Key Points
Question
Is pathologic complete response (pCR) prognostic of oncologic outcomes in soft tissue sarcoma (STS), and what do the long-term results from the Radiation Therapy Oncology Group (RTOG) trial 0630 show?
Findings
Combined long-term results from RTOG 0630 and 9514 show a survival benefit with pCR. Updated results from RTOG 0630 show no increased recurrence with reduced target volumes.
Meaning
Results from this study suggest that pCR may be used to prognosticate outcomes; updated results from RTOG 0630 suggest that reduced target volumes are appropriate for preoperative image-guided radiotherapy.
This ancillary analysis of 2 randomized clinical trials of patients receiving neoadjuvant chemoradiotherapy or preoperative image-guided radiotherapy examines the prognostic significance of pathologic complete response on survival outcomes in patients with soft tissue sarcoma receiving neoadjuvant chemoradiotherapy.
Abstract
Importance
Pathologic complete response (pCR) may be associated with prognosis in patients with soft tissue sarcoma (STS).
Objective
We sought to determine the prognostic significance of pCR on survival outcomes in STS for patients receiving neoadjuvant chemoradiotherapy (CT-RT) (Radiation Therapy Oncology Group [RTOG] 9514) or preoperative image-guided radiotherapy alone (RT, RTOG 0630) and provide a long-term update of RTOG 0630.
Design, Setting, and Participants
RTOG has completed 2 multi-institutional, nonrandomized phase 2 clinical trials for patients with localized STS. One hundred forty-three eligible patients from RTOG 0630 (n = 79) and RTOG 9514 (n = 64) were included in this ancillary analysis of pCR and 79 patients from RTOG 0630 were evaluated for long-term outcomes.
Intervention
Patients in trial 9514 received CT interdigitated with RT, whereas those in trial 0630 received preoperative RT alone.
Main Outcomes and Measures
Overall and disease-free survival (OS and DFS) rates were estimated by the Kaplan-Meier method. Hazard ratios (HRs) and P values were estimated by multivariable Cox model stratified by study, where possible; otherwise, P values were calculated by stratified log-rank test. Analysis took place between December 14, 2016, to April 13, 2017.
Results
Overall there were 42 (53.2%) men; 68 (86.1%) were white; with a mean (SD) age of 59.6 (14.5) years. For RTOG 0630, at median follow-up of 6.0 years, there was 1 new in-field recurrence and 1 new distant failure since the initial report. From both studies, 123 patients were evaluable for pCR: 14 of 51 (27.5%) in trial 9514 and 14 of 72 (19.4%) in trial 0630 had pCR. Five-year OS was 100% for patients with pCR vs 76.5% (95% CI, 62.3%-90.8%) and 56.4% (95% CI, 43.3%-69.5%) for patients with less than pCR in trials 9514 and 0630, respectively. Overall, pCR was associated with improved OS (P = .01) and DFS (HR, 4.91; 95% CI, 1.51-15.93; P = .008) relative to less than pCR. Five-year local failure rate was 0% in patients with pCR vs 11.7% (95% CI, 3.6%-25.1%) and 9.1% (95% CI, 3.3%-18.5%) for patients with less than pCR in 9514 and 0630, respectively. Histologic types other than leiomyosarcoma, liposarcoma, and myxofibrosarcoma were associated with worse OS (HR, 2.24; 95% CI, 1.12-4.45).
Conclusions and Relevance
This ancillary analysis of 2 nonrandomized clinical trials found that pCR was associated with improved survival in patients with STS and should be considered as a prognostic factor of clinical outcomes for future studies.
Trial Registration
ClinicalTrials.gov Identifiers: RTOG 0630 (NCT00589121); RTOG 9514 (NCT00002791)
Introduction
Neoadjuvant radiotherapy (RT) and/or chemotherapy (CT) have been commonly used to treat soft tissue sarcoma (STS) of extremity and trunk.1,2,3,4,5 The most objective reliable measure of this in vivo sensitivity to neoadjuvant therapy is the extent of pathologic response. Treatment-induced pathologic necrosis has been shown to be an independent prognostic factor in patients with osteosarcoma and Ewing sarcoma, although not all studies have conclusively demonstrated the correlation of histologic response with treatment and survival in these patients.6,7,8 However, in patients with extremity or truncal STS, the prognostic effect of histologic response to therapy is much less clear, with only a few published studies offering conflicting results.9,10,11 These studies, through single-institution retrospective reviews, were limited by either their relatively small study populations or by the heterogeneity of their treatment regimens.
In this study, we sought to determine the prognostic significance of treatment-induced histologic response in STS in a large homogeneous group of patients receiving a uniform regimen of neoadjuvant RT and/or CT. Patients enrolled in previous Radiation Therapy Oncology Group (RTOG) sarcoma studies (trials 9514 and 0630) received a relatively uniform treatment regimen (either preoperative CT or RT) with primary end points reported.4,5 Long-term outcomes of the RTOG trial 9514 have been reported,5 and long-term outcomes of the RTOG trial 0630 are updated in this article. Tissue specimens taken from both pretreatment diagnostic biopsies and surgical specimens were centrally reviewed by one of the authors (D.L.), blinded to clinical outcomes. We report how pathologic response rates correlated with oncologic outcomes for patients with trunk and extremity STS.
Methods
In this article, we report long-term (5-year) disease and survival outcomes and adverse events (AEs) of RTOG trial 0630 and the unplanned analysis of histologic response for RTOG trials 0630 and 9514 that was approved by the NRG Oncology Ancillary Projects Committee (the trial protocols for each study are available in Supplement 1 and Supplement 2). The primary objective was to correlate percentage of tumor viability after surgery with survival and disease outcomes. Secondary objectives were to compare local and central reads of histologic type, histologic grade, and R status. Tissue samples from pretreatment biopsy and final pathologic analysis after neoadjuvant CT-RT (RTOG 9514) or RT alone (RTOG 0630 cohort B) were submitted to RTOG for central pathologic review. Without knowledge of clinical outcomes, the study pathology co-chair (D.L.) reclassified all histologic types into World Health Organization classifications (4th version). The RTOG trial 0630 local histologic grading was converted from a 4-grade to 3-grade system using French Federation of Cancer Centers Sarcoma Group grading systems criteria. For assessment of posttreatment response to therapy, microscopic slides from 1 entire cut surface of a cross-sectional slab of tumor were required to be submitted, similar to what is routinely done with osteosarcoma. The reason for this is that when pathologists sample posttreatment STS, they often selectively take only what appears to be grossly viable. Thus, the histologic slides tend to underestimate the amount of nonviable tumor. Posttreatment tumors showed 4 major histologic changes: viable tumor, fibrotic/hyalinized areas, necrotic tumor, and hemorrhage. The presence of only scattered markedly degenerated cells was regarded as nonviable tumor cells. By examining all histologic slides from an entire cut surface, we estimated the percentages of the 4 histologic patterns to determine the percentage of viable tumor. All 4 histologic patterns were tabulated such that the sum of their individual percentages equaled 100%. Percentages of viable neoplasm from each resected specimen were segregated into 5 categories (none [0%], 1%-24%, 25%-50%, 51%-75%, and >75%) for analysis.
For the RTOG trial 0630 long-term update and ancillary project, rates of local, regional, and distant failure and second primary tumor were estimated by the cumulative incidence method, to account for the competing risk of death, and rates of distant disease-free, disease-free, and overall survival (OS) were estimated by the Kaplan-Meier method. For the ancillary project, hazard ratios were estimated by the Cox model stratified by study and compared by Akaike Information Criterion (AIC). Only patients with complete data for all potential covariates (age, gender, race, Zubrod performance status, disease location, disease size, histologic grade, time to surgery, R status, histologic type, and percentage tumor viability) were included in multivariable analysis. In addition, κ statistics were calculated to evaluate the agreement of local and central review of histologic type, histologic grade, and R status. Full statistical details can be found in the eAppendix in Supplement 3.
Results
Long-term Disease-Associated Outcome End Points in Patients of RTOG Trial 0630
The RTOG trial 0630 opened to patient accrual in March 2008. Cohort A closed in January 2010 with 12 patients enrolled. Results from cohort A will not be presented. Cohort B closed in September 2010 with 86 patients enrolled. Six patients in cohort B were ineligible per protocol criteria and 1 patient did not start protocol therapy, leaving 79 analyzable patients. Analysis took place between December 14, 2016, to April 13, 2017.
Patient and tumor characteristics were previously reported.4 Patients were predominantly male (42 [53.2%]) and 72 were White (89.9%), 61 with Zubrod 0 (77.2%), and the median age was 61 years. The most common histologic finding was liposarcoma (23 [29.1%]), with proximal upper leg the most common primary site (33 [41.8%]). Median tumor size was 10.5 cm, 60 (75.9%) were T2b, and 49 (62.0%) were histologic grades 3 to 4 (4-grade system).
Median follow-up for surviving patients was 6.0 years compared with 3.6 years at the time of initial report2; only 4 of 79 patients were censored with less than 5 years of follow-up. Thirty-two patients have died, with 7 new deaths since the initial report.2 The estimated 5-year OS was 62.1% (95% CI, 51.2%-73.0%). There have been 6 in-field LR and 5 patients who did not have surgery for a total of 11 local failures (LFs) with only 1 new since the initial report. The estimated 5-year LF rate was 12.7% (95% CI, 6.5%-21.1%). There have been 35 distant failures (DF), with only 1 new since the initial report. The estimated 5-year DF rate is 45.3% (95% CI, 33.8%-56.0%). The estimated 5-year disease-free survival (DFS) and distant disease-free survival (DDFS) rates were 47.5% (95% CI, 36.4%-58.6%) and 52.1% (95% CI, 40.9%-63.3%), respectively. Outcomes per the CAN-NCIC-SR2 trial definitions of failure are shown in Table 1. Figure 1 shows all disease recurrence and survival outcomes. In addition, minimal toxic effects were added to the previous report (please see long-term toxic effects in eTables 1 and 2 in Supplement 3) and there were no treatment-related deaths. The long-term results from RTOG 0630 have established that the reduced target volumes are appropriate for preoperative image-guided RT.
Table 1. Outcomes in RTOG 0630 per CAN-NCIC-SR2 Trial Definitions.
| Year | Estimate, % (95% CI)a | Cumulative failures, No. | At risk, No. |
|---|---|---|---|
| Local recurrence | |||
| 0 | 100 (NA) | 0 | 73 |
| 1 | 95.6 (90.6-100) | 3 | 63 |
| 2 | 94.0 (88.2-99.7) | 4 | 57 |
| 3 | 92.2 (85.7-98.8) | 5 | 50 |
| 4 | 92.2 (85.7-98.8) | 5 | 45 |
| 5 | 92.2 (85.7-98.8) | 5 | 43 |
| Total | NA | 6 | NA |
| Regional/distant recurrence | |||
| 0 | 100 (NA) | 0 | 73 |
| 1 | 70.9 (60.5-81.4) | 21 | 50 |
| 2 | 65.3 (54.2-76.3) | 25 | 46 |
| 3 | 56.7 (45.2-68.2) | 31 | 39 |
| 4 | 56.7 (45.2-68.2) | 31 | 37 |
| 5 | 55.1 (43.6-66.7) | 32 | 36 |
| Total | NA | 32 | NA |
| Progression-free survival | |||
| 0 | 100 (NA) | 0 | 73 |
| 1 | 67.0 (56.2-77.8) | 24 | 48 |
| 2 | 61.5 (50.3-72.7) | 28 | 44 |
| 3 | 53.0 (41.5-64.5) | 34 | 37 |
| 4 | 51.5 (40.0-63.1) | 35 | 35 |
| 5 | 50.1 (38.5-61.6) | 36 | 34 |
| Total | NA | 39 | NA |
Abbreviations: CAN-NCIC-SR2, National Cancer Institute of Canada Sarcoma2; NA, not applicable; RTOG, Radiation Therapy Oncology Group.
Kaplan-Meier estimate of the percentage event-free.
Figure 1. Estimated Outcomes per RTOG 0630 (Panels A, B, and D) and CAN-NCIC-SR2 Definitions (Panel C).
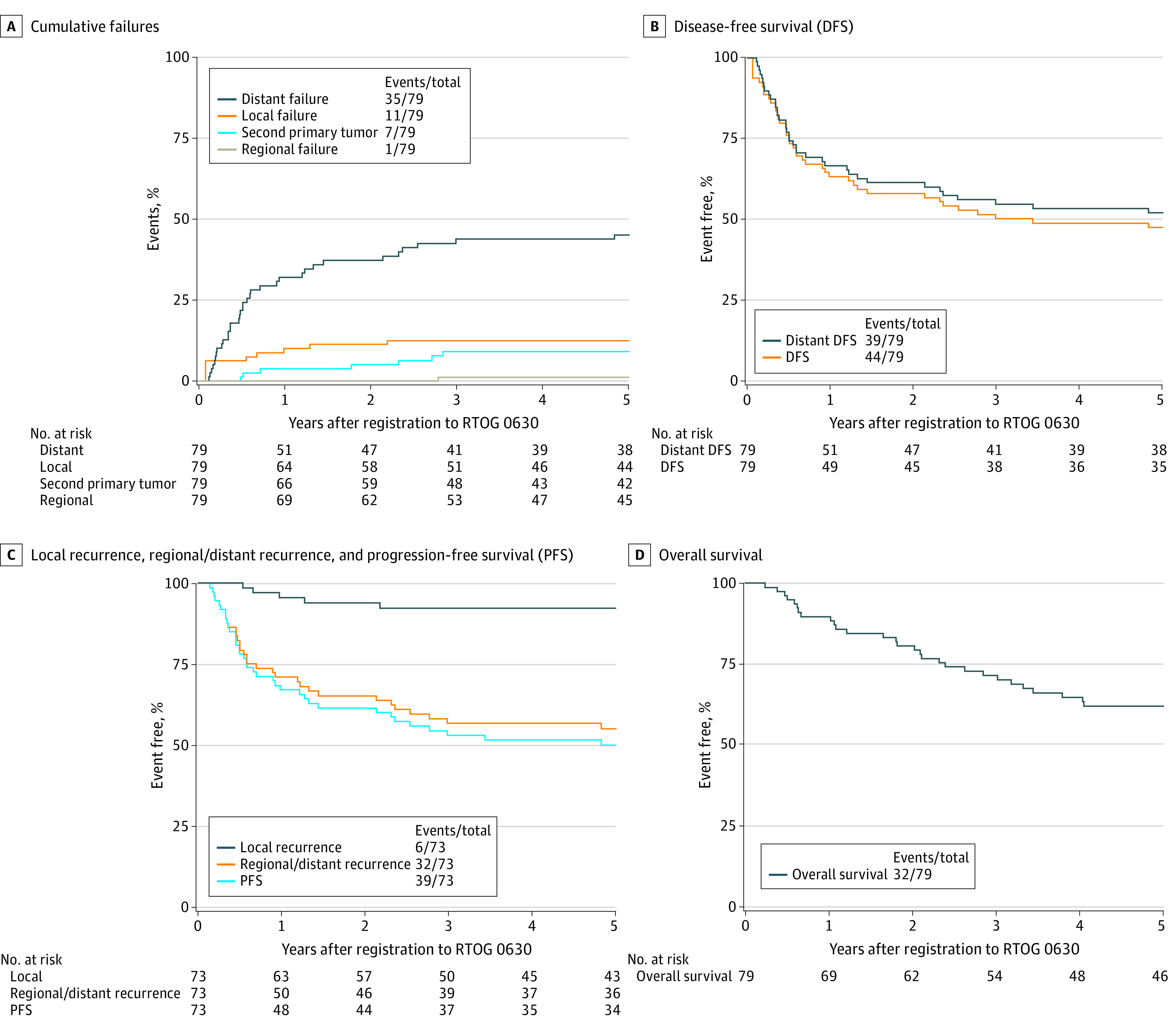
CAN-NCIC-SR2 indicates National Cancer Institute of Canada Sarcoma2; RTOG, Radiation Therapy Oncology Group.
Association Between Pathologic Tumor Viability and Clinical Outcomes in Patients From RTOG 0630 and RTOG 9514
One hundred forty-three eligible patients from RTOG 0630 (n = 79) and RTOG 9514 (n = 64) were included in this analysis. Patient characteristics are shown in Table 2. The most common histologic diagnoses per local pathologists were liposarcoma (38 [26.6%]), undifferentiated pleomorphic sarcoma (36 [25.2%]), undifferentiated spindle cell sarcoma (17 [21.7%]), and leiomyosarcoma (31 [11.9%]). On central review, the most common histologic diagnoses were undifferentiated pleomorphic sarcoma (42 [29.4%]), liposarcoma (23 [16.1%]), myxofibrosarcoma (20 [14.0%]), and leiomyosarcoma (15 [10.5%]). Per local grading, 27 (18.9%) were grade 1, 42 (29.4%) were grade 2, and 71 (49.7%) were grade 3, compared with 13 (9.1%), 33 (23.1%), and 85 (59.4%) per central review.
Table 2. Patient and Tumor Characteristics, Surgery, and Tumor Viability.
| Characteristic | No. (%) | ||
|---|---|---|---|
| RTOG 0630 (n = 79) | RTOG 9514 (n = 64) | Total (n = 143) | |
| Age, median (IQR) [range], y | 61 (51-71) [24-88] | 45.5 (36-53.5) [21-75] | 54 (42-65) [21-88] |
| Sex | |||
| Female | 37 (46.8) | 28 (43.8) | 65 (45.5) |
| Male | 42 (53.2) | 36 (56.3) | 78 (54.5) |
| Race | |||
| White, non-Hispanic | 68 (86.1) | 52 (81.3) | 120 (83.9) |
| All others | 11 (13.9) | 12 (18.8) | 23 (16.1) |
| Zubrod performance status | |||
| 0 | 61 (77.2) | 46 (71.9) | 107 (74.8) |
| 1 | 18 (22.8) | 18 (28.1) | 36 (25.2) |
| Disease location | |||
| Upper extremity | 11 (13.9) | 7 (10.9) | 18 (12.6) |
| Lower extremity | 62 (78.5) | 42 (65.6) | 104 (72.7) |
| Other (ie, hip, buttocks) | 6 (7.6) | 15 (23.4) | 21 (14.7) |
| Disease size (longest diameter, cm) | |||
| Median (IQR) [range], y | 10.5 (6.8-16) [3.5-30] | 15 (11.85-20) [8.2-55] | 12.5 (9.5-18.0) [3.5-55.0] |
| Histologic findings (local) | |||
| Epithelioid sarcoma | 0 | 1 (1.6) | 1 (0.7) |
| Extraskeletal myxoid chondrosarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Leiomyosarcoma | 9 (11.4) | 8 (12.5) | 17 (11.9) |
| Liposarcoma, NOS | 23 (29.1) | 14 (21.9) | 37 (25.9) |
| Liposarcoma, myxoid/round cell | 1 (1.3) | 0 | 1 (0.7) |
| Malignant peripheral nerve sheath tumor | 1 (1.3) | 1 (1.6) | 2 (1.4) |
| Myxofibrosarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Synovial sarcoma | 4 (5.1) | 3 (4.7) | 7 (4.9) |
| Unclassified sarcoma | 3 (3.8) | 6 (9.4) | 9 (6.3) |
| Undifferentiated pleomorphic sarcoma | 20 (25.3) | 16 (25.0) | 36 (25.2) |
| Undifferentiated spindle cell sarcoma | 16 (20.3) | 15 (23.4) | 31 (21.7) |
| Histologic findings (central review) | |||
| Epithelioid sarcoma | 0 | 1 (1.6) | 1 (0.7) |
| Extraskeletal myxoid chondrosarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Leiomyosarcoma | 8 (10.1) | 7 (10.9) | 15 (10.5) |
| Liposarcoma, myxoid/round cell | 13 (16.5) | 6 (9.4) | 19 (13.3) |
| Liposarcoma, pleomorphic | 1 (1.3) | 0 | 1 (0.7) |
| Liposarcoma, well-differentiated/dedifferentiated | 3 (3.8) | 0 | 3 (2.1) |
| Low-grade fibromyxoid sarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Malignant peripheral nerve sheath tumor | 1 (1.3) | 3 (4.7) | 4 (2.8) |
| Myxofibrosarcoma | 17 (21.5) | 3 (4.7) | 20 (14.0) |
| Rhabdomyosarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Sclerosing epithelioid fibrosarcoma | 1 (1.3) | 0 | 1 (0.7) |
| Synovial sarcoma | 4 (5.1) | 3 (4.7) | 7 (4.9) |
| Unclassified sarcoma | 3 (3.8) | 6 (9.4) | 9 (6.3) |
| Undifferentiated pleomorphic sarcoma | 18 (22.8) | 24 (37.5) | 42 (29.4) |
| Undifferentiated spindle cell sarcoma | 0 | 6 (9.4) | 6 (4.2) |
| Unknown | 7 (8.9) | 5 (7.8) | 12 (8.4) |
| Histologic grade (local) | |||
| G1 | 27 (34.2) | 0 | 27 (18.9) |
| G2 | 29 (36.7) | 13 (20.3) | 42 (29.4) |
| G3 | 20 (25.3) | 51 (79.7) | 71 (49.7) |
| Unknown | 3 (3.8) | 0 | 3 (2.1) |
| Histologic grade (central review) | |||
| G1 | 13 (16.5) | 0 | 13 (9.1) |
| G2 | 21 (26.6) | 12 (18.8) | 33 (23.1) |
| G3 | 38 (48.1) | 47 (73.4) | 85 (59.4) |
| Unknown | 7 (8.9) | 5 (7.8) | 12 (8.4) |
| Surgery | |||
| No | 5 (6.3) | 3 (4.7) | 8 (5.6) |
| Yes | 74 (93.7) | 61 (95.3) | 135 (94.4) |
| Amputation | |||
| No. | 74 | 61 | 135 |
| No | 73 (98.6) | 56 (91.8) | 129 (95.6) |
| Yes | 1 (1.4) | 5 (8.2) | 6 (4.4) |
| R status (local) | |||
| No. | 74 | 61 | 135 |
| R0 | 54 (73.0) | 56 (91.8) | 110 (81.5) |
| R1 | 18 (24.3) | 3 (4.9) | 21 (15.6) |
| R2 | 2 (2.7) | 0 | 2 (1.5) |
| Unknown | 0 | 2 (3.3) | 2 (1.5) |
| R status (central review) | |||
| No. | 74 | 61 | 135 |
| R0 | 56 (75.7) | 56 (91.8) | 112 (83.0) |
| R1 | 17 (23.0) | 3 (4.9) | 20 (14.8) |
| Unknown | 1 (1.4) | 2 (3.3) | 3 (2.2) |
| Tumor viability (central review) | |||
| No. | 74 | 61 | 135 |
| 0% | 14 (18.9) | 14 (23.0) | 28 (20.7) |
| 1%-24% | 22 (29.7) | 19 (31.1) | 41 (30.4) |
| 25%-50% | 10 (13.5) | 13 (21.3) | 23 (17.0) |
| 51%-75% | 6 (8.1) | 2 (3.3) | 8 (5.9) |
| 76%-100% | 20 (27.0) | 3 (4.9) | 23 (17.0) |
| Unknown | 2 (2.7) | 10 (16.4) | 12 (8.9) |
Abbreviations: NOS, not otherwise specified; RTOG, Radiation Therapy Oncology Group.
Among 135 patients who had surgery, the R status per local read was R0 in 110 (81.5%), R1 in 21 (15.6%), and R2 in 2 (1.5%), compared with 112 (83.0%), 20 (14.8%), and 0% per central review (Table 2). Per central review, 28 (20.7%) had 0% tumor viability or a pathologic complete response (pCR), 41 (30.4%) had 1% to 24% tumor viability, 23 (17.0%) had 25% to 50% tumor viability, 8 (5.9%) had 51% to 75% tumor viability, and 23 (17.0%) had greater than 75% tumor viability. In RTOG 9514 and 0630 the rates of 0% tumor viability were 14 (23.0%) and 14 (18.9%), respectively.
The local and central review of histologic types matched for 70 of 118 patients; the κ statistic was 0.51 (95% CI, 0.41-0.61), indicating moderate agreement (eTable 3 in Supplement 3). The local and central review of histologic grade matched for 86 of 128 patients; the κ statistic was 0.43 (95% CI, 0.31-0.56), indicating moderate agreement (eTable 4 in Supplement 3). The local and central review of R status matched for 125 of 131 patients. The κ statistic was 0.83 (95% CI, 0.71-0.96), indicating almost perfect agreement (eTable 5 in Supplement 3).
Figure 2A shows OS by percentage tumor viability and trial. There were no deaths in either study in the 0% tumor viability group resulting in a 100% 5-year OS. Five-year survival rates for greater than 0% tumor viability were 76.5% (95% CI, 62.3%-90.8%) in RTOG 9514 and 56.4% (95% CI, 43.3%-69.5%) in RTOG 0630. Patients with greater than 0% tumor viability had significantly worse OS in both univariate (P = .002) and multivariable analysis (P = .01) after adjustment for disease size, histologic grade, and histologic type (Table 3). Hazard ratios for tumor viability could not be estimated because there were no events in the 0% tumor viability group. The significance of tumor viability in multivariable results was robust to the selection of the final model (eTable 6 in Supplement 3).
Figure 2. Participant Clinical Outcomes by Trial and Posttreatment Tumor Viability.
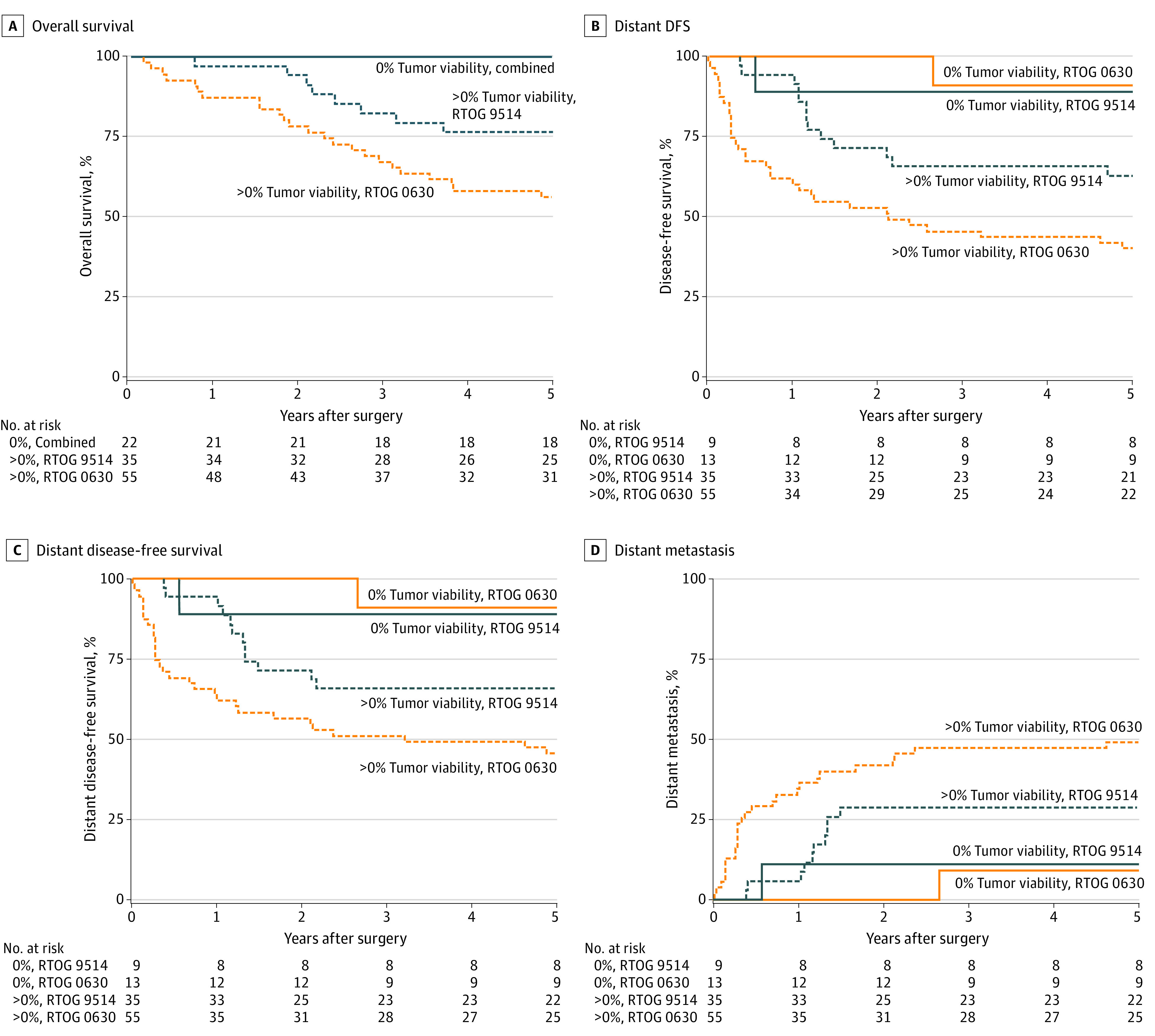
Estimated rates of (A) overall survival; B, disease-free survival; C, distant disease-free survival; and D, distant metastasis by posttreatment tumor viability.
Table 3. Multivariable Analysis of 112 Participants.
| End point | Variablea | HR (95% CI) | P value |
|---|---|---|---|
| Overall survival (37 events) | Size, cm (>20 vs ≤20) | 2.51 (1.12-5.59) | .03 |
| Grade (2-3 vs 1) | 3.49 (0.80-15.30) | .10 | |
| Histology (others vs leio or lipo or myxofib) | 2.24 (1.12-4.45) | .02 | |
| Tumor viability (>0% vs 0%) | Cannot estimate | .01b | |
| Disease-free survival (51 events) | Zubrod PS (1 vs 0) | 1.64 (0.87-3.10) | .13 |
| Location (other vs lower/upper extremity) | 1.93 (0.85-4.40) | .12 | |
| Size, cm (>16 vs ≤16) | 2.63 (1.42-4.88) | .002 | |
| Histology (others vs lipo or myxofib) | 2.42 (1.23-4.76) | .01 | |
| Tumor viability (>0% vs 0%) | 4.91 (1.51-15.93) | .008 | |
| Distant disease-free survival (47 events) | Zubrod PS (1 vs 0) | 2.02 (1.06-3.87) | .03 |
| Location (other vs lower/upper extremity) | 2.15 (0.94-4.91) | .07 | |
| Size, cm (>16 vs ≤16) | 2.78 (1.49-5.18) | .001 | |
| Histology (others vs leio or lipo or myxofib) | 1.78 (0.94-3.38) | .08 | |
| Tumor viability (>0% vs 0%) | 4.33 (1.32-14.14) | .02 | |
| Distant metastasis (40 events) | Zubrod PS (1 vs 0) | 2.45 (1.25-4.82) | .009 |
| Location (other vs lower/upper extremity) | 2.25 (0.92-5.49) | .08 | |
| Size, cm (>16 vs ≤16) | 2.74 (1.40-5.33) | .003 | |
| Tumor viability (>0% vs 0%) | 4.09 (1.25-13.36) | .02 |
Abbreviations: HR, hazard ratio; lipo, liposarcoma; myxofib, myxofibrosarcoma; PS, performance status; leio, leiomyosarcoma.
All tumor viability data, 94% of grade data, and 94% of histologic data were based on central review; all other data were from the treating institution.
There were no events in the 0% tumor viability group so the hazard ratio and P value cannot be estimated by the Cox model. The P value comes from the log-rank test stratified by study, size, grade, and histologic findings.
Figure 2B shows DFS by posttreatment tumor viability and trial. The 5-year DFS rates in RTOG 9514 patients with 0% tumor viability and greater than 0% tumor viability were 88.9% (95% CI, 68.4%-100.0%) and 62.7% (95% CI, 46.6%-78.8%), respectively. The 5-year DFS rates in RTOG 0630 patients with 0% tumor viability and greater than 0% tumor viability were 90.9% (95% CI, 73.9%-100.0%) and 40.0% (95% CI, 27.1%-52.9%), respectively. In univariate analysis, greater than 0% tumor viability was associated with a 5-fold increase in the hazard of DFS failure (HR, 5.11; 95% CI, 1.59-16.41; P = .006). After adjustment for Zubrod performance status, disease location, disease size, and histologic type, the estimated hazard ratio was 4.91 (95% CI, 1.51-15.93) with P = .008 (Table 3). The significance of posttreatment tumor viability in multivariable results was robust to the selection of the final model (eTable 7 in Supplement 3).
Figure 2C shows DDFS by percentage of tumor viability and trial. The 5-year DDFS rates in RTOG 9514 patients with 0% tumor viability and greater than 0% tumor viability were 88.9% (95% CI, 68.4%-100.0%) and 65.7% (95% CI, 50.0%-81.4%), respectively. The 5-year DDFS rates in RTOG 0630 patients with 0% tumor viability and greater than 0% tumor viability were 90.9% (95% CI, 73.9%-100.0%) and 45.5% (95% CI, 32.3%-58.6%), respectively. In univariate analysis, greater than 0% tumor viability was associated with a 4-fold increase in the hazard of DDFS failure (HR, 4.41; 95% CI, 1.37-14.21; P = .01). After adjustment for Zubrod performance status, disease location, disease size, and histologic type, the estimated hazard ratio was 4.33 (95% CI, 1.32-14.14; P = .02) (Table 3). The significance of posttreatment tumor viability in multivariable results was robust to the selection of the final model (eTable 8 Supplement 3).
Figure 2D shows distant metastasis by percentage tumor viability and trial. The 5-year distant metastasis (DM) rates in RTOG 9514 patients with 0% tumor viability and greater than 0% tumor viability were 11.1% (95% CI, 0.5%-40.6%) and 28.6% (95% CI, 14.7%-44.1%), respectively. The 5-year DM rates in RTOG 0630 patients with 0% tumor viability and greater than 0% tumor viability were 9.1% (95% CI, 0.4%-34.7%) and 49.1% (95% CI, 35.2%-61.6%), respectively. In univariate analysis, greater than 0% tumor viability was associated with a nearly 4-fold increase in the hazard of DM (HR, 3.72; 95% CI, 1.15-12.09; P = .03). After adjustment for Zubrod performance status, disease location, and disease size, the estimated hazard ratio was 4.09 (95% CI, 1.25-13.36; P = .02) (Table 3). The significance of posttreatment tumor viability in multivariable results was robust to the selection of the final model (eTable 9 in Supplement 3).
In both studies, the 5-year LF rate for patients with 0% tumor viability was 0%. For patients with greater than 0% tumor viability, the 5-year LF rates were 11.7% (95% CI, 3.6%-25.1%) and 9.1% (95% CI, 3.3%-18.5%) in RTOG 9514 and 0630, respectively. Patients with greater than 0% tumor viability did not have significantly more LF in univariate analysis (P = .08). Hazard ratios for posttreatment tumor viability could not be estimated because there were no events in the 0% tumor viability group. Multivariable analysis is not possible due to the limited number of events (11).
Discussion
We previously reported that at a median follow-up of 3.6 years, preoperative image-guided RT with margin reduction could significantly reduce radiation-related late morbidities (subcutaneous fibrosis, edema, and joint stiffness)4 compared with the preoperative arm results of the CAN-NCIC-SR2 randomized trial (10.5% vs 37%, P < .001).2 No marginal recurrence was found in the reduced volume. The estimated 2-year LR-free, R/DR-free, and PFS rates per the CAN-NCIC-SR2 trial definitions were 94.0% (95% CI, 88.2%-99.7%), 65.3% (95% CI, 54.2%-76.3%), and 61.5% (95% CI, 50.3%-72.7%), respectively. The estimated 2-year OS rate was 80.6% (95% CI, 71.8%-89.4%). In RTOG 0630, at median follow-up of 6.0 years, we observed only 1 new in-field LR compared with the initial report.4 The long-term disease end point results from this multi-institutional prospective phase 2 clinical trial have further proven that the parameters used, namely reductions of longitudinal CTV margin to 3 cm for intermediate- or high-grade soft tissue sarcoma of 8 or more cm or to 2 cm for low-grade soft tissue sarcomas or tumors smaller than 8 cm, are appropriate for preoperative image-guided radiotherapy.
To date, the significance of treatment-induced of tumor viability in STS has not been well defined. Of note, the pathology co-chair (D.L.) has detailed the methodology in a prior publication,10 in which we concluded that it is reproducible. Data are mixed on the prognostic value of pathologic response as a marker for oncologic outcomes for preoperative therapy for STS of the trunk and extremity.9,10,11 The combined analysis of RTOG 9514 and 0630 shows that pathologic response is prognostic for cancer-related outcomes. In this study pCR, defined as 0% tumor viability in the final specimen after neoadjuvant treatment, was associated with improved survival outcomes in patients with STS treated with either neoadjuvant RT or CT-RT from the long-term update of these 2 multicenter prospective studies.
In this series, 21% of patients had 0% tumor viability noted on central review. In RTOG 9514 and 0630 the rates of 0% tumor viability were 23% and 19%, respectively. Importantly, single institutional series with similar treatment cohorts to RTOG 9514 and 0630 showed lower pCR rates with preoperative therapy. One analysis11 evaluated preoperative chemoradiation for intermediate to high-grade extremity and trunk STS. Similar to RTOG 9514, patients received 6 cycles of mesna, doxorubicin, ifosfamide, and dacarbazine chemotherapy interdigitated with 44 Gy RT. The authors noted that 9% experienced 0% tumor viability. Another study12 evaluated patients with grades 1 to 3 STS of the extremity and trunk who received preoperative radiation alone (median dose, 50 Gy) and similarly found a 9% pCR rate.
In the current analysis, there were no deaths in either RTOG study in the 0% tumor viability group resulting in a 100% 5-year OS. Five-year survival rates for greater than 0% tumor viability were 76.5% in RTOG 9514 and 56.4% in RTOG 0630. The LF rate was 0% for patients with pCR vs 5-year 11.7% and 9.1% for patients with less than pCR in RTOG studies 9514 and 0630, respectively. Unlike this study, single-institution series were unable to correlate post-treatment tumor viability rates with oncologic outcomes.11,12 Importantly, hyalinization/fibrosis was found to be a favorable predictor of RFS and OS.12 Correlation with hyalinization/fibrosis and disease outcomes is warranted in future studies.
Furthermore, some studies10,11,13 suggest that patients with low posttreatment tumor viability rates are at higher risk of developing or dying from metastatic disease. This did not prove true in our series. In RTOG 9514, 5-year distant metastatic rates for patients with 0% tumor viability and greater than 0% tumor viability were 11.1% and 28.6%, respectively. In RTOG 0630, 5-year distant metastatic rates for patients with 0% tumor viability and greater than 0% tumor viability were 9.1% and 49.1%, respectively. The current study clearly shows that those with 0% tumor viability are significantly less likely to develop distant metastatic disease than those who have greater than 0% tumor viability on final pathologic findings, suggesting that this is a reasonable marker to assess therapeutic efficacy.
Data are mixed as to whether histologic findings can be correlated with outcomes. Although studies suggest a clear correlation between pCR and outcomes for Ewing sarcoma and osteosarcoma,6,7,8 the prognostic effect of histologic response to therapy is less clear for truncal or extremity STS.9,10,11 Review of RTOG 9514 and 0630 showed that histologic findings could predict outcomes. In our series, leiomyosarcoma, liposarcoma, and myxofibrosarcoma were associated with better OS, whereas liposarcoma and myxofibrosarcoma were associated with better DFS. Myxoid histologies have been shown to be quite responsive to therapy.14 These data suggest that histologic findings were prognostic of outcomes and should be taken into account for tailoring therapy and further clinical trial stratification.
Tumor response noted on imaging could provide further biomarker data that could be correlated with pathologic response and disease outcomes.15,16 Treatment-related changes in tumor size and contrast enhancement noted on imaging for gastrointestinal stromal tumors were shown by Choi et al.17 This protocol was adapted for high-risk STS of the trunk and extremities and found to predict treatment response and disease-related outcomes.17,18 Although we did not correlate pathologic and imaging response to oncologic outcomes in this study, we believe that further assessment is warranted in future clinical trials.
Strengths and Limitations
To our knowledge, this is the first report of the prognostic significance of pathologic response in patients with STS who received preoperative therapy with chemotherapy and/or radiation from RTOG studies 9514 and 0630. A strength of this study include analysis of 2 multicenter, prospective phase 2 RTOG clinical trials. Several limitations exist. First, this was an ancillary analysis of a small number of patients rather than a prespecified end point of RTOG 9514 and 0630. Second, correlation of hyalinization/fibrosis to oncologic outcomes could have further strengthened this study. Third, assessment of imaging and pathologic response in relation to disease outcomes was not performed and would provide valuable and potentially prognostic biomarker information. Finally, further clarity is required on which histologic types may benefit from treatment intensification and personalized therapy.
Conclusions
The long-term update of disease outcome from the multi-institutional preoperative image-guided radiotherapy clinical trial (RTOG 0630) has further suggested that the target volumes used in this study are appropriate for preoperative image-guided RT. Furthermore, the ancillary analysis with RTOG studies 9514 and 0630 has demonstrated that pCR was associated with improved survival outcomes in patients with STS who receive preoperative therapy. To this end, pCR should be considered a prognostic factor for clinical outcomes in future STS clinical trials.
Trial Protocol (RTOG 0630)
Trial Protocol (RTOG 9514)
eFigure 1. Flow Diagram
eAppendix. Report
eTable 1. Late Toxicities of Interest at 1-5 Years in RTOG 0630
eTable 2. Treatment-Related Late Adverse Events Ocurring in At Least 5% of Patients in RTOG 0630 (n=75)
eTable 3. Comparison of Local and Central Review Histology
eTable 4. Comparison of Local and Central Review Histologic Grade
eTable 5. Comparison of Local and Central Review R Status
eTable 6. Alternative Multivariable Models for Overall Survival (n=112; 37 events)
eTable 7. Alternative Multivariable Models for Disease-Free Survival (n=112; 51 events)
eTable 8. Alternative Multivariable Models for Distant Disease-Free Survival (n=112; 47 events)
eTable 9. Alternative Multivariable Models for Distant Metastasis (n=112; 40 events)
Data Sharing Statement
References
- 1.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48-53. doi: 10.1016/j.radonc.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241. doi: 10.1016/S0140-6736(02)09292-9 [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119(10):1878-1884. doi: 10.1002/cncr.27951 [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: Results of radiation therapy oncology group RTOG-0630 trial. J Clin Oncol. 2015;33(20):2231-2238. doi: 10.1200/JCO.2014.58.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer. 2010;116(19):4613-4621. doi: 10.1002/cncr.25350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picci P, Böhling T, Bacci G, et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J Clin Oncol. 1997;15(4):1553-1559. doi: 10.1200/JCO.1997.15.4.1553 [DOI] [PubMed] [Google Scholar]
- 7.Lewis IJ, Nooij MA, Whelan J, et al. ; MRC BO06 and EORTC 80931 collaborators; European Osteosarcoma Intergroup . Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99(2):112-128. doi: 10.1093/jnci/djk015 [DOI] [PubMed] [Google Scholar]
- 8.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790. doi: 10.1200/JCO.2002.20.3.776 [DOI] [PubMed] [Google Scholar]
- 9.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203-3209. doi: 10.1200/JCO.2001.19.13.3203 [DOI] [PubMed] [Google Scholar]
- 10.Lucas DR, Kshirsagar MP, Biermann JS, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: clinicopathological correlation. Oncologist. 2008;13(4):451-458. doi: 10.1634/theoncologist.2007-0220 [DOI] [PubMed] [Google Scholar]
- 11.Mullen JT, Hornicek FJ, Harmon DC, et al. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer. 2014;120(23):3676-3682. doi: 10.1002/cncr.28945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer IM, Hornick JL, Barysauskas CM, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group Response Score. Int J Radiat Oncol Biol Phys. 2017;98(2):375-383. doi: 10.1016/j.ijrobp.2017.02.087 [DOI] [PubMed] [Google Scholar]
- 13.Henshaw RM, Priebat DA, Perry DJ, Shmookler BM, Malawer MM. Survival after induction chemotherapy and surgical resection for high-grade soft tissue sarcoma. Is radiation necessary? Ann Surg Oncol. 2001;8(6):484-495. doi: 10.1007/s10434-001-0484-8 [DOI] [PubMed] [Google Scholar]
- 14.de Vreeze RS, de Jong D, Haas RL, Stewart F, van Coevorden F. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J Radiat Oncol Biol Phys. 2008;72(5):1480-1487. doi: 10.1016/j.ijrobp.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 15.Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer. 2013;49(4):875-883. doi: 10.1016/j.ejca.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi M, Kharofa J, Zambrano EV, et al. Increase in tumor size on MRI after neoadjuvant treatment is associated with greater pathologic necrosis and poor survival in patients with soft tissue sarcoma. J Interv Oncol. 2013;2:109. [Google Scholar]
- 17.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753-1759. doi: 10.1200/JCO.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- 18.Stacchiotti S, Verderio P, Messina A, et al. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer. 2012;118(23):5857-5866. doi: 10.1002/cncr.27624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol (RTOG 0630)
Trial Protocol (RTOG 9514)
eFigure 1. Flow Diagram
eAppendix. Report
eTable 1. Late Toxicities of Interest at 1-5 Years in RTOG 0630
eTable 2. Treatment-Related Late Adverse Events Ocurring in At Least 5% of Patients in RTOG 0630 (n=75)
eTable 3. Comparison of Local and Central Review Histology
eTable 4. Comparison of Local and Central Review Histologic Grade
eTable 5. Comparison of Local and Central Review R Status
eTable 6. Alternative Multivariable Models for Overall Survival (n=112; 37 events)
eTable 7. Alternative Multivariable Models for Disease-Free Survival (n=112; 51 events)
eTable 8. Alternative Multivariable Models for Distant Disease-Free Survival (n=112; 47 events)
eTable 9. Alternative Multivariable Models for Distant Metastasis (n=112; 40 events)
Data Sharing Statement


