Abstract
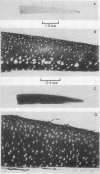
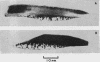
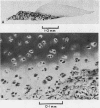
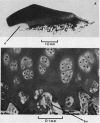
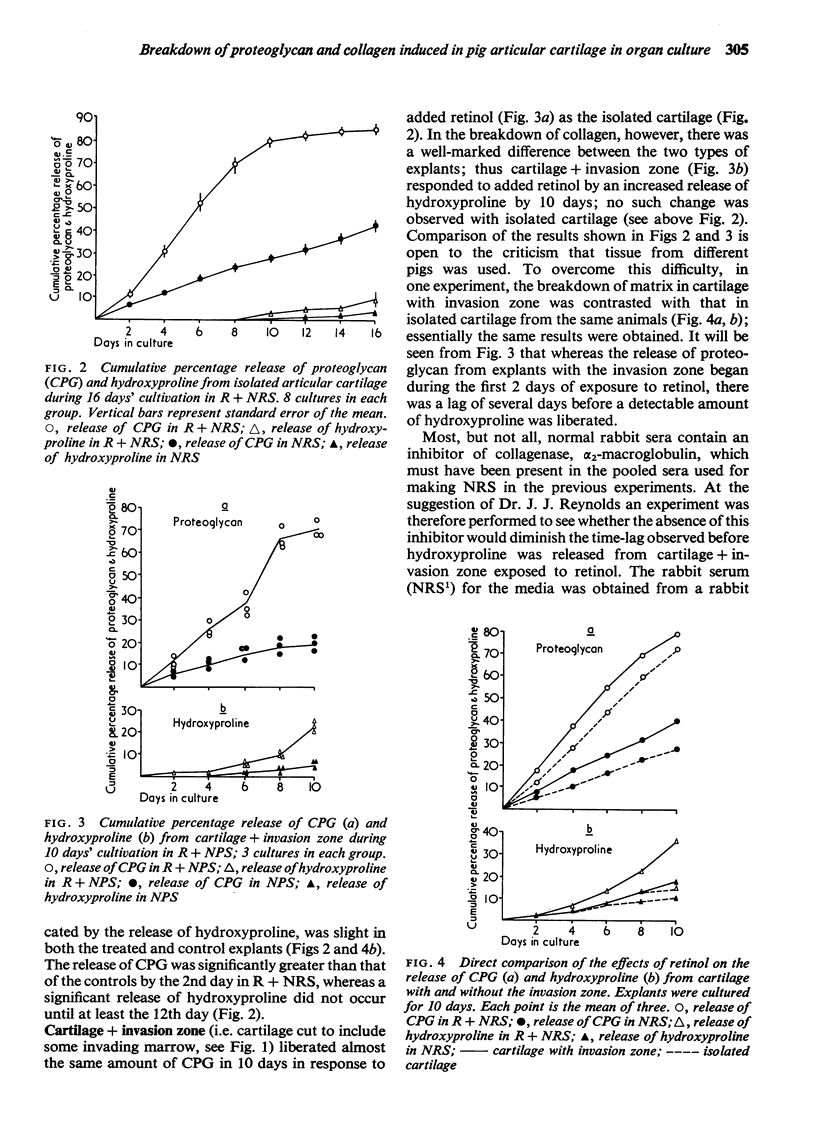
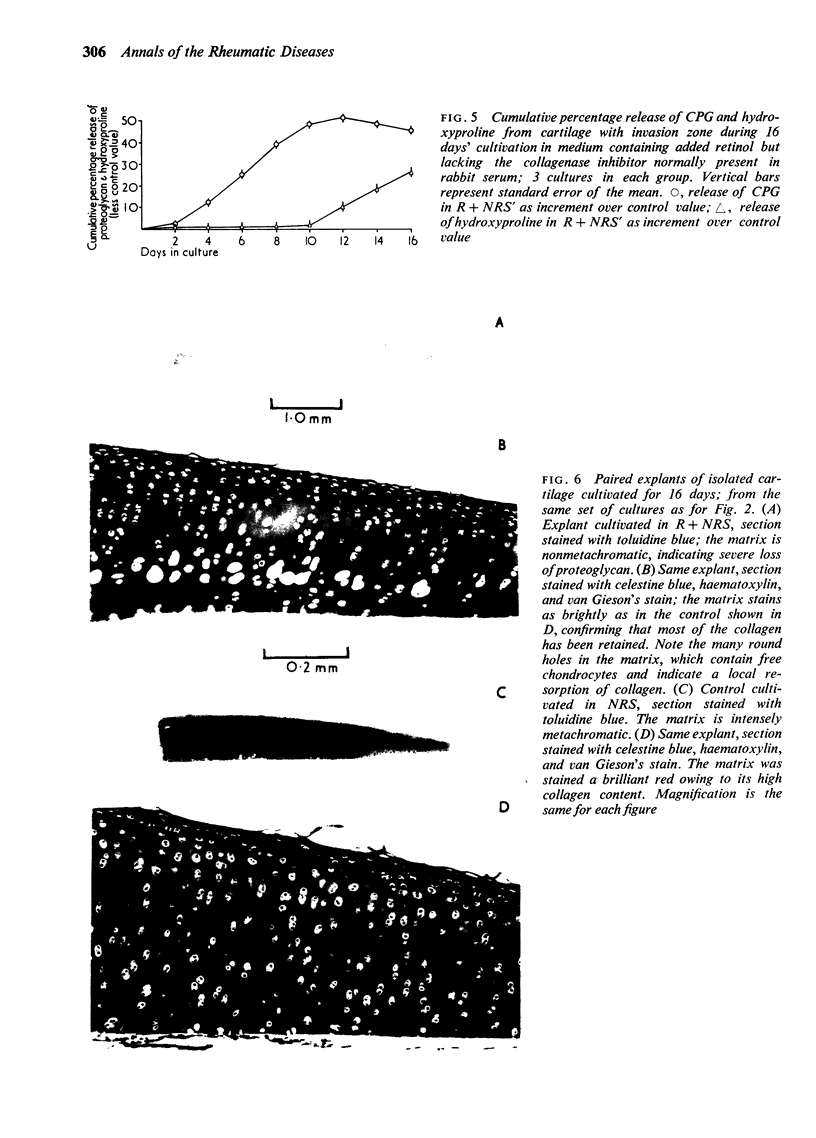
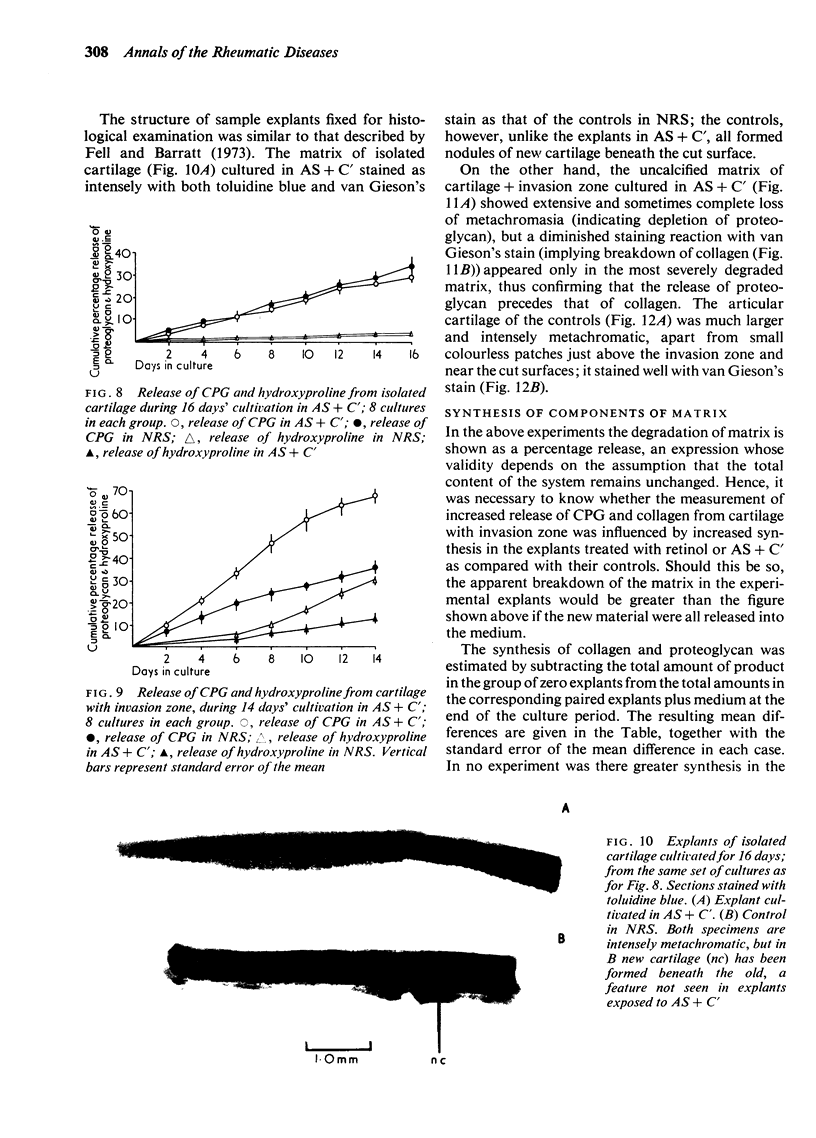
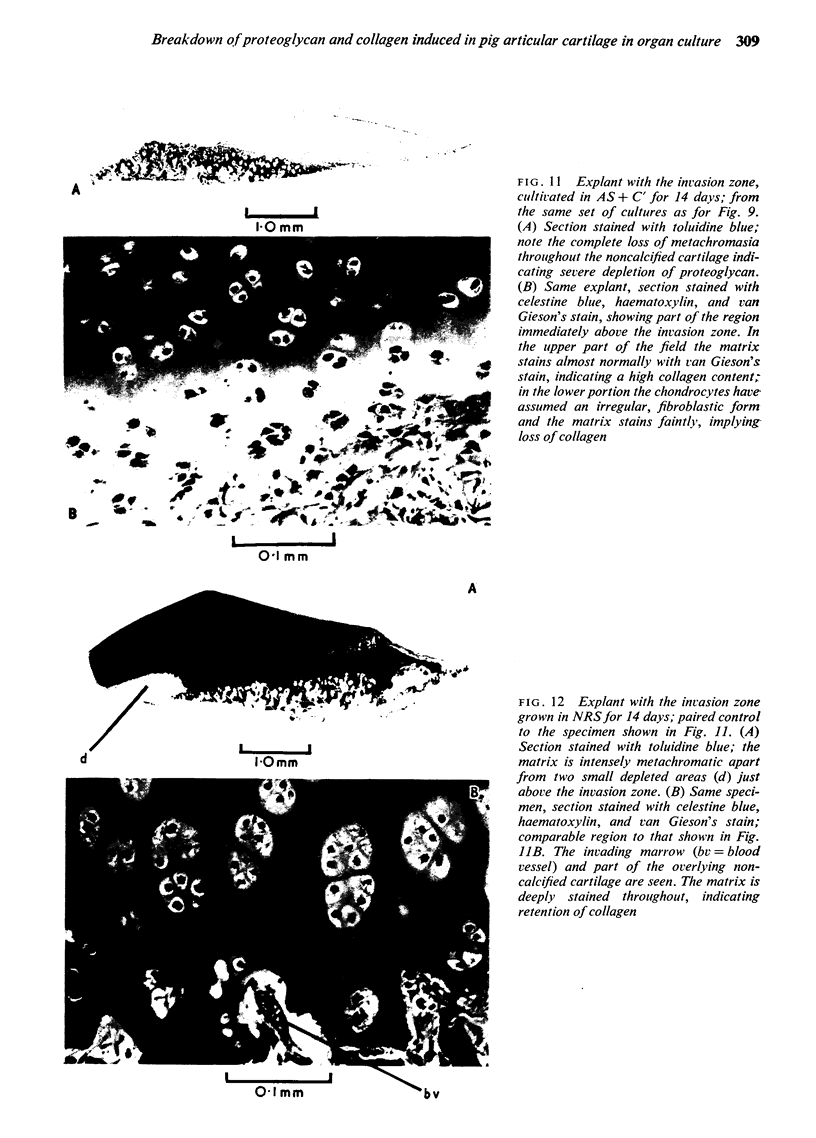
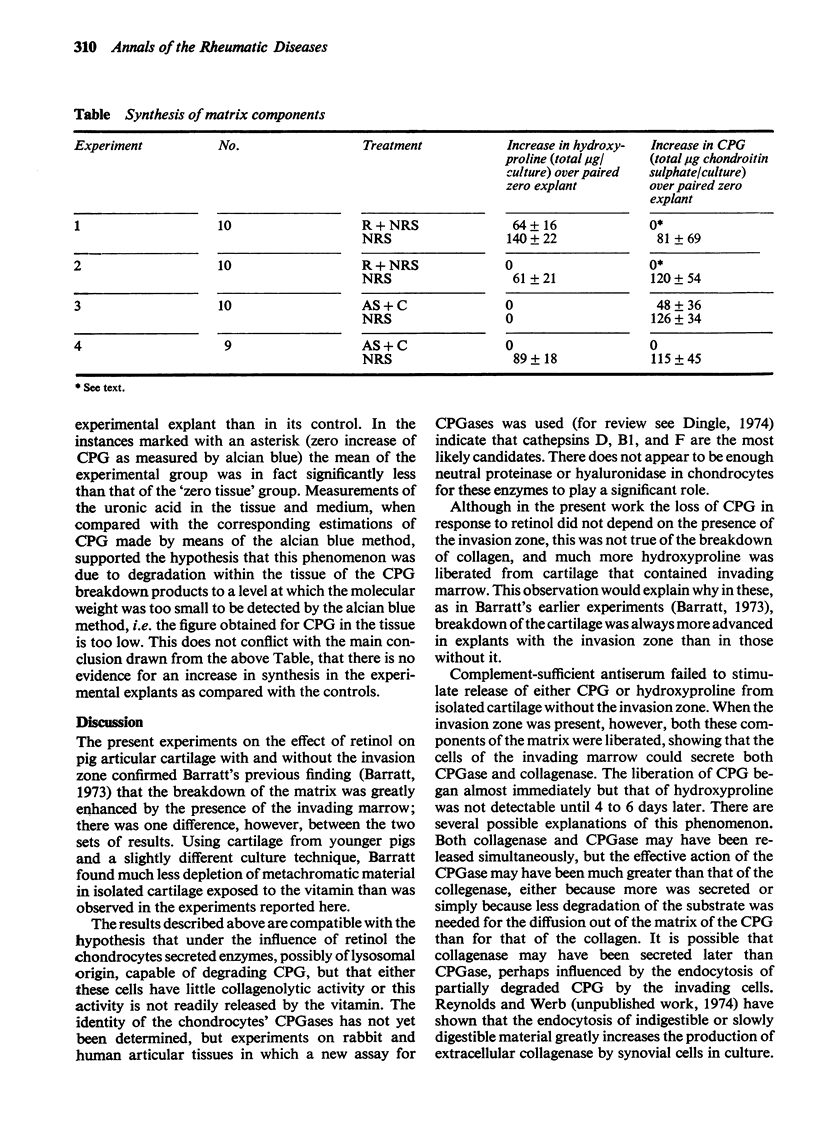
Explants of articular cartilage from young pigs were maintained in organ culture for 10--16 days, and degradation of matrix was induced by retinol or complement-sufficient antiserum. The percentage breakdown of proteoglycan and collagen (as hydroxyproline release) was measured. The response of the cartilage depended on whether or not the explants were cut so as to include some of the invading marrow ('invasion zone'). In media containing retinol, cartilage lost up to three-quarters of its proteoglycan whether the invasion zone was present or not, but very little of its collagen unless this region was included. In the presence of complement-sufficient anti-serum, however, cartilage without the invasion zone was virtually unaffected, but both proteoglycan and hydroxyproline were released when invasion zone was included; here proteoglycan release began almost immediately, but there was a time-lag of 6--8 days before a substantial amount of hydroxyproline appeared in the medium. Histological examination of sample explants from the experiments supported the biochemical findings. The possible significance of the results in relation to rheumatoid arthritis is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Barratt M. E. The role of soft connective tissue in the response of pig articular cartilage in organ culture to excess of retinol. J Cell Sci. 1973 Jul;13(1):205–219. doi: 10.1242/jcs.13.1.205. [DOI] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B., Barratt M. E. The role of soft connective tissue in the breakdown of pig articular cartilage cultivated in the presence of complement-sufficient antiserum to pig erythrocytes. I. Histological changes. Int Arch Allergy Appl Immunol. 1973;44(3):441–468. doi: 10.1159/000230951. [DOI] [PubMed] [Google Scholar]
- Gnädinger M. C., Itoi M., Slansky H. H., Dohlman C. H. The role of collagenase in the alkali-burned cornea. Am J Ophthalmol. 1969 Sep;68(3):478–483. doi: 10.1016/0002-9394(69)90718-1. [DOI] [PubMed] [Google Scholar]
- Hook C. W., Brown S. I., Iwanij W., Nakanishi I. Characterization and inhibition of corneal collagenase. Invest Ophthalmol. 1971 Jul;10(7):496–503. [PubMed] [Google Scholar]
- Poole A. R., Barratt M. E., Fell H. B. The role of soft connective tissue in the breakdown of pig articular cartilage cultivated in the presence of complement-sufficient antiserum to pig erythrocytes. II. Distribution of immunoglobulin G (IgG). Int Arch Allergy Appl Immunol. 1973;44(3):469–488. doi: 10.1159/000230952. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Whiteman P. The quantitative measurement of Alcian Blue-glycosaminoglycan complexes. Biochem J. 1973 Feb;131(2):343–350. doi: 10.1042/bj1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]