Key Points
Questions
What are the rates of posttonsillectomy bleeding in pediatric patients, and what is its predicted distribution?
Findings
This retrospective cohort study of 96 415 children who underwent tonsillectomy found that the predicted 5th, 50th, and 95th quantiles for bleeding after a tonsillectomy procedure were 1.17%, 1.97%, and 4.75%, respectively.
Meaning
The findings of this retrospective cohort study provide estimated bleeding rates to guide surgeons in self-monitoring their individual frequency and improving quality outcomes for pediatric patients after tonsillectomy.
Abstract
Importance
The American Academy of Otolaryngology–Head and Neck Surgery Foundation has recommended yearly surgeon self-monitoring of posttonsillectomy bleeding rates. However, the predicted distribution of rates to guide this monitoring remain unexplored.
Objective
To use a national cohort of children to estimate the probability of bleeding after pediatric tonsillectomy to guide surgeons in self-monitoring of this event.
Design, Settings, and Participants
This retrospective cohort study used data from the Pediatric Health Information System for all pediatric (<18 years old) patients who underwent tonsillectomy with or without adenoidectomy in a children’s hospital in the US from January 1, 2016, through August 31, 2021, and were discharged home. Predicted probabilities of return visits for bleeding within 30 days were calculated to estimate quantiles for bleeding rates. A secondary analysis included logistic regression of bleeding risk by demographic characteristics and associated conditions. Data analyses were conducted from August 7, 2022 to January 28, 2023.
Main Outcomes and Measures
Revisits to the emergency department or hospital (inpatient/observation) for bleeding (primary/secondary diagnosis) within 30 days after index discharge after tonsillectomy.
Results
Of the 96 415 children (mean [SD] age, 5.3 [3.9] years; 41 284 [42.8%] female; 46 954 [48.7%] non-Hispanic White individuals) who had undergone tonsillectomy, 2100 (2.18%) returned to the emergency department or hospital with postoperative bleeding. The predicted 5th, 50th, and 95th quantiles for bleeding were 1.17%, 1.97%, and 4.75%, respectively. Variables associated with bleeding after tonsillectomy were Hispanic ethnicity (OR, 1.19; 99% CI, 1.01-1.40), very high residential Opportunity Index (OR, 1.28; 99% CI, 1.05-1.56), gastrointestinal disease (OR, 1.33; 99% CI, 1.01-1.77), obstructive sleep apnea (OR, 0.85; 99% CI, 0.75-0.96), obesity (OR,1.24; 99% CI, 1.04-1.48), and being more than 12 years old (OR, 2.48; 99% CI, 2.12-2.91). The adjusted 99th percentile for bleeding after tonsillectomy was approximately 6.39%.
Conclusions and Relevance
This retrospective national cohort study predicted 50th and 95th percentiles for posttonsillectomy bleeding of 1.97% and 4.75%. This probability model may be a useful tool for future quality initiatives and surgeons who are self-monitoring bleeding rates after pediatric tonsillectomy.
This retrospective national cohort study examines bleeding after tonsillectomy among US children to estimate its probability and to provide guidance to surgeons who are self-monitoring bleeding frequency.
Introduction
Tonsillectomy is among the most common pediatric surgical procedures, often indicated for recurrent tonsillitis or obstructive sleep-disordered breathing,1,2,3,4,5,6 such as obstructive sleep apnea (OSA), which affects 1% to 5% of children from 2 to 8 years of age.7,8 Given the frequency of this procedure and the potential for associated comorbidities, clinicians should frequently monitor outcomes after pediatric tonsillectomy.9,10,11,12
In its 2019 tonsillectomy clinical practice guidelines,8 the American Academy of Otolaryngology–Head and Neck Surgery Foundation recommended that clinicians self-monitor posttonsillectomy bleeding rates. Research suggests that as many as one-third of deaths after tonsillectomy are associated with bleeding events. Furthermore, primary and secondary posttonsillectomy hemorrhage represent among the most frequent reasons for hospital readmission after surgery. The recognized challenges from bleeding after tonsillectomy emphasize the importance of periodic monitoring to ensure acceptable rates of this complication. However, surgeons lack reliable estimates of posttonsillectomy bleeding rates to compare with assessments of their individual data.
The Pediatric Health Information Systems (PHIS) database contains deidentified patient information from more than 49 not-for-profit children’s hospitals in the US. Prior research has been conducted across a variety of pediatric subspecialities given the extensive nature of this resource. This large data set is well suited to evaluating rates of postoperative complications that reflect population trends. The primary objective of this study was to estimate a probability distribution of bleeding after pediatric tonsillectomy. A secondary objective was to identify patient-specific risk factors for bleeding revisits to the emergency department (ED) or hospital after pediatric tonsillectomy. We hypothesized that the median rate for posttonsillectomy bleeding would be approximately 2%.
Methods
This study was granted exemption from review by the University of Texas Southwestern Institutional Review Board because it used only external deidentified data; informed consent was also waived. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
We performed a retrospective national cohort study using PHIS records of children who met the inclusion criteria: age 0 to 17 years; had undergone tonsillectomy with or without adenoidectomy from January 1, 2016, to August 31, 2021; and were discharged home. The primary outcome of interest was a revisit to the ED or hospital (inpatient/observation) for bleeding (primary/secondary diagnosis) within 30 days after index discharge.
The PHIS database is a comparative pediatric database that includes clinical and resource utilization data for inpatient, ambulatory surgery, emergency department, and observation unit patient encounters from more than 49 not-for-profit children’s hospitals in the US. Participating hospitals provide deidentified encounter data, including patient demographic characteristics, diagnosis and procedure codes per the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), length of stay, and discharge information. The PHIS is maintained by the Children’s Hospital Association, which in a combined effort with participating hospitals, reviews the data bimonthly to provide quarterly quality reports.13
Tonsillectomy cases were identified using ICD-10 codes 0CTPXZZ, 0CTP0ZZ, 0C5P0ZZ, 0C503ZZ, 0C5PXZZ, 0CBP0ZZ, 0CBP3ZZ, and 0CBPXZZ. Tonsillectomy with adenoidectomy cases were identified using ICD-10 codes 0CTQXZZ, 0CTQ0ZZ, 0C5Q0ZZ, 0C5Q3ZZ, 0C5QXZZ, 0CBQ0ZZ, 0CBQ3ZZ, and 0CBQXZZ (adenoidectomy procedures); and ICD-10 codes 0CTPXZZ, 0CTP0ZZ, 0C5P0ZZ, 0C503ZZ, 0C5PXZZ, 0CBP0ZZ, 0CBP3ZZ, and 0CBPXZZ (tonsillectomy procedures). The specific Current Procedural Terminology codes were 42820, 42821, 42825, and 42826.
Patients with posttonsillectomy bleeding were defined as those patients who returned to the ED or were re-admitted to the hospital and were ascribed a primary or secondary ICD-10 diagnosis code of J95.830, ie, “postprocedural hemorrhage of a respiratory system organ or structure following a respiratory system procedure.”
Also recorded from each patient’s PHIS record were: age (years); sex (male/female); race and ethnicity (Asian, non-Hispanic Black [henceforth Black], Hispanic, non-Hispanic White [henceforth White], or other); primary payer (government, private, other); prematurity (<37 weeks gestation); cardiovascular disease (heart and great vessel malformation, endocardium disease, cardiomyopathy, conduction disorder, dysrhythmia, device, or transplantation); gastrointestinal disease (congenital anomaly, chronic liver disease/cirrhosis, inflammatory bowel disease, device, transplantation, or other); neurological disorder (brain/spinal cord malformation, intellectual disability, central nervous system degeneration/disease/disorder, infantile cerebral palsy, epilepsy, occlusion of cerebral artery, muscular dystrophy/myopathy, or movement disease/device); genetic variation (chromosomal anomaly); complex condition (≥1 complex chronic condition); trisomy 21 (yes/no); OSA (yes/no); chronic tonsillitis (yes/no); older than 12 years (yes/no); American Academy of Pediatrics age category (ie, neonate [<30 days], infant [≥30 days and <1 year], early childhood [≥1 year and <5 years], late childhood (≥5 years and <13 years], adolescent [≥13 years and ≤17 years]); urban residence (per zip code); and Opportunity Index (very low, low, moderate, high, very high), a composite metric based on education, health, and environment data based on US census data.14
Statistical Analysis
The association of posttonsillectomy bleeding was evaluated with each variable in a univariate analysis using unadjusted logistic regression. Odds ratios (ORs) and 99% CIs were reported for each variable. The probability of posttonsillectomy bleeding was modeled with a multivariable logistic regression, with a generalized estimating equation to account for potential clustering by hospital. Variables that exhibited P values < .25 in the univariate analysis were added to the model for the dependent variable (posttonsillectomy bleeding). Because of the large sample size and no issues of overfitting, all statistically and clinically relevant variables that entered the model were retained. Calibration was assessed by plotting a scatter plot smoother and overlaying a diagonal line that represented the line of perfect calibration.15,16 The distribution of the predicted probability of posttonsillectomy bleeding was calculated and graphically displayed.
Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from August 7, 2022, to January 28, 2023, using SAS Enterprise Guide, version 8.3 (SAS Institute). Missing values were managed by listwise deletion. The ORs and adjusted ORs were used as measures of effect size.
Results
A search of the PHIS database identified 96 415 children (mean [SD] age, age, 5.2 [3.9] years; 41 284 [42.8%] female and 55 119 (57.2%) male; 1829 [1.9%] Asian, 20 183 [20.9%] Black, 18 542 [19.2%] Hispanic, 46 954 [48.7%] White, and 8907 [9.2%] individuals of other race and ethnicity) who had undergone pediatric tonsillectomy during the study period. Of the total, 2100 patients (2.2%; median [IQR] age, 4 [2-7] years) revisited the ED or hospital with postoperative hemorrhage.
The government was the primary payer for most patients (n = 55 665; 57.7%). The distribution of the Opportunity Index included more children in the very low (n = 21 383; 22.2%) than the very high category (n = 16 680; 17.3%). More than half (n = 53 356; 55.3%) of the children had been diagnosed with OSA. The following variables were different in the univariate analysis for bleeding: race and ethnicity (2.2% White vs 1.6% Black; 2.7% Hispanic vs 2.2% White), primary payer (3.2% other payer vs 2.1% government), complex chronic condition, gastrointestinal disease, chronic tonsillitis, diabetes, and the proportion older than 12 years of age. Table 1 presents the full cross-tabulations.
Table 1. Bleeding Rates After Pediatric Tonsillectomy, by Patient Characteristic.
| Characteristic | Total, No. (%) | Bleeding rate (%) | OR (99% CI) |
|---|---|---|---|
| Age ≥12 y | |||
| No | 87 416 (90.7) | 1.91 | 1 [Reference] |
| Yes | 8999 (9.3) | 4.80 | 2.59 (2.22-2.99) |
| Age group | |||
| Infant | 173 (0.2) | 1.73 | 1 [Reference] |
| Early childhood | 55 464 (57.5) | 1.64 | 0.95 (0.21-4.25) |
| Late childhood | 33 913 (35.2) | 2.44 | 1.42 (0.32-6.38) |
| Adolescent | 6865 (7.1) | 5.21 | 3.12 (0.69-14.07) |
| Sex | |||
| Female | 41 284 (42.8) | 2.10 | 0.94 (0.84-1.05) |
| Male | 55 119 (57.2) | 2.24 | 1 [Reference] |
| Race and ethnicity | |||
| Asian | 1829 (1.9) | 2.62 | 1.20 (0.81-1.76) |
| Black | 20 183 (20.9) | 1.64 | 0.74 (0.63-0.88) |
| Hispanic | 18 542 (19.2) | 2.71 | 1.24 (1.07-1.43) |
| White | 46 954 (48.7) | 2.20 | 1 [Reference] |
| Other | 8907 (9.2) | 2.08 | 0.94 (0.81-1.16) |
| Payer | |||
| Government | 55 665 (57.7) | 2.13 | 1 [Reference] |
| Private | 37 201 (38.6) | 2.16 | 1.02 (0.90-1.15) |
| Other | 3549 (3.7) | 3.16 | 1.50 (1.16-1.94) |
| Opportunity Index | |||
| Very low | 21 383 (22.2) | 2.04 | 1 [Reference] |
| Low | 19 725 (20.5) | 2.10 | 1.03 (0.86-1.23) |
| Moderate | 17 504 (18.2) | 2.23 | 1.10 (0.91-1.31) |
| High | 15 634 (16.2) | 2.30 | 1.13 (0.94-1.36) |
| Very high | 16 680 (17.3) | 2.58 | 1.27 (1.06-1.51) |
| Missing data | 5489 (5.7) | 1.26 | 0.6 (0.44-0.85) |
| Urban zip code | |||
| No | 13 741 (14.3) | 1.87 | 1 [Reference] |
| Yes | 81 271 (84) | 2.24 | 1.20 (1.01-1.43) |
| Complex chronic condition | |||
| No | 79 319 (82.3) | 2.12 | 1 [Reference] |
| Yes | 17 096 (17.7) | 2.45 | 1.16 (1.01-1.34) |
| Premature birth | |||
| No | 95 785 (99.3) | 2.18 | 1 [Reference] |
| Yes | 630 (0.7) | 1.75 | 0.80 (0.36-1.75) |
| Cardiovascular disease | |||
| No | 94 063 (97.6) | 2.16 | 1 [Reference] |
| Yes | 2352 (2.4) | 2.81 | 1.31 (0.94-1.81) |
| Gastrointestinal disease | |||
| No | 92 897 (96.4) | 2.15 | 1 [Reference] |
| Yes | 3518 (3.6) | 2.84 | 1.33 (1.02-1.74) |
| Neurologic disorder | |||
| No | 93 756 (97.2) | 2.18 | 1 [Reference] |
| Yes | 2659 (32.8) | 2.11 | 0.97 (0.68-1.37) |
| Genetic variation | |||
| No | 89 597 (92.9) | 2.16 | 1 [Reference] |
| Yes | 6818 (7.1) | 2.45 | 1.14 (0.92-1.41) |
| Trisomy 21 | |||
| No | 91 601 (95.0) | 2.17 | 1 [Reference] |
| Yes | 4814 (5.0) | 2.39 | 1.10 (0.86-1.42) |
| Obstructive sleep apnea | |||
| No | 43 059 (44.7) | 2.24 | 1 [Reference] |
| Yes | 53 356 (55.3) | 2.13 | 0.95 (0.85-1.06) |
| Chronic tonsillitis | |||
| No | 94 654 (98.2) | 2.16 | 1 [Reference] |
| Yes | 1761 (1.8) | 3.29 | 1.54 (1.09-2.19) |
| Obesity | |||
| No | 85 329 (88.5) | 2.05 | 1 [Reference] |
| Yes | 11 086 (11.5) | 3.18 | 1.57 (1.35-1.83) |
| Died (after index stay) | 0 (0) | 0 | NA |
Abbreviations: NA, not applicable; OR, odds ratio.
A probability distribution model of bleeding included age, sex, race and ethnicity, payer type, Opportunity Index, urban residence, gastrointestinal disease, cardiovascular disease, genetic variation, OSA, chronic tonsillitis, and obesity. The model showed that adolescent age, sex, Hispanic ethnicity, other insurance payer, residing in a very high opportunity zip code, gastrointestinal disease, and obesity were associated with increased bleeding risk. Table 2 presents all ORs and 99% CIs. Black race was associated with a decreased risk of bleeding (OR, 0.78; 99% CI, 0.65-0.93). The variable that showed the smallest confidence interval—suggesting a high precision of effect-size measurement—was residing in a very high Opportunity Index area (OR, 1.28; 99% CI, 1.05-1.56).
Table 2. Multivariable Analysis for Bleeding Revisits After Pediatric Tonsillectomy.
| Variable | Odds ratio (99% CI) |
|---|---|
| Age ≥12, y | |
| No | 1 [Reference] |
| Yes | 2.48 (2.12-2.91) |
| Sex | |
| Female | 0.91 (0.81-1.03) |
| Male | 1 [Reference] |
| Race and ethnicity | |
| Asian | 0.92 (0.74-1.15) |
| Black | 0.78 (0.65-0.93) |
| Hispanic | 1.19 (1.01-1.40) |
| White | 1 [Reference] |
| Other | 0.92 (0.74-1.15) |
| Payer | |
| Government | 1 [Reference] |
| Private | 0.99 (0.87-1.14) |
| Other | 1.60 (1.21-2.12) |
| Opportunity Index | |
| Very low | 1 [Reference] |
| Low | 1.02 (0.85-1.23) |
| Moderate | 1.09 (0.90-1.31) |
| High | 1.13 (0.93-1.38) |
| Very high | 1.28 (1.05-1.56) |
| Urban zip code | |
| No | 1 [Reference] |
| Yes | 1.15 (0.95-1.39) |
| Cardiovascular disease | |
| No | 1 [Reference] |
| Yes | 1.23 (0.87-1.73) |
| Gastrointestinal disease | |
| No | 1 [Reference] |
| Yes | 1.33 (1.01-1.77) |
| Genetic variation | |
| No | 1 [Reference] |
| Yes | 1.07 (0.84-1.35) |
| Obstructive sleep apnea | |
| No | 1 [Reference] |
| Yes | 0.85 (0.75-0.96) |
| Chronic tonsillitis | |
| No | 1 [Reference] |
| Yes | 1.33 (0.93-1.91) |
| Obesity | |
| No | 1 [Reference] |
| Yes | 1.24 (1.04-1.48) |
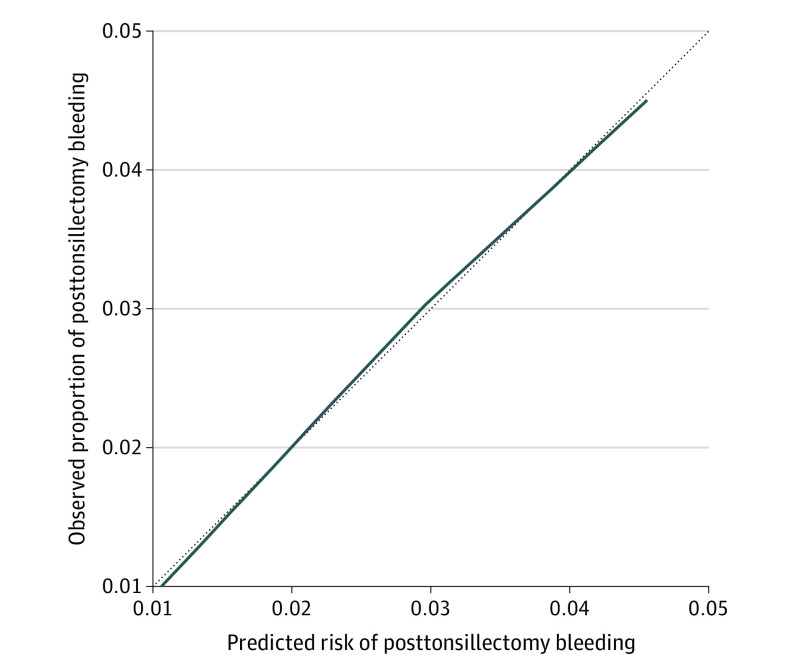
Figure 1 shows a calibration plot that indicates an adequate fit of the linear model. The smooth line is close to the diagonal with minimal nonsystematic deviation, indicating a fairly calibrated model.
Figure 1. Calibration Plot of Prediction Model for Bleeding After Pediatric Tonsillectomy.
The dotted diagonal line represents the line of perfect calibration. The solid line represents predicted risk vs observed probability.
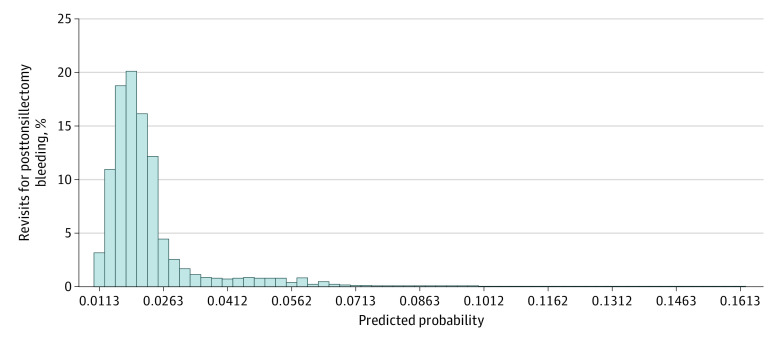
Figure 2 shows the probability distribution in graphic form. The median predicted probability of revisits for posttonsillectomy bleeding across all patients was 1.97%. The 5th percentile of the distribution was 1.17%; the 95th percentile, 4.75%; and the 99th percentile, 6.39%. The number of children with hematologic disorders accounted for fewer than 1.00% of patients and had no bearing on these findings. We did not perform additional sensitivity analyses.
Figure 2. Distribution of the Predicted Probability of Bleeding After Pediatric Tonsillectomy.
Discussion
This retrospective national cohort study was performed to estimate the probability of posttonsillectomy bleeding in children, and to guide surgeon self-monitoring of postoperative bleeding frequency. The probability distribution estimated that revisits for posttonsillectomy bleeding occurred at a median rate of 1.97%, and 6.39% at the 99th percentile.
The updated clinical practice guideline for tonsillectomy in children8 recommends that clinicians determine their primary and secondary posttonsillectomy bleeding rates annually or more often. The addition of this key action statement highlights current trends aimed at improving surgical quality and outcomes. During the iterative process of writing the guideline, nonotolaryngologists were surprised to learn that many otolaryngologists do not know their individual or institutional posttonsillectomy bleeding rates.17,18 A challenge to knowing this information is the difficulty with capturing posttonsillectomy bleeding occurrences. Surgeons who belong to large institutions may have access to quality reports from third-party organizations. Pediatric otolaryngologists participating in the PHIS system can build custom reports and compare their rates of posttonsillectomy bleeding with data from other PHIS institutions. Unfortunately, many tonsillectomy procedures are performed by physicians without access to robust reporting frameworks. For these surgeons, measuring their tonsillectomy bleeding rate requires a substantial effort. Furthermore, even if they can determine a frequency of events, there are no establilshed ranges with which to compare their rates. This current study fills an important quality and safety knowledge gap by providing estimations of the probability of pediatric posttonsillectomy bleeding.19,20,21
As in other series, a posttonsillectomy bleeding case was defined as a patient who requires a return visit to the ED or hospital for bleeding.13,22,23,24,25 This definition does not capture any minor bleeding that may be resolved at home. Although this approach underestimates the natural bleeding rate, minor self-resolving bleeding episodes minimally affect quality and safety outcomes. The purpose of measuring the bleeding rate is to capture clinically important events. Although some revisits for bleeding resolve without surgical intervention, from a time and cost perspective, these are still important. Severe bleeding with prolonged recovery is also likely to be captured by these visits. Therefore, using ED and hospital revisits is a useful and objective measure of posttonsillectomy bleeding.26
In this adjusted model, the median (range) bleeding rate was 1.97% (1.17% at the 5th percentile to 4.75% at the 95th percentile). A previous meta-analysis27 showed a mean posttonsillectomy bleeding rate of 4.2%, which corresponds to the 95th percentile in our adjusted model. Additional investigations explored bleeding rates by technique27,28,29; electrocautery use has been associated with a rate of 4.9%, whereas harmonic scalpel (11.3%) and laser (5.3%) use have been associated with higher bleeding rates. Given the differences in outcomes among tonsillectomy techniques, technique is an important consideration for surgeons that was not explored in this series. Other studies have shown posttonsillectomy bleeding rates ranging from 2% to 5%.30,31 Most investigations have used similar definitions of tonsil bleeding (ie, return visits) and have included both single-institution data and large databases. A previous PHIS study found a bleeding rate of 1.58%32; however, it did not create a probability model, which we have shown can be useful for providing an important perspective to surgeons when self-monitoring their rates.
Older age was a risk factor for posttonsillectomy bleeding on univariate analysis but not on multivariable modeling. Previous studies have consistently found older age to be associated with an increased rate of posttonsillectomy hemorrhage.7,33,34,35,36 Although the mechanism is unclear, there is a hypothesis that the tonsillar fossa heals by secondary intention, and bleeding can occur when the wound sloughs. A relatively dry oropharynx (from inadequate hydration) can compound the propensity for bleeding.37,38 Older children and adolescents who tend to maintain hydration status well enough to prevent gross signs of dehydration may have relative dehydration, hence more secondary bleeding than younger children who present earlier with dehydration.39,40,41 Conversely, the tonsil bleed rate may be associated with the larger feeding vessels and surface area in adolescents, making hemostasis less likely if bleeding occurs. Regardless, this and other data show that increasing age has been associated with more revisits for tonsil bleeding; therefore, any evaluation of tonsillectomy bleeding rates should consider patient age.
Compared with White children, Hispanic children had a higher risk (OR, 1.19) and Black children had a lower risk (OR, 0.78) of posttonsillectomy bleeding. There are few previous studies on the associations of race and ethnicity with tonsil bleeding. Large registry studies have suggested that White children have a higher risk of bleeding.42,43 Additional local studies should examine this issue, but different indications may play a role; eg, patients with OSA have a decreased risk of bleeding, and OSA may be more prevalent among Black patients than White patients.44 In our study, Hispanic patients had a higher risk of revisits for bleeding—a novel finding, but one that may have been associated with ED utilization patterns rather than higher bleeding rates.45
Children residing in a very high opportunity area (per the Opportunity Index14) had a greater risk of bleeding (OR, 1.28). Higher ED utilization rate being a risk for revisits runs counter to the findings for social status and opportunity given the finding that children from higher opportunity areas had higher bleeding rates.46,47,48 This may be a consequence of resource availability, which may allow these children to revisit the ED or hospital more easily than their counterparts in lower-opportunity areas. Previous evidence has shown chronic tonsillitis to be associated with higher bleeding rates, and higher-income children to disproportionately undergo tonsillectomy procedures for infectious indications instead of obstructive sleep disorder breathing.49,50,51 The association of socioeconomic status with posttonsillectomy bleeding needs further investigation at the local level.22,52
Patients with gastrointestinal disorders also had increased bleeding risk (OR, 1.33), a result that could be multifactorial. Laryngopharyngeal reflux is thought to be a contributor to chronic tonsilitis. Gastrointestinal disease can contribute to feeding problems, increasing the risk of poor oral intake after surgery, and both chronic tonsillitis and dehydration may increase the risk of bleeding.53 Finally, obesity was associated with an increased risk, albeit with a small effect size (OR, 1.24; 99% CI, 1.04-1.48). Ongoing exploration is warranted for these secondary findings.
Strengths and Limitations
The strengths of this study were the large sample size, multiple institutions, diverse settings, and regression analysis to create a model of bleeding that likely represents the larger pediatric tonsillectomy population. Despite these strengths, there are several key limitations. The PHIS captures approximately 15% of pediatric hospital admissions and up to 40% of complex admissions (eg, complex cardiac surgery). However, this sample may not be representative of all pediatric tonsillectomy practices. Surgeons or practices may have bleeding rates outside of this model owing to variations in the patient population, indications for treatment, and/or other factors not yet identified. This study did not account for all risk factors for bleeding, eg, surgical technique, resident surgeon involvement, and history of bleeding disorders. Additionally, the PHIS database does not distinguish between tonsillectomy and tonsillotomy procedures. Although a lower bleeding rate may be expected with tonsillotomy,54 there have also been study findings suggesting that a different bleeding rate is not evident.55
This study’s goal was not to determine the predictive causes of bleeding but rather to build a probability model of bleeding quantiles that can be used for rate comparisons. With this in mind, the proposed ranges identified by this study may prove to adequately apply to both tonsillectomy and tonsillotomy in future studies. Considering that the mean rates concur with those found by previous studies, the quantiles produced should have some external validity. Finally, there were inevitably some patients with posttonsillectomy hemorrhage who were not captured by revisits because they did not revisit the ED or hospital or because they presented to a facility not tracked by PHIS.
These study findings can be generalized in several ways. First, the probability model can serve as a tool for individuals and institutions to compare posttonsillectomy hemorrhage rates. This is especially true for those who do not have access to the PHIS or other large registries. Second, the study proposes a definition that can be measured and compared with other groups—namely, tracking revisits for bleeding (ED or hospital observation/admission). Third, the model provides data that can be used in hypothesis testing for future studies, especially in generating sample size power analyses.56,57
Conclusions
This retrospective national cohort study of US children who had undergone a tonsillectomy procedure estimated the median posttonsillectomy bleeding rate to be 1.97% and the 99th percentile of bleeding rates to be 6.39%. In addition to providing estimates for quality improvement initiatives, this model provides a useful tool for surgeons to self-monitor their individual bleeding rate after pediatric tonsillectomy procedures.
Data Sharing Statement
References
- 1.Randall DA. Current indications for tonsillectomy and adenoidectomy. J Am Board Fam Med. 2020;33(6):1025-1030. doi: 10.3122/jabfm.2020.06.200038 [DOI] [PubMed] [Google Scholar]
- 2.Farhood Z, Ong AA, Discolo CM. PANDAS: a systematic review of treatment options. Int J Pediatr Otorhinolaryngol. 2016;89:149-153. doi: 10.1016/j.ijporl.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 3.Adil EA, Medina G, Cunningham MJ. Differentiating tonsil cancer from benign tonsillar hypertrophy. J Pediatr. 2018;197:309-309.e1. doi: 10.1016/j.jpeds.2018.02.048 [DOI] [PubMed] [Google Scholar]
- 4.Lee HH, Dalesio NM, Lo Sasso AT, Van Cleve WC. Impact of clinical guidelines on revisits after ambulatory pediatric adenotonsillectomy. Anesth Analg. 2018;127(2):478-484. doi: 10.1213/ANE.0000000000003540 [DOI] [PubMed] [Google Scholar]
- 5.Paradise JL, Bluestone CD, Bachman RZ, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children: results of parallel randomized and nonrandomized clinical trials. N Engl J Med. 1984;310(11):674-683. doi: 10.1056/NEJM198403153101102 [DOI] [PubMed] [Google Scholar]
- 6.Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Rockette HE, Kurs-Lasky M. Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics. 2002;110(1 Pt 1):7-15. doi: 10.1542/peds.110.1.7 [DOI] [PubMed] [Google Scholar]
- 7.Kou YF, Mitchell RB, Johnson RF. A cross-sectional analysis of pediatric ambulatory tonsillectomy surgery in the United States. Otolaryngol Head Neck Surg. 2019;161(4):699-704. doi: 10.1177/0194599819844791 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg. 2019;160(1_suppl)(suppl):S1-S42. doi: 10.1177/0194599818801757 [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Celestin J, Lockey RF. Pediatric sleep apnea syndrome: an update. J Allergy Clin Immunol Pract. 2016;4(5):852-861. doi: 10.1016/j.jaip.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Roland PS, Rosenfeld RM, Brooks LJ, et al. ; American Academy of Otolaryngology–Head and Neck Surgery Foundation . Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1)(suppl):S1-S15. doi: 10.1177/0194599811409837 [DOI] [PubMed] [Google Scholar]
- 11.Patel HH, Straight CE, Lehman EB, Tanner M, Carr MM. Indications for tonsillectomy: a 10 year retrospective review. Int J Pediatr Otorhinolaryngol. 2014;78(12):2151-2155. doi: 10.1016/j.ijporl.2014.09.030 [DOI] [PubMed] [Google Scholar]
- 12.Kou YF, Wang C, Shah GB, Mitchell RB, Johnson RF. Tonsillectomy outcomes among children with mental health disorders in the United States. Otolaryngol Head Neck Surg. 2020;162(5):754-760. doi: 10.1177/0194599820910115 [DOI] [PubMed] [Google Scholar]
- 13.Amoils M, Chang KW, Saynina O, Wise PH, Honkanen A. Postoperative complications in pediatric tonsillectomy and adenoidectomy in ambulatory vs inpatient settings. JAMA Otolaryngol Head Neck Surg. 2016;142(4):344-350. doi: 10.1001/jamaoto.2015.3634 [DOI] [PubMed] [Google Scholar]
- 14.Diversity Data Kids. Child Opportunity Index. Accessed February 23, 2023. https://www.diversitydatakids.org/child-opportunity-index
- 15.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143-152. doi: 10.1002/sim.4780030207 [DOI] [PubMed] [Google Scholar]
- 16.Copas JB. Plotting p against x. Appl Stat. 1983;25-31. doi: 10.2307/2348040 [DOI] [Google Scholar]
- 17.Chorney SR, Weinberger R, Weintraub AY, Buzi A. Post-tonsillectomy hemorrhage and the diagnosis of occult pediatric coagulopathies. Laryngoscope. 2021;131(6):E2069-E2073. doi: 10.1002/lary.29244 [DOI] [PubMed] [Google Scholar]
- 18.Krishna P, Lee D. Post-tonsillectomy bleeding: a meta-analysis. Laryngoscope. 2001;111(8):1358-1361. doi: 10.1097/00005537-200108000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Windfuhr JP, Verspohl BC, Chen YS, Dahm JD, Werner JA. Post-tonsillectomy hemorrhage: some facts will never change. Eur Arch Otorhinolaryngol. 2015;272(5):1211-1218. doi: 10.1007/s00405-014-3025-3 [DOI] [PubMed] [Google Scholar]
- 20.Williams JD, Pope TH Jr. Prevention of primary tonsillectomy bleeding: an argument for electrocautery. Arch Otolaryngol. 1973;98(5):306-309. doi: 10.1001/archotol.1973.00780020318005 [DOI] [PubMed] [Google Scholar]
- 21.Walner DL, Karas A. Standardization of reporting post-tonsillectomy bleeding. Ann Otol Rhinol Laryngol. 2013;122(4):277-282. doi: 10.1177/000348941312200411 [DOI] [PubMed] [Google Scholar]
- 22.Gilani S, Bhattacharyya N. Revisit rates for pediatric tonsillectomy: an analysis of admit and discharge times. Ann Otol Rhinol Laryngol. 2020;129(2):110-114. doi: 10.1177/0003489419875758 [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya N. Ambulatory pediatric otolaryngologic procedures in the United States: characteristics and perioperative safety. Laryngoscope. 2010;120(4):821-825. doi: 10.1002/lary.20852 [DOI] [PubMed] [Google Scholar]
- 24.Brigger MT, Brietzke SE. Outpatient tonsillectomy in children: a systematic review. Otolaryngol Head Neck Surg. 2006;135(1):1-7. doi: 10.1016/j.otohns.2006.02.036 [DOI] [PubMed] [Google Scholar]
- 25.Gabalski EC, Mattucci KF, Setzen M, Moleski P. Ambulatory tonsillectomy and adenoidectomy. Laryngoscope. 1996;106(1 Pt 1):77-80. doi: 10.1097/00005537-199601000-00015 [DOI] [PubMed] [Google Scholar]
- 26.Levy E, Kuperman A, Sela E, et al. Utility of the pediatric bleeding questionnaire in predicting posttonsillectomy bleeding. Otolaryngol Head Neck Surg. 2021;1945998211061474. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Kim BG, Kim DH, Hwang SH. Efficacy of pillar suture for post-tonsillectomy morbidity in children: a meta-analysis. Braz J Otorhinolaryngol. 2021;87(5):583-590. doi: 10.1016/j.bjorl.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reusser NM, Bender RW, Agrawal NA, Albright JT, Duncan NO, Edmonds JL. Post-tonsillectomy hemorrhage rates in children compared by surgical technique. Ear Nose Throat J. 2017;96(7):E7-E11. doi: 10.1177/014556131709600702 [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Rodman C, Worobetz NE, Johnson J, Elmaraghy C, Chiang T. Topical biomaterials to prevent post-tonsillectomy hemorrhage. J Otolaryngol Head Neck Surg. 2019;48(1):45. doi: 10.1186/s40463-019-0368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury K, Tewfik TL, Schloss MD. Post-tonsillectomy and adenoidectomy hemorrhage. J Otolaryngol. 1988;17(1):46-49. [PubMed] [Google Scholar]
- 31.Shay S, Shapiro NL, Bhattacharyya N. Revisit rates and diagnoses following pediatric tonsillectomy in a large multistate population. Laryngoscope. 2015;125(2):457-461. doi: 10.1002/lary.24783 [DOI] [PubMed] [Google Scholar]
- 32.Leung P, DeVore EK, Kawai K, et al. Does ibuprofen increase bleed risk for pediatric tonsillectomy? Otolaryngol Head Neck Surg. 2021;165(1):187-196. doi: 10.1177/0194599820970943 [DOI] [PubMed] [Google Scholar]
- 33.Gonçalves AI, Rato C, de Vilhena D, Duarte D, Lopes G, Trigueiros N. Evaluation of post-tonsillectomy hemorrhage and assessment of risk factors. Eur Arch Otorhinolaryngol. 2020;277(11):3095-3102. doi: 10.1007/s00405-020-06060-1 [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharyya N. Benchmarks for the durations of ambulatory surgical procedures in otolaryngology. Ann Otol Rhinol Laryngol. 2011;120(11):727-731. doi: 10.1177/000348941112001106 [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Walsh J, Tunkel DE, Boss EF, Ryan M, Lee AH. Frequency of post-tonsillectomy hemorrhage relative to time of day. Laryngoscope. 2020;130(7):1823-1827. doi: 10.1002/lary.28302 [DOI] [PubMed] [Google Scholar]
- 36.Wei JL, Beatty CW, Gustafson RO. Evaluation of posttonsillectomy hemorrhage and risk factors. Otolaryngol Head Neck Surg. 2000;123(3):229-235. doi: 10.1067/mhn.2000.107454 [DOI] [PubMed] [Google Scholar]
- 37.Johnson RF, Chang A, Mitchell RB. Nationwide readmissions after tonsillectomy among pediatric patients: United States. Int J Pediatr Otorhinolaryngol. 2018;107:10-13. doi: 10.1016/j.ijporl.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 38.Hession-Laband E, Melvin P, Shermont H, Murphy JM, Bukoye B, Amin M. Reducing readmissions post-tonsillectomy: a quality improvement study on intravenous hydration. J Healthc Qual. 2018;40(4):217-227. doi: 10.1097/JHQ.0000000000000143 [DOI] [PubMed] [Google Scholar]
- 39.Khoury H, Azar SS, Boutros H, Shapiro NL. Preoperative predictors and costs of 30-day readmission following inpatient pediatric tonsillectomy in the United States. Otolaryngol Head Neck Surg. 2021;165(3):470-476. doi: 10.1177/0194599820980709 [DOI] [PubMed] [Google Scholar]
- 40.Mahant S, Keren R, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network . Variation in quality of tonsillectomy perioperative care and revisit rates in children’s hospitals. Pediatrics. 2014;133(2):280-288. doi: 10.1542/peds.2013-1884 [DOI] [PubMed] [Google Scholar]
- 41.Rothschild MA, Catalano P, Biller HF. Ambulatory pediatric tonsillectomy and the identification of high-risk subgroups. Otolaryngol Head Neck Surg. 1994;110(2):203-210. doi: 10.1177/019459989411000210 [DOI] [PubMed] [Google Scholar]
- 42.Cooper JN, Koppera S, Boss EF, Lind MN. Differences in tonsillectomy utilization by race/ethnicity, type of health insurance, and rurality. Acad Pediatr. 2021;21(6):1031-1036. doi: 10.1016/j.acap.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 43.Dhaduk N, Rodgers A, Govindan A, Kalyoussef E. Post-tonsillectomy bleeding: a national perspective. Ann Otol Rhinol Laryngol. 2021;130(8):941-947. doi: 10.1177/0003489420987438 [DOI] [PubMed] [Google Scholar]
- 44.Kou YF, Sakai M, Shah GB, Mitchell RB, Johnson RF. Postoperative respiratory complications and racial disparities following inpatient pediatric tonsillectomy: a cross-sectional study. Laryngoscope. 2019;129(4):995-1000. doi: 10.1002/lary.27405 [DOI] [PubMed] [Google Scholar]
- 45.Dai X, Ryan MA, Clements AC, et al. The effect of language barriers at discharge on pediatric adenotonsillectomy outcomes and healthcare contact. Ann Otol Rhinol Laryngol. 2021;130(7):833-839. doi: 10.1177/0003489420980176 [DOI] [PubMed] [Google Scholar]
- 46.Boss EF, Marsteller JA, Simon AE. Outpatient tonsillectomy in children: demographic and geographic variation in the United States, 2006. J Pediatr. 2012;160(5):814-819. doi: 10.1016/j.jpeds.2011.11.041 [DOI] [PubMed] [Google Scholar]
- 47.Chorney SR, Dailey JF, Zur KB. Pediatric adenoidectomy in the very young child and indications for postoperative inpatient admission. Int J Pediatr Otorhinolaryngol. 2020;130:109796. doi: 10.1016/j.ijporl.2019.109796 [DOI] [PubMed] [Google Scholar]
- 48.Raol N, Zogg CK, Boss EF, Weissman JS. Inpatient pediatric tonsillectomy: does hospital type affect cost and outcomes of care? Otolaryngol Head Neck Surg. 2016;154(3):486-493. doi: 10.1177/0194599815621739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharyya N, Shapiro NL. Associations between socioeconomic status and race with complications after tonsillectomy in children. Otolaryngol Head Neck Surg. 2014;151(6):1055-1060. doi: 10.1177/0194599814552647 [DOI] [PubMed] [Google Scholar]
- 50.Billings KR, Somani SN, Lavin J, Bhushan B. Polysomnography variables associated with postoperative respiratory issues in children <3 years of age undergoing adenotonsillectomy for obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2020;137:110215. doi: 10.1016/j.ijporl.2020.110215 [DOI] [PubMed] [Google Scholar]
- 51.Kieran S, Gorman C, Kirby A, et al. Risk factors for desaturation after tonsillectomy: analysis of 4092 consecutive pediatric cases. Laryngoscope. 2013;123(10):2554-2559. doi: 10.1002/lary.23956 [DOI] [PubMed] [Google Scholar]
- 52.Edmonson MB, Eickhoff JC, Zhang C. A population-based study of acute care revisits following tonsillectomy. J Pediatr. 2015;166(3):607-12.e5. doi: 10.1016/j.jpeds.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 53.Lauder G, Emmott A. Confronting the challenges of effective pain management in children following tonsillectomy. Int J Pediatr Otorhinolaryngol. 2014;78(11):1813-1827. doi: 10.1016/j.ijporl.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 54.Zhang LY, Zhong L, David M, Cervin A. Tonsillectomy or tonsillotomy?: a systematic review for paediatric sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2017;103:41-50. doi: 10.1016/j.ijporl.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 55.Acevedo JL, Shah RK, Brietzke SE. Systematic review of complications of tonsillotomy versus tonsillectomy. Otolaryngol Head Neck Surg. 2012;146(6):871-879. doi: 10.1177/0194599812439017 [DOI] [PubMed] [Google Scholar]
- 56.Diercks GR, Comins J, Bennett K, et al. Comparison of ibuprofen vs acetaminophen and severe bleeding risk after pediatric tonsillectomy: a noninferiority randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2019;145(6):494-500. doi: 10.1001/jamaoto.2019.0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stokes W, Swanson RT, Schubart J, Carr MM. Postoperative bleeding associated with ibuprofen use after tonsillectomy: a meta-analysis. Otolaryngol Head Neck Surg. 2019;161(5):734-741. doi: 10.1177/0194599819852328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement