Abstract
Aims
To expand the evidence base for the clinical use of metformin, we conducted a meta-analysis of randomized controlled trials (RCTs) comparing the efficacy and safety of metformin versus insulin with respect to short-term neonatal outcomes.
Methods
A comprehensive search of electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) was performed. Two reviewers extracted the data and calculated pooled estimates by use of a random-effects model. In total, 24 studies involving 4355 participants met the eligibility criteria and were included in the quantitative analyses.
Results
Unlike insulin, metformin lowered neonatal birth weights (mean difference − 122.76 g; 95% confidence interval [CI] − 178.31, − 67.21; p < 0.0001), the risk of macrosomia (risk ratio [RR] 0.68; 95% CI 0.54, 0.86; p = 0.001), the incidence of neonatal intensive care unit admission (RR 0.73; 95% CI 0.61, 0.88; p = 0.0009), and the incidence of neonatal hypoglycemia (RR 0.65; 95% CI 0.52, 0.81; p = 0.0001). Subgroup analysis based on the maximum daily oral dose of metformin indicated that metformin-induced neonatal birth weight loss was independent of the oral dose.
Conclusions
Our meta-analysis provides further evidence that metformin is a safe oral antihyperglycemic drug and has some benefits over insulin when used for the treatment of gestational diabetes, without an increased risk of short-term neonatal adverse outcomes. Metformin may be particularly useful in women with gestational diabetes at high risk for neonatal hypoglycemia, women who want to limit maternal and fetal weight gain, and women with an inability to afford or use insulin safely.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00592-022-02016-5.
Keywords: Gestational diabetes mellitus, Insulin, Metformin, Neonatal outcomes, Randomized controlled trials
Introduction
Gestational diabetes mellitus (GDM) is a common complication during pregnancy and is defined as any glucose intolerance that occurs or is diagnosed for the first time during pregnancy [1]. GDM develops in about 5% to 14% of all pregnancies and is associated with certain pregnancy-related complications and a long-term risk of diabetes in both the mother and offspring [2]. With the establishment of the two-child policy and epidemic of obesity in China, the incidence of GDM has been increasing, resulting in a heavy economic burden on the public health care system and individuals [3]. According to the latest data reported by the International Diabetes Federation in 2021, about one in six live births (20 million) is affected by high plasma glucose concentration during pregnancy, and GDM accounts for 83.6% of these cases of hyperglycemia [4].
Women with uncontrolled GDM have higher-risk pregnancies, and some adverse effects of GDM may also affect the fetus, including fetal anomalies, macrosomia (birth weight of > 4000 g), fetal distress, metabolic disorders, growth imbalance, hyperbilirubinemia, and some long-term complications [5]. Traditionally, insulin has been the gold standard for the treatment of GDM because it cannot cross the placenta and allows for precise glucose control. However, insulin therapy has several disadvantages, including the need for multiple injections, risks of hypoglycemia and hyperbilirubinemia, the rising cost of insulin, and the lack of affordability [6]. These disadvantages suggest that current treatment regimens fall short of optimizing outcomes. Metformin is a commonly used oral antihyperglycemic drug in clinical practice with excellent efficacy in terms of glycemic control and weight loss, good tolerance, and a reasonable price [7]. Several organizations currently support its use as an alternative to insulin [8, 9]. However, recent long-term studies of offspring have provided conflicting results. Two follow-up studies of children aged 2 to 9 years whose mothers had gestational diabetes showed that several growth parameters tended to be larger in metformin-exposed offspring than in offspring exposed to insulin. These growth parameters included weight, body mass index, triceps skinfold, waist and arm circumferences and body fat percent, and they were also associated with cardio-metabolic disease in later life [10, 11]. This has slowed the clinical use of metformin as a substitute for insulin in the treatment of GDM.
We therefore performed this updated meta-analysis to compare the efficacy and safety of metformin versus insulin with respect to short-term neonatal outcomes in the treatment of GDM. The objective of our study was to determine whether metformin is superior to insulin in terms of altering neonatal growth outcomes and inducing neonatal adverse outcomes during treatment of GDM. Addressing this issue is particularly important because the number of pregnancies exposed to metformin is increasing worldwide.
Methods
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (CRD42022330187) [12].
Search strategy
A systematic literature search of PubMed, Embase, the Cochrane Library, and Web of Science (last search was updated on 1 May 2022) was performed using prespecified terms (Supplemental Text S1) with no filters and no language or location restrictions. We also searched for additional eligible trials in previously published meta-analyses on related topics.
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) The population comprised pregnant women with GDM, (2) The interventions were metformin (with or without extra insulin treatment) and insulin, (3) The study included one or more neonatal outcomes, and (4) The study design was a randomized controlled trial (RCT). We excluded studies involving pregnant women with pre-existing diabetes, and duplicate studies published in different journals were included only once.
Definitions of neonatal outcomes
The neonatal outcomes included neonatal growth outcomes and neonatal adverse outcomes. The neonatal growth outcomes were birth weight, birth height, macrosomia (≥ 4000 g), large for gestational age (LGA) (birth weight at the > 90th percentile), and small for gestational age (SGA) (birth weight at the < 10th percentile). The neonatal adverse outcomes were neonatal hypoglycemia, admission to the neonatal intensive care unit (NICU), hyperbilirubinemia, respiratory distress syndrome, premature birth, congenital anomalies, abnormal pH of the umbilical cord, abnormal Apgar score at 5 min, neonatal death, neonatal sepsis, and birth trauma.
Data collection and management
The titles, abstracts, citation information, and descriptor terms of the publications identified through the search strategy were screened. Full-text articles of all selected abstracts were obtained, and two reviewers (Bo Sheng and Juan Ni) independently assessed all the full-text articles for eligibility to determine the final study selection. Any disagreements between the two authors were settled by group discussion until a consensus was reached. We designed a data extraction form to collect relevant information including the authors, year of publication, country, number of patients, definition of gestational diabetes, patient characteristics, and interventions.
Risk of bias and quality assessment
We used the Cochrane Collaboration’s tool to assess the risk of bias in terms of the following seven aspects: (1) Random sequence generation (selection bias), (2) Allocation concealment (selection bias), (3) Blinding of participants and personnel (performance bias), (4) Blinding of outcome assessment (detection bias), (5) Incomplete outcome data (attrition bias), (6) Selective reporting (reporting bias), and (7) Other bias. We classified these aspects as low risk of bias, uncertain risk of bias, or high risk of bias.
We assessed the quality of evidence in these studies by using the GRADE profiler (GRADEpro GDT) [13]. The GRADE system was used to assess the study limitations (risk of bias), inconsistency, indirectness, imprecision, and publication bias across the body of evidence to derive an overall summary of the quality of evidence, which was classified each as high, moderate, low, or very low.
Statistical analysis
The standardized mean difference (SMD) was calculated using the mean and standard deviation for continuous variables. The risk ratio (RR) was calculated for dichotomous variables with 95% confidence intervals (CIs). The meta-analysis was performed using Review Manager (RevMan) version 5.4.1 (Nordic Cochrane Centre, Copenhagen, 2014), and Egger’s test was used to assess publication bias through the ‘metafor’ package in R version 3.5.1 [14]. The studies were determined to be heterogenous if I2 > 50% and p < 0.1. A sensitivity analysis was performed by excluding each study one by one to evaluate the credibility of the pooled results. A prespecified subgroup analysis was also performed to explore the sources of heterogeneity. Potential publication bias was assessed by the application of contour-enhanced funnel plots and Egger’s linear regression test at the p < 0.05 level of significance. If publication bias was indicated, we further evaluated the number of missing studies by trim-and-fill analysis and recalculated the pooled risk estimate with the addition of those missing studies. Except where otherwise specified, a p value of < 0.05 was considered statistically significant.
Results
Literature search and study characteristics
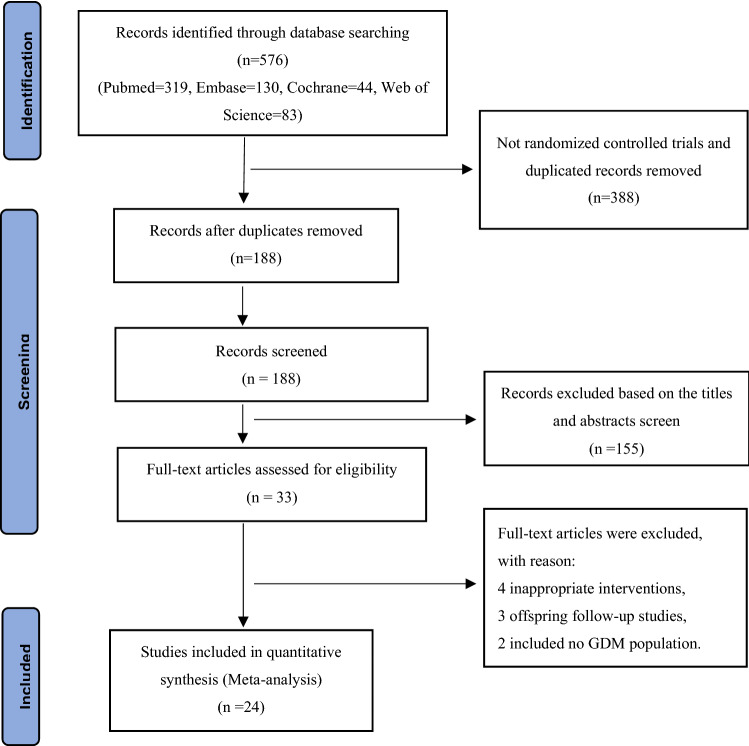
In total, 576 studies were retrieved through PubMed, Embase, the Cochrane Library, and Web of Science. After removal of duplicates and title/abstract screening, 188 trials underwent full text assessment, after which the full set of eligibility criteria was applied. After full text evaluation, 24 studies remained eligible for inclusion in this review. The process of study selection is illustrated in Fig. 1. As shown in Table 1, 24 RCTs involving 4355 patients with GDM were included to estimate the impact of metformin versus insulin on neonatal outcomes [15–38]. The earliest study began in 2001, and the latest study was completed in 2021. Five studies each were conducted in Iran [23, 30–32, 35],Egypt [15, 18, 20, 22, 37], and Pakistan [16, 17, 21, 27, 38]; three in Finland [26, 28, 34]; and one each in Australia [19], India [36], Spain [29], Brazil [33], New Zealand [24], and the USA [25]. In this meta-analysis, we mainly focused on the daily oral dose of metformin in pregnant women with GDM. Three studies among the 24 RCTs did not report the dose of metformin [19, 23, 30], and the remaining 21 studies were included for further subgroup analysis.
Fig. 1.
PRISMA flow diagram
Table 1.
Main characteristics of RCTs included in the meta-analysis
| Author, year | Country | Study period | Participants | Participants enrolled | Dose | No. of Neonatal outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
| Metformin | Escalating to Insulin§ | Insulin | Metformin (mg/day) | Insulin (units/kg/day) | |||||
| Abdullah, 2021 | Egypt | Oct 2019 to Apr 2021 | Women aged 21–35 yr; Singleton; Gestational age, 20–28 wks | 94 | 5 | 100 | 500–2500 | 0.7 | 7 |
| Ainuddin, 2014 | Pakistan | Dec 2008 to Dec 2010 | Women aged 20–46 yr; Singleton; Gestational age, 20–36 wks | 43 | 32 | 75 | 500–2500 | 0.9 | 15 |
| Arshad, 2017 | Pakistan | 2010 to 2012 | NR | 25 | NR | 25 | 1500 | 08–0.9 | 5 |
| Ashoush, 2016 | Egypt | Jan 2013 to Nov 2014 | Gestational age, 26–32 wks | 47 | 11* | 48 | 1000–2500 | 0.7 | 8 |
| Barrett, 2013 | Australia | NR | Singleton | 236 | 97 | 242 | NR | NR | 13 |
| Eid, 2018 | Egypt | Mar 2016 to Jun 2017 | Women aged 18–42 yr; Singleton; Gestational age, 22–30 wks | 113 | 2 | 116 | 500–2500 | 0.5 | 17 |
| Ghomian, 2018 | Iran | NR | Women aged 18–40 yr; Singleton; Gestational age. 24–28 wks | 143 | 30 | 143 | NR | NR | 6 |
| Gamal, 2018 | Egypt | Feb 2016 to Jan 2017 | NR | 58 | 5* | 58 | 1500–2500 | 1.0 | 3 |
| Hassan, 2012 | Pakistan | Dec 2008 to Dec 2010 | Singleton; Gestational age, 20–35 wks | 75 | 18* | 75 | 500–3000 | NR | 10 |
| Hamadani, 2017 | Pakistan | NR | Singleton | 30 | NR | 30 | 500–2000 | NR | 2 |
| Huhtala, 2020 | Finland | Jun 2006 to Dec 2010 | NR | 110 | 23* | 110 | 500–2000 | NR | 6 |
| Ijas, 2010 | Finland | Jun 2005 to Jun 2009 | Singleton; Gestational age, 12–34 wks | 32 | 15* | 50 | 750–2250 | NR | 12 |
| Jahanshahi, 2020 | Iran | 2017 to 2018 | Singleton; Gestational age, 20–34 wks | 30 | 3 | 30 | NR | NR | 2 |
| Picón-César, 2021 | Spain | Oct 2016 to June 2019 | Women aged 18–45 yr; Singleton; Gestational age, 14–35 wks | 70 | 24* | 97 | 425–2500 | 0.3 | 15 |
| Mesdaghinia, 2013 | Iran | NR | Women aged 18–45 yr; Singleton; Gestational age, 24–34 wks | 100 | 22 | 100 | 500–2500 | 0.5 | 13 |
| Moore, 2007 | USA | 2001 to 2004 | Gestational age, 24–30 wks | 32 | 0 | 31 | 500–2000 | 0.7 | 6 |
| Niromanesh, 2012 | Iran | Dec 2010 to Jan 2012 | Women aged 18–40 yr; Singleton; Gestational age, 20–34 wks | 80 | 11* | 80 | 500–2500 | 0.7 | 14 |
| Rowan, 2008 | New Zealand | NR | Women aged 18–45 yr; Singleton; Gestational age, 20–33 wks | 363 | 168* | 370 | 500–2500 | NR | 15 |
| Ruholamin, 2014 | Iran | 2011 | Women aged 18–45 yr. Singleton; Gestational age,t 24–33 wks | 50 | 2 | 50 | 500–1500 | 0.2 | 13 |
| Saleh, 2016 | Egypt | Nov 2012 to Dec 2014 | Gestational age, 26–34 wks | 67 | NR | 70 | 500–3000 | 0.7–1 | 12 |
| Somani, 2016 | India | Feb 2014 to Jul 2015 | Women aged 18–35 yr. Singleton; Gestational age, 24–34 wks | 32 | 1 | 33 | 500–2000 | NR | 11 |
| Spaulonci, 2013 | Brazil | Nov 2007 to Jan 2010 | Singleton | 47 | 12* | 47 | 1700–2250 | 0.4 | 11 |
| Tertti, 2013 | Finland | Jun 2006 to Dec 2010 | Singleton; Gestational age, 22–34 wks | 110 | 23* | 110 | 500–2000 | NR | 12 |
| Wasim, 2019 | Pakistan | Feb 2016 to Dec 2017 | Singleton; Gestational age, 22–34 wks | 137 | 34* | 141 | 1000–2500 | 0.7–0.8 | 11 |
NR No Reported
§ indicates glycemic control is not achieved by maximum metformin dose, and insulin is added
* represents the participants are included in the metformin group for pooled analysis
Supplemental Fig. S1 provides a summary of the risk of bias for each included study. No selection bias, attrition bias, or selective bias was present in any of the RCTs, indicating relatively high quality. Because insulin was given by injection and metformin was given orally, all the included studies involved open allocation, which did not affect the short-term neonatal outcomes because these were all objective. The quality of the evidence (GRADE) for the neonatal outcomes of interest, including neonatal birth weight, macrosomia, LGA, SGA, birth height, NICU admission, and neonatal hypoglycemia, was very low to moderate. The GRADE system evidence for the above outcomes and reasons for upgrade and downgrade are shown in Table 2.
Table 2.
Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) summary of neonatal outcomes of meta-analysis
| Metformin vs. insulin for gestational diabetes mellitus Patient population: patients with gestational diabetes mellitus Intervention: metformin Comparison: insulin | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects | RR/SMD (95%CI) | No. of Participants (Studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with Insulin | Risk with Metformin | |||||
| Birthweight |
SMD -0.33 (-0.5 to -0.17) |
4174 (22 RCTs) |
⨁⨁◯◯ Low |
Most of researches have limitations in methodology Unexplained heterogeneity |
||
| Macrosomia | 137 per 1,000 | 93 per 1,000 |
RR 0.68 (0.54 to 0.86) |
3484 (20 RCTs) |
⨁⨁⨁◯ Moderate |
Researches have limitations in methodology |
| LGA | 188 per 1,000 | 162 per 1,000 |
RR 0.86 (0.73 to 1.02) |
2843 (12 RCTs) |
⨁⨁⨁◯ Moderate |
Most of researches have limitations in methodology |
| SGA | 75 per 1,000 | 75 per 1,000 |
RR 1.00 (0.77 to 1.30) |
2812 (12 RCTs) |
⨁⨁◯◯ Low |
Most of researches have limitations in methodology Unexplained heterogeneity |
| Birth Height |
SMD -0.09 (-0.27 to -0.08) |
1084 (3 RCTs) |
⨁◯◯◯ Very low |
Researches have limitations in methodology Very few RCTs lead to imprecision of estimate Unexplained heterogeneity |
||
| NICU admission | 207 per 1000 | 151 per 1000 |
RR 0.73 (0.61 to 0.88) |
3527 (18 RCTs) |
⨁⨁⨁◯ Moderate |
Most of researches have limitations in methodology |
| Hypoglycemia | 164 per 1000 | 107 per 1000 |
RR 0.65 (0.54 to 0.84) |
3670 (20 RCTs) |
⨁⨁◯◯ Low |
Most of researches have limitations in methodology 2. There is a possibility of publication bias in these studies |
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of the effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of the effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
RCTs Randomized controlled trials; CI confidence interval; RR risk ratio; SMD Std mean difference
Neonatal birth weight and macrosomia
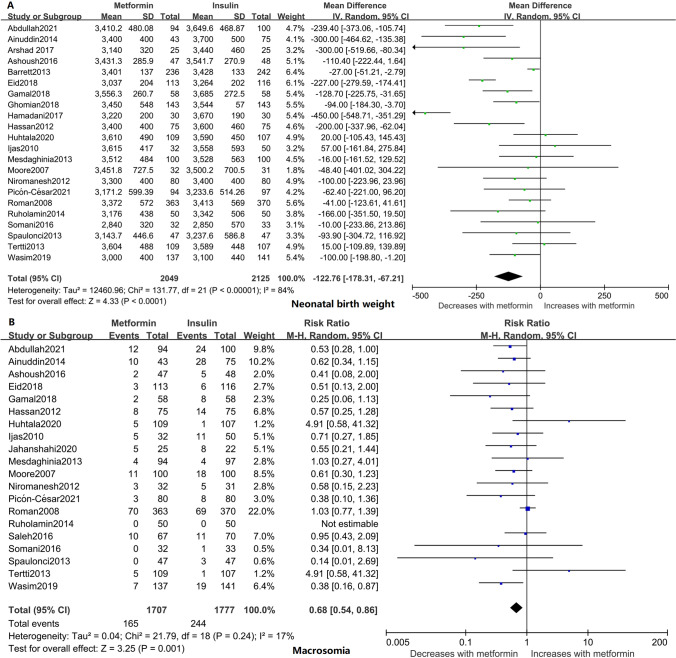
Twenty-two studies involving 4174 neonates reported the neonatal birth weight. [15–19, 21–23, 25–38] The results indicated that the birth weights of neonates whose mothers were treated with metformin were significantly lower than those of neonates whose mothers were treated with insulin during pregnancy (95% CI − 178.31, − 67.21; I2 = 84%; p < 0.0001) (Fig. 2A). On average, metformin-exposed neonates weighed 122.76 g less than those whose mothers received insulin. Similar to the birth weight in the metformin-exposed group, metformin also lowered the risk of macrosomia by 30% compared with the insulin-exposed group based on 20 studies (RR 0.75; 95% CI 0.54, 0.86; I2 = 17%; p = 0.001) (Fig. 2B) [15, 17, 18, 20, 22, 24–38].
Fig. 2.
Forest plots for neonatal growth outcomes. A Neonatal birth weight. B Macrosomia
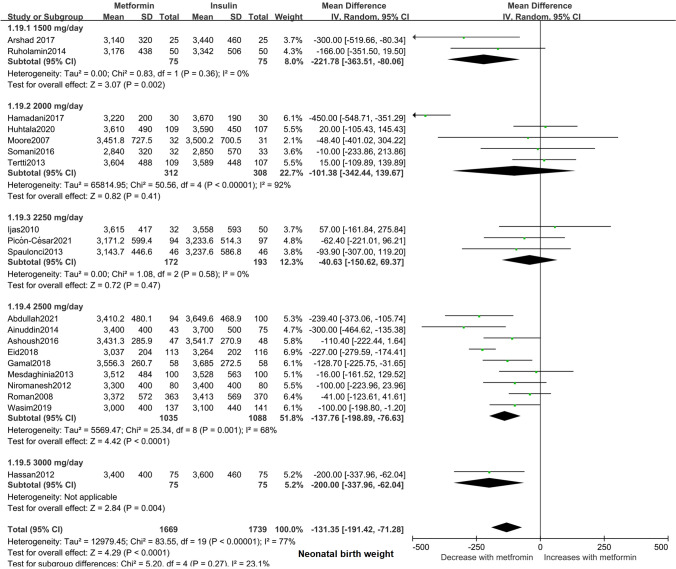
To explore potential source of heterogeneity among the studies, we carried out several sensitivity analyses (Supplemental Fig. S2). Nevertheless, significant heterogeneity (I2 = 74%) was still present among the studies after we excluded one study from the analysis [21]. Next, 20 studies involving 3408 neonates were included in a subgroup analysis of birth weight [15–18, 21, 22, 24–35, 37, 38], and we found that the neonates whose mothers were treated with a maximum oral dosage of metformin of 1500 mg/day (95% CI − 363.51, − 80.06; I2 = 0%; p = 0.002), 2500 mg/day (95% CI − 198.89, − 76.63; I2 = 68%; p < 0.001), and 3000 mg/day (95% CI − 337.96, − 62.04; p = 0.004) had obviously lower birth weights than those of neonates whose mothers were treated with insulin. However, the birth weight of neonates born to mothers treated with a maximum oral dosage of metformin of 2000 mg/day (95% CI − 342.44, 139.67; I2 = 92%; p = 0.41) and 2250 mg/day (95% CI − 150.62, 69.37; I2 = 0%; p = 0.47) showed no significant difference between the groups (Fig. 3).
Fig. 3.
Forest plot for subgroup analysis of neonatal birth weight. Data are expressed as mean difference (random-effects model) and 95% CI
To assess the potential publication bias of neonatal birth weight, we used the ‘metafor’ package of R software for Egger’s test. Our results showed that the funnel plot of neonatal birth weight was asymmetrical (Supplemental Fig. S3A), and Egger’s test indicated possible publication bias (p = 0.008) (Supplemental Table S1). Next, we used trim-and-fill analysis to recalculate our pooled risk estimate; the results suggested no publication bias (p = 0.28), and the funnel plot also became symmetrical (Supplemental Fig. S3B).
Other neonatal growth outcomes
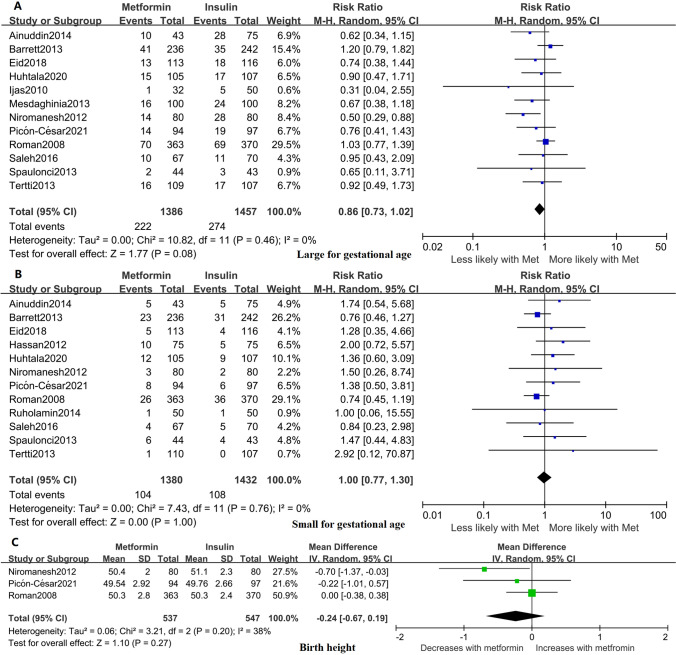
Twelve studies reported the frequency of LGA and SGA [17, 19, 20, 22, 24, 26, 28, 29, 31–34], and three studies reported the neonatal height [24, 29, 31]. The results suggested no difference in the risk of being born LGA (RR 0.86; 95% CI 0.73, 1.02; I2 = 0%; p = 0.08), the risk of being born SGA (RR 1.00; 95% CI 0.77, 1.30; I2 = 0%; p = 1.0), or the neonatal height (95% CI − 0.67, 0.19; I2 = 38%; p = 0.27) between metformin and insulin exposure (Fig. 4). No evidence of publication bias was observed by Egger’s test in other neonatal growth outcomes (Supplemental Fig. S3 and Table S1).
Fig. 4.
Forest plots for other neonatal growth outcomes. A Large for gestational age (LGA). B Small for gestational age (SGA). C Neonatal birth height
Neonatal adverse outcomes
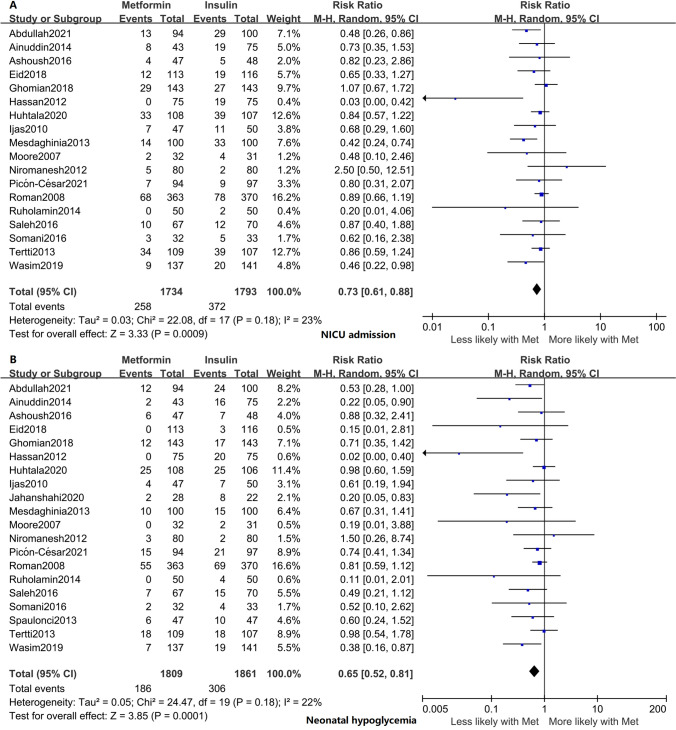
Eighteen studies involving 3527 neonates reported the incidence of NICU admission [15, 17, 19, 20, 22–29, 31, 32, 34–36, 38], and the results indicated a lower incidence in metformin-exposed than insulin-exposed neonates (RR 0.73; 95% CI, 0.61, 0.88; I2 = 23%; p = 0.0009) (Fig. 5A). Moreover, 20 studies involving 3670 neonates were included in the analysis of neonatal hypoglycemia [15, 17, 18, 20, 22–36, 38]. The results showed that insulin-exposed neonates had a higher incidence of hypoglycemia than metformin-exposed neonates (RR 0.65; 95% CI 0.52, 0.81; I2 = 22%; p = 0.0001) and that metformin lowered the risk of neonatal hypoglycemia by 45% compared with the insulin-exposed group (Fig. 5B). We used contour-enhanced funnel plots and Egger’s linear regression test to assess the potential publication bias of NICU admission and neonatal hypoglycemia (Supplemental Fig. S3D and S3F). Egger’s test indicated no publication bias for NICU admission, but neonatal hypoglycemia was associated with possible publication bias (p = 0.006) (Supplemental Table S1). We used trim-and-fill analysis to recalculate our pooled risk estimate of neonatal hypoglycemia, which suggested no publication bias (p = 0.71) (Supplemental Fig. S3E).
Fig. 5.
Forest plots for neonatal adverse outcomes. A NICU admission. B Neonatal hypoglycemia
There were no significant differences in the other neonatal adverse outcomes, including respiratory distress syndrome (14 studies) (RR 0.71; 95% CI, 0.51, 0.99; I2 = 0%; p = 0.07), an abnormal Apgar score at 5 min (15 studies) (RR 0.00; 95% CI − 0.15, 0.16; I2 = 59%; p = 0.95), hyperbilirubinemia (9 studies) (RR 0.88; 95% CI 0.69, 1.12; I2 = 0%; p = 0.29), congenital anomalies (9 studies) (RR 0.73; 95% CI 0.44, 1.22; I2 = 0%; p = 0.23), preterm birth (11 studies) (RR 1.08; 95% CI 0.78, 1.50; I2 = 22%; p = 0.63), an abnormal pH of the umbilical cord (5 studies) (RR 0.01; 95% CI − 0.00, 0.01; I2 = 0%; p = 0.14), neonatal death (10 studies) (RR 0.52; 95% CI 0.13, 2.18; I2 = 0%; p = 0.37), neonatal sepsis (4 studies) (RR 0.71; 95% CI 0.34, 1.45; I2 = 0%; p = 0.34), and birth trauma (6 studies) (RR 0.92; 95% CI 0.57, 1.49; I2 = 0%; p = 0.74) (Supplemental Figs. S4 and S5).
Discussion
In this systematic review and meta-analysis, we found that neonates exposed to metformin in utero weighed less at birth than those whose mothers were exposed to insulin. The risk of macrosomia is substantially lower (by 30%) when GDM is treated with metformin than with insulin, and there is no concomitant increase in the risk of being born SGA or LGA. Despite being born at lower average birth weights, neonates of metformin-treated women do not have an increased incidence of neonatal adverse outcomes. In contrast, metformin significantly lowers the risk of neonatal hypoglycemia and the incidence of NICU admission.
It is well accepted that the fetuses of obese women with GDM have a higher risk of developing macrosomia than those of women with GDM of normal weight [39]. Some recent meta-analyses showed that weight gain during pregnancy was significantly lower in women with GDM who received metformin than in those who received insulin [4, 7]. Whether metformin-induced weight loss in women with GDM leads to a significant reduction in the incidence of fetal macrosomia remains unclear. Our results provide evidence that metformin can also effectively control neonatal birth weight and reduce the incidence of fetal macrosomia. In particular, there is growing evidence that macrosomia is likely to be associated with shoulder dystocia, brachial plexus injury, delayed motor development, and a higher risk of obesity or diabetes later in life [7, 40]. Moderate neonatal birth weight control may effectively reduce and avoid some complications related to macrosomia, especially for pregnant women with GDM. To explore the relationship between neonatal birth weight and the oral dose of metformin, we performed a subgroup analysis of neonatal birth weight based on the maximum daily oral dose of metformin. We found that a maximum oral dosage of metformin of 1500, 2500, and 3000 mg/day was associated with neonatal birth weight loss, but there was no significant difference in an oral dosage of metformin of 2000 and 2250 mg/day. These results suggest that metformin-induced neonatal birth weight loss occurs independently of the oral dose of metformin. This is consistent with the previous finding that a low dosage of metformin (< 1000 mg/day), but not a high dosage, had significant efficacy for body mass index control or weight loss in adolescents [41].
Macrosomic fetuses in women with diabetes develop a unique pattern of overgrowth involving central deposition of subcutaneous fat in the abdominal and interscapular areas with skeletal growth remaining largely unaffected [40, 42]. During early gestation, the embryo expresses very low levels of organic cation transporters, making metformin likely to be safe in the first trimester. However, metformin can easily cross the placenta via organic cation transporters in the second and third trimesters and may reach near-maternal concentrations in the fetus [43]. In addition to lowering blood glucose concentration, metformin has a variety of intracellular effects including inhibition of mitochondrial respiration and effects on the nutrient-sensing pathway by both adenosine monophosphate-activated protein kinase and mammalian target of rapamycin mechanisms [44–47]. Moreover, in the Metformin in Women with Type 2 Diabetes in Pregnancy (MiTy) trial, the lower neonatal adiposity in the metformin group led to a lower incidence of fetal macrosomia [48]. Therefore, the significant metformin-induced reduction in the incidence of macrosomia may be related to the inhibition of fetal fatty acid synthesis. This effect of metformin differentiates its dose-dependent hypoglycemic effect, the underlying mechanism of which remains to be explored.
In accordance with previous meta-analyses [7, 51, 52], the incidence of NICU admission and hypoglycemia were also significantly reduced in our study. The rates of NICU admission are mainly influenced by fetal physiologic compromise, including preterm birth, hypoglycemia, respiratory distress syndrome, and neonatal jaundice. In our meta-analysis, the infants born to mothers treated with insulin needed additional management for hypoglycemia, which is partly associated with an increase in NICU admission. Neonatal hypoglycemia is one of the most common metabolic disorders of the newborn and is due to hyperinsulinemia of the fetus in response to maternal hyperglycemia in utero [49]. Fetal hypoglycemia can also lead to more serious complications such as seizures and serious brain injury [50]. Notably, metformin significantly lowered the risk of neonatal hypoglycemia by 44% in our meta-analysis, and it may reduce the risk of neonatal brain injury. The use of metformin may not harm the fetus during pregnancy and may be safer in the neonatal period with potentially beneficial effects.
A major strength of our meta-analysis is our provision of a complete overview of the effect of maternal metformin exposure on neonatal growth outcomes and neonatal adverse outcomes. We included 24 studies, which is a higher number than included in previous analyses; additionally, all of these studies were RCTs, which greatly reduced the likelihood of recall and selection biases. Moreover, a subgroup analysis by the different daily doses of metformin for treatment of GDM and an investigation of the relationship between the maternal oral dose of metformin and neonatal birth weight were carried out for the first time. Furthermore, we assessed potential publication bias by contour-enhanced funnel plots and Egger’s test, the results of which suggested that our results regarding neonatal outcomes were not affected by publication bias. This increases the confidence in our findings.
Our study has several limitations that merit further discussion. First, the possibility of confounding factors in several studies cannot be completely ruled out. For example, women who had poor glycemic control with metformin and required extra insulin therapy were included in the metformin-treated group in some studies, which might cause selection bias. However, the proportion of metformin-treated women requiring insulin supplementation ranged from 8.6% to 46.8% (average, 16.2%) of the total metformin-treated women. Moreover, these patients used a lower total insulin dose than those treated with insulin alone. Therefore, we believe that such selection bias may not have influenced the overall outcomes of the studies. Second, data on neonatal growth outcomes and neonatal adverse outcomes were unavailable or incompletely reported in most of the included studies, restricting us from performing a more detailed relevant analysis and obtaining more comprehensive results. Finally, although subgroup and sensitivity analyses were performed to explore the potential sources of heterogeneity in neonatal birth weight, the cause of the high heterogeneity remains unclear.
In conclusion, the results of this meta-analysis add to the evidence that metformin may be particularly useful in women with GDM at high risk for neonatal hypoglycemia, women who want to limit maternal and fetal weight gain, or women with an inability to afford or use insulin safely. Metformin can effectively lower neonatal birth weight and the incidences of macrosomia, neonatal hypoglycemia, and NICU admission compared with insulin without an increased risk of neonatal adverse outcomes. Whether the effect of metformin on neonatal birth weight is associated with the oral dose of metformin requires further investigation in large-scale trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Text S1. Database search strategies. (A) PubMed. (B) Ovid Embase. (C) Web of Science. (D) Cochrane Library. (DOCX 21 kb)
Table S1. The Egger’s test of neonatal outcomes in the meta-analysis. (DOCX 11 kb)
Fig. S1. Summary of risk of bias for each included study. +, low risk of bias; ?, unclear risk; -, high risk. (TIF 4812 kb)
Fig. S2. Leave-one-out sensitivity analysis of neonatal birth weight. Data are expressed as mean difference (random-effects model) and 95% CI. (PDF 936 kb)
Fig. S3. Funnel plots to assess publication bias of neonatal outcomes. (A) Neonatal birth weight. (B) Neonatal birth weight after trim-and-fill analysis. (C) Macrosomia. (D) Neonatal hypoglycemia. (E) Neonatal hypoglycemia after trim-and-fill analysis. (F) NICU admission. (G) LGA. (H) SGA. (I) Neonatal birth height. (PDF 873 kb)
Fig. S4. Forest plots for neonatal adverse outcomes. (A) Respiratory distress syndrome. (B) Hyperbilirubinemia. (C) Abnormal pH of umbilical cord. (F) Preterm birth. (PDF 2197 kb)
Fig. S5. Forest plots for neonatal adverse outcomes. (A) Apgar score at 5 minutes. (B) Congenital anomalies. (C) Neonatal death. (D) Neonatal sepsis. (E) Birth trauma. (PDF 2421 kb)
Acknowledgements
We thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Author contributions
BS, JN, and BL: Conception of research protocol, study design, literature review, data extraction and interpretation. BS: Drafting of the manuscript. HL and XML: Manuscript revision and project development. GGJ: Data analysis and quality assessment. All authors have read and approved the manuscript.
Funding
No specific funding was received for this study.
Availability of data and materials
Data will be available upon request of the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Standard Statement
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
All procedures in this study were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
As the corresponding author, I confirm on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Footnotes
This article belongs to the topical collection Pregnancy and Diabetes, managed by Antonio Secchi and Marina Scavini.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Sheng, Email: shengbo119@126.com.
Juan Ni, Email: nijuankiki@163.com.
Bin Lv, Email: hxlvbin@163.com.
Guoguo Jiang, Email: 89983314@qq.com.
Xuemei Lin, Email: xuemeilinscu@163.com.
Hao Li, Email: lihao0510@163.com.
References
- 1.Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev. 2009;3:1–57. doi: 10.1002/14651858.CD003395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canningham FG, Leveno KJ, Bloom SL, et al. Williams obstetrics. 25. New York: McGraw-Hill; 2019. pp. 1104–1121. [Google Scholar]
- 3.Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94(3):322–332. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butalia S, Gutierrez L, Lodha A, et al. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabet Med. 2017;34(1):27–36. doi: 10.1111/dme.13150. [DOI] [PubMed] [Google Scholar]
- 6.Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2014;9(3):e92485. doi: 10.1371/journal.pone.0092485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao LX, Shi WT, Han YX. Metformin versus insulin for gestational diabetes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34(16):2741–2753. doi: 10.1080/14767058.2019.1670804. [DOI] [PubMed] [Google Scholar]
- 8.Society of Maternal-Fetal Medicine Publications Committee (2018) SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 218(5): B2–B4 [DOI] [PubMed]
- 9.Feig DS, Berger H, Donovan L, et al. Diabetes canada clinical practice guidelines expert committee. Diabetes Pregnancy Can J Diabetes. 2018;42:S255–S282. doi: 10.1016/j.jcjd.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU)—body composition at 2 years of age. Diabetes Care. 2011;34(10):2279–2284. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open. 2018;6(1):e000456. doi: 10.1136/bmjdrc-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3 rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team (2018) R: a language and environment for statistical computing. Version 3.5.1. Vienna, R Foundation for Statistical Computing.
- 15.Ahmed AKH, Galal SKH, Abdelaziz EA. Metformin versus insulin in gestational diabetes. Med J Cairo Univ. 2021;89(6):2525–2532. [Google Scholar]
- 16.Arshad R, Khanam S, Shaikh F, et al. Feto-maternal outcomes and glycemic control in metformin versus insulin treated gestational diabetics. Pak J Med Sci. 2017;33(5):1182–1187. doi: 10.12669/pjms.335.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainuddin J, Karim N, Hasan AA, et al. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract. 2015;107(2):290–299. doi: 10.1016/j.diabres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Ashoush S, El-Said M, Fathi H, et al. Identification of metformin poor responders, requiring supplemental insulin, during randomization of metformin versus insulin for the control of gestational diabetes mellitus. J Obstet Gynaecol Res. 2016;42(6):640–647. doi: 10.1111/jog.12950. [DOI] [PubMed] [Google Scholar]
- 19.Barrett HL, Gatford KL, Houda CM, et al. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment. Diabetes Care. 2013;36(3):529–536. doi: 10.2337/dc12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleh HS, Abdelsalam WA, Mowafy HE, et al. Could metformin manage gestational diabetes mellitus instead of insulin? Int J Reprod Med. 2016;2016:3480629. doi: 10.1155/2016/3480629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamadani A, Zahid S, Butt ZB. Metformin versus insulin treatment in gestational diabetes in pregnancy and their effects on neonatal birthweight. Pak J Med Sci. 2017;11:914–916. [Google Scholar]
- 22.Eid SR, Moustafa RSI, Salah MM, et al. Is metformin a viable alternative to insulin in the treatment of gesta- tional diabetes mellitus (GDM)? Comparison of maternal and neonatal outcomes. Egypt Pediatr Assoc Gaz. 2018;66(1):15–21. [Google Scholar]
- 23.Ghomian N, Vahed SHM, Firouz S, et al. The efficacy of metformin compared with insulin in regulating blood glucose levels during gestational diabetes mellitus: a randomized clinical trial. J Cell Physiol. 2019;234(4):4695–4701. doi: 10.1002/jcp.27238. [DOI] [PubMed] [Google Scholar]
- 24.Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 25.Moore LE, Briery CM, Clokey D, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J RAeprod Med. 2007;52(11):1011–1015. [PubMed] [Google Scholar]
- 26.Ijäs H, Vääräsmäki M, Morin-Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG. 2011;118(7):880–885. doi: 10.1111/j.1471-0528.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- 27.Hassan JA, Karim N, Sheikh Z. Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci. 2012;28(3):384–389. [Google Scholar]
- 28.Huhtala MS, Tertti K, Juhila J, et al. Metformin and insulin treatment of gestational diabetes: effects on inflammatory markers and IGF-binding protein-1-secondary analysis of a randomized controlled trial. BMC Pregnancy Childbirth. 2020;20(1):401. doi: 10.1186/s12884-020-03077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picón-César MJ, Molina-Vega M, Suárez-Arana M, et al. Metformin for gestational diabetes study: metformin vs insulin in gestational diabetes: glycemic control and obstetrical and perinatal outcomes: randomized prospective trial. Am J Obstet Gynecol. 2021;225(5):e1–e517. doi: 10.1016/j.ajog.2021.04.229. [DOI] [PubMed] [Google Scholar]
- 30.Jahanshahi M, Shahmirzadi AR, Kashani E, et al. Effects of metformin and insulin therapy regimens on postpartum oral glucose tolerance test results in pregnant women with gestational diabetes mellitus: a comparative study. Horm Mol Biol Clin Investig. 2020;41(4):2020018. doi: 10.1515/hmbci-2020-0018. [DOI] [PubMed] [Google Scholar]
- 31.Niromanesh S, Alavi A, Sharbaf FR, et al. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract. 2012;98(3):422–429. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Mesdaghinia E, Samimi M, Homaei Z, et al. Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med. 2013;4(3):327–333. [PMC free article] [PubMed] [Google Scholar]
- 33.Spaulonci CP, Bernardes LS, Trindade TC, et al. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209(1):e1–e7. doi: 10.1016/j.ajog.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Tertti K, Ekblad U, Koskinen P, et al. Metformin vs. insulin in gestational diabetes: A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab. 2013;15(3):246–251. doi: 10.1111/dom.12017. [DOI] [PubMed] [Google Scholar]
- 35.Ruholamin S, Eshaghian S, Allame Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: a randomized clinical trial. J Res Med Sci. 2014;19(10):970–975. [PMC free article] [PubMed] [Google Scholar]
- 36.Somani PS, Sahana PK, Chaudhuri P, et al. Treatment of gestational diabetes mellitus: insulin or metformin? J Evolution Med Dent. 2016;5(63):4423–4429. [Google Scholar]
- 37.Gamal HE, Elaleem MA, Sadek S, et al. Insulin versus metformin in treatment of gestational diabetes mellitus (randomized controlled clinical trial) Egypt J Hosp Med. 2018;72(1):3753–3761. [Google Scholar]
- 38.Wasim T, Shaukat S, Javaid L, et al. Comparison of metformin and insulin for management of gestational diabetes mellitus: a randomized control trial. Pak J Med Health Sci. 2019;13(3):823–827. [Google Scholar]
- 39.Yogev Y, Langer O. Pregnancy outcome in obese and morbidly obese gestational diabetic women. Eur J Obstet Gynecol Reprod Biol. 2008;137(1):21–26. doi: 10.1016/j.ejogrb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(2):14–20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- 41.Hui F, Zhang Y, Ren T, et al. Role of metformin in overweight and obese people without diabetes: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2019;75(4):437–450. doi: 10.1007/s00228-018-2593-3. [DOI] [PubMed] [Google Scholar]
- 42.Tarry-Adkins JL, Aiken CE, Ozanne SE. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: a systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002848. doi: 10.1371/journal.pmed.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee N, Hebert MF, Prasad B, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos. 2013;41(12):2225–2232. doi: 10.1124/dmd.113.054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(3):607–614. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463–471. doi: 10.1016/j.cmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 47.Jansson N, Rosario FJ, Gaccioli F, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feig DS, Murphy K, Asztalos E, et al. MiTy Collaborative Group. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicenter randomized controlled trial. BMC Pregnancy Childbirth. 2016;16(1):173. doi: 10.1186/s12884-016-0954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kole MB, Ayala NK, Clark MA, et al. Factors associated with hypoglycemia among neonates born to mothers with gestational diabetes mellitus. Diabetes Care. 2020;43(12):e194–e195. doi: 10.2337/dc20-1261. [DOI] [PubMed] [Google Scholar]
- 50.Harding JE, Harris DL, Hegarty JE, et al. An emerging evidence base for the management of neonatal hypoglycemia. Early Hum Dev. 2017;104:51–56. doi: 10.1016/j.earlhumdev.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104(3):353–357. doi: 10.1016/j.diabres.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 52.Jiang YF, Chen XY, Ding T, et al. Comparative efficacy and safety of OADs in management of GDM: network meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2015;100(5):2071–2080. doi: 10.1210/jc.2014-4403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Database search strategies. (A) PubMed. (B) Ovid Embase. (C) Web of Science. (D) Cochrane Library. (DOCX 21 kb)
Table S1. The Egger’s test of neonatal outcomes in the meta-analysis. (DOCX 11 kb)
Fig. S1. Summary of risk of bias for each included study. +, low risk of bias; ?, unclear risk; -, high risk. (TIF 4812 kb)
Fig. S2. Leave-one-out sensitivity analysis of neonatal birth weight. Data are expressed as mean difference (random-effects model) and 95% CI. (PDF 936 kb)
Fig. S3. Funnel plots to assess publication bias of neonatal outcomes. (A) Neonatal birth weight. (B) Neonatal birth weight after trim-and-fill analysis. (C) Macrosomia. (D) Neonatal hypoglycemia. (E) Neonatal hypoglycemia after trim-and-fill analysis. (F) NICU admission. (G) LGA. (H) SGA. (I) Neonatal birth height. (PDF 873 kb)
Fig. S4. Forest plots for neonatal adverse outcomes. (A) Respiratory distress syndrome. (B) Hyperbilirubinemia. (C) Abnormal pH of umbilical cord. (F) Preterm birth. (PDF 2197 kb)
Fig. S5. Forest plots for neonatal adverse outcomes. (A) Apgar score at 5 minutes. (B) Congenital anomalies. (C) Neonatal death. (D) Neonatal sepsis. (E) Birth trauma. (PDF 2421 kb)
Data Availability Statement
Data will be available upon request of the corresponding author.