Synopsis
Cardiovascular complications associated with SARS-CoV-2 infection are common and lead to high mortality in the acute phase and high morbidity in the chronic phase impacting an individual's quality of life and health outcomes. Patients afflicted with COVID-19 infection display an increased risk for myocarditis, dysrhythmia, pericarditis, ischemic heart disease, heart failure, and thromboembolism. Although cardiovascular complications are reported across all patients with COVID-19, hospitalized patients with severe infection are most vulnerable. The underline pathobiology remains poorly defined albeit complex. Following current guidelines in decision-making for evaluation and management in addition to the beginning or returning exercise is recommended.
Keywords: COVID-19, Cardiovascular complications COVID-19, Post-acute sequelae of SARS-CoV-2 infection, Cardiac, myocarditis, dysrhythmia, heart failure
Key Points
-
•
Cardiovascular complications are a common manifestation of acute and post-acute COVID-19 infection.
-
•
Complications include cardiomyopathy, myocardial infarction, arrhythmias, heart failure, and deep venous thrombosis.
-
•
Pathophysiology remains poorly defined and complex.
-
•
No single study has proven a distinct treatment for post COVID-19 associated cardiovascular disease; therefore, it is recommended to follow established guidelines for treating specific cardiovascular conditions.
-
•
Cardiac rehabilitation program is advised for individuals who meet the established criteria for cardiac rehabilitation.
Introduction
In the initial three years of the pandemic, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, has infected more than 600 million individuals causing over 6 million deaths.1 COVID-19 can target multiple systems in the body, including the cardiovascular (CV) system. Multisystemic involvement can be present during the acute phase of the infection and may continue or develop even long-term. This extended presentation of COVID-19 is referred to long COVID, long-haul COVID, and Post-acute sequela of SARS-CoV-2 infection (PASC).2 CV manifestations associated with COVID-19 are diverse. The spectrum includes symptoms ranging from dyspnea, impaired activity tolerance, palpitations, and chest pain, to more complex syndromes such as cardiomyopathy, myocardial infarction, arrhythmias, heart failure, and deep venous thrombosis.3 The global burden from CVD include nearly 18 million death. 4 Due to the COVID-19 pandemic, this number is to growth, aggravating heart disease's incidence, prevalence, and economic load. The epidemiology of CVD in the setting of prevalent COVID-19 is an area of active investigation, with a particular focus on establishing the paradigm of disease course and its impact on CV health outcomes.
Background And Discussion
Acute cardiovascular manifestations of COVID-19
Acute myocardial injury, defined by an elevation of serum cardiac troponins in the absence of electrocardiographic or echocardiographic evidence of acute ischemia, has been described as the most common and early cardiac abnormality during the acute phase of COVID-19. Initial studies at the pandemic's beginning reported incidences ranging from 1%-100%.5 More recent studies have placed the incidence of acute myocardial injury in the setting of COVID-19 at ∼31%.6 The reported differences may reflect the significant heterogeneity of the definitions used and the population studied. Despite the difference in reported incidence, all studies showed that levels of cardiac troponin in COVID-19 correlate with clinical disease severity, inflammatory and prothrombotic markers, older age, and the presence of CV risk factors. Moreover, elevated troponin levels were found to be associated with increased in-hospital mortality and arrhythmias.5 , 6
Mechanisms of cardiac injury during the acute phase of COVID-19 include direct and indirect routes. The unique affinity of SARS-CoV-2 for the host angiotensin-converting enzyme 2 (ACE2) receptor raises the possibility of direct viral infection of the vascular endothelium and myocardium. 7 SARS-CoV-2 viral particles have been identified in cardiomyocytes obtained from heart biopsies, 1 supporting the notion of virus invasion. 8 In addition, SARS-CoV-2 can invade endothelial cells and cause endotheliitis. Furthermore, the cytokine storm triggered by the immune response against SARS-CoV-2 might also cause direct cardiac injury. In this regard, a multicenter pathology study found inflammatory cardiac changes, including interstitial macrophage infiltration affecting 86% of the studied patients. 9
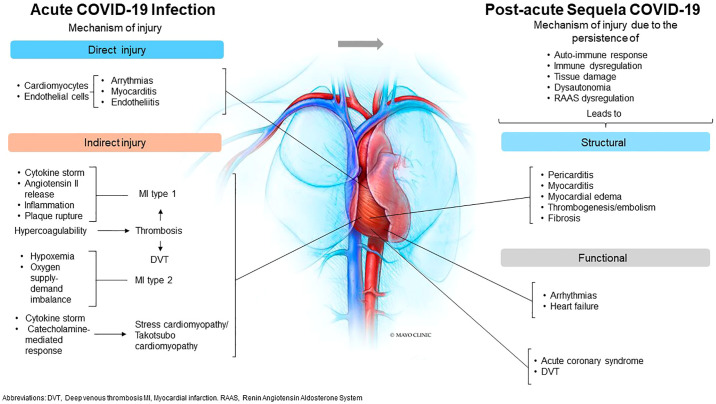
Several lines of evidence also support an indirect mechanism for cardiac injury. Namely, respiratory failure may induce hypoxic injury with an acute imbalance in myocardial oxygen supply without atherothrombosis (type 2 myocardial infarction). 10 In addition, overwhelming cytokine release and excessive Angiotensin II activity may lead to endothelial activation, increased endothelial permeability, vascular inflammation, and inflammation-induced hypercoagulability with intracoronary thrombosis (type I myocardial infarction). 11 In fact, histopathologic and immunohistochemical analysis of COVID-19 cases have shown cardiac fibrin microthrombi and megakaryocytes within the cardiac microvasculature, suggesting that thrombosis may play an important role in myocardial injury during the disease.11 , 12 In addition, atherosclerotic plaque instability and rupture, increased metabolic demand, and reduced cardiac reserve with myocardial dysfunction has also been described as potential mechanisms of cardiac injury.13 (See Figure 1 )
Figure 1.
Summary of potential mechanisms involved in the acute and post-acute cardiac complications of COVID-19 infections
During the acute phase of the infection, myocarditis and arrhythmias are associated with direct viral injury of the myocardium. In contrast, indirect injuries associated with exaggerated inflammatory and hypercoagulability state are postulated to be involved in ischemic events. Long-term complications are linked with persistent autoimmune and inflammatory responses leading to structural changes and functional abnormalities.
Central illustration Courtesy of [Mayo Foundation for Medical Education and Research, Rochester, Minnesota]; with permission
The incidence of myocardial infarction in patients with acute COVID-19 infection remains unclear. A precise diagnosis of ST-elevation myocardial infarction (STEMI) is difficult since COVID-19 can cause myocarditis, coronary spasm, and stress cardiomyopathy, all mimicking STEMI. Moreover, during the initial stages of the pandemic, significant concerns regarding the safety of hospital personnel delayed diagnostic and treatment procedures such as percutaneous coronary interventions, limiting an accurate diagnosis of the acute ischemic coronary syndrome.14 It has been reported that approximately 40% of COVID-19 patients with STEMI did not have obstructive coronary disease suggesting oxygen supply-demand imbalance as the pathophysiological mechanism. 14
COVID-19 is a significant risk factor for myocarditis, with risk varying by age group. A comprehensive study using an extensive U.S. hospital-based administrative database of healthcare encounters from >900 hospitals found that among patients with myocarditis ∼42% had a history of COVID-19. 15 Although direct myocardial injury is the most common mechanism of viral myocarditis, the exact mechanisms in SARS-CoV-2 infection remain largely unknown.16 , 17
Cardiac arrhythmias are associated with high morbidity and mortality among patients hospitalized with COVID-19 infection, with only half of them reported surviving hospital discharge. Atrial fibrillation was the most common occurring in approximately 80% of patients with arrhythmias, followed by bradyarrhythmia (∼23%) and ventricular arrhythmias (∼21%).18 Patients with a high burden of comorbidities and preexisting CVD, including congestive heart failure (HF) and coronary artery disease, were more predisposed to develop arrhythmias during COVID-19 infection. 18 Multiple mechanisms have been hypothesized for the development of arrhythmias, including COVID-19-associated electrolyte disturbances such as hypomagnesemia or hypokalemia. Other influencing factors involve systemic inflammation and viral-induced injury in the cardiac conduction system. 18
HF in patients with COVID-19 may be precipitated by acute illness, acute hemodynamic stress (e.g., acute cor pulmonale), or acute myocardial injury.19 Patients with a previous history of HF or preexisting heart disease were at risk of acute decompensation, extended hospital stays, increased risk of mechanical ventilation, and mortality. 20 However, 22 % of patients with a new diagnosis of HF during acute infection did not have history or risk factors for CVD. 20
The presence of Takotsubo cardiomyopathy, a form of non-ischemic, stress-induced cardiomyopathy (also known as Gebrochenes-Herz syndrome, transient apical ballooning syndrome, apical ballooning cardiomyopathy, and broken-heart syndrome),21 has also been demonstrated in COVID-19 patients. It is associated with higher cardiac and inflammatory biomarkers, critical illness, lower ejection fraction, and higher mortality. 22 The mechanism may involve the overwhelming stress caused by the infection with a catecholamine surge, the overactive immune response from cytokine storm, and the development of coronary vasculature dysfunction. 22
Patients with SARS-CoV-2 infection appear to have a high risk for thromboembolic complications of up to 71%.14 Localized clot formation rather than diffuse thrombi is also a unique feature in COVID-19 and is seen primarily in the lung vessels.14 Multiple studies have reported widespread procoagulant/hypercoagulable states, including thrombotic microangiopathy, endothelial dysfunction, bleeding disorder, and thrombosis. The proposed mechanism involves the cytokine storm from excessive inflammation causing endothelial injury and platelet and coagulation factors activation. 23 However, we should remember the traditional Virchow's triad: stasis of flow due to immobilization, intravascular vessel wall damage, and a hypercoagulable state, all present in COVID-19 infection.24
Post-acute cardiovascular manifestations of COVID-19
The post-acute sequelae or long-term CV complications of COVID-19 are defined as persistent or new symptoms 4 or more weeks after the initial infection. Symptoms and signs may include chest pain, palpitations, impaired activity intolerance, dyspnea, fatigue, and orthostatic manifestations. The seriousness of these symptoms can be variable and are present in individuals without a history of CVD (5-29% of patients). 25 , 26 Specific CV syndromes can also be present and are associated with more severe CV complications of COVID-19 and comprise pericardial effusion, cardiac injury leading to myocarditis, myocardial infarction, left or right ventricular dysfunction, arrhythmias, congestive heart failure, and nonspecific findings on imaging.27, 28, 29, 30, 31 In fact, 60% of patients present active myocardial inflammation on cardiac magnetic resonance (CRM).25 , 26
A recent study analyzed a cohort of more than 150,000 individuals with COVID-19 using a national healthcare database from the US Department of Veterans Affairs. 32 Compared with 5.8 million historical and 5.6 million contemporary controls, individuals that experienced COVID-19 infection are at increased risk of ischemic and non-ischemic heart disease, myocarditis, pericarditis, arrhythmias, HF, and thromboembolic events. 32 In this study, CV complications were reported in hospitalized and non-hospitalized patients, although the seriousness of the CVD was directly associated with the severity of the initial COVID-19 infection. 32 Furthermore, CV sequela of COVID-19 was present in all age groups, either sex, races, in patients without a history of CVD, and in younger patients without CVD risk factors including hypertension, obesity, hyperlipidemia, and diabetes.32
Table 1 summarizes CV complications and reported prevalence during the acute and post-acute infection. A significant variability in the frequency of specific symptoms or cardiac syndromes across published studies is present, mainly due to differences in cohort characteristics, study design, and SARS-CoV-2 variants. 27 , 28 , 33
Table 1.
| Acute | Post-acute sequelae |
|---|---|
| Asymptomatic heart disease Myocarditis 1.4-42% Pericarditis 3.2% Stress-induced Cardiomyopathy 2-4% Myocardial Infarction (Type I Type 2) 7-40% Venous thromboembolism 15-21% Arrhythmia 18-44% Atrial Fibrillation/flutter 6.6-80% Tachycardia Bradycardia 12.8 -22.6% Ventricular arrhythmia 20.7% Heart failure 22-33% Left ventricular dysfunction 10-16% Right ventricular dysfunction 16-39% Myocardial injury ∼31%. Cardiogenic shock |
Symptoms |
| Chest Pain 5-20% Palpitations 10-20% Effort intolerance 12-19% Dyspnea 18-62% Fatigue 32-36% Orthostatic intolerance 10% | |
| Cardiovascular Syndromes | |
| Pericardial effusion 1-30% Cardiac injury Myocarditis 4-60% Myocardial Infarction 17-23% Heart Failure 10-52% Left Ventricle dysfunction 3-18% Right Ventricle dysfunction 10-14% Arrhythmias Atrial Fibrillation 18% Ventricular tachycardia/fibrillation 4-6% Pericarditis 14% Venous thromboembolism 15-21% Myocardial edema and myocardial abnormalities on cardiac magnetic resonance (up to 78%) |
Mechanisms for continuing cardiac injury post-acute infection, are likely multifactorial. Long-term functional and structural cardiac damage due to inflammation associated with viral persistence in the heart tissue, immune dysregulation, and the development of autoimmune response to cardiac antigens, have been proposed as potential mechanisms. 34 The perseverant inflammation may cause tissue damage and myocardial fibrosis leading to decreased ventricular compliance, impaired myocardial perfusion, increased myocardial stiffness, reduced contractility, and potential arrhythmias.30 , 33 , 34 Neurophysiological mechanisms, such as central nervous system sensitization, have also been proposed as a potential factor to explain CV symptoms such as fatigue, dyspnea, orthostatic intolerance, and impaired activity tolerance in PASC patients. 35 In addition, it has been shown that PASC patients had a lower peak oxygen uptake (VO2peak) 30% below predicted, suggesting that abnormal ventilation, circulatory limitations, and deconditioning could be responsible for below-normal cardiorespiratory fitness and explaining some of the cardiac symptoms present in those patients. 36
Evaluation And Management
Considering the high prevalence of CV complications in the post-acute phase of COVID-19, it is highly recommended to perform a comprehensive CV screening in individuals with either CVD risk factors or CV complications during the acute phase. A comprehensive approach for management and follow-up care of CVD associated with COVID-19 has been presented. 33 In brief, the initial evaluation of the patient should include:
-
•
A comprehensive review of COVID-19 past medical history.
-
•
A thorough assessment of lingering symptoms.
-
•
Assessment of mental, physical, and cognitive health.
-
•
Review of risk factors for CVD (obesity, hyperglycemia/diabetes mellitus, hyperlipidemia, tobacco use, sleep apnea, sedentarism, poor nutrition, and stress).
-
•
Assessment of previous tests, cardiology evaluations, and current CV history.
-
•
Evaluation of current medications focusing on those prescribed for CV conditions and medications with potential CV effect (beta-blockers, calcium channel blockers, ACE inhibitors, anti-arrhythmic agents, anticoagulants, antiplatelet agents, antihistaminics, cold decongestant medications).
A complete CV system examination and laboratory and cardiac tests should be requested to diagnose, treat, and exclude serious pulmonary, thromboembolic, and cardiac complications. These include complete blood count, electrolytes, troponin level, brain natriuretic peptide, D-dimer, C-reactive protein, sedimentation rate, lipid panel, liver function tests, glucose, creatinine, ECG, echocardiogram, functional capacity testing (stress testing, echo stress test, 6-min walking test). Ambulatory cardiac monitoring (Holter, event monitor) should be requested when palpitations, orthostatic intolerance, and arrhythmias are present. Additional testing is recommended if significant abnormalities are found, including CRM and cardiac computed tomography angiography (CTA). Invasive coronary angiography may be indicated for high-risk individuals. Referral to specialist clinics, such as arrhythmia clinic and autonomic dysfunction clinic, is highly recommended. In addition, referral to appropriate services such as physical and occupational therapy, psychology, and social services will provide the necessary support to alleviate the overall burden of the disease. 2 , 33 , 34
In the absence of a distinct proven treatment for CV complications associated with post-acute COVID-19, controlling CVD risk factors, and managing specific CV disorders using current guidelines are recommended. 34 , 36 Management of viral myocarditis and stress cardiomyopathy should be based on therapy for ventricular dysfunction and arrhythmic risk. Maintaining adequate heart rate control is the preferred strategy when managing atrial fibrillation.34 , 36 Primary percutaneous interventions (PCI) remained the standard of care for STEMI. Guideline-directed medical therapy for long-term care should be administrated, including beta blockers, antiplatelet agents, nitrates, statin, and heparin therapy.37
Exercise interventions
Currently, no evidence-based guidelines for returning or starting an exercise program for patients with CV symptoms associated with COVID-19 have been established. In this regard, NIH recommendations are valuable in guiding the acute and subacute management of activities and exercises in the setting of COVID-19. 38 In brief, NIH guidelines recommend the following:
-
•
Patients with asymptomatic or mild illness should not exercise for ten days after symptoms onset or positive test.
-
•
Patients with moderate disease (O2 saturations >94% at room air) should not exercise for ten days after the resolution of symptoms.
-
•
Patients with severe illness (O2 saturations <94% and respiratory rate > 30 breaths/min on room air) requiring hospitalization or supplemental oxygen should refrain from exercises for at least 14 days after resolution of the initial COVID-19 infection symptoms, with a physician or advance practitioner assessing patient symptoms prior to starting an exercise program.
-
•
Patients with myocardial injury, acute coronary syndrome, and arrhythmias require cardiology clearance before exercising and referral to cardiac rehabilitation (CR).
In addition to the NIH recommendations, patients with COVID-19 related myocarditis should not start any CR or exercise program for 3-6 months. In this group of patients, if the left ventricular ejection fraction (LVEF) is within normal and no arrhythmias are present, a lighter exercise program can be started three months post-infection. However, if the patient displays a low LVEF, they should be reevaluated six months before any exercise activity decision.36 , 39
Growing clinical experience recommended that eligible individuals who meet the established criteria for CR should be referred to specialized CR programs. 40 Qualified patients include those with evidence of an acute coronary event in the setting of COVID-19 with or without coronary intervention, new myocardial dysfunction or valve disease that required surgical intervention, worsening HF due to COVID-19, and heart transplant following COVID-19.40 The multidisciplinary and comprehensive approach to CR provided the desirable environment to support individuals with CV complications associated with COVID-19. Rehabilitation interventions for individuals with PASC appear to improve certain clinical outcomes, including quality of life, functional exercise capacity, and dyspnea.41
In parallel, exercise recommendations for patients with PASC have been developed based on the comprehensive perspective from multidisciplinary experts in Physical Medicine and Rehabilitation, CR, Cardiology, Internal Medicine, and Pulmonary and Critical Care. 2 , 36 , 39 A complete exercise recommendations and prescription are described in Box 1 .
Box 1. Suggestions for Exercise Prescription.
-
1.
Patients with PASC are diverse in terms of symptom severity, exercise capacity, and perceived functional decline relative to pre-illness abilities. Exercise program individualization is a must.
-
2.
Assessment of exercise capacity with either CPX or the 6-min walk test is advised.
-
3.
Patients with significant functional limitations benefit from physical and/or occupational therapy before beginning a formal exercise program.
-
4.
Start with a small dose of exercise with a gradual progression of duration and intensity.
-
5.
Stop exercise if clinically significant symptoms occur, such as chest pain, palpitations, excessive HR, severe dyspnea, lightheadedness/dizziness, pre-syncope/syncope, excessive fatigue, peripheral edema, headache, and tunnel vision.
-
6.
Participation in supervised exercise in a cardiopulmonary rehabilitation program (center-based, home-based, or hybrid approach) is highly desirable. However, reimbursement may not be available, and some rehabilitation staff may not be adequately prepared to treat patients with PASC.
-
7.Aerobic exercise prescription specifics based on the acronym FITT-P (frequency, intensity, type, time, progression) are as follows:
-
1.Frequency: daily; consider multiple brief (<10 min) daily sessions.
-
2.Intensity: begin with lower intensity (Borg perceived exertion ratings [RPE] of 10-11 on the 6- to 20-point scale, HR <60% of maximum).
-
3.Type: consider starting with non-weight-bearing modes of exercise for individuals with limited capacities and/or orthostatic symptoms: recumbent stationary cycle, recumbent stepper, rowing ergometer.
-
4.Time: conservative duration at first: >5-10 min.
-
5.Progression: Time: increase by 1-3 min/session, as tolerated with a goal duration of 30-45 min; intensity: gradually progress to moderate intensity (RPE 12-13, HR >80% of maximum); and further progress to moderate to higher intensity (RPE 14-17, HR >80% of maximum), as tolerated.
-
1.
-
8.Resistance exercise prescription specifics based on the FITT-Pacronym:
-
1.Frequency: 2-3 sessions/wk on nonconsecutive days.
-
2.Intensity: perform 10-15 slow repetitions of each exercise, RPE 11-13 or 40-60% of one repetition maximum.
-
3.Time: one to three sets of 8-10 exercises for the major muscle groups; initial focus should be on the lower extremities and body core for patients with POTS-like symptoms. For these patients, gradually add other exercises for the major muscle groups.
-
4.Type: elastic bands, hand weights, body weight, and weight training machines.
-
5.Progression: gradual progression of repetitions and resistance, as tolerated.
-
1.
Abbreviations: CPX, cardiopulmonary exercise testing; HR, heart rate; PASC, post-acute sequelae COVID-19 syndrome; POTS, postural orthostatic tachycardia syndrome.
From: [ Smer A, Squires RW, Bonikowske AR, Allison TG, Mainville RN, Williams MA. Cardiac Complications of COVID-19 Infection and the Role of Physical Activity. J Cardiopulm Rehabil Prev. Jul 15 2022;doi:10.1097/hcr.0000000000000701] with permission
Summary
CV complications are a common manifestation of acute and PASC infection. They span several CV disorders, including myocardial injury, acute coronary syndromes, myocarditis, cardiomyopathy, arrhythmias, HF, and deep venous thrombosis. The risk and burden of CVD among those who survive the disease are substantial. Studies are necessary to elucidate the interaction between SARS-CoV-2 and the CV system and the biological processes that result in cardiac dysfunction. Evidence for the treatment of CV complications associated with SARS-CoV-2 is lacking and CV manifestations are currently managed following established guidelines developed prior COVID -19 pandemia. Understanding and refining diagnosis, treatment, and exercise interventions are imperative to prevent and manage CV sequela and the progression of CVD in high-risk patients in the context of a lengthy pandemic.
Clinics Care Points
-
•
At present, no evidence-based guidelines for the management of post-acute cardiovascular complications associated with COVID-19 are available.
-
•
Accordingly, implementation of established guidelines for the treatment of specific cardiovascular conditions is recommended.
-
•
Patients who meet the established criteria for cardiac rehabilitation, should be referred to specialized programs. Others may benefit from an individualized and gradually progressive exercise program as recommended by a multidisciplinary panel of experts.
Footnotes
Betsy Medina-Inojosa, M.D. Email: medinainojosa.betsy@mayo.edu. Mayo Clinic,200 First Street SW, Rochester, MN 55905
Disclosure: All authors declare no conflict of interest.
References
- 1.Bearse M., Hung Y.P., Krauson A.J., et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34(7):1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteson J.H., Azola A., Barry J.T., et al. Multi‐disciplinary collaborative consensus guidance statement on the assessment and treatment of cardiovascular complications in patients with post‐acute sequelae of SARS‐CoV‐2 infection (PASC) Pm & R. 2022;14(7):855. doi: 10.1002/pmrj.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., Liu F., Wang L. COVID-19 and cardiovascular diseases. Journal of molecular cell biology. 2021;13(3):161–167. doi: 10.1093/jmcb/mjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. Journal of the American college of cardiology. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smilowitz N.R., Jethani N., Chen J., et al. Myocardial injury in adults hospitalized with COVID-19. Circulation. 2020;142(24):2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe A.S., Cleland J.G., Katus H.A. Myocardial injury in severe COVID-19 infection. European heart journal. 2020;41(22):2080–2082. doi: 10.1093/eurheartj/ehaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weckbach L.T., Curta A., Bieber S., et al. Myocardial Inflammation and Dysfunction in COVID-19–Associated Myocardial Injury. Circulation: Cardiovascular Imaging. 2021;14(1) doi: 10.1161/CIRCIMAGING.120.011713. [DOI] [PubMed] [Google Scholar]
- 9.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. European Heart Journal. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation. 2021;143(10):1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 12.Bois M.C., Boire N.A., Layman A.J., et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation. 2021;143(3):230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giustino G., Pinney S.P., Lala A., et al. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J Am Coll Cardiol. 2020;76(17):2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehmer T.K., Kompaniyets L., Lavery A.M., et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbidity and Mortality Weekly Report. 2021;70(35):1228. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escher F., Pietsch H., Aleshcheva G., et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27(1):251–261. doi: 10.1007/s10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coromilas E.J., Kochav S., Goldenthal I., et al. Worldwide survey of COVID-19–associated arrhythmias. Circulation: Arrhythmia and Electrophysiology. 2021;14(3) doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bader F., Manla Y., Atallah B., Starling R.C. Heart failure and COVID-19. Heart failure reviews. 2021;26(1):1–10. doi: 10.1007/s10741-020-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Garcia J., Jaladanki S., Rivas-Lasarte M., et al. New heart failure diagnoses among patients hospitalized for COVID-19. Journal of the American College of Cardiology. 2021;77(17):2260–2262. doi: 10.1016/j.jacc.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templin C., Ghadri J.R., Diekmann J., et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. New England Journal of Medicine. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 22.Techasatian W., Nishimura Y., Nagamine T., et al. Characteristics of Takotsubo cardiomyopathy in patients with COVID-19: Systematic scoping review. American heart journal plus: cardiology research and practice. 2022 doi: 10.1016/j.ahjo.2022.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acharya Y., Alameer A., Calpin G., Alkhattab M., Sultan S. A comprehensive review of vascular complications in COVID-19. Journal of Thrombosis and Thrombolysis. 2021:1–8. doi: 10.1007/s11239-021-02593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acanfora D., Acanfora C., Ciccone M.M., et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses. 2021;13(10):1904. doi: 10.3390/v13101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang W.-T., Toh H.S., Liao C.-T., Yu W.-L. Cardiac involvement of COVID-19: a comprehensive review. The American Journal of the Medical Sciences. 2021;361(1):14–22. doi: 10.1016/j.amjms.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkodaymi M.S., Omrani O.A., Fawzy N.A., et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clinical Microbiology and Infection. 2022;28(5):657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groff D, Sun A, Ssentongo AE, et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Network Open. 2021;4(10):e2128568-e2128568. [DOI] [PMC free article] [PubMed]
- 29.Satterfield B.A., Bhatt D.L., Gersh B.J. Cardiac involvement in the long-term implications of COVID-19. Nature Reviews Cardiology. 2022;19(5):332–341. doi: 10.1038/s41569-021-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavelle M.P., Desai A.D., Wan E.Y. Arrhythmias in the COVID-19 patient. Heart Rhythm O2. 2022;3(1):8–14. doi: 10.1016/j.hroo.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghantous E., Szekely Y., Lichter Y., et al. Pericardial Involvement in Patients Hospitalized With COVID‐19: Prevalence, Associates, and Clinical Implications. Journal of the American Heart Association. 2022;11(7) doi: 10.1161/JAHA.121.024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nature medicine. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. European heart journal. 2022;43(11):1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. bmj. 2021:372. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 35.Goudman L., De Smedt A., Noppen M., Moens M. Is Central Sensitisation the Missing Link of Persisting Symptoms after COVID-19 Infection? Journal of clinical medicine. 2021;10(23):5594. doi: 10.3390/jcm10235594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smer A., Squires R.W., Bonikowske A.R., Allison T.G., Mainville R.N., Williams M.A. Cardiac Complications of COVID-19 Infection and the Role of Physical Activity. J Cardiopulm Rehabil Prev. 2022 doi: 10.1097/HCR.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. Circulation. 2014;130(25):e344-e426. [DOI] [PubMed]
- 38.Panel C-TG. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Published 2022. Accessed November 28, 2022. [PubMed]
- 39.Vanichkachorn G., Newcomb R., Cowl C.T., et al. Post-COVID-19 Syndrome (Long Haul Syndrome): Description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin Proc. 2021;96(7):1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor R.S., Dalal H.M., McDonagh S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. 2022;19(3):180–194. doi: 10.1038/s41569-021-00611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fugazzaro S., Contri A., Esseroukh O., et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int J Environ Res Public Health. 2022;19(9) doi: 10.3390/ijerph19095185. [DOI] [PMC free article] [PubMed] [Google Scholar]