Abstract
Aims
Current ultrasound (US) Doppler techniques cannot demonstrate the vascularization of the dermis. The purpose of this study was to investigate whether the new Superb Vascular Imaging (SMI) and Microvascular Flow (MV-Flow) techniques improve the detection of normal dermis vessels. SMI and MV-Flow were compared side-by-side to conventional power-Doppler (PD) imaging.
Methods
By using US, 50 healthy volunteers were evaluated at level of five body areas: forehead, forearm, palm, buttock, and thigh. Two off-site operators evaluated the images to assess the difference between SMI and PD imaging and between MV-Flow and PD imaging in terms of dermis flow amount. A 0–3 scoring system was adopted.
Results
SMI scored grade 0 in 0% of body areas, grade 1 in 58%, grade 2 in 33%, and grade 3 in 9%. In comparison with SMI, PD scored grade 0 in 38% of body areas, grade 1 in 56%, grade 2 in 6%, and grade 3 in 0%. MV-Flow scored grade 0 in 0% of body areas, grade 1 in 52%, grade 2 in 43%, and grade 3 in 6%. Comparted to MV-Flow, PD scored grade 0 in 53% of body areas, grade 1 in 34%, grade 2 in 13%, and grade 3 in 0%. The difference in terms of sensitivity was statistically significant for all the body areas investigated.
Conclusions
We found both SMI and MV-Flow to be superior to PD imaging and capable to demonstrate normal vascularization of the dermis.
Keywords: Skin, Dermis, Dermatology ultrasound, Doppler ultrasound, Vascular flow
Introduction
The dermis has a mesodermal origin and is dominated by packages of organized collagen, providing the supporting structure to the skin [1]. It includes blood vessels, lymphatics, nerves, hair follicles, and sweat glands [2, 3]. The strong vascular network serves a number of vital functions such as nutritional support for tissues and homeostasis [2, 4, 5]. The dermis includes the thinner, superficial layer of the papillary dermis and the thicker and more profound layer of the reticular dermis [2, 6]. A deeper plexus, made by relatively larger vessels, mostly veins, is located at the interface of the dermis and subcutis, fed by branches of the large subcutaneous arteries [5]. Vessels’ caliber in the reticular dermis ranges from 50 to 150 μm [7]. The border between papillary and reticular dermis hosts a superficial plexus that supplies the dermal papillae through a candelabra-like capillary system [6, 8]. This superficial plexus is located 1 to 1.5 mm below the skin surface and consists of vessels less than 50 μm in caliber. The two dermal plexuses are connected each other by direct, vertical, arborizing channels [7].
Dermis vessels are usually not detectable in normal subjects using conventional color-Doppler and power-Doppler (PD) imaging [5, 6, 9, 10]. This is due to the small size of the vessels and to their slow velocity, commonly equal to or less than 2 cm/s. recently, several ultrasound (US) companies have developed advanced technologies for the US imaging of the microvasculature. In our study, we prospectively evaluated if these new techniques could allow detecting more flow signals in comparison with PD. Consequently, we prospectively compared PD with Superb Microvascular Imaging (SMI, Canon Medical Systems, Tokyo, Japan) or with Microvascular Flow (MV-Flow, Samsung Medison Co Ltd, Seoul, South Korea) in the assessment of dermal vessels in healthy subjects.
Materials and methods
The study was developed as a single-center, prospective experience on healthy adult volunteers. The study protocol was approved by our institutional review board and was conducted according to Good Clinical Practice and the Declaration of Helsinki. All participants provided a written informed consent. Inclusion criteria were age above 18 years old and absence of any skin abnormality at both physical inspection and US exploration. Fifty Caucasian subjects were enrolled between September 2020 and December 2021. There were 29 males and 21 females, aged 20–78 years (mean, 46 years old).
Thirty studies were obtained on an Aplio i800 system (Canon Medical Systems Corporation), equipped with a 22-MHz linear probe. Twenty other studies were carried out using an RS85 system Prestige (Samsung Medison Co Ltd, Seoul, South Korea), using a 22-MHz linear probe.
Two operators acquired each one the images of 25 subjects. Scans were taken at level of five body areas: forehead, dorsal aspect of the forearm, hand palm, buttock, and anterior aspect of the thigh. These anatomical sites have different dermal skin and echogenicity, representing a combination of sun-exposed and non-sun exposed areas and including a glabrous skin site (hand palm). A large amount of gel was employed, without ever using any stand-off pad. Care was taken to avoid motion of the operator’s hand or the transducer, which was placed gently above the gel layer. The beam focus was located at the dermis-hypodermis edge. The color gain was never changed between the two techniques. In each body area, a SMI or the MV-Flow scan was first obtained. Once that a clear SMI or MV-Flow scan showing dermis vessels was taken, the image was freeze on the split-screen and then a PD scan was quickly obtained. The operator froze the PD acquisition and went back to the frame showing the largest amount of flow signal, storing it in the scanner archive (Figs. 1, 2).
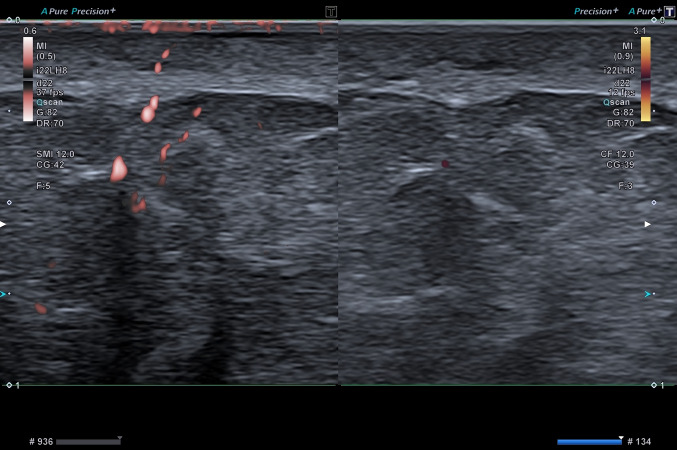
Fig. 1.
49-year-old asymptomatic male. Dermis vascularization in the thigh at SMI (left part of the image) and at PD (right part of the image). SMI detects significantly more flows than PD
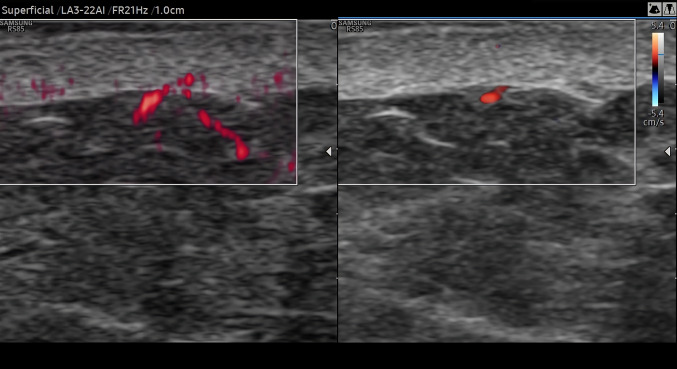
Fig. 2.
49-year-old asymptomatic male. Dermis vascularization in the thigh at MV-Flow (left part of the image) and at PD (right part of the image). MV-Flow detects significantly more flows than PD
Two off-site reviewers evaluated the split-screen images of each anatomic site of all subjects, comparing SMI with PD and MV-Flow with PD. A subjective, semi-quantitative side-by-side comparison was made by consensus, grading the flow signals as absent (grade 0), scarce (grade 1), moderate (grade 2), and strong (grade 3) [11]. Care was taken to exclude from this retrospective assessment the subcutaneous vessels as well as the color artifacts. In case of disagreement between the two reviewers, a third operator was involved. The McNemar test was employed to compare the sensitivity the different techniques in the detection of flow signals for each anatomical site. A p-value less than 0.05 was regarded as statistically significant.
Results
SMI scored grade 0 in 0 out of 150 total body areas of 30 subjects, grade 1 in 87 out of 150 body areas, grade 2 in 49 of 150 body areas, and grade 3 in 14 of 150 body areas. In comparison, PD scored grade 0 in 57 out of 150 total body areas, grade 1 in 84 out of 150 body areas, grade 2 in 9 of 150 body areas, and grade 3 in 0 of 150 body areas. MV-Flow scored grade 0 in 0 out of 100 total body areas of 20 subjects, grade 1 in 52 out of 100 body areas, grade 2 in 43 of 100 body areas, and grade 3 in 6 of 100 areas. In comparison, PD scored grade 0 in 53 out of 100 total body areas, grade 1 in 34 out of 100 body areas, grade 2 in 13 of 100 body areas, and grade 3 in 0 of 100 body areas (Table 1). The difference in terms of sensitivity was statistically significant for all the body areas investigated. In none of the cases PD scored better than SMI or than MV-Flow.
Table 1.
Color scoring at SMI and PD in 30 subjects and at MV-Flow and PD in 20 subjects at level of 5 different anatomic areas
| Scoring | Body Areas | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Forehead | Forearm | Palm | Buttock | Thigh | ||||||
| SMI | PD | SMI | PD | SMI | PD | SMI | PD | SMI | PD | |
| Grade 0 | 0/30 | 7/30 | 0/30 | 11/30 | 0/30 | 10/30 | 0/30 | 14/30 | 0/30 | 15/30 |
| Grade 1 | 20/30 | 22/30 | 19/30 | 17/30 | 17/30 | 18/30 | 15/30 | 13/30 | 16/30 | 14/30 |
| Grade 2 | 8/30 | 1/30 | 8/30 | 2/30 | 10/30 | 2/30 | 11/30 | 3/30 | 12/30 | 1/30 |
| Grade 3 | 2/30 | 0/30 | 3/30 | 0/30 | 3/30 | 0/30 | 4/30 | 0/30 | 2/30 | 0/30 |
| Scoring | Forehead | Forearm | Palm | Buttock | Thigh | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MV-Flow | PD | MV-Flow | PD | MV-Flow | PD | MV-Flow | PD | MV-Flow | PD | |
| Grade 0 | 0/20 | 13/20 | 0/20 | 10/20 | 0/20 | 10/20 | 0/20 | 11/20 | 0/20 | 9/20 |
| Grade 1 | 10/20 | 7/20 | 11/20 | 6/20 | 9/20 | 7/20 | 8/20 | 6/20 | 13/20 | 8/20 |
| Grade 2 | 9/20 | 0/20 | 9/20 | 4/20 | 8/20 | 3/20 | 11/20 | 3/20 | 6/20 | 3/20 |
| Grade 3 | 1/20 | 0/20 | 0/20 | 0/20 | 3/20 | 0/20 | 1/20 | 0/20 | 1/20 | 0/20 |
Discussion
SMI technology applies a clutter suppression algorithm, separating flow signals from the motion artifacts arising from nearby structures [12]. This allows preserving the low-flow components, which are removed by conventional wall filters in color- and PD imaging, while displaying flow signals with a high spatial resolution and high frame rates. SMI uses higher frame rates than PD, more than 50 Hz, and lower pulse repetition frequencies ranging between 220 and 234 Hz [12]. Although, by now less investigated than SMI, also MV-Flow has the potential display minute vessels with slow blood flow velocities. MV-Flow is characterized by high tissue suppression to reduce tissue noise signals, suppression of flash artifacts (due to its advanced filter), compound images, and high sensitivity.
In the evaluation of thyroid or breast nodules with US the difficulty resides in the small caliber of the vessels and in the slow flow of the blood inside them. In the assessment of the skin vascular network there are two additional obstacles. The first one is represented by the probe pressure, even if the operator handles it as gently as possible. The second difficulty is the closeness of the vessels to the footprint of the transducer. The uppermost portion of the US field-of-view is compressed, for a length that depends on the transducer quality and on the emission frequency [13–15]. All these aspects explain the difficulty in studying the normal vascularization of the skin with US.
A number of strategies have been proposed to improve the ultrasound display of superficial, slow flows at color- and PD imaging. These options include optimization of scanning setting sensitivity (small color box, high transmission frequency, low pulse repetition frequency, low or null wall filter, high color gain) [9, 16], interposition of a gel stand-off pad [13], use ultra-high frequencies [17], injection of contrast medium microbubbles [18]. Due to the intrinsic limitations of Doppler techniques, however, none of these options allows an adequate representation of the normal dermis vessels.
SMI and MV-Flow allow preserving the low-flow components, which are removed by conventional wall filters in color- and PD imaging, while displaying flow signals with a higher spatial resolution and higher frame rates [19–21]. In our study PD imaging was unable to detect dermis flows, showing no or low vascular signals. SMI and MV-Flow allowed to collect significantly more flows compared side-by-side to PD. SMI and MV-Flow has been tested in a number of superficial and abdominal anatomical sites [11, 19–26]. Govind and coworkers found that SMI is more sensitive than color-Doppler imaging in demonstrating microvenous reflux in limbs with venous disease and SMI [27]. İslamoğlu and Uysal employed SMI to assess plaques of cicatricial alopecia [28]. Ávila de Almeida et al. [29] reported a case of glomus tumor evaluated with SMI. Dermatology is a growing field of application of US [9, 10, 16]. Our study proved that SMI and MV-Flow are by far more sensitive than PD in detecting normal dermal flows. In reality, the difference is probably even higher than what found in our study. In SMI and MV-Flow the color signal is visible only in the vessels while in PD color typically bleeds out of the lumen. This blooming artifact partially compensated on the images at the intrinsic poor sensitivity of PD. Despite this, SMI and MV-Flow were significantly more sensitive than PD.
In most of our cases PD showed subcutaneous vessels approaching the dermis-hypodermis border and then appearing as “cut”, without any continuation in the dermis itself. SMI and MV-Flow, instead, allowed to seen the vessels entering the dermis and branching inside it. At least in the deeper portion of the dermis layer, SMI and MV-Flow could detect a number of vessel while, even using these two technologies, it was hard to demonstrate the vascularization of the more superficial portion of the dermis.
The availability of techniques capable to sensitively demonstrate normal and abnormal dermis flows may be quite useful in clinical practice, being employed in initial diagnosis, activity status assessment, response to treatment evaluation, and prognostic formulation of a number of dermatology scenarios. These include morphea, psoriasis, vasculitides, suppurative hidradenitis, burns, keloid scars, and surgical flaps [7, 30, 31].
Our study has a number of limitations. Firstly, it was conducted in a single center, and the number of cases was relatively small. Secondly, the choice of the anatomical sites to be sampled was somehow arbitrary. However, since the difference between SMI and PD imaging performance was so marked, it is our opinion that similar results would have been obtained also if scanning other anatomical locations. Thirdly, although care was taken to obtain adequately matching scans between SMI and PD and between MV-Flow and PD, these scans could not be perfectly identical each other. The presence of color artifacts, particularly when using SMI could have theoretically interfered on the images analysis. However, as stated above, care was always taken to exclude artifacts from this retrospective assessment of the scans. It is of note that these artifacts were always seen at the top of the field of view, near the epidermis entrance of the echoes, so they were always easy to be excluded from our analysis. Finally, we did not make any attempt to differentiate dermis vessels into arteries and veins. This aspect was not relevant for the study purpose and, additionally, the vessels we investigated were too small to obtain an adequate spectral sampling. Another limitation of the present study was the use of a semi-quantitative score. This scoring system can be employed to assess the amount of flow signals. With color-Doppler, PD, and the new microvascular technologies such as SMI and MV-Flow [11]. However, these scores posing the problem of inter-reader reproducibility. Automatic quantification of the number of colored pixels within the box, using options such as Canon’s Vascular Index, may allow instead an objective quantification of the vascular flows, all will be probably preferred in the future [32].
Conclusion
New microvascular imaging techniques such as SMI and MV-Flow allow reliable display of normal dermis flows while conventional PD imaging basically cannot. Even if set to detect slow flows, conventional Doppler techniques show absent or minimal flow signals within the normal dermis. Instead newer vascular tool are capable to detect significant more flows in a side by side comparison with PD. This opens new perspectives in the assessment of dermal abnormalities.
Abbreviations
- MV-Flow
Microvascular flow
- PD
Power Doppler
- SMI
Superb microvascular imaging
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors declare that there are no financial or other relations that could lead to a conflict of interest.
Ethical statement
This study was approved by the local research ethics committee and all the subjects enrolled were informed about the examinations and the procedure, and their written consents were obtained before the US examination.
Footnotes
Awarded as best oral paper at the 28th National Congress of the SIUMB, Rome, November 14-16, 2021.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12:390–399. [PubMed] [Google Scholar]
- 2.Barcaui EO, Carvalho AC, Piñeiro-Maceira J, et al. Study of the skin anatomy with high-frequency (22 MHz) ultrasonography and histological correlation. Radiol Bras. 2015;48:324–329. doi: 10.1590/0100-3984.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González Díaz CP. Characterization of dermatological lesions by ultrasound. Rev Colomb Radiol. 2014;25:4006–4014. [Google Scholar]
- 4.Wortsman X. Ultrasound in dermatology: why, how, and when? Semin Ultrasound CT MRI. 2013;34:177–195. doi: 10.1053/j.sult.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Daly SM, Leahy MJ. ’Go with the flow’: a review of methods and advancements in blood flow imaging. J Biophotonics. 2013;6:217–255. doi: 10.1002/jbio.201200071. [DOI] [PubMed] [Google Scholar]
- 6.Wortsman X, Wortsman J, Carreno L, et al (2013) Sonographic anatomy of the skin, appendages, and adjacent structures. Wortsman X (ed), Springer, 15–35
- 7.Morita TCAB, Trés GFS, Criado RFJ, et al. Update on vasculitis: an overview and dermatological clues for clinical and histopathological diagnosis - part I. An Bras Dermatol. 2020;95:355–371. doi: 10.1016/j.abd.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braverman IM. The role of blood vessels and lymphatics in cutaneous inflammatory processes: an overview. Br J Dermatol. 1983;109(Suppl 25):89–98. [PubMed] [Google Scholar]
- 9.Catalano O, Wortsman X. Dermatology ultrasound. Imaging technique, tips and tricks, high-resolution anatomy. Ultrasound Q. 2020;36:321–327. doi: 10.1097/RUQ.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 10.Gaitini D (2013) Introduction to color Doppler ultrasound of the skin. In: Dermatologic ultrasound with clinical and histologic correlations. Wortsman X (ed), Springer, 3–14
- 11.Ayaz E, Ayaz M, Önal C, et al. seeing the unseen: evaluating testicular vascularity in neonates by using the Superb Microvascular Imaging ultrasound technique. J Ultrasound Med. 2019;38:1847–1854. doi: 10.1002/jum.14882. [DOI] [PubMed] [Google Scholar]
- 12.Bayramoglu Z, Kandemirli SG, Sarı ZNA, et al. Superb Microvascular Imaging in the evaluation of pediatric Graves disease and Hashimoto thyroiditis. J Ultrasound Med. 2020;39:901–909. doi: 10.1002/jum.15171. [DOI] [PubMed] [Google Scholar]
- 13.Corvino A, Sandomenico F, Corvino F, et al. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of the skin. J Ultrasound. 2019;23:45–53. doi: 10.1007/s40477-019-00376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hangiandreou NJ. AAPM/RSNA physics tutorial for residents. Topics in US: B-mode US: basic concepts and new technology. Radiographics. 2003;23:1019–1033. doi: 10.1148/rg.234035034. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence JP. Physics and instrumentation of ultrasound. Crit Care Med. 2007;35(8 Suppl):S314–S322. doi: 10.1097/01.CCM.0000270241.33075.60. [DOI] [PubMed] [Google Scholar]
- 16.Jin W, Kim GY, Park SY, et al. The spectrum of vascularized superficial soft-tissue tumors on sonography with a histopathologic correlation: Part 1, benign tumors. AJR. 2010;195:439–445. doi: 10.2214/AJR.09.3832. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimatsu H, Hayashi A, Yamamoto T, et al. Visualization of the “intradermal plexus” using ultrasonography in the dermis flap: a step beyond perforator flaps. Plast Reconstr Surg Glob Open. 2019;7:e2411. doi: 10.1097/GOX.0000000000002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chami L, Lassau N, Chebil M, et al. Imaging of melanoma: usefulness of ultrasonography before and after contrast injection for diagnosis and early evaluation of treatment. Clin Cosmet Investig Dermatol. 2011;4:1–6. doi: 10.2147/CCID.S13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubinsky TJ, Revels J, Wang S, et al. Comparison of Superb Microvascular Imaging with color flow and power Doppler imaging of small hepatocellular carcinomas. J Ultrasound Med. 2018;37:2915–2924. doi: 10.1002/jum.14654. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Wei X, Zhao S, Huang H, Wang W, Qiu J, Chen X, Cheng C, Tian Z, Rychik J. Characterization of placental microvascular architecture by MV-Flow imaging in normal and fetal growth-restricted pregnancies. J Ultrasound Med. 2021;40:1533–1542. doi: 10.1002/jum.15531. [DOI] [PubMed] [Google Scholar]
- 21.Malho AS, Ximenes R, Ferri A, Bravo-Valenzuela NJ, Araujo Júnior E (201) MV-Flow and LumiFlow: new Doppler tools for the visualization of fetal blood vessels. Radiol Bras 54:277–278. doi: 10.1590/0100-3984.2020.0109 [DOI] [PMC free article] [PubMed]
- 22.Ates F, Durmaz MS, Yorulmaz A, et al. Quantitative assessment of bladder wall vascularity index in children with acute cystitis using superb microvascular imaging. J Ultrasound. 2022;25:27–33. doi: 10.1007/s40477-020-00549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park AY, Kwon M, Woo OH, et al. A prospective study on the value of ultrasound microflow assessment to distinguish malignant from benign solid breast masses: association between ultrasound parameters and histologic microvessel densities. Korean J Radiol. 2019;20:759–772. doi: 10.3348/kjr.2018.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Z-Z, Huang Y-H, Shen H-L, et al. Clinical applications of Superb Microvascular Imaging in the liver, breast, thyroid, skeletal muscle, and carotid plaques. J Ultrasound Med. 2019;38:2811–2820. doi: 10.1002/jum.15008. [DOI] [PubMed] [Google Scholar]
- 25.Zhan J, Diao XH, Jin JM, et al. Superb Microvascular Imaging—a new vascular detecting ultrasonographic technique for avascular breast masses: a preliminary study. Eur J Radiol. 2016;85:915–921. doi: 10.1016/j.ejrad.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Ünal ÖF, BayramoĞlu Z, Adaletlİ İ. Evaluation of periarticular soft tissues in patients with juvenile idiopathic arthritis by superb microvascular imaging and shear wave elastography. Arch Rheumatol. 2020;35:264–273. doi: 10.46497/ArchRheumatol.2020.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govind D, Thomas KN, Hill BG, et al. Microvenous reflux in the skin of limbs with superficial venous incompetence. Ultrasound Med Biol. 2018;44:756–761. doi: 10.1016/j.ultrasmedbio.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 28.İslamoğlu ZGK, Uysal E. A preliminary study on ultrasound techniques applied to cicatricial alopecia. Skin Res Technol. 2019;25:810–814. doi: 10.1111/srt.12725. [DOI] [PubMed] [Google Scholar]
- 29.Ávila de Almeida C, Guarçoni S, Leverone A, Nakamura R, Marchiori E, Canella C. Characterization of a glomus tumor using 33-MHz ultrasound and superb microvascular imaging. Skin Res Technol. 2021;27:466–468. doi: 10.1111/srt.12972. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Furst DE, Jin H, et al. High-frequency ultrasound of the skin in systemic sclerosis: an exploratory study to examine correlation with disease activity and to define the minimally detectable difference. Arthritis Res Ther. 2018;20:181. doi: 10.1186/s13075-018-1686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen CK, Ghatak S, Gnyawali SC, et al. Cutaneous imaging technologies in acute burn and chronic wound care. Plast Reconstr Surg. 2016;138(3 Suppl):119S–S128. doi: 10.1097/PRS.0000000000002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corvino A, Varelli C, Cocco G, Corvino F, Catalano O. Seeing the unseen with superb microvascular imaging: ultrasound depiction of normal dermis vessels. J Clin Ultrasound. 2022;50:121–127. doi: 10.1002/jcu.23068. [DOI] [PubMed] [Google Scholar]