Abstract
The brown dog tick Rhipicephalus sanguineus (sensu lato) in the southeastern Mediterranean region and the Middle East is difficult to identify due to the presence of multiple mitochondrial DNA haplogroup lineages. The purpose of this study was to clarify the identity of the “southeastern Europe” lineage of this tick species complex. Our research shows that female ticks of the “southeastern Europe” lineage correspond to the morphology of R. rutilus Koch, 1844 as found in type-material at the Museum für Naturkunde Berlin in Germany. We characterised the complete mitogenomes of R. rutilus, R. turanicus Pomerantsev, 1940 and Rhipicephalus sanguineus (Latreille, 1806) in order to improve our understanding of the phylogenetic relationships among species within the R. sanguineus (sensu lato) complex. The material associated with the morphology of R. rutilus was previously labelled as the “southeastern Europe” lineage and found in Israel and Egypt, including Lower Egypt and the Nile Delta, where the original type-material was collected. Based on the morphology, genetic identity, and geographical distribution of the species, we conclude that the name R. rutilus is correctly linked to the “southeastern Europe” lineage of R. sanguineus (sensu lato).
Keywords: Brown dog tick, Mitochondrial DNA, Morphology, Israel, Egypt, Species identity
Graphical abstract
Highlights
-
•
Morphology of extant material matches the holotype of Rhipicephalus rutilus Koch, 1844.
-
•
Mitogenome and phylogenetic analysis of Rhipicephalus rutilus.
-
•
Rhipicephalus rutilus is Rhipicephalus sanguineus (sensu lato) “southeastern Europe” lineage.
-
•
Mitogenomes robustly resolve the phylogeny of the Rhipicephalus sanguineus (sensu lato) species complex.
1. Introduction
In 1820 two young naturalists, Dr Wilhelm Hemprich and Dr Christian Ehrenberg, conducted a research expedition to Egypt and neighbouring regions. For an overview of their route and localities, see e.g. Bradley (1968). The expedition was supported by the Prussian government, and brought back an enormous amount of material for the Berlin Museum (Hemprich and Ehrenberg, 1828). Hemprich died from malaria during their travels in 1825. Ehrenberg soon returned to Berlin to become one of the foremost experts on microscopic life and micropaleontology at the time. This expedition was analogous to efforts to rediscover ancient Egyptian culture by Napoleon at the time of his invasion of Egypt in 1798. The French scientific expedition to Egypt was described in major volumes between years 1809 and 1829 in the “Description de l’Egypte” (Audouin, 1826).
Both expeditions brought ticks from the region back to Europe. Napoleon’s French expedition gave us Rhipicephalus linnaei (Audouin, 1826) – the tropical brown dog tick (Šlapeta et al., 2021). The Hemprich-Ehrenberg expedition yielded Rhipicephalus specimens that were subsequently named by the German arachnologist Carl Ludwig Koch as Rhipicephalus rutilus Koch, 1844 and Rhipicephalus limbatus Koch, 1844, both of which are members of the Rhipicephalus sanguineus (sensu lato) species complex (Camicas et al., 1998). While the type of R. linnaei was permanently lost, the types of R. rutilus and R. limbatus are held in the Museum für Naturkunde Berlin, Germany (Moritz & Fischer, 1981; Šlapeta et al., 2022). The availability of such historical museum material, together with new regional surveys using morphological and genetic tools over the past 10 years, enables the elucidation of their modern identity (Dantas-Torres et al., 2013; Chitimia-Dobler et al., 2017; Hornok et al., 2017; Senbill et al., 2022).
Rhipicephalus rutilus Koch, 1844 was described from a female collected in Egypt on an unknown host. Koch (1847) provided a more detailed description of the same specimen, accompanied by an illustration. Neumann (1897) re-described the species and added a second female specimen from Port Natal (now Durban); however the specimen could not be located in the Museum für Naturkunde Berlin, Germany by us. On the basis of what we now know about the species, the identification of that specimen must be considered as unlikely. Neumann (1911) divided R. sanguineus into three subspecies and considered R. rutilus to be a synonym of R. sanguineus sanguineus. Since that time R. rutilus has either been considered as a synonym of R. sanguineus (Zumpt, 1950; Floch and Fauran, 1959 (as rutibus [sic]), Morel and Vassiliades, 1962; Pegram et al., 1987; Keskin, 2009; Walker et al., 2000; Tucker, 2017; Aziz et al., 2018; Ramzan et al., 2020), or as incertae sedis (Guglielmone and Nava, 2014).
The aim of the present study was to clarify the identity of the “southeastern Europe” lineage of R. sanguineus (sensu lato). We show that female ticks that were genetically assigned to the “southeastern Europe” lineage of R. sanguineus (sensu lato) correspond to the morphology of the R. rutilus type-material. We characterised the complete mitochondrial genome (mitogenome) of R. rutilus, R. turanicus, and R. sanguineus (sensu stricto) in order to improve our understanding of phylogenetic relationships among species within the Rhipicephalus sanguineus (sensu lato) species complex.
2. Materials and methods
2.1. Available material and morphological identification
Ticks from several cats, a dog and a hedgehog were collected in Jerusalem, Israel in 2017 (Power et al., 2021). Ticks were collected together with fleas and stored in individual tubes with 70% ethanol. All ticks were observed under a stereo microscope (SMZ-2B, Nikon, Australia) and photographed using a digital microscope (VHX-6000, KEYENCE Inc., Japan) and identified using published keys and guides (Walker et al., 2000). A specimen of R. sanguineus (sensu stricto) (#208, referred to here as P8/22-208) was collected in 2021 from a dog in Györköny, Hungary. The holotype (ZMB 1093) of R. rutilus was observed (JŠ) at the Museum für Naturkunde Berlin, Germany and photographed on the museum’s equipment for specimen digitalisation with a DSLR camera.
2.2. DNA isolation and amplification of the partial mitochondrial cox1 gene
DNA was isolated from ticks stored in 70% (v/w) ethanol as previously described by Šlapeta et al. (2022). Briefly, dried ticks with incisions in their idiosoma were subjected to total tick genomic DNA (gDNA) isolation with the Monarch Genomic DNA Purification Kit (New England Biolabs, Australia). Exoskeletons were retained and preserved in 70% (v/w) ethanol. Extracted gDNA was stored at −20 °C.
The ∼600 nucleotide (nt) fragment of the mitochondrial DNA (mtDNA) cytochrome c oxidase subunit 1 (cox1) gene was amplified using the primer sets S0725/S0726, as described in Chandra et al. (2019) and/or Šlapeta et al. (2022). MyTaq™ Red Mix (Bioline, Australia) was used for DNA amplifications in 30 μl reactions, with 2 μl of template gDNA. All reactions included PCR-grade water (ddH2O) as a no-template control. The PCR reactions were performed in a T100™ Thermal Cycler (BioRad, Australia) and the PCR products were sequenced at Macrogen Ltd. (South Korea).
2.3. Genome skimming of R. rutilus, R. turanicus and R. sanguineus (sensu stricto) and assembly of mitogenome from next generation sequence data
The gDNA isolated from three adult Rhipicephalus spp. ticks was used for genome skimming via next-generation sequencing (NGS) using a NEBNext® DNA Library Prep Kit followed by NGS using 150 bp paired-end Illumina sequencing system at a depth of 1 Gb of raw sequence data (Novogene, Singapore). The whole mtDNA (IZ12-T3: JS6028; IZ13-T1: JS6029; P8/22-208: JS6373) was assembled from FastQ data using the MITObim pipeline (Hahn et al., 2013) (https://github.com/chrishah/MITObim) as previously described (Šlapeta et al., 2022). The complete circular mtDNA (mitogenomes) were aligned with all available complete mtDNA sequences of species of the R. sanguineus (sensu lato) group in CLC Main Workbench 21 (CLC bio, Qiagen, Australia).
2.4. Phylogenetic analysis
Sequence alignments were constructed using the CLC Main Workbench 21 (CLC bio, Qiagen, Australia). Evolutionary analyses including selection of models were conducted in MEGA 11 (Tamura et al., 2021). Whole mitogenome sequence alignments were included for newly obtained mitogenomes as well as all available mitogenomes from within the R. sanguineus (sensu lato) complex (as of December 20, 2022). The nucleotide mitogenome sequence alignment (15,029 sites) was produced with the aid of amino acid sequence translation. The phylogeny was reconstructed using selected best model, Maximum Likelihood method and General Time Reversible (GTR) model with a discrete Gamma distribution among sites (+G), and the rate variation model was allowed for some sites to be evolutionarily invariable (+I). The percentage of replicate trees in which the associated taxa clustered together was used for a bootstrap test (200 replicates). The cox1 gene phylogeny included all newly obtained sequences as well as all cox1 sequences belonging to the R. sanguineus (s.l.) “southeastern Europe” lineage available in GenBank. Sequences from R. microplus (Canestrini, 1897) and R. australis Fuller, 1899 served as outgroups. The cox1 phylogenetic tree based on nucleotide alignment was inferred by using the GTR + G + I for cox1 alignment. There was a total of 1001 positions in the final dataset. The percentage of replicate trees in which the associated taxa clustered together was calculated for the bootstrap test (1000 replicates).
2.5. Voucher material and sequence data deposition
The tick voucher specimens were deposited at the Australian National Insect Collection, CSIRO, Canberra, Australian Capital Territory, Australia, under the following accession numbers: R. turanicus (ANIC 48 006 603, ANIC 48 006 604, ANIC 48 006 606, ANIC 48 006 608 to ANIC 48 006 611); R. rutilus (ANIC 48 006 605, ANIC 48 006 607); H. adleri (ANIC 48 006 612); and R. sanguineus (ANIC 48 006 613). Raw FastQ sequence data were deposited at SRA NCBI BioProject: PRJNA917775. The nucleotide sequence data including three assembled mitogenomes generated in this study were deposited in GenBank (NCBI): OQ184022-OQ184024 (complete mtDNA) and OQ180895-OQ180904 (cox1). All sequence data, photographic documentation and associated supplementary material and additional data are available at LabArchives (https://dx.doi.org/10.25833/qt1c-z916).
3. Results
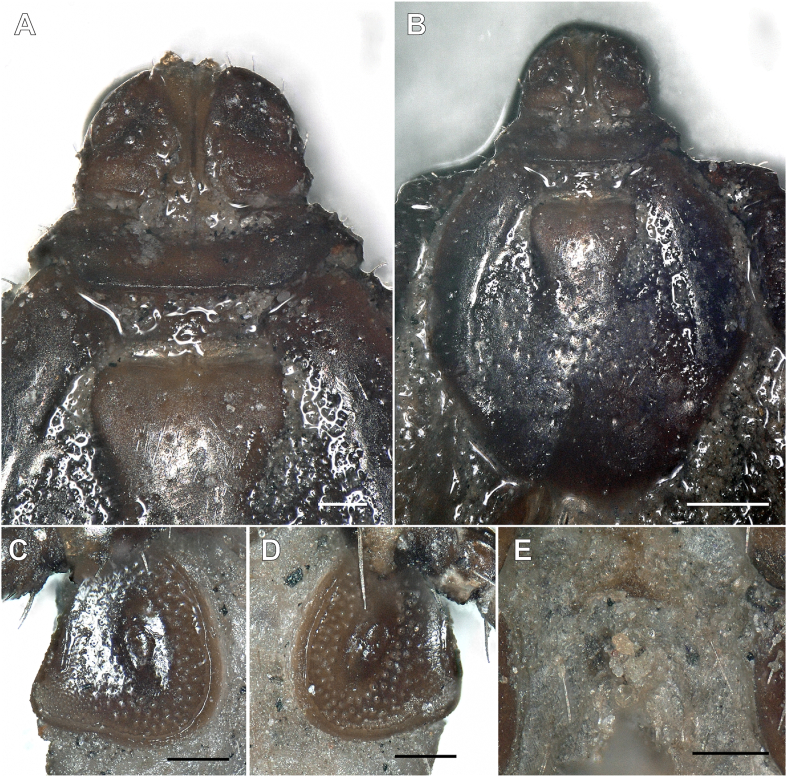
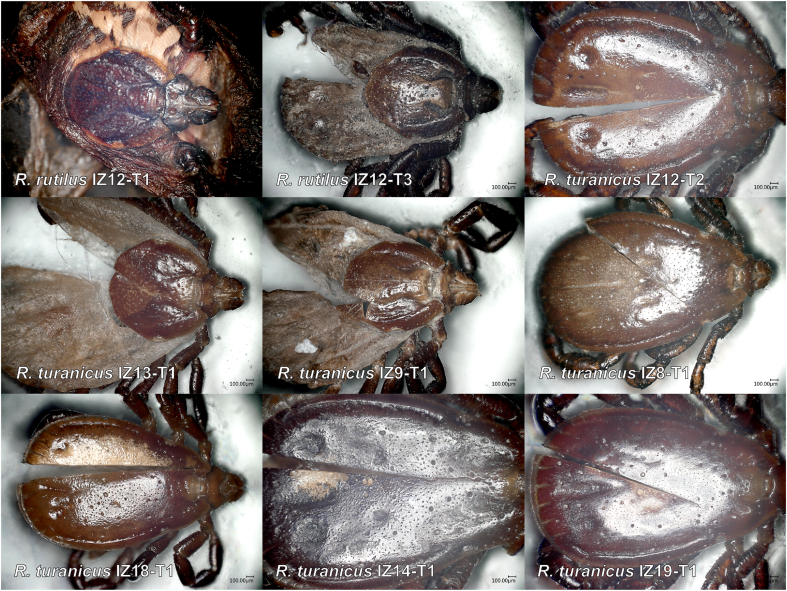
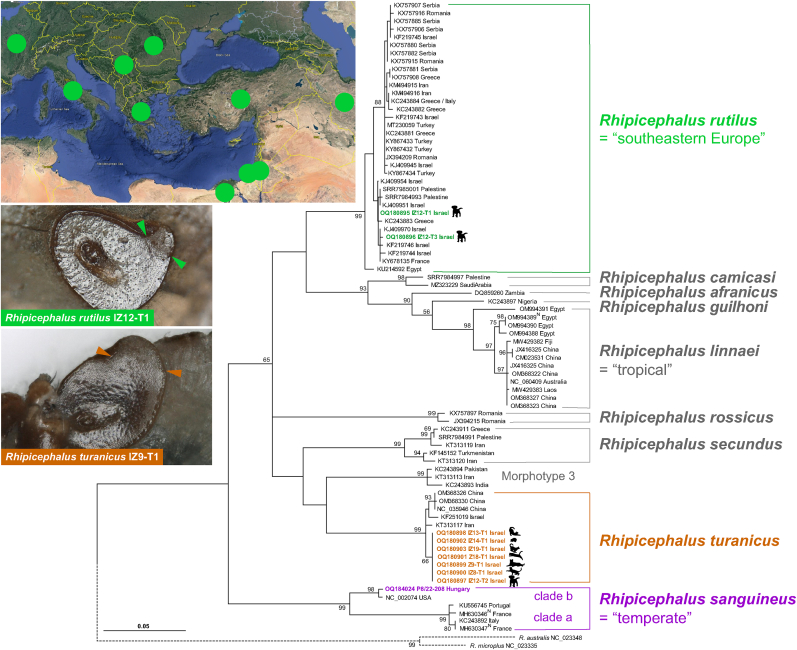
Morphological investigation revealed the presence of two female brown dog ticks, Rhipicephalus sanguineus (sensu lato) (IZ12-T1, IZ12-T3), whose spiracles were similar to those reported by Dantas-Torres et al. (2013) as “Rhipicephalus sp. Morphotype 1” with a narrow, short, but distinct dorsal projection (Fig. 1, Fig. 2). The remaining Rhipicephalus spp. ticks exhibited spiracles that had a broad dorsal projection and were identified as R. turanicus. The recently re-established species R. secundus Feldman-Muhsam, 1952 is morphologically similar to R. turanicus, and the scutal punctation was examined to differentiate the two species from each other (Fig. 3). The presence of numerous large punctations across the scutum suggested a R. turanicus identification (Fig. 3). The additional single tick was identified as Haemaphysalis adleri Feldman-Muhsam, 1951 (Table 1).
Fig. 1.
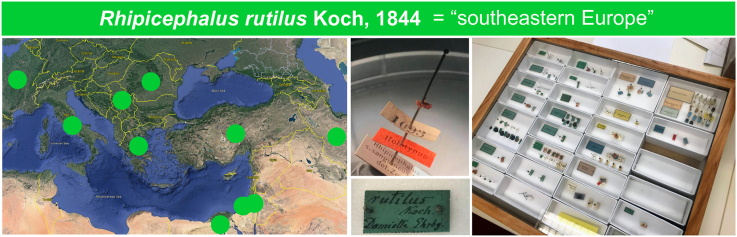
Rhipicephalus rutilus engorged adult female (IZ12-T1, ANIC 48 006 605) collected from a dog from Israel. A Basis capitulum with palps. B Scutum with eyes. C, D Spiracles. E Genital pore. Scale-bars: 100 μm (A, C, D, E); 300 μm (B).
Fig. 2.
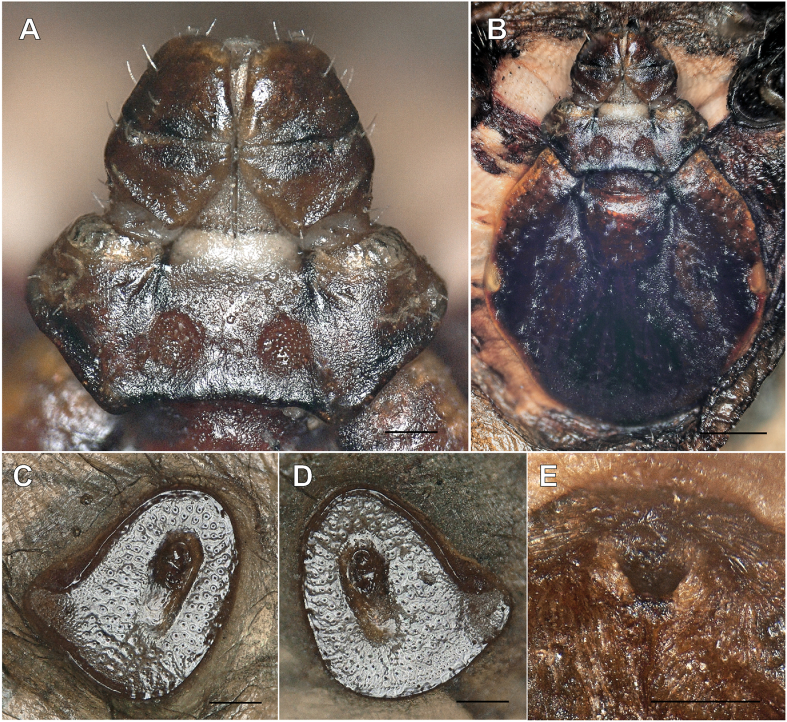
Rhipicephalus rutilus unengorged adult female (IZ12-T3, ANIC 48 006 607) collected from a dog from Israel. A Basis capitulum with palps. B Scutum with eyes. C, D Spiracles. E Genital pore (obscured by debris). Scale-bars: 100 μm (A, C, D, E); 500 μm (B).
Fig. 3.
Scutum of Rhipicephalus rutilus and Rhipicephalus turanicus from Israel. Note the incision to the scutum and alloscutum is an artefact, as it was part of the process to isolate DNA from these specimens. Scale-bars: 100 μm.
Table 1.
Summary of ticks and hosts from Jerusalem, Israel.
| ID# | ANIC number | Tick species | Tick stage and sex | Host, age | Date | Sequence data |
|---|---|---|---|---|---|---|
| IZ08-T1 | ANIC 48 006 603 | Rhipicephalus turanicus | Unengorged male | Cat, NA | April 3, 2017 | OQ180900 |
| IZ09-T1 | ANIC 48 006 604 | Rhipicephalus turanicus | Unengorged female | Cat, NA | April 4, 2017 | OQ180899 |
| IZ12-T1 | ANIC 48 006 605 | Rhipicephalus rutilus | Engorged female | Dog, 6 weeks | April 30, 2017 | OQ180895 |
| IZ12-T2 | ANIC 48 006 606 | Rhipicephalus turanicus | Unengorged male | OQ180897 | ||
| IZ12-T3 | ANIC 48 006 607 | Rhipicephalus rutilusa | Unengorged female | OQ180896, OQ184022 | ||
| IZ13-T1 | ANIC 48 006 608 | Rhipicephalus turanicusa | Unengorged female | Cat, 1 year | May 4, 2017 | OQ180898, OQ184023 |
| IZ14-T1 | ANIC 48 006 609 | Rhipicephalus turanicus | Unengorged male | Hedgehog, NA | April 30, 2017 | OQ180902 |
| IZ18-T1 | ANIC 48 006 610 | Rhipicephalus turanicus | Unengorged male | Cat, 1.5 years | May 10, 2017 | OQ180901 |
| IZ19-T1 | ANIC 48 006 611 | Rhipicephalus turanicus | Unengorged male | Cat, 7 month | May 11, 2017 | OQ180903 |
| IZ20-T1 | ANIC 48 006 612 | Haemaphysalis adleri | Engorged female | Cat, 1 year | May 11, 2017 | OQ180904 |
Notes: ANIC, Australian National Insect Collection; dog, Canis lupus familiaris; cat, Felis catus; hedgehog, Erinaceus concolor; NA, not known.
Mitogenome available.
The presence of a “Rhipicephalus sp. Morphotype 1” specimen prompted us to review its identity. “Rhipicephalus sp. Morphotype 1” was originally associated with partial mtDNA sequences from Italy and Greece, later identified as an “East Mediterranean” lineage by Hornok et al. (2017) from Romania, Greece, Serbia and Israel, and as the “southeastern Europe” lineage by Chitimia-Dobler et al. (2017) from Egypt (Nava et al., 2018; Bakkes et al., 2020; Šlapeta et al., 2021). This lineage from “southeastern Europe” forms a well-supported phylogenetic clade based on partial cox1 (Fig. 4). The general morphology, and specifically the shape of the spiracles (IZ12-T1, IZ12-T3), closely resemble the R. rutilus holotype (ZMB 1093) held at the Museum für Naturkunde Berlin, Germany (Fig. 5) (Koch, 1844, 1847). The holotype was collected in Damiette (= Damietta, Egypt) by Ehrenberg.
Fig. 4.
Phylogenetic analysis of Rhipicephalus rutilus based on cox1 sequences. The phylogenetic tree was reconstructed using Maximum Likelihood and GTR + G + I model with bootstrap support (> 50%) shown. The newly obtained sequences and the labels are colour-coded. Accession numbers accompanied by (N) indicate sequence from neotype reference material. The taxonomy is indicated on the right of the tree. The dotted line indicates the outgroups (R. australis, R. microplus). The inset map shows the countries (circles) where R. rutilus has been detected based on cox1 (map courtesy of Google Earth: SIO, NOAA, U.S. Navy, NSA, GEBCO. Image Landsat/Copernicus). Female spiracle of R. rutilus (IZ12-T1, ANIC 48 006 605) and R. turanicus (IZ09-T1, ANIC 48 006 604) shown with arrows indicating the start of the dorsal process.
Fig. 5.
Holotype female of Rhipicephalus rutilus as illustrated by Koch (1847). On the right are photographs of the holotype held at the Museum für Naturkunde Berlin, Germany (ZMB 1093) in dorsal and lateral view. A close up of the left spiracle is shown in the inset, note the short narrow process. The 1847 illustration is provided courtesy of the Biodiversity Heritage Library (biodiversitylibrary.org). The specimen label and view of the pinned voucher ZMB 1093 is shown as is at the Museum für Naturkunde Berlin, Germany in the lower part.
To verify our morphological investigation, we obtained ten cox1 partial sequences from ticks collected from Israel (Table 1). Phylogenetic analysis with related cox1 sequences from Rhipicephalus species and lineages confirmed the grouping of R. sanguineus (sensu lato) (IZ12-T1, IZ12-T3) sequences with the “southeastern Europe” lineage that we consider to represent R. rutilus (Fig. 4). The cox1 sequences from R. turanicus were grouped within the haplogroup of other samples of R. turanicus (Fig. 4). Using partial cox1, the R. rutilus clade was sister group to a strongly supported (93%) monophyletic clade consisting of cox1 sequences from R. linnaei, R. camicasi, R. guilhoni and R. afranicus, but this sister relationship was unsupported (< 50%). Similarly, the placement of the R. turanicus partial cox1 sequence clade was unsupported (< 50%) within other clades. To improve the phylogenetic resolution, we attempted to obtain complete mtDNA.
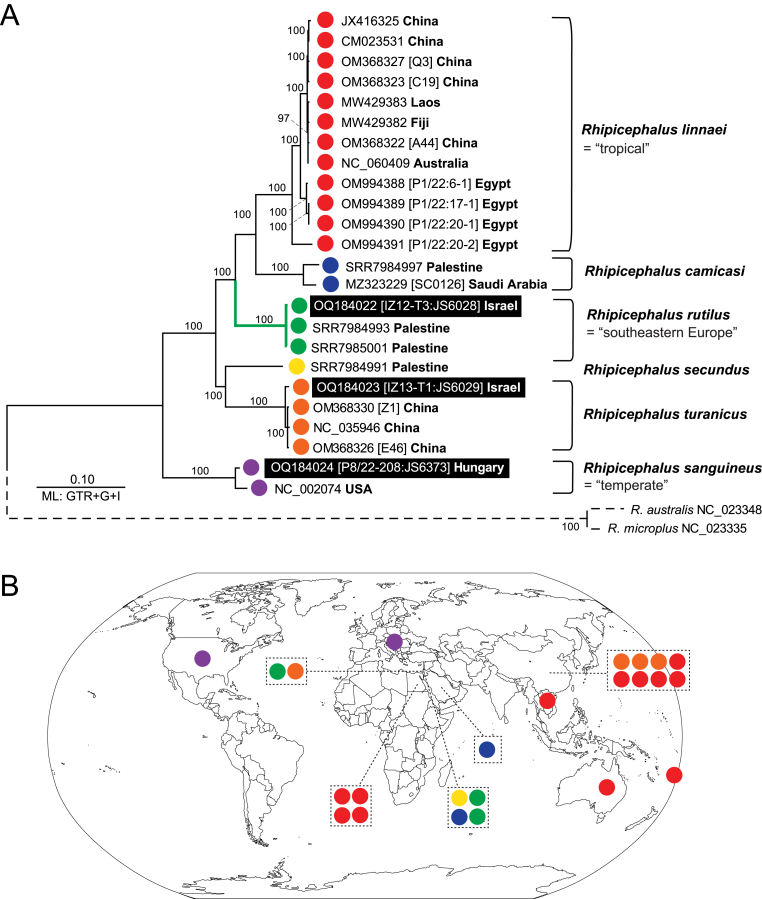
Mitogenomes were assembled from low pass whole genome sequencing (genome skimming) reads for R. rutilus (IZ12-T3: JS6028) and R. turanicus (IZ13-T1: JS6029) from Israel. To complement the work, we generated whole genome sequence approach and mitogenome assembly for R. sanguineus (sensu stricto) (P8/22-208: JS6373) from Hungary. Phylogenetic analysis using complete mitogenomes (mtDNA) yielded a robust and highly resolved tree with R. rutilus strongly supported (100%) as the sister clade to R. linnaei and R. camicasi (Fig. 6A). Similarly, R. turanicus was also strongly supported as a monophyletic clade (100%) that is sister (100% support) to the mitogenome of R. secundus (Fig. 6A). With these three newly obtained mitogenomes, there are currently 24 complete mitogenomes available for six species within the R. sanguineus (sensu lato) species complex, namely R. sanguineus, R. linnaei, R. rutilus, R. turanicus, R. secundus and R. camicasi (Fig. 6B).
Fig. 6.
Mitogenome phylogeny of Rhipicephalus rutilus with related species within the Rhipicephalus sanguineus (sensu lato) species complex. A The phylogenetic tree was reconstructed using Maximum Likelihood and GTR + G + I model with bootstrap support (> 50%) shown. The three newly obtained mitogenomes are on a black background. Tree taxonomy is indicated on the right of the tree and is colour-coded. The dotted line indicates the outgroup (R. australis, R. microplus). B World map with country locations for 24 mitogenomes currently available for Rhipicephalus sanguineus (sensu lato) species complex.
4. Discussion
This study took advantage of ectoparasites that were collected during a study that focused on fleas (Ctenocephaldes felis, Ctenocephalides canis) and their carriage of Bartonella and Rickettsia in Israel (Power et al., 2021). Out of the 33 animals (28 cats, 4 dogs and 1 hedgehog) there were six cats (21%), one dog (25%) and one hedgehog (100%) with ticks that remained unprocessed (Power et al., 2021). Initial morphological inspection of the tick specimens indicated that 11 belonged to the genus Rhipicephalus and one to the genus Haemaphysalis. The majority of the Rhipicephalus ticks exhibited features (i.e. shape of spiracles) that suggested R. turanicus, with several specimens from a dog suggesting R. sanguineus (sensu lato) (Table 1). The tick belonging to the genus Haemaphysalis was identified as H. adleri. While originally described from the golden jackal (Canis aureus) from Israel, the present engorged female was found on a cat and confirms previous findings (Feldman-Muhsam, 1951; Salant et al., 2014). In Israel, recent study into ticks and tick-borne pathogens has demonstrated the common occurrence of both R. sanguineus (sensu lato) and R. turanicus on dogs and cats (Salant et al., 2014; Mumcuoglu et al., 2022a). It was already believed that R. sanguineus (sensu lato) is the “Rhipicephalus sp. Morphotype 1” or “southeastern Europe” lineage because of the scarce sequence records available in public databases (Zemtsova et al., 2016; Hornok et al., 2017). This “southeastern Europe” lineage is now well recognised from neighbouring Egypt, including Lower Egypt and the Nile Delta (Abdullah et al., 2016; Chitimia-Dobler et al., 2017; Senbill et al., 2022).
Carl Ludwig Koch provided the following description of a specimen collected during the Hemprich-Ehrenberg expedition to Egypt: “R. rutilus. Flach, oval; Thorax sehr fein punktiert, roth; Hinderleib etwas dunkel menningroth. Beine rostroth. Länge 1 1/8''''. Weibchen. Männchen: unbekannt. Vaterland: Aegypten.” [R. rutilus. Flat, oval; Thorax very finely dotted, red; Abdomen somewhat dark, lead oxide red colour. Rusty legs. Length 1 1/8‴. Female. Male: unknown. Origin: Egypt] (Koch, 1844). The length is presented in the historic length unit Paris line (ligne, 1‴ = 2.2558 mm) implying that the given length was 2.54 mm. The ZMB 1093 label recorded reveals further details about the locality where the material was collected – Damiette (= Damietta, a port city in the Nile Delta in Lower Egypt) (Moritz and Fischer, 1981). Based on Bradley’s (1968) account of the expedition, it was probably collected in the winter or spring of 1823. The morphological identity of the species R. rutilus was doubtful and traditionally believed to represent R. sanguineus, so the name was treated as a junior synonym of the latter. Re-evaluation of the type-material of R. rutilus revealed the key characteristic that links it with the extant material, beyond the general description by Koch (1844) and illustration by Koch (1847). Specifically, the spiracle on female ZMB 1093 held at the Museum für Naturkunde Berlin, Germany (Fig. 5) has the shape and the distinct dorsal process matching the spiracles photographed in our modern material from dogs in Israel and in ticks from Italy and Greece as shown in figure 2f in Dantas-Torres et al. (2013) from dogs. The genital pore of the female holotype could not be observed due to the position of the mounting pin used to display the specimen, which unfortunately pierces the genital region. Older tick specimens in the Berlin Museum were often mounted pinned and dried like insects, and only later was it usual to put the whole (undamaged) specimen in alcohol.
One can consider morphological features of the males of R. rutilus. Using the molecular evidence, we can link the features illustrated in figure 1g-i in Dantas-Torres et al. (2013) and figure 7a,b in Hornok et al. (2017) as the current best morphological features of R. rutilus, in particular the long, extended dorsal process that tapers towards the narrow opening at the tip as the prominent feature.
Our understanding of the distribution of the “southeastern Europe” lineage and thus R. rutilus primarily comes from sequence records generated over the past 10 years. Ticks on Egyptian dogs have been characterised multiple times. Chitimia-Dobler et al. (2017) characterised ticks from dogs found in Luxor, Upper Egypt, and found both R. linnaei and R. rutilus. Šlapeta et al. (2022) found only R. linnaei and R. turanicus on dogs in Esna, a town about 60 km south of Luxor along the River Nile. In Lower Egypt, only R. rutilus genetic records exist from ticks on dogs from Cairo and Alexandria (Abdullah et al., 2016; Senbill et al., 2022). The “southeastern Europe” lineage itself was recognised in 2017 based on material from the Balkan region in Europe and the eastern Mediterranean including Türkiye and Israel (Chitimia-Dobler et al., 2017; Hornok et al., 2017). This cox1 haplogroup representing R. rutilus has been reported from Italy as the “Rhipicephalus sp. Morphotype 1” sensu Dantas-Torres et al. (2013) under the GenBank accession number KC243884. A more puzzling record that will require verification is a cox1 under the GenBank accession number KY678135 obtained from a specimen collected from a dog in France with no further history about the dog (Duron et al., 2017). There are also two cox1 records (GenBank: KM494915, KM494915) from ticks collected from dogs in Iran that have not yet been published. A recent study into Rhipicephalus spp. on sheep and goats from Iran recorded three distinct Rhipicephalus spp. haplogroups, besides R. bursa (Hosseini-Chegeni et al., 2019). The authors originally suggested the presence of R. sanguineus (sensu lato), R. sanguineus (sensu stricto), and R. turanicus based on cox1. However, our reanalysis investigating whether the R. sanguineus (sensu lato) haplogroup could be R. rutilus provides new evidence that these three Iranian haplogroups on livestock are R. turanicus, R. secundus and as yet unknown species that was previously referred to as “Rhipicephalus sp. Morphotype 3” sensu Dantas-Torres et al. (2013); with none of them being R. rutilus or R. sanguineus. It is possible that R. rutilus will prove to be restricted to dogs in Iran and hence absent from livestock (Hosseini-Chegeni et al., 2019). Dogs are the main host of R. rutilus. Taking available cox1 sequences as evidence of host association, R. rutilus was also found on a cat (KJ409951) and a hedgehog (KJ409970) from Israel.
Using the evidence documented above – the morphology, genetic identity and geographical distribution – we believe we are justified to link R. rutilus with what is recognised as the “southeastern Europe” lineage of R. sanguineus (sensu lato). To our knowledge there is no older available name for this species (Camicas et al., 1998; Guglielmone et al., 2015 and updates). Attempting to extract DNA non-destructively from a 200-year-old specimen is not impossible (Ivanova et al., 2006; Chandra et al., 2021), but in our opinion the risk of permanently damaging the R. rutilus holotype is too high and the existing collateral and morphological evidence presented here is sufficient.
To complement our work on mitogenomes, we have made available the first mitogenome of R. sanguineus from Europe. Phylogenetic analysis confirmed previously demonstrated divergence of two well-defined clades (a + b) of R. sanguineus (Hornok et al., 2017). The complete mitogenome from the Hungarian specimen is almost identical to the reference mitogenome of R. sanguineus from the USA, but partly distinct from the neotype material cox1 partial sequences (Nava et al., 2018). Hornok et al. (2017) have already reported that these two mtDNA lineages coexist in certain sampling sites such as Piacenza in Italy and Zagreb in Croatia.
The identity of brown dog ticks within the south-eastern Mediterranean regions or/and the Middle East is still far from completely understood. It seems that R. sanguineus (sensu stricto) is largely absent. Species such as R. linnaei, R. rutilus and R. camicasi need to be considered together with those related to R. turanicus and R. secundus (Chandra et al., 2022; Mumcuoglu et al., 2022b; Okely et al., 2022; Šlapeta et al., 2022). Morphological differentiation is feasible if large series of locally collected ticks are assembled and compared, but such differentiation is not without its limitations (e.g. Hornok et al., 2017). Establishing good reference material, both morphological and genetic, will be of particular value when comparing studies from different regions since these ticks are vectors of serious disease agents and the role of different species to be competent vectors is masked by the incomplete record of the species identity. Mitogenomes are easy to obtain and cost-effective, in fact if obtained via genome skimming, they enable screening for pathogen DNA as well (Ravi et al., 2019; Jia et al., 2020; Kneubehl et al., 2022; Šlapeta et al., 2022; Kelava et al., 2023). Adoption of the formal available name R. rutilus, together with a permanent genomic reference, eliminates the need for informal names such as “Morphotype 1” or “southeastern Europe” in future studies.
5. Conclusions
Based on the morphology, genetic identity, and geographical distribution of the species, we conclude that the name R. rutilus is correctly linked to the “southeastern Europe” lineage of R. sanguineus (sensu lato). We are justified to call material previously labelled as the “southeastern Europe” with a formal name R. rutilus for which, we provide molecular reference in form of complete mitogenome.
Funding
No specific funding to declare.
Ethical approval
Not applicable.
CRediT authorship contribution statement
Jan Šlapeta: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Bruce Halliday: Data curation, Investigation, Resources, Writing – review & editing. Jason A. Dunlop: Data curation, Investigation, Resources, Writing – review & editing. Yaarit Nachum-Biala: Investigation, Writing – review & editing. Harold Salant: Investigation, Writing – review & editing. Sajjad Ghodrati: Investigation, Data curation, Writing – review & editing. David Modrý: Resources, Supervision, Writing – review & editing. Shimon Harrus: Resources, Supervision, Writing – review & editing. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the facilities, scientific and technical assistance with Artemis HPC provided by the Sydney Informatics Hub, Core Research Facilities of the University of Sydney.
Data availability
The tick voucher specimens were deposited at the Australian National Insect Collection, CSIRO, Canberra, Australian Capital Territory, Australia, under the following accession numbers: R. turanicus (ANIC 48 006 603, ANIC 48 006 604, ANIC 48 006 606, ANIC 48 006 608 to ANIC 48 006 611); R. rutilus (ANIC 48 006 605, ANIC 48 006 607); H. adleri (ANIC 48 006 612); and R. sanguineus (ANIC 48 006 613). Raw FastQ sequence data were deposited at SRA NCBI BioProject: PRJNA917775. The nucleotide sequence data including three assembled mitogenomes generated in this study were deposited in GenBank (NCBI): OQ184022-OQ184024 (complete mtDNA) and OQ180895-OQ180904 (cox1). All sequence data, photographic documentation and associated supplementary material and additional data are available at LabArchives (https://dx.doi.org/10.25833/qt1c-z916).
References
- Abdullah H.H., El-Molla A., Salib F.A., Allam N.A., Ghazy A.A., Abdel-Shafy S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of rickettsioses in Egypt. Vet. World. 2016;9:1087–1101. doi: 10.14202/vetworld.2016.1087-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouin V. Histoire naturelle; 1826. Explication sommaire des planches dʼarachnides de lʼÉgypte et de la Syrie. Description de lʼÉgypte ou recueil des observations et des recherches qui ont été faites en Égypte pendant lʼexpédition de lʼarmée française (Band 5,1,1: Texte 1): Histoire naturelle; p. 186. (Band 5,2,1: Planches 1) Pl. 9. [Google Scholar]
- Aziz S., Shah S.F., Amin F., Khan M.A., Ahmad M. Taxonomic study of arthropod pests of livestock in district Peshawar, Khyber Pakhtunkhwa. Pak. J. Life Soc. Sci. 2018;16:85–96. [Google Scholar]
- Bakkes D.K., Chitimia-Dobler L., Matloa D., Oosthuysen M., Mumcuoglu K.Y., Mans B.J., Matthee C.A. Integrative taxonomy and species delimitation of Rhipicephalus turanicus (Acari: Ixodida: Ixodidae) Int. J. Parasitol. 2020;50:577–594. doi: 10.1016/j.ijpara.2020.04.005. [DOI] [PubMed] [Google Scholar]
- Bradley J.C. The Hemprich-Ehrenberg expedition to Egypt and Asia Minor of 1820–25. Deutsche Entomol. Z. 1968;15:107–109. [Google Scholar]
- Camicas J.-L., Hervy J.-P., Adam F., Morel P.C. Répartition. Orstom; Paris: 1998. Les Tiques du Monde (Acarida, Ixodida). Nomenclature, Stades Décrits, Hôtes. [Google Scholar]
- Chandra S., Smith K., Alanazi A.D., Alyousif M.S., Emery D., Šlapeta J. Rhipicephalus sanguineus sensu lato from dogs and dromedary camels in Riyadh, Saudi Arabia: Low prevalence of vector-borne pathogens in dogs detected using multiplexed tandem PCR panel. Folia Parasitol. (Praha) 2019;66:7. doi: 10.14411/fp.2019.007. [DOI] [PubMed] [Google Scholar]
- Chandra S., Halliday B., Šlapeta J. Museum material of Rhipicephalus sanguineus sensu Roberts (1965) collected in 1902–1964 from Australia is identical to R. sanguineus sensu lato tropical lineage at the mitochondrial DNA 12S rRNA level. Med. Vet. Entomol. 2021;35:315–323. doi: 10.1111/mve.12495. [DOI] [PubMed] [Google Scholar]
- Chandra S., Alanazi A.D., Šlapeta J. Mitochondrial genome of Rhipicephalus cf. camicasi Morel, Mouchet & Rodhain, 1976 from a camel (Camelus dromedarius Linnaeus) in Riyadh, Saudi Arabia. Folia Parasitol. (Praha) 2022;69 doi: 10.14411/fp.2022.005. [DOI] [PubMed] [Google Scholar]
- Chitimia-Dobler L., Langguth J., Pfeffer M., Kattner S., Kupper T., Friese D., et al. Genetic analysis of Rhipicephalus sanguineus sensu lato ticks parasites of dogs in Africa north of the Sahara based on mitochondrial DNA sequences. Vet. Parasitol. 2017;239:1–6. doi: 10.1016/j.vetpar.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Latrofa M.S., Annoscia G., Giannelli A., Parisi A., Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O., Binetruy F., Noel V., Cremaschi J., McCoy K.D., Arnathau C., et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017;26:2905–2921. doi: 10.1111/mec.14094. [DOI] [PubMed] [Google Scholar]
- Feldman-Muhsam B. A note on East Mediterranean species of the genus Haemaphysalis. Bull. Res. Counc. Isr. 1951;1:96–107. [Google Scholar]
- Floch H., Fauran P. Sur les Ixodides, autres que ceux du genre Amblyomma en Guyane et aux Antilles Françaises. Acarologia. 1959;1:393–407. [Google Scholar]
- Guglielmone A.A., Nava S. Names for Ixodidae (Acari: Ixodoidea): Valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names – with notes on confusions and misidentifications. Zootaxa. 2014;3767:1–256. doi: 10.11646/zootaxa.3767.1.1. [DOI] [PubMed] [Google Scholar]
- Guglielmone, A.A., Sánchez, M.E., Franco, L.G., Nava, S., Rueda, L.M., Robbins, R.G., 2015. Hard ticks (Acari: Ixodida: Ixodidae): a non-profit open-access web portal for original descriptions of tick species (valid and invalid), dubious and uncertain names, and selected nomina nuda and updates. Instituto Nacional de Tecnologia Agropecuaria, Argentina. http://rafaela.inta.gob.ar/nombresgarrapatas/. (Accessed 3 January 2022).
- Hahn C., Bachmann L., Chevreux B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads - a baiting and iterative mapping approach. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemprich F.W., Ehrenberg C.G. Ernst Siegfried Mittler; Berlin, Posen und Bromberg: 1828. Naturgeschichfliche Reisen durch Nord-Afrika und West-Asien in den Jahren 1820 bis 1825 von Dr. F.W. Hemprich und Dr. C.G. Ehrenberg. Erster Band, Erste Abteilung. Historischer Teil. [Google Scholar]
- Hornok S., Sándor A.D., Tomanović S., Beck R., DʼAmico G., Kontschán J., et al. East and west separation of Rhipicephalus sanguineus mitochondrial lineages in the Mediterranean Basin. Parasites Vectors. 2017;10:39. doi: 10.1186/s13071-017-1985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Chegeni A., Nasrabadi M., Sadat Hashemi-Aghdam S., Oshaghi M.A., Lotfi A., Telmadarraiy Z., Sedaghat M.M. Molecular identification of Rhipicephalus species (Acari: Ixodidae) parasitizing livestock from Iran. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2019;30:448–456. doi: 10.1080/24701394.2018.1546298. [DOI] [PubMed] [Google Scholar]
- Ivanova N.V., DeWaard J.R., Hebert P.D.N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes. 2006;6:998–1002. [Google Scholar]
- Jia N., Wang J., Shi W., Du L., Sun Y., Zhan W., et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell. 2020;182:1328–1340.e13. doi: 10.1016/j.cell.2020.07.023. [DOI] [PubMed] [Google Scholar]
- Kelava S., Mans B.J., Shao R., Barker D., Teo E.J.M., Chatanga E., et al. Seventy-eight entire mitochondrial genomes and nuclear rRNA genes provide insight into the phylogeny of the hard ticks, particularly the Haemaphysalis species, Africaniella transversale and Robertsicus elaphensis. Ticks Tick Borne Dis. 2023;14 doi: 10.1016/j.ttbdis.2022.102070. [DOI] [PubMed] [Google Scholar]
- Keskin A. MSc Thesis, Institute of Sciences, Gaziosmanpaşa University; Turkey: 2009. Amasya Ilinde Bulunan Sert Kenelerin (Acari: Ixodidae) Faunistik Açidan Incelenmesi. [Google Scholar]
- Kneubehl A.R., Munoz-Leal S., Filatov S., de Klerk D.G., Pienaar R., Lohmeyer K.H., et al. Amplification and sequencing of entire tick mitochondrial genomes for a phylogenomic analysis. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-23393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C.L. Systematische Übersicht über die Ordnung der Zecken. Arch. Naturges. 1844;10:217–239. [Google Scholar]
- Koch C.L. C.H. Zeh [etc.]; Nürnberg: 1847. Übersicht des Arachnidensystems. [Google Scholar]
- Morel P.C., Vassiliades G. Les Rhipicephalus du groupe sanguineus: espèces Africaines (Acariens: Ixodoidea) Rev. Elev. Med. Vet. Pays Trop. 1962;15:343–386. [Google Scholar]
- Moritz M., Fischer S.-C. Die typen der arachniden-sammlung des zoologischen Museums Berlin. IV. Ixodei. Mitt. Zool. Mus. Berlin. 1981;57:341–364. https://onlinelibrary.wiley.com/doi/10.1002/mmnz.19810570205 [Google Scholar]
- Mumcuoglu K.Y., Arslan-Akveran G., Aydogdu S., Karasartova D., Kosar A., Savci U., et al. Pathogens in ticks collected in Israel: II. Bacteria and protozoa found in Rhipicephalus sanguineus sensu lato and Rhipicephalus turanicus. Ticks Tick Borne Dis. 2022;13 doi: 10.1016/j.ttbdis.2022.101986. [DOI] [PubMed] [Google Scholar]
- Mumcuoglu K.Y., Estrada-Peña A., Tarragona E.L., Sebastian P.S., Guglielmone A.A., Nava S. Reestablishment of Rhipicephalus secundus Feldman-Muhsam, 1952 (Acari: Ixodidae) Ticks Tick Borne Dis. 2022;13 doi: 10.1016/j.ttbdis.2022.101897. [DOI] [PubMed] [Google Scholar]
- Nava S., Beati L., Venzal J.M., Labruna M.B., Szabó M.P.J., Petney T., et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis. 2018;9:1573–1585. doi: 10.1016/j.ttbdis.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Neumann G. Revision de la Famille des Ixodidès. Mem. Soc. Zool. Fr. 1897;10:324–422. [Google Scholar]
- Neumann, L.G., 1911. Acarina. Ixodidae. Das Tierreich. 26 . pp. 1-169, xii–xvi.
- Okely M., Chen Z., Anan R., Gad-Allah S. Updated checklist of the hard ticks (Acari: Ixodidae) of Egypt, with notes of livestock host and tick-borne pathogens. Syst. Appl. Acarol. 2022;27:811–838. [Google Scholar]
- Pegram R.G., Clifford C.M., Walker J.B., Keirans J.E. Clarification of the Rhipicephalus sanguineus group (Acari, Ixodoidea, Ixodidae). I. R. sulcatus Neumann, 1908 and R. turanicus Pomerantsev, 1936. Syst. Parasitol. 1987;10:3–26. [Google Scholar]
- Power R.I., Calvani N.E.D., Nachum-Biala Y., Salant H., Harrus S., Šlapeta J. Adaptation of gltA and ssrA assays for diversity profiling by Illumina sequencing to identify Bartonella henselae, B. clarridgeiae and B. koehlerae. J. Med. Microbiol. 2021;70 doi: 10.1099/jmm.0.001400. [DOI] [PubMed] [Google Scholar]
- Ramzan M., Naeem-Ullah U., Bokhari S.H.M., Saba S., Khan K.A., Saeed S. Checklist of the tick (Acari: Argasidae, Ixodidae) species of Pakistan. Vet. Ital. 2020;56:221–236. doi: 10.12834/VetIt.1721.9077.1. [DOI] [PubMed] [Google Scholar]
- Ravi A., Ereqat S., Al-Jawabreh A., Abdeen Z., Abu Shamma O., Hall H., et al. Metagenomic profiling of ticks: Identification of novel rickettsial genomes and detection of tick-borne canine parvovirus. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant H., Mumcuoglu K.Y., Baneth G. Ectoparasites in urban stray cats in Jerusalem, Israel: Differences in infestation patterns of fleas, ticks and permanent ectoparasites. Med. Vet. Entomol. 2014;28:314–318. doi: 10.1111/mve.12032. [DOI] [PubMed] [Google Scholar]
- Senbill H., Tanaka T., Karawia D., Rahman S., Zeb J., Sparagano O., Baruah A. Morphological identification and molecular characterization of economically important ticks (Acari: Ixodidae) from North and North-Western Egypt. Acta Trop. 2022;231 doi: 10.1016/j.actatropica.2022.106438. [DOI] [PubMed] [Google Scholar]
- Šlapeta J., Chandra S., Halliday B. The “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826) Int. J. Parasitol. 2021;51:431–436. doi: 10.1016/j.ijpara.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Šlapeta J., Halliday B., Chandra S., Alanazi A.D., Abdel-Shafy S. Rhipicephalus linnaei (Audouin, 1826) recognised as the “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato: Neotype designation, redescription, and establishment of morphological and molecular reference. Ticks Tick Borne Dis. 2022;13 doi: 10.1016/j.ttbdis.2022.102024. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker N.S.G. University of Florida; 2017. Permethrin-etofenprox cross-resistance status in the brown dog tick, Rhipicephalus sanguineus sensu lato (Latreille) and prevalence of pathogens in peridomestic populations. MSc Thesis. [Google Scholar]
- Walker J., Keirans J., Horak I. Cambridge University Press; Cambridge: 2000. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. [Google Scholar]
- Zemtsova G.E., Apanaskevich D.A., Reeves W.K., Hahn M., Snellgrove A., Levin M.L. Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exp. Appl. Acarol. 2016;69:191–203. doi: 10.1007/s10493-016-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumpt F. Preliminary study to a revision of the genus Rhipicephalus Koch. Moçambique. 1950;60:57–169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The tick voucher specimens were deposited at the Australian National Insect Collection, CSIRO, Canberra, Australian Capital Territory, Australia, under the following accession numbers: R. turanicus (ANIC 48 006 603, ANIC 48 006 604, ANIC 48 006 606, ANIC 48 006 608 to ANIC 48 006 611); R. rutilus (ANIC 48 006 605, ANIC 48 006 607); H. adleri (ANIC 48 006 612); and R. sanguineus (ANIC 48 006 613). Raw FastQ sequence data were deposited at SRA NCBI BioProject: PRJNA917775. The nucleotide sequence data including three assembled mitogenomes generated in this study were deposited in GenBank (NCBI): OQ184022-OQ184024 (complete mtDNA) and OQ180895-OQ180904 (cox1). All sequence data, photographic documentation and associated supplementary material and additional data are available at LabArchives (https://dx.doi.org/10.25833/qt1c-z916).