Abstract
The influence of hypoperfusion on cognition in patients with Moyamoya disease (MMD) is unclear. This study investigated cognitive function changes in MMD patients without stroke and illustrated the relationship between cognitive impairment and hypoperfusion. We prospectively performed a structured battery of seven neurocognitive tests on 115 adult MMD patients without stroke and 82 healthy controls. Hemodynamic assessment was performed using dynamic susceptibility contrast-enhanced MRI. The best subset regression (BSR) strategy was used to identify risk factors. Global cognition (MoCA), speed of information processing (TMT-A), executive function (TMT-B), visuospatial function (CDT), and verbal memory (CAVLT) were significantly poorer in MMD patients without stroke than in healthy controls. The TMT-B score significantly correlated with cerebral blood flow (CBF) in the bilateral lateral frontal lobes, centrum semiovale, and temporal lobes. The TMT-A and CAVLT scores significantly correlated with CBF in the left centrum semiovale (L-CSO) and temporal lobes. According to the BSR results, age, education, white matter lesions, and hypoperfusion of the L-CSO were risk factors for cognitive impairment. Hypoperfusion leads to multiple cognitive impairments in MMD patients without stroke. The perfusion of particular areas may help evaluate the cognitive function of MMD patients and guide therapeutic strategies.
Keywords: Cognitive impairment, Moyamoya disease, hypoperfusion, non-stroke, risk factor
Introduction
Moyamoya disease is a cerebrovascular condition involving stenosis changes of the distal carotid artery and unusual reticular vessel development in the skull base.1,2 Initial symptoms include transient ischemic attacks (TIAs), cerebral infarction, hemorrhage, and headache. 3 Revascularization is currently the primary treatment for MMD. Surgical revascularization is generally recommended in children regardless of the severity of ischemia because their brain is still developing. In adult MMD, revascularization is recommended in patients with ischemic symptoms and hemodynamic impairment. It is still controversial whether and when surgical intervention should be performed in patients with mild symptoms or hemodynamic stability. 4 However, the influence of long-term hypoperfusion on cognitive function is an important yet often neglected aspect concerning surgical indication.
Neuropsychological impairment has been recognized in patients with MMD,5–8 but the current definition of ischemic symptoms and the hemodynamic impairment effect do not consider cognitive function. At present, how hypoperfusion affects cognitive function is still unknown. Few studies have examined the correlation between cognitive dysfunction and long-term hypoperfusion (excluding stroke), which is important to elucidate the hemodynamic mechanism of cognitive impairment in patients with MMD at an early stage. 9 Hence, in this study, we applied dynamic susceptibility contrast-enhanced MRI (DSC-MRI) and a structured battery of seven neurocognitive tests to comprehensively evaluate cognitive impairment in MMD patients without stroke and investigate whether hypoperfusion in specific regions is associated with cognitive impairment.
Methods
Patients
This study was approved by the Ethics Committee of our Hospital (Approval Number: KY-2018-6-60) and guided by the Declaration of Helsinki. Written informed consent was obtained from both patients and healthy controls. A total of 115 patients with MMD treated at our department between 2018 and 2021 were enrolled. All patients met the following inclusion criteria: (1) diagnosis by digital subtraction angiography (DSA) according to guidelines in 2012 4 ; (2) age >18 years; (3) right handedness; (4) no history of surgery; (5) no recent stroke events in the cerebral cortex, basal ganglia, brainstem, or cerebellum, as observed through MRI screening, and small cerebral infarctions with lesion diameter <8 mm in the white matter. 8 Patients who met the following criteria were excluded: (1) neurological deficits due to psychiatric illness and severe systemic diseases; (2) severe dyskinesia or language disorder.
Additionally, we recruited 82 healthy volunteers matched for age, sex, and educational level who had not been diagnosed cerebrovascular diseases as the control cohort.
Neuropsychological assessment
A trained cognitive psychologist (Hou-Di Zhang) performed all the tests. The Montreal Cognitive Assessment (MoCA) was used to assess overall cognitive impairment, with a cutoff of 26 and 1-point correction for persons educated for ≤12 years.10–12 Daily living ability was assessed using the Activity of Daily Living Scale (ADL).13,14 The 17-item Hamilton Rating Scale for Depression (HAMD-17) was used to assess depression.15,16 Visuospatial praxis was evaluated using the clock drawing test (CDT). 17 The Chinese auditory verbal learning test (CAVLT) was used to measure transient and delayed memory. 18 Trail Making Test A (TMTA) and B (TMTB) were used to assess the speed of information processing19,20 and executive function, 21 respectively.
Radiological examination
All patients underwent MRI and DSA within 10 days of neuropsychological assessment. Stroke was excluded on MRI. The bilateral cerebral hemispheres of patients were staged based on Suzuki staging. White matter damage was estimated using fluid-attenuated inversion recovery. The Fezakas scale was used to score deep white matter hyperintensity and periventricular hyperintensity. 22,23
All static images were reviewed by two experienced readers (Drs. Xu and Zhang) blinded to the clinical information. Any differences in observations were resolved by consensus.
Hemodynamic examination
We used DSC-MRI to assess the cerebral hemodynamic status. The examination was performed using a MAGNETOM Skyra 3 T MRI scanner (Siemens, Germany) with a 20-channel head coil.24,25 The acquired DSC-MRI images were processed using a post-processing workstation (Syngo Via 20, Siemens) and analyzed using the MR Neuro-Perfusion software. All analyses were performed by the same neuroradiologist (Hong-Tao Zhang), who was blinded to the neuropsychological findings.
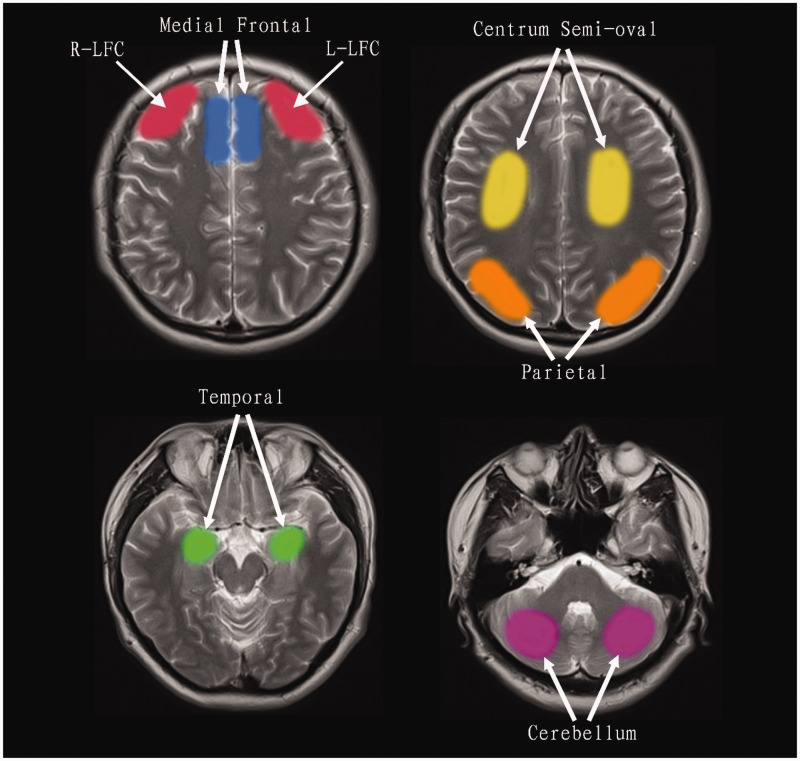
Regional cerebral blood flow (rCBF) values were evaluated in each patient by manually drawing a series of regions of interest (ROIs) over the cerebral cortex.25–27 The selected ROIs to assess rCBF were the lateral frontal, medial frontal, centrum semiovale (CSO), parietal cortex, temporal cortex, and cerebellum on both sides to assess rCBF (Figure 1).27–29 The method of ROI mapping is described in our previous research report. 25
Figure 1.
Regions of interest (ROIs) included the left (L) and right (R) lateral frontal cortex (LFC), medial frontal cortex, centrum semi-oval, parietal cortex, temporal cortex, and cerebellum.
Statistical analysis
All statistical analyses were performed using the SPSS software (version 25.0) and R statistical software (version 4.1.2). Pearson’s χ2 test was used to compare categorical variables. The normality of the data was investigated using the Kolmogorov–Smirnov test. The Mann–Whitney U-test and independent-sample t-test were used to compare continuous data from the skewness and normal distributions, respectively. Multiple linear regression analysis was used to analyze the relationship between cognitive scores, age, educational level, and rCBF. The variance inflation factor (VIF) was calculated to determine multicollinearity. Variables with a VIF >10, considered severely collinear, were excluded from further analyses. The best subset regression (BSR) strategy was used to construct the logistic regression model, and the adjusted R-square was used to select the optimal model from all possible subsets. Continuous data are expressed as mean ± SD. The level of significance was set at p < 0.05.
Results
Patient characteristics
In this study, 115 adult MMD patients without stroke and 82 healthy controls were enrolled. There were no significant differences in sex, age, or years of education between the groups (Table 1).
Table 1.
Characteristics and clinical information of participants.
| MMD (n = 115) Mean ± SD | HC (n = 82) Mean ± SD | P | |
|---|---|---|---|
| Sex (F:M) | 58:57 | 39:43 | 0.691 |
| Age, y | 39.29 ± 11.82 | 40.44 ± 11.63 | 0.701 |
| Education, y | 12.94 ± 2.80 | 12.67 ± 3.67 | 0.165 |
| Medical history, n(%) | |||
| Diabetes mellitus | 12 (10.4) | 7 (8.5) | 0.656 |
| Hypertension | 47 (40.9) | 23 (28.0) | 0.064 |
| Hyperlipidemia | 20 (17.4) | 10 (12.2) | 0.317 |
F: female; M: male; SD: standard deviation; MMD: Moyamoya disease.
Among the enrolled MMD patients, 47 had a history of hypertension, and other vascular risk factors included diabetes, hyperlipidemia, and hyperhomocysteinemia in 12, 20, and 26 patients, respectively. TIA was the most common initial symptom (79 cases, 68.7%); other patients had headache (9 cases, 7.8%) or were asymptomatic (27 cases, 23.4%). The neurological status at admission was graded according to the mRS score: 4 patients were grade 0, and 111 patients were grade 1. According to the Fazekas grading criteria, 64 patients were classified as grade 0, and the rest (51 cases) were classified as grade 1. The details of the Suzuki stage are presented in Table 2.
Table 2.
Subgroup risk factor and rCBF analysis.
| MMD (MOCA ≥ 26) | MMD (MOCA < 26) | P | ||
|---|---|---|---|---|
| Sex (F:M) | 21:17 | 37:40 | 0.467 | |
| Age, y | 34.42 ± 10.59 | 41.69 ± 11.71 | 0.003 | |
| Education, y | 13.89 ± 2.56 | 12.47 ± 2.80 | 0.013 | |
| Clinical presentations | 0.614 | |||
| TIA | 24 | 55 | ||
| Headache | 4 | 5 | ||
| Asymptomatic | 10 | 17 | ||
| Medical history, n(%) | ||||
| Diabetes mellitus | 4 | 8 | 1.000 | |
| Hypertension | 12 | 35 | 0.155 | |
| Hyperlipidemia | 5 | 15 | 0.400 | |
| Hyperhomocysteinemia | 8 | 18 | 0.779 | |
| Fazekas classification | 0.02 | |||
| 0 | 27 | 37 | ||
| 1 | 11 | 40 | ||
| Time from first onset to admission(months) | 11.92 ± 16.59 | 22.01 ± 41.19 | 0.174 | |
| Suzuki stage | ||||
| Left | 0.296 | |||
| 1–2 | 9 | 29 | ||
| 3–4 | 17 | 31 | ||
| 5–6 | 12 | 17 | ||
| Right | 0.835 | |||
| 1–2 | 13 | 22 | ||
| 3–4 | 18 | 41 | ||
| 5–6 | 7 | 14 | ||
| mRs | 0.202 | |||
| 0 | 3 | 1 | ||
| 1 | 35 | 76 | ||
| rCBF of ROI | ||||
| L-LFC | 1.44 ± 0.25 | 1.34 ± 0.30 | 0.026 | |
| L-MFC | 1.58 ± 0.36 | 1.56 ± 0.36 | 0.369 | |
| L-CSO | 0.60 ± 0.15 | 0.51 ± 0.13 | 0.002 | |
| L-PC | 1.41 ± 0.40 | 1.33 ± 0.33 | 0.277 | |
| L-TC | 1.27 ± 0.43 | 1.13 ± 0.32 | 0.216 | |
| R-LFC | 1.48 ± 0.34 | 1.41 ± 0.32 | 0.234 | |
| R-MFC | 1.58 ± 0.32 | 1.57 ± 0.29 | 0.909 | |
| R-CSO | 0.56 ± 0.12 | 0.50 ± 0.13 | 0.019 | |
| R-PC | 1.38 ± 0.30 | 1.33 ± 0.33 | 0.166 | |
| R-TC | 1.34 ± 0.42 | 1.21 ± 0.33 | 0.108 | |
rCBF: relative cerebral blood flow: CBF values of the associated cortex/ipsilateral cerebellum; L: left; r: right, LFC: lateral frontal cortex; MFC: medial frontal cortex; CSO: centrum semi-oval; PC: parietal cortex; TC temporal cortex.
Neuropsychological assessment of MMD patients and healthy controls
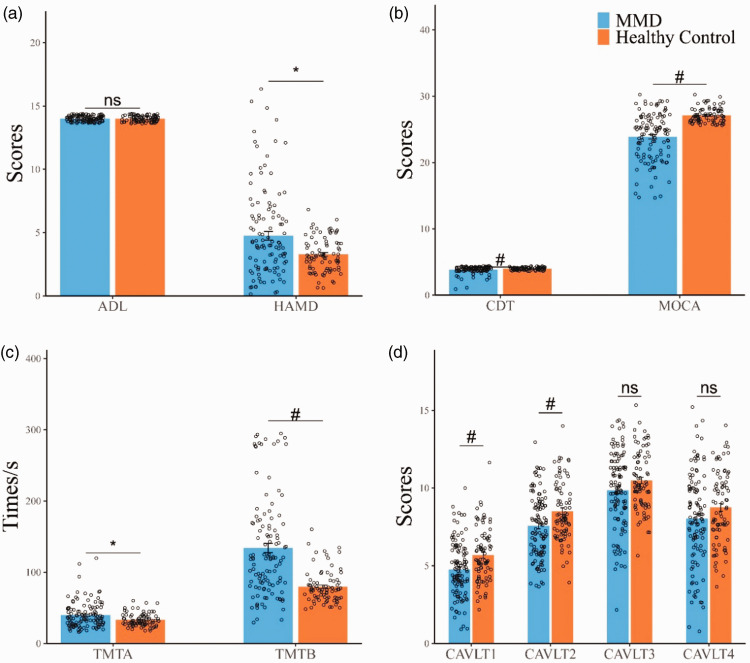
The test performances of the participants are summarized in Table 3 and Figure 2. The ADL scores were not abnormal in MMD patients compared with the healthy controls. The HAMD test showed that 28 patients (24.3%) were possibly depressed, but none were diagnosed with depression. The mean HAMD scores were significantly higher in patients (4.75 ± 3.66) than in controls (3.28 ± 1.32).
Table 3.
Neuropsychological assessments.
| MMD (n = 115) Mean ± SD | HC (n = 82) Mean ± SD | P | |
|---|---|---|---|
| MOCA | 23.88 ± 3.55 | 27.12 ± 1.08 | <0.001 |
| ADL | 14.00 ± 0 | 14.00 ± 0 | 1 |
| HAMD | 4.75 ± 3.66 | 3.28 ± 1.32 | 0.019 |
| TMTA | 39.71 ± 18.06 | 33.11 ± 9.03 | 0.025 |
| TMTB | 134.01 ± 69.85 | 79.73 ± 23.11 | <0.001 |
| CDT | 3.84 ± 0.52 | 4 ± 0 | 0.003 |
| CAVLT1 | 4.75 ± 1.74 | 5.68 ± 1.78 | 0.001 |
| CAVLT2 | 7.57 ± 1.98 | 8.51 ± 1.93 | 0.002 |
| CAVLT3 | 9.83 ± 2.41 | 10.49 ± 1.86 | 0.147 |
| CAVLT10 | 8.03 ± 2.93 | 8.76 ± 2.33 | 0.111 |
MOCA: Montreal Cognitive Assessment, ADL: The activity of Daily Living Scale; HAMD: The 17-item HamiltonRating Scale for Depression; TMTA: Trail Making Test A; TMTB: Trail Making Test B; CDT: clock drawn test; CAVLT1-3: Chinese Auditory Verbal Learning Test (transient memory); CAVLT10: Chinese Auditory Verbal Learning Test (delayed memory).
Figure 2.
(a,b,c,d) Comparison of cognitive function between MMD and healthy controls. Compared with the healthy controls matched by gender, age and education years, the adult MMD without stroke had a wide range of cognitive impairment, including MOCA, HAMD, CDT, TMTA, TMTB, CAVLT1, CAVLT2. Ns: no significant difference; *:0.01<p < 0.05: #p ≤ 0.01.
The MoCA scores were significantly lower (p < 0.001) in MMD patients than in healthy controls (23.88 ± 3.55 vs. 27.12 ± 1.08). Of the 115 patients, 77 (67.0%) were considered to have cognitive impairment.
In addition, the scores of TMTA (p = 0.025), reflecting information processing speed, and TMTB (p < 0.001), reflecting executive function, were both significantly higher in the MMD group than in the control group. Scores of transient memory (CAVLT1 and CAVLT2, both p < 0.01) and visuospatial praxis (CDT, p = 0.019) were lower in the MMD group than in the control group. CAVLT3 and CAVLT10 scores were lower in the MMD group than in the control group, although not significantly.
Multiple linear regression analysis of cognitive score and cerebral blood flow
Multiple linear regression was used to analyze the relationship between cognitive scores and rCBF in different regions to avoid confounding factors. The B value represents the regression coefficient of rCBF after adjusting for age and educational level. The TMTB score negatively correlated with rCBF in the bilateral lateral frontal lobes, CSO, and temporal cortex. The TMTA score negatively correlated with rCBF in the bilateral CSO and left temporal cortex. CAVLT1, CAVLT2, CAVLT3, and CAVLT10 scores positively correlated with rCBF in the left CSO (L-CSO) and temporal cortex. Additionally, CAVLT3 score positively correlated with the left lateral frontal lobe, and CAVLT10 score positively correlated with the right CSO (R-CSO). However, no correlation was observed between CDT scores and ROIs (Table 4).
Table 4.
Correlation coefficient between rCBF and scores/times of cognitive scale.
| ROI | Cognitive scale | B | P |
|---|---|---|---|
| L-LFC | TMTB | −45.78 | 0.009 |
| CAVLT3 | 1.61 | 0.04 | |
| L-CSO | TMTA | −31.58 | 0.008 |
| TMTB | −114.92 | 0.001 | |
| CAVLT1 | 3.7 | 0.001 | |
| CAVLT2 | 4.23 | 0.001 | |
| CAVLT3 | 4.40 | 0.005 | |
| CAVLT10 | 4.06 | 0.022 | |
| L-TC | TMTA | −10.26 | 0.02 |
| TMTB | −33.00 | 0.011 | |
| CAVLT1 | 3.7 | 0.001 | |
| CAVLT2 | 1.36 | 0.005 | |
| CAVLT3 | 1.34 | 0.021 | |
| CAVLT10 | 1.31 | 0.046 | |
| R-LFC | TMTB | −38.29 | 0.011 |
| R-CSO | TMTA | −39.37 | 0.003 |
| TMTB | −125.43 | 0.001 | |
| CAVLT10 | 3.93 | 0.046 | |
| R-TC | TMTB | −38.02 | 0.003 |
Multiple linear regression analysis was used to analyze the relationship between cognitive score and rCBF in different region. The B value represents the regression coefficient of the rCBF after adjusting age and education level.
rCBF: relative cerebral blood flow: CBF values of the associated cortex/ipsilateral cerebellum; L: left; R: right; LFC: lateral frontal cortex; MFC: medial frontal cortex; CSO: centrum semi-oval; PC: parietal cortex; TC: temporal cortex.
Association between cognitive impairment and hypoperfusion
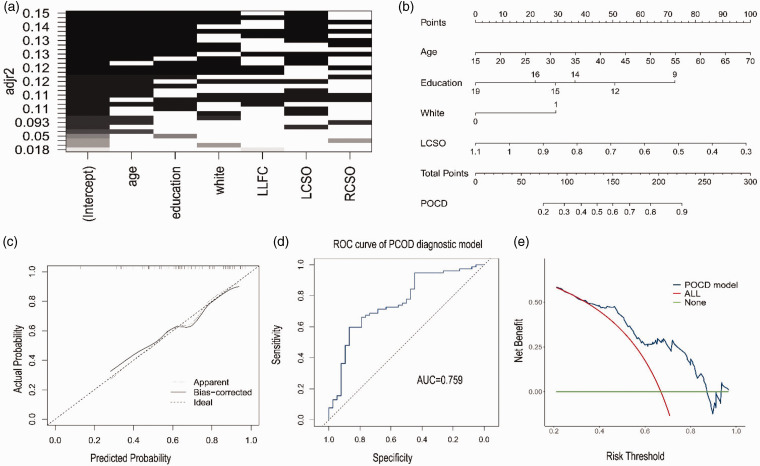
To assess the overall cognitive function, we further divided the patients with MMD into the normal (MoCA score ≥ 26) and cognitive impairment groups (MoCA score < 26). A comparison of clinical information and perfusion data is shown in Table 2. We subsequently incorporated variables with p < 0.1 into the BSR. None of the variables met the multicollinearity criterion (VIF > 10). The optimal subset was determined according to the adjusted maximum value of R-squared. Four variables, namely age, education, white matter lesions (Fazekas scale), and rCBF of the L-CSO, were finally included as predictors. A column chart was then constructed (Figure 3(a)). A nomogram was established based on the BSR. Age had the greatest influence on cognitive impairment, followed by rCBF of L-CSO, education, and white matter damage. The risk of cognitive decline in patients with MMD positively correlated with the sum of the scores for the four predictive variables in the prediction model (Figure 3(b)). As shown in the calibration plot, internal validation of the model revealed a good predictive performance for MMD patients with cognitive impairment, with a mean absolute error of 0.025 (Figure 3C). Moreover, the area under the ROC curve was 0.759 (95% CI, 0.66–0.85; Figure 3(d)). The clinical decision curve suggests that the prediction model has a highnet clinical benefit (Figure 3(e)).
Figure 3.
Risk factors analysis of cognitive function. (a) The optimal subset is determined according to the adjusted maximum value of R-squared. Age, education, white matter (Fezakas scale) and rCBF of left centrum semi-oval (LCSO) were selected as potential risk factors of cognitive impairment. (b) Characteristics in the nomogram to predict probability of cognitive function in MMD patients without stroke. (c) Calibration curve of the predictive nomogram for probability of cognitive function in MMD patients without stroke. (d) The area under the curve (AUC) of a receiver operating curve (ROC) and (e) Decision curve analysis (DCA) was used to assess net clinical benefit of the model.
Discussion
The present study, which has the largest sample size compared to previous studies, analyzed the correlation between cognitive function and hypoperfusion in 115 non-stroke adult patients with MMD. The results show that long-term hypoperfusion leads to multiple cognitive domain impairments in MMD patients, such as information processing speed, executive function, visual-spatial function, and memory. Cerebral hypoperfusion in specific brain regions (the lateral frontal lobes, CSO, and temporal cortex) is closely associated with cognitive impairment. Moreover, using the BSR strategy, age, education, white matter damage, and rCBF of the L-CSO were identified as potential risk factors for cognitive impairment in MMD.
Previous studies have documented cognitive decline in some adult patients with MMD, with incidence of 23%–71.4%.5–8,26,30 The cognitive impairment is mainly manifested in intelligence, memory, and executive function.6,8,31–33 Different results may be related toethnicity, age, and grouping or evaluation criteria. However, a core question is whether cognitive impairment in MMD can be attributed to progressive cerebral hypoperfusion or irreversible cerebrovascular events, such as cerebral infarction. This difference is of great therapeutic value because low cerebral perfusion may be ameliorated by cerebral revascularization, unlike severe cerebral infarction. Previous studies have shown that revascularization can prevent further decline in cognitive function in adults 34 and children 35 and even improve it in some patients. 36 Our findings suggest that patients with MMD have cognitive impairment in different domains under long-term hypoperfusion conditions, especially in terms of information processing speed, executive function, visual-spatial function, and immediate memory.
Furthermore, our results reveal that the long-term hypoperfusion state affects the cognitive function of patients with MMD earlier than the occurrence of stroke events and traditional ischemic symptoms. Few studies have been conducted on cognitive impairment in MMD caused by hypoperfusion. Karzmark’s research showed that 23% of stroke-free MMD patients had significant cognitive impairment. 8 Executive function, mental efficiency, and word finding were most frequently impaired, whereas memory was relatively intact, and 37% of patients showed emotional distress. Our study showed a 67% incidence of cognitive impairment according to MoCA. The main cognitive impairments were related to information processing speed, executive ability, transient memory, visuospatial ability, and emotional distress. These results revealed early manifestations of cognitive impairment in MMD that could be used as evidence of brain function damage caused by long-term hypoperfusion. Different memory results may be related to different criteria and research methods used for assessing cognitive impairment.6,8,32,33 Regardless of the outcome differences, these studies suggest that cognitive impairment occurs in a high proportion of MMD patients without stroke.
In our study, 7.8% had a headache, and 23.5% were asymptomatic. One-third of patients without typical ischemic symptoms had stable hemodynamics. There has been controversy over whether surgery should be performed in these patients, but the need of cognitive assessment has been overlooked. The introduction of cognitive impairment into surgical indications may lead to significant changes in the indications for treatment of these patients. However, the unification of criteria and assessment methods for cognitive impairment may be the next important step.
Recent investigations have shown an association between the frontal lobe and executive function, 29 and between the temporal lobe and memory function. 37 This study focused on the relationship between brain regions and cognitive function. The application of perfusion technology in MMD is well documented. The results indicate that the decrease in perfusion in the bilateral centrum semiovale and left temporal cortex is related to executive function, speed of information processing, and auditory-verbal memory. The decreased perfusion in the bilateral lateral frontal cortex and the right temporal cortex is also related to executive function. We found that perfusion in the left hemisphere had a greater effect on cognitive function than did perfusion in the right hemisphere, consistent with previous research. 38 Notably, previous studies have suggested that cognitive impairment in these areas may be caused by stroke. Our findings indicate that long-term hypoperfusion can induce cognitive impairment. Cognitive function changes in MMD occur gradually with a decrease in intracranial perfusion and not immediately after a stroke. It also is a certain reference value for other types of chronic ischemic disease, such as intracranial atherosclerotic stenosis (ICAS), for which there is no definite indication for surgery or intervention in patients with ICAS, 39 especially in mild ICAS. However, different conclusions may be drawn if cognitive improvement is considered as the clinical trial endpoint. We can provide necessary references for surgical indications in patients with no-stroke MMD and other chronic hypoperfusion cerebrovascular disease through cognitive evaluations, because cerebral hypoperfusion is potentially reversible through surgical intervention before more serious cerebrovascular events take place.
According to the comparison between the abnormal (MoCA score <26) and normal (MoCA score ≥26) groups, the nomogram showed that age, rCBF in the L-CSO, education, and white matter damage were risk factors for cognitive impairment. Age had the greatest influence, followed by rCBF of the L-CSO, education, and white matter lesions. Previous studies have shown that cognitive impairment is closely related to age and white matter lesions.29,32 Higher education can also provide experiential knowledge, which may partly compensate for the cognitive impact of MMD. 31 However, the influence of L-CSO hypoperfusion on cognition has not drawn sufficient attention. As the communication center of fibers and the dominant hemisphere, the CSO significantly impacts executive function, information processing speed, and memory, thus having the greatest effect on cognition.
Limitations
Our study had some limitations. First, obtaining a demographically adjusted standard for part of the cognitive scale is difficult. Second, ethnic and population biases may have been introduced. Third, follow-up data on cognitive function after surgery were not included.
Conclusion
Cognitive impairment caused by hypoperfusion occurs earlier than that caused by stroke in patients with MMD. Our study provides evidence of cognitive impairment in non-stroke patients with MMD, in terms of information processing speed, executive function, visual-spatial function, and memory. Cerebral hypoperfusion in specific brain regions is closely associated with cognitive impairment, especially CSO. Older Age, lower education, white matter damage, and hypoperfusion of the L-CSO are potential risk factors for cognitive impairment in non-stroke patients with MMD.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from China's National Natural Science Foundation (Grant No.82172021 and 82171280).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: XXS: first author; design and conceptualisation the study, acquisition of data, analysis of the data and drafting of the manuscript. HDZ: co-first author; responsible for the cognitive scale measurement of patients, drafting of the manuscript. FHG; JLX; HTZ: acquisition of data, analysis of the data. LH;ZXZ; BL; FBH:critically revising the article. LD;CH: corresponding author; drafting and revision for intellectual content; modify the manuscript.
ORCID iD: Jia-Li Xu https://orcid.org/0000-0002-3651-4383
References
- 1.Scott RM, Smith ER. Disease and Moyamoya syndrome. N Engl J Med 2009; 360: 1226–1237. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki J, Takaku A. Cerebrovascular ‘Moyamoya’ disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20: 288–299. [DOI] [PubMed] [Google Scholar]
- 3.Duan L, Bao X-Y, Yang W-Z, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke 2012; 43: 56–60. [DOI] [PubMed] [Google Scholar]
- 4.Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of Moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 2012; 52: 245–266. [DOI] [PubMed]
- 5.Festa JR, Schwarz LR, Pliskin N, et al. Neurocognitive dysfunction in adult Moyamoya disease. J Neurol 2010; 257: 806–815. [DOI] [PubMed] [Google Scholar]
- 6.Karzmark P, Zeifert PD, Tan S, et al. Effect of Moyamoya disease on neuropsychological functioning in adults. Neurosurgery 2008; 62: 1048–1051; discussion 1051–1042. [DOI] [PubMed] [Google Scholar]
- 7.Kronenburg A, van den Berg E, van Schooneveld MM, et al. Cognitive functions in children and adults with Moyamoya vasculopathy: a systematic review and meta-analysis. J Stroke 2018; 20: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karzmark P, Zeifert PD, Bell-Stephens TE, et al. Neurocognitive impairment in adults with Moyamoya disease without stroke. Neurosurgery 2012; 70: 634–638. [DOI] [PubMed] [Google Scholar]
- 9.Kazumata K, Tokairin K, Ito M, et al. Combined structural and diffusion tensor imaging detection of ischemic injury in Moyamoya disease: relation to disease advancement and cerebral hypoperfusion. J Neurosurg 2020; 134: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Cavanagh J, Sheridan M, et al. Factor structure of the Montreal cognitive assessment in Parkinson disease. Int J Geriatr Psychiatry 2020; 35: 188–194. [DOI] [PubMed] [Google Scholar]
- 11.Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal cognitive assessment (MoCA) screening superior to the mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int Psychogeriatr 2019; 31: 491–504. [DOI] [PubMed] [Google Scholar]
- 12.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009; 73: 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM. Assessment of older people self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–186. [PubMed] [Google Scholar]
- 14.Lahav O, Katz N. Independent older adult's IADL and executive function according to cognitive performance. OTJR (Thorofare N J) 2020; 40: 183–189. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CH, Park C, McIntyre RS. Early improvement in hamd-17 and hamd-7 scores predict response and remission in depressed patients treated with fluoxetine or electroconvulsive therapy. J Affect Disord 2019; 253: 154–161. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto S, Mogi N, Umegaki H, et al. The clock drawing test as a valid screening method for mild cognitive impairment. Dement Geriatr Cogn Disord 2004; 18: 172–179. [DOI] [PubMed] [Google Scholar]
- 18.Leung AW, Cheng SK, Mak AK, et al. Functional gain in hemorrhagic stroke patients is predicted by functional level and cognitive abilities measured at hospital admission. NeuroRehabilitation 2010; 27: 351–358. [DOI] [PubMed] [Google Scholar]
- 19.Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, et al. Assessing the impact of physical exercise on cognitive function in older medical patients during acute hospitalization: secondary analysis of a randomized trial. PLoS Med 2019; 16: e1002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossoni ED, Kael MA. The Trail Making Test A and B: a technical note on structural nonequivalence. Percept Mot Skills 1994; 78: 625–626. [DOI] [PubMed] [Google Scholar]
- 21.Tombaugh T. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–214. [DOI] [PubMed] [Google Scholar]
- 22.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 t in Alzheimer’ dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 23.Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008; 64: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao PG, Han C, Zuo ZW, et al. Clinical assessment of cerebral hemodynamics in Moyamoya disease via multiple inversion time arterial spin labeling and dynamic susceptibility contrast-magnetic resonance imaging: a comparative study. J Neuroradiol 2017; 44: 273–280. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZW, Han C, Zhao F, et al. Collateral circulation in Moyamoya disease: a new grading system. Stroke 2019; 50: 2708–2715. [DOI] [PubMed] [Google Scholar]
- 26.Calviere L, Catalaa I, Marlats F, et al. Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in european adults with moyamoya disease. Clinical article. J Neurosurg 2010; 113: 753–759. [DOI] [PubMed] [Google Scholar]
- 27.Kazumata K, Tokairin K, Sugiyama T, et al. Association of cognitive function with cerebral blood flow in children with moyamoya disease. J Neurosurg Pediatr 2019; 11: 1–7. [DOI] [PubMed] [Google Scholar]
- 28.Kazumata K, Tha KK, Tokairin K, et al. Brain structure, connectivity, and cognitive changes following revascularization surgery in adult Moyamoya disease. Neurosurgery 2019; 85: E943–E952. [DOI] [PubMed] [Google Scholar]
- 29.Calviere L, Ssi Yan Kai G, Catalaa I, et al. Executive dysfunction in adults with Moyamoya disease is associated with increased diffusion in frontal white matter. J Neurol Neurosurg Psychiatry 2012; 83: 591–593. [DOI] [PubMed] [Google Scholar]
- 30.Roder C, Haas P, Fudali M, et al. Neuropsychological impairment in adults with Moyamoya angiopathy: preoperative assessment and correlation to mri and h2(15)o pet. Neurosurg Rev 2020; 43: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 31.He S, Duan R, Liu Z, et al. Characteristics of cognitive impairment in adult asymptomatic moyamoya disease. BMC Neurol 2020; 20: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, He S, Xu Z, et al. Association between white matter impairment and cognitive dysfunction in patients with ischemic Moyamoya disease. BMC Neurol 2020; 20: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Z, Wen YJ, Huang Z, et al. Different aspects of cognitive function in adult patients with Moyamoya disease and its clinical subtypes. Stroke Vasc Neurol 2020; 5: 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeifert PD, Karzmark P, Bell-Stephens TE, et al. Neurocognitive performance after cerebral revascularization in adult moyamoya disease. Stroke 2017; 48: 1514–1517. [DOI] [PubMed] [Google Scholar]
- 35.Matsushima Y, Aoyagi M, Masaoka H, et al. Mental outcome following encephaloduroarteriosynangiosis in children with Moyamoya disease with the onset earlier than 5 years of age. Childs Nerv Syst 1990; 6: 440–443. [DOI] [PubMed] [Google Scholar]
- 36.Baek HJ, Chung SY, Park MS, et al. Preliminary study of neurocognitive dysfunction in adult Moyamoya disease and improvement after superficial temporal artery-middle cerebral artery bypass. J Korean Neurosurg Soc 2014; 56: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichenbaum H. On the integration of space, time, and memory. Neuron 2017; 95: 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron 2014; 83: 1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahab F, Liu M, Rahman HA, et al. Recurrent hemispheric stroke syndromes in symptomatic atherosclerotic internal carotid artery occlusions: the carotid occlusion surgery study randomized trial. Neurosurgery 2020; 87: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]