Abstract
Introduction
In type 2 diabetes (T2D), key barriers to optimal glycaemic control include lack of persistence with treatment, reduced medication adherence and therapeutic inertia. This study aimed to assess the impact of these barriers in obese adults with type 2 diabetes treated with a GLP-1 receptor agonist (GLP-1RA) and compare them against other glucose-lowering agents in a real-world setting.
Methods
A retrospective study was conducted using electronic medical records from 2014 to 2019 for adults with T2D at the Valencia Clínico-Malvarrosa Department of Health (Valencia, Spain). Four study groups were established: all GLP-1RA users, SGLT2i users, insulin users and other glucose-lowering agent users (miscellany group). To account for imbalance between groups, propensity score matching (PSM) including age, gender and pre-existing cardiovascular disease was performed. Chi-square tests were used for comparisons between groups. Time to first intensification was calculated using competing risk analysis.
Results
Among the 26,944 adults with T2D, 7392 individuals were selected following PSM, with 1848 patients in each group. At 2 years, GLP-1RA users were less persistent than non-users (48.4% versus 72.7%, p < 0.0001) but more adherent (73.8% versus 68.9%, respectively, p < 0.0001). A greater proportion of persistent GLP-1RA users than non-persistent users exhibited reduced HbA1c (40.5% versus 18.6%, respectively, p < 0.0001), but no differences in cardiovascular outcomes and death were found. Overall, therapeutic inertia was observed in 38.0% of the study population. The large majority of GLP-1RA users received treatment intensification, whereas only 50.0% of GLP-1RA non-users were intensified.
Conclusion
Under real-life conditions, obese adults with T2D persistently treated with GLP-1RA showed improved glycaemic control. Despite benefits, persistence with GLP-1RA was limited after 2 years. Additionally, therapeutic inertia occurred in two out of three study participants. Strategies to facilitate medication adherence, persistence and treatment intensification in people with T2D should be made a priority in order to achieve and maintain glycaemic targets and improve outcomes in this population.
Trail registration
Study registered in clinicaltrials.org with the identifier NCT05535322.
Keywords: Glucagon-like peptide-1 receptor agonists (GLP-1RA), Medication adherence, Persistence with treatment, Real-world study, Therapeutic inertia, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| Glucagon-like peptide-1 receptor agonists (GLP-1 RA) with proven cardiorenal benefits are recommended in individuals with type 2 diabetes and obesity. |
| In type 2 diabetes, key barriers to optimal glycaemic control include lack of persistence with treatment, reduced medication adherence and therapeutic inertia. While the effect of persistence is known for some conventional glucose-lowering drugs, little is known about these measures for GLP-1RA or how they compare to other drugs. |
| The aim of the study was to evaluate these measures in type 2 diabetes GLP-1RA users with obesity and compare them against the users of other glucose-lowering agents. It is important to understand trends in diabetes prescriptions to identify issues that can be addressed to improve treatment outcomes. |
| What was learned from the study? |
| Under real-life conditions, persistence at 2 years with GLP-1RA was low compared to other glucose-lowering agents (48.4% versus 72.7%, p < 0.0001). Among HbA1c-tested GLP-1RA users, a greater proportion of 2-year-persistent individuals showed improved glycaemic control (40.5% versus 18.6%, respectively, p < 0.0001); however, no differences in cardiovascular outcomes or all-cause death were found between 2-year-persistent and non-persistent individuals. Therapeutic inertia was found in 38.0% of the whole study population. |
| Strategies to reinforce medication adherence, persistence and treatment intensification for patients with type 2 diabetes should be made a priority in order to achieve and maintain therapeutic goals and improve outcomes. |
Introduction
Type 2 diabetes is a chronic, lifelong condition that can cause micro- and macro-vascular complications with increased morbidity and mortality associated with a deterioration of glycaemic control, placing significant burdens on patients and health care systems [1]. Optimal glycaemic control is required to reduce the incidence and progression of chronic complications [2]. Unfortunately, many individuals with type 2 diabetes fail to achieve the recommended glycaemic control target [3]. Key barriers to optimal glycaemic control include non-adherence to diabetes therapy and lack of persistence with treatment over time, which represent an area of concern in the management of type 2 diabetes [4]. Indeed, reduced treatment persistence and adherence have been associated with inadequate glycaemic control and, consequently, with an increase in demand on healthcare resources [5, 6].
Persistence with treatment is the duration between treatment initiation and discontinuation despite continuing to be prescribed the medication [7]. Persistence with antidiabetic treatment has been shown to play an important role in glycaemic control, being associated not only with better clinical outcomes but also with economic benefits [8, 9]. Treatment adherence, defined as compliance with the prescribed dosing schedule during the observation period, can be indirectly quantified by assessing the proportion of days within a treatment period that are covered by the amount of medication that was accessed by the patient [10]. A level of adherence at or above 80% has been considered sufficient for effective type 2 diabetes treatment, since that level has been associated with a reduction in hospitalizations and mortality [10].
Over the last decade, an increased number of glucose-lowering drugs have become available to treat type 2 diabetes, allowing for more individualized management of the disease. Of these, glucagon-like peptide-1 receptor agonists (GLP-1RA) are incretin mimetics that have been shown to reduce HbA1c while inducing substantial weight loss with a low risk of hypoglycaemia [11]. GLP-1RA represents the first recommended injectable therapy after the failure of oral glucose-lowering agents, even before starting basal insulin therapy [11]. In addition, clinical trials and real-world studies have demonstrated that GLP-1RA can reduce cardiovascular risk and chronic kidney disease progression [12–15].
Individuals with type 2 diabetes receiving GLP-1RA may discontinue treatment because of gastrointestinal side effects that can be associated with these drugs, a lack of efficacy, patient expectations or other reasons [8]. As a result, it is important to monitor long-term patient persistence and adherence to identify problems in managing type 2 diabetes pharmacologically [9]. To the same extent, the non-intensification of treatment after suboptimal glycaemic control (therapeutic inertia) should also be evaluated considering its impact on the management and control of chronic conditions such as diabetes [16]. Persistence, adherence and therapeutic inertia are not as well studied for the newer GLP-1RA drugs as compared with other conventional antidiabetic therapies.
The aim of this work was to compare, under real-world conditions, treatment persistence, adherence and therapeutic inertia in type 2 diabetes people treated with GLP-1RA against users of other glucose-lowering drugs. We also analysed whether persistent GLP-1RA users experienced fewer cardiovascular outcomes and lower all-cause mortality than non-persistent patients. Finally, we assessed whether the type of GLP-1RA and administration schedule (weekly versus daily) influenced adherence and persistence.
Methods
Study Design and Data Sources
This work represents a real-world retrospective cohort study using electronic databases. We evaluated the population of the Department of Health of Valencia Clínico Malvarrosa (approximately 340,000 patients). Data retrieval was performed over a 6-year period between January 2014 and December 2019.
Participants´ data were extracted from ABUCASIS, an electronic database of the Consellería de Sanitat Universal I Salut Pública, which contains longitudinal data from primary care medical records, including clinical histories (SIA) and pharmacological prescriptions (GAIA). Laboratory data were also obtained from ABUCASIS.
A purpose-built database was created with anonymized patient data in which each record corresponded to a single patient.
The study strictly complied with the current personal data protection regulations and was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the Ethical Review Board of the INCLIVA Research Institute and the Valencia Clinic University Hospital [INC-LIR-2020-01 (AR_GLP1) (133/20)]. The study was registered in clinicaltrials.org with the identifier NCT05535322.
Population
All identified adults with type 2 diabetes registered in the Population Information System (SIP) of the Valencian Community and corresponding to the Valencia Clínico–La Malvarrosa Health Department who were diagnosed before the end of the study period and had at least one glucose-lowering drug prescription were included (Fig. 1). Type 2 diabetes diagnosis was defined according to the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM, ICD-10-CM) codes.
Fig. 1.
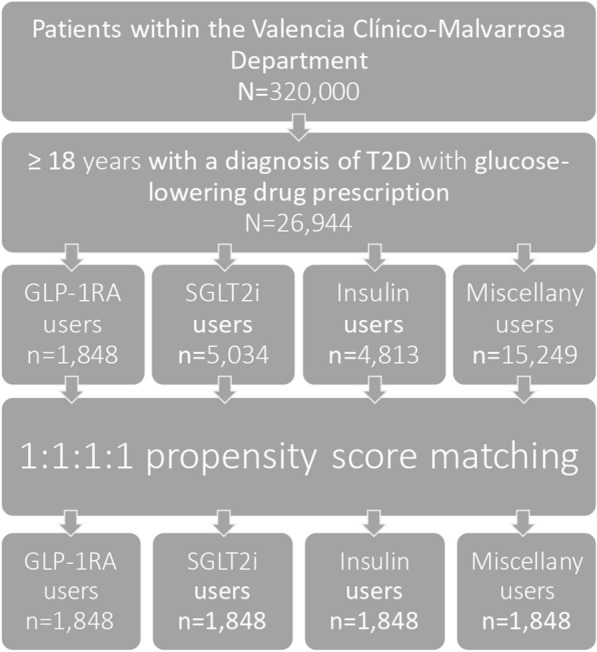
Flow chart of the study population. GLP-1RA: GLP-1 receptor agonists, SGLT2i: SGLT2 inhibitors, T2D: type 2 diabetes
Study Groups
Four groups were established according to which glucose-lowering agents were prescribed independently of other antidiabetic drugs concurrently prescribed: (a) all GLP-1RA users (GLP-1RA group), (b) SGLT2 inhibitor users with no GLP-1RA prescription (SGLT2i group), (c) insulin users with no GLP-1RA or SGLT2i prescriptions (insulin group) and (d) users of other glucose-lowering agents (miscellany group) (Fig. 1). In Spain, there is only reimbursement of the GLP-1RA cost for people with type 2 diabetes who have a BMI ≥ 30.0 kg/m2. Therefore, GLP-1RA users in this study were type 2 diabetes individuals with obesity.
Variables Included in the Analysis
Data on age, gender, prescription of antidiabetic agents and registered clinical outcomes (death, cardiovascular events, heart failure hospitalizations, severe hypoglycaemia requiring hospitalization) and HbA1c values were collected and analysed. The exposure to each antidiabetic agent was established using the World Health Organization Anatomical Therapeutic Chemical Classification system.
For the analysis of variables, some definitions were required. (1) Persistence of treatment was defined as the percentage of patients continuing treatment from the time of prescription until evidence of discontinuation. (2) Treatment adherence was inferred from the proportion of days covered (days in which an individual had access to the medication during the prescription period during follow-up), and a level of adherence ≥ 80.0% was considered to constitute adherence to treatment. (3) Therapeutic inertia was defined as non-intensification of treatment once started despite having an HbA1c ≥ 7.5% during follow-up. HbA1c values at baseline and during the study period were collected and analysed.
Statistical Analysis
Results are shown as mean ± SD for continuous variables and number and percentage for categorical variables. p values of < 0.0500 were chosen to indicate statistical significance.
To address the imbalance between the four treatment groups, 1:1:1:1 propensity score matching (PSM) was performed. The following variables were included in a logistic regression model used to estimate propensity scores (PS): age, gender, pre-existing cardiovascular disease (coronary heart disease, stroke, transient ischemic attack, acute myocardial infarction, peripheral vascular disease) and heart failure.
Treatment persistence was defined as the percentage of patients who continued treatment after the prescription date during the study period. Patients were considered persistent until evidence of discontinuation with no gaps between two consecutive prescriptions of more than 30 days for non-insulin agents and more than 3 months for insulin. Treatment adherence was defined as the proportion of days covered (PDC ≥ 0.8) during the treatment period. GLP-1RA users were compared with GLP-1RA non-users for treatment persistence and adherence. In GLP-1RA users, clinical outcomes (death, cardiovascular outcomes, heart failure hospitalization, severe hypoglycaemia requiring hospitalization, reduction in HbA1c and achieving HbA1c ≤ 7.0%) were compared between persistent versus non-persistent individuals during follow-up. Dichotomization of the slope of the linear regression of HbA1c was used as a reduction in HbA1c, and strictly negative slope values were labelled as positive. Treatment persistence and adherence in users treated with daily GLP-1RA were compared to those treated with weekly GLP-1RA. To the same extent, persistence and adherence were compared between users of short-acting versus long-acting GLP-1RA. Chi-square tests were used for comparisons between groups. Therapeutic inertia during follow-up was compared between the four study groups in those with follow-up HbA1c ≥ 7.5%. Time to first intensification with any treatment was calculated using competing risk analysis. Statistical analysis was carried out using R statistical software version 3.6.1.
Results
Data on the patients’ characteristics are detailed in Table 1. Among 26,944 adults with type 2 diabetes, there were 1848 individuals in the GLP-1RA group, 5034 in the SGLT-2i group, 4813 in the insulin group and 15,249 in the miscellany group (Table 1). Gender, age, pre-existing cardiovascular disease and heart failure were significantly different among groups (p < 0.0001) (Table 1). Of note, there were 927 women (50.2%) in the GLP-1RA group versus 2058 (40.9%) in the SGLT2i group. Also, mean age was 68.2 ± 14.75 in the insulin group compared to 57.68 ± 10.74 in the GLP-1RA group. Pre-existing cardiovascular disease and heart failure were more prevalent among participants in the insulin group (34.6% and 14.8%) than in the other groups (25.6% and 8.7% with GLP-1RA, 23.8% and 7.6% with SGLT2i and 18.9% and 4.9% in the miscellany group, respectively) (Table 1). These differences between groups were addressed using PSM. Following PSM, a total of 7392 individuals were selected, corresponding to 1848 patients for each of the four study groups (GLP-1 receptor agonists, SGLT-2 inhibitors, insulin and miscellany groups) (Table 1). There were no significant differences in age (p = 0.9860), pre-existing cardiovascular disease (p = 0.6141) or heart failure (p = 0.3469) between these four groups (Table 1). However, gender differences remained significant after statistical adjustments (p < 0.0010). This was attributed to the insulin group, which deviated from the other groups, making matching only possible for three of the four variables included in PSM (age, gender, pre-existing cardiovascular disease and heart failure) (Table 1).
Table 1.
Patients’ characteristics among treatment groups
| Total | GLP-1RA | SGLT2i | Insulin | Miscellany | p value | |
|---|---|---|---|---|---|---|
| Without PSM | ||||||
| N (%) | 26,944 (100.0) | 1848 (6.9) | 5034 (18.7) | 4813 (17.9) | 15,249 (56.6) | |
| Age (years) (SD) | 64.8 ± 12.90 | 57.7 ± 10.70 | 61.3 ± 11.10 | 68.2 ± 14.70 | 65.8 ± 12.40 | < 0.0001 |
| Gender (female) (n, %) | 12,611 (46.8) | 927 (50.2) | 2058 (40.9) | 2308 (34.6) | 2885 (18.9) | < 0.0001 |
| Cardiovascular disease* (n, %) | 6221 (23.1) | 474 (25.6) | 1198 (23.8) | 1664 (34.6) | 2885 (18.9) | < 0.0001 |
| Heart failure (n, %) | 2003 (7.4) | 160 (8.7) | 383 (7.6) | 712 (14.8) | 748 (4.9) | < 0.0001 |
| Mean follow-up time, years (SD) | 3.17 (2.12) | 2.01 (1.78) | 1.66 (1.24) | 3.62 (2.18) | 3.66 (2.08) | < 0.0001 |
| With PSM | ||||||
| N (n, %) | 7392 (100.0) | 1848 (25.0) | 1848 (25.0) | 1848 (25.0) | 1848 (25.0) | |
| Age (years) (SD) | 57.58 ± 10.86 | 57.68 ± 10.74 | 57.53 ± 10.80 | 57.63 ± 10.87 | 57.49 ± 11.01 | 0.9863 |
| Gender (female) (n, %) | 3624 (49.0) | 927 (50.2) | 937 (50.7) | 823 (44.5) | 937 (50.7) | < 0.0010 |
| Cardiovascular disease* (n, %) | 1830 (24.8) | 474 (25.6) | 447 (24.2) | 444 (24.0) | 465 (25.2) | 0.6140 |
| Heart failure (n, %) | 592 (8.0) | 160 (8.7) | 136 (7.4) | 157 (8.5) | 139 (7.5) | 0.3470 |
| Mean follow-up time, years (SD) | 2.73 (2.08) | 2.01 (1.78) | 1.64 (1.23) | 3.86 (2.17) | 3.40 (2.11) | < 0.0001 |
GLP-1RA GLP-1 receptor agonists, PSM propensity score matching, SGLT2i SGLT2 inhibitors
*Pre-existing cardiovascular disease (coronary heart disease, stroke, transient ischemic attack, acute myocardial infarction, peripeheral vascular disease) and heart failure. p values correspond to comparisons between all four groups
Persistence and Adherence to Treatment
In 7068 individuals after PSM, the overall 1-year rates of persistence and adherence to treatment were 85.6% (6048) and 70.1% (4954), respectively (Table 2). Rates of persistence at 1 year were similar between GLP-1RA users (1414 of 1734; 81.5%) and the SGLT2i group (1442 of 1781; 81.0%) (p = 0.6900), whereas the insulin and miscellany groups had higher 1-year persistence rates near 90.0% for both groups (1591 of 1771 and 1600 of 1782, respectively) (Table 2).
Table 2.
Treatment persistence and adherence
| N (%) | Total 7068 (100.0) |
GLP-1RA 1734 (24.5) |
SGLT2i 1781 (25.2) |
Insulin 1771 (25.1) |
Miscellany 1782 (25.2) |
p value |
|---|---|---|---|---|---|---|
| 1-Year persistence (n, %) | 6048 (85.6) | 1414 (81.5) | 1442 (81.0) | 1591 (89.8) | 1600 (89.8) | < 0.0001 |
| 2-Year persistence (n, %) | 4748 (64.2) | 841 (45.5) | 1115 (60.3) | 1426 (77.2) | 1366 (73.9) | < 0.0001 |
| Adherence (n, %) | 4954 (70.1) | 1280 (73.8) | 1166 (65.5) | 1597 (90.2) | 910 (51.1) | < 0.0001 |
GLP-1RA GLP-1 receptor agonists, SGLT2i SGLT2 inhibitors
p values correspond to comparisons between all four groups
Significant differences in adherence were found between the four study groups (p < 0.0001). The rate of adherence to treatment was higher among the insulin group (1597 of 1771; 90.2%), followed by GLP-1RA users (1280 of 1734; 73.8%) and the SGLT2i group (1166 of 1781; 65.5%). The least adherent to treatment was the miscellany group (910 of 1782; 51.1%) (Table 2).
Persistence and adherence to treatment were compared between GLP-1RA users (n = 1734) versus non-GLP-1RA users (n = 5334) (Table 3). Among individuals on GLP-1RA, the rate of persistence at 1 year was 81.5% (n = 1414) versus 86.9% (n = 4634) among GLP-1RA non-users (p < 0.0001) (Table 3). At 2 years, persistence rates remained lower and dropped more markedly in GLP-1RA users than among non-users (48.4% versus 72.7%, respectively; p < 0.0001) (Table 3). The rate of adherence to treatment was 73.8% (n = 1280) among GLP-1RA users, compared to 68.9% (n = 3674) in non-users (p < 0.0001) (Table 3).
Table 3.
Treatment persistence and adherence among GLP-1RA users versus non-users
| Without HbA1c | With HbA1c | |||||
|---|---|---|---|---|---|---|
| GLP-1RA users (n = 1734) | GLP-1RA non-users* (n = 5334) | p value | GLP-1RA users (n = 985) | GLP-1RA non-users* (n = 3747) | p value | |
| Prescription duration (months) (mean, SD) | 24.8 ± 21.85 | 36.18 ± 25.38 | < 0.0001 | 33.15 ± 21.63 | 43.37 ± 23.70 | < 0.0001 |
| 1-Year persistence (n, %) | 1414 (81.5) | 4634 (86.9) | < 0.0001 | 883 (89.6) | 3493 (93.2) | < 0.0001 |
| 2-Year persistence (n, %) | 840 (48.4) | 3879 (72.7) | < 0.0001 | 652 (66.2) | 3125 (83.4) | < 0.0001 |
| Adherence (n, %) | 1280 (73.8) | 3674 (68.9) | < 0.0001 | 741 (75.2) | 2679 (71.5) | 0.0220 |
| HbA1c % (mean, SD) | – | – | – | 7.5 ± 1.40 | 7.1 ± 1.24 | < 0.0001 |
GLP-1RA GLP-1 receptor agonists, SGLT2i SGLT2 inhibitors
*Individuals in the SGLT2i, insulin and miscellany groups
Among those individuals who had HbA1c measurements during follow-up (n = 4732), mean HbA1c in the GLP-1RA group (n = 985) was 7.5% ± 1.31 and 7.1% ± 1.24 (p < 0.0001) among GLP-1RA non-users (Table 3). GLP-1RA users had a significantly lower persistence rate at 1 year and 2 years than GLP-1RA non-users (89.6% and 66.2% versus 93.2% and 83.4%; respectively) (Table 3). However, GLP-1RA users were found to be more adherent than non-users (75.2% and 71.5%, respectively; p = 0.0220) (Table 3).
2-Year Persistence Among GLP-1RA Users
Of 1734 individuals who were treated with GLP-1RA, 840 (48.4%) were persistent at 2 years (Table 4). No significant differences were found between the persistent and non-persistent groups in the occurrence of death, AMI, stroke or heart failure events at 2 years. No events of severe hypoglycaemia requiring hospitalization were observed (Table 4).
Table 4.
Two-year persistent versus non-persistent individuals among GLP-1RA users
| Persistence | Non-persistence | p value | |
|---|---|---|---|
| Without HbA1c measurement | |||
| N = 1734 (%) | 840 (48.4) | 894 (51.6) | |
| Death (n, %) | 23 (2.8) | 20 (2.3) | 0.6060 |
| Acute myocardial infarction (n, %) | 15 (1.8) | 8 (0.9) | 0.1580 |
| Stroke (n, %) | 23 (2.8) | 13 (1.4) | 0.0880 |
| Heart failure hospitalization (n, %) | 8 (0.9) | 8 (0.9) | 1.0000 |
| Severe hypoglycaemia with hospitalization (n, %) | – | – | – |
| With HbA1c measurement | |||
| N = 985 (%) | 652 (66.2) | 333 (33.8) | |
| Mean HbA1c (SD) | 7.5 ± 1.31 | 7.4 ± 1.55 | 0.1420 |
| HbA1c ≤ 7.0% (n, %) | 355 (54.5) | 159 (47.8) | 0.0540 |
| Reduction in HbA1c (n, %) | 265 (40.6) | 62 (18.6) | < 0.0001 |
GLP-1RA GLP-1 receptor agonists
When analysing only those individuals with registered HbA1c measurements during follow-up (n = 985), we observed that 652 (66.2%) individuals were persistent after 2 years. The differences between persistent versus non-persistent individuals at 2 years were analysed, showing that 265 of 652 (40.6%) persistent individuals exhibited reduced HbA1c versus 62 of 333 (18.6%) non-persistent individuals (p < 0.0001) (Table 4). In addition, 355 of 652 (54.5%) persistent individuals achieved HbA1c values ≤ 7.0%, whereas 159 of 333 (47.8%) non-persistent individuals reached HbA1c levels ≤ 7.0%. However, this trend did not reach statistical significance (p = 0.5400) (Table 4). Similarly, no significant differences were observed in deaths, heart failure hospitalizations or severe hypoglycaemia between these two groups.
Persistence and Type of GP-1RA Among GLP-1RA Users
When evaluating short-acting versus long-acting GLP-1RA users, there were no significant differences in rates of persistence at 1 and 2 years (Table 5). However, the rate of adherence to treatment was significantly higher among individuals treated with long-acting GLP-1RA than among short-acting GLP-1RA users (75.6% versus 55.0%, p < 0.0001; respectively) (Table 5).
Table 5.
Treatment persistence and adherence and type of GLP-1RA
| Short-acting n = 149 |
Long-acting n = 1585 |
p value | |
|---|---|---|---|
| Adherence (n, %) | 82 (55.0) | 1198 (75.6) | < 0.0001 |
| Persistence at 1 year (n, %) | 127 (85.2) | 1287 (81.2) | 0.2697 |
| Persistence at 2 years (n, %) | 78 (52.3) | 762 (48.1) | 0.3617 |
| Daily n = 790 | Weekly n = 944 | p value | |
|---|---|---|---|
| Adherence (n, %) | 526 (66.6) | 754 (79.9) | < 0.0001 |
| Persistence at 1 year (n, %) | 687 (87.0) | 727 (77.0) | < 0.0001 |
GLP-1RA GLP-1 receptor agonists
In a comparison of weekly versus daily GLP-1RA, weekly GLP-1RA users had a significantly higher rate of adherence to treatment than daily GLP-1RA users did (79.9% versus 66.6%, respectively; p < 0.0001) but a significantly lower persistent rate at 1 year (77.0% versus 87.0%, respectively; p < 0.0001) (Table 5).
Therapeutic Inertia
Among 4732 individuals with HbA1c testing during follow-up, 1364 had HbA1c values ≥ 7.5% and were included in the analysis of therapeutic inertia (Table 6). Of those, 848 (61.95%) individuals received treatment intensification during follow-up, whereas therapeutic inertia was observed in 519 (38.0%) (Table 6). The mean time to intensification was 5.06 ± 7.74 months (Table 6).
Table 6.
Therapeutic inertia according to treatment group
| All | GLP-1RA non-users* | GLP-1RA group | SGLT2i group | Insulin group | Miscellany group | |
|---|---|---|---|---|---|---|
| n = 4732 | n = 3747 | n = 985 | n = 1117 | n = 1253 | n = 1377 | |
| HbA1c ≥ 7.5% (n, %) | 1364 (28.8) | 957 (25.5) | 407 (41.3) | 315 (28.2) | 522 (41.7) | 120 (8.7) |
| Therapeutic inertia (n, % of those with HbA1c ≥ 7.5%) | 519 (38.0) | 474 (49.5) | 45 (11.1) | 105 (33.3) | 249 (47.7) | 120 (100.0) |
GLP-1RA GLP-1 receptor agonists, SGLT2i SGLT2 inhibitors
*Individuals in the SGLT2i, insulin and miscellany groups
Therapeutic Inertia According to Treatment Group
Therapeutic inertia was compared between treatment groups (Table 6). Among GLP-1RA users, 407 of 985 individuals (41.3%) were sub-optimally controlled (HbA1c ≥ 7.5%) during follow-up. Of those, the majority received treatment intensification (n = 362; 88.9%), and therapeutic inertia was observed in only 45 individuals (11.1%) (Table 6).
Conversely, among GLP-1RA non-users with HbA1c ≥ 7.5% (957 of 3747; 25.5%), only half received treatment intensification (n = 483; 50.5%) (Table 6). Among GLP-1RA non-users, therapeutic inertia ranged from 33.3% in the SGLT2i group (105 of 315) to 100.0% in the miscellany group (120 of 120). In the insulin group, therapeutic inertia was observed in 249 of 522 individuals (47.7%) (Table 6).
Among individuals who were not optimally controlled and received treatment intensification, mean time to intensification was 4.17 (± 5.24) months in GLP-1RA users, 3.48 (± 5.52) months in the SGLT2i group and 7.62 (± 10.89) months in the insulin group. None of the individuals in the miscellany group with HbA1c ≥ 7.5% (120 of 1377; 8.7%) were intensified (Table 6).
Discussion
In this retrospective cohort study, both treatment persistence and adherence were assessed among GLP-1RA users and other glucose-lowering treatment groups. Therapeutic inertia was also evaluated. Individuals treated with GLP-1RA showed more adherence to treatment but were less persistent as compared to GLP-1RA non-users. The least adherent to treatment were those individuals in the SGLT2i (65.5%) and the miscellany (51.1%) groups. In the GLP-1RA group, no differences in cardiovascular outcomes or all-cause mortality were observed between 2-year-persistent and non-persistent individuals. Among HbA1c-tested GLP-1RA users, a greater proportion of 2-year-persistent individuals had improved glycaemic control than non-persistent ones did. Additionally, weekly GLP-1RA users were more adherent to medication but less persistent than daily GLP-1RA users. Finally, therapeutic inertia was found in 38.0% of the study population as a whole. Interestingly, nearly 89.0% of non-optimally controlled GLP-1RA users received treatment intensification during follow-up, whereas only half of GLP-1RA non-users who did not achieve optimal glycaemic control received intensified therapy.
According to our results, individuals treated with GLP-1RA were the least persistent at 2 years among the four groups examined, including comparisons to both oral agent and insulin users, which is in agreement with previous studies [17–19]. Some of the reasons might be the known adverse effects of GLP-1, which are mainly gastrointestinal, and the injectable route of administration of GLP-1RA prescribed at the time of the study [8]. Nevertheless, adherence to GLP-1RA treatment was superior to the adherence observed in the two groups treated with oral agents. Multiple factors can affect adherence to treatment, including the administration route, modality and device. However, patient's expectations, treatment satisfaction and the outcomes achieved are also relevant and should be considered [9, 20]. In our study, the most treatment-persistent individuals after 1 and 2 years of follow-up were those in the insulin group (89.8% and 77.2%, respectively). A retrospective real-world study conducted in the US that recruited almost 52,000 type 2 diabetes patients treated with two oral antidiabetic agents found that individuals initiating a third oral agent were more persistent than those initiating insulin after a 2-year period [21]. The greater complexity of insulin treatment compared to oral medication, the association of lower persistence rates with type 2 diabetes treatment and the higher risk of hypoglycaemia experienced with insulin may have contributed to these findings [8]. In contrast, our results showed that individuals treated with or initiating insulin not only had higher persistence than the other groups but also showed higher treatment adherence. In our setting, type 2 diabetes insulin users were likely to have more advanced diabetes and perhaps a greater awareness of the condition. This may lead to closer follow-up by medical teams, which may explain these results. Nonetheless, this discrepancy might reflect more direct associations found when evaluating different populations and health care systems.
When comparing persistent versus non-persistent individuals treated with GLP-1RA agents, we found that persistent users had better glycaemic control. This finding is in line with previously published data [22–24]. Indeed, a recent systematic review found that, despite the substantial heterogeneity across the studies included, a larger proportion of persistent GLP-1RA users achieved better glycaemic control than non-persistent users [25]. Interestingly, there seems to be a lack of data examining the occurrence of macrovascular complications and persistence with GLP-1RA treatment among individuals with diabetes. In our work, we compared the incidence of cardiovascular events and all-cause mortality between persistent and non-persistent GLP-1RA users. However, no significant differences were observed between the two groups, perhaps because of a short follow-up period. The type, dosing and administration regimen of GLP-1RA have also been shown to influence adherence and persistence with treatment [26]. In our study, subjects treated with weekly GLP-1RA had a higher rate of adherence than those receiving daily GLP-1RA. Given that treatment complexity is a factor that negatively impacts treatment adherence, this result is consistent with previous published evidence [27]. However, daily GLP-1RA users were slightly more persistent with treatment than those on weekly GLP-1RA, which seems to contradict previous findings [28]. Regarding the relationship between GLP-1RA types, individuals treated with shorter-acting GLP-1RA were less adherent to the drug than those treated with long-acting GLP-1. This could be attributed to long-acting GLP-1RA being more effective than short-acting GLP-1RA in regard to glycaemic and weight control, as shown by a systematic review including 14 trials [29].
A large body of evidence has shown therapeutic inertia to be a multi-factorial and major barrier to optimal glycaemic control that is present in all stages of treatment intensification among people with diabetes [30]. In this work, therapeutic inertia was observed in nearly 40% of the study population. In a previous study conducted in our setting, non-intensification of treatment occurred among 42.0% of the study population. Despite differences in the inclusion criteria and methodology between the two studies, the findings were similar. Significantly, among suboptimally controlled GLP-1RA users, the vast majority received treatment intensification (almost 89.0%), whereas, worryingly, among non-GLP-1 users, nearly half did not receive treatment intensification during follow-up. In our setting, a large proportion of GLP-1RA users are prescribed the GLP-1RA by endocrinologists. Consequently, a better ability to intensify treatment upon failure, presumably because of tighter control, may add to the long-term efficacy of GLP-1RA and improved outcomes for patients. Nonetheless, the present study demonstrates that therapeutic inertia represents a significant issue, particularly among non-GLP-1RA users. Addressing this effect and further research to elucidate the causes are justified.
This large population-based study provides a comprehensive overview of real-life GLP-1RA treatment patterns, highlighting existing barriers to achieving optimal glycaemic control among obese adults with type 2 diabetes from a metropolitan region in southern Europe. However, with regard to potential limitations, we recognize that it is a retrospective study and, as such, is subject to potential missing data or data inaccuracy. At the methodological level, the number of variables incorporated into PSM to balance the comparison between the different treatment groups was limited because of the sample size. A larger sample size would have resulted in a better balance between groups. In fact, other variables which could have had an impact on the outcomes of interest (such as duration of diabetes, treatment provider, BMI or the socio-economic status) were not included and, as a consequence, the results should be interpreted bearing this limitation in mind. With regards to the efficacy of the different types of GLP-1RA, the sample size was also too limited to allow for further sub-analysis. Similarly, both a larger sample size and longer follow-up would have resulted in a higher number of cardiovascular events, perhaps allowing differences to be observed between persistent and non-persistent subjects. A 2-year mean follow up may not have been sufficient to detect any differences in mortality or cardiovascular events limiting these findings. Therefore, further research with a larger sample size and longer follow-up is required. In addition, the definitions of persistence and adherence are subject to potential inaccuracies. This is because they represent indirect measures based on prescription events that are used to infer medication use and may not always reflect patients’ medication-taking behaviour at home. In addition, no data was available to analyse potential reasons for treatment discontinuation and low medication adherence. Finally, the results obtained are from a specific region with a specific population and prescribing policies. They should therefore be viewed with caution when trying to translate to other health systems, avoiding generalizations.
Conclusion
Under real-life conditions, lack of persistence in treatment with GLP-1RA among type 2 diabetes adults with obesity remains a significant concern, particularly since those who were persistent showed improved glycaemic control as compared with non-persistent patients. Additionally, therapeutic inertia represents a major issue in type 2 diabetes, particularly in those individuals who were not treated with GLP-1RA. These findings should contribute to the generation of cost-effective strategies aimed at improving health outcomes of type 2 diabetes individuals in our setting and, potentially, elsewhere. Such strategies should be based on reinforcing persistence and adherence to the prescribed treatment and reducing therapeutic inertia.
Acknowledgements
We thank all the participants of the study. The engagement of all health care professionals who take care of people with type 2 diabetes, particularly those working in primary care settings, in the Department of Health of Valencia Clínico-Malvarrosa (Valencia, Spain) and the daily efforts of patients living with diabetes themselves should also be acknowledged.
Funding
We acknowledge financial support by Novo Nordisk. INCLIVA Research Institute is funding the journal’s Rapid Service Fee.
Author Contributions
All authors contributed to the conception and design of the study; José Miguel Calderón, Inmaculada Sauri and José Luis Trillo contributed to the acquisition and analysis of data; F. Javier Ampudia-Blasco, Ana Palanca and Josep Redón contributed to the interpretation of the results; all authors contributed to the drafting and revision of the text. All authors read and approved the final manuscript.
Disclosures
F. Javier Ampudia-Blasco has served as a consultant/advisor for Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, MannKind Co., Medtronic, Menarini, Merck, Novartis, Novo Nordisk, and Sanofi and as a speaker for Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, LifeScan, Eli Lilly, Madaus, Medtronic, Menarini, Merck, Novartis, Novo Nordisk, and Sanofi and has received grant support from Novo Nordisk and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Compliance with Ethics Guidelines
The study strictly complied with the current personal data protection regulations and was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the Ethical Review Board of the INCLIVA Research Institute and the Valencia Clinic University Hospital [INC-LIR-2020-01 (AR_GLP1) (133/20)]. The need for consent to participate in the study was waived because it was a retrospective study of anonymized data. All authors have consented to publication. This study did not include animal research, clinical trials, or the use of plants.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Ana Palanca, Email: ana.palanca@gmail.com.
F. Javier Ampudia-Blasco, Email: ampudia_fra@gva.es.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. [PubMed]
- 2.American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;16;45(Suppl 1):S83–96. [DOI] [PubMed]
- 3.de Pablos-Velasco P, Parhofer KG, Bradley C, Eschwège E, Gönder-Frederick L, Maheux P, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol. 2014;80(1):47–56. doi: 10.1111/cen.12119. [DOI] [PubMed] [Google Scholar]
- 4.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adher. 2016;22(10):1299–1307. doi: 10.2147/PPA.S106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014;48(5):562–570. doi: 10.1177/1060028014526362. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20(1):52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 7.Cramer JA, Benedict Á, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerci B, Chanan N, Kaur S, Jasso-Mosqueda JG, Lew E. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Therapy. 2019;10(2):437–449. doi: 10.1007/s13300-019-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19–29. doi: 10.2147/CEOR.S117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care. 2017;40(11):1588–1596. doi: 10.2337/dc16-1925. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos AS, Buse JB. Initial injectable therapy in type 2 diabetes: key considerations when choosing between glucagon-like peptide 1 receptor agonists and insulin. Metab Clin Exp. 2019;1(98):104–111. doi: 10.1016/j.metabol.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 13.Longato E, Di Camillo B, Sparacino G, Tramontan L, Avogaro A, Fadini GP. Better cardiovascular outcomes of type 2 diabetic patients treated with GLP-1 receptor agonists versus DPP-4 inhibitors in clinical practice. Cardiovasc Diabetol. 2020;19(1):74. doi: 10.1186/s12933-020-01049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak B, Wintzell V, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, et al. Use of glucagon-like peptide 1 receptor agonists and risk of serious renal events: Scandinavian cohort study. Diabetes Care. 2020;43(6):1326–35. [DOI] [PubMed]
- 15.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 16.Khunti K, Gomes MB, Pocock S, Shestakova MV, Pintat S, Fenici P, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20(2):427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jermendy G, Kiss Z, Rokszin G, Abonyi-Tóth Z, Wittmann I, Kempler P. Persistence to treatment with novel antidiabetic drugs (dipeptidyl peptidase-4 inhibitors, sodium-glucose co-transporter-2 inhibitors, and glucagon-like peptide-1 receptor agonists) in people with type 2 diabetes: a nationwide cohort study. Diabetes Ther. 2018;9(5):2133–2141. doi: 10.1007/s13300-018-0483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(4):1040–1043. doi: 10.1111/dom.13160. [DOI] [PubMed] [Google Scholar]
- 19.Lee DSU, Lee H. Adherence and persistence rates of major antidiabetic medications: a review. Diabetol Metab Syndr. 2022;14(1):12. doi: 10.1186/s13098-022-00785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durden E, Liang M, Fowler R, Panton UH, Mocevic E. The effect of early response to GLP-1 RA therapy on long-term adherence and persistence among type 2 diabetes patients in the United States. JMCP. 2019;25(6):669–680. doi: 10.18553/jmcp.2019.18429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin PA, Wei W, Zhou S, Xie L, Baser O. Outcomes and treatment patterns of adding a third agent to 2 OADs in patients with type 2 diabetes. JMCP. 2014;20(5):501–512. doi: 10.18553/jmcp.2014.20.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–355. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzer-Cohen C, Chodick G, Husemoen LLN, Rhee N, Shalev V, Karasik A. A retrospective database study of liraglutide persistence associated with glycemic and body weight control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):683–696. doi: 10.1007/s13300-019-0583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morieri ML, Avogaro A, Fadini GP. Long-acting injectable GLP-1 receptor agonists for the treatment of adults with type 2 diabetes: perspectives from clinical practice. Diabetes Metab Syndr Obes. 2020;13:4221–34. [DOI] [PMC free article] [PubMed]
- 25.Evans M, Engberg S, Faurby M, Fernandes JDDR, Hudson P, Polonsky W. Adherence to and persistence with antidiabetic medications and associations with clinical and economic outcomes in people with type 2 diabetes mellitus: a systematic literature review. Diabetes Obes Metab. 2022;24(3):377–390. doi: 10.1111/dom.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mody R, Yu M, Nepal B, Konig M, Grabner M. Adherence and persistence among patients with type 2 diabetes initiating dulaglutide compared with semaglutide and exenatide BCise: 6-month follow-up from US real-world data. Diabetes Obes Metab. 2021;23(1):106–115. doi: 10.1111/dom.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;34(3):658–73. [DOI] [PMC free article] [PubMed]
- 28.Uzoigwe C, Liang Y, Whitmire S, Paprocki Y. Semaglutide once-weekly persistence and adherence versus other GLP-1 RAs in patients with type 2 diabetes in a US real-world setting. Diabetes Ther. 2021;12(5):1475–1489. doi: 10.1007/s13300-021-01053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huthmacher JA, Meier JJ, Nauck MA. Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care. 2020;43(9):2303–2312. doi: 10.2337/dc20-0498. [DOI] [PubMed] [Google Scholar]
- 30.Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol. 2019;1(10):2042018819844694. doi: 10.1177/2042018819844694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


