Abstract
BACKGROUND
In a phase 2 study, rucaparib, an inhibitor of poly(ADP-ribose) polymerase (PARP), showed a high level of activity in patients who had metastatic, castration-resistant prostate cancer associated with a deleterious BRCA alteration. Data are needed to confirm and expand on the findings of the phase 2 study.
METHODS
In this randomized, controlled, phase 3 trial, we enrolled patients who had metastatic, castration-resistant prostate cancer with a BRCA1, BRCA2, or ATM alteration and who had disease progression after treatment with a second-generation androgen-receptor pathway inhibitor (ARPI). We randomly assigned the patients in a 2:1 ratio to receive oral rucaparib (600 mg twice daily) or a physician’s choice control (docetaxel or a second-generation ARPI [abiraterone acetate or enzalutamide]). The primary outcome was the median duration of imaging-based progression-free survival according to independent review.
RESULTS
Of the 4855 patients who had undergone prescreening or screening, 270 were assigned to receive rucaparib and 135 to receive a control medication (intention-to-treat population); in the two groups, 201 patients and 101 patients, respectively, had a BRCA alteration. At 62 months, the duration of imaging-based progression-free survival was significantly longer in the rucaparib group than in the control group, both in the BRCA subgroup (median, 11.2 months and 6.4 months, respectively; hazard ratio, 0.50; 95% confidence interval [CI], 0.36 to 0.69) and in the intention-to-treat group (median, 10.2 months and 6.4 months, respectively; hazard ratio, 0.61; 95% CI, 0.47 to 0.80; P<0.001 for both comparisons). In an exploratory analysis in the ATM subgroup, the median duration of imaging-based progression-free survival was 8.1 months in the rucaparib group and 6.8 months in the control group (hazard ratio, 0.95; 95% CI, 0.59 to 1.52). The most frequent adverse events with rucaparib were fatigue and nausea.
CONCLUSIONS
The duration of imaging-based progression-free survival was significantly longer with rucaparib than with a control medication among patients who had metastatic, castration-resistant prostate cancer with a BRCA alteration. (Funded by Clovis Oncology; TRITON3 ClinicalTrials.gov number, NCT02975934.)
Despite recent approvals for treatment of castration-sensitive prostate cancer, the metastatic form of this disease remains lethal.1–3 Poly(ADP-ribose) polymerase (PARP) inhibition leads to the formation of double-stranded DNA breaks that cannot be repaired accurately and are deadly to tumor cells with DNA-repair defects,4–6 a finding that has been validated in several phase 3 clinical trials showing the benefit of PARP inhibitors in adult patients with ovarian, breast, pancreatic, or prostate cancer with deleterious or suspected deleterious BRCA1 or BRCA2 alterations.7–10 PARP inhibitors have shown clinical efficacy in patients with metastatic, castration-resistant prostate cancer associated with alterations in genes encoding DNA damage response, with the greatest efficacy observed in those with BRCA alterations.9,11–13 In the phase 2 TRITON2 study,11 PARP inhibitor rucaparib14 showed a high level of activity in metastatic, castration-resistant prostate cancer associated with a deleterious BRCA alteration in patients who had received previous treatment with a second-generation androgen-receptor pathway inhibitor (ARPI) and taxane-based chemotherapy.
We conducted the open-label, controlled, randomized, phase 3 TRITON3 trial of rucaparib involving men with metastatic, castration-resistant prostate cancer at an earlier stage of treatment to confirm and expand on data from the TRITON2 study. The TRITON3 population consisted of patients with alterations in BRCA or ATM (the gene encoding ATM serine–threonine kinase) who had not received previous chemotherapy for metastatic, castration-resistant disease. To reflect both clinical practice and existing guidelines,1,15,16 we randomly assigned patients to receive either rucaparib or the physician’s choice of docetaxel or a second-generation ARPI (abiraterone acetate or enzalutamide). Here, we report the primary results of the trial.
METHODS
PATIENTS
Adult men with histologically or cytologically confirmed metastatic, castration-resistant prostate cancer and a BRCA or ATM alteration were eligible for enrollment. All the patients had a history of disease progression after treatment with one previous second-generation ARPI (abiraterone acetate, enzalutamide, apalutamide, or an investigational agent) but no chemotherapy for castration-resistant disease. Previous taxane-based chemotherapy for castration-sensitive disease was permitted. After the adoption of an early protocol amendment to reflect evolving clinical practice, patients could have received a qualifying second-generation ARPI for either hormone-sensitive or castration-resistant disease. Full eligibility criteria are provided in the trial protocol, available with the full text of this article at NEJM.org.
The intention-to-treat population consisted of all the patients who had undergone randomization, with a prespecified subgroup that included patients with a BRCA alteration. In this ongoing trial, the visit-cutoff date for the primary results was August 25, 2022.
RANDOMIZATION AND INTERVENTIONS
Patients were randomly assigned in a 2:1 ratio to receive 600 mg of oral rucaparib twice daily or the physician’s choice of docetaxel, abiraterone acetate, or enzalutamide. Doses of all medications are provided in the Supplementary Appendix, available at NEJM.org. The physician’s choice of medication (control group) was prespecified before randomization. Abiraterone acetate or enzalutamide could not be selected if the patient had received either drug before trial initiation.
Stratification factors at randomization included an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a scale from 0 to 5, with a higher score reflecting greater disability), the presence of hepatic metastases (yes or no), and genetic alteration (BRCA1, BRCA2, or ATM). Imaging end points were measured according to the modified Response Evaluation Criteria in Solid Tumor (RECIST), version 1.1, and the criteria of the Prostate Cancer Clinical Trials Working Group 3.17,18 Patients who were assigned to the control group could cross over to receive rucaparib after documented disease progression as confirmed by independent review.
OUTCOMES
The primary efficacy outcome was the median duration of imaging-based progression-free survival according to the prespecified criteria on independent review (see the Methods section in the Supplementary Appendix). Key secondary outcomes were overall survival and objective response according to independent imaging-based review. Additional secondary outcomes included the duration of response according to independent imaging-based and investigator review, the time to progression according to prostate-specific antigen (PSA) testing, a confirmed PSA decrease of at least 50% (PSA50) or 90% (PSA90), the frequency of clinical benefit, and patient-reported outcomes according to several surveys, including the Functional Assessment of Cancer Therapy–Prostate, Brief Pain Inventory–Short Form, and EuroQol Group 5-Dimension 5-Level (EQ-5D-5L) questionnaires. Detailed definitions of primary and secondary outcomes are provided in the Supplementary Appendix.
To assess safety, adverse events were classified according to the terms used in the Medical Dictionary for Drug Regulatory Activities, version 23.0; the severity of toxic events was graded according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 4.03 or higher. All adverse events that occurred during randomized treatment are reported. Additional safety assessments are described in the Supplementary Appendix.
TRIAL OVERSIGHT
The trial was approved by local or national review boards and performed in accordance with Good Clinical Practice guidelines of the International Council for Harmonisation and the Declaration of Helsinki. The trial and subsequent analysis were designed by the sponsor (Clovis Oncology) in consultation with the coordinating investigators and members of the trial steering committee. All the authors had full access to the data, with no restrictive agreements concerning confidentiality between the sponsor and the authors. Representatives of Clovis Oncology provided input regarding the interpretation of the data. The manuscript was written with medical writing assistance funded by Clovis Oncology, with early critical review and input by the authors. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
STATISTICAL ANALYSIS
We determined that a sample size of approximately 400 patients (including approximately 100 patients in the ATM subgroup) in the intention-to-treat population would provide the trial with approximately 90% power to detect a hazard ratio of 0.67 in the rucaparib group as compared with the control group at a two-sided 0.05 significance level. In this calculation, we assumed that the median duration of imaging-based progression-free survival would be 6 months in the control group; in the rucaparib group, we assumed a median duration of 9 months in the intention-to-treat population and 10 months in the BRCA subgroup. We also determined that a sample size of approximately 300 patients in the BRCA subgroup would result in approximately 200 events of disease progression or death, which would provide the trial with approximately 90% power to detect a hazard ratio of 0.60 at a two-sided 0.05 significance level.
Efficacy data were analyzed on an intention-to-treat basis. We used an ordered step-down, multiple-comparisons procedure to test the primary efficacy outcome, first in the BRCA subgroup and then in the intention-to-treat population if statistical significance had been determined (Fig. S1 in the Supplementary Appendix); key secondary outcomes were then tested. After a protocol amendment, interim overall survival replaced overall response as the first secondary outcome in the step-down procedure to reflect the importance of this outcome. The final analysis of overall survival was planned when the data were approximately 70% mature. In the case of nonsignificant results regarding interim analyses of overall survival, significance could not be declared for subsequent outcomes until completion of the final overall survival analysis.
We performed Kaplan–Meier analysis to summarize time-to-event variables. For the primary analysis, we used the stratified hazard ratio from a Cox proportional-hazards model to estimate the hazard ratio between the two treatment groups. A log-rank test was used for treatment-group comparisons. We compared the objective response between treatment groups using the Cochran–Mantel–Haenszel method. Patient-reported outcomes were compared between treatment groups by means of an analysis of covariance, with the treatment as a categorical factor and baseline measurement for the variable as a continuous covariate.
The statistical analysis plan did not include a provision for correcting for multiplicity in testing for additional secondary or exploratory outcomes; therefore, all secondary and exploratory results are reported as point estimates and 95% confidence intervals. The widths of the 95% confidence intervals were not adjusted for multiplicity, so the intervals should not be used in place of hypothesis testing. Additional details regarding the statistical analysis are provided in the protocol and the Supplementary Appendix.
RESULTS
PATIENT CHARACTERISTICS
From February 8, 2017, to February 2, 2022, a total of 4855 patients underwent prescreening or screening at 143 sites in 12 countries. Of these patients, 405 had a deleterious BRCA or ATM alteration and underwent randomization (270 to the rucaparib group and 135 to the control group) (Fig. S2). Baseline genomic, demographic, and disease characteristics were well balanced in the two groups (Table 1). Although the age and genomic features of the patients were generally representative of the population at risk for prostate cancer, men of African descent were under-represented relative to the general population (Table S1). Among the patients who had undergone screening, 302 patients had a BRCA alteration and 103 patients had an ATM alteration. Additional details regarding genomic data are provided in the Supplementary Appendix.
Table 1.
Characteristics of the Patients at Baseline (Intention-to-Treat Population).*
| Characteristic | Rucaparib (N = 270) | Control (N = 135) |
|---|---|---|
| Demographic | ||
| Age | ||
| Median (range) — yr | 70 (45–90) | 71 (47–92) |
| ≥65 yr — no. (%) | 186 (69) | 103 (76) |
| Race — no. (%)† | ||
| White | 199 (74) | 103 (76) |
| Black | 10 (4) | 4 (3) |
| Asian | 4 (1) | 1 (1) |
| Other | 4 (1) | 0 |
| Missing data | 53 (20) | 27 (20) |
| Medical history | ||
| ECOG performance-status score — no. (%)‡ | ||
| 0 | 132 (49) | 68 (50) |
| 1 | 138 (51) | 67 (50) |
| Gene alteration — no. (%)‡ | ||
| BRCA1 | 29 (11) | 15 (11) |
| BRCA2 | 172 (64) | 86 (64) |
| ATM | 69 (26) | 34 (25) |
| Genomic test — no. (%) | ||
| Tissue | 79 (29) | 39 (29) |
| Plasma | 170 (63) | 79 (59) |
| Other | 21 (8) | 17 (13) |
| Distant metastasis — no. (%) | ||
| M0 | 110 (41) | 53 (39) |
| M1 | 112 (41) | 65 (48) |
| MX | 36 (13) | 16 (12) |
| Missing data | 12 (4) | 1 (1) |
| Median PSA (range) — ng/ml | 26.9 (0.1–1247) | 28.8 (0–1039) |
| Metastases on independent imaging-based review — no. (%) | ||
| Bone | 235 (87) | 114 (84) |
| Nodal | 118 (44) | 60 (44) |
| Visceral | 74 (27) | 46 (34) |
| Hepatic metastases — no. (%)‡ | 23 (9) | 11 (8) |
| Gleason score of ≥8 at diagnosis — no. (%)§ | 173 (64) | 96 (71) |
| Measurable disease on independent imaging-based review — no. (%) | 106 (39) | 55 (41) |
| Previous therapies | ||
| Any anticancer therapy — no. (%) | ||
| Second-generation ARPI | ||
| Abiraterone acetate | 150 (56) | 80 (59) |
| Apalutamide | 8 (3) | 1 (1) |
| Enzalutamide | 119 (44) | 61 (45) |
| Docetaxel for hormone-sensitive prostate cancer | 63 (23) | 28 (21) |
| Therapy for castration-resistant prostate cancer — no. (%) | ||
| 0 | 48 (18) | 26 (19) |
| ≥1 | 222 (82) | 109 (81) |
| Assigned control medication — no. (%) | ||
| Docetaxel | NA | 75 (56) |
| Abiraterone acetate | NA | 28 (21) |
| Enzalutamide | NA | 32 (24) |
Control medications were chosen by the treating physician and included docetaxel or a second-generation androgen-receptor pathway inhibitor (ARPI; abiraterone acetate or enzalutamide). ECOG denotes Eastern Cooperative Oncology Group, NA not applicable, and PSA prostate-specific antigen.
Race was reported by the patient. Some data regarding race were missing owing to region-specific privacy laws.
This category was a stratification factor at randomization.
The scale for the Gleason score ranges from 2 to 10, with higher scores indicating a worse prognosis.
In the control group, 75 of 135 patients (56%) received docetaxel. No previous treatment for castration-resistant prostate cancer had been administered to 74 of 405 patients (18%). Included among the patients who had previously received docetaxel for hormone-sensitive disease were 16 of 60 patients (27%) who were assigned to receive an ARPI and 12 of 75 patients (16%) who were assigned to receive docetaxel.
PRIMARY OUTCOME
At 62 months, the median duration of imaging-based progression-free survival was significantly longer in the rucaparib group than in the control group both in the BRCA analysis and in the intention-to-treat analysis. In the BRCA subgroup, 182 of 302 patients (60%) had disease progression or had died. The median duration of imaging-based progression-free survival was 11.2 months (95% confidence interval [CI], 9.2 to 13.8) in the rucaparib group and 6.4 months (95% CI, 5.4 to 8.3) in the control group (hazard ratio, 0.50; 95% CI, 0.36 to 0.69; P<0.001 by log-rank test) (Fig. 1A). In the intention-to-treat population, 258 of 405 patients (64%) had disease progression or had died. The median duration of imaging-based progression-free survival was 10.2 months (95% CI, 8.3 to 11.2) in the rucaparib group and 6.4 months (95% CI, 5.6 to 8.2) in the control group (hazard ratio, 0.61; 95% CI, 0.47 to 0.80; P<0.001 by log-rank test) (Fig. 1B). In the ATM subgroup, 76 of 103 patients (74%) had disease progression or had died; the median duration of imaging-based progression-free survival was 8.1 months (95% CI, 5.5 to 8.3) in the rucaparib group and 6.8 months (95% CI, 4.0 to 10.4) in the control group (hazard ratio, 0.95; 95% CI, 0.59 to 1.52) (Fig. 1C). We verified the proportionality of hazards for the Cox proportional-hazard assumption graphically for the BRCA subgroup and the intention-to-treat population using log–log plots (Fig. S3).
Figure 1. Progression-free Survival in Three Trial Populations.
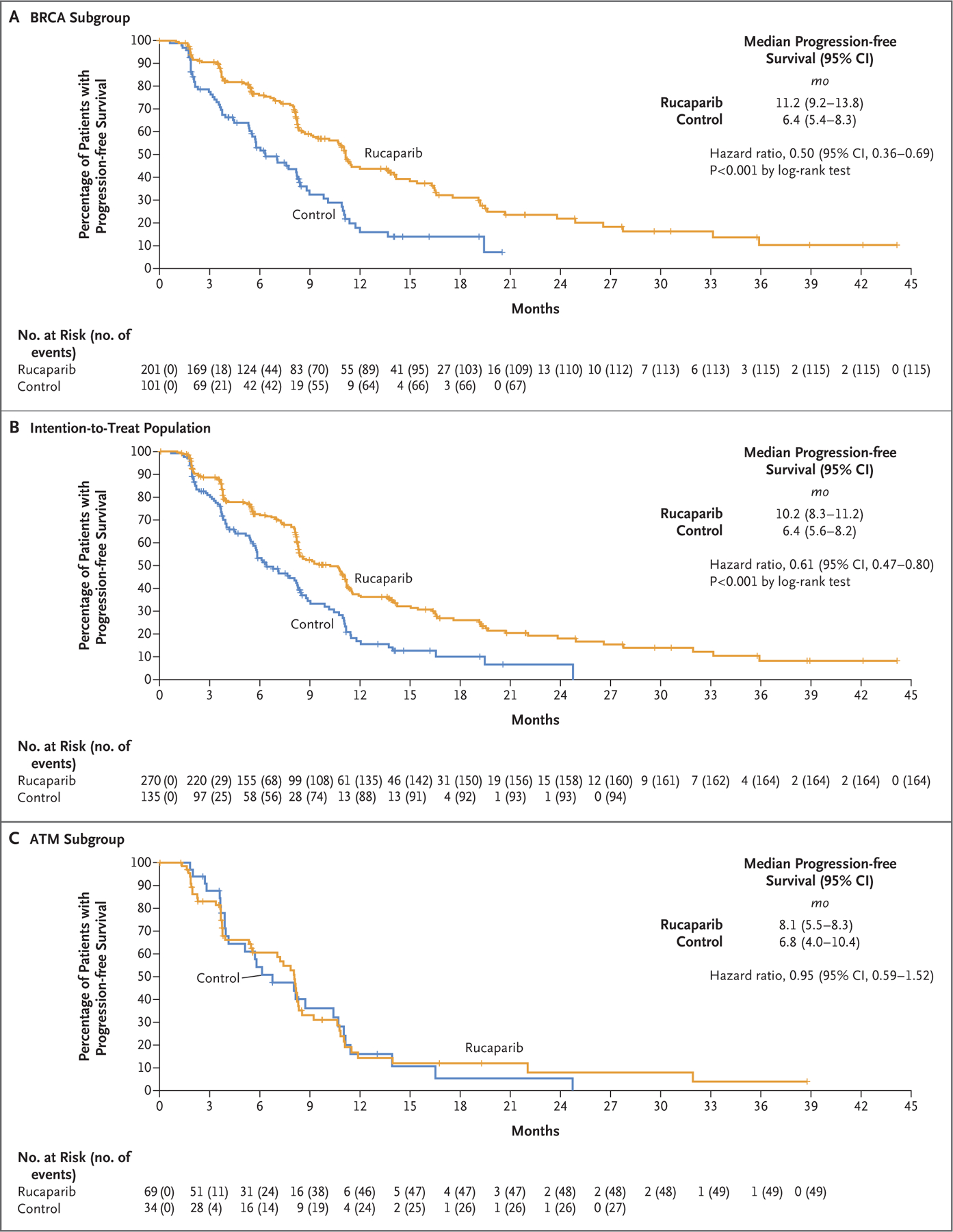
Shown are Kaplan–Meier curves for imaging-based progression-free survival according to independent review in the BRCA subgroup (Panel A), the intention-to-treat population (Panel B), and the ATM subgroup for rucaparib as compared with a control medication (docetaxel or a second-generation androgen-receptor pathway inhibitor [abiraterone acetate or enzalutamide]). Data maturity was 60% in the BRCA subgroup, 64% in the intention-to-treat population, and 74% in the ATM subgroup. The widths of the 95% confidence intervals were not adjusted for multiplicity and cannot be used in place of hypothesis testing. BRCA denotes BRCA1 and BRCA2, and CI confidence interval.
The results of sensitivity analyses for imaging-based progression-free survival to evaluate the effect of data censoring were similar to the results of the primary analysis (Table S2). In addition, the reasons that data regarding imaging-based progression-free survival were censored for patients who had received at least one subsequent anticancer therapy are shown in Table S3.
SECONDARY OUTCOME
At 62 months, an interim analysis of overall survival was conducted along with the analysis of imaging-based progression-free survival. In the BRCA subgroup, 162 of 302 patients had died (data maturity, 54%); the median overall survival was 24.3 months (95% CI, 19.9 to 25.7) in the rucaparib group and 20.8 months (95% CI, 16.3 to 23.1) in the control group (hazard ratio, 0.81; 95% CI, 0.58 to 1.12; P = 0.21 by log-rank test) (Fig. S6A). In the intention-to-treat population, 240 of 405 patients had died (data maturity, 59%) (Fig. S6B). Data for the exploratory ATM subgroup are shown in Figure S6C.
Among 161 of 405 patients (40%) with measurable disease at baseline, the frequency of a confirmed objective response according to independent imaging-based review in the rucaparib group and the control group was 45% (37 of 82 patients) and 17% (7 of 41 patients), respectively, in the BRCA subgroup; 35% (37 of 106 patients) and 16% (9 of 55 patients), respectively, in the intention-to-treat population; and no response (0 of 24 patients) and 14% (2 of 14 patients), respectively, in the ATM subgroup (Table S4). PSA50 and PSA90 responses, the median time to PSA progression, and the median duration of response according to independent imaging-based review are provided in the Supplementary Appendix. Results for investigator-assessed efficacy outcomes (imaging-based progression-free survival, objective response, and duration of response) were generally aligned with the results for independent review (Table S8).
EXPLORATORY OUTCOMES
As of the data cutoff, at least one subsequent anticancer regimen was administered to 162 of 270 patients (60%) in the rucaparib group and to 91 of 135 patients (67%) in the control group; subsequent PARP inhibitor therapy was administered to 8 of 270 patients (3%) and to 81 of 135 patients (60%), respectively, and subsequent platinum therapy to 41 of 270 patients (15%) and to 7 of 135 patients (5%), respectively. After disease progression, 63 of 135 patients (47%) in the control group crossed over to receive rucaparib.
We performed exploratory efficacy analyses of rucaparib as compared with each control treatment. In the BRCA subgroup, the median duration of imaging-based progression-free survival was longer with rucaparib than with docetaxel (11.2 months vs. 8.3 months; hazard ratio, 0.53; 95% CI, 0.37 to 0.77) (Fig. 2A); the median duration was also longer with rucaparib than with abiraterone acetate or enzalutamide (11.2 months vs. 4.5 months; hazard ratio, 0.38; 95% CI, 0.25 to 0.58) (Fig. 2B). Median imaging-based progression-free survival in the rucaparib group as compared with the control group in the intention-to-treat population and the ATM subgroup are shown in Fig. S4A through S4D.
Figure 2. Comparison of Progression-free Survival between Rucaparib and Control Medications in the BRCA Subgroup.
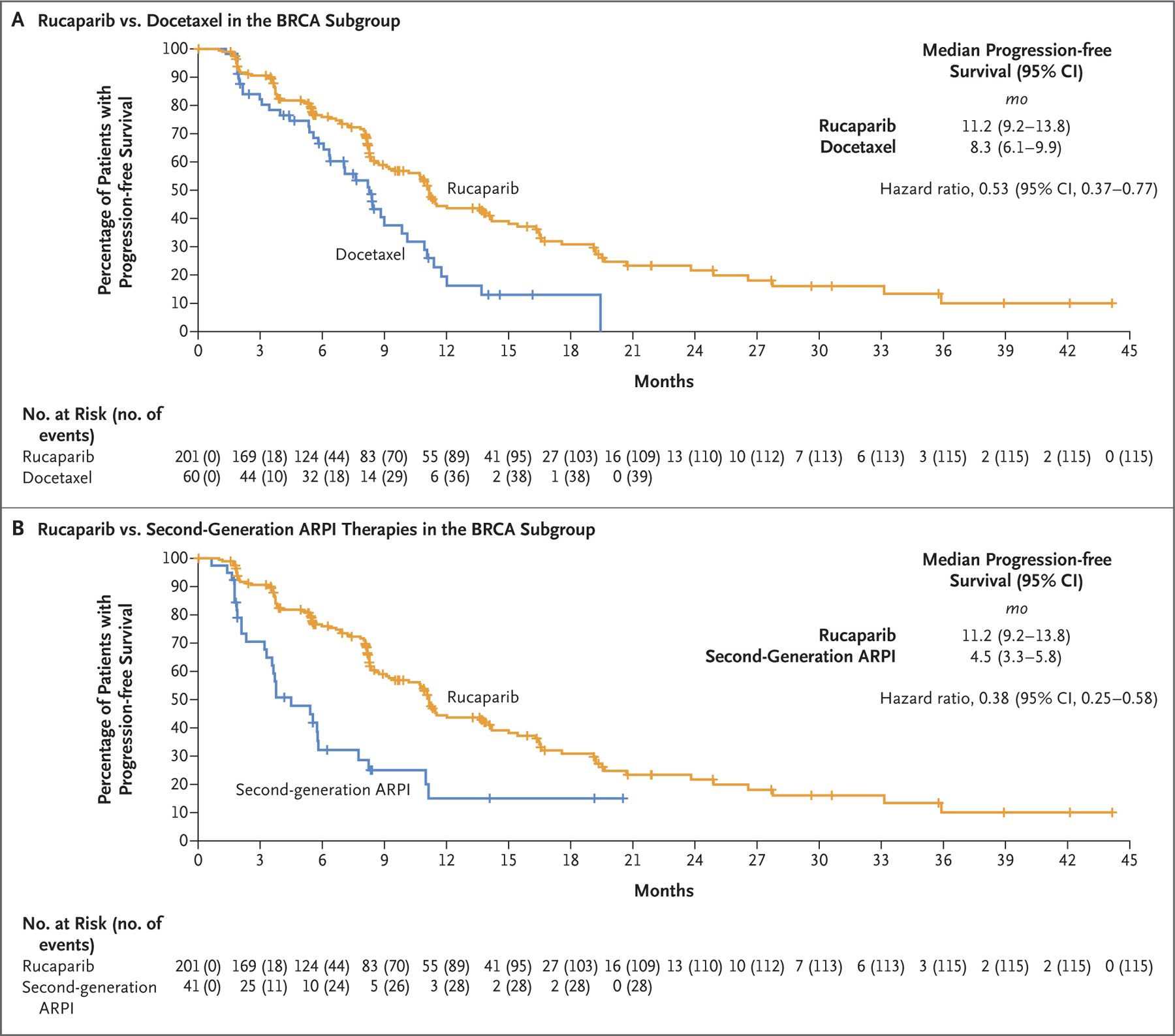
Shown are Kaplan–Meier curves for rucaparib as compared with a control medication (docetaxel or a second-generation androgen-receptor pathway inhibitor [ARPI]) in the BRCA subgroup. The widths of the 95% confidence intervals were not adjusted for multiplicity and cannot be used in place of hypothesis testing.
The results of prespecified exploratory subgroup analyses of imaging-based progression-free survival were similar to those of the primary analysis, with the exception of some subgroups with a small number of patients (e.g., those with BRCA1 alterations and with hepatic metastases) (Fig. 3 and Fig. S5).
Figure 3. Risk of Disease Progression or Death in the BRCA Subgroup, According to Variable.
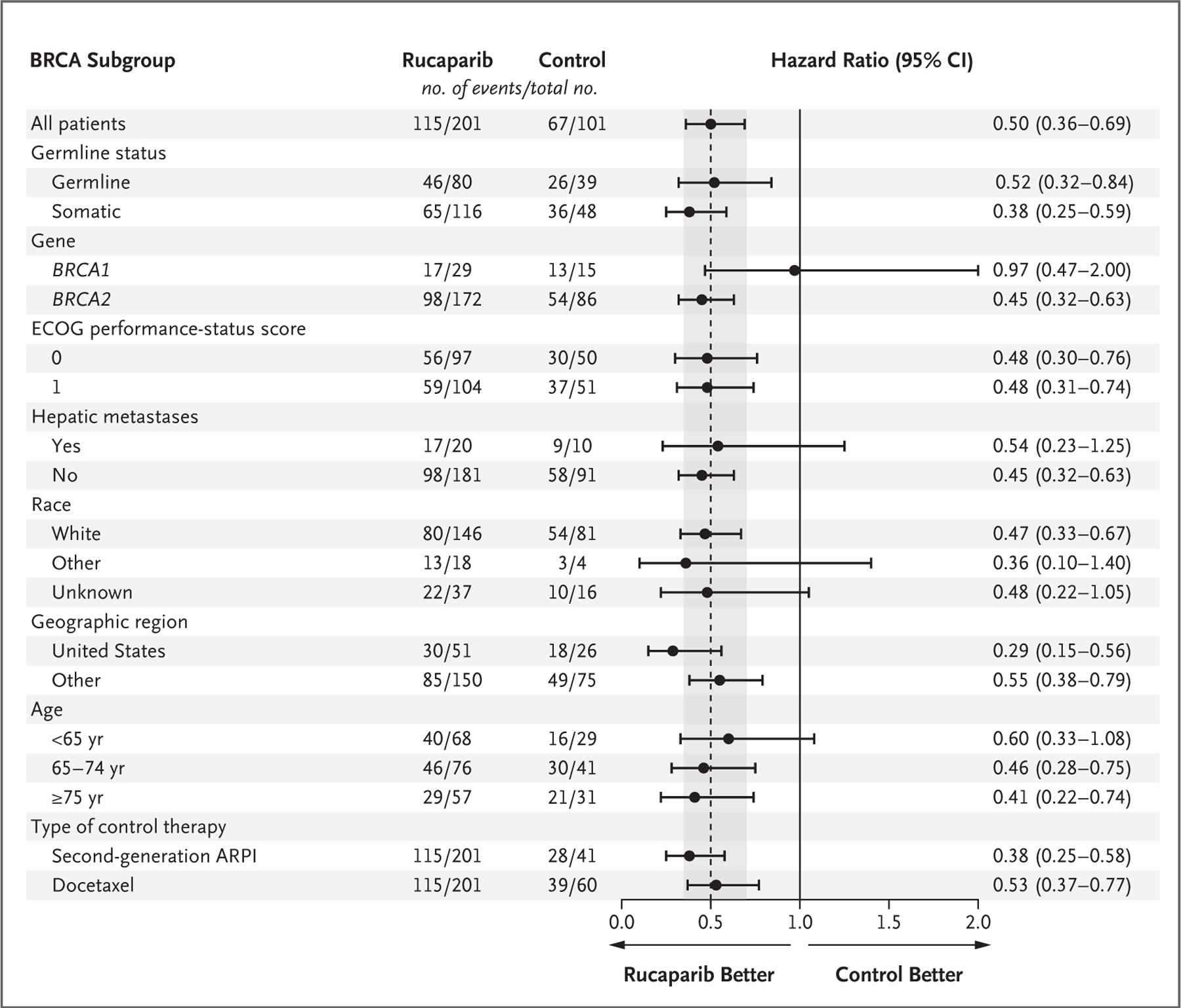
Shown is the risk of imaging-based disease progression or death in the BRCA subgroup according to prespecified variables in the rucaparib group as compared with the control group (second-generation ARPI or docetaxel). ECOG denotes Eastern Cooperative Oncology Group.
PATIENT-REPORTED OUTCOMES
Changes from baseline to week 25 in the score on the Functional Assessment of Cancer Therapy–Prostate questionnaire were similar for rucaparib and control medications in the BRCA subgroup (difference in least-squares means [±SE] for rucaparib as compared with control, 3.1±2.5; 95% CI, −1.8 to 8.1) as well as in the intention-to-treat population (difference, 2.4±2.2; 95% CI, −1.9 to 6.6). Similar results in the two groups were also observed on the Brief Pain Inventory–Short Form questionnaire and on the EQ-5D-5L Visual Analogue Scale.
SAFETY
The safety population, which consisted of all the patients who had received at least one dose of a protocol-specified treatment, included 270 patients in the rucaparib group and 130 patients in the control group. The median treatment duration was 8.3 months (range, 0.2 to 46.0) in the rucaparib group and 5.1 months (range, 0.3 to 30.4) in the control group. In the control group, the median treatment duration was 4.8 months (range, 0.7 to 11.0) with docetaxel and 5.4 months (range, 0.3 to 30.4) with a second-generation ARPI. According to the protocol and established clinical practice, the treatment duration for docetaxel was limited to 10 cycles, with a median of 6 cycles (range, 1 to 10).
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin; the most common adverse events in the control group were fatigue, diarrhea, and neuropathy (Table 2). The most common adverse events of grade 3 or more were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group and fatigue and neutropenia or a decreased neutrophil count in the control group. No cases of myelodysplastic syndrome or acute myeloid leukemia were reported. Interstitial lung disease was reported in 1 patient (<1%) in the rucaparib group; in the control group, pneumonitis was reported in 2 patients (2%), both of whom were receiving docetaxel. Pulmonary embolism occurred in 9 patients (3%) in the rucaparib group and in 9 patients (7%) in the control group; deep-vein thrombosis was reported in 3 patients (1%) and 1 patient (1%), respectively.
Table 2.
Adverse Events (Safety Population).*
| Variable | Rucaparib (N = 270) | Control Medication | ||||||
|---|---|---|---|---|---|---|---|---|
| Docetaxel (N = 71) | Second-Generation ARPI (N = 59) | Total Control (N = 130) | ||||||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| number of patients (percent) | ||||||||
| Any adverse event | 270 (100) | 161 (60) | 71 (100) | 43 (61) | 58 (98) | 26 (44) | 129 (99) | 69 (53) |
| Fatigue | 165 (61) | 19 (7) | 48 (68) | 7 (10) | 34 (58) | 5 (8) | 82 (63) | 12 (9) |
| Nausea | 134 (50) | 7 (3) | 11 (15) | 1 (1) | 14 (24) | 0 | 25 (19) | 1 (1) |
| Anemia or decreased hemoglobin | 126 (47) | 64 (24) | 10 (14) | 1 (1) | 13 (22) | 0 | 23 (18) | 1 (1) |
| Decreased appetite | 96 (36) | 1 (<1) | 15 (21) | 0 | 14 (24) | 2 (3) | 29 (22) | 2 (2) |
| Diarrhea | 83 (31) | 4 (1) | 28 (39) | 2 (3) | 8 (14) | 0 | 36 (28) | 2 (2) |
| Rash† | 78 (29) | 4 (1) | 13 (18) | 1 (1) | 6 (10) | 0 | 19 (15) | 1 (1) |
| Constipation | 74 (27) | 3 (1) | 13 (18) | 1 (1) | 6 (10) | 0 | 19 (15) | 1 (1) |
| Increased ALT or AST | 72 (27) | 14 (5) | 3 (4) | 1 (1) | 3 (5) | 0 | 6 (5) | 1 (1) |
| Vomiting | 65 (24) | 2 (1) | 6 (8) | 1 (1) | 5 (8) | 0 | 11 (8) | 1 (1) |
| Back pain | 60 (22) | 9 (3) | 11 (15) | 3 (4) | 14 (24) | 2 (3) | 25 (19) | 5 (4) |
| Peripheral edema | 54 (20) | 0 | 15 (21) | 0 | 6 (10) | 0 | 21 (16) | 0 |
| Increased creatinine | 51 (19) | 0 | 3 (4) | 0 | 3 (5) | 0 | 6 (5) | 0 |
| Thrombocytopenia or decreased platelet count | 50 (19) | 16 (6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | 49 (18) | 0 | 17 (24) | 4 (6) | 13 (22) | 0 | 30 (23) | 4 (3) |
| Dysgeusia | 45 (17) | 1 (<1) | 15 (21) | 0 | 3 (5) | 0 | 18 (14) | 0 |
| Dyspnea | 44 (16) | 1 (<1) | 12 (17) | 2 (3) | 3 (5) | 0 | 15 (12) | 2 (2) |
| Decreased weight | 40 (15) | 2 (1) | 9 (13) | 0 | 7 (12) | 0 | 16 (12) | 0 |
| Neutropenia or decreased neutrophil count | 37 (14) | 20 (7) | 11 (15) | 10 (14) | 0 | 0 | 11 (8) | 10 (8) |
| Dizziness | 36 (13) | 1 (<1) | 3 (4) | 0 | 8 (14) | 0 | 11 (8) | 0 |
| Headache | 31 (11) | 1 (<1) | 4 (6) | 0 | 5 (8) | 0 | 9 (7) | 0 |
| Limb pain | 30 (11) | 2 (1) | 6 (8) | 0 | 8 (14) | 1 (2) | 14 (11) | 1 (1) |
| Neuropathy‡ | 25 (9) | 0 | 34 (48) | 4 (6) | 2 (3) | 0 | 36 (28) | 4 (3) |
| Cough | 24 (9) | 2 (1) | 11 (15) | 0 | 5 (8) | 0 | 16 (12) | 0 |
| Insomnia | 20 (7) | 0 | 8 (11) | 0 | 6 (10) | 0 | 14 (11) | 0 |
| Hypertension | 16 (6) | 6 (2) | 2 (3) | 1 (1) | 9 (15) | 6 (10) | 11 (8) | 7 (5) |
| Stomatitis | 13 (5) | 2 (1) | 10 (14) | 2 (3) | 0 | 0 | 10 (8) | 1 (1) |
| Hypokalemia | 12 (4) | 3 (1) | 4 (6) | 1 (1) | 6 (10) | 2 (3) | 10 (8) | 3 (2) |
| Alopecia | 5 (2) | 0 | 25 (35) | 1 (1) | 1 (2) | 0 | 26 (20) | 1 (1) |
| Febrile neutropenia | 2 (1) | 2 (1) | 9 (13) | 8 (11) | 0 | 0 | 9 (7) | 8 (6) |
| Serious adverse event or dose adjustment | ||||||||
| Serious adverse event | 78 (29) | NA | 23 (32) | NA | 13 (22) | NA | 36 (28) | NA |
| Interruption of intervention owing to adverse event | 142 (53) | NA | 19 (27) | NA | 12 (20) | NA | 31 (24) | NA |
| Dose reduction owing to adverse event | 104 (39) | NA | 21 (30) | NA | 11 (19) | NA | 32 (25) | NA |
| Discontinuation owing to adverse event | 40 (15) | NA | 23 (32) | NA | 5 (8) | NA | 28 (22) | NA |
| Death from adverse event | 5 (2) | NA | 0 | NA | 3 (5) | NA | 3 (2) | NA |
Listed are adverse events of any grade that were reported in at least 10% of the patients in either group and corresponding adverse events of grade 3 or higher, according to the Common Terminology Criteria for Adverse Events. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
Rash includes acneiform dermatitis, blister, blood blister, bullous dermatitis, contact dermatitis, eczema, erythematous rash, maculopapular rash, palmar–plantar erythrodysesthesia syndrome, papular rash, photosensitivity reaction, pruritic rash, pruritis, psoriasis, skin discoloration, skin exfoliation, skin hyperpigmentation, skin induration, skin lesion, skin toxicity, skin ulcer, and solar dermatitis.
Neuropathy includes neurotoxicity, paresthesia, peripheral motor neuropathy, peripheral neuropathy, peripheral sensory neuropathy, and polyneuropathy.
Adverse events leading to treatment discontinuation were reported in 40 patients (15%) in the rucaparib group and in 28 patients (22%) in the control group. Death from an adverse event during treatment occurred in 5 patients (2%) in the rucaparib group and in 3 patients (2%) in the control group. In the rucaparib group, 1 patient each died from cardiac failure, esophageal perforation, myocardial ischemia, sepsis, and a combination of lower respiratory tract infection and ventricular fibrillation. In the control group, 1 patient each died from coronavirus disease 2019, pneumonia, and an unknown cause. No adverse events that led to death were considered by the investigator to be related to a trial treatment.
DISCUSSION
In this trial involving men with metastatic, castration-resistant prostate cancer, we found that the median duration of imaging-based progression-free survival (the primary outcome) was significantly longer in the rucaparib group than in the control group (11.2 months vs. 6.4 months). Among the control medications, 56% of the patients received docetaxel, which has been a standard therapy for two decades.15,16 The benefit with respect to imaging-based progression-free survival in the rucaparib group was reported both in the BRCA subgroup and in the intention-to-treat population, with the greatest benefit in the BRCA subgroup. In an exploratory analysis in the subgroup of patients with an ATM alteration, the duration of imaging-based progression-free survival was similar in the rucaparib and control groups.
Previous studies involving men with metastatic, castration-resistant prostate cancer have been criticized for their choice of comparator drugs.19,20 For example, in multiple studies — including the PROfound, IMbassador250, and KEYNOTE-641 trials — enzalutamide or abiraterone acetate was used as a comparator in patients with disease that had progressed while the patient was receiving the alternative (or an identical) drug, an approach that does not represent evidence-based standard of care.9,19–22 In contrast, in TRITON3, physicians could choose between docetaxel and a second-generation ARPI that was newly prescribed for the patient, a trial design that provides a more robust treatment comparison. The benefit of rucaparib over docetaxel was striking, given that numerous other studies either did not include docetaxel in the control group or did not show the superiority of the intervention to docetaxel.9,23
At the time of this report, data regarding overall survival were not mature. Among the patients in the control group who had discontinued the trial drug (mostly because of progressive disease), a large percentage (60%) subsequently received a PARP inhibitor. Among patients with measurable disease at baseline, the frequency of an objective response was higher with rucaparib than with the control medication in both the BRCA subgroup and the intention-to-treat population, which confirmed our results in TRITON2.11 In the current trial, we enrolled a smaller number of patients with BRCA1 alterations than with BRCA2 alterations, and the treatment benefit was not conclusive in those with BRCA1 alterations, a finding that was similar to the results of the PROfound phase 3 trial.9 Also, in the current trial, the repeated use of second-generation ARPIs appeared to have only modest activity and was less efficacious than PARP inhibition, a finding that was consistent with the results of previous studies.9,24
Our finding of the limited efficacy of rucaparib in the ATM subgroup was similar to the results of previous clinical trials involving PARP inhibitors.9 As in TRITON2,13 we observed no objective response for rucaparib according to independent imaging-based review in the ATM subgroup.
The strengths of TRITON3 include the relatively large number of patients who were enrolled in the BRCA and ATM subgroups. The trial design allowed for crossover from a control medication to rucaparib in patients who had confirmed progression. After the adoption of a protocol amendment, we included patients with metastatic, castration-sensitive disease who had previously received a second-generation ARPI, a practice that more closely reflects modern prostate cancer treatment after recent drug approvals for such patients.1,3 Several recent phase 2–3 studies have shown the clinical efficacy of a PARP inhibitor combined with a second-generation ARPI as first-line treatment in patients with metastatic, castration-resistant prostate cancer. These studies showed increased benefit particularly in patients with a genetic alteration associated with DNA damage, which highlights a potential benefit of such combination therapy.25–27 Trial limitations include the immaturity of overall survival data and the exploratory nature of some subgroup analyses.
The most frequent adverse events with rucaparib were fatigue, nausea, and anemia or decreased hemoglobin. Treatment interruption or dose reduction may be considered to mitigate these adverse events.14 No cases of myelodysplastic syndrome or acute myeloid leukemia were reported. The risk of thromboembolic events, a side effect that has been associated with olaparib,27,28 was lower than or similar to the risk associated with control medications.
The use of rucaparib resulted in a longer duration of imaging-based progression-free survival than a physician’s choice of docetaxel or a second-generation ARPI in patients with metastatic, castration-resistant prostate cancer in whom treatment with an ARPI had failed.
Supplementary Material
Acknowledgments
Supported by Clovis Oncology. Dr. Abida’s work is supported by a grant (P30-CA008748) from the National Cancer Institute.
We thank the patients and their families and caregivers for their participation in this trial; Clovis Oncology representatives Melanie Dowson, Jowell Go, and Owen Bowles for clinical operations assistance, Andrew Croskery and Peter Morello for publications assistance, and Erin Dominy for clinical development assistance; and Sachi Yim and Kathleen Blake of Ashfield MedComms for medical-writing and other editorial assistance with an earlier version of the manuscript.
Footnotes
Contributor Information
Karim Fizazi, Gustave Roussy Institute, Paris-Saclay University, Villejuif, France
Josep M. Piulats, Institut Català d’Oncologia–Bellvitge Institute for Biomedical Research –CiberOnc, Barcelona, Spain
M. Neil Reaume, Ottawa Hospital Research Institute, Ottawa, Canada
Peter Ostler, Mount Vernon Cancer Centre, Northwood, United Kingdom
Ray McDermott, St. Vincent’s University Hospital and Cancer Trials Ireland, Dublin, Ireland
Joel R. Gingerich, CancerCare Manitoba, Winnipeg, Toronto, Canada
Elias Pintus, Guy’s Hospital,London, United Kingdom
Srikala S. Sridhar, Princess Margaret Cancer Centre, Toronto, Canada
Richard M. Bambury, Cork University Hospital, Wilton, Ireland
Urban Emmenegger, Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Canada
Henriette Lindberg, Herlev University Hospital, Herlev, Denmark
David Morris, Urology Associates, Nashville
Franco Nolè, European Institute of Oncology IRCCS, Milan
John Staffurth, London, Velindre University NHS Trust, Cardiff, United Kingdom
Charles Redfern, Sharp HealthCare, San Diego, CA
María I. Sáez, Medical Oncology Intercenter Unit, Regional and Virgen de la Victoria University Hospitals, IBIMA, Málaga, Spain
Wassim Abida, Genitourinary Oncology Service, Memorial Sloan Kettering Cancer Center, New York
Gedske Daugaard, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
Axel Heidenreich, Universitätsklinikum Köln, Cologne, Germany; Medical University of Vienna, Vienna
Laurence Krieger, Genesis Care, North Shore, Sydney
Brieuc Sautois, University Hospital of Liège, CHU Sart-Tilman, Liège, Belgium
Andrea Loehr, Clovis Oncology, Boulder, CO
Darrin Despain, Clovis Oncology, Boulder, CO
Catherine A. Heyes, Clovis Oncology UK, Cambridge, United Kingdom (C.A.H., S.P.W.) — all in the United Kingdom
Simon P. Watkins, Clovis Oncology UK, Cambridge, United Kingdom (C.A.H., S.P.W.) — all in the United Kingdom
Simon Chowdhury, Guy’s Hospital and Sarah Cannon Research Institute, London, United Kingdom
Charles J. Ryan, University of Minnesota, Minneapolis
Alan H. Bryce, Mayo Clinic, Phoenix, AZ
REFERENCES
- 1.Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the Advanced Prostate Cancer Consensus Conference 2021. Eur Urol 2022;82:115–41. [DOI] [PubMed] [Google Scholar]
- 2.Vogl UM, Beer TM, Davis ID, et al. Lack of consensus identifies important areas for future clinical research: Advanced Prostate Cancer Consensus Conference (APCCC) 2019 findings. Eur J Cancer 2022;160:24–60. [DOI] [PubMed] [Google Scholar]
- 3.Sayegh N, Swami U, Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract 2022;18:45–55. [DOI] [PubMed] [Google Scholar]
- 4.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. [DOI] [PubMed] [Google Scholar]
- 5.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913–7. [DOI] [PubMed] [Google Scholar]
- 6.Ashworth A A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008;26:3785–90. [DOI] [PubMed] [Google Scholar]
- 7.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 8.Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 9.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020; 382:2091–102. [DOI] [PubMed] [Google Scholar]
- 10.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol 2020;38:3763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol 2021;22:1250–64. [DOI] [PubMed] [Google Scholar]
- 13.Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the Phase II TRITON2 study. Clin Cancer Res 2020;26:2487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubraca (rucaparib) tablets Boulder, CO: Clovis Oncology, 2022. (package insert). [Google Scholar]
- 15.National Comprehensive Cancer Network. Prostate cancer, version 1. Clinical practice guidelines in oncology (NCCN guidelines) 2023. (https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf ).
- 16.Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–34. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wambeke S, Vera-Badillo FE, Gyawali B. Controlling the control arm in metastatic castration-resistant prostate cancer trials: best standard of care or the minimum standard of care? J Clin Oncol 2022;40:1518–21. [DOI] [PubMed] [Google Scholar]
- 20.Ardolino LC, Dear R, Armstrong AJ, Gillessen S, Joshua AM. Clinical trials for metastatic castrate-resistant prostate cancer-who is looking after the control patients? Questions for the future. Ann Oncol 2022;33:574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff JN, Liang LW, Kim J, Stenzl A. KEYNOTE-641: a phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Future Oncol 2021;17:3017–26. [DOI] [PubMed] [Google Scholar]
- 22.Powles T, Yuen KC, Gillessen S, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med 2022;28:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudard S, Fizazi K, Sengeløv L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol 2017;35:3189–97. [DOI] [PubMed] [Google Scholar]
- 24.Sartor O, George D, Tombal B, et al. Real-world outcomes of second novel hormonal therapy or radium-223 following first novel hormonal therapy for mCRPC. Future Oncol 2022;18:35–45. [DOI] [PubMed] [Google Scholar]
- 25.Saad F, Armstrong AJ, Thiery-Vuillemin A, et al. PROpel: phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2022;40:Suppl:11. abstract ( 10.1200/JCO.2022.40.6_suppl.011). [DOI] [Google Scholar]
- 26.Hussain MHA, Kocherginsky M, Agarwal N, et al. BRCAAWAY: a randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) with DNA repair defects. J Clin Oncol 2022;40:16:suppl_5018. abstract ( 10.1200/JCO.2022.40.16_suppl.5018). [DOI] [Google Scholar]
- 27.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid 2022;1(9). 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 28.Lynparza (olaparib) tablets Wilmington, DE: AstraZeneca Pharmaceuticals, 2022. (package insert). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


