Abstract
Objective:
Standard-dose methylprednisolone (methyl-Pd) is generally preferred as the first-line treatment in immune thrombocytopenia (ITP) unless there is an urgent indication to increase the platelet value. A significant proportion of patients (around 40%) does not benefit from this treatment. This study investigated whether pretreatment platelet level and other hemogram indices in patients with ITP patients can be used to predict early response to standard-dose methyl-Pd treatment.
Methods:
Patients who received first-line standard-dose methyl-Pd therapy with the diagnosis of primary ITP were included. Patients were categorized as complete responder (CR), responder (R), and non-responder (NR) according to the response status obtained within the first 14 days of treatment. The hemogram indices of the CR, R, and NR groups measured at the start of the treatment were compared retrospectively.
Results:
One hundred forty four patients with ITP were included in the study. The number of patients with NR, R, and CR were 47 (33%), 40 (28%), and 57 (39%), respectively. The mean platelet level of the NR group was lower than responders (R and CR groups) (p=0.002 and p=0.049, respectively). The mean platelet volume (MPV) levels of the NR group were statistically lower than that of the CR group (p=0.018). If MPV ≥10 fL and platelet >12,000/mm³, the probability of an early response with methyl-Pd is higher [sensitivity =98.1% (95% confidence interval (CI) =89.7-99.9%), specificity =45% (95% CI =23.1-68.5%), positive predictive value =82.3% (95% CI =75.7-87.4%), negative predictive value =90% (95% CI =54.9-98.5%)].
Conclusions:
Patients with ITP with low platelet and MPV levels were less responsive to standard-dose methyl-Pd treatment. It may be more appropriate to apply more effective treatments to these patients other than standard-dose methyl-Pd alone.
Keywords: Immune thrombocytopenia, methylprednisolone, treatment, complete blood count indices
Abstract
Amaç:
İmmün trombositopenide (ITP), trombosit değerini artırmak için acil bir endikasyon olmadığı sürece, genellikle birinci basamak tedavi olarak standart doz metilprednizolon (metil-Pd) tercih edilir. Hastaların azımsanmayacak bir bölümü (%40 kadarı) bu tedaviden fayda görememektedir. Bu çalışmada, ITP hastalarında tedaviye başlandığı esnada ölçülen platelet (PLT) sayısı ve diğer hemogram indekslerinin, standart doz metil-Pd tedavisine erken yanıtı öngörmede kullanılıp kullanılamayacağını araştırmak amaçlandı.
Yöntemler:
Primer ITP tanısı ile birinci basamak standart doz metil-Pd tedavisi alan hastalar dahil edildi. Hastalar tedavinin ilk 14 günü içinde elde edilen yanıt durumuna göre tam yanıt veren (CR), yanıt veren (R) ve yanıt vermeyen (NR) olarak kategorize edildi. CR, R ve NR gruplarının tedaviye başlandığı esnada ölçülen hemogram indeksleri retrospektif olarak karşılaştırıldı.
Bulgular:
Yüz kırk dört ITP hastası çalışmaya dahil edildi. NR, R ve CR gruplarının hasta sayısı sırasıyla 47 (%33), 40 (%28) ve 57 (%39) idi. NR grubunun ortalama PLT sayısı yanıt verenlere göre (R ve CR grupları) daha düşüktü (sırasıyla p=0,002 ve p=0,049). NR grubunun ortalama ortalama trombosit hacmi (MPV) değeri, CR grubuna göre istatistiksel olarak düşüktü (p=0,018). Eğer tedavi başlangıcında MPV ≥10 fL ve PLT >12.000/mm³ ise, metil-Pd ile erken yanıt olasılığının yüksek olduğu gözlendi [sensitivite =%98,1 (%95 güven aralığı (GA) =%89,7-99,9), spesifite =%45 (%95 GA =%23,1-68,5), pozitif belirleyicilik değeri =%82,3 (%95 GA =%75,7-87,4), negatif belirleyicilik değeri =%90 (%95 GA =%54,9-98,5)].
Sonuçlar:
Düşük PLT ve MPV seviyelerine sahip ITP hastaları, standart doz metil-Pd tedavisine daha az yanıt vermiştir. Bu hastalara tek başına standart doz metil-Pd tedavisi yerine daha etkili tedavilerin uygulanması daha uygun olabilir.
Keywords: İmmün trombositopeni, metilprednizolon, tedavi, tam kan sayımı indeksleri
INTRODUCTION
Primary immune thrombocytopenia (ITP) is an immunological disease in which the platelet (PLT) count in the peripheral blood is below 100,000/mm³ and the risk of bleeding increases1. It is an acquired disease characterized by isolated thrombocytopenia.
Patients with a PLT level of less than 30,000/mm³ at the time of diagnosis or a thrombocyte level of 30,000-50,000/mm³ and the bleeding should be treated immediately. First-line treatment of the disease is usually corticosteroid (CS) and/or intravenous immunoglobulin (IVIG)2. There are treatment options such as prednisolone, methylprednisolone (methyl-Pd), and dexamethasone in different doses among CS treatments. In clinical practice, the standard-dose (0.8 mg/kg/day) methyl-Pd (equivalent to 1 mg/kg/day prednisolone) is generally preferred as first-line treatment if there is no life-threatening bleeding or the patient does not require emergency surgical intervention. Although the PLT level usually starts to increase within 3-5 days when first-line treatment is started in primary ITP, some patients do not obtain a sufficient response. In almost 40% of patients, an early response cannot be achieved with standard-dose CS therapy3. Patients who do not respond to treatment in the early period are at a high risk of bleeding and are exposed to unnecessary toxicity of CS treatment. Therefore, it is critical to predict patients who will not respond to standard-dose methyl-Pd treatment before starting treatment. There are very few studies in the literature investigating the factors that predict early response to first-line treatment.
Mean platelet volume (MPV) is a component of the hemogram test, which can be performed in most medical centers, and is an indicator of PLT function4. MPV value has been examined as a guiding marker in the differential diagnosis of thrombocytopenia. A value of MPV ≥8.8 fL has acceptable sensitivity and specificity for the diagnosis of over-destructive thrombocytopenia5. In previous studies, it was shown that MPV was found to be low in patients who developed thrombocytopenia due to therapy-related bone marrow suppression6. Very few studies show that MPV predicts response to treatment in ITP, and only one study on this subject has been published in the literature7.
If thrombocytopenia is accompanied by life-threatening bleeding, high-dose methyl-Pd ± IVIG treatments are administered. However, in the absence of bleeding findings, the level of thrombocytopenia alone does not guide the drug selection and dose to be used in the treatment. In routine practice, if the patient’s PLT count is below 30,000/mm³ and there is no life-threatening bleeding, standard-dose CS treatment is used. In other words, whether the PLT level of the patient is 1000/mm³ or 29,000/mm³, the treatment is started at the same CS dose. We planned this study to answer the question “should these patients receive the same treatment?” occupied in our minds. In our study, we investigated whether PLT levels and other hemogram indices measured before starting standard-dose methyl-Pd treatment in primary patients with ITP can be used to predict early response to treatment. The pre-treatment hemogram indices of patients with and without response to first-line methyl-Pd treatment will be compared retrospectively.
MATERIALS and METHODS
Study Population and Design
The information of patients who started methyl-Pd (0.8 mg/kg/day) treatment with the diagnosis of primary ITP in the University of Health Sciences Turkey, Istanbul Sultan 2. Abdulhamid Han Training and Research Hospital and Gulhane Faculty of Medicine Hematology Clinics between 2014 and 2022 were retrospectively analyzed from the hospital registry system and patient follow-up files.
The diagnosis of primary ITP was made after other immune and nonimmune secondary causes of isolated thrombocytopenia were excluded8. The data including age, gender, and treatment response were recorded for each patient. In addition, complete blood count (CBC) indices [PLT, white blood cell (WBC), neutrophil, lymphocytes, monocytes, eosinophil, hemoglobin, mean corpuscular volume (MCV), MPV, and red cell distribution width (RDW)], which were last measured before starting treatment were recorded for each patient.
Those who had a disease or drug use that would affect CBC indices and those who were treated for ITP other than 0.8 mg/kg/day methyl-Pd treatment (dexamethasone, IVIG, high-dose methyl-Pd) were excluded from the study.
In primary patients with ITP, 0.8 mg/kg/day methyl-Pd treatment was started for those with a PLT level below 30,000/mm³ and/or bleeding findings. Patients were categorized as complete responder (CR), responder (R), and non-responder (NR) according to the response status obtained within the first 14 days of methyl-Pd treatment. Those whose PLT count increased over 100,000/mm³ with treatment were accepted as CR, those with levels between 30,000/mm³ and 100,000/mm³ and increased at least 2 times compared to the baseline were accepted as R, and those below 30,000/mm³ were accepted as NR1. All three groups were compared in terms of hemogram indices measured at the start of treatment. The NR group was categorized as unresponsive to treatment, whereas the R and CR groups were categorized as responders. They were compared among themselves in terms of hemogram indices in the unresponsive and responding groups.
CBC was analyzed with automatic hematology analyzers (Beckman-Coulter DXH, Abbott Diagnostics Cell-Dyn Sapphire and Mindray BC-6800).
The study was approved by the local Institutional Review Board and Ethics Committee [Local Ethics Committee of Health Sciences University Hamidiye Scientific Research (decision no: 23/7, date: 13.01.2023)] and was conducted in accordance with the Helsinki Declaration of 2013. Since the study was retrospective, informed consent was not obtained from the patients.
Statistical Analysis
The mean (standard deviation), number, and percentage were used as descriptive statistics. Student’s t-test was used for the pairwise comparison of groups, and the One-way ANOVA test was used for the comparison of three groups. Tukey analysis was performed for the results that were significant in the one-way ANOVA test, and pairwise comparisons were made. Receiver operating characteristic (ROC) analysis was performed for the predictive value of the early response to treatment of the PLT level, and the area under the curve (AUC) was calculated. Specificity, sensitivity, positive predictive value (PPV), and negative predictive values (NPV) were calculated. A p<0.05 was considered statistically significant. The SPSS version 26 program was used for statistical analysis.
RESULTS
The data of 242 patients who started first-line treatment with the diagnosis of primary ITP were obtained. However, 98 patients were excluded from the study (Figure 1). Thus, 144 primary patients with ITP who were started on first-line methyl-Pd treatment (0.8 mg/kg/day) were included in the study.
Figure 1.
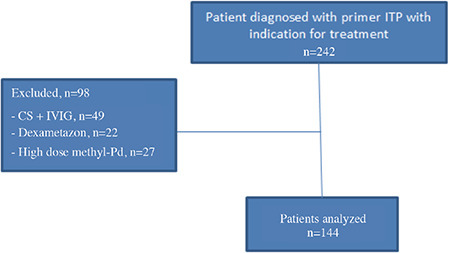
Flowchart of patients.
CS: Corticosteroid, IVIG: Intravenous immunoglobulin, ITP: Immune thrombocytopenia, methyl-Pd: Methylprednisolone
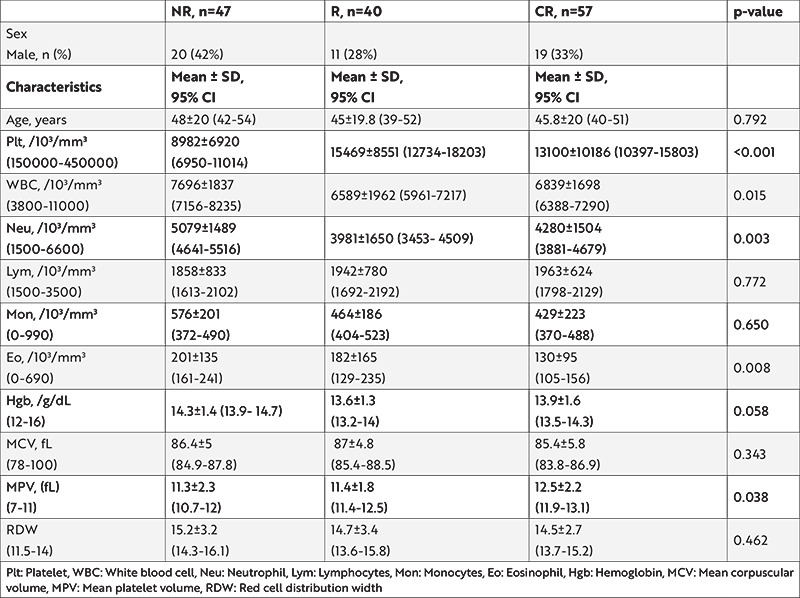
The mean age of the entire study group (n=144) was 46.3±20.0 years. Ninety-four (65%) patients were female and 50 (35%) were male. The number of patients with NR, R, and CR were 47 (33%), 40 (28%), and 57 (39%), respectively. The comparison of the characteristics of the groups is summarized in Table 1. There was no statistically significant difference between age, lymphocytes, monocytes, hemoglobin, MCV, and RDW between all three groups. A statistically significant difference was found between all three groups in terms of PLTs, WBC, neutrophils, eosinophils, and MPV (p<0.05).
Table 1. Comparison of groups in terms of hemogram indices.
ROC curve analysis was performed for PLT, WBC, neutrophils, MPV, and eosinophil (Figure 2). The AUC was 0.650 [p=0.004, 95% confidence interval (CI) =0.557-0.741) and 0.659 (p=0.002, 95% CI =0.559-0.759], respectively for PLT and MPV. AUC was 0.331, 0.313, and 0.395, respectively for WBC, neutrophil, and eosinophil.
Figure 2.
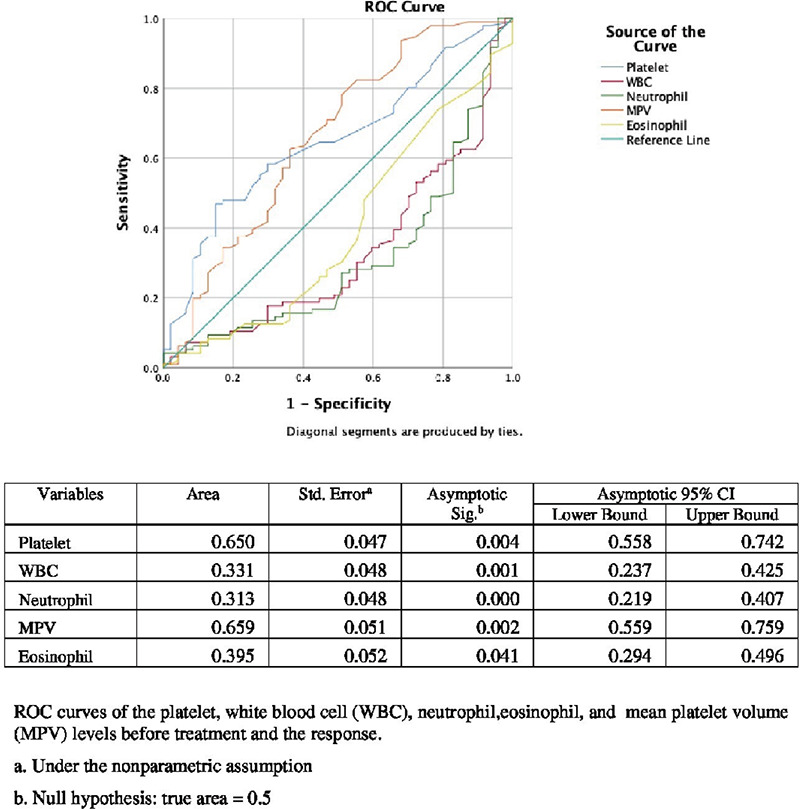
ROC curve analysis for platelet, WBC, neutrophil, MPV, and eosinophil.
ROC: Receiver operating characteristic, WBC: White blood cell, MPV: Mean platelet volume, CI: Confidence interval, Std. error: Standard error
Pre-treatment PLT and MPV levels were compared in pairs between the NR, R, and CR groups. PLT levels of non-responders (NR group) were statistically lower than responders (R and CR groups) (p=0.002 and p=0.049, respectively), however, no statistically significant difference was observed between R and CR in the responder group in terms of PLT level (p=0.393). MPV levels of the NR group were statistically lower than the CR groups (p=0.018), however, no statistically significant difference was observed between the NR and R groups (p=0.409).
Diagnostic accuracy tests were performed to predict early response status with methyl-Pd treatment. Sensitivity and specificity for responders were 51.55% (95% CI =43.2-63.8%) and 74.47% (95% CI =57.4-84.4%), respectively, for PLTs ≥12,000/mm³ [NPV =43% (95% CI =36.4-49.9%), PPV =80% (95% CI =70.8-86.8%)]. Sensitivity and specificity for responders were 97.9% (95% CI =92.6-99.7%) and 22.9% (95% CI =12-37.3%), respectively, for MPV ≥10 fL [NPV =84.6% (95% CI =55.9-96%), PPV =71.5% (68.2-74.6)]. If MPV ≥10 fL and PLT >12,000/mm³, the probability of an early response with standard-dose methyl-Pd is even higher [sensitivity =98.1% (95% CI =89.7-99.9%)], specificity =45% (95% CI =23.1-68.5%), PPV =82.3% (95% CI =75.7-87.4%), NPV =90% (95% CI =54.9-98.5%).
DISCUSSION
We achieved an early response rate of 67% in primary patients with ITP with methyl-Pd treatment at a dose of 0.8 mg/kg/day (28% R + 39% CR). Previous studies show that the early response rate is between 50% and 90%9. Mithoowani et al.10 reported that they achieved a 59% response in the first 14 days with prednisolone equivalent to the standard-dose methyl-Pd. Kim et al.11 reported an early response rate of 76.9% with oral methyl-Pd therapy in 39 adult primary patients with ITP. However, in their study, they applied 10 mg/kg/day intravenous methyl-Pd for 3 days as initial treatment and followed by oral 1 mg/kg/day prednisone treatment11. As it seems, there is a group of patients with ITP who will not benefit from standard-dose methyl-Pd treatment in a substantial proportion. We think that these patients should not be exposed to the unnecessary toxicity of methyl-Pd by recognizing them before treatment and taking alternative treatments other than the standard-dose methyl-Pd. The only problem is not only unnecessary methyl-Pd exposure, but also patients lose time while waiting for the response to treatment, and in this period, they are faced with the risk of bleeding in a thrombocytopenic condition.
In this study, we showed that pretreatment PLT and MPV levels can predict early response to standard-dose methyl-Pd treatment. If the pre-treatment PLT level is above 12,000/mm³, an early response to the treatment will be obtained with a sensitivity of 51.55% and a specificity of 74.47%. It was observed that if the pre-treatment MPV level was ≥10 fL, a response would be obtained with a sensitivity of 97.9% and a specificity of 22.9%. We combined PLT and MPV levels to increase the predictive power of treatment response. We showed that if the patient’s MPV ≥10 fL and PLT ≥12,000/mm³, the probability of an early response with standard-dose methyl-Pd is much higher (sensitivity =98.1%, specificity =45%).
We encountered some publications in the literature on predicting the response to CS treatments in ITP. Tripathi et al.12 reported that the presence of abnormal megakaryocyte morphology (hypolobulated forms and micromegakaryocytes) was associated with unresponsiveness to CS (prednisolone 2 mg/kg/day) treatment. Patients who did not respond to CS treatment could obtain a response when they applied eltrombopag treatment. Since we did not evaluate the megakaryocyte morphology of the patients in our study, we cannot comment on this issue. In our country, Akkuş et al.7 conducted a study on 70 patients with ITP patients and 70 healthy control groups. They found that the MPV level at diagnosis among patients with ITP was significantly higher in first-line treatment responders than in non-responders (11.09 and 9.38 fL, p=0.005). While the MPV levels of those who responded to treatment were significantly higher than the healthy control group, the levels of those who did not respond to the treatment were found to be lower than that of the healthy control group. Based on the results of the study, they suggested that MPV could be used as an indicator of early response to first-line treatment in newly diagnosed adult patients with ITP. Based on the data of this study, it would not be appropriate to comment on methyl-Pd treatment because 51% of the patients received IVIG together CS in the first-line treatment. Additionally, no information was given about the details of CS treatment (methyl-Pd or dexamethasone)7. In this study, we also found that the MPV level of the CR group to be significantly higher than that of the NR group (p=0.018). Eren et al.13 investigated the relationship between neutrophil-lymphocyte ratio (NLR) and response and loss of response to CS treatment in 47 patients with ITP. Because of their study, they could not find a relationship between NLR and response to CS treatment in patients with ITP patients13. Oka et al.14 analyzed the phenotypes of cells in the lymphocyte region of the bone marrow in patients with ITP patients to determine whether the cellular phenotype predicts response to first-line therapy. In 52 newly diagnosed patients with ITP, they observed an abnormal CD4:CD8 ratio (CD4/CD8 ratio <0.4 and 2.3<CD4/CD8 ratio) in 22 patients in the first-line treatment response group. On the other hand, they found a normal CD4:CD8 ratio in all NRs and control groups. Based on the study results, they suggested that the CD4:CD8 ratio, B cells, and NK cells may contribute to the prediction of the therapeutic outcome of patients with ITP14. However, in this study, more than half of the patients received IVIG together with CS as first-line therapy.
CSs are not the only treatment agents of choice for treating primary ITP. IVIG, thrombopoietin receptor agonists (eltrombopag, romiplostim), rituximab, and other immunosuppressive drugs are also used for treating primary ITP15. Al-Samkari and Kuter16 reported that patients with low pretreatment thrombopoietin levels in their serum responded better to thrombopoietin receptor agonists.
The main limitations of our study are its retrospective nature. We would like to compare the response status of patients with a pre-treatment PLT count below 12,000/mm³ and MPV below 10 fL, who received methyl-Pd treatment at a higher dose than the standard dose, with those who received standard-dose treatment. However, we could not do it because we did not have the appropriate number of patients for such a study.
CONCLUSION
In a conclusion, we showed that patients with ITP with low PLT and MPV levels were less responsive to standard-dose CS treatment. Based on the present study results, we think that it may be more appropriate to apply more effective treatments (such as high-dose CS ± IVIG, standard-dose CS + rituximab) to these patients other than standard-dose CS alone. Results from comparative studies involving larger numbers of patients are needed to make very ambitious recommendations. We believe that our study will shed light on future studies.
Footnotes
Ethics
Ethics Committee Approval: The study was approved by the Local Ethics Committee of Health Sciences University Hamidiye Scientific Research (decision no: 23/7, date: 13.01.2023) and was conducted in accordance with the Helsinki Declaration of 2013.
Informed Consent: Since the study was retrospective, informed consent was not obtained from the patients.
Peer-review: Externally and internally peer-reviewed.
Author Contributions
Surgical and Medical Practices: E.K., M.Y., S.S., M.A., M.K.K., Concept: E.K., M.Y., S.S., E.C., M.A., M.K.K., Design: E.K., M.Y., S.S., E.C., M.A., M.K.K., Data Collection and/or Processing: E.K., M.Y., S.S., M.A., M.K.K., Analysis and/or Interpretation: E.K., E.C., Literature Search: E.K., Writing: E.K., E.C., M.K.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–66. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mithoowani S, Arnold DM. First-Line Therapy for Immune Thrombocytopenia. Hamostaseologie. 2019;39:259–65. doi: 10.1055/s-0039-1684031. [DOI] [PubMed] [Google Scholar]
- 4.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61. [PubMed] [Google Scholar]
- 5.Norrasethada L, Khumpoo W, Rattarittamrong E, Rattanathammethee T, Chai-Adisaksopha C, Tantiworawit A. The use of mean platelet volume for distinguishing the causes of thrombocytopenia in adult patients. Hematol Rep. 2019;11:7732. doi: 10.4081/hr.2019.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles KM, Cooke LJ, Richards EM, Baglin TP. Platelet size has diagnostic predictive value in patients with thrombocytopenia. Clin Lab Haematol. 2005;27:370–3. doi: 10.1111/j.1365-2257.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 7.Akkuş E, Fidan Ç, Demirci G, Kuştaş AA, Yüksel M. Mean platelet volume and response to the first line therapy in newly diagnosed adult immune thrombocytopenia patients: a retrospective study. Turk J Med Sci. 2020;50:798–803. doi: 10.3906/sag-1912-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo E, Deane S. Diagnosis and classification of immune-mediated thrombocytopenia. Autoimmun Rev. 2014;13:577–83. doi: 10.1016/j.autrev.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP) Blood. 2005;106:2244–51. doi: 10.1182/blood-2004-12-4598. [DOI] [PubMed] [Google Scholar]
- 10.Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3:e489–96. doi: 10.1016/S2352-3026(16)30109-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, Choi YS, Moon JY, et al. Methylprednisolone versus intravenous immune globulin as an initial therapy in adult primary immune thrombocytopenia. Korean J Intern Med. 2019;34:383–9. doi: 10.3904/kjim.2015.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi AK, Mishra S, Kumar A, Yadav D, Shukla A, Yadav Y. Megakaryocyte morphology and its impact in predicting response to steroid in immune thrombocytopenia. Platelets. 2014;25:526–31. doi: 10.3109/09537104.2013.845875. [DOI] [PubMed] [Google Scholar]
- 13.Eren R, Ünaldı M, Karışmaz A, et al. Neutrophil Lymphocyte Ratio in Estimating Response to Corticosteroid Treatment in Immune Thrombocytopenia Patients. Istanbul Medical Journal. 2019;20:54–7. [Google Scholar]
- 14.Oka S, Ono K, Nohgawa M. Prediction of response to first-line therapy with ITP by flow cytometric analysis of bone marrow lymphocyte phenotypes. Int J Hematol. 2020;111:771–8. doi: 10.1007/s12185-020-02847-4. [DOI] [PubMed] [Google Scholar]
- 15.Samson M, Fraser W, Lebowitz D. Treatments for Primary Immune Thrombocytopenia: A Review. Cureus. 2019;11:e5849. doi: 10.7759/cureus.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol. 2018;93:1501–8. doi: 10.1002/ajh.25275. [DOI] [PubMed] [Google Scholar]


