Abstract
Adipose tissue develops lipids, aberrant adipokines, chemokines, and pro-inflammatory cytokines as a consequence of the low-grade systemic inflammation that characterizes obesity. This low-grade systemic inflammation can lead to insulin resistance (IR) and metabolic complications, such as type 2 diabetes (T2D) and nonalcoholic fatty liver disease (NAFLD). Although the CXC chemokines consists of numerous regulators of inflammation, cellular function, and cellular migration, it is still unknown that how CXC chemokines and chemokine receptors contribute to the development of metabolic diseases (such as T2D and NAFLD) during obesity. In light of recent research, the objective of this review is to provide an update on the linkage between the CXC chemokine, obesity, and obesity-related metabolic diseases (T2D and NAFLD). We explore the differential migratory and immunomodulatory potential of CXC chemokines and their mechanisms of action to better understand their role in clinical and laboratory contexts. Besides that, because CXC chemokine profiling is strongly linked to leukocyte recruitment, macrophage recruitment, and immunomodulatory potential, we hypothesize that it could be used to predict the therapeutic potential for obesity and obesity-related diseases (T2D and NAFLD).
Keywords: Obesity, CXC chemokines, Type 2 diabetes, Nonalcoholic fatty liver disease, Therapeutic potential, Inflammation
Introduction
Obesity is increasing at an alarming rate around the world. Statistics from around the world show an epidemic rise in all age groups. Obesity affects one out of every six children aged 2 to 19, and the rate has more than tripled in the last 20 years. During this time, the prevalence of severe obesity nearly doubled, rising from 4.7 to 9.2% [1]. Moreover, obesity is a significant risk factor for several cancers, including insulin resistance (IR), type 2 diabetes (T2D), immune disorders, cardiovascular diseases (CVD), and nonalcoholic fatty liver disease (NAFLD). Overall, obesity is associated with a reduced life span, higher healthcare costs, and a reduced quality of life [2–6]. The overweight, obese individual has been found to have a spectrum of metabolic abnormalities, oxidative stress, mitochondrial dysfunction, immune dysfunction, and chronic low-grade inflammation [7, 8]. In addition, a recent review study reported that exercise prevented weight gain, weight loss, and maintenance of weight loss in obese individuals. Weight loss has been associated with improvements in the prevalence and severity of several obesity-associated comorbidities, such as IR, inflammation, dyslipidemia, hypertension, the metabolic syndrome, diabetes, pulmonary disease, and CVD [9].
Besides that, it has been shown that chemokines coordinate the recruitment of immune cells during obesity, T2D and CVD in both mice and humans which cause inflammation [10]. It has been suggested that adipocytes in obese adipose tissue recruit neutrophils, which then further promote inflammation. Adipocytes produce adipokines such as leptin and chemokine such as interleukin-8 (IL-8 or CXCL8). CXCL8 is a potent chemoattractant for neutrophils. Once in the adipose tissue, neutrophils can recruit more blood neutrophils by releasing C–X–C motif chemokine ligand 2 (CXCL2), another important neutrophil chemoattractant. This study demonstrated that CXC chemokines play an important role in the inflammation during obesity [11].
There are currently 17 CXC chemokines in humans, most of which plays a role in obesity and obesity-related diseases [12–14]. During obesity, the proinflammatory effects of CXCL1, CXCL5, CXCL8, and CXCL14 mainly lead to tumor cell growth and IR [15–20]. CXCL16/CXCR6 axis plays an important role in the recruitment of NKT (Natural killer T) cell and induce inflammation in NALFD [21, 22]. Moreover, immune cell infiltration is stimulated by CXCL9, CXCL10, and CXCL11 via CXCR3 [23]; T lymphocyte and monocyte recruitment are mostly triggered by CXCL12 and CXCR4 [24]; and T cell recruitment is mainly promoted by CXCL16/CXCR6 [25]. CXCL17 also plays an important role in the development of NAFLD [26]. These molecules play proinflammatory and cytotoxic roles during obesity-induced diabetes. Furthermore, CXCL13 is crucial for the chemotaxis and activation of leukocytes in diabetes [27]. Obesity and disorders related to obesity have been linked to CXC chemokines, which suggests that they play a vital role in the development of obesity-related disorders. As a consequence, CXC chemokines might be prospective therapeutic targets for a number of obesity-related disorders. In this article, we provide an overview of the numerous functions of CXC chemokines ligands and chemokine receptors in obesity and the obesity-related disorders such as T2D and NAFLD.
Methods
This is a narrative review supported by a PubMed and Google Scholar literature search. The search was conducted from August 2022 to February 2023. Additional information is provided in Table 1.
Table 1.
The search strategy summary
| Items | Specification |
|---|---|
| Date of search | August 2022 – February 2023 |
| Databases and other sources searched | PubMed and Google Scholar |
| Search terms used | CXC chemokines (CXCL1 to CXCL17), obesity, type 2 diabetes, nonalcoholic fatty liver diseases, simple steatosis, nonalcoholic steatohepatitis, cirrhosis, liver fibrosis, hepatocellular carcinoma, |
| Timeframe | 1998–2023 |
| Inclusion and exclusion criteria | English language only, original studies and reviews only |
| Selection process | West China Hospital Sichuan University |
| Any additional considerations, if applicable | We considered clinical/ preclinical studies, original studies, as well as data from previously published reviews. |
CXC chemokine family
Chemokines are small peptide mediators composed of a group of small chemotactic cytokine proteins (15 kDa). Different cell types secret these proteins following induction, or they may be constitutively expressed [28]. Chemokines affect the migration, proliferation, angiogenesis, survival, and gene expression of numerous cell types in their respective microenvironments [28–30]. Chemokines can exert these effects via their respective G protein-coupled receptors (GPCRs).
Chemokines are classified into four subfamilies based on the location of the first two cysteines (C) in the main sequence, where “X” signifies an unconserved amino acid. CXC chemokines are further divided into ELR + and ELR- subtypes depending on whether the three-amino-acid motif ELR (glutamic acid, leucine, and arginine) is present or absent before the CXC sequence [31]. CXC chemokine ligands are related to trimeric G-proteins (Gαβγ), and the actions of four different types of Gα subunits determine activation effects inside cells [32–34].
Gαs and Gαi regulate cAMP levels by stimulating and inhibiting adenylate cyclase (AC), respectively; cAMP can further activate protein kinase PKA [34]. Gαq stimulates phosphatidylinositol (PI)-specific phospholipase C (PLC) and the hydrolysis of phosphatidylinositol biphosphate (PIP2), generating twosecond messengers, diacylglycerol (DG) and inositol 1,4,5-triphosphate (IP3); DG and IP3 can activate protein kinase PKC and stimulate intracellular calcium release [33]. Gβγ complex has also been reported to trigger PLC activation. Gα12 exerts its functions primarily through other small monomeric G-proteins [33]. Further downstream of chemokine receptor pathways, phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription (STAT), and nuclear factor kB (NF-kB) cascades represent four major events promoting cell survival and chemotaxis [32].
The common receptor for ELR + CXC chemokines is CXCR2, except for CXCL8, which can also bind to CXCR1. There are three different growth-regulatory oncogenes: CXCL1, CXCL2, and CXCL3. The ELR- family chemokine CXCL14 has been shown to have a high binding affinity for CXCR4, which lets it interact with the CXCL12/CXCR4 axis [35]. Generally, CXCR2-binding ELR+CXC chemokines can enhance angiogenesis by activating CXCR2 on endothelial cells, but CXCR3-binding ELR− CXC chemokines have the inverse effect [35]. The biological roles played by CXC chemokines in obesity and obesity-related disorders such as T2D and NAFLAD are discussed further below.
Role of CXC chemokines in the development of obesity
Previous research has shown that chemokines can aid in progression of morbid obesity by promoting inflammation in various obesity-related disorders. As shown in Table 2, diseases associated with obesity have significantly higher levels of CXC chemokines, which promote obesity and the pathologies linked to it.
Table 2.
Studies on the upregulation of CXC-chemokines in obesity and obesity-related disorders
| Family | Disease/indicators of obesity | Types of study | Abbreviations/ scientific name | Conclusion/effects | References |
|---|---|---|---|---|---|
| ELR+ | Ovarian cancer, Prostate cancer and NAFLD | In vitro, In vivo | Gro-α/CXCL1 |
Angiogenesis in ovarian cancer cells. ↑ promotes prostate cancer progression. ↑ Promote systemic inflammation. ↑ |
[36, 15, 37, 38] |
| Obese oocytes, Metabolic syndrome, Adipose tissue inflammation | In vivo | GROβ/CXCL2 | Promote inflammation. ↑ | [39–41] | |
| Obesity, Cervical cancer | In vitro, In vivo | GROγ/ CXCL3 |
Malignancy-associated capacities such as migration. ↑ Improvement obesity-related comorbidities. ↑ Promote systematic inflammation and insulin resistance. ↑ |
[42–44] | |
| Lymphatic vasculature dysfunction | In vivo | PF4/CXCL4 | PF4 is a promising biomarker with lymphatic defects independent of the presence or absence of obesity. ↑ | [45] | |
| Prostate Lung Colorectal and Ovarian cancer and Obesity | In vivo | GCP-2 /CXCL6 | Worked out as an inflammatory biomarker. ↑ | [46, 47] | |
| NAFLD, Obese obstructive sleep apnea and Metabolic inflammation at the maternal–fetal interface | In vitro, In vivo | IL -8 /CXCL8 | Promote complex inflammation. ↑ | [48–50, 51–54] | |
| NASH, Obesity and Psoriasis with obesity | In vivo | MIG/CXCL9 | Promote Inflammation. ↑ | [55–57] | |
| ELR− |
Obesity, Ovarian Inflammation, Diabetes, Diliary inflammation and NAFLD |
In vitro, In vivo |
IP-10/CXCL10 and I-TAC/ CXCL11 |
Work as an Inhibitor of adipose tissue angiogenesis and promote inflammation. ↑ | [58–62] |
| Breast cancer and Obesity | In vitro, In vivo | SDF-1α/CXCL12 |
Regulating metastasis of breast cancer. ↑ Promote inflammation. ↑ |
[63, 64] | |
| Obesity models and Diabetes | In vitro, In vivo | BRAK/CXCL14 |
CXCL14 promotes the recruitment of M2-type macrophages. ↑ Promote inflammation. ↑ Regulator of glucose metabolism. ↑ Associated with inflammation or lipid metabolism. ↑ |
[65, 66, 19, 67–69] | |
| Obesity, Diabetes and Metabolic Syndrome in Psoriasis | In vivo | SR-PSOX/CXCL16 |
Accumulation and alterations in lipid metabolism. ↑ Associated with breast adipocyte hypertrophy in African American women. ↑ Innate lymphoid cells activation and tissue distribution in obese psoriatic patients. ↑ |
[70–72] |
The ↑ symbol represented up-regulation of CXC-chemokines during obesity and obesity related disorders types 2 diabetes and nonalcoholic fatty liver diseases
CXCL1 and CXCL2
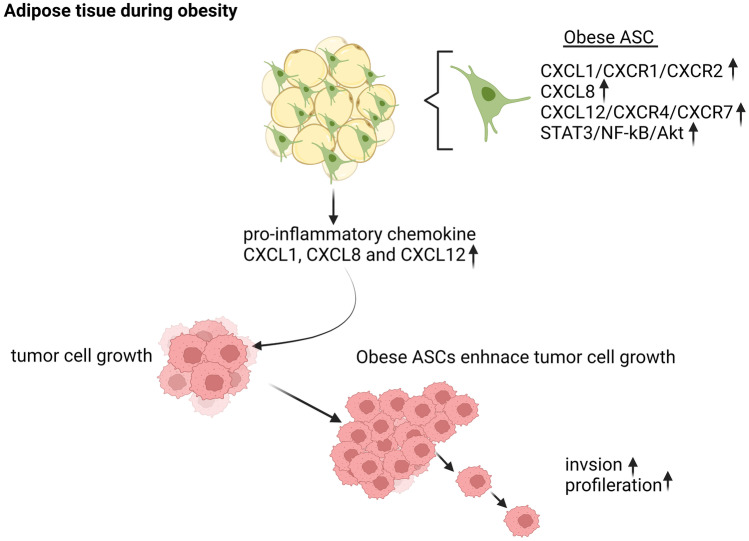
In various adipose tissue depots, chemokines like CXCL1, CXCL5, CXCL8, and CXCL10 are upregulated in obesity. Compared to lean individuals, obese people have significantly higher serum levels of these chemokines. Adipose stromal cells (ASCs) associated with obesity have higher levels of the chemokines CXCL1 and CXCL8, as well as their receptors CXCR1 and CXCR2, which regulate ASCs (CD34bright CD45-CD31-) trafficking and function in the tumor microenvironment ( Fig. 1) [15, 16]. According to Hariharan et al., bladder-derived ASCs secrete CXCL1, which is crucial for migrating bladder cancer cells. Depleting CXCL1 in an obese patient’s conditioned media from ASCs prevented the migration of T24 bladder cancer cells [73]. A recent bioinformatics study found that the protein-protein interaction network in obese people is controlled by the hub genes CXCL1, CXCL2, CXCL8, and CXCL12 [41]. A recent clinical study also found that the proinflammatory genes CXCL1, CXCL12, and CXCL6 were significantly hypomethylated in the blood of obese individuals. This suggests that vascular dysfunction in obese adults may be caused by a systemic hypomethylation and increased expression of immune-related genes [47].
Fig. 1.
The effect of CXCL1, CXCL8 and CXCL12 in obesity on adipose-derived stromal cells and their impact on the tumor cell growth environment. During obesity, pro-inflammatory chemokines CXCL1, CXCL8 and receptors CXCR1 and CXCR2, as well as chemokine CXCL12 signaling via receptors CXCR4 and CXCR7, activate tumor cell growth and invasion pathways (STAT3, NF-kB, and AKT) in adipose stromal cells. Created with BioRender.com
CXCL5 and CXCL8
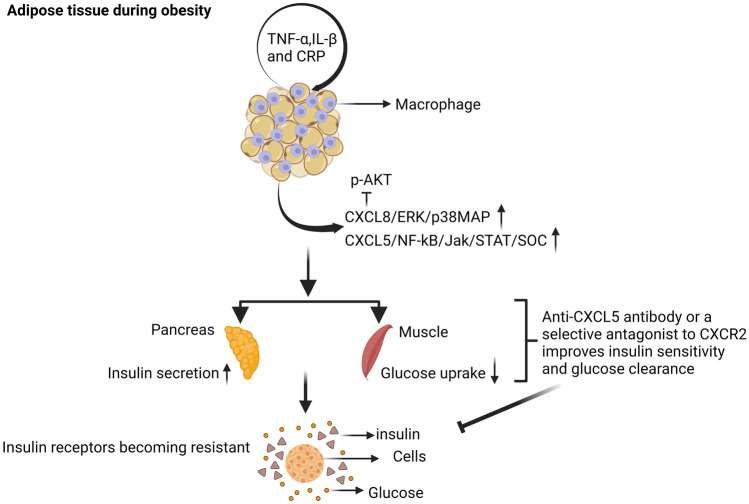
CXC Ligand 5 (CXCL5) is a chemokine that suppresses insulin action in muscles while also promoting IR. It is released by white adipose tissue during obesity [17]. In both mice and humans, circulating CXCL5 and its receptor CXCR2 are significantly increased during obesity [17, 74]. CXCL5 encodes one of the chemokines involved in the recruitment of immune cells. In this context, the adipose tissue of obese patients secretes free chemokines that enhance monocyte chemotaxis and macrophage infiltration [75]. In addition, CXCL5 blocks insulin action in muscle via stimulating the Janus kinase/signal transducers and activators of transcription/ suppressor of cytokine signaling protein (Jak/STAT/SOC) signaling pathway, suggesting its ability to cause in IR. IR patients have a higher CXCL5 concentration than non-IR obese patients. Furthermore, CXCL5 is directly regulated by tumor necrosis factor alpha (TNF-α) in adipose tissue and macrophages via NF-kB activation, indicating that CXCL5 mediates the effects of TNF-α on IR. Significantly inhibition of signaling from CXCR2, the CXCL5 receptor, by injection of a neutralizing anti-CXCL5 antibody or a selective antagonist to CXCR2 improves insulin sensitivity and glucose clearance in insulin-resistant obese mice. Thus, these findings show that CXCL5 promotes IR, and its suppression and/or elimination may be considered as a therapeutic strategy for treating metabolic syndrome (Fig. 2) [17, 74].
Fig. 2.
Effect of CXCL5 and CXCL8 on insulin resistance associated with obesity. CXCL5 is produced in response to TNF-α by adipose tissue-resident macrophages. Adipose tissue-resident macrophages also produce CXCL8 in response to TNF-α, IL-β, and CRP. CXCL5 activates the NF/kB/Jak/STAT/SOC signaling pathways and CXCL8 activates the ERK/p38MAP signaling pathways, which shows that CXCL5 and CXCL8 can induce insulin resistance inhibition of the insulin-induced p-Akt pathway. Inhibition of signaling from CXCR2, by injection of a neutralizing anti-CXCL5 antibody or a selective antagonist to CXCR2 improves both insulin sensitivity and glucose clearance in insulin-resistant obese mice. Created with BioRender.com
Cytokines (i.e. TNF- α, Interleukin 6 (IL-6) and Interleukin-1 beta (IL-1 β) cause inflammation. They are already known to be released by white adipose tissue [76] also several chemokines, including CXCL8 also known Interleukin 8 (IL-8) and CCL2 [77, 78]. Recent studies have found elevated levels of CXCL8 in obese individuals [54, 79–81]. CXCL8 represents the α and β chemokines, respectively, and may contribute to the adipose tissue inflammation via chemotaxis of inflammatory cells such as monocytes/macrophages, neutrophils and mast cells. CXCL8 is secreted by adipocytes, monocytes, macrophages, T-lymphocytes, and endothelial cells [80–82]. Furthermore, references reported that the level of CXCL8 in the medium and CXCL8 mRNA expression were significantly increased in human adipocytes after stimulation with TNF-α, IL-1β, or c-reactive protein (CRP) [18, 83]. CXCL8 is a key adipocytokine that leads to IR in adipocytes by blocking the insulin signal phosphorylates a serine/threonine protein kinase (p-AKT) pathway through the extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (p38MAP) kinase pathways during obesity (Fig. 2) [18]. Stimulation of CXCL8 action could be a target for obesity intervention strategies and complications.
CXCL12 and CXCL14
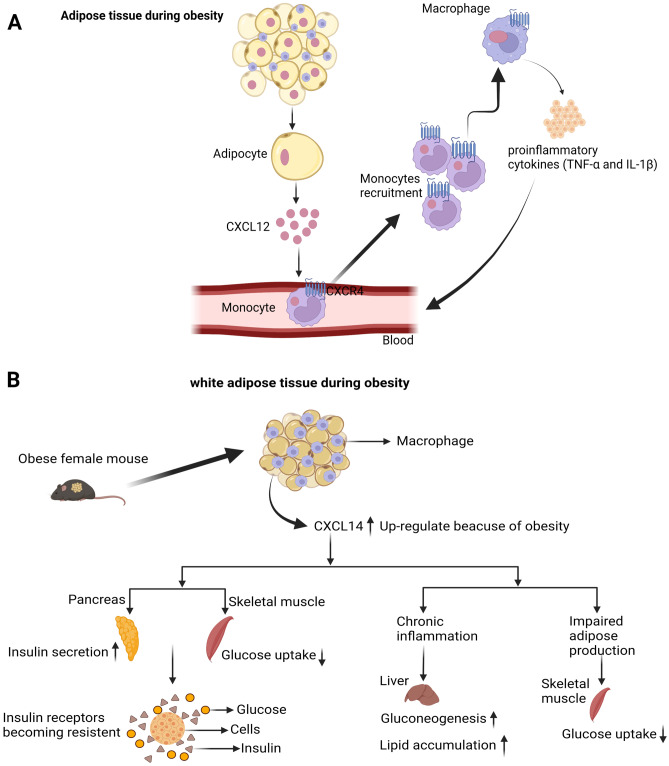
A recent study found that the chemokine CXCL12, which activates tumor cell growth and invasion pathways (STAT3, NF-kB, and AKT) in ASCs obtained from white adipose tissue of obese HiMyc mice via receptors CXCR4 and CXCR7, is responsible for accelerated prostate tumor growth in obesity (Fig. 1) [84]. Also, Su et al. found that CXCL12 signaling in the prostate epithelium from ASCs promotes prostate cancer in obese individuals [85]. Numerous studies have shown that CXCL12 is highly expressed in adipose tissue during obesity, indicating that CXCL12 and its receptors (CXCR4/CXCR7) play a significant role in obesity [41, 47, 85]. CXCL12 has also been identified as an adipokine that stimulates systemic IR, obesity-related inflammation, and macrophage recruitment to adipose tissue (Fig. 3A) [86].
Fig. 3.
CXCL12 -derived macrophage recruitment in adipose tissue and CXCL14’s metabolic regulator functions. A Adipocytes secrete CXCL12 during obesity, which recruits monocytes into adipose tissue via its receptor CXCR4. Mature macrophages, which have been differentiated from monocytes, secrete proinflammatory mediators, which may lead to systemic insulin resistance. B On the basis of the phenotypic abnormalities of CXCL14-deficient female mice fed an HFD, CXCL14 is implicated in obesity-induced insulin resistance. Organs and consequences of CXCL14 action are illustrated schematically. Created with BioRender.com. ↑ arrow symbol shows up-regulated while ↓ arrow show downregulated
CXCL14 (also known as BRAK, BMAC, or Mip-2γ) is found in skeletal muscle, white adipose tissue, and brown adipose tissue, implying that it may be involved in myogenesis, adipogenesis, and metabolic regulation. CXCL14 attracts activated tissue macrophages and dendritic progenitor cells as a chemoattractant [87–93]. CXCL14 promotes visceral obesity and adipose tissue inflammation in animals, leading to an increase in hepatic gluconeogenesis and the development of IR [19, 65, 94–96]. In addition, the obesity-induced upregulation of CXCL14 in white adipose tissue promotes macrophage infiltration and subsequent inflammatory responses. Increased CXCL14 production in high-fat diet (HFD) fed mice modulates the expression of adipokines, including adiponectin, retinol-binding protein-4, and IL-6, thereby promoting gluconeogenesis in the liver and inhibiting glucose uptake in skeletal muscle. It is susceptible that the dramatic increase in macrophages in white adipose tissue and the direct action of CXCL14 on skeletal muscle play significant roles in this diabetic cascade. CXCL14 also indirectly contributes to the fatty liver formation, which significantly affects glucose metabolism (Fig. 3B) [19, 20]. In regards to simply recruiting inflammatory cells to visceral white adipose tissue, these studies demonstrated that CXC chemokines play essential roles in obesity-induced IR and impaired glucose metabolism.
CXC chemokines and T2D
Obesity is associated with higher levels of low-grade chronic inflammation, which predisposes humans to a broad spectrum of comorbidities such as T2D, dyslipidemia, CVD, and NAFLD [97, 98]. T2D, also known as non-insulin-dependent diabetes mellitus, is distinguished by IR and pancreatic-cell (β cells) dysfunction attributable to hyperglycemia [99, 100]. IR impacts the entire diabetes pathophysiology. IR can pursue in the liver, muscles, and adipose tissue. Islet 𝛽 cells produce more insulin to compensate for IR, which may exceed their maximum capacity and result in 𝛽 cell failure [101].
Chronic, low-grade inflammation of adipose tissue in regard to obesity and IR is crucial to the development of T2D. Popov et al. reported that inflammation is associated with impairment of oxidative status, carbohydrate and lipid metabolism in T2D complicated by NAFLD [102]. Many studies have been conducted in both humans and mice on the role of inflammation’s involvement in the development of T2D. In human and mouse adipocytes, multiple pro-inflammatory mediators, such as CXCL1, CXCL10, and monocyte chemoattractant protein (MCP)-1, induce IR [103, 104]. The biological role of CXC chemokines in T2D development is addressed further below.
CXCL1 and CXCL2
According to previous studies, T2D patients exhibited the most significant increases in CXCL1 and CXCL5 levels [105–107]. Craig and colleagues demonstrated that CXCL1 and CXCL5 are up-regulated in obese diabetic mice (db/db) compared to control mice (non-diabetic/non-obese) [108]. CXCL1 is highly expressed in diabetic wounds in rats and humans via the interleukin 17 (IL-17) pathway. In the same study, interleukin (IL-7) inhibitors (Huangbai liniment and berberine) significantly reduced IL-17 expression and its downstream targets, including CXCL1, in diabetic wounds [109]. Similarly, another study demonstrated that CXCL1 and CXCL2 are up-regulated in diabetic wound mice, whereas Cryptotanshinone (inhibitor) significantly decreased CXCL1 and CXCL2 chemokine in Cryptotanshinone mice relative to vehicle mice [110]. In addition, Anuradha et al. found that the number of chemokines found in the plasma of T2D patients increased. These chemokines include CXCL1, CXCL2, CXCL8, CXCL9, CXCL10, and CXCL11. This finding suggests that the chemokine network plays a significant role in the progression of T2D [111]. The bioinformatics analysis of endothelial precursor cells isolated from T2D patients also revealed that CXCL1 chemokine is up-regulated, implying that CXCL1 may play an essential role in the pathophysiology of endothelial precursor cells during T2D and stimulate an inflammatory response, which may be critical for the reduced number and hypofunction of endothelial precursor cells isolated from T2D patients [112]. Moreover, prior studies have demonstrated that the serine-phosphorylated STAT1 and NF-kB (IkKB) pathways, which control the transcription of CXCL1 and CXCL2, are significant contributors to the inflammatory response in β cells associated with islet β cell death in T2D. In both humans and animals with T2D, the CXCL1 and CXCL2 genes are regulated, encoding proteins that promote neutrophils and other CXCR2 + cells to migrate toward secreting tissue [113–115]. Single-cell RNA analysis has demonstrated that in diabetic macular edema, through boosting the production of the pro-inflammatory chemokines CXCL2 and CXCL8, CD14++ monocytes predominate in inducing inflammation. These highly expressed genes for inflammation suggest that immune cells in the blood of diabetic macular edema patients were in a proinflammatory state. This may have led to the destruction of vascular endothelial cells and retinopathy [116]. Nevertheless, the skin tissue from mice with a T2D-like phenotype displayed an up-regulation of the inflammatory gene CXCL2. These results indicate that CXCL2 is strongly associated with inflammation of tissues in mouse models of T2D [117]. In contrast, a transcriptome study found that CXCL2, CXCL3, CXCL5, and CXCL8 are down-regulated in T2D patients’ neutrophils compared to healthy controls, whereas CXCR1 and CXCR2 genes are significantly upregulated in T2D patients’ neutrophils compared to healthy controls. This work demonstrates that circulating neutrophils from T2D patients exhibit aberrant activation at the transcriptome level and that these neutrophils may also have reduced motility due to downregulated chemotaxis, which may help to explain why certain T2D patients have higher infection rates [118].
CXCL8
Previous research indicates that toll-like receptor (TLR)-4 signaling is one of the key pro-inflammatory pathways induced via endogenous or exogenous molecules related to risk or infections. Circulation levels of the classical TLR4 ligand lipopolysaccharide (LPS), which has been recently designated “metabolic endotoxmia”, are high in obese and T2D patients. This situation is also found in obesity/ diabetes model of rodents. [119, 120]. Isolated human islets were stimulated to produce IL-1, CXCL8, and TNF by LPS in a TLR4-dependent manner, whereas β cell viability and function were substantially compromised. CXCL8, which was specifically detected in β cells, stimulated monocyte recruitment, which was completely prevented by CXCL8 neutralization. TLR4 is extremely pathogenic in human islets, causing a complicated multi-cellular inflammatory response that includes β cell failure, chemokine secretion, and macrophages infiltration. The highly elevated TLR4 response in obesity may exacerbate β cell damage and hasten diabetes progression [48].
Cimini and colleagues found that T2D patients had higher CXCL8 levels than non-diabetic subjects, and that CXCL8 concentration correlated with higher IL-6, TNF-α, fasting blood glucose, glycosylated hemoglobin, low-density lipoprotein cholesterol, lower adiponectin, and 25(OH) vitamin concentrations, indicating that T2D patients have a marked elevation of circulating CXCL8, which identifies subjects with worse inflammatory, glycometabolic and lipid profile and lower vitamin D levels [121]. Moreover, reference showed that CXCL8 recruits’ neutrophils and stimulates tissue inflammation through binding to CXCR1 and CXCR2. In both in vitro and in vivo diabetes models, CXCL8 suppression inhibits the activation of CXCR1 and CXCR2, as well as their downstream JAK2/STAT3 and ERK1/2 pathways [122].
CXCL9, CXCL10, and CXCL11
Numerous studies have shown that CXCL9, CXCL10, and CXCL11 and their receptor CXCR3 are up-regulated in T2D patients [123–125]. Blocking the CXCL10/CXCR3 system is considered to be a promising therapeutic target due to the extensive research on the CXCL10/CXCR3 axis role in the immunopathogenesis of diabetes [105]. Additionally, high glucose levels activate the p38 MAP kinase signaling pathway, which in turn induces CXCR3 in CD8+ T cells. Likewise, high glucose levels induced CXCL9,CXCL10, and CXCL11 expression, which promoted and infiltrated CD8+ T cells into the peripheral tissue of diabetics and increased cytotoxicity [123, 126].
CXCL12 and CXCL13
SDF-1 alpha (CXCL12) is a stromal cell-derived factor that has a role in the activation of T lymphocytes and monocytes but not neutrophils. It induces a rapid and transient dramatic increase in intracellular calcium ions and further chemotaxis by activating the receptor CXCR4, which acts as its receptor. CXCL12 can also bind to another receptor, CXCR7, activating the beta-arrestin pathway [105, 127]. Patients with T2D who have the heterozygous SDF-1 3′A genotype (801G/A in the 3′ untranslated region) have higher levels of insulin-dependent adult progenitor cell mobilization, which is known to be involved in angiogenesis and vascular repair. On the other hand, homing of progenitor cells is a factor in the vascular complications of diabetes. This is because patients with the SDF-1 3′A genotype have higher levels of CXCL12 mRNA in their peripheral blood mononuclear cells. Variations in the CXCL12 gene’s genetic makeup may impact the movement of inflammatory cells or defective precursors, which might increase the risk of diabetes disease [128, 129]. Moreover, karimabad et al. reported that injured duct, red blood cells, δ-cells, β cells, and α cells display higher amounts of CXCL12 during T2D and that bone marrow and secondary lymphoid organs recruit immune cells to the blood via the CXCR4 receptor. Together, CXCL12 and CXCR4 contribute to the development of T2D, potentially by increasing B-cell mortality, glomerulonephritis, and microangiopathy [129]. Elevated CXCL12 is associated with disease in the systemic compartment and can be used as a blood biomarker to identify individuals with T2D [130, 131].
CXCL13/CXCR5 has a considerable proinflammatory consequence by itself. CXCL13/CXCR5 signaling is a crucial upstream mediator driving p-ERK, p-AKT, and p-STAT3 cell signaling pathways as well as stimulating the production of inflammatory cytokines TNF- α and IL-6. The chemokine CXCL13 and its receptor CXCR5 in the spinal cord contribute to the pathogenesis of painful diabetic neuropathy [132]. Surprisingly, Jiang et al. reported that CXCL13 promoted bone marrow stromal cell proliferation in high glucose environments, promoting the healing of fractures in diabetic rats [133]. Furthermore, previous studies have showed increased levels of CXCL13 in T2D patients, and this protein has been speculated to be responsible for triggering leukocyte chemotaxis and activation [27, 82, 134].
CXCL14
Obesity affects the majority of T2D patients. Matsushita et al. found that serum CXCL14 levels were independently associated with serum C-peptide and fatty liver index in T2D patients. A high serum C-peptide concentration may reflect IR rather than β cell function in these patients, because CXCL14 displayed simple correlations with obesity-related factors. These findings suggested that serum CXCL14 levels in T2D patients could be used to predict elevated serum C-peptide and hepatic steatosis [66]. Although CXCL14’s cellular receptor and signaling pathway are still unknown, it is postulated to have chemotactic activity toward a wide range of inflammatory mononuclear cells, such as monocytes, neutrophils, dendritic cells, and natural killer cells [135]. In this outcome, the serum CXCL14 concentration in T2D individuals involved has been reported to be elevated in correlation with IR and hepatic steatosis. It is believed that CXCL14 plays an important role in both the immune response and inflammation. This chemokine could be a new biomarker or therapeutic target for IR, hepatic steatosis, and T2D linked to obesity.
CXCL16
CXCL16 is a protein that combines the functions of a scavenger receptor with those of an inflammatory chemokine. This transmembrane protein is composed of an extracellular chemokine domain and a transmembrane mucin stalk [136]. This chemokine domain functions as a recruiter for cells expressing the CXCR6 receptor as well as a scavenger, facilitating oxidized low-density lipoprotein uptake (ox-LDL). Pro-inflammatory stimuli increase CXCL16 expression, which increases ox-LDL uptake and hastens foam cell formation in the vascular endothelium [137]. CXCL16 is a remarkable chemokine that is available in two forms: Soluble CXCL16 links to immune cells that express the CXCR6 receptor and leads them to the site of inflammation [138] and ox-LDL is subsequently internalized by the cell membrane receptor form [139]. According to previous studies, the pancreatic β cell’s autophagy and transcription factor activation in diabetes could be induced by the activation of the CXCL16/ox-LDL pathway in β cells [140, 141]. Similar, Gutwein et al. reported that high levels of oxLDL were associated by increased glomerular CXCL16 expression in diabetic nephropathy [142]. This evidence confirms that oxLDL and CXCL16 have a link during diabetes. Moreover, the membranous CXCL16 can be cleaved to its soluble form by a Disintegrin and Metalloproteinase (ADAM10) [143]. Recent research indicates that ADAM10/CXCL16 upregulation in the pancreatic islets of diabetic mice results in an accumulation of T-cells via an increased NF-kB pathway. Consequently, in diabetic mice, cleaved CXCL16 promoted T-cell recruitment into β cells and enhanced oxidative stress, inflammatory response, and mortality [140]. In addition, recent study demonstrated the processing enzyme ADAM10 and the CXCL16/CXCR6 receptor have a role in the development and progression of proliferative diabetic retinopathy. The researcher accepted that proliferative diabetic retinopathy is mediated by elevated levels of retinal NF-kB, vascular endothelial growth factor, and intercellular adhesion molecule 1 [144]. In a recent study by Tawfik et al. discovered that patients with T2D had much higher levels of CXCL16 in their blood than healthy patients [145]. Likewise, CXCL16 serum levels were elevated in T2D patients with or without coronary artery disease compared to healthy patients [146]. Moreover, patients with diabetes mellitus, with or without gestational diabetes mellitus disease, had elevated blood CXCL16 levels [141, 147]. These studies confirmed that the chemokine CXCL16 is a key part of inflammation and may contribute to the development and progression of T2D. In T2D, serum CXCL16 might be used to monitor inflammation. Figure 4 displays the recent evidence regarding the role of CXC chemokines in the development of T2D and their blockade as a potential therapeutic approach.
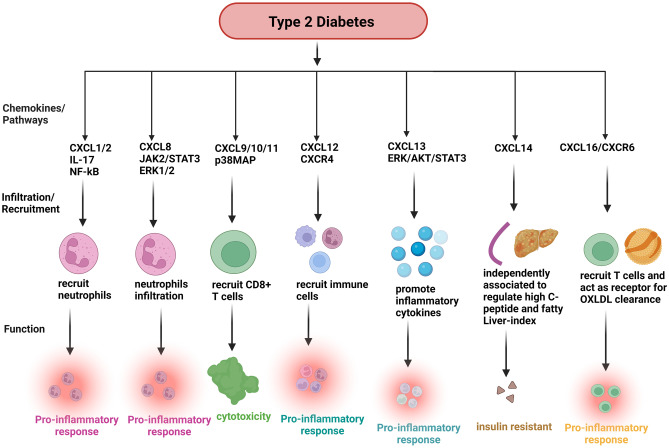
Fig. 4.
The functions of CXC chemokines in T2D. Chemokines mainly control the migration of neutrophils, monocytes, T cells, and leukocytes in T2D. CXCL1 and CXCL2 recruit neutrophils through the IL-17/NF-kB pathway and play a pro-inflammatory role. CXCL8 recruits neutrophiles via the JAK2/STAT3/ERK1/2 pathways to induces pro-inflammatory response. CXCL9, CXCL10, and CXCL11 recruits CD8 + T cells through the p38MAP pathway, which plays an important role in cytotoxicity. CXCL12/CXCR4 axis recruits immune cells, which creates inflammation. CXCL13 recruits promote inflammatory cytokines with the help of ERK/AKT/STAT3 pathways and induces inflammation. In addition, CXCL14 independently associated with serum C-peptide and fatty liver index which induce insulin resistance. CXCL16/CXCR6/ADAM10 recruits T cells via NF-kB pathway and acts as a receptor for oxLDL clearance. Created with BioRender.com
CXC chemokines and NAFLD
The metabolic syndrome, which includes obesity, dyslipidemia, hyperinsulinemia, and IR, is characterized by NAFLD, which is its hepatic manifestation [148, 149]. Because of this, NAFLD is often considered a hepatic manifestation of metabolic syndrome [150]. NAFLD represents a wide range of liver disorders, from simple steatosis (SS) to nonalcoholic steatohepatitis (NASH) [151] ; It is well known that the latter increases the risk of liver cirrhosis and hepatocellular carcinoma (HCC) [152]. SS patients rarely suffer sever disease, but nearly 20% of NASH patients progress to the end-stages of liver disease [153, 154]. The risk of developing cirrhosis and hepatocellular carcinoma is higher in individuals with NASH than in individuals with SS, implying that NASH is a more severe form of liver injury [154–156].
Furthermore, adults with diagnosed NAFLD tend to follow dietary patterns including high fat and sodium with suboptimal micronutrient intake and low physical activity [157]. Abdallah et al. reported that anti-inflammatory dietary patterns showed benefits to NAFLD risk factors, severity markers and inflammatory markers compared to the control diet [158]. These studies demonstrated that NAFLD could be prevented through dietary patterns.
CXCL1 and CXCL2
Regarding the development of NAFLD, a number of reference articles provide a comprehensive summary of key chemokines and their receptors. Chemokine pathophysiological involvement in the development of NAFLD have been widely explored in NAFLD humans and animal models [159–161]. CXCL1 was among the most significantly active genes in the livers of mice following a 3-month HFD with binge ethanol exposure (30-fold in the liver and 5-fold in epididymal adipose tissue) [162]. CXCL1 expression increased significantly in hepatocytes, hepatic stellate cells (HSCs), and liver sinusoidal endothelial cells in the liver [162]. The regulation of CXCL1 by an HFD-plus-ethanol binge is thought to be associated with elevated levels of free fatty acid (FFA) in the liver, which stimulate CXCL1 in hepatocytes via ERK1/2, Jun N-terminal kinase (JNK), and NF-kB. While CXCL1 overexpression exacerbated steatohepatitis in 3-month HFD-fed mice, CXCL1 inhibition with a neutralizing antibody or CXCL1 gene disruption decreased hepatic neutrophil infiltration and injury following an HFD plus ethanol binge [162]. Moreover, In the choline deficient amino acid-defined (CDAA) diet-induced animal NASH model, CXCL1 mRNA levels are enhanced in a TLR4-MyD88-dependent manner, resulting in increased neutrophil infiltration related to hepatic inflammation and fibrosis [163]. In addition, In HFD-fed animals, adenoviral overexpression of CXCL1 induces hepatic neutrophil infiltration, oxidative stress, and hepatocyte mortality, increasing progression from SS to steatohepatitis [37]. Mueller et al. reported that CXCL1 is up-regulated in Ldlr-/-. Leiden mice enhanced hepatic inflammation, but treatment with the multicomponent pharmaceutical product (HC-24) inhibits the development of free cholesterol and has anti-inflammatory actions at the molecular and cellular levels in the liver [38]. Recently, bioinformatic studies of human and animal also demonstrated that CXCL1 and CXCL2 is highly expressed in the NALFD disorder which indicated that CXCL1 and CXCL2 play important role in the live inflammation in human and mice [164, 165]. Moreover, a study demonstrated that CXCL2 activates the TLR4 pathway to recruit neutrophil and macrophage infiltrations in the palmitate-induced fibrosis mouse model [166]. Saiman et al. found that increased hepatic levels of CXCL1 and CXCL2 trigger the recruitment of neutrophils from the periphery after hepatic injury in humans [167]. These data indicate that CXCL1 and CXCL2 have a role in neutrophil recruitment and infiltration as a consequence of hepatic cell dysfunction in the liver, which leads to the development of chronic inflammation in NAFLD.
CXCL5
Previous reports indicated that CXCL5 is essential for neutrophil recruitment and activation via the CXCR2 receptor, as well as hepatocyte proliferation [168, 169]. CXCL5 is linked to neutrophil infiltration and a poor prognosis in HCC. It is considered a therapeutic target in the disease, as treatment with small-interfering RNAs or antibodies against CXCL5 can inhibit tumor growth, proliferation, migration, and invasion [170]. Xu et al. found that CXCL5 was over-expressed in HCC with high metastatic potential, and that CXCL5 could increase HCC migration and invasion, most frequently via autocrine and paracrine mechanisms. Evidence also suggests that the CXCL5-CXCR2-ERK1/2/JNK/p38MAPK pathways may play important roles in HCC migration and invasion, and neutrophil and macrophage recruitment [171]. CXCL5 was identified as a gene target of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in liver cells, and increased levels of CXCL5 transcript and protein were found in fibrotic liver and activated hepatic stellate cells. Previous research demonstrated that MALAT1 expression increases in activated hepatic stellate cells and is regulated by hyperglycemia and insulin in vitro. In addition, MALAT1 expression was found to increase in activated hepatic stellate cells [172]. A recent study showed that CXCL5 activation of the NF-kB pathway in human hepatocyte-derived spheroids and primary rat hepatocytes caused NASH, while livogrit, a CXCL5 inhibitor, showed promise as a haptic therapeutic formulation that could reduce CXCL5 levels in the development of NASH [173]. In Longitudinal studies, CXCL5 chemokine is evaluated in the diet-induced progression of NAFLD to HCC in diet-induced animal model of nonalcoholic fatty liver disease (DIAMOND) [174].
CXCL6
Chemokine CXCL6 (GCP-2) is an essential component of inflammatory cells. It is expressed in the liver and plays a role in the pathogenesis of multiple inflammatory responses and liver fibrosis [175]. CXCL6 dysregulation was closely associated with the activation of liver-infiltrating lymphocytes in the initial stage of hepatitis C-induced fibrosis [176]. Cai et al. showed that activated Kupffer cells (or stellate macrophages) are the source of CXCL6 in fibrotic livers, and that CXCL6 secreted by these macrophages promotes the release of transforming growth factor-beta (TGF-β) by Kupffer cells via the CXCR1/2 epidermal growth factor receptor (EGFR) pathway, which promotes HSCs activation [175]. Moreover, a recent study found that mRNA expression levels of CXCL6, and CXCR1 were high in SS during the pathogenesis of NAFLD via the pro-inflammatory NF-kB pathway [177].
CXCL8 and CXCL9
As evidenced by experimental findings, CXCL8 is a CXC chemokine with capabilities that are simultaneously pro-inflammatory and pro-angiogenesis [178]. Macrophages are an essential element of NAFL and NASH, and research has demonstrated that liver-activated macrophages can create higher levels of CXCL8, hence inducing CXCL8/mir-17 clusters. CXCL8 may enhance neutrophil recruitment in NASH by triggering the AKT/mTOR/STAT signaling pathway. The intrahepatic expression of CXCL8 was also elevated in the blood and liver of NAFL patients. These findings revealed that CXCL8, with the highest ranking in the NAFL stage, may play a significant role in both the NAFL and NASH stages [178, 179]. In addition, inflammatory neutrophil infiltration is distinguished by upregulation of CXCL8, and CXCR1/2, which recruit neutrophils into the liver to produce reactive oxygen species and proteases, resulting in hepatocyte damage [180–182]. For instance, studies have demonstrated that patients with NAFLD have increasing levels of the inflammatory chemokine CXCL8 [49, 179, 183–185] and these recent research suggests that CXCL8 plays a role in the pathophysiology of NAFLD and could be a potential treatment target for NAFLD.
The gene CXCL9 is a member of the chemokine superfamily, which produces secreted proteins that are involved in immune regulation, inflammation, and T cell trafficking. The encoded protein attracts lymphocytes but not neutrophils when it binds to the CXCL3. According to studies, CXCL9 plays a role in a number of pathological processes, including the growth of tumors, immunity, and inflammation [186, 187]. The liver tissues of NASH patients have higher levels of CXCL9 expression. CXCL9 mRNA was discovered to be overexpressed in both NASH and SS mice models, and hepatocytes and sinusoid endothelial cells that released CXCL9 protein were detected in regions where inflammatory cells had infiltrated [188]. In a high-risk cohort of obese adults with NASH without fibrosis, the expression of CXCL9 was upregulated [55]. Another cohort research found that the effects of liver fibrosis were positively connected with blood CXCL9 concentrations in patients with chronic liver disease, which were considerably greater than in healthy control individuals [189]. Patients with chronic hepatitis C virus infection also had higher levels of CXCL9 expression, which was associated with liver fibrosis [190]. In mouse models, the interaction from NAFLD to HCC in male mice was associated with a chronic trend of increased CXCL9 levels [174, 191]. Furthermore, a recent study found that CXCL9 disrupts the Treg/Th17 balance in a mouse model of metabolic-associated fatty liver disease (MAFLD) by activating the p-JNK pathway [192]. Moreover, bioinformatics analysis also demonstrated that CXCL9 is up-regulated in both humans and animals NAFL and NASH disorders [193–195]. CXCL9 is a key factor in chronic liver inflammation, according to these findings; nevertheless, its expression and involvement in NAFLD require additional exploration.
CXCL10 and CXCL12
CXCL10 is produced by a number of cells, including macrophages, monocytes, hepatocytes, hepatic stellate cells, and endothelial cells [196]. In a well-designed and comprehensive study, Ibrahim et al. may have identified the central link between lipotoxicity, and recruitment of macrophages, which promotes inflammatory processes in NASH. The researchers examined the chemokine CXCL10 and mixed lineage kinase 3 (MLK3) in hepatocytes stimulated by palmitic acid or lysophosphatidylcholine (LPC), as well as in an in vivo model of NASH [197]. CXCL10-containing extracellular vesicles (EVs) were increased in treated cells and mice fed a high-calorie (saturated fat, cholesterol, and fructose; FFC) diet. In vitro, CXCL10 bound to EVs exerted a stronger chemotactic effect on macrophages than CXCL10 unbound to EVs. Two significant pathways controlled by MLK3 were discovered by MLK3 deletion or pharmacological inhibition. First, inhibiting MLK3 reduces LPC-induced CXCL10 production, most probably via a p38/signal transducer and activator of transcription 1-dependent pathway; second, MLK3 inhibition diminished CXCL10 trafficking into EVs (with increased intracellular CXCL10 levels in hepatocytes) and LPC-induced EV release, which may be dependent on JNK activation. In conclusion, MLK3-dependent hepatocyte production of CXCL10-containing EVs may represent a crucial interface between lipotoxicity and inflammation generated by macrophages recruited into the liver [197]. Finally, these findings not only emphasize the importance of CXCL10 in driving hepatic inflammation in NASH, but it also suggests the role of EVs in hepatic inflammation, which is a novel field of cell biology that requires further investigation [197]. Furthermore, several studies on CXC10 chemokine and NAFLD indicate that CXCL10 has been recommended as a potential therapeutic target for NAFLD treatment [198–200].
CXCL12 is abundantly expressed in numerous tissues, including the liver, where biliary cells express it [201]. CXCL12 as well as its receptor CXCR4 have aberrant expression that has been linked to NAFLD. CXCR4 regulates cell localization, chemotaxis, activation, migration, division, and differentiation when it binds to its ligand, CXCL12 [202, 203]. CXCL12 is widely generated by sinusoidal endothelial cells in the liver and enhances hematopoietic stem cell migration following chronic liver injury [202]. NASH developments include elevated CXCR4 and CXCL12 protein levels as well as aberrant CD4+ T- cell responses to CXCL12 [202, 204]. During immune surveillance and liver inflammation, liver sinusoidal endothelial cells promote CD4+ T cell recruitment by upregulating peri-vascular CXCL12 production and activating CXCL12/CXCR4-dependent intracellular transport pathways [24]. Moreover, Activation of the CXCL12/CXCR4 axis increased collagen I synthesis and hematopoietic stem cell proliferation in an animal model of CCL4-induced hepatic fibrosis [205].
CXCL16
Prior research has found that CXCL16 is substantially expressed in the livers of patients who suffer from metabolic and inflammatory liver disorders [206, 207] and that CXCL16 inhibition reduced steatohepatitis and liver macrophage infiltration in chronic hepatic damage [208]. In addition, CXCL16 is considered to be a survival and maturation regulator for hepatic NKT cells [209].
In experimental NAFLD, CXCR6 promotes liver inflammation by enhancing the invasion of hepatic NKT cells and inflammatory macrophages [208, 210, 211]. The CXCL16/CXCR6 axis stimulates hepatic NKT cell migration, promoting CCl4-induced liver fibrosis [21, 22]. Indeed, injured hepatocytes exhibited increased CXCL16/CXCR6 axis expression, showing that the CXCL16/CXCR6 interplay is involved in the pathogenesis of NAFLD [208, 212–214].
CXCL17
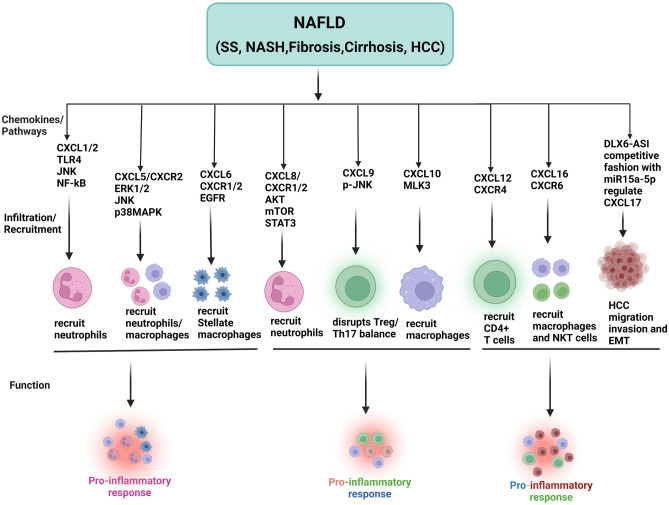
CXCL17 is a newly identified 119-amino acid CXC chemokine; its recently identified receptor, GPR35/CXCR8, is a GPCR involved in metabolic processes [215–217]. It stimulates monocytes, macrophages, dendritic cells, and immature myeloid-derived cells as a chemoattractant [217]. Autophagy inhibition was also associated with increased levels of CXCL17, which enhances cell proliferation and migration in human HCC tissues. Its suppression induces autophagy via nuclear translocation of liver kinase B1, which phosphorylates and activates AMPK, leading to an increase in tumor size and proliferation reduction [218]. In addition, an indicator that can predict the patient’s prognosis in liver cancer is lncRNA called distal-less homeobox 6 antisense 1 (DLX6-AS1) [219], whose down-regulation hinders cancer cells’ tendency to proliferate [220]. Likewise, silencing DLX6-AS1 effectively suppresses the bioactivities of HCC cells [221]. MicroRNAs (miRNAs), such as miR-15a-5p, are also crucial in the development of liver cancer. Interestingly, miR-15a-5p levels that are higher can inhibit the proliferation of liver cancer cells as well as other tumor-promoting features [222, 223]. There have been some studies on the roles of HCC-derived exosomes in human cancers. For instance, in HCC cells co-cultured with HCC-derived exosomes, increased migration, invasion, and epithelial-mesenchymal transition (EMT), as well as decreased E-cadherin and elevated vimentin levels, have been observed [224]. The M2-polarized macrophages and the miR-15a-5p/CXCL17 axis make it possible for DLX6-AS1 in HCC-derived exosomes to induce cancer. DLX6-AS1 suppresses miR-15a-5p, which stimulates the polarization of M2 macrophages and the invasion and metastasis of HCC. The silencing of CXCL17 can inhibit migration, invasion, and EMT. Finally, DLX6-AS1 in HCC-derived exosomes modulates CXCL17 by binding to miR-15a-5p in a competitive fashion. This induces M2 macrophage polarization, which in turn promotes HCC migration, invasion, and EMT [26]. Furthermore, Li et al. demonstrated that CXCL17 may positively regulate CD68+ macrophage accumulation while negatively regulating CD4+ T cell infiltration in HCC tumors, suggesting that CXCL17 production is connected with adverse immune infiltration and may be a key target for anti-HCC therapy [225]. Figure 5 illustrates the recent evidence regarding the role of CXC chemokines in the pathogenesis of NAFLD and their blockade as a potential therapeutic approach.
Fig. 5.
The role of CXC chemokines in the NAFLD. CXCL1 and CXCL2 recruit neutrophils via TLR- 4, JNK and NF-kB signaling, which induce inflammation. CXCL5 recruits neutrophiles through the ERK1/2/JNK and p38MAPK pathways, which plays an important role in the pro-inflammatory process. CXCL6 and receptor CXCR1 and CXCR2 recruits stellate macrophages by EGFR mechanism as a result pro-inflammatory response occur, CXCL8 and its receptors, CXCR1 and CXCR2, recruit neutrophils through the AKT/mTOR/STAT3 pathways, causing hepatocyte injury and inflammation. CXCL9 disrupts the Treg/Th17 via the p-JNK pathway, which acts as a pro-inflammatory signal. CXCL10 recruits the macrophage chemotaxis via MLK3 mechanism and produces a pro-inflammatory response. CXCL12 and its receptor CXCR4 recruit CD4+ T cells in the results of liver injury. CXCL6 and its receptor, CXCR6/CXCR6 axis, primarily recruit macrophages and NKT cells and produce proinflammatory response. DLX6-AS1 in HCC-derived exosomes modulates CXCL17 by binding to miR-15a-5p in a competitive fashion, which in turn promotes HCC migration, invasion, and EMT. Created with BioRender.com
Conclusions and future perspectives
We attempted to explain the expression, molecular mechanisms, sources, and key functions of CXC chemokines in obesity and disorders associated with obesity, such as T2D and NAFLD. In particular, suppressing the CXCR2 pathway prevents the development of IR and inflammation, which may help to improve the prognosis of disorders associated with obesity and inflammation. CXC chemokines suppression in an obese patient’s conditioned media from ASCs prevented cancer cell migration. Moreover, the CXC chemokines linkage may contribute to the immunopathogenesis of diabetes. On the other side, this axis’ suppression can decrease the risk of immunological rejection. This could be a possible therapeutic target for diabetes patients. Inhibition of the CXC chemokines can inhibit tumor growth, proliferation, migration, and invasion during HCC, making it a potential target for therapeutic intervention.
Nevertheless, our understanding of the complex communication system between CXC chemokines and their receptors is still inadequate, which could hinder the creation of innovative therapeutics for obesity, T2D, and NAFLD. If possible, the role of each CXC chemokine and chemokine receptor should be adequately and instantly addressed to ensure translation into potential clinical implications. As a result, further clinical and pre-clinical studies are required to investigate the molecular mechanism and ascertain whether an anti-inflammatory strategic approach targeting specific CXC chemokines and chemokine receptors could be a promising therapeutic approach to prevent the progression of obesity, T2D, and NAFLD.
Acknowledgements
This study was financially supported by West China Hospital Sichuan University. The authors gratefully acknowledge Jing Zhao for her excellent language support. BioRender.com was used to create the figures.
Author contributions
All authors contributed to the conception, drafting, illustration, and final revision of the work. Each author has reviewed and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32070671, 32270690), the Covid-19 research projects of West China Hospital Sichuan University (Grant no. HX-2019-nCoV-057) as well as the regional innovation cooperation between Sichuan and Guangxi Provinces (2020YFQ0019).
Declarations
Conflict of interest
The authors say that they have no commercial or financial ties that could be seen as a conflict of interest in the study we did. Because of this, there were no conflicts of interest in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amin Ullah, Email: amindawar225@gmail.com.
Bairong Shen, Email: bairong.shen@scu.edu.cn.
References
- 1.World health organization. Key facts about obesity and overweight. Available online: https://www.who.int/news-room/factsheets/detail/obesity-and-overweight. Accessed 21 Dec 2022.
- 2.Lopez KN, Baker-Smith C, Karamlou T, Gallegos FN, Pasquali S, Patel A, et al. Addressing social determinants of health and mitigating health disparities across the lifespan in congenital heart disease: a scientific statement from the american heart association. J Am Heart Assoc. 2022;11:1–21. doi: 10.1161/JAHA.122.025358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes,and related disorders. Immunity. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhtiyari M, Kazemian E, Kabir K, Hadaegh F, Aghajanian S, Mardi P, et al. Contribution of obesity and cardiometabolic risk factors in developing cardiovascular disease: a population – based cohort study. Sci Rep. 2022;12:1–10. doi: 10.1038/s41598-022-05536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarus E, Edward H. Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;3:100026. doi: 10.1016/j.obpill.2022.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan A, Ross HM, Parra NS, Chen SL, Chauhan K, Wang M, et al. Risk prevention and health promotion for non-alcoholic fatty liver diseases (NAFLD) Livers. 2022;2:264–82. doi: 10.3390/livers2040022. [DOI] [Google Scholar]
- 7.Zhang J, Shi Y. In search of the holy grail: toward a unified hypothesis on mitochondrial dysfunction in age-related diseases. Cells. 2022;11:1906. doi: 10.3390/cells11121906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: a chronic low-grade inflammation and its markers. Cureus. 2022;14:1–11. doi: 10.7759/cureus.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celik O, Yildiz BO. Obesity and physical exercise. Minerva Endocrinol. 2021;46:131–44. doi: 10.23736/S2724-6507.20.03361-1. [DOI] [PubMed] [Google Scholar]
- 10.Cinkajzlová A, Mráz M, Haluzík M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J Endocrinol. 2021;252:R1–22. doi: 10.1530/JOE-21-0159. [DOI] [PubMed] [Google Scholar]
- 11.Uribe-Querol E, Rosales C. Neutrophils actively contribute to obesity-associated inflammation and pathological complications. Cells. 2022;11:1883. doi: 10.3390/cells11121883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheurlen KM, Snook DL, Alfieri T, Littlefield AB, George B, Seraphine C et al. Obesity hormones and itaconate mediating inflammation in human colon cancer cells – Another lead to early-onset colon cancer? Heliyon. 2023:e13132. [DOI] [PMC free article] [PubMed]
- 13.Lu X, Wang Z, Ye D, Feng Y, Liu M, Xu Y, et al. The role of CXC chemokines in cardiovascular diseases. Font Pharmacol. 2022;12:3830. doi: 10.3389/fphar.2021.765768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh J, Jun M, Yang H, Lee B. Cellular and molecular mechanisms and effects of berberine on obesity-induced inflammation. Biomedicine. 2022;10:1739. doi: 10.3390/biomedicines10071739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-ning-tapia EM, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:1167. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavey C, Lazennec G, Iankova I, Teyssier J, Lagarrigue S, Clape C, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metabol. 2009;9:339–49. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobashi C, Asamizu S, Ishiki M, Iwata M, Usui I, Yamazaki K, et al. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J Inflamm. 2009;6:1–6. doi: 10.1186/1476-9255-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nara N, Nakayama Y, Okamoto S, Tamura H, Kiyono M, Muraoka, et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem. 2007;282:30794–803. doi: 10.1074/jbc.M700412200. [DOI] [PubMed] [Google Scholar]
- 20.Hara T, Nakayama Y. CXCL14 and insulin action. Vitam Horm. 2009;80:107–23. doi: 10.1016/S0083-6729(08)00605-5. [DOI] [PubMed] [Google Scholar]
- 21.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev/Immunology. 2014;14:181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28. doi: 10.1189/JLB.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokunaga R, Zhang W, Naseem M, Puccini A, BergerSoni MD, Shivani, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev. 2019;63:40–7. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann K, Erben U, Kruse N, Wechsung K, Schumann M. Chemokine transfer by liver sinusoidal endothelial cells contributes to the recruitment of CD4 + T cells into the murine liver. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0123867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabrouk N, Tran T, Sam I, Pourmir I, Gruel N, Tartour E. CXCR6 expressing T cells: Functions and role in the control of tumors. Front Immunol. 2022;1–9. [DOI] [PMC free article] [PubMed]
- 26.Wang L, Lin J, Ma X, Xu D, Shi C, Wang W, et al. Exosomal DLX6-AS1 from hepatocellular carcinoma cells induces M2 macrophage polarization to promote migration and invasion in hepatocellular carcinoma through microRNA-15a-5p / CXCL17 axis. J Experimental Clin Cancer Res. 2021;40:1–16. doi: 10.1186/s13046-021-01973-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Ramne S, Drake I, Ericson U, Nilsson J, Orho-melander M, Engström G, et al. Identification of inflammatory and disease-associated sugar and sugar-sweetened beverages and their role in type 2 diabetes risk. Nutrients. 2020;12:1–15. doi: 10.3390/nu12103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MC, Mayo KH. Chemokines from a structural perspective. Int J Mol Sci. 2017;18:2088. doi: 10.3390/ijms18102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluchino N, Mamillapalli R, Moridi I, Tal R, Taylor HS. G-protein-coupled receptor CXCR7 is overexpressed in human and murine endometriosis. Reprod Sci. 2018;25:1168–74. doi: 10.1177/1933719118766256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattermann K, Mentlein R. An Infernal Trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Ann Anat. 2012;195:1–8. doi: 10.1016/j.aanat.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:0–10. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Mellado M, Rodr M, Mart C. Chemokine signaling and functional response: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- 33.Neves SR. G protein pathways. Science. 2002;296:1–5. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 34.Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006;147:46–S55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanegashima K, Suzuki K, Nakayama Y, Tsuji K, Shigenaga A, Otaka A, et al. CXCL14 is a natural inhibitor of the CXCL12 – CXCR4 signaling axis. FEBS Lett. 2013;587:1731–5. doi: 10.1016/j.febslet.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Ouh Y, Cho HW, Lee JK, Choi SH. CXC chemokine ligand 1 mediates adiponectin-induced angiogenesis in ovarian cancer. Tumor Biol. 2019;4:1–10. doi: 10.1177/1010428319842699. [DOI] [PubMed] [Google Scholar]
- 37.Hwang S, He Y, Xiang X, Seo W, Kim S-J, Ren JM, Id T, Hwang O, He S, Xiang Y. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology. 2020;72:412–29. doi: 10.1002/hep.31031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller AM, Kleemann R, Gart E, Duyvenvoorde W, Van, Verschuren L, Caspers M, et al. Cholesterol accumulation as a driver of hepatic inflammation under translational dietary conditions can be attenuated by a multicomponent medicine. Front Endocrinol. 2021;12:1–14. doi: 10.3389/fendo.2021.601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, et al. Obesity modulates inflammation and lipid metabolism oocyte gene expression: a single-cell transcriptome perspective. J Clin Endocrinol Metab. 2017;102:2029–38. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobos L, Alqahtani S, Xia L, Coltellino V, Kishman R, McIlrath D, et al. Comparison of silver nanoparticle-induced inflammatory responses between healthy and metabolic syndrome mouse models. J Toxicol Environ Health. 2021;83:249–68. doi: 10.1080/15287394.2020.1748779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen J, Wang L. Identification of key genes and their association with immune infiltration in adipose tissue of obese patients: a bioinformatic analysis. Adipocyte. 2022;11:401–12. doi: 10.1080/21623945.2022.2104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuente-Hernandez Marcela Angelica De la. Alanis-Manriquez EC, Ferat-Osorio E, Rodriguez-Gonzalez A, Lagunas LA-PKV-SJM-Z, Lagunas VM. Molecular changes in adipocyte-derived stem cells during their interplay with cervical cancer cells. Cell Oncol. 2022;45:85–101. doi: 10.1007/s13402-021-00653-6. [DOI] [PubMed] [Google Scholar]
- 43.Herrero-Aguayo V, Sáez-Martínez P, López-Cánovas JL, Prados-Carmona JJ, Alcántara-Laguna MD, López FL, et al. Dysregulation of components of the inflammasome machinery after bariatric surgery: novel targets for a chronic disease. J Clin Endocrinol Metab. 2021;19:e4917–34. [DOI] [PubMed]
- 44.Deiuliis JA, Oghumu S, Duggineni D, Zhong J, Rutsky J, Banerjee A, et al. CXCR3 modulates obesity-induced visceral adipose inflammation and systemic insulin resistance. Obesity. 2014;22:1264–74. doi: 10.1002/oby.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma W, Gil HJ, Escobedo N, Benito-Martín A, Ximénez-Embún P, Muñoz J, et al. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight. 2020;5:1–18. doi: 10.1172/jci.insight.135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldridge RC, Wentzensen N, Pfeiffer RM, Brinton LA, Patricia Hartge C, Guillemette TJ, Kemp, Ligia A, Pinto BT. Endogenous estradiol and inflammation biomarkers: potential interacting mechanisms of obesity-related disease. Cancer Causes Control. 2021;31:309–20. doi: 10.1007/s10552-020-01280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali MM, Naquiallah D, Qureshi M, Mirza MI, Hassan C, Masrur M, et al. DNA methylation profile of genes involved in inflammation and autoimmunity correlates with vascular function in morbidly obese adults. Epigenetics. 2022;17:93–109. doi: 10.1080/15592294.2021.1876285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He W, Rebello O, Savino R, Terracciano R, Schuster-Klein C, Guardiola B, et al. TLR4 triggered complex inflammation in human pancreatic islets. Mol Basis Dis. 2019;1865:86–97. doi: 10.1016/j.bbadis.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 49.Auguet T, Bertran L, Binetti J, Aguilar C, Martínez S, Sabench F, et al. Relationship between IL-8 circulating levels and TLR2 hepatic expression in women with morbid obesity and nonalcoholic steatohepatitis. Int J Mol Sci. 2020;21:1–15. doi: 10.3390/ijms21114189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, et al. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010;155:35–43. doi: 10.1016/j.trsl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Ballak DB, Essen P, Van, Diepen JA, Van, Jansen H, Hijmans A. MAP3K8 (TPL2/COT) affects obesity-induced adipose tissue inflammation without systemic effects in humans and in mice. PLoS ONE. 2014;9:2–9. doi: 10.1371/journal.pone.0089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas M, Cecilia B, Biniyam W, Marcus T, Marcus C. Association between obesity and periodontal risk indicators in adolescents. Int J Pediatr Obes. 2011;6:264–70. doi: 10.3109/17477166.2010.495779. [DOI] [PubMed] [Google Scholar]
- 53.Yang X, Li M, Haghiac M, Catalano PM, Mouzon SH, Reserve CW. Causal relationship between obesity-related traits and TLR4- driven responses at the maternal–fetal interface. Diabetologia. 2017;59:2459–66. doi: 10.1007/s00125-016-4073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima RS, Mattos RT, Medeiros NI, Kattah FM, Julya R, Nascimento S, et al. CXCL8 expression and methylation are correlated with anthropometric and metabolic parameters in childhood obesity. Cytokine. 2021;143:155538. doi: 10.1016/j.cyto.2021.155538. [DOI] [PubMed] [Google Scholar]
- 55.Subudhi S, Drescher HK, Dichtel LE, Bartsch LM, Chung RT, Hutter MM, et al. Distinct hepatic gene-expression patterns of NAFLD in patients with obesity. Hepatol Commun. 2022;6:77–89. doi: 10.1002/hep4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harakeh S, Kalamegam G, Pushparaj PN, Al-Hejin A, Alfadul SM, Al Amri T, et al. Chemokines and their association with body mass index among healthy Saudis. Saudi J Biol Sci. 2020;27:6–11. doi: 10.1016/j.sjbs.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte GV, Boeira V, Correia T, Porto-Silva L, Cardoso T, Macedo MN, et al. Osteopontin, CCL5 and CXCL9 are independently associated with psoriasis, regardless of the presence of obesity. Cytokine. 2015;74:287–92. doi: 10.1016/j.cyto.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Hueso L, Ortega R, Selles F, Yun N, Ortega J, Civera M, et al. Upregulation of angiostatic chemokines IP-10 / CXCL10 and I-TAC / CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J Obes. 2018;1–12. [DOI] [PubMed]
- 59.Ruebel M, Shankar K, Gaddy D, Lindsey F, Badger T, Andres A et al. Maternal obesity is associated with ovarian inflammation and up-regulation of early growth response factor (Egr)-1. Am J Physiol Endocrinol Metab. 2016;1–27. [DOI] [PubMed]
- 60.Lee H, Park J, Kang J, Kawada T, Yu R, Han I. Cytokine chemokine and chemokine receptor gene expression in the mesenteric adipose tissue of KKAy mice. Cytokine. 2009;46:160–5. doi: 10.1016/j.cyto.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Ibet G, Vannan M, Eksteen D, Reyes B. NLRP3 receptor contributes to protection against experimental antigen-mediated cholangitis. Biosci Rep. 2020;40:1–9. doi: 10.1042/BSR20200689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolfs MGM, Gruben N, Rensen SS, Verdam FJ, Greve JW, Driessen A, et al. Determining the association between adipokine expression in multiple tissues and phenotypic features of non-alcoholic fatty liver disease in obesity. Nutr Diabetes. 2015;5:1–7. doi: 10.1038/nutd.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arendt LM, Mccready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Tumor Stem Cell Biol. 2013;73:6080–93. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng H, Zhang H, Zhu H. Blocking CXCR7-mediated adipose tissue macrophages chemotaxis attenuates insulin resistance and inflammation in obesity. Biochem Biophys Res Commun. 2016;479:649–55. doi: 10.1016/j.bbrc.2016.09.158. [DOI] [PubMed] [Google Scholar]
- 65.Cereijo R, Cairo AG-NM, Eizirik DL, Marta Giralt FV. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adoptation. Cell Metabol. 2018;28:750–63. doi: 10.1016/j.cmet.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Matsushita Y, Hasegawa Y, Takebe N, Onodera K, Shozushima M, Oda T, et al. Serum C-X-C motif chemokine ligand 14 levels are associated with serum C-peptide and fatty liver index in type 2 diabetes mellitus patients. J Diabetes Investig. 2021;12:1042–9. doi: 10.1111/jdi.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryyti R, Pemmari A, Peltola R, Hämäläinen M, Moilanen Effects of lingonberry (vaccinium vitis-idaea L.) supplementation on hepatic gene expression in high-fat diet fed mice. Nutrients. 2021;13:1–23. doi: 10.3390/nu13113693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaume Padilla1 NTJSL. Zhang H, Cui J, Zuidema MY, Hill CZ. Vascular transcriptional alterations produced by juvenile obesity in Ossabaw swine. Physiol Genomics. 2013;16:1–42. doi: 10.1152/physiolgenomics.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.RyytiID R, Hamalainen M, Peltola R, Moilanen E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS One. 2020;15:1–17. doi: 10.1371/journal.pone.0232605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mcpherson KC, Shields CA, Poudel B, Johnson AC, Taylor L, Stubbs C, et al. Altered renal hemodynamics is associated with glomerular lipid accumulation in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol. 2020;318:911–21. doi: 10.1152/ajprenal.00438.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denis GV, Sebastiani P, Andrieu G, Tran AH, Strissel KJ, Frank L, Lombardi Relationships among obesity, type 2 diabetes and plasma cytokines in african american women. Obesity. 2018;25:1916–20. doi: 10.1002/oby.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schielke L, Zimmermann N, Hobelsberger S, Steininger J, Strunk A, Blau K, et al. Metabolic syndrome in psoriasis is associated with upregulation of CXCL16 on monocytes and a dysbalance in innate lymphoid cells. Front Immunol. 2022;13:1–10. doi: 10.3389/fimmu.2022.916701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hariharan N, Ashcraft KA, Svatek RS, Livi CB, Wilson D, Kaushik D, et al. Adipose tissue-secreted factors alter bladder cancer cell migration. J Obes. 2018;2018:1–10. doi: 10.1155/2018/9247864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chavey C, Fajas L. Drives obesity to diabetes, and further. Aging. 2009;1:674–7. doi: 10.18632/aging.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arias N, Aguirre L, Fernández-Quintela A, M González AL, Miranda J, Macarulla MT, et al. MicroRNAs involved in the browning process of adipocytes. J Physiol Biochem. 2015;72:509–21. doi: 10.1007/s13105-015-0459-z. [DOI] [PubMed] [Google Scholar]
- 76.Juge-aubry CE, Somm E, Giusti V, Chicheportiche R, Verdumo C, Burger D, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist upregulation in obesity and inflammation. Diabetes. 2003;52:1–7. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 77.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. PNAS. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruun JM, Pedersen SB, Metabolism C, Amtssygehus A. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab. 2000;86:1267–73. doi: 10.1210/jcem.86.3.7264. [DOI] [PubMed] [Google Scholar]
- 79.Sindhu S, Kochumon S, Thomas R, Bennakhi A, Al-Mulla F, Ahmad R. Enhanced adipose expression of interferon regulatory factor (IRF)-5 associates with the Signatures of metabolic inflammation in diabetic obese patients. Cell. 2020;9:1–20. doi: 10.3390/cells9030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straczkowski M, Dzienis-straczkowska S, Ste A, Kowalska I, Szelachowska M, Kinalska IDA. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor alpha system. J Clin Endocrinol Metab. 2015;87:4602–6. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- 81.David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines. 2016;4:1–15. doi: 10.3390/vaccines4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 83.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/S0303-7207(01)00394-X. [DOI] [PubMed] [Google Scholar]
- 84.Saha A, Ahn S, Blando J, Su F, Kolonin MG. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives myc-induced prostate cancer in obese mice. Cancer Res. 2018;77:5158–68. doi: 10.1158/0008-5472.CAN-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su F, Daquinag AC, Ahn S, Saha A, Dai Y, Zhao Z, et al. Progression of prostate carcinoma is promoted by adipose stromal cell-secreted CXCL12 signaling in prostate epithelium. Precision Oncol. 2021;26:1–10. doi: 10.1038/s41698-021-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim D, Kim J, Yoon JH, Ghim J, Yea K. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 2014;57:1456–65. doi: 10.1007/s00125-014-3237-5. [DOI] [PubMed] [Google Scholar]
- 87.Schaerli P, Willimann K, Ebert LM, Walz A, Moser B, Bern C. Cutaneous CXCL14 targets blood precursors to epidermal niches for langerhans cell differentiation. Immunity. 2005;23:331–42. doi: 10.1016/j.immuni.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Shurin GV, Ferris R, Tourkova IL, Lokshin A, Balkir L, Collins B, et al. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J Immunol. 2005;174:1–10. doi: 10.4049/jimmunol.174.9.5490. [DOI] [PubMed] [Google Scholar]
- 89.Shellenberger TD, Wang M, Gujrati M, Jayakumar A, Strieter RM, Burdick MD, et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–70. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- 90.Cao X, Zhang W, Wan T, He L, Chen T, Yuan Z, et al. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 γ chemoattractant for human neutrophils and dendritic cells. J Immunol. 2000;165:1–10. doi: 10.4049/jimmunol.165.5.2588. [DOI] [PubMed] [Google Scholar]
- 91.Frederick MJ, Henderson Y, Xu X, Deavers MT, Sahin AA, Wu H, et al. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am J Pathol. 2000;156:1937–50. doi: 10.1016/S0002-9440(10)65067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sleeman MA, Fraser JK, Murison JG, Kelly SL, Prestidge RL, Palmer DJ, et al. B cell- and monocyte-activating chemokine (BMAC), a novel non-ELR α-chemokine Matthew. Int Immunol. 2000;12:677–89. doi: 10.1093/intimm/12.5.677. [DOI] [PubMed] [Google Scholar]
- 93.Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Ii KC, Azam M, et al. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem Biophys Res Commun. 1999;706:703–6. doi: 10.1006/bbrc.1999.0257. [DOI] [PubMed] [Google Scholar]
- 94.Hara T, Tanegashima K. Pleiotropic functions of the CXC-type chemokine CXCL14 in mammals. J Biochem. 2012;151:469–76. doi: 10.1093/jb/mvs030. [DOI] [PubMed] [Google Scholar]
- 95.Meuter S, Schaerli P, Roos RS, Brandau O, Bo MR, Andrian UH, Von, et al. Murine CXCL14 is dispensable for dendritic cell function and localization within peripheral tissues. Mol Cell Biol. 2007;27:983–92. doi: 10.1128/MCB.01648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanegashima K, Okamoto S, Nakayama Y, Taya C, Shitara H, Ishii R. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. POLS ONE. 2010;5:1–9. doi: 10.1371/journal.pone.0010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21:201–23. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastanias P, Mackenzie K, Robinson S, Wang W. Medical complications resulting from severe obesity. Psychiatr Care Severe Obes. 2017;49–73.
- 99.Butler AE, Janson J, Bonner-weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 100.Ashcroft FM, Rorsman P. Diabetes mellitus and the β-cell: the last ten years. Cell. 2012;148:1160–71. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leea B-C. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2015;1842:446–62. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Popov SS, Kryl’skii ED, Shulgin KK, Raskina EA, Popova TN, Pashkov AN, et al. Inflammation is associated with impairment of oxidative status, carbohydrate and lipid metabolism in type 2 diabetes complicated by non-alcoholic fatty liver disease. Minerva Endocrinol. 2022;47:304–13. doi: 10.23736/S2724-6507.20.03257-5. [DOI] [PubMed] [Google Scholar]
- 103.Kumar M, Roe K, Nerurkar PV, Orillo B, Thompson KS, Verma S. Reduced immune cell infiltration and increased pro-inflammatory mediators in the brain of type 2 diabetic mouse model infected with west nile virus. J Neuroinflamm. 2014;11:1–17. doi: 10.1186/1742-2094-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Badr G, Badr BM, Mahmoud MH, Mohany M, Rabah DM, Garraud O. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1 α, MIP-2, KC, CX3CL1 and TGF- β in wounded tissue. Immunology. 2012;13:1–9. doi: 10.1186/1471-2172-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sajadi SMA, Hossein Khoramdelazad, Gholamhossein Hassanshahi HR, Hosseini J, Mahmoodi M, Arababadi MK, Derakhshan R, et al. Plasma levels of CXCL1 (GRO-alpha) and CXCL10 (IP-10) are elevated in type 2 diabetic patients: evidence for the involvement of inflammation and angiogenesis/angiostasis in this disease state. Clin Lab. 2013;59:133–7. doi: 10.7754/Clin.Lab.2012.120225. [DOI] [PubMed] [Google Scholar]
- 106.Hakimizadeh E, Shamsizadeh A, Nazari M, Arababadi MK, Rezaeian M, Jamali RV, Poor Z, Nahideh M, Khorramdelazad H, Darakhshan S. Increased circulating levels of CXC chemokines is correlated with duration and complications of the disease in type-1 diabetes: a study on iranian diabetic patients. Clin Lab. 2013;59:531–7. doi: 10.7754/Clin.Lab.2012.120518. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi K, Ohara M, Sasai T, Homma H, Nagasawa K, Takahashi T, et al. Serum CXCL1 concentrations are elevated in type 1 diabetes mellitus, possibly reflecting activity of anti-islet autoimmune activity. Diab/Metab Res Rev. 2011;27:830–3. doi: 10.1002/dmrr.1257. [DOI] [PubMed] [Google Scholar]
- 108.Nunemaker CS, Chung HG, Verrilli GM, Corbin KL, Upadhye A, Sharma PR. Increased circulating levels of CXC chemokines is correlated with duration and complications of the disease in type-1 diabetes: a study on iranian diabetic patients. Clin Lab. 2013;222:267–76. doi: 10.7754/clin.lab.2012.120518. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J, Zhou R, Deng L, Cao G, Zhang Y, Xu H, et al. Huangbai liniment and berberine promoted wound healing in high-fat diet / streptozotocin-induced diabetic rats. Biomed Pharmacother. 2022;150:112948. doi: 10.1016/j.biopha.2022.112948. [DOI] [PubMed] [Google Scholar]
- 110.Song M, Chen L, Zhang L, Li C, Wake J. Cryptotanshinone enhances wound healing in type 2 diabetes with modulatory effects on inflammation, angiogenesis and extracellular matrix remodelling. Pharm Biol. 2020;58:845–53. doi: 10.1080/13880209.2020.1803369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anuradha R, Saravanan M, Chandrakumar D, Menon A, Thiruvengadam K, Nutman TB, et al. Helminth infection modulates systemic pro- inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis. 2020;14:e0008101. doi: 10.1371/journal.pntd.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen Z, Chen Q, Ying H, Ma Z, Bi X, Li X, et al. Identification of differentially expressed genes in the endothelial precursor cells of patients with type 2 diabetes mellitus by bioinformatics analysis. Exp Ther Med. 2020;19:499–510. doi: 10.3892/etm.2019.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cnop M, Welsh N, Jonas J, Jo A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:97–107. doi: 10.2337/diabetes.54.suppl_2.S97. [DOI] [PubMed] [Google Scholar]
- 114.Pedersen SS, Prause M, Williams K, Barrès R. Butyrate inhibits IL-1β-induced inflammatory gene expression by suppression of NF- κB activity in pancreatic beta cells. J Biol Chem. 2022;298:1–31. doi: 10.1016/j.jbc.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, et al. NF-kB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306:131–49. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma P, Zhang P, Chen S, Shi W, Ye J, Chen S. Immune cell landscape of patients with diabetic macular edema by single-cell RNA analysis. Font Pharmacol. 2021;12:1–14. doi: 10.3389/fphar.2021.754933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leguina-ruzzi A, Valderas JP. BLT2 expression improves skin integrity and protects from alterations caused by hyperglycemia in type 2 diabetes. Dermato-Endocrinol. 2017;9:1–12. doi: 10.1080/19381980.2016.1267078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Q, Zhou W, Wang Y, Huang J, Hui X, Zhou Z, et al. Abnormal peripheral neutrophil transcriptome in newly diagnosed type 2 diabetes patients. J Diabetes Res. 2020;2020:1–10. doi: 10.1155/2020/9519072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cani PD, Bibiloni R, Knauf C, Neyrinck AM, Delzenne NM. Changes in gut microbiota control metabolic diet – induced obesity and diabetes in mice. Diabetes. 2008;57:1–12. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 120.Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cimini FA, Barchetta I, Porzia A, Mainiero F, Costantino C, et al. Circulating IL–8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017;54:961–7. [DOI] [PubMed]
- 122.Cui S, Zhu Y, Du J, Khan MN, Wang B, Wei J, et al. CXCL8 antagonist improves diabetic nephropathy in male mice of diabetes and attenuates high glucose-induced mesangial injury. Endocrinology. 2017;158:1671–84. doi: 10.1210/en.2016-1781. [DOI] [PubMed] [Google Scholar]
- 123.Tang W, Lv Q, Zou XCJ, Shi ZLY. CD8 + T cell-mediated cytotoxicity toward schwann cells promotes diabetic peripheral neuropathy. Cell Physiol Biochem. 2013;32:827–37. doi: 10.1159/000354485. [DOI] [PubMed] [Google Scholar]
- 124.Kochumon S, Arefanian H, Sindhu S, Al-Mulla F, Ahmad JT. Adipose tissue steroid receptor RNA activator 1 (SRA1) expression is associated with obesity, insulin resistance, and inflammation. Cells. 2021;10:2602. doi: 10.3390/cells10102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ascaso P, Palanca A, Martinez-Hervás S, Sanz MJ, Ascaso JF, Piqueras L, et al. Peripheral blood levels of CXCL10 are a useful marker for diabetic polyneuropathy in subjects with type 2 diabetes. Int J Clin Pract. 2021;75:e14302. doi: 10.1111/ijcp.14302. [DOI] [PubMed] [Google Scholar]
- 126.Santopaolo M, Sullivan N, Thomas AC, Alvino VV, Nicholson LB, Gu Y, et al. Activation of bone marrow adaptive immunity in type 2 diabetes: rescue by co-stimulation modulator abatacept. Font Immunol. 2021;12:609406. doi: 10.3389/fimmu.2021.609406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.N Y, GR MM, GH A. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iran Red Crescent Med J. 2011;13:751–2. [PMC free article] [PubMed] [Google Scholar]
- 128.Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor – dependent signaling. Mol Med. 2008;14:301–8. doi: 10.2119/2007-00052.Humpert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karimabad MN, Hassanshahi G. Significance of CXCL12 in type 2 diabetes mellitus and its associated complications. Inflammation. 2014;38:710–7. doi: 10.1007/s10753-014-9981-3. [DOI] [PubMed] [Google Scholar]
- 130.Lu C, Ma J, Su J, Wang X, Liu W, Ge X. Serum stromal cell-derived factor-1 levels are associated with diabetic kidney disease in type 2 diabetic patients. Endocr J. 2021;68:1101–7. doi: 10.1507/endocrj.EJ21-0039. [DOI] [PubMed] [Google Scholar]
- 131.Nätynki A, Leisti P, Tuusa J, Varpuluoma O, Ukkola O, Junttila J, et al. Use of gliptins reduces levels of SDF-1 / CXCL12 in bullous pemphigoid and type 2 diabetes, but does not increase autoantibodies against BP180 in diabetic patients. Front Immunol. 2022;13:1–13. doi: 10.3389/fimmu.2022.942131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu S, Liu X, Xiong H, Wang W, Liu Y, Yin L, et al. CXCL13/CXCR5 signaling contributes to diabetes-induced tactile allodynia via activating pERK, pSTAT3, pAKT pathways and pro-inflammatory cytokines production in the spinal cord of male mice. Brain Behav Immun. 2019;80:711–4. doi: 10.1016/j.bbi.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 133.Jiang H, Yicun W, Meng J, Chen S, Wang J, Qiu Y, et al. Effects of transplanting bone marrow stromal cells transfected with CXCL13 on fracture healing of diabetic rats. Cell Physiol Biochem. 2018;49:123–33. doi: 10.1159/000492848. [DOI] [PubMed] [Google Scholar]
- 134.Wu C, Chen X, Shu J, Lee C. Whole-genome expression analyses of type 2 diabetes in human skin reveal altered immune function and burden of infection. Oncotarget. 2017;8:34601–9. doi: 10.18632/oncotarget.16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm. 2016;13:1–8. doi: 10.1186/s12950-015-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adamski V, Mentlein R, Lucius R, Synowitz M, Held-feindt J, Hattermann K. The chemokine receptor CXCR6 evokes reverse signaling via the transmembrane chemokine CXCL16. Int J Mol Sci. 2017;18:1468. doi: 10.3390/ijms18071468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xia Y, Entman ML, Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension. 2013;62:1129–37. doi: 10.1161/HYPERTENSIONAHA.113.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang Y, Henderson FC, Yi Q, Lei Q, Li Y, Chen N. Chemokine CXCL16 expression suppresses migration and invasiveness and induces apoptosis in breast cancer cells. Mediat Inflamm. 2014;2014:1–9. doi: 10.1155/2014/478641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang H, Liao M, Zhao W, Zheng X, Xu F, Wang H, et al. CXCL16 / ROCK1 signaling pathway exacerbates acute kidney injury induced by ischemia-reperfusion. Biomed Pharmacother. 2018;98:347–56. doi: 10.1016/j.biopha.2017.12.063. [DOI] [PubMed] [Google Scholar]
- 140.Abdel-bakky MS, Alqasoumi A, Altowayan WM, Amin E, Darwish MA. Resveratrol inhibited ADAM10 mediated CXCL16-cleavage and T-cells recruitment to pancreatic β -cells in type 1 diabetes mellitus in mice. Pharmaceutics. 2022;14:594. doi: 10.3390/pharmaceutics14030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao L, Wu F, Jin L, Lu T, Yang L, Pan X, et al. Serum CXCL16 as a novel marker of renal injury in type 2 diabetes mellitus. PLoS One. 2014;9:e87786. doi: 10.1371/journal.pone.0087786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gutwein P, Abdel-bakky MS, Doberstein K, Schramme A, Beckmann J, Schaefer L, et al. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. Mol Med. 2009;13:3809–25. doi: 10.1111/j.1582-4934.2009.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kato T, Hagiyama M, Ito A. Renal ADAM10 and 17: their physiological and medical meanings. Front Cell Dev Biol. 2018;6:153. doi: 10.3389/fcell.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.El-Asrar AMA, Nawaz MI, Ahmad A, Alexandra De Zutter M, Mairaj Siddiquei MB, Allegaert E, Gikandi PW, et al. Evaluation of proteoforms of the transmembrane chemokines CXCL16 and CX3CL1, their receptors, and their processing metalloproteinases ADAM10 and ADAM17 in proliferative diabetic retinopathy. Front Immunol. 2021;11:601639. doi: 10.3389/fimmu.2020.601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tawfik MS, Abdel-messeih PL, Nosseir NM, Heba HM. Circulating CXCL16 in type 2 diabetes mellitus egyptian patients. J Radiation Res Appl Sci. 2020;14:9–15. [Google Scholar]
- 146.Zhou F, Wang J, Wang K, Zhu X, Pang R, Li X, et al. Serum CXCL16 as a novel biomarker of coronary artery disease in type 2 diabetes mellitus: a pilot study. Ann Clin Lab Sci. 2016;46:184–9. [PubMed] [Google Scholar]
- 147.Lekva T, Michelsen AE, Aukrust P, Cecilie M, Roland P, Henriksen T, et al. CXC chemokine ligand 16 is increased in gestational diabetes mellitus and preeclampsia and associated with lipoproteins in gestational diabetes mellitus at 5 years follow-up. Diabetes Vasc Dis Res. 2017;14:525–33. doi: 10.1177/1479164117728011. [DOI] [PubMed] [Google Scholar]
- 148.Brandon J, Perumpail MAK, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–76. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 150.Younossi ZM. Nonalcoholic fatty liver disease. Zobair M Younossi. 1999;1:57–62. doi: 10.1007/s11894-999-0088-1. [DOI] [PubMed] [Google Scholar]
- 151.Angulo P. Non-alcoholic fatty liver disease. Med Progress. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 152.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–9. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 153.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 154.Adams LA, Lymp JF, Sauver JST, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 155.Day CP, James OF, Steatohepatitis A tale of two hits? Gastroenterology. 1998;114:842–5. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 156.CP D Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 157.Silva HE, Da, Arendt BM, Noureldin SA, Therapondos G, Chb MBM, Guindi M, et al. A cross-sectional study assessing dietary intake and physical activity in canadian patients with nonalcoholic fatty liver disease vs healthy controls. J Acad Nutr Dietetics. 2014;114:1181–94. doi: 10.1016/j.jand.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Abdallah J, Assaf S, Das A, Hirani V. Effects of anti–inflammatory dietary patterns on non– alcoholic fatty liver disease: a systematic literature review. Eur J Nutr. 2023;1–16. [DOI] [PMC free article] [PubMed]
- 159.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–94. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 160.Seki Y-SR. Ekihiro. Chemokines and chemokine receptors in the development of NAFLD. Obes Fat Liver Liver Cancer. 2018;1061:45–53. [DOI] [PubMed]
- 161.Nagata N, Chen G, Xu L, Ando H. An update on the chemokine system in the development of NAFLD. Medicina. 2022;58:761. doi: 10.3390/medicina58060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang B, Xu M, Zhou Z, Cai Y, Li M, Wang W, et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology. 2015;62:1070–85. doi: 10.1002/hep.27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang L, Miura K, Zhang B, Matsushita H, Yang YM, Liang S, et al. TRIF differentially regulates hepatic steatosis and inflammation/fibrosis in mice. Cell Mol Gastroenterol Hepatol. 2017;3:469–83. doi: 10.1016/j.jcmgh.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gart E, Duyvenvoorde W, Van, Caspers MPM, Trigt N, Van, Snabel J, Menke A, et al. Intervention with isoleucine or valine corrects hyperinsulinemia and reduces intrahepatic diacylglycerols, liver steatosis, and inflammation in ldlr – / –. Leiden mice with manifest obesity-associated NASH. FASEB J. 2022;36:1–18. doi: 10.1096/fj.202200111R. [DOI] [PubMed] [Google Scholar]
- 165.Dai W, Sun Y, Jiang Z, Du K, Xia N, Zhong G. Key genes associated with non-alcoholic fatty liver disease and acute myocardial infarction. Med Sci Monit. 2020;26:1–11. doi: 10.12659/MSM.922492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ogawa Y, Imajo K, Honda Y, Kessoku T, Tomeno W. Palmitate-induced lipotoxicity is crucial for the pathogenesis of nonalcoholic fatty liver disease in cooperation with gut-derived endotoxin. Sci Rep. 2018;1–14. [DOI] [PMC free article] [PubMed]
- 167.Saiman Y, Friedman SL. The role of chemokines in acute liver injury. Front Physiol. 2012;3:1–12. doi: 10.3389/fphys.2012.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bisset LR, Schmid P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 169.Hamirani YS, Katz R, Nasir K, Zeb I, Blaha MJ, Blumenthal RS, et al. Association between inflammatory markers and Liver Fat: the multi-ethnic study of atherosclerosis. J Clin Exp Cardiol. 2014;5:1–7. doi: 10.4172/2155-9880.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia J, Xu X, Huang P, He M, Wang X. The potential of CXCL5 as a target for liver cancer-what do we know so far? Expert Opin Ther Targets. 2015;19:141–6. doi: 10.1517/14728222.2014.993317. [DOI] [PubMed] [Google Scholar]
- 171.Xu X, Huang P, Yang B, Wang X, Xia J. Roles of CXCL5 on migration and invasion of liver cancer cells. J Transl Med. 2014;12:1–11. doi: 10.1186/1479-5876-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Leti F, Legendre C, Still CD, Chu X, Petrick A, Gerhard GS, et al. Altered expression of MALAT1 lncRNA in nonalcoholic steatohepatitis fibrosis regulates CXCL5 in hepatic stellate cells. Transl Res. 2018;190:25–39. doi: 10.1016/j.trsl.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Balkrishna A, Gohel V, Kumari P, Manik M, Bhattacharya K, Dev R. Livogrit prevents methionine-cystine deficiency induced nonalcoholic steatohepatitis by modulation of steatosis and oxidative stress in human hepatocyte-derived spheroid and in primary rat hepatocytes. Bioengineered. 2022;13:10811–26. doi: 10.1080/21655979.2022.2065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mirshahi F, Aqbi HF, Cresswell K, Saneshaw M, Coleman C, Jacobs T, et al. Longitudinal studies can identify distinct inflammatory cytokines associated with the inhibition or progression of liver cancer. Liver Int. 2019;40:468–72. doi: 10.1111/liv.14323. [DOI] [PubMed] [Google Scholar]
- 175.Cai X, Li Z, Zhang Q, Qu Y, Xu M, Wan X, et al. CXCL6-EGFR-induced kupffer cells secrete TGF‐β1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/ EZH2 pathway in liver fibrosis. J Cell Mol Med. 2018;22:5050–61. doi: 10.1111/jcmm.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ivan Bieche T, Asselah I, Laurendeau D, Vidaud C, Degote V, Paradise P, Bedossa D-C, Valla P, Marcellinc M, Vidauda ALaboratoire. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology. 2005;332:130–44. doi: 10.1016/j.virol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 177.Chen M, Xing J, Pan D, Peng X, Gao P. Chinese herbal medicine mixture 919 syrup alleviates nonalcoholic fatty liver disease in rats by inhibiting the NF- κ B pathway. Biomed Pharmacother. 2020;128:110286. doi: 10.1016/j.biopha.2020.110286. [DOI] [PubMed] [Google Scholar]
- 178.Tuncer L, Özbek H, Topal C, Uygan S. The serum levels of IL-1\beta, IL-6,IL-8 and TNF-\alpha in nonalcoholic fatty liver. Turk J Med Sci. 2003;33:1–7. [Google Scholar]
- 179.Pan X, Kaminga AC, Liu A, Wen SW, Chen J, Luo J. Chemokines in non-alcoholic fatty liver disease: a systematic review and network meta-analysis. Font Immunol. 2020;11:1–11. doi: 10.3389/fimmu.2020.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Casilli F, Bianchini A, Gloaguen I, Biordi L, Alesse E, Festuccia C, et al. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem Pharmacol. 2005;69:385–94. doi: 10.1016/j.bcp.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 181.Nojima H, Konishi T, Freeman CM, Schuster RM, Japtok L, Kleuser B, et al. Chemokine receptors, CXCR1 and CXCR2, differentially regulate exosome release in hepatocytes. PLoS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0161443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: Prevention of reperfusion injury. PNAS. 2004;101:11791–6. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wong AM, Ding X, Wong AM, Yu J, Kahn M, Wong N, et al. Unique molecular characteristics of NAFLD-associated liver cancer accentuate beta-catenin/ TNFRSF19-mediated immune evasion. J Hepatol. 2022;77:410–23. doi: 10.1016/j.jhep.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 184.Ipsen DH, Agerskov RH, Klaebel JH, Lykkesfeldt J, Nyborg PT. The development of nonalcoholic steatohepatitis is subjected to breeder dependent variation in guinea pigs. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-021-82643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Youssry S, Kamel MA. Effect of folate supplementation on immunological and autophagy markers in experimental nonalcoholic fatty liver disease. Eur Cytokine Netw. 2019;30:135–43. doi: 10.1684/ecn.2019.0437. [DOI] [PubMed] [Google Scholar]
- 186.Ikeda A, Aoki N, Kido M, Iwamoto S, Nishiura H, Maruoka R, et al. Progression of autoimmune hepatitis is mediated by IL-18-producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology. 2014;60:224–36. doi: 10.1002/hep.27087. [DOI] [PubMed] [Google Scholar]
- 187.Song XI, Shen Y, Lao Y, Tao Z, Zeng J, Wang J, et al. CXCL9 regulates acetaminophen –induced liver injury via CXCR3. Exp Ther Med. 2019;18:4845–51. doi: 10.3892/etm.2019.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Semba T, Nishimura M, Nishimura S, Ohara O, Ishige T, Ohno S, et al. The FLS (fatty liver shionogi) mouse reveals local expressions of lipocalin-2, CXCL1 and CXCL9 in the liver with non-alcoholic steatohepatitis. Gastroenterology. 2013;13:1–8. doi: 10.1186/1471-230X-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tacke F, Zimmermann HW, Berres M, Trautwein C, Wasmuth HE. Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int. 2011;6:840–9. doi: 10.1111/j.1478-3231.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 190.Ángeles M, Sousa J, Zaida A, Moreno G, Tenor DP, Medrano LM, et al. CXCL9–11 polymorphisms are associated with liver fibrosis in patients with chronic hepatitis C: a cross–sectional study. Clin Trans Med. 2017;6:0–9. doi: 10.1186/s40169-017-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Y, Huang J, Tian Z, Zhou Y. The role of CXC cytokines as biomarkers and potential targets in hepatocellular carcinoma. Math Biosci Eng. 2019;17:1381–95. doi: 10.3934/mbe.2020070. [DOI] [PubMed] [Google Scholar]
- 192.Li L, Xia Y, Ji X, Wang H, Zhang Z, Lu P, et al. MIG/CXCL9 exacerbates the progression of metabolic-associated fatty liver disease by disrupting Treg/Th17 balance. Exp Cell Res. 2021;407:112801. doi: 10.1016/j.yexcr.2021.112801. [DOI] [PubMed] [Google Scholar]
- 193.Zhu M, Li M, Zhou W, Yang Y, Li F, Zhang L, et al. Qianggan extract improved nonalcoholic steatohepatitis by modulating lncRNA / circRNA immune ceRNA networks. BMC Complement Altern Med. 2019;19:1–13. doi: 10.1186/s12906-019-2577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang W, Liu X, Wei P, Ye F, Chen Y, Shi L, et al. SPP1 and CXCL9 promote non-alcoholic steatohepatitis progression based on bioinformatics analysis and experimental studies. Front Med. 2022;9:1–9. doi: 10.3389/fmed.2022.862278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng J, Wu H, Zhang Z, Yao S. Dynamic co–expression modular network analysis in nonalcoholic fatty liver disease. Hereditas. 2021;158:31. doi: 10.1186/s41065-021-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu Z, Zhang X, Lau J, Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert Rev Mol Med. 2016;18:e16. doi: 10.1017/erm.2016.16. [DOI] [PubMed] [Google Scholar]
- 197.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Nathan W, Harrison SA, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2017;63:731–44. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He W, Huang C, Zhang X, Wang D, Chen Y, Zhao Y, et al. Identification of transcriptomic signatures and crucial pathways involved in non-alcoholic steatohepatitis. Endocrine. 2021;73:52–64. doi: 10.1007/s12020-021-02716-y. [DOI] [PubMed] [Google Scholar]
- 199.Jiang H, Mao T, Liu Y, Tan X, Sun Z, Cheng Y, et al. Protective effects and mechanisms of yinchen linggui zhugan decoction in HFD-induced nonalcoholic fatty liver disease rats based on network pharmacology and experimental verification. Front Pharmacol. 2022;13:1–14. doi: 10.3389/fphar.2022.908128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Luo N, Yang C, Zhu Y, Chen Q, Zhang B. Diosmetin ameliorates nonalcoholic steatohepatitis through modulating lipogenesis and inflammatory response in a STAT1/CXCL10-dependent manner. J Agric Food Chem. 2021;69:655–67. doi: 10.1021/acs.jafc.0c06652. [DOI] [PubMed] [Google Scholar]
- 201.Bigorgne AE, Delbos LB, Naveau S, Dagher I, Prévot S, Gasselin ID, et al. Obesity-induced lymphocyte hyperresponsiveness to chemokines: a new mechanism of fatty liver inflammation in obese mice. Gastroenterology. 2008;134:1459–69. doi: 10.1053/j.gastro.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 202.Boujedidi H, Robert O, Bignon A, Anne-Marie Cassard-Doulcier M-LR, Gary-Gouy H, Hemon P, et al. CXCR4 dysfunction in non-alcoholic steatohepatitis in mice and patients. Clin Sci. 2015;128:257–67. doi: 10.1042/CS20130833. [DOI] [PubMed] [Google Scholar]
- 203.Yu Z, Han-bo CAO, Wen-jun LI, Li Z. The CXCL12 (SDF-1)/ CXCR4 chemokine axis: oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Nat Med. 2018;16:801–10. doi: 10.1016/S1875-5364(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 204.Li Y, Li N, Liu J, An X. Gr-1high Ly6G + myeloid-derived suppressor cells and their role in a murine model of non-alcoholic steatohepatitis Yue. Am J Transl Res. 2020;12:2827–42. [PMC free article] [PubMed] [Google Scholar]
- 205.Wang S, Gao S, Li Y, Qian X, Luan J, Lv X. Emerging importance of chemokine receptor CXCR4 and its ligand in liver disease. Front Cell Dev Biol. 2021;9:1–15. doi: 10.3389/fcell.2021.716842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang H, Shao Y, Zhang S, Xie A, Ye Y, Shi L, et al. CXCL16 deficiency attenuates acetaminophen- induced hepatotoxicity through decreasing hepatic oxidative stress and inflammation in mice. Acta Biochim Biophys Sin. 2017;49:1–9. doi: 10.1093/abbs/gmw112. [DOI] [PubMed] [Google Scholar]
- 207.Mcmahan RH, Porsche CE, Edwards MG, Rosen HR. Free fatty acids differentially downregulate chemokines in liver sinusoidal endothelial cells:insights into non-alcoholic fatty liver disease. PLoS One. 2016;11:1–14. doi: 10.1371/journal.pone.0159217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wehr A, Baeck C, Ulmer F, Gassler N, Hittatiya K, Luedde T, et al. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6 + NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu H, Zhang Q, Chen G. CXCR6 deficiency ameliorates ischemia-reperfusion injury by reducing the recruitment and cytokine production of hepatic NKT cells in a mouse model of non-alcoholic fatty liver disease. Int Immunopharmacol. 2019;72:224–34. doi: 10.1016/j.intimp.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 211.Wehr A, Baeck C, Heymann F, Niemietz M, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J Immunol. 2013;190:5226–36. doi: 10.4049/jimmunol.1202909. [DOI] [PubMed] [Google Scholar]
- 212.Jiang L, Yang M, Li X, Wang Y, Zhou G, Zhao J. CXC motif ligand 16 promotes nonalcoholic fatty liver disease progression via hepatocyte – stellate cell crosstalk. J Clin Endocrinol Metab. 2018;103:3974–85. doi: 10.1210/jc.2018-00762. [DOI] [PubMed] [Google Scholar]
- 213.Ma KL, Wu Y, Zhang Y, Wang GH, Hu ZB, Ruan XZ. Activation of the CXCL16 / CXCR6 pathway promotes lipid deposition in fatty livers of apolipoprotein E knockout mice and HepG2 cells. Am J Transl Res. 2018;10:1802–16. [PMC free article] [PubMed] [Google Scholar]
- 214.Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, Gaar J, Van De, Vroomen M, et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2017;0:1–11. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 215.José L, Maravillas-Montero AM, Burkhardt, Peter A, Hevezi, Christina D, Carnevale, Martine J. Smit and AZ. GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. 2016;194:29–33. doi: 10.4049/jimmunol.1401704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, et al. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350:74–81. doi: 10.1016/j.bbrc.2006.08.194. [DOI] [PubMed] [Google Scholar]
- 217.Pisabarro MT, Leung B, Kwong M, Corpuz R, Frantz GD, Chiang N, et al. Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol. 2006;176:2069–73. doi: 10.4049/jimmunol.176.4.2069. [DOI] [PubMed] [Google Scholar]
- 218.Wang L, Li H, Zhen Z, Yu W, Zeng H, Li L, et al. CXCL17 promotes cell metastasis and inhibits autophagy via the LKB1-AMPK pathway in hepatocellular carcinoma. Gene. 2018;690:129–36. doi: 10.1016/j.gene.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 219.Long J, Bai Y, Yang X, Lin J, Yang X, Wang D, et al. Construction and comprehensive analysis of a ceRNA network to reveal potential prognostic biomarkers for hepatocellular carcinoma. Cancer Cell Int. 2019;19:1–12. doi: 10.1186/s12935-019-0817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu D, Zheng Z, Zhang Y, Fan S, Zhang Z, Wang Y. Down-regulated lncRNA DLX6-AS1 inhibits tumorigenesis through STAT3 signaling pathway by suppressing CADM1 promoter methylation in liver cancer stem cells. J Exp Clin Cancer Res. 2019;38:1–17. doi: 10.1186/s13046-019-1239-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 221.Zhang L, He X, Jin T, Li G, Jin Z. Long non-coding RNA DLX6-AS1 aggravates hepatocellular carcinoma carcinogenesis by modulating miR-203a / MMP-2 pathway. Biomed Pharmacother. 2017;96:884–91. doi: 10.1016/j.biopha.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 222.Long J, Jiang C, Liu B, Fang S, Kuang M. MicroRNA-15a-5p suppresses cancer proliferation and division in human hepatocellular carcinoma by targeting BDNF. Tumor Biol. 2015;37:5821–8. doi: 10.1007/s13277-015-4427-6. [DOI] [PubMed] [Google Scholar]
- 223.Li Y, Lin Q, Chang SUE, Zhang R, Wang J. Vitamin D3 mediates miR –15a–5p inhibition of liver cancer cell proliferation via targeting E2F3. Oncol Lett. 2020;20:292–8. doi: 10.3892/ol.2020.11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF- β / smad signaling pathway. Onco Targets Ther. 2019;12:6897–905. doi: 10.2147/OTT.S209413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li L, Yan J, Xu J, Liu C, Zhen Z, Chen H, et al. CXCL17 expression predicts poor prognosis and correlates with adverse immune infiltration in hepatocellular carcinoma. PLoS One. 2014;9:e110064. doi: 10.1371/journal.pone.0110064. [DOI] [PMC free article] [PubMed] [Google Scholar]