Abstract
Background
Cor triatriatum sinistra (CTS) is a rare condition where the left atrium (LA) is divided by a thin membrane into an upper and lower chamber. Incidentally, the diagnosis is made in late adulthood, usually because of a favourable variant such as in our patient who presented with partial CTS.
Case summary
We present the case of a 62-year-old female who presented with COVID-19. She was known for longstanding symptoms of dyspnoea on exertion as well as a minor stroke several years ago. Computed tomography on admission suggested there was a mass in the LA but transthoracic echocardiography and cardiac magnetic resonance imaging revealed the diagnosis of partial CTS in which the superior compartment received pulmonary venous drainage from the right lung and the left-sided pulmonary veins drained into the inferior chamber. Since there were signs of chronic pulmonary oedema she successfully underwent balloon dilatation of the membrane resulting in remission of symptoms and normalization of the pressure in the accessory chamber.
Discussion
Partial CTS is a rare variant of CTS. Since part of the pulmonary veins drains in the lower chamber of the LA (and thereby unload the right ventricle), it is a favourable variant and patients may present later in life when membrane orifices calcify or it may be discovered as an incidental finding. In some patients requiring intervention, balloon dilatation of the membrane may be considered as an alternative to surgical removal of the membrane by thoracotomy.
Keywords: Case report, Cor triatriatum sinistra, Partial, COVID, CMR, Adult congenital heart disease
Learning points.
Partial cor triatriatum is a rare variant of cor triatriatum sinistra (CTS) in which part of the pulmonary veins drain normally and unload the right ventricle.
In this variant of CTS, patients may be free of symptoms until late adulthood.
If symptoms are severe, surgical removal of the atrial membrane (or in selected cases catheter-based intervention) is usually favourable.
Introduction
Cor triatriatum sinistra (CTS) is a rare cardiac anomaly with an estimated prevalence of 0.1–0.4% of patients with congenital cardiac disease.1 In the classic variant, the left atrium (LA) is bisected by a fibromuscular membrane into a proximal compartment receiving all the pulmonary venous return and a distal part including the atrial appendage and the mitral valve vestibule or the ‘true’ atrium.2 It commonly presents in infancy or early childhood with a clinical picture mimicking mitral stenosis. The degree of pulmonary venous congestion and hypertension depends on the degree of left atrial obstruction. Most severe cases may result in mortality during infancy. Some patients, however, may remain asymptomatic until adolescence or even late adulthood because of an incomplete membrane or other favourable conditions. Cor triatriatum sinistra is frequently associated with congenital abnormalities including atrial septal defects (ASDs) and unroofed coronary sinus3,4 which both allow decompression to the right atrium.
Timeline
| 10 years prior to presentation | Longstanding symptoms of dyspnoea on exertion NYHA 2, orthopnoea, as well as palpitations |
| 2 years prior to presentation | Ischaemic stroke |
| At presentation | Unclear diagnosis, possible tumour or thrombus in the left atrium based on computed tomography. Initiation of therapeutic anticoagulation |
| 22 days after presentation | Cardiac magnetic resonance imaging confirms the diagnosis of partial cor triatriatum sinistra. Anticoagulation is terminated |
| 11 months after presentation | A successful balloon dilatation is performed. Follow-up transthoracic ultrasound demonstrated a reduction of the mean pressure in the accessory chamber from 17 to 10 mmHg with laminar flow across the membrane |
| 13 months after presentation | At the outpatient clinic, she has no symptoms anymore |
Case summary
We present a 62-year-old female known with an ischaemic stroke 2 years prior to her presentation. She had symptoms of dyspnoea on exertion (New York Heart Association functional classification; NYHA 2) and orthopnoea for more than 10 years as well as episodes of palpitations. A 2-day outpatient cardiac monitor did not show evidence of atrial fibrillation at the time of her cryptogenic stroke. She was admitted with COVID-19 pneumonia. On physical examination, she was afebrile with a normal breathing rate. The pulse rate was 61 b.p.m., blood pressure 95/63 mmHg, and oxygen saturation 95% under 6 L/min oxygen. Physical examination revealed normal heart sounds without murmurs. Bilateral lung crackles were heard. Computed tomography (CT) (non-triggered, slice thickness 0.625 mm) at presentation (see Supplementary material online, Figure S1) showed pulmonary consolidations mainly in the left lower lobe. The right lung was less affected by COVID-associated consolidations but demonstrated interlobular septal thickening—suggestive of pulmonary oedema—which were mostly absent in the left lung. In addition, CT indicated the presence of a left atrial mass (48 × 46 × 42 mm) with a small dense configuration in the periphery of the lesion suggestive of calcification (Figure 1). Based on the CT, the lesion was highly suspicious for thrombus or tumour (myxoma, lymphoma, or metastasis). Therapeutic nadroparin was initiated and transthoracic echocardiography (TTE) was planned, identifying the abnormality as a partly anechoic, partly hypoechoic structure in the roof of the LA next to the interatrial septum. Although there was no colour Doppler flow in the anomaly, a small turbulent jet emerged from the lesion (Figure 2A and B, Supplementary material online, Video S1A). Contrast-enhanced echocardiography using Luminity contrast agent (Lantheus Medical Imaging) showed enhancement of the lesion. The combination of findings practically ruled out a solid mass and suggested the diagnosis of CTS. To confirm the diagnosis and to rule out thrombosis in the LA (since contrast echocardiography was of insufficient quality), cardiac magnetic resonance (CMR) imaging was performed 22 days later. Cardiac magnetic resonance showed a normal-sized right ventricle with preserved ejection fraction. In the LA, a linear density was seen subdividing the LA into superior and inferior compartments both with the signal intensity of blood (Figure 3A, Supplementary material online, Video S2A). The superior compartment received pulmonary venous drainage from the right lung and the left-sided pulmonary veins drained into the inferior chamber matching with partial CTS. On CMR multiple fenestrations were seen in the membrane. No thrombus (or tumour) was detected in the atrial compartments. Cardiac magnetic resonance perfusion images demonstrated clearly delayed perfusion of the right lung and the accessory left atrial chamber (Figure 4A, Supplementary material online, Video S3A). Thereupon anticoagulation was terminated as it was determined that the appearance of mass on CT and echocardiography was caused by a slow flow phenomenon and not by thrombus. After she recovered from her COVID-19 pneumonia, she was discharged from the hospital. At the outpatient clinic, TTE was repeated which did not demonstrate elevated pulmonary artery pressures during rest and exercise but at invasive measurements mild pulmonary arterial hypertension was diagnosed with elevated pulmonary artery pressure (PAP) in the right pulmonary artery (31/23/26 mmHg; systolic; diastolic; mean) and slightly elevated PAP in the left pulmonary artery (33/17/24 mmHg). Transoesophageal echocardiography clearly demonstrated the fibromuscular membrane with a mean trans-membrane gradient of 11 mmHg increasing to 16 mmHg with passive leg raise (Figure 5A, Supplementary material online, Video S4A and B). The patient was discussed with the heart team. Since there is experience in our hospital with transcatheter balloon dilatation of CTS and she was deemed an eligible candidate, she underwent percutaneous dilatation of the atrial membrane to relieve the obstruction. After dilatation, the mean gradient in the accessory chamber was reduced from 17 to 10 mmHg resulting in non-turbulent flow between the proximal and distal LA compartments. Two months later she was free of symptoms without any complaints of dyspnoea. On TTE laminar flow was observed between the left atrial compartments (Figure 6A, Supplementary material online, Video S5A) with a maximum gradient of 2 mmHg.
Figure 1.
Chest computed tomography revealing the ‘mass’ in the left atrium. The arrow points to a small jet of negative contrast. The bright spot in the membrane is highly suggestive of calcification.
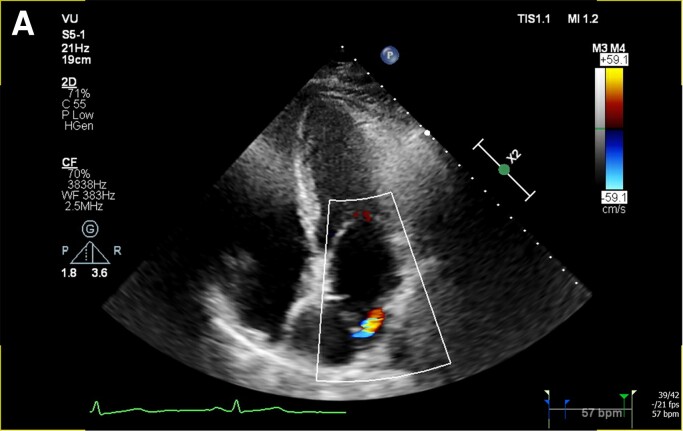
Figure 2.
Transthoracic echocardiography image (A) and video (see Supplementary material online, Video S2A) of the fibromuscular membrane in the apical four chamber with turbulent Doppler flow coming from the interatrial membrane. Video of the CTS in the parasternal long axis (see Supplementary material online, Video S2B).
Figure 3.
Cardiac magnetic resonance image (A) and video (see Supplementary material online, Video S2A) of the cor triatriatum. Both left atrial compartments have the same signal intensity (of blood), ruling out thrombus. There is flow visible between the accessory chamber and the true left atrium.
Figure 4.
Cardiac magnetic resonance perfusion image (A) and video (see Supplementary material online, Video S3A) showing the passage of contrast through the cardiac compartments. The perfusion to the accessory chamber is evidently delayed compared with the true left atrium.
Figure 5.
Transoesophageal echocardiography images showing the intra-atrial membrane (see Supplementary material online, Video S4A) with turbulent flow between the atrial chambers (Supplementary material online, Video S4B) with a maximum gradient of 16 mmHg (A).
Figure 6.
Transthoracic echocardiography image (A) and video (see Supplementary material online, Video S5A) of the cor triatrium sinistra after successful balloon dilatation demonstrating non-turbulent, laminar flow between the compartments.
Discussion
We present a case of partial CTS which is an extremely uncommon congenital defect only published a few times before.5–7 In this variant, one or more pulmonary veins connect to the true LA, thereby significantly reducing the transpulmonary pressure gradient suspending the onset of symptoms. We hypothesize that progressive calcification of the membrane orifice(s) and concomitant chronic right-sided pulmonary congestion provoked symptoms of dyspnoea in our patient. At first sight, CTS may be confused with dilated coronary sinus/pulmonary veins on echocardiography or with malignancy or thrombosis. However, by carefully scanning the lesion for colour Doppler jets or by administration of an ultrasound contrast-enhancing agent, the diagnosis can be established by echocardiography alone. Transoesophageal echocardiography is superior to TTE due to enhanced visualization of the LA and its membrane. Computed tomography (with adequate electrocardiogram triggering) usually is diagnostic for CTS but cases of partial CTS—as illustrated in our patient—may be overlooked due to delayed passage of venous contrast into the accessory chamber giving it the appearance of a solid mass. In retrospect, we noticed a small jet of negative contrast on CT images (Figure 1) caused by mixing of native blood from the accessory chamber into the contrast-enhanced blood of the true LA. Although only a few cases have been presented using CMR imaging in CTS,8,9 it is very well suited to establish the diagnosis. Cardiac magnetic resonance is also considered the gold standard for the quantification of chamber volumes and function, which can be used to evaluate whether there is right ventricular overload. Finally, it can establish whether thrombosis is present in the accessory chamber (due to slow flow) or exclude malignancy. Cardioembolic stroke is a regular finding in CTS and potential mechanisms of thrombus formation include increased prevalence of atrial fibrillation, stagnation of blood flow in the accessory compartment, and paradoxical embolization due to associated ASD.10 In our patient, anticoagulation was terminated when CMR (and later transoesophageal echocardiography) did not detect intracardiac thrombus. However, since our patient had a prior ischaemic stroke and once experienced palpitations, we think her anticoagulation should not have been terminated as it was very likely her stroke was of cardioembolic origin due to either atrial fibrillation or stagnation of blood in the accessory chamber. There are currently no guidelines on how to manage the risk of thromboembolism in CTS but we think there should be a very low threshold to initiate anticoagulation in case an embolic event occurs in a patient with CTS even in the absence of documented atrial fibrillation or visualized atrial thrombus. The treatment of CTS depends on the level of symptoms. An incidental finding of an atrial membrane without a pressure gradient requires no treatment. Surgical intervention involving the removal of the atrial membrane may be needed in patients with severe obstruction, and it has been shown to provide satisfactory early and long-term survival with a low probability of re-intervention.11 Alternatively, in selected cases, catheter-based intervention may be feasible but long-term follow-up results are unknown, and in subjects with atrial membrane calcification, dilatation of the orifice might not be successful.12
Conclusion
We have described an exceptionally rare case of partial CTS presenting later in life. Our patient successfully underwent balloon dilatation of the membrane after which she was free of symptoms.
Supplementary Material
Contributor Information
Bob von Bartheld, Department of Cardiology, St Jansdal Hospital, Wethouder Jansenlaan 90, P.O. Box 138, Harderwijk 3840 AC, The Netherlands.
Mischa Rijnierse, Department of Cardiology, Amsterdam University Medical Center, PO Box 7057, Amsterdam, 1081 HV, The Netherlands.
Ramon van Loon, Department of Cardiology, Amsterdam University Medical Center, PO Box 7057, Amsterdam, 1081 HV, The Netherlands.
Lilian Meijboom, Department of Radiology and Nuclear Medicine, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Vrije Universiteit Amsterdam, PO Box 7057, Amsterdam, 1081 HV, The Netherlands.
Lead author biography
 Dr. Bob von Bartheld works as a cardiologist at St. Jansdal Hospital in Harderwijk, the Netherlands. He obtained a PhD in 2016 and is working as an imaging cardiologist with degrees in echocardiography, cardiac computed tomography and cardiac MRI.
Dr. Bob von Bartheld works as a cardiologist at St. Jansdal Hospital in Harderwijk, the Netherlands. He obtained a PhD in 2016 and is working as an imaging cardiologist with degrees in echocardiography, cardiac computed tomography and cardiac MRI.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
References
- 1. Jegier W, Gibbons JE, Wiglesworth F. Cor triatriatum: clinical, hemodynamic and pathological studies: surgical correction in early life. Pediatrics 1963;31:255–267. [PubMed] [Google Scholar]
- 2. Sankhyan LK, Anderson RH, Chowdhury UK, George N, Vaswani P, Pandey NN, et al. Surgical management of divided atrial chambers. J Card Surg 2021;36:4267–4279. [DOI] [PubMed] [Google Scholar]
- 3. Chen Q, Guhathkurta S, Vadalapali G, Nalladaru Z, Easthope RN, Sharma AK. Cor triatriatum in adults: three new cases and a brief review. Tex Heart Inst J 1999;26:206–210. [PMC free article] [PubMed] [Google Scholar]
- 4. Rudienė V, Hortshoj CMS, Glavckaite S, Zakarkaite D, Petrulionienie Z, Gumbiene L, et al. Cor triatriatum sinistrum diagnosed in the adulthood: a systematic review. Heart 2019;105:1197–1202. [DOI] [PubMed] [Google Scholar]
- 5. Buchholz S, Jenni R. Doppler echocardiographic findings in 2 identical variants of a rare cardiac anomaly, ‘subtotal’ cor triatriatum: a critical review of the literature. J Am Soc Echocardiogr 2001;14:846–849. [DOI] [PubMed] [Google Scholar]
- 6. Kariyanna PT, Warrier N, Hegde S, Kats Y. A rare case of subtotal cor triatriatum with anomalous pulmonary venous return in an adult. Research 2016;3:1518. [Google Scholar]
- 7. Sharma A, Biradar B, Malhi AS, Kumar S. Subtotal cor triatriatum on dual-source computed tomography. Ann Thorac Surg 2019;107:e213. [DOI] [PubMed] [Google Scholar]
- 8. Elagha AA, Fuisz AR, Weissman G. Cardiac magnetic resonance imaging can clearly depict the morphology and determine the significance of cor triatriatum. Circulation 2012;126:1511–1513. [DOI] [PubMed] [Google Scholar]
- 9. Minoiu C, Meduri A, Merlino B, Savino G, Marano R, Bonomo L. Cor triatriatum: the role of cardiac-MR in establishing a correct diagnosis. In: European Congress of Radiology. 2015.
- 10. Amara RS, Lalla R, Jeudy J, Nam Hong S. Cardioembolic stroke in a young male with cor triatriatum sinister: a case report. Eur Heart J Case Rep 2020;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saxena P, Burkhart HM, Schaff HV, Daly R, Joyce LD, Dearani PS. Surgical repair of cor triatriatum sinister: the Mayo Clinic 50-year experience. Ann Thorac Surg 2014;97:1659–1663. [DOI] [PubMed] [Google Scholar]
- 12. Li WW, Koolbergen DR, Bouma BJ, Hazekamp MG, de Mol BA, de Winter RJ. Cathether-based interventional strategies for cor triatriatum in the adult–feasibility study through a hybrid approach. BMC Cardiovasc Disord 2015;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.