Abstract
Purpose
SGA infants with fetal growth restriction have reduced ability to adapt themselves to the postnatal life because of certain epigenetic changes in cardiac function. The aim of the present study is to assess and compare the cardiac functions of fetal growth restricted SGA newborns to the term stable AGA newborns, and evaluate any differences in the cardiac functions during the postnatal transitional circulation.
Method
This observational study was conducted at a multispecialty tertiary care hospital in Western India from June to November 2021. The newborns were evaluated using bedside echocardiography at 24–48 h and repeat screening after 48 h. The echocardiographic assessment of the systolic function was done using EF, FS, FAC and TAPSE; diastolic function using E/A wave ratio and global functioning using LV MPI.
Result
Twnety-four babies were included in cases and 30 in the control arm of the study. Maternal and newborn characteristics were comparable between the two groups. FS, EF for left ventricle and TAPSE, FAC for right ventricular systolic function were significantly lower in SGA group (p = 0.02, 0.02, 0.00 and 0.01; respectively). The current study revealed a lower tricuspid E/A ratio and higher mitral E/A ratio with a significant difference beyond 48 h in the first week of life (p value 0.00). Left ventricular MPI was significantly higher in SGA infants compared to AGA infants during two subsequent readings in immediate newborn period with p values 0.01 and 0.02 respectively. The subgroup analysis revealed that fetal growth-restricted neonates with absent end-diastolic flow had a greater impact on ventricular functions.
Conclusion
Present study showed a significant systolic and diastolic dysfunction during initial newborn period in growth restricted SGA infants.
Keywords: EF Ejection fraction, FS Fractional shortening, FAC Fractional area change, TAPSE tricuspid annular plane systolic excursion, MPI Myocardial performance Index, AGA appropriate for gestational age, SGA small for gestational age
Introduction
The normal transition of the fetus to the neonate involves significant hemodynamic changes followed by improved oxygenation and systemic blood flow delivering oxygen to the target organs as required after cessation of placental circulation. Clinical assessment of hemodynamic well-being of the newborn involves assessment of capillary filling time, mean blood pressure, peripheral pulses, urine output, serum lactate measurement and core to peripheral temperature difference. Although the above clinical parameters hold paramount importance on the bedside, but sadly none of these parameters evaluate the hemodynamics directly. In fact, dependence on only the bedside parameters and clinical judgement not only leads to subjective variation in clinician’s assessment but sometimes may delay the important early recognition and assessment of vital pathological conditions [1–6].
Functional echocardiography and point of care ultrasound give a wholesome hemodynamic status of the newborn by objective assessment of systolic and diastolic function. Various objective parameters on bedside echocardiography include fractional shortening, ejection fraction, tricuspid annular plane systolic excursion (TAPSE), fractional area change, myocardial performance index, and mitral and/or tricuspid E–A wave ratio (E/A ratio) [3, 7–10].
Thus, in addition to the above standard clinical assessment, a bedside functional echocardiography and POCUS [11–15] can help in evaluating hemodynamics objectively. It can support the clinical acumen to make timely and accurate diagnosis and initiate early targeted medical intervention, and hence may help in reducing the long-term morbidity and mortality [11, 16–19].
Small for gestational age (SGA) infants are at a higher risk of neonatal morbidity and mortality. It has been postulated that SGA infants, particularly those with fetal growth restriction, have reduced ability to adapt to postnatal life. The reason lies in the fact that growth restricted fetuses have certain epigenetic changes in cardiac function and adaptation which has consequences in the immediate newborn period and also noted to have future implications on the long term cardiovascular effects. These manifest later in adulthood with increased risk for cardiovascular diseases and this phenomenon is now known as Barker’s hypothesis [20, 21].
Therefore, as compared to appropriate for gestational age (AGA) infants, SGA babies particularly with fetal growth restriction are likely to have certain important hemodynamic differences which increase their propensity for altered risk for morbidity and have future implications as well. The aim of the present study is to assess and compare the cardiac functions in stable growth restricted small for gestational age newborns to the term stable appropriate for gestational age newborns, and evaluate any differences in the cardiac functional during the postnatal transitional circulation.
Methods
This observational study was conducted at a multispecialty tertiary care hospital in Western India between June 2021 and November 2021. The study was approved by the Institutional Ethics Committee. The stable in-born term fetal growth-restricted SGA neonates (≥ 38 weeks gestation and birth weight less than 10th percentile with evidence of fetal growth restriction or ≥ 38 weeks gestation with birth weight less than 3rd centile) were included in the study. While stable term AGA (≥ 38 weeks and birth weight between 10th percentile to 90th percentile) in-born infants were enrolled as the control group. Gestational age was calculated based on the last menstrual period of mothers. Evidence of fetal growth restriction was defined based on the Doppler velocity abnormality. The current definition of Fetal growth restriction is based on the Delphi criteria [22]. Neonates requiring NICU admission and those with syndromic associations were excluded from the study. Informed consents were taken from the parents.
The enrolled newborns were evaluated using bedside echocardiography 24–48 h after birth and subsequently before discharge. Echocardiography assessment was done by a senior neonatal fellow trained in advanced neonatal echocardiography. Echocardiography was performed in a quiet setting with no medications being given for sedation. The first analysis was done 24–48 h after birth and the next analysis was done beyond 48 h, before discharge from the post-natal wards.
Echocardiography was performed using Acuson X 300, Siemens Medical Solutions, Inc. USA machine using a 4–8 MHz transducer and means of two consecutive heart beats were calculated. The echocardiographic assessment was done under three major categories: (1) assessing the systolic function using fractional shortening, ejection fraction, fractional area change, tricuspid annular plane systolic excursion, (2) the diastolic function using E/A wave ratio and (3) assessment of right and left ventricular outputs and left ventricle myocardial performance index using pulse wave Doppler. A subgroup analysis of the fetal growth-restricted neonates according to the type of umbilical artery Doppler changes was done to assess the effect of abnormal Doppler’s on the ventricular functions.
Statistical analysis
The statistical package for social sciences (SPSS for Windows version 22, Chicago, IL, USA) was used for data analysis. Descriptive analysis in the form of means and standard deviations was performed for continuous variables. The comparison between the groups and the subgroup analysis was done using an independent T test. A p value of < 0.05 was considered statistically significant.
Results
During the 6 months of the study period, a total of 71 term SGA infants (born at ≥ 38 weeks of gestation with a birth weight less than 10th centile on Intergrowth-21 I newborn size growth standard charts) were born. Of the 71 babies, 41 were excluded because they did not meet the inclusion criteria. Out of the 30 stable SGA neonates, 6 constitutionally small neonates with birth weight between 10th and 3rd centile with no evidence of fetal growth restriction were excluded from the study. Thus, a total of 24 neonates with fetal growth restriction (FGR-SGA) were included in the study (refer Fig. 1). 30 term stable newborns were included in the control arm of the study.
Fig. 1.
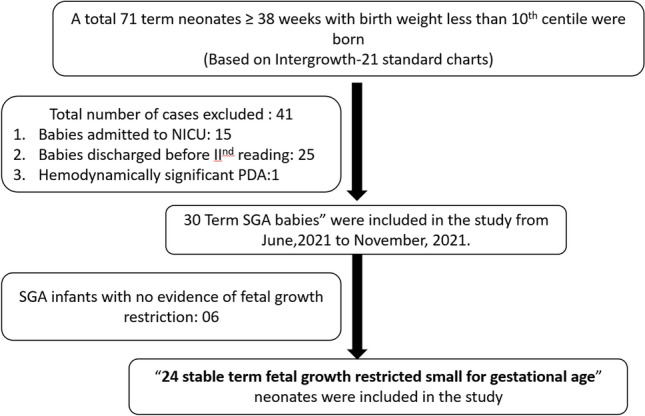
Flow diagram of patients enrolled during the study period
The maternal characteristics of the two groups were comparable (Table 1). The mean gestational age at birth was 38.9 ± 0.87 and 38.37 ± 0.81 weeks in the control and cases cohorts, respectively (p value = 0.02). The majority of the mothers were multigravida in both the groups with a larger number of primigravida in the SGA group (p value 0.13).
Table 1.
A Maternal characteristics of the study population, (B) neonatal Characteristics of the study population
| A | ||||
|---|---|---|---|---|
| Parameters | Term SGA (n = 24) | Term AGA (n = 30) | p value | |
| Gestational age | Mean ± SD | 38.37 ± 0.81 | 38.9 ± 0.87 | 0.02 |
| Parity | ||||
| Primigravida | N (%) | 12 (50%) | 09 (30%) | 0.13 |
| Multigravida | N (%) | 12 (50%) | 21 (70%) | 0.13 |
| USG findings | ||||
| Normal USG | N (%) | None | 30 (100%) | – |
| Fetal growth restriction (< 3rd centile) | ||||
| 11 (45.8%) | 00 (none) | – | ||
| Umbilical artery Doppler abnormal only | 04 (16.6%) | 00 (none) | – | |
| Umbilical artery with MCA doppler abnormal | 09 (37.5%) | 00 (none) | – | |
| Umbilical artery Doppler | ||||
| PI > 95TH Centile | N (%) | 04/13 (30.8%) | NA | – |
| AEDF | 07/13 (53.9%) | NA | ||
| REDF | 02/13 (15.3%) | NA | ||
| Delivery | ||||
| LSCS | N (%) | 17 (70.8%) | 13 (43.4%) | 0.09 |
| Indication for LSCS | ||||
| Elective | N/TOTAL LSCS (%) | None | 04/13 (31%) | – |
| PIH | 09/17 (52.94%) | None | – | |
| Fetal distress | 02/17 (11.76%) | 03/13 (23%) | 0.32 | |
| PROM | 04/17 (23.52%) | 01/13 (7.6%) | 0.08 | |
| (NPL/DTA/CPD) | 02/17 (11.78%) | 05/13 (38.4%) | 0.00 | |
| B | p Value | |||
| Sex | ||||
| Male | N (%) | 10 (41.7%) | 13 (43.3%) | 0.90 |
| Female | 14 (58.3%) | 17 (56.7%) | 0.90 | |
| Birth weight (grams) | Mean ± SD (range) | 2006.45 ± 189 (1520–2300) | 3014.44 ± 313 (2550–3640) | < 0.0001 |
| Length (cm) | Mean ± SD (range) | 44.70 ± 3.10 (40–50) | 47.7 ± 2.00 (43–52) | < 0.0001 |
| BSA (m2) | Mean ± SD (range) | 0.15 (0.13–0.17) | 0.19 (0.18–0.22) | < 0.0001 |
| Resuscitation | ||||
| None | N (%) | 20 (83.3%) | 27 (90%) | 0.47 |
| Physical stimulation | 03 (12.5%) | 03 (10%) | 0.77 | |
| Bag and mask ventilation | 01 (4.2%) | None | – | |
| APGAR score (5 min) | Mean (range) | 8.41 ± 0.82 (6–9) | 8.93 ± 0.25 (8–9) | 0.00 |
| DCC (Yes’%) | % | 79.2% | 93.3% | 0.12 |
| Enrollment (hours of life) | Mean ± SD | 30.70 ± 4.11 h | 32.60 ± 4.22 h | 0.08 |
| Heart rate | Mean ± SD (range) | 136.54 ± 13.66 (115–170) | 133.62 ± 14.55 (124–166) | 0.44 |
PIH pregnancy induced hypertension, PROM premature rupture of membrane, MSL Meconium stained liquor, NPL Non-progress of labor, DTA Deep transverse arrest, CPD Cephalo-pelvic disproportion, IUGR Intrauterine growth restriction, MCA middle cerebral artery, BSA body surface area, DCC delayed cord clamping
Of the total 24 FGR-SGA infants, 70.8% (17/24) were delivered by caesarean section (C-section). The most common indication for the C-section in the FGR-SGA group was pregnancy-induced hypertension (52%) while in the AGA group elective C-section was common. Antenatal USG in the FGR-SGA group was suggestive of fetal growth restriction with estimated fetal weight less than 3rd centile in 11 (45.8%) neonates and umbilical artery doppler was abnormal in 13 neonates (54.2%). Of the 13 cases, 4 neonates had raised pulsatility index greater than 95th centile, 7 cases had absent end-diastolic flow (AEDF) and 2 cases had reverse end-diastolic flow (REDF). 9 (37.5%) neonates with umbilical artery doppler changes had MCA doppler pulsatility index less than 5th centile with reversal of cerebral placental ratio (CPR) (Table 1).
Both the groups had undergone the initial enrollment at similar hours after birth with no significant difference. Similar sex distribution was noted in both groups. AGA neonates had significantly higher birth weight, length and BSA. The majority of the neonates didn’t require any resuscitation in both groups. APGAR scores were comparable among the two groups. The average heart rate between the two groups was similar (Table 1).
Assessment of the systolic function
Fractional shortening (FS) was lower in the FGR-SGA group. The difference in the first readings (at 24–48 h) between the two groups was not significant, however the 2nd reading beyond 48 h of life was significantly lower in FGR-SGA infants compared to AGA infants (p = 0.02). Similarly, the ejection fraction was also lower in the FGR-SGA group, where 2nd evaluation beyond 48 h revealed a significant difference (p value 0.02) (Table 2). Both, the velocity time integral (VTI) and left ventricle output were lower in the FGR-SGA arm, with no significant difference.
Table 2.
Comparison of the systolic function and Left ventricular Myocardial Performance Index
| Parameters | Term SGA (n = 24) Mean ± SD |
Term AGA (n = 30) Mean ± SD |
p value |
|---|---|---|---|
| Fractional shortening (%) | |||
| 1st Reading | 22.12 ± 9.11 | 25.02 ± 5.30 | 0.12 |
| 2nd Reading | 25.58 ± 8.50 | 30.11 ± 5.28 | 0.02 |
| Ejection fraction (%) | |||
| 1st Reading | 57.77 ± 9.90 | 58.39 ± 9.47 | 0.15 |
| 2nd Reading | 61.91 ± 9.42 | 66.89 ± 7.72 | 0.02 |
| Aortic root diameter (mm) | 6.49 ± 0.66 | 7.36 ± 0.94 | 0.00 |
| Left VTI* (cm/systole) | |||
| 1st Reading | 9.9 ± 1.59 | 10.37 ± 1.85 | 0.43 |
| 2nd Reading | 11.52 ± 2.02 | 12.06 ± 3.12 | 0.86 |
| LVO(ml/kg/min)$ | |||
| 1st Reading | 202.45 ± 48.20 | 218.13 ± 45.82 | 0.23 |
| 2nd Reading | 247.7 ± 55.24 | 268.26 ± 52.06 | 0.17 |
| Fractional area change (%) | |||
| 1st reading | 19.34 ± 9.23 | 24.72 ± 8.11 | 0.02 |
| 2nd reading | 21.98 ± 8.74 | 27.07 ± 6.14 | 0.01 |
| TAPSE@ | |||
| 1st reading | 6.59 ± 0.91 | 7.74 ± 1.25 | 0.00 |
| 2nd reading | 7.60 ± 1.03 | 8.51 ± 0.80 | 0.00 |
| Pulmonary valve diameter(mm) | 6.76 ± 0.77 | 8.65 ± 0.85 | 0.00 |
| Right VTI# | |||
| 1st reading | 10.49 ± 1.79 | 11.40 ± 2.92 | 0.13 |
| 2nd reading | 10.60 ± 2.50 | 12.26 ± 1.67 | 0.70 |
| RVO(ml/kg/min)^ | |||
| 1st reading | 245.87 ± 79.40 | 275.76 ± 62.84 | 0.12 |
| 2nd reading | 278.04 ± 72.22 | 324.16 ± 58.81 | 0.02 |
| LV Myocardial Performance Index (PWa Doppler) | |||
| 1st Reading | 0.27 ± 0.05 | 0.23 ± 0.07 | 0.01 |
| 2nd Reading | 0.29 ± 0.07 | 0.24 ± 0.06 | 0.02 |
*Velocity time integral
$Left ventricular output
@Tricuspid annular plane systolic excursion
#Right velocity time integral
^Right ventricular output
aPulse wave
Fractional area change (FAC) was significantly lower in the FGR-SGA cohort as compared to the AGA cohort, and it persisted similarly in subsequent evaluations (p value 0.02, 0.01 respectively) (Table 2). TAPSE values were also significantly lower in the FGR-SGA group as compared to the AGA group at 1st and 2nd evaluations (p = 0.00 and 0.00 respectively) (Table 2) (See Figs. 2, 3).
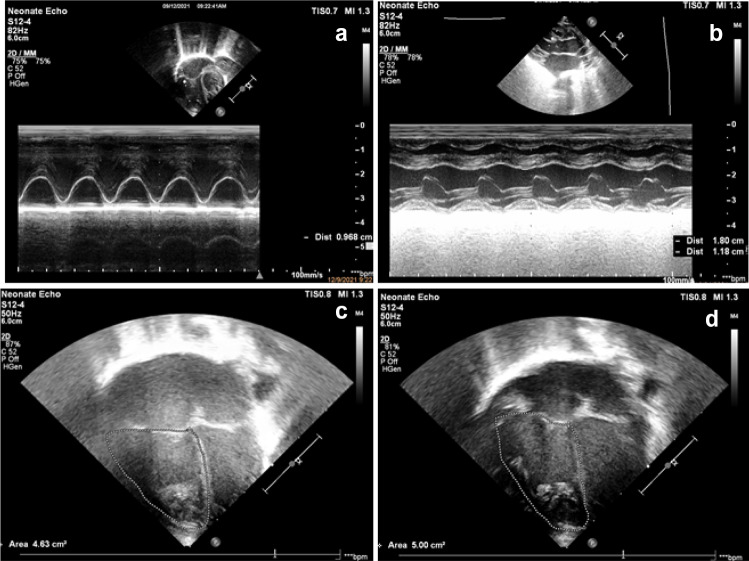
Fig. 2.
Images of systolic functional parameters in echocardiography. a Tricuspid annular plane systolic excursion (TAPSE) shown in M-mode. b Fractional shortening (FS) in PLAX using M-mode (LVEDD: 1.80 cms; LVESD: 1.8 cms). c Fractional area change in 4 chamber view- End-systole area(area: 4.63 cm2). d Fractional area change- End Diastolic area (Area: 5.00 cm2). FS LVEDD-LVESD/LVEDD, FAC EDV-ESV/EDV
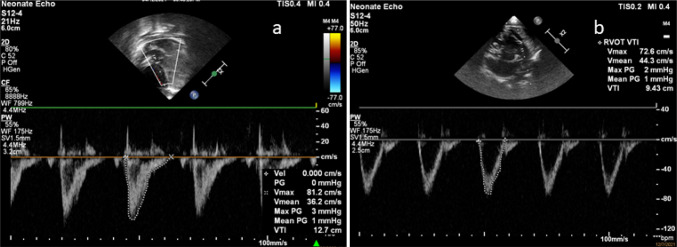
Fig. 3.
Image showing Left ventricular and right ventricular output. a Left ventricular velocity time Integral measured in apical view with LVOT using PW Doppler (VTI): shown in the figure as 12.7 cm. b Right ventricular velocity time Integral measured in short-axis view using PW doppler (VTI): shown in the figure as 9.3 cm. Stroke volume = cross sectional area × velocity time integral
There was no statistically significant difference in the right ventricular output (RVO) or right outflow tract VTI between the two groups on the first assessment. However, in the subsequent assessment, a significantly lower RVO was noted in FGR-SGA infants (p value 0.02).
Myocardial performance index (MPI) measured by pulse wave Doppler
MPI was performed using a pulse wave Doppler for assessing the global dysfunction. The current study included MPI by conventional 2D echocardiography which revealed a significantly higher MPI value in the FGR-SGA cohort (p value of 0.01 and 0.02 on the 1st and 2nd evaluations, respectively) (Table 2).
Assessment of the diastolic function
There was no significant difference in the tricuspid E/A ratio between the 2 cohorts (p value 0.08 on 1st evaluation and 0.41 on the 2nd evaluation). However, the mitral valve E/A wave ratio was significantly higher (p value 0.001) in the FGR-SGA infants as compared to term AGA infants in the 2nd evaluation (Table 3). This was suggestive of mild subclinical left ventricular diastolic dysfunction (See Fig. 4).
Table 3.
Comparison of the diastolic functions
| Parameters | Term SGA (n = 24) Mean ± SD |
Term AGA (n = 30) Mean ± SD |
p value |
|---|---|---|---|
| RV@ E (cm/s) | |||
| 1st Reading | 42.78 ± 9.7 | 46.04 ± 11.60 | 0.73 |
| 2nd Reading | 51.74 ± 8.4 | 52.04 ± 10.93 | 0.91 |
| RV@ A(cm/s) | |||
| 1st Reading | 55.00 ± 8.9 | 54.91 ± 11.63 | 0.56 |
| 2nd reading | 55.32 ± 8.3 | 54.86 ± 11.07 | 0.86 |
| RV@ E/A | |||
| 1st Reading | 0.84 ± 0.12 | 0.84 ± 0.15 | 0.08 |
| 2nd Reading | 0.93 ± 0.06 | 0.94 ± 0.05 | 0.41 |
| LV^E (cm/s) | |||
| 1st Reading | 49.14 ± 8.05 | 49.97 ± 9.60 | 0.73 |
| 2nd Reading | 51.92 ± 8.65 | 53.84 ± 8.41 | 0.41 |
| LV^A (cm/s) | |||
| 1st Reading | 43.81 ± 8.3 | 48.57 ± 11.41 | 0.24 |
| 2nd Reading | 47.17 ± 7.6 | 53.56 ± 9.08 | 0.08 |
| LV^E/A | |||
| 1st Reading | 1.14 ± 0.21 | 1.05 ± 0.20 | 0.12 |
| 2nd Reading | 1.10 ± 0.10 | 1.01 ± 0.07 | 0.00 |
@Right ventricle
^Left ventricle
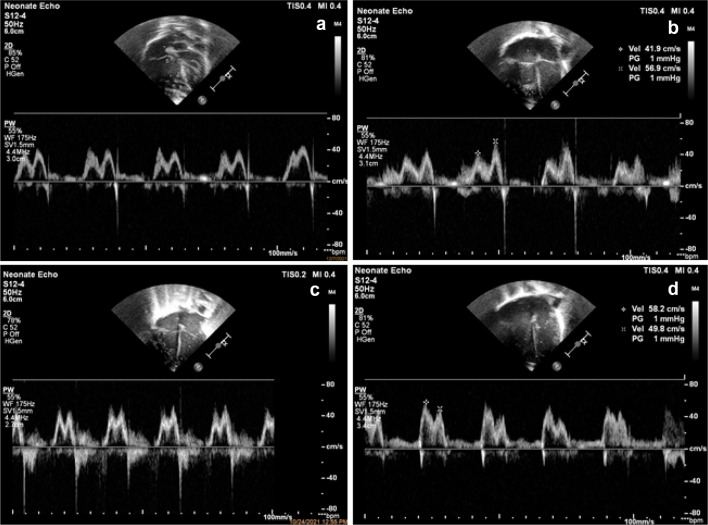
Fig. 4.
Images of diastolic functional parameters in echocardiography. a Apical 4 chamber view PW Doppler across Tricuspid valve showing Normal E/A ratio. b Apical 4 chamber view PW Doppler across Tricuspid valve showing Abnormal E/A ratio. c Apical 4 chamber view PW Doppler across Mitral Valve showing Normal E/A ratio. d Apical 4 chamber view PW doppler across Mitral Valve showing Abnormal E/A ratio
Subgroup analysis based on umbilical artery Doppler waveform
In the current study, neonates born with fetal growth restriction with various umbilical artery doppler changes like raised PI (> 95th centile), AEDF and REDF were compared with each other for the ventricular functions.
FAC and TAPSE values for RV systolic function were lower in neonates with AEDF/REDF as compared to neonates with only raised umbilical artery PI above 95th centile but the difference was not statistically significant in the current study. FS and EF as a marker of LV systolic function were significantly lower in both echocardiographic assessments for AEDF/REDF group. This infers to a more severe left ventricular systolic dysfunction in growth-restricted neonates with AEDF/REDF.
LVO and RVO were also lower in the AEDF/REDF group, however, the difference was not significant. Diastolic dysfunction measured as altered E/A ratio was similar with no significant difference between the two groups. LVMPI was higher in severely growth-restricted fetuses with AEDF/REDF as compared to growth-restricted fetuses with PI > 95th centile (Table 4).
Table 4.
Comparison of ventricular function in FGR SGA based on the type of waveform pattern of Umbilical artery Doppler
| Echocardiography parameters | Reading | Fetal growth restriction with umbilical artery Doppler changes | p value | |
|---|---|---|---|---|
| PI > 95TH centile Mean ± SD (n = 11) |
AEDF/REDF Mean ± SD (n = 13) |
|||
| Fractional area change | 1st Reading | 20.64 ± 4.09 | 15.97 ± 8.5 | 0.32 |
| 2nd Reading | 23.60 ± 3.60 | 16.77 ± 6.33 | 0.07 | |
| TAPSE | 1st Reading | 6.42 ± 0.63 | 6.28 ± 0.54 | 0.59 |
| 2nd Reading | 7.50 ± 0.79 | 7.25 ± 1.25 | 0.68 | |
| Fractional shortening | 1st Reading | 24.00 ± 10.51 | 17.44 ± 8.22 | 0.28 |
| 2nd Reading | 31.00 ± 11.60 | 20.11 ± 3.87 | 0.02 | |
| Ejection fraction | 1st Reading | 55.95 ± 2.36 | 52.26 ± 12.88 | 0.27 |
| 2nd Reading | 67.05 ± 7.55 | 51.51 ± 10.90 | 0.02 | |
| Left Ventricular Output | 1st Reading | 196.50 ± 24.74 | 195.00 ± 43.19 | 0.96 |
| 2nd Reading | 243.75 ± 46.30 | 229.72 ± 44.80 | 0.61 | |
| Right Ventricular output | 1st Reading | 261.25 ± 55.60 | 222.22 ± 63.80 | 0.31 |
| 2nd Reading | 284.25 ± 65.50 | 262.33 ± 72.2 | 0.57 | |
| Left Ventricular Myocardial Performance Index | 1st Reading | 0.26 ± 0.07 | 0.30 ± 0.06 | 0.21 |
| 2nd Reading | 0.28 ± 0.08 | 0.32 ± 0.08 | 0.28 | |
| RV E/A ratio | 1st Reading | 0.81 ± 0.05 | 0.81 ± 0.21 | 0.98 |
| 2nd Reading | 0.95 ± 0.03 | 0.93 ± 0.07 | 0.63 | |
| LV E/A ratio | 1st Reading | 1.08 ± 0.07 | 1.21 ± 0.04 | 0.29 |
| 2nd Reading | 1.09 ± 0.12 | 1.13 ± 0.20 | 0.19 | |
Discussion
The major proportion of FGR-SGA born worldwide is from South Asia and African countries. In 2010, 32.4 million infants were born SGA worldwide, of which 27% were from low and middle-income countries. The prevalence of SGA in India is 46.9%, the third-highest in the world [23, 24]. SGA infants can be broadly classified into two types: (1) constitutionally small but otherwise normal who were SGA at birth due to factors like maternal weight, nutrition, height, parity and ethnicity and (2) SGA associated with fetal growth restriction with or without intrauterine umbilical artery doppler changes [25].
Fetal growth restriction in SGA babies is defined as, “SGA with abnormal Doppler indices such as umbilical artery pulsatility index above the 95th centile or mean uterine artery PI above 95th centile.” As per the previous studies, during the antenatal assessment of fetal well-being using antenatal doppler, a higher proportion of placental pathology and fetal growth-restricted SGA and AGA were observed if the doppler was abnormal, with the OR of 4.46, 95% CI 1.55, 13.22. Of the individual Doppler types, abnormal MCA Doppler was significantly associated with placental pathology and FGR compared to a normal MCA Doppler OR = 20.7, 95% CI 2.54, 447.1 [26]. Similar to this, in the present study a higher proportion of FGR SGA had MCA doppler changes (37.5%) and 4 cases showed only umbilical artery Doppler changes. In the present study, 45.8% of the SGA FGR cases had normal Doppler’s although EFW was below 3rd centile. A possible explanation for the presence of normal Doppler despite fetal growth restriction is partially attributed to some clinically unnoticed histopathological findings in the placenta. Parra-Saavedra et al. [27] showed that 78% of late-onset SGA births with normal umbilical artery Doppler had histological placental abnormalities as did for the 22% of AGA births.
The most common risk factor for SGA in the present study was pregnancy-induced hypertension. Population-based studies from several countries have shown that pregnancy-induced hypertension and chronic hypertension, are among the most common medical conditions associated with increased propensity for SGA and fetal growth restriction [28]. The abnormal cardiac functions in term FGR-SGA babies are mainly attributed to pressure and volume overload secondary to the state of chronic hypoxia, undernutrition and placental vascular resistance. In a normal heart, any condition leading to pressure overload produces hypertrophy, however in the fetal stage, with a background of chronic hypoxia and undernutrition, myocardial hypertrophy fails to occur. Instead, the intrauterine chronic stress leads to more spherical cavity and changes similar to dilated cardiomyopathy [29]. Hence, the heart is not as efficient in generating normal cardiac output leading to altered systolic and diastolic function in the immediate newborn period. This alteration due to growth restriction has been linked to long term consequences in childhood. In the present study, EF and FS was lower than controls with a significant difference in the second reading (p value: 0.02).
Previously an echocardiographic assessment was done in the umbilical artery ligated rat model with IUGR. The study showed a significant lower EF and FS (both, p value < 0.05). Similar to this, fetal echocardiographic assessment of a human fetus with placental insufficiency revealed a significantly lower FS compared to a normal growing fetus (32.0 ± 5.0 in controls vs 20.0 ± 5.20; p value < 0.05) [30, 31].
In this study, the LVO was recorded to be lower in FGR-SGA infants as compared to the AGA infants, but the difference was not statistically significant (p value:0.23). Similar to the present study, previous studies have shown lower LV stroke volume in SGA babies with heterogeneity among the studies in attaining a statistically significant result. In one of the previous studies, mitral annular peak systolic velocity (“Sm”) was assessed using tissue doppler imaging. The study showed significantly lower “Sm” velocities in FGR infants as compared to AGA infants (4.5 vs 5.0; p value < 0.001). Thus, based on the fact that EF, FS and LVO are lower in SGA babies as compared to their AGA counterparts, we can say that LV systolic function is deranged initially during the transitional circulation in growth-restricted fetus [32, 33].
In the current study, TAPSE and FAC as markers for RV systolic function were significantly lower in the FGR group. Similar, a study assessed the RV function using tissue doppler imaging, which revealed a lower tricuspid annular peak systolic velocity in FGR-SGA when compared with AGA infants [34]. As tissue Doppler imaging was unavailable for the current study, we assessed TAPSE using M- mode and FAC by conventional 2D echocardiography. The current study results were similar to TDI findings of the previous studies [16, 35]. Thus, based on the above parameters, it can be interpreted that RV systolic function is altered during the first week in growth-restricted neonates.
The current study revealed a lower tricuspid E/A ratio than AGA infants and mitral E/A ratio higher than AGA infants with a significant difference beyond 48 h in the first week of life. As per the evidence, however, there is a lot of heterogeneity regarding the E/A ratio in growth-restricted fetuses in the previous studies where some authors have shown a reduced ratio, whereas others have shown a higher E/A ratio [36]. In similarity to the present study, Fouzas et al. [35] showed a higher mitral E/A ratio in growth-restricted neonates on the 2nd day and 5th post-natal day. However, the difference was not statistically significant.
An altered E/A ratio is a sign of diastolic dysfunction which correlates mainly to abnormal ventricular relaxation. In the majority of the FGR SGA infants, some degree of intrauterine hypoxia does occur during the fetal stage. Due to abnormal placentation, chronic in-utero malnutrition and hypoxic states, there is a decrease in cardiac sarcomere protein and an increase in accumulation of glycogen and collagen for compensating the loss of myocardial cells. This results in increased rigidity and decrease in compliance leading to difficulty in diastolic relaxation and ventricular filling leading to diastolic dysfunction. However, to date, there is mixed evidence supporting the above physiological alteration leading to diastolic dysfunction in these neonates [37].
Myocardial performance index (MPI) has evolved over the years as a useful tool for assessing the combined systolic and diastolic function together. As per the previous studies and after technical refinement to improve the reproducibility of the index, a modified MPI has evolved. The modified MPI uses MV and AV regions as a landmark for calculating three-time intervals, namely, Isovolumetric contraction, Isovolumetric relaxation and ejection time. Using the modified MPI, normal left MPI has a range from 0.22 to 0.66 [38]. In the current study, left MPI has been in the normal range, but with higher values in FGR as compared to AGA infants. Similar to the present finding, many studies have documented a higher MPI than fetuses with normal growth at term gestation. The reason for higher MPI is the prolongation of isovolumetric relaxation time and decrease in the ejection time which reflect a deranged fetal cardiac function in the intrauterine environment [39].
In the current study, a subgroup analysis was done to see the effect of severity of the Doppler changes during the antenatal scans and their effect on the hemodynamics in the immediate postnatal period. The present study showed neonates who had fetal growth restriction with AEDF/REDF were associated with significantly lower FS and EF (p-value: 0.02 for both) compared to the neonates with only raised pulsatility index above 95th centile. Similarly, these neonates (AEDF/REDF) had a lower LVO, RVO, FAC and TAPSE as compared to less severely growth-restricted fetuses. They also demonstrated a higher MPI in the current study in the neonates with AEDF/REDF in the umbilical artery.
Previous studies were done in fetuses and animal studies have positively correlated the severity of Doppler abnormality to changes in ductus venosus. Recent literature has shown persistent higher MPI in the fetuses severely growth-restricted with worsening umbilical artery doppler changes. Hence, studies in the fetal stage support the findings of the current study [40–42]. However, the current study to the best of our knowledge is probably the first study where the type of the UA Doppler changes have been correlated with postnatal ventricular dysfunction.
Limitations
Although the current study showed some significant differences between the two cohorts, there are several limitations. Firstly, follow up of SGA neonates during their infancy may reveal serial changes in echocardiography, if any persists beyond one week. The second major limitation was the lack of blinding of the neonatologist assessing the ventricular function in the two cohorts. Thirdly, a larger sample size would have yielded a more generalizable result.
Conclusion
Based on the current study, it is clear that SGA infants with fetal growth have mild to moderate subclinical systolic and diastolic ventricular dysfunction which clearly emphasizes their reduced ability to adapt to the postnatal life secondary to cardiac remodeling due to chronic in-utero growth restriction.
Declarations
Funding
The authors declare that no funds, grants, or other support were received for conducting this study and preparation of this manuscript.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author contributions
All authors made substantial contributions to the conception or design of the work. Acquisition, analysis and interpretation of the data was done by Arjun Verma. The first draft of the manuscript was written by Arjun Verma. Gauri Oka and Yogen Singh revised critically for important intellectual content. Pradeep Suryawanshi approved the version to be published. All authors read and approved the final manuscript.
Ethics approval
This observational study was approved by Institutional ethics committee of Bharati Vidyapeeth medical college and certificate was granted on 7th May, 2021 under the reference number: BVDUMC/IEC/20.
Consent to participate
Written informed consent was obtained from the parents.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the individual patient data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time and central peripheral temperature difference. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F168–F173. doi: 10.1136/adc.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brillantino C, Rossi E, Minelli R, et al. A rare case of renal tumor in children: clear cell sarcoma. G Chir. 2019;40(3):217–224. [PubMed] [Google Scholar]
- 3.Vitale V, Rossi E, Di Serafino M, et al. Pediatric encephalic ultrasonography: the essentials. J Ultrasound. 2018 doi: 10.1007/s40477-018-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillantino C, Rossi E, Bifano D, et al. An unusual onset of pediatric acute lymphoblastic leukemia. J Ultrasound. 2020 doi: 10.1007/s40477-020-00461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botta F, Raimondi S, Rinaldi L, et al. Association of a CT-based clinical and radiomics score of non-small cell lung cancer (NSCLC) with lymph node status and overall survival. Cancers. 2020;12:1432. doi: 10.3390/cancers12061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brillantino C, Rossi E, Minelli R, Bifano D, Baldari D, Pizzicato P, Zeccolini R, Zeccolini M. Mediastinal thymoma: a difficult diagnosis in the pediatric age. Radiol Case Rep. 2021;16(9):2579–2585. doi: 10.1016/j.radcr.2021.06.035.PMID:34285726;PMCID:PMC8278152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans N. Echocardiography on neonatal intensive care units in Australia and New Zealand. J Paediatr Child Health. 2000;36:169–171. doi: 10.1046/j.1440-1754.2000.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Ünlüer EE, Karagöz A, Akoglu H, Bayata S. Visual estimation of bedside echocardiography ejection fraction by emergency physicians. West J Emerg Med. 2014;15:221–226. doi: 10.5811/westjem.2013.9.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaergaard J, Kjaer A, Flachskampf FA, Foster E, Pellikka PA. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr. 2006;7:340–348. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Ishii M, Eto G, Tei C, Tsutsumi T, Hashino K, Sugahara Y, et al. Quantitation of the global right ventricular function in children with normal heart and congenital heart disease: a right ventricular myocardial performance index. Pediatr Cardiol. 2000;21:416–421. doi: 10.1007/s002460010100. [DOI] [PubMed] [Google Scholar]
- 11.Minella R, Minelli R, Rossi E, et al. Gastroesophageal and gastric ultrasound in children: the state of the art. J Ultrasound. 2020 doi: 10.1007/s40477-020-00471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brillantino C, Rossi E, Baldari D, et al. Duodenal hematoma in pediatric age: a rare case report. J Ultrasound. 2020 doi: 10.1007/s40477-020-00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brillantino C, Rossi E, Pirisi P, et al. Pseudopapillary solid tumour of the pancreas in paediatric age: description of a case report and review of the literature. J Ultrasound. 2021 doi: 10.1007/s40477-021-00587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufano A, Flammia RS, Antonelli L, Minelli R, Franco G, Leonardo C, Cantisani V. The value of contrast-enhanced ultrasound (CEUS) in differentiating testicular masses: a systematic review and meta-analysis. Appl Sci. 2021;11:8990. doi: 10.3390/app11198990. [DOI] [Google Scholar]
- 15.Santarsiere M, Rumolo M, Menna BF, et al. A rare case of bilateral testicular metastasis from ileocecal NET: multiparametric US detection. J Ultrasound. 2022 doi: 10.1007/s40477-022-00657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumolo M, Santarsiere M, Menna BF, et al. Color Doppler and microvascular flow imaging to evaluate the degree of inflammation in a case of hidradenitis suppurativa. J Vasc Ultrasound. 2022 doi: 10.1177/15443167211066491. [DOI] [Google Scholar]
- 17.Sehgal A, McNamara PJ. Does point of care functional echocardiography enhances cardiovascular care in the NICU? J Perinatol. 2008;28(11):729–735. doi: 10.1038/jp.2008.100. [DOI] [PubMed] [Google Scholar]
- 18.Kluckow M, Seri I, Evans N. Functional echocardiography: an emerging clinical tool for the neonatologist. J Pediatr. 2007;150:125–130. doi: 10.1016/j.jpeds.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 19.Skinner JR. Echocardiography on the neonatal unit: a job for the neonatologist or the cardiologist? Arch Dis Child. 1998;78:401–402. doi: 10.1136/adc.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy WE, Nicol JL. The Barker hypothesis confirmed: association of low birth weight with all-cause natural deaths in young adult life in a remote Australian Aboriginal community. J Dev Orig Health Dis. 2019;10(1):55–62. doi: 10.1017/S2040174417000903. [DOI] [PubMed] [Google Scholar]
- 21.Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121(22):2427–2436. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- 22.Molina LCG, Odibo L, Zientara S, Običan SG, Rodriguez A, Stout M, Odibo AO. Validation of Delphi procedure consensus criteria for defining fetal growth restriction. Ultrasound Obstet Gynecol. 2002;56(1):61–66. doi: 10.1002/uog.20854. [DOI] [PubMed] [Google Scholar]
- 23.Narang A, Chaudhari MK, Kumar P. Small for gestational age babies: Indian scene. Indian J Paediatr. 1997;64(2):221–224. doi: 10.1007/BF02752452. [DOI] [PubMed] [Google Scholar]
- 24.Black RE. Global prevalence of small for gestational age births. Nestle Nutr Inst Workshop Ser. 2015;81:1–7. doi: 10.1159/000365790. [DOI] [PubMed] [Google Scholar]
- 25.Lausman A, Kingdom J, Gagnon R, Basso M, Bos H, Crane J, Davies G, Delisle M, Hudon L, Menticoglou S, Mundle W, Ouellet A, Pressey T, Pylypjuk C, Roggensack A, Sanderson F. Intrauterine growth restriction: screening, diagnosis, and management. J Obstet Gynaecol Can. 2013;35:741–748. doi: 10.1016/S1701-2163(15)30865-3. [DOI] [PubMed] [Google Scholar]
- 26.Curtin WM, Millington KA, Ibekwe TO, Ural SH. Suspected fetal growth restriction at 37 weeks: a comparison of doppler and placental pathology. Biomed Res Int. 2017 doi: 10.1155/2017/3723879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra-Saavedra M, Crovetto F, Triunfo S, et al. Placental findings in late-onset SGA births without Doppler signs of placental insufficiency. Placenta. 2014;34(12):1136–1141. doi: 10.1016/j.placenta.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Yang H, Sun X, Li G. Risk factors and complications of small for gestational age. Pak J Med Sci. 2019;35(5):1199–1203. doi: 10.12669/pjms.35.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verburg B, Jaddoe V, Wladimiroff J, Hofman A, Witteman J, Steegers E. Fetal hemodynamic adaptive changes related to intrauterine growth the generation R study. Circulation. 2008;117:649–659. doi: 10.1161/circulationaha.107.709717. [DOI] [PubMed] [Google Scholar]
- 30.Makikallio K, Vuolteenaho O, Jouppila P, Rasanen J. Ultrasonographic and biochemical of human fetal cardiac dysfunction in placental insufficiency. Circulation. 2002;105:2058–2063. doi: 10.1161/01.CIR.0000015505.24187.FA. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, Zhao D, Chen CK, Yap CH. Echocardiographic assessment of fetal cardiac function in the uterine artery ligation rat model of IUGR. Pediatr Res. 2021;90(4):801–808. doi: 10.1038/s41390-020-01356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altın H, Karaarslan S, Karataş Z, Alp H, Şap F, Baysal T. Evaluation of cardiac functions in term small for gestational age newborns with mild growth retardation: a serial conventional and tissue Doppler imaging echocardiographic study. Early Hum Dev. 2012;88(9):757–764. doi: 10.1016/j.earlhumdev.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Xu J, Xu T, Fan J, Tao S. Left ventricular systolic function of newborns with asphyxia evaluated by tissue Doppler imaging. Pediatr Cardiol. 2009;30:741–746. doi: 10.1007/s00246-009-9421-6. [DOI] [PubMed] [Google Scholar]
- 34.Mirza H, Sabina T, Almira K, Zijo B, Emina V, Verica M, Refet G. Right ventricular systolic longitudinal function in infants: correlation of TAPSE with gestational age and body weight. J Pediatr Neonatal Individ Med. 2018;7(2):e070216. [Google Scholar]
- 35.Fouzas S, Karatza A, Davlouros P, Chrysis D, Alexopoulos D, Dimitriou G. Neonatal cardiac dysfunction in intrauterine growth restriction. Pediatr Res. 2014 doi: 10.1038/pr.2014.22. [DOI] [PubMed] [Google Scholar]
- 36.Naujorks AA, Zielinsky P, Beltrame PA, Castagna RC, Petracco R, Busato A, et al. Myocardial tissue Doppler assessment of diastolic function in the growth restricted fetus. Ultrasound Obstet Gynecol. 2009;34:68–73. doi: 10.1002/uog.6427. [DOI] [PubMed] [Google Scholar]
- 37.Comas M, Crispi F, Cruz-Martinez R, Martinez JM, Figueras F, Gratacós E. Usefulness of myocardial tissue Doppler vs conventional echocardiography in the evaluation of cardiac dysfunction in early-onset intrauterine growth restriction. Am J Obstet Gynecol. 2010;203(1):45.e1–45.e457. doi: 10.1016/j.ajog.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez-Andrade E, López-Tenorio J, Figueroa-Diesel H, et al. A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet Gynecol. 2005;26(3):227–232. doi: 10.1002/uog.1959. [DOI] [PubMed] [Google Scholar]
- 39.Api O, Emeksiz M, Api M, Uǧurel V, Unal O. Modified myocardial performance index for evaluation of fetal cardiac function in pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33:51–57. doi: 10.1002/uog.6272. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe S, Hashimoto I, Saito K, et al. Characterization of ventricular myocardial performance in the fetus by tissue Doppler imaging. Circ J. 2009;73:943–947. doi: 10.1253/circj.CJ-08-0529. [DOI] [PubMed] [Google Scholar]
- 41.Crispi F, Valenzuela-Alcaraz B, Cruz-Lemini M, Gratacós E. Ultrasound assessment of fetal cardiac function. Aust J Ultrasound Med. 2013;16(4):158–167. doi: 10.1002/j.2205-0140.2013.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crispi F, Gratacós E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther. 2013;32:47–64. doi: 10.1159/000338003. [DOI] [PubMed] [Google Scholar]