOI] [PubMed] [Google Scholar]
- 37.Shu S.L., Yang Y., Allen C.L., Hurley E., Tung K.H., Minderman H., Wu Y., Ernstoff M.S. Purity and yield of melanoma exosomes are dependent on isolation method. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2019.1692401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S., Chu W., Lai R., Lim S., Hui J., Toh W. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Maes C., Stockmans I., Moermans K., Van Looveren R., Smets N., Carmeliet P., Bouillon R., Carmeliet G. Soluble VEGF isoforms are essential for establishingepiphyseal vascularization and regulating chondrocyte development and survival. J. Clin. Investig. 2004;113(2):188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solchaga L.A., Penick K., Porter J.D., Goldberg V.M., Caplan A.I., Welter J.F. FGF‐2 enhances the mitotic and chondrogenic potentials of human adult bone marrow‐derived mesenchymal stem cells. J. Cell. Physiol. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 42.Lee J., Lee J.-Y., Chae B.-C., Jang J., Lee E., Son Y. Fully dedifferentiated chondrocytes expanded in specific mesenchymal stem cell growth medium with FGF2 obtains mesenchymal stem cell phenotype in vitro but retains chondrocyte phenotype in vivo. Cell Transplant. 2017;26(10):1673–1687. doi: 10.1177/0963689717724794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neuss S., Becher E., Wöltje M., Tietze L., Jahnen‐Dechent W. Functional expression of HGF and HGF receptor/c‐met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cell. 2004;22(3):405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 44.Marquez-Curtis L.A., Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu D.-Y., Yeh W.-L., Huang S.-M., Tang C.-H., Lin H.-Y., Chou S.-J. Osteopontin increases heme oxygenase–1 expression and subsequently induces cell migration and invasion in glioma cells. Neuro Oncol. 2012;14(11):1367–1378. doi: 10.1093/neuonc/nos262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon D.S., Lee K.-M., Kim S.-H., Kim S.H., Jung Y., Kim S.H., Park K.H., Choi Y., Ryu H.A., Choi W.J. Synergistic action of IL-8 and bone marrow concentrate on cartilage regeneration through upregulation of chondrogenic transcription factors. Tissue Eng. 2016;22(3–4):363–374. doi: 10.1089/ten.tea.2015.0425. [DOI] [PubMed] [Google Scholar]
- 47.Bobacz K., Sunk I., Hofstaetter J., Amoyo L., Toma C., Akira S., Weichhart T., Saemann M., Smolen J. Toll‐like receptors and chondrocytes: the lipopolysaccharide‐induced decrease in cartilage matrix synthesis is dependent on the presence of toll‐like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 2007;56(6):1880–1893. doi: 10.1002/art.22637. [DOI] [PubMed] [Google Scholar]
- 48.Gong R., Rifai A., Ge Y., Chen S., Dworkin L.D. Hepatocyte growth factor suppresses proinflammatory NFκB activation through GSK3β inactivation in renal tubular epithelial cells. J. Biol. Chem. 2008;283(12):7401–7410. doi: 10.1074/jbc.M710396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attur M.G., Dave M.N., Stuchin S., Kowalski A.J., Steiner G., Abramson S.B., Denhardt D.T., Amin A.R. Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum. 2001;44(3):578–584. doi: 10.1002/1529-0131(200103)44:3<578::AID-ANR106>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Liu L., Gu H., Liu H., Jiao Y., Li K., Zhao Y., An L., Yang J. Protective effect of resveratrol against IL-1β-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-κB signaling pathway: an “in vitro study”. Int. J. Mol. Sci. 2014;15(4):6925–6940. doi: 10.3390/ijms15046925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boomsma R.A., Geenen D.L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu S., Lu C., Han Q., Li J., Du Z., Liao L., Zhao R.C. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology. 2011;279(1–3):189–195. doi: 10.1016/j.tox.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Hung S.-C., Pochampally R.R., Chen S.-C., Hsu S.-C., Prockop D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cell. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 54.Fagiani E., Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Nagy J.A., Dvorak A.M., Dvorak H.F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 56.Kwon H.M., Hur S.-M., Park K.-Y., Kim C.-K., Kim Y.-M., Kim H.-S., Shin H.-C., Won M.-H., Ha K.-S., Kwon Y.-G. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharmacol. 2014;63(1):19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 57.García-Fernández L. Osteochondral Tissue Engineering; 2018. Osteochondral Angiogenesis and Promoted Vascularization: New Therapeutic Target; pp. 315–330. [DOI] [PubMed] [Google Scholar]
- 58.Wan S., Bao D., Li J., Lin K., Huang Q., Li Q., Li L. Extracellular vesicles from hypoxic pretreated urine-derived stem cells enhance the proliferation and migration of chondrocytes by delivering miR-26a-5p. Cartilage. 2022;13(2) doi: 10.1177/19476035221077401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen K., Duan A., Cheng J., Yuan T., Zhou J., Song H., Chen Z., Wan B., Liu J., Zhang X. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205–5p/PTEN/AKT pathway. Acta Biomater. 2022;143:173–188. doi: 10.1016/j.actbio.2022.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y., Ming J., Li Y., Li B., Deng M., Ma Y., Chen Z., Zhang Y., Li J., Liu S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discovery. 2021;7(1):1–15. doi: 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott K., Cohen D., Hays M., Nielson D., Grinstaff M., Lawson T., Snyder B., Boyan B., Schwartz Z. Regulation of inflammatory and catabolic responses to IL-1β in rat articular chondrocytes by microRNAs miR-122 and miR-451. Osteoarthritis Cartilage. 2021;29(1):113–123. doi: 10.1016/j.joca.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Scott K.M., Cohen D.J., Boyan B.D., Schwartz Z. miR‐122 and the WNT/β‐catenin pathway inhibit effects of both interleukin‐1β and tumor necrosis factor‐α in articular chondrocytes in vitro. J. Cell. Biochem. 2022;123(6):1053–1063. doi: 10.1002/jcb.30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao W., Ning Y., Xu H.-J., Zou W.-Z., Hu J., Liu X.-Z., Yang Y., Li Z.-H. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin. Sci. 2019;133(18):1955–1975. doi: 10.1042/CS20181064. [DOI] [PubMed] [Google Scholar]
- 64.Patton M.C., Zubair H., Khan M.A., Singh S., Singh A.P. Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival. J. Cell. Biochem. 2020;121(1):828–839. doi: 10.1002/jcb.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciardiello C., Migliorino R., Leone A., Budillon A. Large extracellular vesicles: size matters in tumor progression. Cytokine Growth Factor Rev. 2020;51:69–74. doi: 10.1016/j.cytogfr.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin‐Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno S., Tapparo M., Collino F., Chiabotto G., Deregibus M.C., Soares Lindoso R., Neri F., Kholia S., Giunti S., Wen S. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng. 2017;23(21–22):1262–1273. doi: 10.1089/ten.tea.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorgun C., Ceresa D., Lesage R., Villa F., Reverberi D., Balbi C., Santamaria S., Cortese K., Malatesta P., Geris L. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs) Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.