Thromboembolism has been noted to be an important complication in hospitalized patients with COVID-19 from the beginning of the pandemic. A National Institutes of Health–sponsored, randomized, placebo-controlled trial was done to determine if extended thromboprophylaxis after hospital discharge would reduce deaths and thromboembolic events. The trial was done during 2021 to 2022, spanning the period when the Omicron and Delta variants were circulating and when there was a decreasing rate of hospitalization with COVID-19.
Visual Abstract. Thromboprophylaxis After COVID-19 Hospitalization.
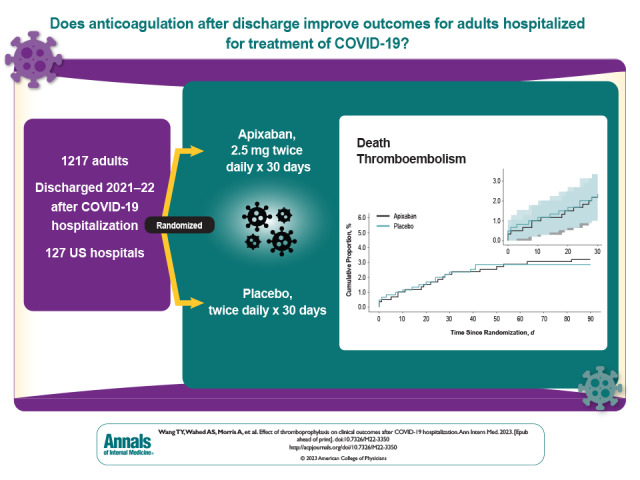
Thromboembolism has been noted to be an important complication in hospitalized patients with COVID-19 from the beginning of the pandemic. A National Institutes of Health–sponsored, randomized, placebo-controlled trial was done to determine if extended thromboprophylaxis after hospital discharge would reduce deaths and thromboembolic events. The trial was done during 2021 to 2022, spanning the period when the Omicron and Delta variants were circulating and when there was a decreasing rate of hospitalization with COVID-19.
Abstract
Background:
Patients hospitalized with COVID-19 have an increased incidence of thromboembolism. The role of extended thromboprophylaxis after hospital discharge is unclear.
Objective:
To determine whether anticoagulation is superior to placebo in reducing death and thromboembolic complications among patients discharged after COVID-19 hospitalization.
Design:
Prospective, randomized, double-blind, placebo-controlled clinical trial. (ClinicalTrials.gov: NCT04650087)
Setting:
Done during 2021 to 2022 among 127 U.S. hospitals.
Participants:
Adults aged 18 years or older hospitalized with COVID-19 for 48 hours or more and ready for discharge, excluding those with a requirement for, or contraindication to, anticoagulation.
Intervention:
2.5 mg of apixaban versus placebo twice daily for 30 days.
Measurements:
The primary efficacy end point was a 30-day composite of death, arterial thromboembolism, and venous thromboembolism. The primary safety end points were 30-day major bleeding and clinically relevant nonmajor bleeding.
Results:
Enrollment was terminated early, after 1217 participants were randomly assigned, because of a lower than anticipated event rate and a declining rate of COVID-19 hospitalizations. Median age was 54 years, 50.4% were women, 26.5% were Black, and 16.7% were Hispanic; 30.7% had a World Health Organization severity score of 5 or greater, and 11.0% had an International Medical Prevention Registry on Venous Thromboembolism risk prediction score of greater than 4. Incidence of the primary end point was 2.13% (95% CI, 1.14 to 3.62) in the apixaban group and 2.31% (CI, 1.27 to 3.84) in the placebo group. Major bleeding occurred in 2 (0.4%) and 1 (0.2%) and clinically relevant nonmajor bleeding occurred in 3 (0.6%) and 6 (1.1%) apixaban-treated and placebo-treated participants, respectively. By day 30, thirty-six (3.0%) participants were lost to follow-up, and 8.5% of apixaban and 11.9% of placebo participants permanently discontinued the study drug treatment.
Limitations:
The introduction of SARS-CoV-2 vaccines decreased the risk for hospitalization and death. Study enrollment spanned the peaks of the Delta and Omicron variants in the United States, which influenced illness severity.
Conclusion:
The incidence of death or thromboembolism was low in this cohort of patients discharged after hospitalization with COVID-19. Because of early enrollment termination, the results were imprecise and the study was inconclusive.
Primary Funding Source:
National Institutes of Health.
Several studies have shown that hospitalization is associated with an increased risk for venous thromboembolism (VTE), both during the hospitalization and after discharge. Patients hospitalized with an acute infection of SARS-CoV-2, the virus responsible for COVID-19, and particularly those requiring treatment in an intensive care setting, exhibit an increased risk for arterial thromboembolism and VTE. Anticoagulant therapy is recommended to prevent thromboembolic complications and improve outcomes for all patients hospitalized with COVID-19, although the recommended dose varies depending on the severity of the illness (1–3). Reports about the frequency of thromboembolic events after discharge from the hospital vary (4–6), and certain patients at highest risk for postdischarge thromboembolism may benefit from an extended course of thromboprophylaxis.
In addition to the acute manifestations associated with COVID-19, up to 17% of patients with COVID-19 may develop chronic symptoms, particularly respiratory and neurocognitive symptoms along with an inferior quality of life, called postacute sequelae of COVID-19 (PASC) (7). Studies have suggested that microvascular thrombosis may contribute to the development of PASC, raising the question of whether an extended course of anticoagulant therapy after hospitalization would prevent PASC.
We conducted a prospective, randomized, double-blind, clinical trial (ACTIV-4C [COVID-19 Thrombosis Prevention Trials: Post-hospital Thromboprophylaxis]) to determine whether a course of extended thromboprophylaxis beginning at the time of discharge from hospital through 30 days would decrease the frequency of thromboembolism and death. We also assessed health-related quality of life to investigate whether extended thromboprophylaxis would affect PASC development.
Methods
This was an adaptive, multistage prospective, randomized, double-blind, placebo-controlled trial designed to compare the effectiveness and safety of various antithrombotic strategies for patients discharged after hospitalization for COVID-19. This article describes stage 1 of this protocol, which randomly assigned study participants to either 2.5 mg of apixaban twice daily or matching placebo; because of the waning COVID-19 pandemic, other stages were not initiated. Outcomes were compared after blinded, central adjudication. The trial protocol was developed by the trial chair, principal investigator, and a protocol development committee that included representatives from the National Heart, Lung, and Blood Institute (NHLBI). The study protocol (available at Annals.org) was approved by a centralized institutional review board (Western Institutional Review Board-Copernicus Group), and local institutional review boards where applicable. An independent data and safety monitoring board (DSMB) was assigned by the NHLBI and met monthly to monitor the trial for efficacy and safety. A full list of study personnel is provided in the Appendix.
Study Population
A total of 127 hospitals in the United States were activated for the ACTIV-4C trial. Potential study participants were older than 18 years and hospitalized for 48 hours or longer with a SARS-CoV-2 infection (positive polymerase chain reaction, antigen, or point-of-care test results within 2 weeks of the hospital admission date). Patients who had an existing indication for either therapeutic or prophylactic dose anticoagulation at hospital discharge, those with a contraindication to antithrombotic therapy (for example, ischemic stroke, intracranial bleed, or neurosurgery within 3 months, known bleeding within the past 30 days, or known major surgery within 14 days), as well as those with an inherited or active acquired bleeding disorder were excluded. Platelet counts were required to be greater than 50 × 109 cells/L and hemoglobin greater than 80 g/L. In addition, patients who were pregnant, were prison inmates, had renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2), had a life expectancy less than 90 days, or were unwilling or unable to provide informed consent were excluded. Full inclusion and exclusion criteria are outlined in the study protocol.
The thromboprophylaxis regimen used during hospitalization was left to the discretion of the patient's hospital providers. Patients could be enrolled in clinical trials investigating thromboprophylaxis strategies during hospitalization as long as these studies did not require anticoagulant therapy to continue after discharge from the hospital.
Study Procedures
Participants were randomly assigned in a 1:1 ratio to receive 2.5 mg of apixaban or matching placebo twice daily for 30 days and were stratified on the basis of concomitant use of an antiplatelet agent (for example, aspirin or clopidogrel) and the World Health Organization (WHO) COVID-19 severity score (<5 or ≥5) (8). Before hospital discharge, enrolled participants were provided a 30-day supply of their blinded study therapy. Postdischarge follow-up visits were done centrally using a combination of telephone or e-mail surveys conducted by call centers at the University of Illinois Chicago and Duke Clinical Research Institute. The first follow-up at postdischarge day 2 confirmed study medication initiation. Subsequent follow-up visits at days 10, 20, and 30 confirmed study medication adherence and assessed clinical outcomes. Two additional encounters occurred at days 45 and 90 to determine risk for thromboembolic complications after completion of the study intervention. Participant-reported events triggered a telephone call from a research pharmacist to confirm the event, determine the type and severity of the event, check if medical attention was required, and if so, obtain the contact information for the treating clinician. Medical records were obtained by the trial monitors for all events considered possible components of the primary efficacy or safety end points during the 30-day treatment period or that raised any safety concerns over the additional 60-day posttreatment follow-up. Medical records were reviewed by the Clinical Endpoints Committee, who adjudicated events using standardized criteria. Both the medical monitors and the Clinical Endpoints Committee were unaware of randomized drug assignment.
Study End Points
The primary end point was the composite of all-cause mortality, venous thrombosis, and arterial thrombosis within 30 days of randomization. Thrombosis end points included new, symptomatic deep venous thrombosis, pulmonary embolism, thrombosis of other veins (for example, cerebral sinus and splanchnic veins), ischemic stroke, myocardial infarction, and other arterial thrombosis (for example, mesenteric or acute limb ischemia). The primary safety end points included major bleeding (9) and clinically relevant nonmajor bleeding (10) defined according to the International Society on Thrombosis and Haemostasis.
Prespecified secondary end points included the composite of the primary end point at days 45 and 90 after randomization, all venous thromboembolic events (including deep venous thrombosis, pulmonary embolism, splanchnic venous thrombosis, and cerebral venous sinus thrombosis) at day 30, and all arterial thromboembolic events (including stroke, myocardial infarction, mesenteric thrombosis, and peripheral arterial thromboembolism) at day 30. After interim results from the ACTIV-4A (Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 ACUTE) trial showed a potential benefit of antithrombotic strategies on patient-reported quality of life (11) among patients hospitalized with COVID-19, on 20 April 2022, the Protocol Development Committee, with DSMB approval, added a further 2 secondary end points: the composite end point of EuroQoL Group 5-Dimension (EQ-5D) index score and mortality at day 30 and at day 90 after randomization. Detailed definitions for each end point are described in the protocol; the addition of the quality-of-life end points are described in Appendix 5 of the protocol. Details about the randomization process are outlined (available at Annals.org).
Sample Size Estimation
The primary analysis was an intention-to-treat comparison of a composite end point of all-cause mortality and venous and arterial thromboembolic events within 30 days after randomization between intervention and placebo groups. This binary primary end point was used to power the study. Contemporary placebo-controlled trials of posthospitalization prophylactic anticoagulation for medically ill patients had reported a 2% to 4% composite event rate for VTE, myocardial infarction, stroke, and cardiovascular death (12, 13), whereas emerging clinical experience suggested that patients recently hospitalized with COVID-19 had even higher event rates. Assuming an anticipated composite event rate of 4% for the no anticoagulant group; a 35% risk reduction effect size; and applying a group-sequential, 2-sample, 2-sided Z test for proportions with pooled SD to test the primary hypothesis at an overall significance level α of 0.05 and 80% power, we estimated a sample size of 5320 (2660 participants assigned to each group), accounting for a 5% possible loss to follow-up rate.
Statistical Analysis
The detailed statistical analysis plan is provided (available at Annals.org). Baseline characteristics of the participants randomly assigned to the 2 groups were described using frequencies (percentages) for categorical variables and medians with 25th and 75th percentiles for continuous variables. All participants randomly assigned to the treatment groups were included in the primary analysis. Follow-up began at the time of randomization, and the primary end point at day 30 was compared between the 2 study groups using a 2-sample z statistic for the difference in proportions having the composite event in the anticoagulant and placebo groups. Composite event proportions were also modeled using a log-binomial regression model with treatment group as the independent variable and adjusting for trial stratification variables (that is, antiplatelet use and WHO ordinal scale score). In addition, unadjusted event rates for each treatment group and the relative risk with CIs were calculated. Kaplan–Meier cumulative incidence curves were also calculated to allow visualization of the patterns of time-to-first events. A sensitivity analysis was done as a modified intention-to-treat analysis, excluding all randomly assigned participants who did not initiate treatment. The proportions of 30- and 90-day safety end points (International Society on Thrombosis and Haemostasis–defined major and clinically relevant nonmajor bleeding) between the 2 groups were compared. The proportion of participants in each assigned treatment group who experienced each safety event and the relative risk were calculated.
Secondary outcomes were planned to be formally tested if the primary hypothesis was statistically significant at the 2-sided α level of 0.05. The 30- and 90-day quality-of-life composite end points were analyzed using a proportional odds model. The covariates in the model included the participant's age, sex, D-dimer level within 72 hours before discharge, body mass index, antiplatelet usage, WHO severity score, and the randomized treatment. The EQ-5D index scores were described, with death designated as a score of 0 per the EQ-5D user guide (14). Statistical methods for the testing of secondary end points not related to quality-of life followed the same procedures described earlier for the primary end point. Cumulative incidence of the primary composite efficacy end point within 30 days were described for protocol-specified subgroups: age, sex, race, ethnicity, body mass index, D-dimer levels, length of hospital stay, and WHO severity score. Baseline data were also collected to stratify patients by the International Medical Prevention Registry on Venous Thromboembolism VTE risk score, a risk assessment tool for identification of hospitalized medical patients at high risk for VTE (13, 15).
Analyses were done with SAS, version 9.4 (SAS Institute), and R, version 4.0.5 (R Core Development Team).
Role of the Funding Source
The sponsors were not involved in the design or conduct of the study, nor in the analysis of the data or the decision to submit the manuscript.
Results
Study Population
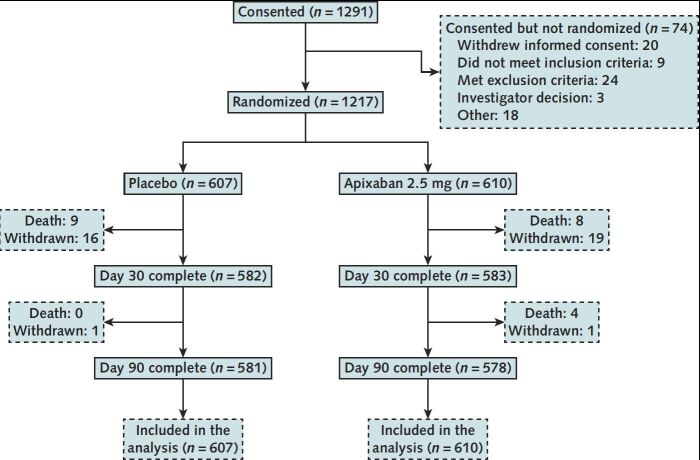
Between 15 February 2021 and 23 June 2022, a total of 1291 participants provided informed consent and 1217 were randomly assigned across 107 U.S. hospitals, with 610 and 607 participants randomly assigned to apixaban and placebo groups, respectively (Figure 1). On 15 June 2022, the DSMB met and found no safety concerns preventing study continuation but also noted the lower than expected event rate and the waning incidence of COVID-19 hospitalizations in the United States and worldwide. On 21 June 2022, the NHLBI and study leadership, while agreeing with the DSMB that the benefit from antithrombotic therapy in hospitalized patients with COVID-19 after hospital discharge remained an important question, made the determination that the lower-than-expected event rate and the markedly reduced study accrual rate after the peak of COVID-19 hospitalizations in January 2022 would prevent this study from answering its main scientific question promptly. As a result, enrollment to both groups was terminated on 23 June 2022. Participants already enrolled and randomly assigned were instructed to continue their assigned treatments and follow-ups, including the protocol-prescribed 90-day follow-up.
Figure 1. CONSORT flow diagram.
A total of 1291 participants provided informed consent; 1217 were randomly assigned across 107 hospitals in the United States, with 610 and 607 participants randomly assigned to apixaban and placebo groups, respectively. CONSORT = Consolidated Standards of Reporting Trials.
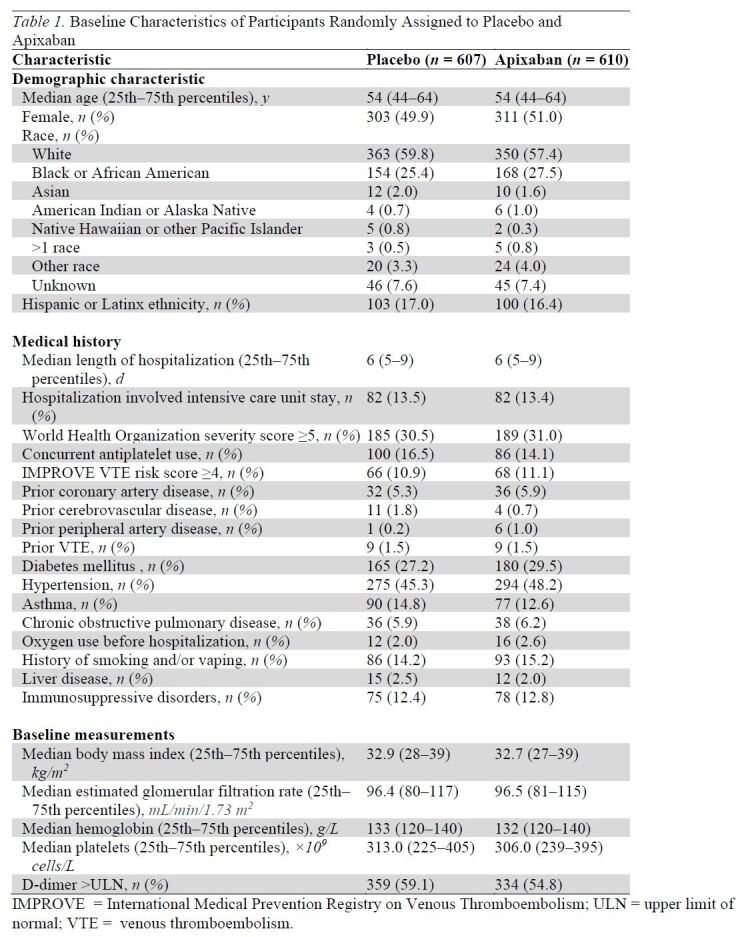
Median age of randomized participants was 54 years (interquartile range, 43 to 64 years), 50.4% were women, 26.5% self-identified as Black, and 16.7% self-identified as Hispanic (Table 1). Approximately half of the participants were recruited during the Delta variant wave of COVID-19 (July to October 2021), and approximately 15% were recruited during the early Omicron variant wave (January to March 2022). The remainder were enrolled either before the Delta variant (15%) or between the Delta and Omicron variants (20%). Among those randomly assigned, 30.7% had a WHO score of 5 or greater and 15.3% had concurrent antiplatelet medication use. At hospital discharge, 16.4% were prescribed antiplatelet therapy (93% aspirin). The median body mass index was 32.8 kg/m2, 56.9% had an elevated D-dimer result within 72 hours of discharge, and 11.0% had an International Medical Prevention Registry on Venous Thromboembolism VTE risk prediction score greater than 4. All baseline characteristics were balanced between treatment groups among randomized participants (Table 1).
Table 1.
Baseline Characteristics of Participants Randomly Assigned to Placebo and Apixaban
Primary and Secondary End Points
The last 30-day follow-up visit was completed on 26 August 2022. By day 30, thirty-six (3%) participants were lost to follow-up, of which 35 withdrew consent; by day 90, thirty-seven (3%) participants were lost to follow-up, all of whom withdrew consent (Figure 1).
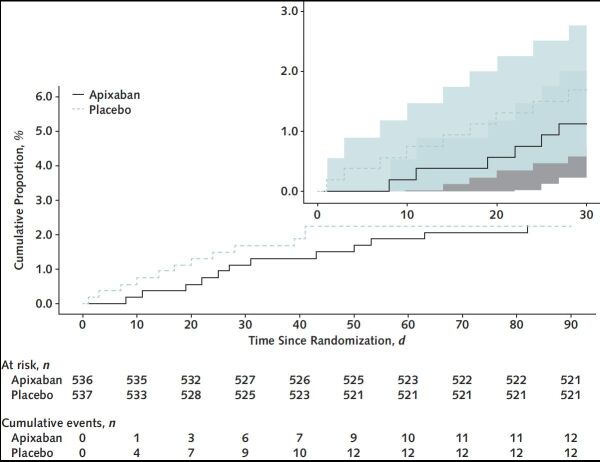
For the intention-to-treat analysis, the primary composite end point of 30-day all-cause mortality, arterial thromboembolism, or VTE occurred in 13 participants (2.13% [95% CI, 1.14 to 3.62]) in the apixaban group and 14 participants (2.31% [CI, 1.27 to 3.84]) in the placebo group (Table 2). Kaplan–Meier curves for the 2 groups are shown in Figure 2. Event rates for individual components of this composite end point, as well as results for the composite primary outcome at days 45 and 90, are shown in Table 2. Bleeding events were uncommon and are shown in Table 2. Event rates for the primary composite end point for the prespecified subgroups are shown in Appendix Figure 1.
Table 2.
Primary and Secondary End Point Comparisons of Participants Randomly Assigned to Apixaban Versus Placebo
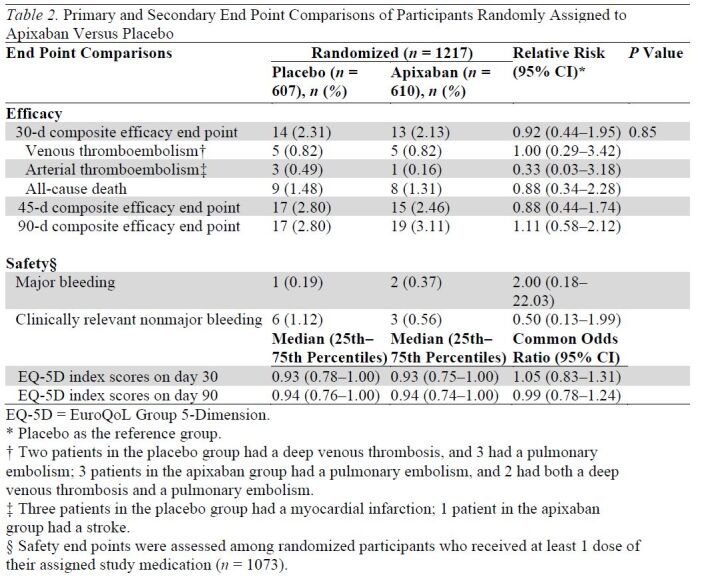
Figure 2. Primary composite efficacy end point within 90 days.
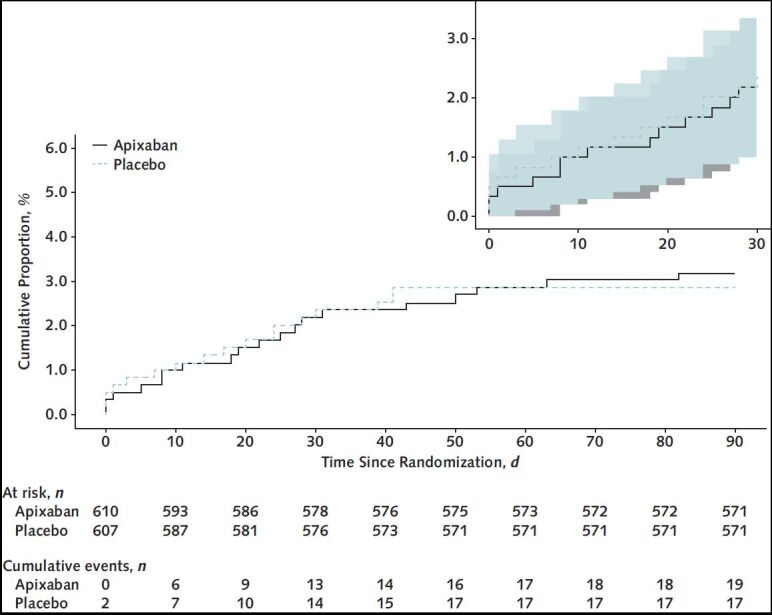
Kaplan–Meier curves of the primary composite efficacy end point within 90 d for participants randomly assigned to apixaban versus placebo (inset shows 30-d event curves). The 95% CIs are shown in the inset.
Appendix Figure 1. Primary composite efficacy end point by prespecified subgroups.
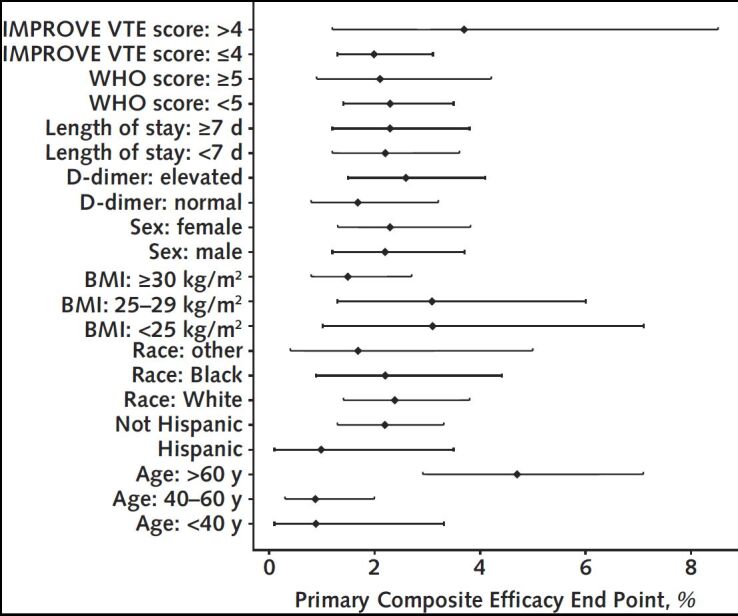
BMI = body mass index; IMPROVE = International Medical Prevention Registry on Venous Thromboembolism; VTE = venous thromboembolism; WHO = World Health Organization.
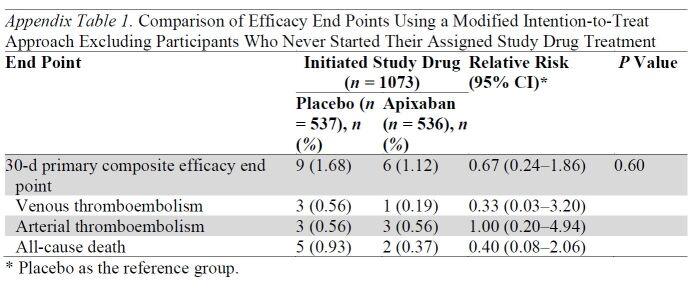
Among randomized participants, 74 (12.1%) assigned to apixaban and 70 (11.5%) assigned to placebo did not initiate the study drug. Efficacy analyses repeated using a modified intention-to-treat approach, excluding participants who did not start their assigned study drug, are shown in Appendix Table 1 and Appendix Figure 2.
Appendix Table 1.
Comparison of Efficacy End Points Using a Modified Intention-to-Treat Approach Excluding Participants Who Never Started Their Assigned Study Drug Treatment
Appendix Figure 2. Primary composite efficacy end point within 90 days using modified intention-to-treat approach.
The median EQ-5D index scores at day 30 were 0.93 (25th to 75th percentiles, 0.75 to 1.00) in the apixaban group and 0.93 (25th to 75th percentiles, 0.78 to 1.00) in the placebo group (Table 2). At day 90, the median results for the index scores were similar to the day 30 results: 0.94 (25th to 75th percentiles, 0.74 to 1.00) in the apixaban group and 0.94 (25th to 75th percentiles, 0.76 to 1.00) in the placebo group (Table 2). Similar results were seen using the modified intention-to-treat approach, with common odds ratios of 1.07 (CI, 0.85 to 1.35) and 1.03 (CI, 0.81 to 1.30) for 30 and 90 days, respectively.
Study Drug Adherence and Serious Adverse Events
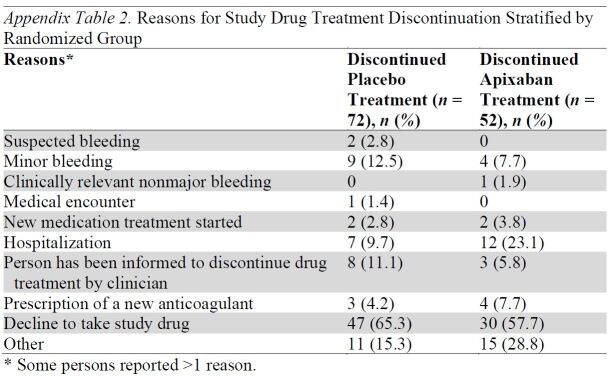
In participants who started the study drug treatment, 8.5% and 11.9% in the apixaban and placebo groups, respectively, permanently discontinued the treatment before 30 days. Reasons for treatment discontinuation are shown in Appendix Table 2. Among those randomly assigned, 105 participants reported a total of 137 serious adverse events, with 54 (8.9%) participants in the apixaban group and 51 (8.4%) in the placebo group reporting at least 1 serious adverse event.
Appendix Table 2.
Reasons for Study Drug Treatment Discontinuation Stratified by Randomized Group
Discussion
This multicenter, prospective, double-blind, randomized, clinical trial investigating an extended course of thromboprophylaxis with apixaban for 30 days at the time of discharge from the hospital in patients hospitalized with COVID-19 was interrupted early because of a lower than anticipated event rate and a declining rate of COVID-19 hospitalizations in the United States. As a consequence, the results were imprecise, and the study was inconclusive.
Several studies early in the course of the pandemic had suggested patients with COVID-19 had a higher risk for thromboembolism both during and after hospitalization (6, 16). In addition, during autopsies of patients who died with COVID-19, a high burden of microvascular thrombosis was found as well as macrovascular thromboembolic events that were clinically unsuspected before death (17, 18). Many patients hospitalized with COVID-19 also had additional risk factors for VTE and were likely to be more sedentary after hospital discharge (19). On the basis of these observations, we estimated the primary end point rate would be 4% in the placebo-treated participants by day 30 after hospital discharge. In contrast, we found a lower rate of 2.3% for the composite primary outcome, suggesting that the increased risk for thromboembolic complications encountered during hospitalization is not as pronounced after hospital discharge.
For the primary composite end point of VTE, arterial thromboembolism, or all-cause mortality, the results were highly compatible for apixaban compared with placebo, with estimates of efficacy ranging from a 56% reduction to a 95% increase in risk. Similar results were seen for the secondary efficacy outcomes as well as the safety outcomes. These results are consistent with prior studies in non–COVID-19 hospitalized medical patients, which did not find a benefit for the routine use of extended thromboprophylaxis in patients after discharge from the hospital (12, 13). We did not restrict our study to prespecified subgroups with a higher risk for thromboembolic complications after hospital discharge because our intent was to have the study apply to all patients hospitalized with COVID-19, given the initial concerns that these patients were at higher risk for thromboembolic complications.
To our knowledge, the ACTIV-4C trial is the first study to investigate whether an extended course of anticoagulant therapy in patients hospitalized with COVID-19 might improve quality-of-life scores after discharge from the hospital. Microvascular complications have been identified as a potential mechanism contributing to the development of PASC (20), suggesting that extended anticoagulation may mitigate symptoms. The EQ-5D assesses health in 5 domains (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression) scored as a single index measure of health to provide an assessment of quality of life (21). Although the results were inconclusive because the study was closed early, the EQ-5D scores were similar between the apixaban and placebo groups at days 30 and 90.
In contrast to our results, the MICHELLE (Medically Ill Hospitalized Patients for COVID-19 THrombosis Extended ProphyLaxis With Rivaroxaban ThErapy) trial found that extended thromboprophylaxis with 10 mg of rivaroxaban per day for 35 days after hospital discharge resulted in improved clinical outcomes compared with no extended thromboprophylaxis (22). Patients enrolled in that study had to have an increased risk for VTE, defined as an elevated modified International Medical Prevention Registry on Venous Thromboembolism VTE score of 2 to 3 with a D-dimer level of >500 μg/L using local laboratory criteria, or a score of 4 or more independent of the D-dimer level at hospital discharge. The primary end point of MICHELLE included symptomatic and asymptomatic VTE, detected by screening all patients with computed tomography pulmonary angiography and bilateral lower limb venous Doppler ultrasound; symptomatic arterial thromboembolism; and cardiovascular death at day 35. Excluding patients with asymptomatic VTE, the MICHELLE trial reported a rate of the composite outcome of symptomatic VTE, arterial thromboembolism, and cardiovascular death of 5.66% in the control group (9 events in 159 control patients) compared with 0.63% in the rivaroxaban group (1 event in 159 patients). In contrast, we found a lower rate of the composite primary outcome of symptomatic VTE, arterial thromboembolism, and all-cause mortality in the control group (2.31%; 14 events in 607 control participants) and a slightly higher rate in the apixaban group (2.13%; 13 events in 610 patients) compared with MICHELLE. Patients enrolled in the MICHELLE trial were more likely to be in the intensive care unit (51.9% of participants in MICHELLE compared with 13.5% of participants in ACTIV-4C), although more than 30% of our study participants had a WHO severity score of 5 or greater, defined as severe disease, and 56.9% had an elevated D-dimer result before discharge from the hospital.
Strengths of our study design include the use of strategies to minimize the need for direct contact with patients during a pandemic, the inclusion of symptomatic outcomes only in the primary end point, and the incorporation of a validated quality-of-life survey tool. Direct patient contact occurred only at the time of enrollment and randomization, while the patient was still hospitalized, with all follow-up occurring in the outpatient setting done through either electronic or telephone contact.
Our study also has several limitations. The lower-than-expected rate of the composite primary outcome was unexpected. Estimates early during the pandemic suggested that the rate of the composite outcome would be higher than what had previously been seen in studies of extended thromboprophylaxis in hospitalized medical patients. One factor likely contributing to this lower rate was the introduction of vaccines for SARS-CoV-2 as our study began, associated with a milder form of the disease and a decreased risk for hospitalization and death (23, 24). Our enrollment period spanned the peaks of infection associated with the Delta and Omicron variants of COVID-19 in the United States during the study, which have also been associated with differences in severity of illness (24, 25). Most patients who were hospitalized with COVID-19 after the vaccines became available were unvaccinated persons (26). Reasons provided by persons who elected to not be vaccinated against COVID-19 (27) are similar to those provided by persons who elect to not participate in clinical research studies (28).
The ACTIV-4C study was terminated early because of a lower-than-expected primary event rate and slow enrollment because of decreasing rates of hospitalization with COVID-19. As a consequence, the study results are imprecise, and the study was inconclusive. For the primary composite end point of VTE, arterial thromboembolism, or all-cause mortality at 30 days, the results were highly compatible, with estimates of efficacy ranging from a 56% reduction to a 95% increase in risk for apixaban compared with placebo.
Supplementary Material
Appendix: Study Teams and Personnel
Protocol Development Committee: Thomas Ortel* (Trial Chair), Tracy Wang* (Trial PI), Alison Morris* (Trial Co-Chair), Lisa Baumann Kreuziger*, Steve Wisniewski†, Mark Geraci*, Abdus Wahed*, Kevin Anstrom*, Eric Leifer*, Jerry Krishnan*, John Quigley*, Gervasio Lamas*, Alexandra Weissman*, Jose Lopez-Sendon*, M. Margaret Knudson*, Deborah Siegal*, Keith Hoots†, Andrei Kindzelski*.
Patient Stakeholder Advisory Group: Renee Leverty† (Lead Coordinator), Heather Ann Brown†, Guadalupe Andrade†, Nita Jain†, Franz Feierbach†, Karen Serwatkewich†, Mary Ann Wolf†.
Duke University: Thomas Ortel* (Trial Chair), Tracy Wang* (Trial PI); Operations/Safety: Kevin Anstrom*, Rachel Olson†, Annie Pennella*, Teresa Atwood†, Kelly Lindblom†, Ann Schutte†, Allegra Stone†, Michael Morse†, Jason Lang†; Call Center: Tina Harding†, Amanda Harrington†, Susan Rogers†, Juan Collazo†, Nikki Kandray†, Meaghan Beauchaine†, Jakela Anderson†, Danielle Britto†, Kimberly Chavis†, Tasha Denning†, Stephanie Garcia†, Angela Pinnix†, Tashyia Webb†, Labriah Wilson†; Unblinding team: Vanessa Blumer†, Elias Pratt†, Molly Daugherty†; Patient Stakeholder Advisory Group coordination: Renee Leverty†, Ann Burnett†.
University of Pittsburgh: Steve Wisniewski† (Coordinating Center Co-PI), Mark Geraci* (Coordinating Center Co-PI), Abdus Wahed* (Trial Biostatistician); DCC and UPitt Operations Team Members: Johanna Carmel Egan†, Emily George†, Edvin Music†, Alana Alameida†, Jake Schreiber†, Frank Sciurba†, Elyse Perkins†, Stephen Wisniewski†; Medical Monitor Team: Joshua Hulbert† (coordinator), Daniel Kass†, Michael M. Myerburg; Statistical Analysis: Lingyun Lyu*; Data Management (SOCAR): Sophie de Brouwer†, Bridget-Anne Kirwan†, Emilie Perrin†.
University of Illinois, Chicago: Jerry Krishnan* (Research Communication Center PI), Nancy Shapiro* (Research Communication Center Co-I), Sharon Hasek†, Barbara Predki†, Miriam Martinez†, Sunni Barbera†, Jennifer Sculley†, Jennifer Peterson†, Sai Illendula†, Julie DeLisa†, Lauren Castro†, Nina Huynh†, Ellen Uppuluri†, James Lee†, Lauren Speakman†, Niha Idrees†, Conny Mei†, Erin Pozzolano†, Tara Driscoll†.
Labcorp: Damir Skific†, Joseph Collins†, Aletha Hanna†, Sheri Mersc†, Sorina Simtion†.
Clinical Endpoints Committee: Brendan Everett* (Chair), Sheila Hegde†, Yuri Kim†, Jean-Philippe Galanaud†, Gregoire Le Gal†, Natalia Rost†, Sean van Diepen†, Aneesh Singhal†.
Data Safety Monitoring Board: Richard C. Becker† (Chair), Gregory del Zoppo†, Robert Glynn†, Peter Henke†, Richard Holubkov†, Kim Kerr†, Agnes Lee†, Hannah Lipman†, Fedor Lure†, Sara Vesely†, Danielle Wenner†.
NIH/NHLBI: Keith Hoots†, Andrei Kindzelski*, Antonello Punturieri†, Ben Sakovich†, Jeff Snyder†, DJ Milliken†, John Bucheimer†, Traci Heath Mondoro†, Gail Weinmann†, James Troendle†, Eric Leifer*, Jungnam Joo*, Lisa G. Berdan†.
CONNECTS Steering and Executive Committees: Neil Aggarwal†, Gordon Bernard†, Samuel Morris Brown†, Cliff Callaway†, Sean Collins†, Mary Cushman†, David Douglas†, Serpil Erzurum†, Ann Farrell†, Annetine Gelijns†, Adit Ginde†, Simone Glynn†, David Goff†, Rachel Harrigan†, Robert Harrington†, Judy Hochman†, Mary Homer†, Robert Johnson†, Nigel Key†, James Kiley†, Walter Koroshetz†, Cliff Lane†, Lisa LaVange†, Gilbert ‘Lynn' Marks†, Peter Marks†, George Mensah†, Diane Nugent†, Thomas Ortel*, Amy Patterson†, Yves Rosenberg†, Matt Shotwell†, Toby Silverman†, Catherine Stoney†, Clinton Wright†, Clyde Yancy†.
RTI: Tracy Nolen†, Sonia Thomas†, Michelle Walter†, Greta Herring†, Amy Kendrick†, Jen Deese†, Taegen Sullivan†.
ACTIV-4C Investigators
UNC Chapel Hill: Raj Kasthuri* (Primary Investigator); Micah Mooberry†, Stephan Moll†, Yasmina Abajas† (Co-Investigators); Amy Brightwood†, Katie Stanford†, Laura Finerty† (Lead Study Coordinators).
Baptist Health Lexington: Andrew Alexander* (Primary Investigator); Lesia Chaffins†, Marianna Nercesian† (Lead Study Coordinators).
Duke Medical Center: Lana Wahid* (Primary Investigator); Rowena Dolor†, Grace Dreyer†, Tatyana Der†, Emily Ko†, Andrea Archibald†, Valerie Renard†, Neil Stafford† (Co-Investigators); Nkiruka Azuogalanya†, Oluwayemisi Mohammed† (Lead Study Coordinators).
University of Illinois at Chicago (UIC): John Quigley* (Primary Investigator); Charles McPherson†, Peggy Choye†, Keri Kim† (Co-Investigators); Neha Atal† (Lead Study Coordinator).
Mount Sinai Medical Center: Gervasio Lamas* (Primary Investigator); Ana Mon† (Lead Study Coordinator).
OSF Little Company of Mary Medical Center (OSF LCM): M. Bassel Atassi* (Primary Investigator); Shamila Garg† (Co-Investigator); James Vrame†, Trevor Murawski† (Lead Study Coordinators).
Wake Forest: Peter J. Miller* (Primary Investigator); Ashish Khanna†, Hinna Wadhwani†, Kinchit Shah†, Mhorys Pickmans†, Ryan Mayes† (Co-Investigators); Brandon Reeves†, Lynnette (Lynne) Harris† (Lead Study Coordinators).
Tallahassee Memorial HealthCare: Janice Lawson* (Primary Investigator); Adam Oliver†, Jianghao Zhu†, Leah Weinstein†, Matthew Lawson†, Narlin Beaty†, Rachel Monroe†, Ryan Johnson† (Co-Investigators); Brittany Stith†, Marissa Ciesla† (Lead Study Coordinators).
UTHouston: Bela Patel* (Primary Investigator); David McPherson†, Luis Ostrosky†, Mary Ayad†, Meghana Gore†, Nithin Thomas† (Co-Investigators); Idorenyin Udoh-Bradford†, Jaicey Johnson†, Pamela Nichols†, Talha Mubashir† (Lead Study Coordinators).
Rhode Island Hospital: Ralph Rogers† (Primary Investigator); Eleftherios Mylonakis† (Co-Investigator); Allyson Sherman-Roe†, Julia Fisher† (Lead Study Coordinators).
Versiti/MCW: Lisa Baumann Kreuziger* (Primary Investigator); Amer Al Homssi†, Haisam Abid†, Lindsay Hammons† (Co-Investigators); Shannon Broaddrick† (Lead Study Coordinator).
Torrance Medical Center: James McKinnell† (Primary Investigator); Brian Sherman†, Jamie Kagihara†, Richard Huynh† (Co-Investigators); Herna Joy Gonzalez† (Lead Study Coordinator).
Montefiore Medical Center: Henny Billett† (Primary Investigator); Melissa Suchanek†, Noelle Townsend†, Veronica Sadler† (Lead Study Coordinators).
Monument Health Clinical Research: Matthew Anderson† (Primary Investigator); Amber Beer†, Crystal Gruetzmacher† (Lead Study Coordinators).
UPMC Presbyterian: Alexandra Weissman* (Primary Investigator); Andrew Schoenling†, Brian Malley†, Brian McVerry†, Brian Rosborough†, Christopher Franz†, Daniel Schloss†, David Barton†, David Huang†, Emily Brant†, Faraaz Shah†, Florian Mayr†, Georgios Kitsios†, Ghady Haidar†, Ian Barbash†, Kaveh Moghbeli†, Meghan Fitzpatrick†, Nadine Talia†, Varun Shetty†, William Bain† (Co-Investigators); Emily Berryman†, Marissa Marcin†, Matthew Gilliam†, Sara DiFiore† (Lead Study Coordinators).
Indiana University Health Methodist Hospital: Warren Gavin† (Primary Investigator); Tony Ren† (Co-Investigator); Molly Stearns† (Lead Study Coordinator).
Florida Heart Center: Prasad Chalasani† (Primary Investigator), Sarah Testa† (Lead Study Coordinator).
WK Physicians/TriState Medical: Anthony Stuart† (Primary Investigator); Ambrosia Holsapple†, Jennifer Prime† (Co-Investigators); Carrie Kay†, Shannon Saksa† (Lead Study Coordinators).
Savannah Health Services, LLC DBA Memorial Health University Medical Center: Mary Downing† (Primary Investigator); Alta Castellino†, Pechoka Sanders† (Lead Study Coordinators).
The University of Chicago Medicine: Jonathan Paul† (Primary Investigator); Amanda Walborn†, Gene Kim†, Mary Acosta†, Matt Cochran†, Nathan Kong†, Nikhil Gupta†, Sean Patrick Pinney† (Co-Investigators); Cynthia Arevalo†, Karli Molignoni† (Lead Study Coordinators).
Miriam Hospital: Ralph Rogers† (Primary Investigator); Eleftherios Mylonakis† (Co-Investigator); Allyson Sherman-Roe†, Julia Fisher† (Lead Study Coordinators).
Tower Health Medical Group: Daniel Forman† (Primary Investigator); Andrew Rettew†, Erik Rupard†, Nicole Agostino† (Co-Investigators); Julie Sheidy†, Renee McLin† (Lead Study Coordinators).
McLaren Center for Research Innovation: Mark Zainea† (Primary Investigator); Adam Bykowski†, Akarsh Parekh†, Christopher Provenzano†, Don Tait†, Melissa Ianitelli†, Nicole Prentice-Gaytan†, Ryan Malek†, Shelley Schendel†, Vasim Lala†, Victor Hunyadi†, Zeinab Saghir† (Co-Investigators); Emily Paschall† (Lead Study Coordinator).
Lifebridge Health: Paul Gurbel† (Primary Investigator); Cescelle Barbour†, Harjit Rahi†, Mehrnaz Ighani†, Sarensa Palikhey†, Seanne Eacho† (Lead Study Coordinators).
Anne Arundel Medical Center: Barry Meisenberg† (Primary Investigator); Caroline Wolfe-Ralph†, Kathy Gray†, Susan Cranford† (Lead Study Coordinators).
East Carolina University/Vidant Health: James Powell† (Primary Investigator); Nakeydia Bryant† (Lead Study Coordinator).
Barnes Jewish: Kristen Sanfilippo† (Primary Investigator); Benjamin Buettner†, Florence Almiron-Torralba†, James Mendoza†, James Watson†, Monalisa Mullick†, Monique Bruinsma†, Natalia Brito Rivera†, Nathan Martin†, Zachary Morgan† (Co-Investigators); Kimberly Kramer† (Lead Study Coordinators).
University of Florida: Kartikeya Cherabuddi† (Primary Investigator); Lisa Merck† (Co-Investigator); Amy Holland†, Cindy Montero†, Nastasia James†, Steven Fowler† (Lead Study Coordinators).
New York University Langone Health: Shari Brosnahan† (Primary Investigator), Lira Gutierrez† (Lead Study Coordinator).
AtlantiCare Regional Medical Center: Aileen Boa-Hocbo†, Anthony Macchiavelli†, Brendan Kelly† (Primary Investigators); Alison Hallam†, Jacqueline White† (Lead Study Coordinators).
Vanderbilt University Medical Center: Benjamin Tillman† (Primary Investigator); Celia Nunez†, Nikki Bratcher† (Lead Study Coordinators).
Ohio State University: Carlos Malvestutto†, Eric Kraut† (Primary Investigator); Abigail Bartosic†, Bethany Hill† (Lead Study Coordinators).
Dignity Health- St Josephs: Omar Gonzalez† (Primary Investigator and Co-Investigator); Connie Hillis†, Samer Salama† (Lead Study Coordinators).
Indiana University Health Bloomington Hospital: Allison Kaderabek† (Primary Investigator); Kim Deckard†, Mark Peabody† (Lead Study Coordinators).
Fanciscan Health Michigan City: Dafer AL-Haddadin†, Michelle Vasquez†, Kyle Widmer† (Primary Investigator); Michelle Sheets† (Lead Study Coordinator).
Maine Medical Center/Maine Medical Partners Adult Hospital Medicine: Daniel Meyer† (Primary Investigator), Terilee Gerry† (Lead Study Coordinator).
Capital Health System: Marc Whitman† (Primary Investigator); Aldrin Amistoso†, Allison Mullarney†, Amber Jezierski†, Rajashree Anandakrishnan† (Co-Investigators); Shannon David†, Soraya Sanchez† (Lead Study Coordinators).
University of Texas Southwestern Medical Center: Sonja Stutzman† (Primary Investigator), Maria Denbow† (Lead Study Coordinator).
NYU Langone Hospital–Brooklyn: Aaron Lord† (Primary Investigator); Marilyn Chacko† (Co-Investigator); Maria Cotrina† (Lead Study Coordinator).
Jamaica Hospital Medical Center: Khalid Gafoor† (Primary Investigator), Kelly Cervellione† (Lead Study Coordinator).
Minneapolis VA Health Care System: David Macdonald†, Ken Kunisaki† (Primary Investigators); Ken Kunisaki† (Co-Investigator); Miranda Hassler† (Lead Study Coordinator).
Loyola University Medical Center (LUMC): Anthi Katsouli† (Primary Investigator); Amir Darki†, Gabriel Edwardo Diaz†, Xixi Luo† (Co-Investigators); Jean Del Priore†, Nancy Schoenecker† (Lead Study Coordinator).
MultiCare Institute for Research & Innovation: Vinay Malhotra† (Primary Investigator), Denise Quinn† (Lead Study Coordinator).
McLaren Bay Region: Elizabeth Pionk† (Primary Investigator); Lisa Winters†, Marci Roberts†, Stephanie Bruma†, Tanya Gardner-Mosley† (Lead Study Coordinators).
Central Arkansas Veterans Health Care System: Mohammed Moursi† (Primary Investigator), Sandi Brock† (Lead Study Coordinator).
Mercy Hospital Buffalo: Raed Alnaji† (Primary Investigator); Sharavanan Thevanayagam† (Co-Investigator); Michael Oyanbadejo†, Savannah Christie† (Lead Study Coordinators).
Baylor Scott and White Medical Center–College Station: Timothy Byrd†, Monish Sheth† (Primary Investigators); Chaitanya Reddy† (Co-Investigator); Dao Marvin†, Sharla Russel†, Wanda Fikes† (Lead Study Coordinators).
Temple University Hospital: Michael Bromberg† (Primary Investigator); Daniel Sacher†, Ekamjeet Randhawa†, Erin Narewski†, Jamie Lee Garfield†, Janpreet Mokha†, Jonathan Galli†, Kartik Shenoy†, Maria Elena Vega Sanchez†, Melinda Darnell†, Navjot Kaur†, Oisin O'Corragain†, Parag Desai†, Sean Duffy† (Co-Investigators); Julie Juhas† (Lead Study Coordinator).
Pen Bay Medical Center: Robert Stein† (Primary Investigator); Caroline Knight†, Stacia Kozidis† (Lead Study Coordinators).
Queens Medical Center: Kuo-Chiang Lian† (Primary Investigator); Brent Tatsuno†, Qi Jie Nicholas Leo†, Stephanie Guo†, Todd Seto†, Ynhu Le†, Young Soo Rho† (Co-Investigators); Michael Yee† (Lead Study Coordinator).
Medical College of Georgia/Augusta University: Jose Vazquez† (Primary Investigator); Caroline Hamilton†, Christos Hatzigeorgiou†, Daniel Anderson†, David Walsh†, Joshua Eudy† (Co-Investigators); Aprile Osborn†, Ashton Bowles† (Lead Study Coordinators).
University at Buffalo: Alberto Monegro† (Primary Investigator); Catherine Wrona† (Lead Study Coordinator).
Wake Med: Judson Williams† (Primary Investigator), Lindsay Boole† (Co-Investigator), Michael Shauberger† (Lead Study Coordinator).
Ochsner Clinic Foundation: Mark Effron† (Primary Investigator), Shannon Williams† (Lead Study Coordinator).
UT Rio Grande Valley (UTRGV): Timothy Heath† (Primary Investigator); Andrew Dentino†, Cesar Gutierrez†, Fatimah Bello†, Jessica Martin† (Co-Investigators); Erik Hinojosa† (Lead Study Coordinator).
UT Health East Texas (UT Tyler): Chiagozie Nwasuruba†, Steven Idell† (Primary Investigators); Arnulfo Duarte†, Christine Kang†, Lindsey Stockton†, Megan Devine†, Omer Chowdhury†, Patti Olusola†, Ross Terada† (Co-Investigators); Christopher Herrick†, Elizabeth Dohanich†, Rebekah Hibbard† (Lead Study Coordinators).
Renown Health: Farah Madhani-Lovely† (Primary Investigator); Alanna Jacobs†, Katie Buckley†, Vivian Cruz† (Lead Study Coordinators).
Summa Health: Michael Tan† (Primary Investigator); Ellen Cicero†, Laura Barker† (Lead Study Coordinators).
UCSF at Zuckerberg San Francisco General Hospital: M. Margaret Knudson* (Primary Investigator); Brigid Donovan†, Vagn Peterson† (Co-Investigators); Brenda Nunez-Garcia† (Lead Study Coordinator).
Christus St. Vincent Regional Medical Center: Theresa Ronan† (Primary Investigator), Estevan Apodaca† (Co-Investigator), Tamara Flys† (Lead Study Coordinator).
Henry Ford Hospital: Scott Kaatz† (Primary Investigator); Christopher Lewandowski†, Joseph Miller†, Matthew George†, Patrick Agunwa†, Vinay Shah† (Co-Investigators); Kathleen Wilson†, Youssef Annous† (Lead Study Coordinators).
CAMC Clinical Trials Center: Stephen Lewis† (Primary Investigator), Tamejiro Takubo† (Co-Investigator), Mariah Preast† (Lead Study Coordinator).
Christus St. Patrick Hospital: Mahmood Tashfeen† (Primary Investigator); Jami Gardner†, Mike Brunet† (Lead Study Coordinators).
University of Arkansas for Medical Sciences: Nikhil Meena† (Primary Investigator), Alicia DeAguero† (Lead Study Coordinator).
Reid Physician Associates: John McGinty† (Primary Investigator), Kristi McGuire† (Lead Study Coordinator).
Western Michigan University Homer Stryket M.D. School of Medicine: Thomas Blok† (Primary Investigator), Kelly Becker† (Co-Investigator).
Baystate Health: Paul Pirraglia† (Primary Investigator); Lynn Perez†, Victoria Cobb† (Lead Study Coordinators).
Oregon Health and Science University: Akram Khan† (Primary Investigator); Abinaya Srikanthan†, Emmanuel Mills†, Jose Pena†, Olivia Krol† (Lead Study Coordinators).
Hope Tower at Jersey Shore University Medical Center: David Kountz† (Primary Investigator); Christopher Ullo†, Kristine Ende†, Maria Madsen†, Maureen Kinsley† (Lead Study Coordinators).
Adventist HealthCare Shady Grove Medical Center: Jonathan Cohen† (Primary Investigator); Opeyemi Araromi†, Patricia Szymkowiak† (Lead Study Coordinators).
Truman Medical Center: Paramdeep Baweja† (Primary Investigator), Mary Reed† (Lead Study Coordinator).
West Virginia University: Troy Krupica† (Primary Investigator); Erica Blystone†, Jennifer Elyse Krupp† (Lead Study Coordinators).
Kettering Medical Center: Harvey Hahn† (Primary Investigator), Daniel Geyer† (Lead Study Coordinator).
MidMichigan Medical Center-Midland: John Blamoun† (Primary Investigator); Brittney Gainforth†, Michele Rabidue† (Lead Study Coordinators).
UW Medicine Valley Medical Center: Michael Previti† (Primary Investigator), Dione Froman† (Lead Study Coordinator).
Boston University: Naomi Hamburg† (Primary Investigator); Elizabeth Klings†, Fitzgerald Shepherd†, Leili Behrooz†, Mark Sloan†, Rena Zheng†, Robert Eberhardt† (Co-Investigators); Daniel Mompoint† (Lead Study Coordinator).
Emory: Manila Gaddh† (Primary Investigator); Aaron Gluth†, Anna Von†, Jason Lucas† (Co-Investigators); Ananya Hooda†, Stephanie Whitten† (Lead Study Coordinators).
Mercury Street Medical Group: John Pullman† (Primary Investigator), Misty Johnson† (Lead Study Coordinator).
Valleywise Health Medical Center: Michael White†, Julie Freiman† (Primary Investigators); Mary Mulrow†, Deborah Gannon† (Lead Study Coordinators).
BayCare Hospital: Leslie Miller† (Primary Investigator), Delia Johnson† (Lead Study Coordinator).
St. Luke's University Health Network: Douglas Corwin† (Primary Investigator); Eric Rau†, Heather Cunningham†, Margaret Clement† (Lead Study Coordinators).
Centura Health: Kathryn Springer† (Primary Investigator), Sheryl Giambartolomei† (Lead Study Coordinator).
Baylor Scott and White-Round Rock: MaryAnn Tran† (Primary Investigator), William Tobleman† (Co-Investigator), Carmen Sutton† (Lead Study Coordinator).
St. Anthony North Health Campus: Hailey Holland†, Sharry Veres† (Primary Investigators); Leslie Lafferty†, Sheryl Giambartolomei† (Lead Study Coordinators).
University of Mississippi Medical Center: Matthew Kutcher† (Primary Investigator); Galbraith James† (Co-Investigator); Lucia Solis†, Simon Barinas† (Lead Study Coordinators).
Erlanger Health System: Sandeep Rajan† (Primary Investigator); Jensen Hyde† (Co-Investigator); Cassidy Shell†, Debbie Dyer†, Tina Higginbotham† (Lead Study Coordinators).
The University of Texas Health Science Center at Tyler: Chiagoze Nwasuruba† (Primary Investigator); Arnulfo Duarte†, Christine Kang†, Lindsey Stockton†, Megan Devine†, Omer Chowdhury†, Ross Terada† (Co-Investigators); Elizabeth Dohanich†, Rebekah Hibbard† (Lead Study Coordinators).
Conemaugh Memorial Medical Center: Khandakar Hussain† (Primary Investigator), Theresa McCreary† (Lead Study Coordinator).
Christus St. Elizabeth Hospital: Waqar Ahmad† (Primary Investigator); Jami Gardner†, Mike Brunet† (Lead Study Coordinators).
Coney Island Hospital: George Juang† (Primary Investigator and Lead Study Coordinator).
Lutheran Medical Group: Vicente Rodriguez† (Primary Investigator), Terrie Ross† (Lead Study Coordinator).
Stony Brook University Hospital: Wadie Bahou† (Primary Investigator), Lisa Malone† (Lead Study Coordinator).
BayCare Hospital–Winter Haven: Kollagunta Chandrasekhar† (Primary Investigator), Amanda Steadham† (Lead Study Coordinator).
Saint Anthony Hospital: Rebecca Vogel† (Primary Investigator); Leslie Lafferty†, Sheryl Giambartolomei† (Lead Study Coordinators).
University of Cincinnati: Jean Elwing† (Primary Investigator); Arun Jose†, Rachel Foot† (Co-Investigators); Nicole Hummel† (Lead Study Coordinator).
Cook County Health (CCH): Saurabh Malhotra† (Primary Investigator); Antonopoulos Pete†, Katayoun Rezai†, Michael Hoffman†, Poushali Bhattacharjee† (Co-Investigators); Arlet Nedeltcheva† (Lead Study Coordinator).
RUSH University Medical Center: Shivi Jain† (Primary Investigator); Deborah Katz†, Irena Medenica†, Irene Dehghan-Paz†, Jamile Shammo†, Joseph Thomas†, Melissa Larson†, Parameswaran Venugopal†, Sefer Gezer†, Seo-Hyun Kim† (Co-Investigators); Maaridge Fariduddin†, Patty Nelson†, Victoria Provido† (Lead Study Coordinators).
The Woodlands Center For Respiratory & Sleep Research: Moshin Bajwa† (Primary Investigator), Christie Cain† (Lead Study Coordinator).
Virginia Mason Medical Center: David Aboulafia† (Primary Investigator); Colette Lambert†, Kara Turner† (Lead Study Investigators).
Snake River Research: Richard Nathan† (Primary Investigator), Jennifer Morrison† (Lead Study Coordinator).
Texas Health Frisco: Daniella Angulo Thompson† (Primary Investigator); Shelley Long†, Tara Craig† (Lead Study Coordinators).
Texas Health Harris Methodist: Cheryl McDonald† (Primary Investigator); Patricia Snelus†, Tara Craig† (Lead Study Coordinators).
Stanford University School of Medicine: Alexandra Gordon† (Primary Investigator); Angelica Pritchard†, Yasmin Jazayeri† (Lead Study Coordinators).
CHRISTUS Trinity Clinic Pulmonary Medicine: Suman Sinha† (Primary Investigator), Amanda Baker† (Lead Study Coordinator).
UT Houston–LBJ Hospital: Bela Patel* (Primary Investigator); Cherian Sujith†, Mary Ayad†, Rosa Maria Estrada-Y-Martin† (Co-Investigators); Elizabeth Vidales†, Idorenyin Udoh-Bradford† (Lead Study Coordinators).
UT Houston–MHH-SW: Bela Patel* (Primary Investigator); Mary Ayad†, Murad Aslam†, Pratik Doshi†, Raj Akula† (Co-Investigators); Idorenyin Udoh-Bradford†, Jaicey Johnson†, Pamela Nichols† (Lead Study Coordinators).
Great El Monte Community Hospital (National Institute of Clinical Research): Nagasamudra Ashok†, Alam Didar Ul† (Primary Investigators); Barbara Lepthien† (Lead Study Coordinators).
HealthCare System 2000s-Garden Grove Hospital: Michael Dao† (Primary Investigator); Barbara Lepthien†, Kyra Cloutier† (Lead Study Coordinators).
Triple O Research: Olayemi Osiyemi† (Primary Investigator); Alexandra Vargas†, Christina Campbell†, Jose Menajovsky-Chaves†, Stephanie Martinez† (Co-Investigators); Myriam Izquierdo† (Lead Study Coordinator).
Mazur, Statner, Dutta, Nathan PC: Ramesh Nathan† (Primary Investigator), Lucinda Van Anglen† (Lead Study Coordinator).
Westchester Medical Center: Mathew Bronstein† (Primary Investigator), Christopher Hall† (Lead Study Coordinator).
Lahey Hospital and Medical Center: Deborah Gannon† (Lead Study Coordinator).
St. Francis Medical Center: Elizabeth Kleiner† (Primary Investigator), Anna Blanco†, Jenifer Bunn† (Lead Study Coordinators).
Penrose Hospital: Michael Roshon† (Primary Investigator); Anna Blanco†, Jenifer Bunn† (Lead Study Coordinators).
Cleveland Clinic Foundation: Keith McCrae† (Primary Investigator), Angela Knight† (Lead Study Coordinator).
Louisiana State University Health Sciences Center: Matthew Lammi† (Primary Investigator), Connie Romaine† (Lead Study Coordinator).
Emanate Health Queen of the Valley Hospital: Mallu Reddy† (Primary Investigator), Barbara Lepthien† (Lead Study Coordinator).
Dartmouth Hitchcock Medical Center: Monic Drescher† (Primary Investigator); Deborah Ornstein†, Howard Leung†, Jose Mercado†, Marshall Ward†, Richard Zuckerman†, Stanislav Henkin† (Co-Investigators); Haley Johnson† (Lead Study Coordinator).
St. Louis University: Stephanie Windish† (Primary Investigator), Mary Lesko† (Lead Study Coordinator).
CHI St Elizabeth Hospital: Paul Gobbo† (Primary Investigator); Corey Godfrey†, Mike Miriovsky† (Lead Study Coordinator).
Hawaii Pacific Health: Wade Kyono† (Primary Investigator); Micah Tong†, Steven Migdol† (Lead Study Coordinators).
Ochsner Clinic Foundation–Baton Rouge: Mark Effron† (Primary Investigator), Shannon Williams† (Lead Study Coordinator).
James A. Haley Veteran's Hospital: Arzu Ilercil† (Primary Investigator), Fabio Leonelli† (Co-Investigator).
Ascension St John Tulsa: Nicholas Hanna† (Primary Investigator); Anderson Mehrle† (Co-Investigator); Melanie Arnold† (Lead Study Coordinator).
Ascension St John Bartlesville: Nicholas Hanna† (Primary Investigator); Jenny Polasek†, Mary Harris†, Melanie Arnold† (Lead Study Coordinators).
Newark Beth Israel Medical Center: Alice Cohen† (Primary Investigator); Maya Shah†, Sari Jacoby† (Co-Investigators); Arjun Gadhiya†, Cynthia Horta†, Mitali Pradham† (Lead Study Coordinators).
* Author.
† Nonauthor contributor.
Footnotes
This article was published at Annals.org on 21 March 2023.
* For members of the ACTIV-4C Study Group, see the Appendix.
References
- 1. Schulman S , Sholzberg M , Spyropoulos AC , et al; International Society on Thrombosis and Haemostasis. ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost. 2022;20:2214-25. [PMID: ] doi: 10.1111/jth.15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cuker A , Tseng EK , Schünemann HJ , et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis for patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients. Blood Adv. 2022;6:4975-82. [PMID: ] doi: 10.1182/bloodadvances.2022007940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes GD , Burnett A , Allen A , et al. Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2022;54:197-210. [PMID: ] doi: 10.1007/s11239-022-02643-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts LN , Whyte MB , Georgiou L , et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347-50. [PMID: ] doi: 10.1182/blood.2020008086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patell R , Bogue T , Koshy A , et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342-6. [PMID: ] doi: 10.1182/blood.2020007938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannis D , Allen SL , Tsang J , et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838-47. [PMID: ] doi: 10.1182/blood.2020010529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson EJ , Williams DM , Walker AJ , et al; OpenSAFELY Collaborative. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13:3528. [PMID: ] doi: 10.1038/s41467-022-30836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. COVID-19 therapeutic trial synopsis. Accessed at https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis on 28 October 2022.
- 9. Schulman S , Kearon C ; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 10. Kaatz S , Ahmad D , Spyropoulos AC , et al; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 11. Greenstein YY, Hubel K, Venugopal V, et al. Preliminary analysis of the association between therapeutic-dose anticoagulation and long term outcomes from the ACTIV-4a and ATTACC multiplatform adaptive trial of hospitalized patients with COVID-19 [Abstract]. Presented at American Thoracic Society International Conference 2022, San Francisco, California, 18 May 2022. doi: 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A5051 [DOI]
- 12. Cohen AT , Harrington RA , Goldhaber SZ , et al; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-44. [PMID: ] doi: 10.1056/NEJMoa1601747 [DOI] [PubMed] [Google Scholar]
- 13. Spyropoulos AC , Ageno W , Albers GW , et al; MARINER Investigators. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118-27. [PMID: ] doi: 10.1056/NEJMoa1805090 [DOI] [PubMed] [Google Scholar]
- 14. EuroQol Research Foundation. EQ-5D-5L user guide: basic information on how to use the EQ-5D-5L instrument. Version 3.0. Accessed at https://euroqol.org/publications/user-guides/ on 28 October 2022.
- 15. Spyropoulos AC , Anderson FA Jr , FitzGerald G , et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-14. [PMID: ] doi: 10.1378/chest.10-1944 [DOI] [PubMed] [Google Scholar]
- 16. Salisbury R , Iotchkova V , Jaafar S , et al. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv. 2020;4:6230-9. [PMID: ] doi: 10.1182/bloodadvances.2020003349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wichmann D , Sperhake JP , Lütgehetmann M , et al. Autopsy findings and venous thromboembolism in patients with COVID-19. A prospective cohort study. Ann Intern Med. 2020;173:268-77. [PMID: ] doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falasca L , Nardacci R , Colombo D , et al. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807-15. [PMID: ] doi: 10.1093/infdis/jiaa578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Bakel BMA , van den Heuvel FMA , Vos JL , et al. High levels of sedentary time in patients with COVID-19 after hospitalisation. J Clin Med. 2022;11. [PMID: ] doi: 10.3390/jcm11041110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahamed J , Laurence J . Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest. 2022;132. [PMID: ] doi: 10.1172/JCI161167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pickard AS , Law EH , Jiang R , et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22:931-41. [PMID: ] doi: 10.1016/j.jval.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 22. Ramacciotti E , Barile Agati L , Calderaro D , et al; MICHELLE Investigators. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399:50-9. [PMID: ] doi: 10.1016/S0140-6736(21)02392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plumb ID , Feldstein LR , Barkley E , et al. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection - United States, June 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:549-55. [PMID: ] doi: 10.15585/mmwr.mm7115e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adjei S , Hong K , Molinari NM , et al. Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods - United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1182-9. [PMID: ] doi: 10.15585/mmwr.mm7137a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hyams C , Challen R , Marlow R , et al; AvonCAP Research Group. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom. Lancet Reg Health Eur. 2023;25:100556. [PMID: ] doi: 10.1016/j.lanepe.2022.100556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Havers FP , Pham H , Taylor CA , et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults 18 years or older in 13 US states, January 2021 to April 2022. JAMA Intern Med. 2022;182:1071-81. [PMID: ] doi: 10.1001/jamainternmed.2022.4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troiano G , Nardi A . Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245-51. [PMID: ] doi: 10.1016/j.puhe.2021.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dassum SR , Ferguson R , Woods P , et al. Patient-reported reasons for non-participation in a COVID-19 therapeutics clinical trial: findings from a multi-center investigation. Contemp Clin Trials. 2023;126:107082. [PMID: ] doi: 10.1016/j.cct.2023.107082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.