Abstract
Background: Limited data exist to support the use of rocuronium continuous infusions in the intensive care unit (ICU). Objective: To evaluate the dosing and monitoring of adult patients who received rocuronium for hypoxemic respiratory failure during the Coronavirus Disease 2019 (COVID-19) pandemic. Methods: This was a retrospective, single-center study from March 1, 2020 to May 31, 2020. We identified all adult patients admitted to any ICU who received rocuronium via continuous infusion. Patients were excluded if they received rocuronium for <6 hours. The main outcome of this study was to determine the median rocuronium maintenance continuous infusion rate in the ICU. Secondary outcomes of this study included the initial continuous infusion rate, duration of therapy, cumulative dose, frequency and median of rocuronium boluses, time to resolution of neuromuscular blockade, and the relationship between the hourly administration rates of rocuronium and train-of-four (TOF) assessments. Results: Seventy-one patients and 97 paralytic infusions were included. Fifty-nine patients (83%) were positive for SARS CoV-2. Of the 97 rocuronium infusions, the median dose at initiation was 3 (3–5) mcg/kg/min and duration of infusion was 45 (23.6–92.5) hours. The median continuous infusion maintenance rate was 4.3 (2.8–7.2) mcg/kg/min. There was a negligible correlation between the dose of rocuronium and the TOF results (r = .04). A total of 1775 TOFs were assessed, of which 46.2% were over-paralyzed, 35.7% well-paralyzed, and 18.1% under-paralyzed. Conclusions: The initial and maintenance infusion doses in our analysis were lower than what have been previously referenced.
Keywords: rocuronium, acute respiratory distress syndrome, neuromuscular blocking agent
Introduction
Acute respiratory distress syndrome (ARDS) is an inflammatory form of acute lung injury, characterized by hypoxia, reduced lung compliance, increased work of breathing, and respiratory failure.1 Despite the controversy surrounding the use of neuromuscular blocking agents (NMBAs) in ARDS, the 2013 Society of Critical Care Medicine (SCCM) guidelines recommend early administration of NMBAs by continuous intravenous infusion in critically ill patients with ARDS and a partial pressure of oxygen (PaO2)/the fraction of inspired oxygen (FiO2) < 150.2 For patients with ARDS secondary to COVID-19, it is recommended by SCCM and the National Institutes of Health to consider using low tidal volume ventilation, higher positive end-expiratory pressure strategy, and intermittent boluses or even continuous infusions of NMBAs if ventilator dyssynchrony persists.3,4
Rocuronium is a non-depolarizing NMBA, approved to be used during rapid sequence intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.5 The only absolute contraindication to rocuronium is an allergy to the drug itself. The package insert recommends initial and maintenance continuous infusion rates of 10 to 12 and 4 to 16 mcg/kg/min, respectively.5 The duration of action may be prolonged in patients with hepatic or renal failure, myasthenia gravis, and carcinomatosis.5 The package insert recommends monitoring of twitch response by using a peripheral nerve stimulator.5 Monitoring a patient’s train-of-four (TOF) is recommend during the use of rocuronium to assess efficacy as well as avoid excessive drug administration.5
The increased demand of healthcare supplies and medications during the COVID-19 pandemic has contributed to shortages of several critical drugs.6 A drug shortage can be defined as a limited supply of a specific drug which adversely impacts the day-to-day drug preparation process by the pharmacy.7 The etiologies of drug shortages are numerous and include manufacturing discontinuation, natural disaster, quality issues, and increases in demand, among others.8 Recently, the United States Food and Drug Administration announced limitations in the supply of several medications commonly used in the intensive care unit (ICU) (i.e., vecuronium bromide, cisatracurium besylate, ketamine, midazolam, hydromorphone, and etomidate).6
The use of rocuronium continuous infusion in the ICU is not well established. A small retrospective analysis demonstrated the use of rocuronium continuous infusion in the ICU among patients with organ dysfunction resulted in prolonged recovery time.9 At our institution, cisatracurium is the preferred NMBA. Due to a shortage of cisatracurium, our Pharmacy and Therapeutics committee approved the use of rocuronium as a therapeutic alternative. The objective of this study was to evaluate the dosing and monitoring of adult patients who received rocuronium in the ICU for hypoxemic respiratory failure during the COVID-19 pandemic.
Methods
We performed a single-center, retrospective analysis at Brigham and Women’s Hospital, a 793-bed tertiary academic medical center in Boston, MA. This study was deemed exempt by the Partners institutional review board. Patients who received continuous infusion rocuronium between March 1, 2020 and May 31, 2020 were identified using a hospital reporting system. Patients were included if they were ≥18 years old, mechanically ventilated via endotracheal tube, and received rocuronium via continuous infusion for ≥6 hours in the ICU. A rocuronium infusion was defined as an individual rocuronium infusion over 6 hours, which was separated by at least 24 hours from another rocuronium infusion without a documented TOF <4/4 between the 2 infusions. Patients could have multiple rocuronium infusions during their ICU admission. All statistical analyses were performed based on the total number of infusions.
Baseline characteristics were collected at the time of first rocuronium initiation, and included patient age, gender, body weight (actual, dosing, and ideal), Acute Physiology and Chronic Health Evaluation (APACHE) II score, cause of respiratory failure, ventilator metrics, and presence of hepatic or renal dysfunction. Acute kidney injury (AKI) was defined as an increase in serum creatinine by ≥ .3 mg/dL within 48 hours or an increase in serum creatinine of ≥1.5 times baseline.10 The presence of chronic kidney disease (CKD) of any stage was documented. Hepatic dysfunction was classified as the presence of cirrhosis, portal hypertension, or hepatitis documented in the patient’s electronic health record.
The primary outcome of this study was to determine the median rocuronium maintenance continuous infusion rate (mcg/kg/min) in the ICU. Secondary outcomes included additional rocuronium metrics, such as initial continuous infusion rate (mcg/kg/min), duration of therapy, cumulative dose, frequency and median dose of rocuronium boluses, time to resolution of neuromuscular blockade, and the relationship between the hourly administration rates of rocuronium and TOF assessments. Two post hoc analyses were performed to compare outcomes in patients with or without COVID-19 and in patients with or without organ dysfunction, defined as AKI, CKD, or hepatic dysfunction.
At our institution, all orders for NMBAs are written for the infusion to be titrated to ventilator synchrony as an efficacy measure in addition to TOF to monitor safety (standard 1 to 2 twitches to achieve 85% to 90% blockade). Per our institution’s TOF monitoring guideline, TOF assessment should be conducted every 15 minutes following a bolus dose and/or initiation of continuous infusion whenever clinically feasible. TOF is re-evaluated every hour until patient is clinically stable and the desired level of neuromuscular blockade is attained and then may be assessed every 2 hours. Our institutional guidelines for sedatives, analgesics, and NMBAs encourage bolus doses prior to the start of infusions and as needed clinically. Our rocuronium guideline recommends generally a starting dose of 3 to 5 mcg/kg/min with a range of 0 to 20 mcg/kg/min. When rocuronium is ordered, the dose range, initial rate, dose titration, and decision of whether to administer a bolus dose are up to the discretion of the provider. While there are no defaults within the order, a selection of 0 to 12 mcg/kg/min for the dose range and either 3, 5, or 8 mcg/kg/min as an initial rate are readily available for providers to select within the electronic medical record system. Titration typically occurs at increments of .5 to 1 mcg/kg/min every 60 minutes to achieve ventilator synchrony along with goal TOF. The initial infusion rate was defined as the rocuronium infusion rate at hour 1 and the maintenance infusion rate defined as rocuronium infusion rate from hour 2 until the end of the infusion event. TOF assessments are performed and documented by the ICU nurses at either the posterior tibial nerve, ulnar nerve, or facial nerve.
All TOF assessments were documented and classified as over-paralyzed (TOF = 0), well-paralyzed (TOF = 1 to 2), or under-paralyzed (TOF = 3 to 4).11 Resolution of neuromuscular blockade is not protocolized in our institutional guideline but was collected when available and defined as the time to return to a TOF of 4/4. The following adverse reactions were recorded based on documentation in the patient medical record by ICU team: myopathy, neuropathy, malignant hyperthermia, anaphylaxis, and bronchospasm.
Descriptive statistics were performed to summarize patient demographics. Continuous variables were presented as medians with interquartile ranges (IQRs). The Pearson correlation test was performed to determine the relationship between the hourly administration rate of rocuronium and TOF assessment. Pearson correlation coefficients were interpreted as follows: > .8 very strong, .6–.8 strong, .4–.6 moderate, .2–.4 weak, and <.2 negligible correlation.12 The Mann–Whitney U test was used to compare COVID-19 to non-COVID-19 patients. Microsoft Excel® (version 16.37) was used to perform data analysis.
Results
Of 72 patients screened, 71 patients were included in the analysis. One patient was excluded due to administration of rocuronium for less than 6 hours. Baseline demographics can be found in Table 1. Chronic kidney disease, AKI, and liver dysfunction were reported among 11 (15.5%), 38 (53.5%), and 2 (2.8%) patients, respectively. Most patients (83%) had respiratory failure secondary to COVID-19. All patients in our analysis had at least 1 TOF assessed and documented.
Table 1.
Baseline Characteristics.
| Characteristic | Rocuronium patients (N = 71) |
|---|---|
| Reasons for respiratory failurea | |
| COVID-19 | 59 (83.1) |
| Non-COVID-19 related | 12 (16.9) |
| Medical | 9 (12.7) |
| Trauma | 1 (1.4) |
| Airway protection | 1 (1.4) |
| Malignancy | 1 (1.4) |
| Age, yearsb | 56 (47-63) |
| Malea | 51 (71.8) |
| Dosing weight, kgb | 85 (72.9-103) |
| Past medical historya | |
| Chronic kidney disease | 11 (15.5) |
| Acute kidney injury | 38 (53.5) |
| Liver dysfunction | 2 (2.8) |
| APACHE IIb | 21 (17-26) |
| Extracorporeal membrane oxygenation a | 10 (14.1) |
| Ventilator metrics at initiation of paralytics: b | |
| FiO2, % | 70 (51.2-90) |
| PaO2, mmHg | 82 (70-104) |
| PaO2/FiO2 | 127.6 (104.2-168.5) |
| Tidal volume/ideal body weight, mL/kg | 6 (5.6-6.4) |
Abbreviations: APACHE II, The Acute Physiology and Chronic Health Evaluation II; COVID-19, Coronavirus-19; FiO2, Fraction of inspired oxygen; PaO2, Partial pressure of oxygen.
an (%),
bMedian (IQR).
We observed a total of 97 rocuronium infusions which included 6653 total hours of administration. None of the patients received any other NMBAs prior to rocuronium infusions.
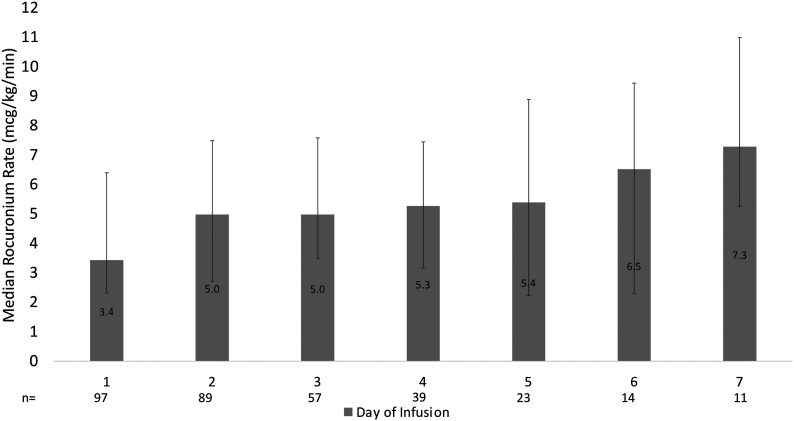
The median rocuronium maintenance continuous infusion rate was 4.3 (2.8–7.2) mcg/kg/min. Of the 97 infusions, 49 (50.5%) received at least 1 bolus prior to initiation of the infusion (64 bolus doses given) with median dose of 60 mg (.7 mg/kg). Forty-two (43%) received at least 1 additional bolus at any point during the infusion (80 bolus doses given) with median dose of 80 mg (.94 mg/kg). The median initial continuous infusion rate was 3 (3–5) mcg/kg/min. Figure 1 displays the median rocuronium infusion rates over time for the first 7 days of infusion. The median rocuronium cumulative dose on day 1 (3.4 mg [2.3–6.4]) was significantly lower than the median cumulative dose on day 7 (7.3 mg [5.3–11]; P = .002). The median cumulative dose and duration of each infusion were 1.16 (.4–2.5) g and 45.1 (23.6–92.5) hours, respectively. Additionally, 68 (94.4%) patients continued rocuronium infusion for more than 1 day. Among those patients, the median rocuronium infusion rates in the first and last day of infusion were 3.2 [2.3–4.8] and 4.7 [2.5–7.8] mcg/kg/min, respectively (P = .01). Forty-seven (69.1%) patients had a higher continuous infusion rate (mcg/kg/min) during their last day of infusion in comparison to the first day.
Figure 1.
The median rocuronium maintenance infusion rates for the first 7-day of infusion.
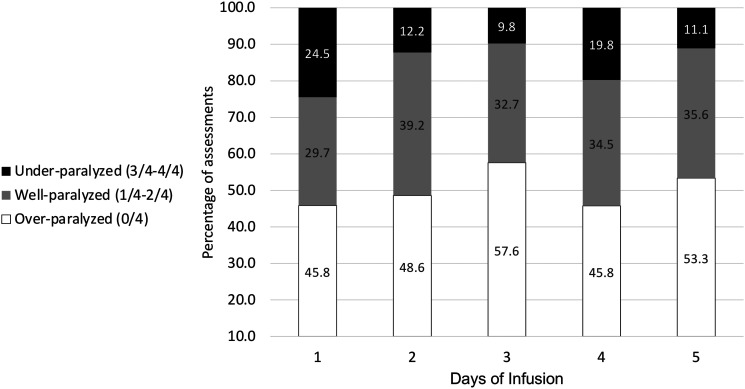
Overall, a total of 1775 TOFs were assessed, of which 820 (46.2%) were over-paralyzed, 635 (35.7%) well-paralyzed, and 320 (18.1%) under-paralyzed. Daily breakdown of TOF assessments for the first 5 days of infusion is shown in Figure 2. There was an average of 5 TOF assessments per patient per day during the first 5 days of infusion. We observed a negligible correlation between the hourly administration rate of rocuronium and the associated TOF assessment (r = .04). The median rocuronium maintenance infusion rate for TOF 1 and 2 were 5 (3–8) and 4 (2–7) mcg/kg/min, respectively.
Figure 2.
TOF assessments for the first 5-day of infusion.
Resolution of neuromuscular blockade was assessed in 36 infusions with a median time to resolution of 6.2 (2.7–16.5) hours. No documented adverse reactions were found among all infusions. Patients with COVID-19 had a significantly greater cumulative dose and duration compared to those without COVID-19 (Table 2). Co-administration of an aminoglycoside was documented in 1 (1.4%) patient, and magnesium and corticosteroids in 32 (45.1%) and 13 (18.4%) patients, respectively.
Table 2.
Comparison of COVIDs-19 and non-COVID-19 outcomes.
| COVID-19 (n = 59) | Non-COVID-19 (n = 12) | P-value | |
|---|---|---|---|
| Number of infusion events | 85 | 12 | N/A |
| Rocuronium maintenance continuous infusion rate, mcg/kg/mina | 4.9 (2.84-7.61) | 3.6 (2.27-4.17) | .03 |
| Initial continuous infusion rate, mcg/kg/mina | 3 (3-5) | 3 (3-5) | N/A |
| Duration of therapy, hoursa | 53.2 (27.49-92.51) | 23.7 (13.85-92.21) | .06 |
| Cumulative dose, grama | 1.19 (.47-2.74) | .33 (.15-4.82) | .01 |
| PaO2/FiO2a | 118.5 (100-162.13) | 179 (134.35-355) | .02 |
| ARDS classificationb | |||
| Mild | 4 (7) | 1 (8) | 1 |
| Moderate | 39 (66) | 5 (24) | .19 |
| Severe | 15 (25) | 2 (17) | .71 |
| >300 | 1 (2) | 4 (33) | .002 |
Abbreviations: ARDS, acute respiratory distress syndrome; FiO2, Fraction of inspired oxygen; PaO2, Partial pressure of oxygen.
aMedian (IQR),
bn (%).
Patients without organ dysfunction received a significantly (P = .05) higher rocuronium maintenance continuous infusion rate with a median of 5.4 (2.94–8.08) mcg/kg/min in comparison to patients with at least 1 type of organ dysfunction (AKI, CKD, or liver dysfunction) with a median of 4.4 (2.5–7.3) mcg/kg/min. Additionally, there was a trend toward a prolonged median time to neuromuscular blockade resolution in patients with organ dysfunction compared to patients without organ dysfunction (10 [3–6.36] hours vs 3.4 [2.25–7.3] hours; P = .059).
Discussion
Our study included 97 rocuronium infusions in 71 critically ill patients. We observed that patients received lower initial and maintenance rocuronium doses than previously reported in the literature.5,13 In addition, our results showed a negligible correlation between the hourly administration rate of rocuronium and TOF assessment. We also observed an increase in dose over time when comparing infusion rates on day 1 and day 7.
While the rocuronium package insert recommends an initial dose of 10 to 12 mcg/kg/min with subsequent doses titrated to response, and the 2002 SCCM guidelines recommend a maintenance dose of 10 to 12 mcg/kg/min, very little evidence exists to guide the use of rocuronium in critically ill patients.2,5,13 Our patients received significantly lower initial and maintenance infusion rates than previously recommended or described by Groetzinger et al.9 In comparison to Groetzinger et al., the patients in our analysis had a higher PaO2/FiO2 ratio, which may explain the lower doses used in our study. Despite using significantly lower initial and maintenance doses, almost half of the TOF assessments in our study were classified as over-paralyzed.11 Although patients with organ dysfunction received significantly lower rocuronium maintenance infusion rates, these patients had a longer, albeit not statistically significant, time to neuromuscular blockade resolution, possibly due to rocuronium accumulation. These findings are similar to those reported by to Groetzinger et al. with a median of 10 hours in both studies among patients with organ dysfunction.9
Rocuronium has a half-life of approximately 1.4 to 2.4 hours, and the administration of a loading dose may result in a more rapid achievement of therapeutic concentrations.5 An initial bolus dose was administered in only half of the infusions in our analysis, while subsequent bolus doses were administered in over 40% of infusions. Further studies evaluating the role of loading doses or alternative dosing strategies of neuromuscular blockade in critically ill patients (i.e., bolus vs continuous) are needed.
The 2016 SCCM guidelines recommend using TOF for monitoring patients receiving NMBAs in addition to clinical assessment, such as patient–ventilator synchrony.2 Utilization of the TOF assessment poses many challenges in critically ill patients, including nursing staff education and training, the subjectivity of TOF results, and possible scenarios that interfere with accuracy, such as peripheral edema.14 Baumann and colleagues15 concluded patients on NMBAs should not be solely monitored by TOF but rather in conjunction with additional clinical assessments. Our institutional guidelines for the management of patients with COVID-19 requiring neuromuscular blockade suggest targeting a TOF of 1/4 to 2/4 twitches in addition to ventilator synchrony, noting the considerable variability of TOF monitoring. Despite the lack of data among ICU patients and the potential of negligible correlation between the hourly administration rate of rocuronium and TOF assessment (r = .04) based on our findings, the use of the TOF may help clinicians to utilize the lowest effective dose to achieve ventilator synchrony and to avoid over-paralysis. Dose minimization of NMBAs is important in all patients to try to decrease the risk of complications, such as severe myopathies. This may be even more important in critically ill patients with multiple risk factors, such as co-administration with corticosteroids or the presence of hepatic or renal dysfunction. As more data have emerged regarding the possible benefits of corticosteroid administration in patients with COVID-19, the risk of corticosteroid-induced myopathy should be strongly considered when administering NMBAs in patients with COVID-19 in particular.16
Patients with COVID-19 had a significantly greater cumulative dose, continuous infusion rates, and duration of rocuronium compared to those without COVID-19 (Table 2). While the differences between ARDS related to COVID-19 and unrelated to COVID-19 are still debated, patients in our analysis with COVID-19 had significantly lower PaO2/FiO2 ratios (P = .02), possibly indicating more severe lung injury.17,18 The frequent NMBA administration among COVID-19 ARDS patients was also found in 2 large observational studies.19,20 NMBAs might result in a reduction of barotrauma and self-induced lung injury.21
Tachyphylaxis to NMBAs, indicated by the need for dose titrations to maintain the same twitch response, has been previously reported in the literature.22,23 Previous studies have demonstrated an increase in the maintenance dose requirements for atracurium and cisatracurium when administrated as continuous infusion over 192 hours and 174 hours, respectively.24 Our results suggest tachyphylaxis may occur with rocuronium administration in critically ill patients; however, the number of infusions was much lower on day 7 (n = 11) in comparison to day 1 (n = 97). For patients who continued rocuronium infusion for more than 1 day, the infusion rate during the last day of infusion was statistically higher in comparison to the first day. This finding could also be due to possible tachyphylaxis or overall clinical improvement. Other studies investigating rocuronium tachyphylaxis are warranted.
There are several limitations of this study. First, this was a single-center retrospective study, which might come with unavoidable confounding variables. Second, there was inconsistent documentation of TOF assessment and neuromuscular blockade resolution was not obtained for all infusions. Third, some outcomes such as duration of ventilation and length of ICU/hospital stay were not assessed as the objective of our study was to evaluate the dosing and monitoring of rocuronium in the ICU. Fourth, due to the retrospective nature of this study, we were unable to assess ventilator synchrony and the indication for additional bolus dosing during the infusion. Fifth, rocuronium down titration was not assessed in over-paralyzed patients. Last, adverse reactions were evaluated based on documentation in the patient medical record by the ICU team, which may have resulted in an underestimation of adverse reactions.
Conclusion
The initial and maintenance continuous infusion rates of rocuronium in critically ill patients at our institution were lower than what have been previously described. Despite this, almost half of the TOF assessments in our cohort were classified as over-paralyzed. Patients initiated on rocuronium may require significantly lower doses than recommended in order to achieve ventilator synchrony and avoid excessive paralysis. Future research evaluating the use of rocuronium in the ICU may be warranted.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Mohammed Aldhaeefi https://orcid.org/0000-0002-5985-9149
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562-572. doi: 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 2.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically Ill patient: Crit Care Med. 2016;44(11):2079-2103. doi: 10.1097/CCM.0000000000002027 [DOI] [PubMed] [Google Scholar]
- 3.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. Published online2020:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available athttps-www.covid19treatmentguidelines.nih.gov. Accessed June 29, 2020. [PubMed] [Google Scholar]
- 5.Zemuron (rocuronium) [Package insert]. Dublin, Ireland: Organon (Ireland) Ltd., Swords, Co. Ltd. Available athttps://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020214s034lbl.pdf. Accessed August 1, 2020. [Google Scholar]
- 6.US Food and Drug Administration . What is New Related to Drugs. Silver Spring, MD: US Food and Drug Administration; 11/6/2020. [Google Scholar]
- 7.Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75(21):1742-1750. doi: 10.2146/ajhp180441 [DOI] [PubMed] [Google Scholar]
- 8.Drug Shortages- Root Causes and Potential Solutions A Report by the Drug Shortages Task Force. Silver Spring, MD: US Food and Drug Administration; 2019. Accessed June 29, 2020. [Google Scholar]
- 9.Groetzinger LM, Hutchins AT, Rivosecchi RM. An evaluation of continuous infusion rocuronium for sustained neuromuscular blockade in critically Ill adults. Ann Pharmacother. 2020;55:106002802096673. doi: 10.1177/1060028020966731 [DOI] [PubMed] [Google Scholar]
- 10.Notice. Kidney Int Suppl. 2012;2(1):1. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 11.Bouju P, Tadié J-M, Barbarot N, et al. Clinical assessment and train-of-four measurements in critically ill patients treated with recommended doses of cisatracurium or atracurium for neuromuscular blockade: a prospective descriptive study. Ann Intensive Care. 2017;7(1):10. doi: 10.1186/s13613-017-0234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans JD.Straightforward Statistics for the Behavioral Sciences. Pacific Grove, CA: Pacific: Grove- BrooksCole Pub Co; 1996.pdf. [Google Scholar]
- 13.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically Ill patient: Crit Care Med. 2002;44(11):2079-2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 14.Strange C, Vaughan L, Franklin C, Johnson J. Comparison of train-of-four and best clinical assessment during continuous paralysis. Am J Respir Crit Care Med. 1997;156(5):1556-1561. doi: 10.1164/ajrccm.156.5.9701079 [DOI] [PubMed] [Google Scholar]
- 15.Baumann MH, McAlpin BW, Brown K, et al. A prospective randomized comparison of train-of-four monitoring and clinical assessment during continuous ICU cisatracurium paralysis. Chest. 2004;126(4):1267-1273. doi: 10.1378/chest.126.4.1267 [DOI] [PubMed] [Google Scholar]
- 16.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19 — Preliminary report. N Engl J Med. 2020;384:693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camporota L, Vasques F, Sanderson B, Barrett NA, Gattinoni L. Identification of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Respir Med. 2020;8(8):752-754. doi: 10.1016/S2213-2600(20)30279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102. doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19–associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LUNG SAFE investigators . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. J Am Med Assoc. 2016;315(8):788-800. [DOI] [PubMed] [Google Scholar]
- 21.Laurent P, Jean-Marie F, Arnaud G, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107-1116. [DOI] [PubMed] [Google Scholar]
- 22.Fink H, Luppa P, Mayer B, et al. Systemic inflammation leads to resistance to atracurium without increasing membrane expression of acetylcholine receptors: Anesthesiology. 2003;98(1):82-88. doi: 10.1097/00000542-200301000-00016 [DOI] [PubMed] [Google Scholar]
- 23.Gangneung Asan Hospital. Gangneung G-do, Republic of Korea. Hyun D-M, Kim Y-M, et al. Resistance to non-depolarizing neuromuscular blocking agents. Asian Pac J Health Sci. 2020;7(1):74-80. doi: 10.21276/apjhs.2020.7.1.14 [DOI] [Google Scholar]
- 24.Kanji S, Barletta JF, Janisse JJ, Kruse JA, Devlin JW. Tachyphylaxis associated with continuous cisatracurium versus pancuronium therapy. Pharmacotherapy. 2002;22(7):823-830. doi: 10.1592/phco.22.11.823.33625 [DOI] [PubMed] [Google Scholar]