Abstract
Cancer is a disease that results from the uncontrolled proliferation and growth of cells. Due to early detection methods, there is a decrease in death rates in many types of cancer. However, among the causes of death worldwide, cancer still ranks second after cardiovascular diseases. Therefore, cancer research has focused mainly on developing more effective treatments to reduce deaths from cancer. With a better understanding of the molecular mechanisms in cancer cells, advances in cancer treatment have evolved and changed. The main priority of research is to develop treatment modalities with the highest response rate and less side effects. In this context, immunotherapies have started a new era in cancer treatments. In this review, an overview of the future of next-generation treatment methods is presented by including the most preferred immunotherapy methods.
Keywords: Cancer, immunotherapy, CAR-T-cell therapy, monoclonal antibody, mRNA vaccine
Abstract
Kanser, hücrelerin kontrolsüz şekilde çoğalması ve büyümesi sonucu oluşan bir hastalıktır. Erken teşhis yöntemleri sayesinde birçok kanser türünde ölüm oranlarında düşüş yaşanmaktadır. Ancak tüm dünyada ölüm nedenleri arasında kanser, kardiyovasküler hastalıklardan sonra halen ikinci sıradadır. Bu nedenle, kanser araştırmalarının çoğu kanserden ölümleri azaltmak için daha etkin tedaviler geliştirmeye odaklanmıştır. Kanser hücresindeki moleküler mekanizmaların daha da iyi anlaşılmasıyla kanser tedavisindeki gelişmeler, zaman içinde gelişmiş ve değişmiştir. Araştırmaların temel önceliği en yüksek yanıt oranına ve en düşük yan etkiye sahip tedavi yöntemleri geliştirmektir. İmmünoterapiler bu bağlamda kanser tedavilerinde yeni bir dönemi başlatmıştır. Bu derlemede en çok tercih edilen immünoterapi yöntemlerine yer verilerek yeni nesil tedavi yöntemlerinin geleceğine dair genel bir bakış açısı sunulmuştur.
Keywords: Kanser, immünoterapi, CAR-T-hücre tedavisi, monoklonal antikor, mRNA aşısı
INTRODUCTION
Cancer is a disease of cells that proliferate uncontrolled. Some features distinguish cancer cells from healthy cells: maintaining proliferative signaling, being insensitive to growth suppressive signals, resistance to cell death, providing unlimited replication capability, promoting angiogenesis, stimulating invasion and metastasis, reprogramming environmental and cellular metabolism, and evading immune destruction1. Because to their genetic mutations, cancer cells are not caught in cell cycle control mechanisms and they escape from apoptosis2. The role of environmental factors in carcinogenesis is critical and genetic alterations. These factors include physical carcinogens such as ionizing and ultraviolet radiation; chemical carcinogens such as smoking, alcohol consumption and asbestos exposure, and dietary consumption of arsenic and aflatoxin. Biological carcinogens, including infections from certain bacteria, viruses or parasites are also among the causes of cancer deaths; approximately one-third of those are related to smoking, alcohol consumption, high body mass index, unhealthy diet, and insufficient physical activity3,4.
Today, despite early detection methods and advanced therapies, cancer still is a global health problem with a high incidence and mortality rate. According to the World Health Organization, it is estimated that approximately 10 million deaths and one in 6 deaths in 2020 are caused by cancer. Breast, colorectal, lung, cervical, and thyroid cancers are most common among women while lung, prostate, colorectal, stomach, and liver cancer are most common among men. While the most common cancer types in terms of new cases diagnosed in 2020 breast, lung, colon and rectum, prostate, skin and stomach, lung, colon and rectum, liver, stomach, and breast cancers are the most common causes of cancer deaths in 20205.
Although there is a decline in death rates in many cancer types due to effective treatment methods, most of the cancer research is focused on developing better therapies to reduce the deaths. Cancer treatment has evolved and changed with a better understanding of the molecular mechanisms underlying cancer. With an increasing number of cancer patients, significant challenges arise worldwide. However, the search for treatment with the highest response rate and fewer side effects continues apace6. There are different types of cancer treatments used in the clinic: surgery, radio-/chemotherapy, hormone therapy, photodynamic therapy, targeted therapy, stem cell transplant, hyperthermia, and immunotherapy7. These types of treatments are often used in combination because of their resistance mechanisms in cancer.
This review predicts the future of new-generation cancer therapeutics considering current research and to discuss the factors that may be effective in determining national and international roadmaps.
IMMUNOTHERAPIES
Immunotherapy has become an advanced treatment strategy among the different therapeutic options in various malignancies, including hematological and solid tumors.
In immunotherapies, a patient’s own immune system is used to fight cancer, to pave the way for more specific and effective treatments. In comparison to chemotherapy, having comparably fewer side effects, cancer immunotherapy is a promising tool for those with different malignancies8. Current immunotherapy therapeutics include inhibitors of immune checkpoint, monoclonal antibodies (mAbs), mRNA vaccines, and adoptive cell transfer in the form of chimeric antigen receptor (CAR)-T cell therapies (Figure 1)9.
Figure 1.
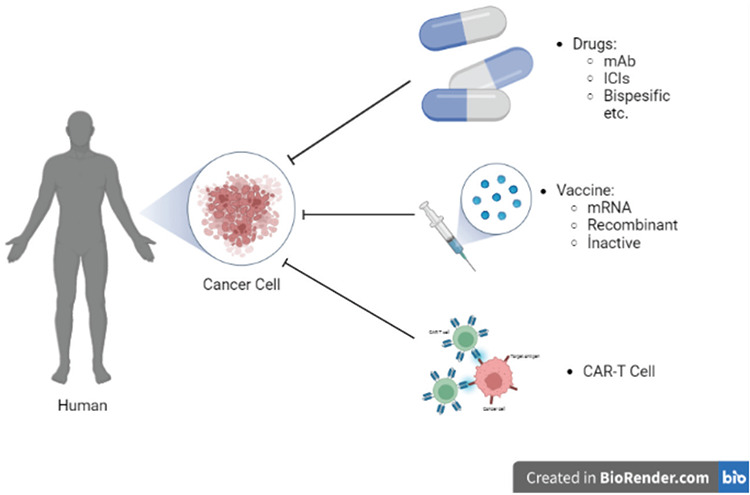
Current advances and future prospects in cancer immunotherapies.
ICI: Immune checkpoint inhibitors, mAb: Monoclonal antibody
Cancer immunotherapy is classified as passive or active immunotherapy based on the immune response. Passive immunotherapy uses agents that increase the existing anti-tumor response, including lymphocytes, cytokines, or mAbs. Active immunotherapy includes methods such as vaccination, non-specific immunomodulation, or activation of the immune system by targeting particularly designed antigen receptors to tumor cells10. Contrary to the successful immunotherapy approaches, limiting factors restrict the activation of tumor-specific immune responses, such as intratumoral heterogeneity, poor production and function of tumor-specific CD8 T-cells, shortage of appropriate neoantigens with defective processing, and antigen presentation11. Resistance mechanisms to immune response are mainly related to T-cell immune checkpoint pathways. Thus, investigating molecular pathways and exploring new immune checkpoints can reduce the immune response evasion. To enhance the response to immunotherapeutics, it is crucial to find strategies that will increase arrival to the tumor site, enhance T-cell continuity and proliferation, reduce immunosuppression and prevent T-cell depletion12.
Monoclonal Antibodies (mAbs) for Cancer Therapy
mAbs produced by B lymphocytes or synthetically are proteins that bind to a specific molecular target9. Anti-cancer effects have been achieved in preclinical models and patient studies by designing humanized mAbs against appropriate targets13. Recently, mAbs have been highly preferred cancer therapeutics as they are substantially specific and have lower cytotoxic effects14.
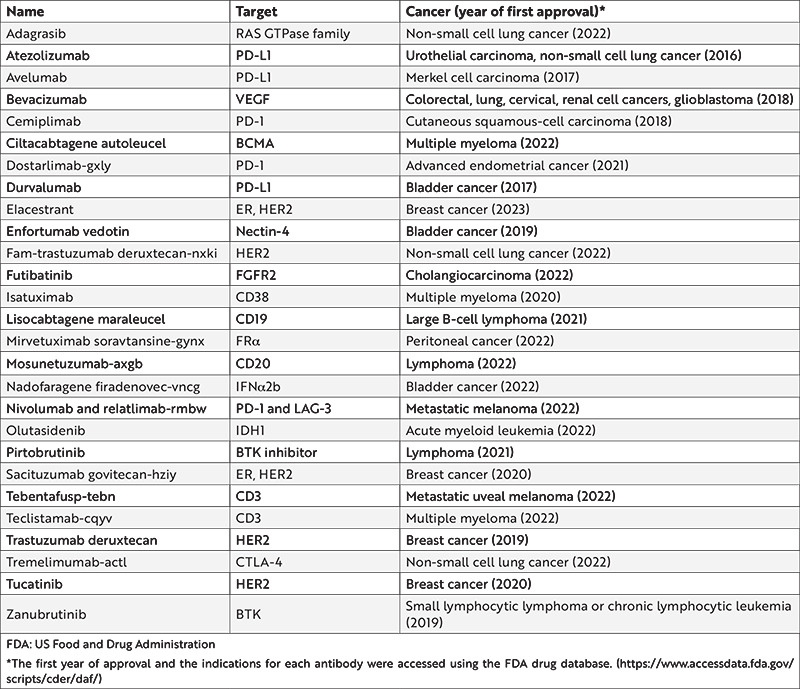
mAbs are one of the rapidly developing immunotherapy produced in the pharmaceutical industry. There are more than 22 US Food and Drug Administration (FDA)-approved immunotherapeutic drugs for oncological diseases, in which several of them are presented in Table 1. Studies have shown that the overall survival of cancer patients could be improved by mAbs15. Based on these studies, recovery has been identified through many anti-cancer mechanisms, such as antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), promotion of apoptosis, and suppression of cell proliferation16. Hybridoma technology was established in 1975 by Köhler and Milstein to develop therapeutic mAbs. Within the scope of this technology, production is performed using immunized mouse spleen cells with the ability to produce antibodies and immortal cancer B-cell myeloma cells17. Although hybridoma technology-based mAbs have a great advantage in having low aggregation and high antigen binding in vivo, murin mAbs have a short half-life as well as low biological activity and effector function onset18. The developed anti-rejection monoclonal antibody muromonab-CD3 is the first therapeutic monoclonal antibody approved for clinical use by the FDA in 198519.
Table 1. FDA-approved immunotherapeutic drugs for different cancer types.
With recent developments, the most preferred method to develop mAbs for use in clinical applications is antibody engineering. The best examples of these studies are the design of murine mAbs as humanized, fully human, chimeric, and bispecific antibodies using cloning and sequencing methods. The development of a fully human mAb has been achieved with the phage display method and transgenic animal technology20. Compared to murine mAb, the constant region found in humanized and human mAbs has reduced immunogenicity, improve effector functions, and significantly prolong serum half-life21.
mAbs are applied for cancer therapy as immune checkpoint inhibitors (ICIs), antibody drug conjugates, and bispesific T-cell linkages. It is also used for targeting pro-tumorigenic compounds and direct tumor cells. The mechanisms of action of targeted mAbs are receptor blocking, ligand blocking, as well as CDC, antibody-dependent cellular phagocytosis, and ADCC, which occur with the activation of host immune system components. In 1997, the first anti-cancer chimeric mAb, anti-CD20 rituximab, has been approved by the FDA for use for treating patients with non-Hodgkin lymphoma22.
mAbs are conjugated with strong chemotherapy methods to form antibody-drug conjugates (ADCs). Considering the large number of mAbs evaluated in ongoing clinical trials, it could be hypothesized that the market share of ADCs will gradually increase. The development of new cancer-targeted mAbs, and the design of new linkers to enable controlled drug release will further advance ADC technologies.
Immune Checkpoint Inhibitors
As cancer cells can evade the tumor-reactive T-cell response, it is necessary to increase the effectiveness of anti-tumor immune responses23. Recent advances in understanding T-cell immunobiology has been particularly influential in identifying therapeutic plans against the immune escape mechanisms of tumors. Therefore, one of the most promising therapeutic modalities for patients recently has been immune checkpoint inhibition24. T-cell activation plays an important role in the regulation of anti-tumor immunity. The immune system includes several checkpoint pathways that focus on T-cell activation. Significant molecules in checkpoint arrangements include T-cell surface molecules, T-cell immunoglobulin, and mucin domains. Expression of these molecules causes an excessive immune response. Therefore, they are important targets for cytotoxic T-cells to attack cancer cells and abolish inhibition25. ICIs are mAbs that can block immune cell receptors or immune checkpoints. Tumor cells tend to overexpress ligands that activate these inhibitory receptors. Therefore, the T-cells evades the immune response and multiplies uncontrollably. The programmed cell death protein 1 (PD-1) and T-cell surface molecule CTLA-4 are examples of the immune checkpoint systems that are in the center of antibody development for blockage. CTLA-4, when activated, transmits inhibitory signals that block the proliferation of T-cells and secretion of cytokine IL-2 for maturation. The CTLA-4 inhibitor ipilimumab26 positively affected the survival rate in a clinical trial on stage III and IV melanoma patients and became the first ICB drug to receive FDA approval in 201127.
PD-1, unlike CTLA-4, suppresses T-cell activity by promoting T-cell depletion. Nivolumab, the first PD-1 drug to target immune checkpoint blockade, was approved by the FDA in 2014. Pembrozilumab, an anti-PD-1 immune checkpoint blocker, and cemiplimab are other drugs. The latest FDA-approved immune checkpoint blocking drugs, including atezolizumab and durvalumab, target the PD-1 ligand, PD-L1, thereby providing the same inhibition of PD-1 activation with a different chemical approach28.
With recent studies, different targets such as lymphocyte activation gene-3 (LAG-3), V-domain Ig suppressor of T-cell activation, T-cell immunoglobulin and mucin domain-containing-3 (TIM-3), ITIM domain (TIGIT), and T-cell immunoglobulin are identified as new immune checkpoints29. In March 2022, Opdualag, a fixed-dose combination of programmed death receptor-1 blocking antibody nivolumab and LAG-3 blocking antibody relatlimab, received FDA approval30.
CAR-T-Cell Therapy
In the last decade, chimeric antigen receptor-T cells have become a new form of as a cell-based immunotherapy in which T-cells from cancer patients are genetically modified and are used to target tumors. With the recent developments in genetic engineering, a chimeric receptor is expressed and cancer cells with potent anti-tumor effects are targeted. CAR-T gained much attention as a novel treatment option for particularly hematological cancers recently31.
CAR-T-cell therapy uses patients’ autologous T-cells to generate a tumor antigen-specific CAR ex vivo, and then infused CAR-T-cells back into patients32. In more recent studies, nanocarriers loaded with gene editing tools and CAR genes have been promising for leukemia regression by inducing CAR-T-cells in vivo33. Currently, most clinical studies involving CAR-T-cells are early phase studies in B-cell malignancies. The most common target is CD19, mostly alone, but more recently along with other antigen targets34.
Because of CD19 expression, blood cancers are the most amenable disease group to occur in the future of CAR-T-cell therapies. High level of tumor expression of the target antigen, the ease of accessing tumor cells via blood and lymphatics and the tolerability of the non-tumor effect of B-cell aplasia on the target make CD19 a good candidate for targeted therapy35. In addition to CD19, BCMA is the other antigen that CAR-T-cell treatment is approved by FDA36.
CAR-T-cell therapy has shown effective clinical results, particularly against B-cell acute lymphoblastic leukemia. However, its effects are limited in solid tumors due to tumor histopathological features, lack of tumor-specific antigens, immunosuppressive tumor microenvironment, and potentially life-threatening tumor toxicity37. Nevertheless, scientists attempt to be made to overcome some of these barriers, particularly by engineering CAR-T agents38. As research in CAR-T therapies expands, promising results will continue to emerge alongside challenges. Thus, CAR-T will continue to positively influence, direct, and influence its potential.
Cancer Vaccine
Cancer vaccines are designed to induce an immune response against tumor antigens. Although it has been long years of research and development, only a small group of cancer vaccines have been transferred into clinical use39. The success of cancer vaccine depends on diverse factors, including the microenvironment of the tumor, the type of antigens used, several vaccine formulations and the immune makeup of the tumor40. Cancer vaccines can be used preventively or therapeutically41. The first preventive cancer vaccines were against viral infections associated with cancer development. Hepatitis B virus (HBV) is the major cause of hepatocellular carcinoma, and HBV vaccine was first licensed in 1986 in the US. Since then, it has become available in many countries around the world. For long-term immunity against chronic HBV infection, three doses of the vaccine are recommended42.
Several cancers are associated with human papillomavirus (HPV), including cervical, oropharyngeal, anal, penile, and vulvovaginal cancers43. Recently, there are three approved HPV vaccines available since 2006, and men and women over 11 years of age are recommended for vaccination to prevent HPV-related disorders. However, currently there is no preventive vaccine for non-viral cancers approved for use in humans. In part, this is due to a lack of tumor-associated antigens (TAAs) along with a risk of autoimmunity on healthy tissues because of cross-reactivity. However, safer TAAs are now tested in therapeutic vaccine trials without causing autoimmune reactions44.
Additionally, pre-clinical and clinical trials of mRNA vaccines as a therapeutic strategy against cancer are based on years of research45. mRNA vaccines have been a rapidly growing area of research as they have good tolerability, degradability, non-integration into the host genome, non-infectious potential, and the potential to induce both cell-mediated immunity and humoral46. The use of mRNA vaccines along with other immunotherapeutic treatment modalities, such as oncolytic viruses, ICIs, and adoptive cell transfer, has increased therapeutic success47.
Decades of research have proven that cancer vaccines can indeed improve systemic tumor regression and lasting remission. However, the reasons for its failure in clinical practice include loss of antigen from the tumor, loss of MHC, inclusion of soluble factors or immunosuppressive cells in the microenvironment, and lack of a strong anti-tumor immune response. Additionally, cancer vaccines have limited clinical application as they cannot induce T-cell responses with high enough avidity to efficiently eradicate tumors. It may be due to its own immune avoidance and escape mechanisms, including the inability to induce research that advances our immunological understanding and is on the verge of using it to develop rational and effective cancer vaccines is promising for the future. The success of any cancer vaccine as a target relies on overcoming the immunosuppressive tumor microenvironment and transforming “cold” tumors into “hot” tumors, thereby inducing a potent tumor-specific immune response that can kill cancer cells. In the future, with the investigation of new target antigens, adjuvants, and delivery systems, current challenges will be overcomed effectively48.
CONCLUSION
Recently, immunotherapy has become a remarkable cancer treatment method with the development of CAR-T-cells, ICIs, and cancer vaccines. In preclinical studies, it has been determined that the combination therapy of CAR-T-cells and ICI is effective on various malignancies and is more effective than the treatments used alone49. For this reason, it is thought that it will be a promising method for their use in clinical studies. Antibody-based cancer therapy has also been highly effective in the clinic. There are different mAb constructs, there are different mAb structures, such as mAbs for clinical trials with optimized pharmacokinetic properties, and mAbs conjugated with small molecule drugs. With the increase in studies on understanding the mechanisms, problems such as resistance to treatment, identification of potential targets, analysis of biological systems, and individual variations will be minimized. With the use of different immunotherapy approaches in the future, cancer patients will have a treatment method with the highest immune response rate and the lowest side effects.
As new genomic and molecular therapies are discovered, it is increasingly important to construct and accurately interpret molecular tumor profiles to deliver effective cancer therapy. However, the remarkable development of molecular techniques and the precision of the information obtained with these tools are important. To convert these molecular profiles to clinical benefit, clinical cases and results obtained from molecular analysis need to be discussed. Artificial intelligence-based clinical decision support tools might be a solution for this problem in the near future50.
Footnotes
Ethics
Peer-review: Externally peer-reviewed.
Author Contributions
Concept: B.Y., Design: B.Y., Literature Search: Z.D., K.T., T.K., Writing: Z.D., K.T., T.K., B.Y.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–13. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;149:778–89. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 4.Cancer [Internet]. [cited 2022 Nov 2]. [Internet] https://www.who.int/news-room/ fact-sheets/detail/cancer.
- 5.Cancer Today [Internet]. [cited 2022 Nov 2]. [Internet] https://gco.iarc.fr/today/home.
- 6.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 7.Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. doi: 10.3322/caac.21731. [DOI] [PubMed] [Google Scholar]
- 8.Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review) Int J Oncol. 2019;54:407–19. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krzyszczyk P, Acevedo A, Davidoff EJ, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 2018;6:79–100. doi: 10.1142/S2339547818300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24:16–24. doi: 10.1007/s12282-016-0688-1. [DOI] [PubMed] [Google Scholar]
- 11.Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365–86. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafeez U, Gan HK, Scott AM. Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr Opin Pharmacol. 2018;41:114–21. doi: 10.1016/j.coph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Esteva FJ. Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. Oncologist. 2004;9:4–9. doi: 10.1634/theoncologist.9-suppl_3-4. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Peng Z, Liu C, et al. Current Status and Future Perspective of Immunotherapy in Gastrointestinal Cancers. Innovation (Camb) 2020;1:100041. doi: 10.1016/j.xinn.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Si Y, Melkonian AL, Curry KC, et al. Monoclonal antibody-based cancer therapies. Chin J Chem Eng. 2021;30:301–7. [Google Scholar]
- 17.Tomita M, Tsumoto K. Hybridoma technologies for antibody production. Immunotherapy. 2011;3:371–80. doi: 10.2217/imt.11.4. [DOI] [PubMed] [Google Scholar]
- 18.Zaroff S, Tan G. Hybridoma technology: the preferred method for monoclonal antibody generation for in vivo applications. Biotechniques. 2019;67:90–2. doi: 10.2144/btn-2019-0054. [DOI] [PubMed] [Google Scholar]
- 19.Kaunitz JD. Development of Monoclonal Antibodies: The Dawn of mAb Rule. Dig Dis Sci. 2017;62:831–2. doi: 10.1007/s10620-017-4478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldmann H. Human Monoclonal Antibodies: The Benefits of Humanization. Methods Mol Biol. 2019;1904:1–10. doi: 10.1007/978-1-4939-8958-4_1. [DOI] [PubMed] [Google Scholar]
- 21.Chiu ML, Gilliland GL. Engineering antibody therapeutics. Curr Opin Struct Biol. 2016;38:163–73. doi: 10.1016/j.sbi.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel) 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Töpfer K, Kempe S, Müller N, et al. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Wang G, Hou X, et al. Adverse and unconventional reactions related to immune checkpoint inhibitor therapy for cancer. Int Immunopharmacol. 2022;108:108803. doi: 10.1016/j.intimp.2022.108803. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Chen J. Current status and future directions of cancer immunotherapy. J Cancer. 2018;9:1773–81. doi: 10.7150/jca.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–46. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Ledford H. Melanoma drug wins US approval. Nature. 2011;471:561. doi: 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 28.Huang Q, Zheng Y, Gao Z, Yuan L, Sun Y, Chen H. Comparative Efficacy and Safety of PD-1/PD-L1 Inhibitors for Patients with Solid Tumors: A Systematic Review and Bayesian Network Meta-analysis. J Cancer. 2021;12:1133–43. doi: 10.7150/jca.49325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohsenzadegan M, Bavandpour P, Nowroozi MR, et al. The Potential of T Cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Designing Novel Immunotherapy for Bladder Cancer. Endocr Metab Immune Disord Drug Targets. 2021;21:2131–46. doi: 10.2174/1871530321666210310142141. [DOI] [PubMed] [Google Scholar]
- 30.FDA approves Opdualag for unresectable or metastatic melanoma | FDA [Internet]. [cited 2022 Nov 2]. Available from: [Internet] https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma.
- 31.Melenhorst JJ, Chen GM, Wang M, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602:503–9. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miliotou AN, Papadopoulou LC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol. 2018;19:5–18. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- 33.Smith TT, Stephan SB, Moffett HF, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–20. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Home - ClinicalTrials.gov [Internet]. [cited 2022 Nov 2]. Available from: [Internet] https://clinicaltrials.gov/
- 35.Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27:1419–31. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20:531–50. doi: 10.1038/s41573-021-00189-2. [DOI] [PubMed] [Google Scholar]
- 38.Ma S, Li X, Wang X, et al. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int J Biol Sci. 2019;15:2548–60. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15:28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancer Vaccines - Cancer Research Institute (CRI) [Internet]. [cited 2022 Nov 2]. [Internet] https://www.cancerresearch.org/treatment-types/cancer-vaccines.
- 42.Stasi C, Silvestri C, Voller F. Hepatitis B vaccination and immunotherapies: an update. Clin Exp Vaccine Res. 2020;9:1–7. doi: 10.7774/cevr.2020.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao CI, Francoeur AA, Kapp DS, Caesar MAP, Huh WK, Chan JK. Trends in Human Papillomavirus-Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001- 2017. JAMA Netw Open. 2022;5:e222530. doi: 10.1001/jamanetworkopen.2022.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–24. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 46.Kowalzik F, Schreiner D, Jensen C, Teschner D, Gehring S, Zepp F. mRNA-Based Vaccines. Vaccines (Basel) 2021;9:390. doi: 10.3390/vaccines9040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.III HAB, Patel MR, Cho DC, et al. A phase 1, open-label, multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with pembrolizumab in subjects with unresectable solid tumors (Keynote-603) Journal of Global Oncology. 2019;5:93. [Google Scholar]
- 48.Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer Vaccines, Adjuvants, and Delivery Systems. Front Immunol. 2021;12:627932. doi: 10.3389/fimmu.2021.627932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell. 2019;36:471–82. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoeben A, Joosten EAJ, van den Beuken-van Everdingen MHJ. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers (Basel) 2021;13:242. doi: 10.3390/cancers13020242. [DOI] [PMC free article] [PubMed] [Google Scholar]


