Abstract
Background
Image-guided percutaneous thermal ablation is an established treatment option for early-stage lung cancer in medically inoperable patients but carries a high risk of pleura-related complications, particularly pneumothorax.
Objective
This study aimed to determine if image-guided transbronchial microwave ablation (tMWA) is a feasible approach to treat peripheral stage 1 lung cancer.
Method
A prospective, single-arm, multicenter study sought to enroll 40 adults who were medically inoperable or declined surgery for peripheral stage 1 lung tumors (≤20 mm). Ablation was performed using navigational bronchoscopy and a flexible MWA probe, guided by cone-beam CT with augmented fluoroscopy. Follow-up at 1, 6, and 12 months included CT imaging of the ablation zone and possible tumor recurrence, adverse events (AEs), pulmonary function, and quality of life.
Results
Across 2 sites, 11 tumors (10 NSCLC, 1 carcinoid) were treated in 10 enrolled patients. Median tumor diameter was 13 × 14 mm (7–19 mm) and median minimum ablative margin was 11 mm (5–19 mm). Technical success and technique efficacy were achieved in all patients. No tumor recurrence was seen during 12-month follow-up. No pneumothorax, pleural effusion, or bronchopleural fistula were noted. Minor AEs included scant hemoptysis, pain, cough, and dyspnea. Two serious AEs occurred ≤30 days of ablation and included a COPD exacerbation (day 9) and a death of unknown cause (day 15). The death led the sponsor to halt enrollment. Pulmonary function and quality-of-life indices remained stable.
Conclusions
Image-guided tMWA is a technically feasible approach for peripheral early-stage lung cancer but warrants further evaluation of safety and efficacy in larger cohorts.
Keywords: Augmented fluoroscopy, Cone-beam computed tomography, Electromagnetic navigation bronchoscopy, Lung cancer, Microwave ablation
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Most patients are diagnosed with advanced-stage disease, but with the advent of low-dose computed tomography (CT) screening, the proportion of lung cancers discovered at an early stage is expected to increase [2]. Non-small cell lung cancer (NSCLC) makes up 80% of all lung cancers [3]. The preferred treatment for early-stage NSCLC is surgery [4], but curative-intent surgery rates vary greatly, with county-specific frequencies across the USA ranging from 13% to 92% [5]. In addition to unfavorable socio-economic and health care delivery factors that lower surgery rates, most lung cancer patients are over 65 years old and/or considered medically inoperable due to frailty or comorbidities [3, 4]. The most common nonsurgical treatment, stereotactic body radiation therapy (SBRT), is associated with a high rate of local control but typically requires multiple fractions, holds risks for radiation injury and decreased pulmonary function, and traditionally cannot be repeated for local recurrence [4]. For patients who decline or are not candidates for SBRT, the recommended alternative is CT-guided percutaneous thermal ablation [4]. Radiofrequency ablation (RFA) is the most thoroughly studied option [6, 7], but more recently, microwave ablation (MWA) has gained interest because relative to RFA, it generates faster, larger, and higher-temperature ablation zones, has less heat-sink effect, and can penetrate high-impedance tissue, such as lung [8].
Percutaneous MWA is effective for early-stage NSCLC and pulmonary metastases [9, 10, 11, 12], but is associated with a high rate of pleura-related complications, limiting its wider adoption [13]. In a meta-analysis, percutaneous MWA procedures showed a pneumothorax rate of 34% (11% requiring intervention) and a pleural effusion rate of 10%, with similar rates for percutaneous RFA [14]. The possibility of a lower complication rate for a transbronchial versus transthoracic approach [15, 16], together with the development of more sophisticated navigational bronchoscopic and imaging modalities, has spurred interest in transbronchial thermal ablation of lung cancers [17, 18]. Preliminary studies with transbronchial-delivered RFA of early-stage NSCLC have provided promising short-term results [19, 20], but clinical experience with transbronchial MWA (tMWA) remains limited to retrospective single-center observations and conference proceedings [21, 22, 23]. Preclinical studies have assessed the radiological and histological aspects of tMWA-induced lung tissue damage and recovery [24, 25, 26, 27]. Here, we present a prospective feasibility study on the safety and short-term efficacy of tMWA guided by cone-beam CT (CBCT) with augmented fluoroscopy (AF) for the ablation of peripheral stage 1 lung cancer [28].
Materials and Methods
Study Design
This prospective, multicenter, single-arm feasibility study intended to include 40 patients in up to 10 centers in the USA, but due to an unexpected patient death, the sponsor (Ethicon, Inc.) halted enrollment. Although the independent data and safety monitoring board (DSMB) approved the study to continue with added precautions, the sponsor decided not to resume enrollment, but to complete follow-up of treated patients and pursue new clinical studies. This report details the results from the first 10 patients enrolled at two centers. The study was performed in accordance with FDA Regulations (21 CFR Parts 50, 54, 56, and 312), the ICH tripartite guideline for Good Clinical Practice (1996), and the Declaration of Helsinki (2013). Protocols and informed consent forms were approved by Institutional Review Boards (IRBs) from both sites (Western IRB study 1186401; Mayo Clinic IRB study 18-006626). An independent external DSMB oversaw safety. The study was registered on ClinicalTrials.gov (identifier NCT03603652).
Patients were informed of all available standard-of-care treatment options. A multidisciplinary team (including radiation oncology and thoracic surgery) performed eligibility screening and investigators sought written consent from eligible patients. Patients aged 18 or older who had histopathologically confirmed ≤20 mm primary lung cancer (stage 1 as confirmed with positron emission tomography, endobronchial ultrasound staging, and brain magnetic resonance imaging) located in the outer two-thirds of the lung but ≥1 cm from the pleura, and who were either medically inoperable or who declined surgery, were considered for enrollment. All tumors were biopsied via guided bronchoscopy with real-time imaging (rEBUS and/or CBCT). All patients underwent CT at screening (≤30 days of ablation) for tumor measurement and planning purposes. Key exclusion criteria included uncorrectable coagulopathy, platelet count below 50,000 mm3, and prior pneumonectomy or bronchiectasis; a complete list of inclusion and exclusion criteria is detailed in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000528820).
After the procedure, per study protocol, patients were observed in the hospital for up to 23 h and followed up at months 1, 6, and 12 with conventional CT to assess the ablation zone and evidence of local tumor recurrence or progression. Adverse events (AEs), Eastern Cooperative Oncology Group (ECOG) performance status, pulmonary function tests, health-related quality of life (HRQOL) questionnaires (QLQ-C30 and QLQ-LC13), and visual numeric pain rating scales were prospectively collected.
Ablation Procedures
All procedures were performed by a pulmonologist or thoracic surgeon proficient in navigational bronchoscopy with CBCT and trained on the NEUWAVETM FLEX MWA System (Ethicon Inc., Raritan, NJ, USA). The NEUWAVETM flexible probe (outer diameter of 1.9 mm) creates oval-shaped ablations via a single microwave source with a 100 W microwave power amplifier operating at 2.45 GHz and a CO2-based cooling system. An interventional radiologist was present for all but one procedure (additional tumor in an already enrolled patient). A hybrid operating room was used with a ceiling- or floor-mounted C-arm system with CBCT (Allura Xper FD20, Philips, Eindhoven, the Netherlands, or Discovery IGS 740, GE Healthcare, Chicago, IL, USA). ENB was performed using superDimensionTM 7.1 (Medtronic, Minneapolis, MN, USA) in 9 of 11 procedures. In 2 procedures, technical issues arose with the ENB device, and a steerable catheter was used without ENB, with CBCT with augmented fluoroscopy guidance alone (Edge, Medtronic, Minneapolis, MN, USA).
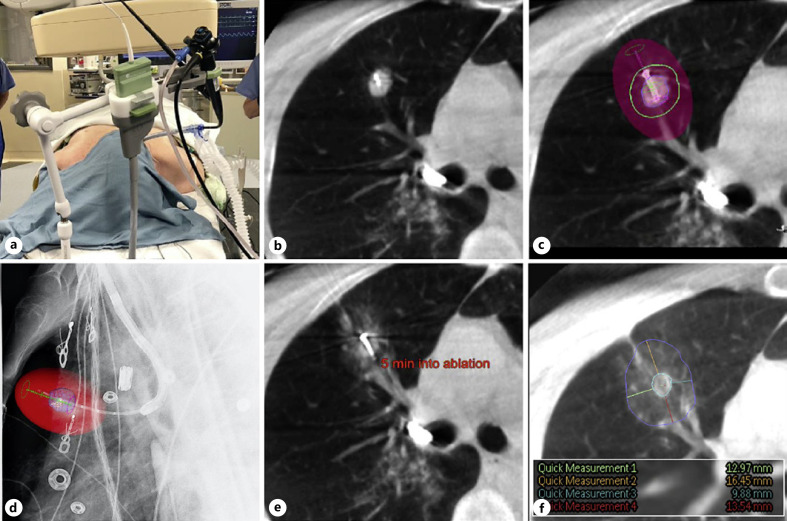
Procedures were performed under general anesthesia with specific measures to prevent atelectasis [29]; antibiotic coverage was given. A CBCT scan was performed to measure and segment the target tumor for AF just prior to the initiation of the guided bronchoscopy procedure. Disease stage was reconfirmed using endobronchial ultrasound to ensure the clearest assessment of the local control rate of the new technology: if rapid on-site evaluation confirmed the absence of metastatic disease in lymph nodes, guided bronchoscopy commenced. Once the virtual target was reached, the position of the catheter relative to the tumor was verified using AF in multiple projection angles; if necessary, the catheter position was adjusted. Once CBCT scans confirmed accurate positioning of the catheter, the bronchoscope was secured into the scope holder (Fig. 1a). Under real-time AF, the ablation probe was placed into the tumor, the sharp tip allowing it to be placed across the airway wall without the need for accessory instruments. CBCT scans were repeated as necessary to ensure proper position (Fig. 1b, c). Subsequently, AF was used to retract the catheter proximally 5 cm, while keeping the probe in place, to avoid heating the catheter during ablation (Fig. 1d). If the probe could not be placed directly into the tumor, “bracketing” was used: the probe was consecutively positioned on opposite sides of the tumor to perform two (or more) overlapping ablations covering a single tumor.
Fig. 1.
Patient positioning and images of the ablation procedure as obtained by CBCT with AF and Philips Lung Suite software for real-time measurements. An operating room photograph (a) and 5 consecutive CBCT or AF images from 1 representative patient who was treated for a 14 × 13 mm tumor in the upper lobe of the right lung are shown. a NeuWave bronchoscope holder with integrated attachment for the MWA catheter. This was used during all CBCT scans and for the ablation itself. b Axial CBCT image showing the probe positioned within the tumor. c Axial CBCT image illustrating ablation planning: colored spheres indicate the target tumor (blue), the minimal desired margin (green), and the final estimated ablation zone (red shaded area). d Lateral augmented fluoroscopic image confirming the position of the probe: the colored spheres indicate the target tumor (blue) and the final estimated ablation zone (red shaded area). e Axial CBCT image, taken 5 min into a 10-min ablation showing early ground glass changes consistent with ablated lung tissue. f Axial CBCT image, taken 10 min after completion of the ablation, showing the final ablation zone (blue circle) and margin measurements which confirm that adequate margins were achieved (colored lines, inset shows enlarged snapshot of measurements of the margin in mm).
After confirming final probe position using CBCT, the time and power were set. Dedicated ablation planning software (Lung Suite, Philips), which provided 3-dimensional visualization of the estimated ablation zone, including setting of custom margins (Fig. 1b, c), was used in most procedures. Ablation zone estimates were based on data collected in ex vivo lung tissue by the sponsor (product instructions for use) [30].
During ablation, CBCT was performed at the half-way point (Fig. 1e) and a final CBCT assessment of the ablation zone was completed 10 min post-ablation to assess the ablative margin (distance between the edge of the tumor and the ablation zone edge), with a desired minimum margin of 10 mm (Fig. 1f) [13, 31]. The ablation zone was indicated by ground-glass changes surrounding the tumor.
Endpoints
The primary endpoints were technical success and technique efficacy, defined as “complete ablation of the tumor including adequate surrounding tissue margin” as determined by the investigator 10 min post-ablation with CBCT and at the month-1 visit with conventional CT, respectively. These are standard definitions used to report outcomes in image-guided tumor ablation [7, 9, 11, 32, 33, 34]. Investigators completed a device user experience survey after each procedure.
Secondary endpoints included the rates of AEs and serious adverse events (SAEs) reported within 30 days of the procedure, as well as tumor recurrence, hospital readmission, and length of hospital stay. Additional endpoints were exploratory and included HRQOL questionnaires that were longitudinally assessed using the validated European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-LC13 guidelines, and ECOG performance status [35]. Pain was assessed using numeric pain rating scales. Pulmonary function was measured at screening and at 6- and 12-month follow-up.
Data Analysis
Given the limited sample size, summaries for primary and secondary endpoints were descriptive. For longitudinal assessment of HRQOL, a score difference (vs. baseline) of >10 points was considered a minimal clinically important difference [36]. For pulmonary function tests, relative changes (%) from baseline were evaluated using the Radiation Therapy Oncology Group scoring system for pulmonary toxicity [37].
Results
Study Patients and Ablation Procedures
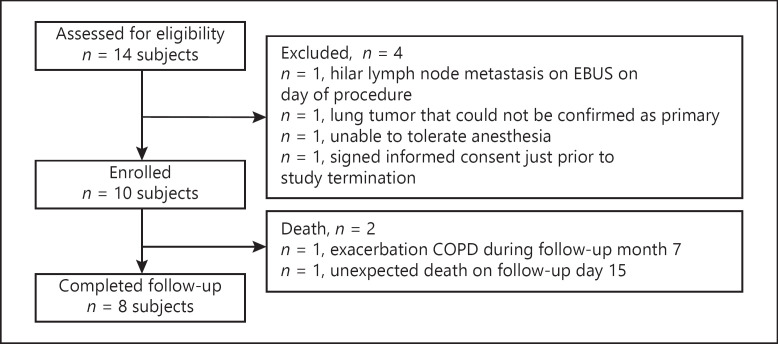
Ten out of 14 screened patients were enrolled at two centers between June 2018 and December 2018 (Fig. 2). Patient characteristics and baseline tumor parameters are detailed in Table 1. Nine out of 10 tumors were diagnosed as NSCLC and one as a carcinoid tumor. Of the 10 patients enrolled, 6 had tumors deemed operable, but the patients chose not to have surgery (5 NSCLC and 1 carcinoid). Six tumors were located in the upper lobes. One patient presented with an additional 7 × 7 mm primary NSCLC lung tumor approximately 9 months after the original ablation, which was considered a synchronous primary by the site's multidisciplinary team; following IRB and DSMB approval, the tumor was ablated and included for efficacy and safety analyses.
Fig. 2.
CONSORT diagram. Eligibility screening was performed by a multidisciplinary team and included medical/surgical history, American Society of Anesthesiology (ASA) score, ECOG status, CT characterization of target tumor, pulmonary function tests, and laboratory analyses.
Table 1.
Patient characteristics (N = 10) and tumor parameters (N = 11)
| Characteristic | |
|---|---|
| BMI, median (range), kg/m2 | 25.5 (22.8, 35.5) |
| Smoking status, n (%) | |
| Current smoker | 3 (30) |
| Former smoker | 6 (60) |
| Nonsmoker | 1 (10) |
| Radiation history, n (%) | |
| Yes | 1 (10) |
| No | 9 (90) |
| Comorbidities, n (%) | |
| Hypertension | 8 (80) |
| Hyperlipidemia/hypercholesterolemia | 8 (80) |
| Gastroesophageal reflux disease | 5 (50) |
| Depression | 4 (40) |
| COPD/emphysema | 3 (30) |
| Cerebrovascular accident | 2 (20) |
| Myocardial infarction/coronary artery disease | 2 (20) |
| Hypothyroidism | 2 (20) |
| ECOG performance score, median (range) | 1 (0, 2) |
| Pulmonary function, median (range) | |
| FEV1, mL | 1,910 (850, 2,910) |
| FEV1, % predicted | 78 (52, 126) |
| FVC, mL | 2,905 (1,270, 4,460) |
| FVC, % predicted | 84 (55, 121) |
| FEV/FVC | 71.5 (47, 97) |
| TLC, mL | 5,435 (3,560, 6,670) |
| TLC, % predicted | 90.5 (73, 107) |
| FRC, mL | 2,870 (1,820, 4,390) |
| FRC, % predicted | 103.5 (74, 135) |
| DLCO, mL/min/mm Hg | 13.45 (4.1, 27.9) |
| DLCO, % predicted | 61.5 (29, 125) |
| Tumor type, n (%) | |
| NSCLC (medically inoperable) | 4 (36) |
| NSCLC (medically operable; declined surgery) | 6 (55) |
| Other (“carcinoid, medically operable; declined surgery”) | 1 (9) |
| Tumor sub-type, n (%) | |
| Squamous cell carcinoma | 4 (36) |
| Adenocarcinoma | 5 (45) |
| Large cell carcinoma | 0 (0) |
| Other (“carcinoid,” “poorly differentiated non-small cell carcinoma”) | 2 (18) |
| Tumor location, n (%) | |
| Right upper lobe (RUL) | 4 (36) |
| Right middle lobe (RML) | 0 (0) |
| Right lower lobe (RLL) | 2 (18) |
| Left upper lobe (LUL) | 2 (18) |
| Left lower lobe (LLL) | 2 (18) |
| Other (“superior segment of the RLL”) | 1 (9) |
| Distance from pleura, median (range), mm | 18 (10, 39) |
| Largest diameter, median (range), mm | 14 (7, 19) |
| Smallest diameter, median (range), mm | 13 (7, 19) |
Eight of 10 treated patients completed the study as planned. One patient died unexpectedly on post-procedure day 15, as detailed below; a second patient died during month 7 due to a chronic obstructive pulmonary disease (COPD) exacerbation.
Patient-level details of all 11 procedures are shown in online supplementary Table S2. Overall, tMWA was delivered for a median total time of 10 min (10–20 min) per procedure with individual ablations delivered for a median of 9 min (2–10 min), a median maximal power of 100 W (80–100 W), and a median maximal temperature of 93.7°C (82.9–100.4°C). The maximum temperature is measured by a single thermocouple, located outside the antenna area but within the ablation zone, proximal of the long marker band (approximately 1.8 cm from the tip of the probe) and is the highest temperature reached during or immediately after the ablation. The median smallest and largest ablation zone diameters were 38 mm (18–55 mm) and 54 mm (43–65 mm), and the median smallest and largest ablation margins were 11 mm (5–19 mm) and 26 mm (10–34 mm), respectively. Procedures lasted for a median of 104 min (58–264 min), inclusive of navigation. Probe placement required a median time of 15 min (9–43 min). In each procedure, 1 CBCT was performed pre-ablation, followed by a median of 5 (1–12) CBCTs during probe positioning/confirmation and ablation, and a median of 1 (1–3) CBCTs post-ablation. The median total radiation dose was 14.6 mSv (9.6–35.5 mSv). A single probe was repositioned to perform overlapping ablations in 6/11 procedures (55%), with a median of 1 (0–3) repositions per procedure. The median distance between the probe and the center of the tumor was 3 mm (1–7 mm).
Technical Success and Technique Efficacy
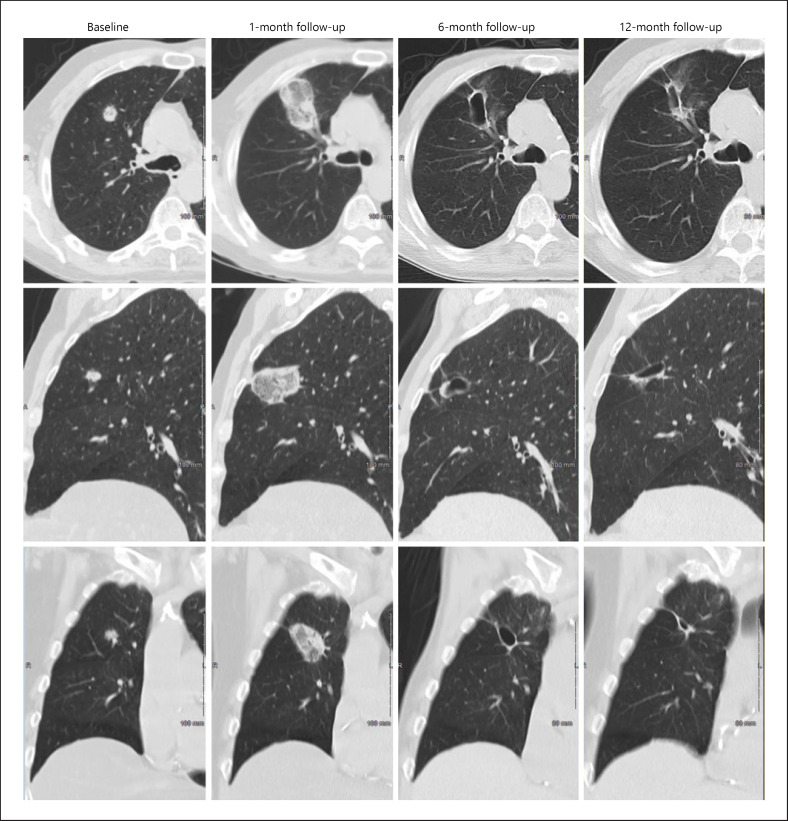
Technical success and technique efficacy rates were both 100% (11/11 tumors and 9/9 tumors, respectively). Serial CT images of a representative patient (Fig. 3) illustrate technique efficacy at 1 month, and a progressive involution of a thin-walled cavitary post-ablation zone, which is commonly seen 6 and 12 months after percutaneous thermal ablation [38, 39].
Fig. 3.
Serial CT images showing the evolution of an ablated target tumor over 12-month follow-up. The axial (top row), sagittal (middle row), and coronal (lower row) CT chest images from 1 representative patient from the study, at baseline (screening visit) (column 1) and at the 1-month (column 2), 6-month (column 3), and 12-month visit (column 4), are shown. Baseline images show a 14 × 13 mm target tumor in the upper lobe of the right lung. At month 1, the tumor is completely covered by the ablation zone and surrounded by a circumferential ablation margin (technique efficacy). At month 6 and 12, a small cavitary post-ablation zone is seen that contracts progressively over time, with no tumor recurrence evident.
The qualitative device user experience survey showed that investigators were able to easily pass the probe through the extended working channel; investigators reported that the system was straightforward to use and performed as expected, while CBCT and AF imaging allowed users to confidently identify the probe and tumor and evaluate the ablation zone.
Secondary and Exploratory Endpoints
All patients were discharged within 24 h. One patient was readmitted on post-procedure day 9 for an acute exacerbation of COPD and discharged home after 2 days. No patient showed evidence of local tumor recurrence on 12-month follow-up CT scan.
At baseline, HRQOL parameters were consistent with reference values reported for stage 1-2 lung cancer patients (online suppl. Table S3) [40]. The median score changes did not reach a minimal clinically important difference, except for small improvements in QLQ-C30 Global Health Status and QLQ-LC13 Dyspnea at month 12 and a discreet deterioration for QLQ-C30 Fatigue at month 6 and 12 (online suppl. Fig. S1). A borderline deterioration for QLQ-C30 pain at month 12 (online suppl. Fig. S1) was reflected neither in QLQ-LC13 pain scores nor in the self-reported numeric pain rating assessments which remained stable (median [range] scores of 0 [0, 10] at pre-procedure, versus 0 [0, 8] on day 30, not shown). ECOG performance status remained stable, showing ECOG status 0-1 in 90% of patients at the pre-procedure visit and in 100% of available patients at the 12-month visit (not shown). Pulmonary function tests demonstrated that indices (expressed as % change from baseline) overall remained stable or slightly improved, at month 6 and 12 (online suppl. Fig. S2). Table 2 summarizes all endpoints.
Table 2.
Study endpoints and outcomes
| Endpoint | Outcome |
|---|---|
| Primary endpoints | |
| Technical success | 100% (11/11 tumors) |
| Technique efficacy | 100% (9/9 tumors) |
| Secondary endpoints | |
| Tumor recurrence | 0% (0/11 tumors) |
| Length of hospital stay | 100% discharged within 24 h (11/11 procedures) |
| Hospital readmission <30 days | 1 readmission within 30 days (1/11 ablations, 9%) |
| COPD exacerbation, with hospital discharge after 2 days | |
| AEs <30 days | 35 minor AEs |
| No pneumothorax, bronchial fistula, or pleural effusion | |
| SAEs <30 days | 2 SAEs: |
| Day 9, COPD exacerbation, probably procedure-related and unlikely device-related | |
| Day 15, unexpected death of unknown cause, probably procedure-related and possibly device-related | |
| Exploratory endpoints | |
| Quality of life (QLQ-C30, QLQ-LC13) | No MCID in standardized scores between screening and month 12, except: |
| Discreet improvement QLQ-C30 Global Health Status | |
| Discreet improvement QLQ-LC13 Dyspnea | |
| Discreet deterioration QLQ-C30 Fatigue | |
| Pain (numeric pain rating scale) | Pain scores low and stable between procedure day and day 30 |
| ECOG performance status | Performance status “good” and stable between procedure day and month 12 |
| Pulmonary function | Indices stable or slightly improved between screening visit and month 6, 12 |
COPD, chronic obstructive pulmonary disease; MCID, minimal clinically important difference.
Safety
No patient developed pneumothorax, pleural effusion, or bronchopleural fistula. Within the first 30 days, 37 AE occurred in 9 subjects (90%). The vast majority of AEs were mild and expected. These included scant hemoptysis, chest wall pain, cough, and dyspnea (Table 3); one AE of pain, one of fatigue, and one of procedural hemorrhage were considered moderate in severity. Of these AEs, 19% (7/37 events) were at least “possibly” related to the procedure and device, 68% (25/37 events) to the procedure only, and 3% (1/37) to the device only. Two AEs were serious and occurred in two subjects (20%). One SAE concerned a patient who was admitted on post-procedure day 9 with a COPD exacerbation that resolved during a 2-day hospital admission; this SAE was considered moderate in severity, probably procedure-related and unlikely device-related. Several weeks after the 6-month visit, this patient died due to a COPD exacerbation that was considered unrelated to procedure or device by the treating investigator and the independent DSMB. This patient had regular COPD exacerbations prior to the ablation, and the frequency of exacerbations did not increase post-ablation.
Table 3.
AEs within first 30 days of 11 ablation procedures in 10 patients
| Description | Events, N1 |
|---|---|
| Scant hemoptysis | 6 |
| Cough, dyspnea | 6 |
| Post-ablation syndrome2 | 3 |
| Musculoskeletal chest/shoulder pain | 3 |
| Headache | 2 |
| Other minor3 | 9 |
| Procedural hemorrhage NBS grade 2 | 1 |
| Exacerbation of COPD4 | 1 |
| Sudden death4 | 1 |
NBS, Nashville Bleeding Scale.
Each event occurred once in a patient.
Defined as ≥2 of the following symptoms within 3 weeks of the procedure: fever, chills, fatigue, nausea, anorexia, and chest/shoulder pain.
Pyrexia, nausea, pneumonia, dysphagia, chest discomfort, anxiety, depression, hypomagnesemia, and back pain.
SAE.
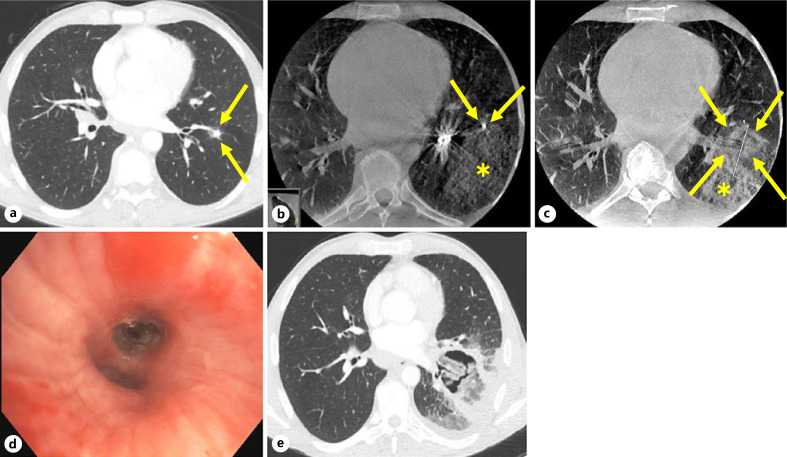
The second SAE was severe and concerned an unexpected death on post-procedure day 15 in a patient who had a medical history of chronic lymphocytic leukemia and peripheral arterial disease with bilateral lower extremity stents, and was being treated with aspirin and clopidogrel, which were both withdrawn 7 days prior to the procedure. Navigation to the tumor was difficult and prolonged and associated with limited parenchymal hemorrhage which was noted on fluoroscopy and CBCT, and bleeding into the airway which resolved after a combination of cold saline and bronchoscopic tamponade (Fig. 4). A total of 100–150 mL of cold saline, mucus, and blood were collected; an estimated blood loss of 25 mL was noted in the anesthesia and operative records. This incident would be classified as grade 2 on the Nashville Bleeding Scale [41]. Ultimately, the ablation probe was verified to be adjacent to the target, and a single ablation was performed (Fig. 4). The patient recovered uneventfully and was discharged 24 h later, per protocol. On post-procedure day 2, the patient was evaluated in the emergency room for pleuritic pain and after a chest X-ray was unrevealing for significant abnormalities, was discharged from the emergency room with steroids for possible post-ablation syndrome. On post-procedure day 3, the patient resumed antiplatelet medication secondary to complaints of ongoing claudication. In a follow-up phone call on post-procedure day 8, the patient stated that symptoms had gradually improved from the week prior. On post-procedure day 14, the patient was seen at an outside emergency room for chest pain, shortness of breath, and mild self-limited hemoptysis and was discharged the same day; CT angiogram of the chest showed a cavitary post-ablation tumor but no pseudoaneurysm, pulmonary embolism, or other vascular abnormalities (Fig. 4). The next day, the patient suffered an out-of-hospital sudden death. Per Emergency Medical Services (EMS) reports, witnesses saw the patient collapse at a gas station, falling forward, and striking his face on the concrete. The patient was found to be in asystole with blood in the nasal cavity. The EMS performed manual and mechanical chest compressions with a Lucas device and had multiple failed attempts at endotracheal intubation. Upon arrival at the emergency department, endotracheal intubation was again attempted, during which there was a large amount of blood noted in the posterior pharynx. After 30 min of resuscitative efforts, the patient was pronounced dead. An autopsy was offered; however, the family declined. After review of all materials available, the DSMB considered this event possibly related to the device and probably related to the study procedure.
Fig. 4.
CT images of the patient who died unexpectedly 15 days post-ablation. The following are shown. a Baseline axial CT image taken at the screening visit, showing an 8 × 8 mm target tumor in the left lower lobe (arrows). b Axial CBCT image taken before ablation, showing the tip of the ablation probe (arrows) and limited parenchymal hemorrhage (asterisk). c Axial CBCT image 10 min after ablation, showing the tumor completely covered by the ablation zone (arrows) (estimation of ablation zone is difficult due to adjacent hemorrhage), with stable parenchymal hemorrhage (asterisk). d Bronchoscopic image taken after probe removal at the conclusion of the ablation procedure, showing intact bronchi and no blood. e Axial CT angiogram image taken on day 14 post-ablation at an outside hospital's emergency room showing a cavitary post-ablation tumor but no pseudoaneurysm, pulmonary embolism, or other vascular anomalies.
Discussion
This is the first trial to prospectively evaluate tMWA of stage 1 peripheral lung cancers. Although limited to 11 tumors, the study shows that when used in combination with CBCT and AF, tMWA achieved local control at 1 month (100% technique efficacy) and no local tumor recurrence up to 12 months. Efficacy outcomes from this small trial are encouraging, considering that percutaneous MWA of pulmonary tumors achieves 1-month technique efficacy rates of 80–96% [9, 10, 11] and that SBRT and percutaneous ablation of stage 1 NSCLC achieve 1-year local tumor control rates of 93–97% and 69–93%, respectively [6, 33, 42, 43, 44, 45]. The 12-month follow-up in our trial also indicated that, similar to SBRT and percutaneous RFA, tMWA allows patients to maintain lung function and quality of life [6, 7, 46]. All patients tolerated the initial procedure well and were discharged within 24 h of the procedure. No events of pneumothorax or bronchopleural fistula were noted in any patient. Minor complications included scant hemoptysis, and a single event of procedural hemorrhage in relation to the ENB procedure, which was noted on fluoroscopy and CBCT imaging.
While the definite cause of death in 1 patient remains unknown, several medical and procedural factors may have contributed to this event. These include multiple serious comorbid medical conditions in a patient on dual antiplatelet therapy, coupled with a lengthy bronchoscopic navigation procedure. This was further complicated by some local bleeding during navigation which, although both the physician and the DSMB considered that the device functioned as intended, may have augmented the energy delivery, thereby resulting in a larger than expected ablation zone. Additional studies are warranted to determine if tMWA will emerge as a promising technique when appropriate safeguards are in place. Therefore, we recommend to either abandon or modify the procedure if navigation is prolonged, or if bleeding occurs that either requires intervention or that obscures radiographic visualization of the tumor.
In 3 out of 10 patients, minor AEs were consistent with post-ablation syndrome, a well-known, benign, and self-limited constellation of symptoms that occurs in up to two-thirds of patients undergoing percutaneous thermal ablation [11, 39, 47]. The clinical definitions of this syndrome vary greatly between studies, and the incidence and severity depend on the tumor size and ablation volume [39, 47, 48]. We defined post-ablation syndrome as two or more specific symptoms occurring within 3 weeks post-ablation (Table 3); consistent with previous studies, all symptoms were mild and transient [32, 48]. The observed respiratory AEs of cough, dyspnea, and COPD exacerbation may in part be attributed to pulmonary comorbidities in this population and to the heightened risk for bronchoscopic procedures to trigger these symptoms.
Even though this study was limited by a small cohort, the multicenter prospective approach ensured a standardized procedural protocol and rigorous follow-ups. In addition to delivering preliminary evidence on efficacy, this study defined critical steps for the design of future tMWA trials. Future clinical tMWA studies will have safeguards in place around length of bronchoscopic navigation, intraprocedural bleeding, and perioperative management of anticoagulants or antiplatelet agents.
Given the importance of achieving adequate ablative margins, technical success and technique efficacy should be determined using standardized imaging protocols as well as consistent and stringent criteria for the CT assessment of ablative margins. Additionally, a 6-month positron emission tomography scan may help detect early tumor recurrence. Both navigation bronchoscopy and CBCT with AF were required adjuncts for successful and safe outcomes of the procedure, and expertise in these areas is considered a prerequisite. The use of CBCT imaging software to plan and confirm the ablation zone and margins was beneficial and should be considered an integral part of the tMWA procedure. With respect to probe design, the sharp tip of the FLEX probe was advantageous in being able to puncture the airway wall and penetrate the tumor without the need for accessory tools.
When performed by a team experienced in CBCT-guided navigational bronchoscopy and interventional radiology, tMWA is a feasible treatment option for peripheral stage 1 NSCLC. Transbronchial MWA has a favorable procedural tolerance profile, does not sacrifice lung function or overall QOL, and patients are typically discharged within a day of a single procedure. These findings support recent early observations with tMWA [21, 22, 23] but warrant evaluation of safety and efficacy in larger patient cohorts with early-stage NSCLC and oligometastatic disease. The advent of robotic navigation bronchoscopy platforms brings improved reach, stability, and precision to the diagnosis and successful ablation of peripheral lung cancers [17, 18]. In addition to the aforementioned safeguards, future tMWA studies should consider including robotic bronchoscopy platforms, which may result in more on-target ablations, shorter navigation time, fewer ablations per target tumor, and fewer CBCT scans/fluoroscopy with less radiation exposure [49, 50, 51].
Conclusion
Image-guided tMWA is a technically feasible approach for peripheral early-stage lung cancer but warrants further evaluation of safety and efficacy in larger cohorts.
Statement of Ethics
The study was performed in accordance with FDA Regulations (21 CFR Parts 50, 54, 56, and 312), the ICH tripartite guideline for Good Clinical Practice (1996), and the Declaration of Helsinki (2013). Protocols and informed consent forms were approved by Institutional Review Boards (IRBs) from both sites (Western IRB study 1186401; Mayo Clinic IRB study 18-006626). Subjects provided written informed consent prior to participation in the study.
Conflict of Interest Statement
Michael Pritchett reports personal fees from Medtronic, BodyVision, Intuitive Surgical, Philips, Biodesix, AstraZeneca, Johnson & Johnson, Noah Medical, United Therapeutics, Actelion, Pfizer, Ambu, and Boston Scientific. Janani Reisenauer receives funding for a research grant sponsored by Intuitive Surgical and consults for Vergent, Elucent Medical, Noah Medical, and AstraZeneca. Ryan Kern is member of the scientific advisory board at VisionAir Solutions. David Wilson is a consultant at Johnson & Johnson and reports consultancy fees from Vergent, Boston Scientific, HeptaMed, Preora, Phillips, AstraZeneca, Auris Health, Pinnacle Biologics, VIDA Diagnostics, Galil Medical, CSA Inc., and Glaxo Smith Kline. Philippe Szapary and Erin Meyers are full-time employees of Johnson & Johnson. Paul Laeseke is a consultant at Johnson & Johnson; shareholder, consultant, and holder of a sponsored research agreement with HistoSonics; shareholder and consultant at Elucent Medical; shareholder at McGinley Orthopedic Innovations; and holder of a sponsored research agreement with Siemens.
Funding Sources
The work was funded by Ethicon, Inc. (Raritan, NJ, USA). Ethicon contributed to the study design, analysis and interpretation of data, reviewed the manuscript, and funded medical writing support, in the form of literature, medical writing, data preparation, and editorial services.
Author Contributions
Michael Pritchett, Janani Reisenauer, and Ryan Kern contributed to the acquisition and analysis and interpretation of data. David Wilson contributed to the conception and design of the study and to the writing of the manuscript. Erin Meyers and Philippe Szapary contributed to the design of the study and the formal analysis of data. Paul Laeseke contributed to the conception and design of the study. All authors contributed to the writing of the manuscript, critically reviewed the manuscript for intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Data Availability Statement
The authors confirm that the data supporting the conclusions of this study are available within the manuscript, the online supplementary material. The data, study protocol, and statistical analysis plan are available at https://clinicaltrials.gov/ct2/show/NCT03603652. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgment
Medical writing support, in the form of literature, medical writing, data preparation, and editorial services, was provided by An Billiau, MD PhD, Celsus Medical Writing.
Funding Statement
The work was funded by Ethicon, Inc. (Raritan, NJ, USA). Ethicon contributed to the study design, analysis and interpretation of data, reviewed the manuscript, and funded medical writing support, in the form of literature, medical writing, data preparation, and editorial services.
References
- 1.International Agency for Research on Cancer | World Health Organization Accessed January 18th 2021. Available from (January 18th 2021) https://gco.iarc.fr/today/fact-sheets-cancers.
- 2.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020 Feb 6;382((6)):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Surveillence epidemiology and end results program. Available from https://seer.cancer.gov/csr/1975_2017/ (accessed January 18, 2021)
- 4.Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC) ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28((Suppl l_4)):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 5.Sineshaw HM, Sahar L, Osarogiagbon RU, Flanders WD, Yabroff KR, Jemal A. County-level variations in receipt of surgery for early-stage non-small cell lung cancer in the United States. Chest. 2020 Jan;157((1)):212–222. doi: 10.1016/j.chest.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015 Oct 1;121((19)):3491–3498. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palussière J, Chomy F, Savina M, Deschamps F, Gaubert JY, Renault A, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018 Aug 24;13((1)):91. doi: 10.1186/s13019-018-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010 Aug;21((8 Suppl l)):S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi J, Ding M, Shi Y, Wang T, Cui D, Tang X, et al. Comparison study of computed tomography-guided radiofrequency and microwave ablation for pulmonary tumors a retrospective, case-controlled observational study. Thorac Cancer. 2018 Oct;9((10)):1241–1248. doi: 10.1111/1759-7714.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogl TJ, Naguib NNN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NEA. Microwave ablation therapy clinical utility in treatment of pulmonary metastases. Radiology. 2011 Nov;261((2)):643–651. doi: 10.1148/radiol.11101643. [DOI] [PubMed] [Google Scholar]
- 11.Healey TT, March BT, Baird G, Dupuy DE. Microwave ablation for lung neoplasms a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017 Feb;28((2)):206–211. doi: 10.1016/j.jvir.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Egashira Y, Singh S, Bandula S, Illing R. Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors a retrospective single-center experience. J Vasc Interv Radiol. 2016 Apr;27((4)):474–479. doi: 10.1016/j.jvir.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Qi J, Li B, Bie ZX, Li YM, Li XG. Risk prediction of pneumothorax in lung malignancy patients treated with percutaneous microwave ablation development of nomogram model. Int J Hyperthermia. 2021;38((1)):488–497. doi: 10.1080/02656736.2021.1902000. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Z, Wang Y, Zhang J, Zheng J, Li W. A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol. 2019 Mar;16((3)):302–314. doi: 10.1016/j.jacr.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Huo YR, Chan MV, Habib AR, Lui I, Ridley L. Pneumothorax rates in CT-Guided lung biopsies a comprehensive systematic review and meta-analysis of risk factors. Br J Radiol. 2020 Apr 1;93((1108)):20190866. doi: 10.1259/bjr.20190866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch EE, Pritchett MA, Nead MA, Bowling MR, Murgu SD, Krimsky WS, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019 Mar;14((3)):445–458. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Krimsky WS, Pritchett MA, Lau KKW. Towards an optimization of bronchoscopic approaches to the diagnosis and treatment of the pulmonary nodules a review. J Thorac Dis. 2018 Jun;10((Suppl 14)):S1637–S1644. doi: 10.21037/jtd.2018.04.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabath BF, Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis. 2019 Jun;11((6)):2628–2638. doi: 10.21037/jtd.2019.01.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koizumi T, Tsushima K, Tanabe T, Agatsuma T, Yokoyama T, Ito M, et al. Bronchoscopy-guided cooled radiofrequency ablation as a novel intervention therapy for peripheral lung cancer. Respiration. 2015;90((1)):47–55. doi: 10.1159/000430825. [DOI] [PubMed] [Google Scholar]
- 20.Xie F, Zheng X, Xiao B, Han B, Herth FJF, Sun J. Navigation bronchoscopy-guided radiofrequency ablation for nonsurgical peripheral pulmonary tumors. Respiration. 2017;94((3)):293–298. doi: 10.1159/000477764. [DOI] [PubMed] [Google Scholar]
- 21.Lau K, Spiers A, Pritchett M, Krimsky W. P1.05-06 bronchoscopic image-guided microwave ablation of peripheral lung tumours early results. J Thorac Oncol. 2018;13((10)):S542. [Google Scholar]
- 22.Chan JWY, Lau RWH, Ngai JCL, Tsoi C, Chu CM, Mok TSK, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance a novel technique and initial experience with 30 cases. Transl Lung Cancer Res. 2021;10((4)):1608–1622. doi: 10.21037/tlcr-20-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau K, Lau R, Baranowski R, Ng C. Late Breaking Abstract bronchoscopic microwave ablation of peripheral lung tumors. Eur Respir J. 2021;58((Suppl 65)):OA230. [Google Scholar]
- 24.Durick NA, Laeseke PF, Broderick LS, Lee FT, Jr, Sampson LA, Frey TM, et al. Microwave ablation with triaxial antennas tuned for lung results in an in vivo porcine model. Radiology. 2008 Apr;247((1)):80–87. doi: 10.1148/radiol.2471062123. [DOI] [PubMed] [Google Scholar]
- 25.Sebek J, Kramer S, Rocha R, Yu KC, Bortel R, Beard WL, et al. Bronchoscopically delivered microwave ablation in an in vivo porcine lung model. ERJ Open Res. 2020 Oct;6((4)):00146–2020. doi: 10.1183/23120541.00146-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan HB, Wang XY, Sun JY, Xie FF, Zheng XX, Tao GY, et al. Flexible bronchoscopy-guided microwave ablation in peripheral porcine lung a new minimally-invasive ablation. Transl Lung Cancer Res. 2019 Dec;8((6)):787–796. doi: 10.21037/tlcr.2019.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoneda KY, Herth F, Spangler T, Raina S, Panescu D. Long-term survival results following endobronchial RF ablation in a healthy-porcine model. Annu Int Conf IEEE Eng Med Biol Soc. 2020 Jul;2020:5252–5258. doi: 10.1109/EMBC44109.2020.9176238. [DOI] [PubMed] [Google Scholar]
- 28.Pritchett MA, Schampaert S, de Groot JAH, Schirmer CC, van der Bom I. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchology Interv Pulmonol. 2018 Oct;25((4)):274–282. doi: 10.1097/LBR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchett MA, Bhadra K, Calcutt M, Folch E. Virtual or reality divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis. 2020 Apr;12((4)):1595–1611. doi: 10.21037/jtd.2020.01.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuwave medical Inc Flexible ablation probes Instructions for Use
- 31.Vogl TJ, Nour-Eldin NEA, Albrecht MH, Kaltenbach B, Hohenforst-Schmidt W, Lin H, et al. Thermal ablation of lung tumors focus on microwave ablation. Rofo. 2017 Sep;189((9)):828–843. doi: 10.1055/s-0043-109010. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SN, Charboneau JW, Dodd GD, 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation proposal for standardization of terms and reporting criteria. Radiology. 2003 Aug;228((2)):335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Chen J, Zhang J, Sun L, Zhuang Y. Radiofrequency ablation of primary non-small cell lung cancer a retrospective study on 108 patients. J BUON. 2019;24((4)):1610–1618. [PubMed] [Google Scholar]
- 34.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation standardization of terminology and reporting criteria - a 10-year update. J Vasc Interv Radiol. 2014 Nov;25((11)):1691.e4–1705.e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fayers PM, BKA, Groenvold M, curran D, Bottomley A, Group . ObotEQoL. 3rd ed. 2001. The EORTC QLQ-C30 scoring manual; p. p.. [Google Scholar]
- 36.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998 Jan;16((1)):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 37.Stone B, Mangona VS, Johnson MD, Ye H, Grills IS. Changes in pulmonary function following image-guided stereotactic lung radiotherapy neither lower baseline nor post-SBRT pulmonary function are associated with worse overall survival. J Thorac Oncol. 2015 Dec;10((12)):1762–1769. doi: 10.1097/JTO.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 38.Casal RF, Tam AL, Eapen GA. Radiofrequency ablation of lung tumors. Clin Chest Med. 2010 Mar;31((1)):151–163. doi: 10.1016/j.ccm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies effectiveness, CT findings, and safety in 50 patients. Radiology. 2008 Jun;247((3)):871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 40.Scott NW, Fayers PM, Aaronson NK. EORTC QLQ-C30 reference values. 2008 [Google Scholar]
- 41.Folch EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, et al. Standardized definitions of bleeding after transbronchial lung biopsy a Delphi consensus statement from the Nashville Working Group. Chest. 2020 Jul;158((1)):393–400. doi: 10.1016/j.chest.2020.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer a preliminary study. J Med Imaging Radiat Oncol. 2013 Aug;57((4)):466–474. doi: 10.1111/1754-9485.12068. [DOI] [PubMed] [Google Scholar]
- 43.Hiraki T, Gobara H, Iishi T, Sano Y, Iguchi T, Fujiwara H, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg. 2007 Nov;134((5)):1306–1312. doi: 10.1016/j.jtcvs.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, et al. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer NRG oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015 Nov 15;93((4)):757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hara R, Itami J, Kondo T, Aruga T, Uno T, Sasano N, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer. 2006 Mar 15;106((6)):1347–1352. doi: 10.1002/cncr.21747. [DOI] [PubMed] [Google Scholar]
- 46.Nestle U, Adebahr S, Kaier K, Gkika E, Schimek-Jasch T, Hechtner M, et al. Quality of life after pulmonary stereotactic fractionated radiotherapy (SBRT) results of the phase II STRIPE trial. Radiother Oncol. 2020 Jul;148:82–88. doi: 10.1016/j.radonc.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Liu BD, Zhi XY. Expert consensus on image-guided radiofrequency ablation of pulmonary tumors-2015 edition. Transl Lung Cancer Res. 2015 Jun;4((3)):310–321. doi: 10.3978/j.issn.2218-6751.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodd GD, 3rd, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors postablation syndrome. AJR Am J Roentgenol. 2005 Jul;185((1)):51–57. doi: 10.2214/ajr.185.1.01850051. [DOI] [PubMed] [Google Scholar]
- 49.Chen AC, Pastis NJ, Jr, Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, et al. Robotic bronchoscopy for peripheral pulmonary lesions a multicenter pilot and feasibility study (BENEFIT) Chest. 2021 Feb;159((2)):845–852. doi: 10.1016/j.chest.2020.08.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalchiem-Dekel O, Connolly JG, Lin IH, Husta BC, Adusumilli PS, Beattie JA, et al. Shape-sensing robotic-assisted bronchoscopy in the diagnosis of pulmonary parenchymal lesions. Chest. 2021 Aug 9; doi: 10.1016/j.chest.2021.07.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benn BS, Romero AO, Lum M, Krishna G. Robotic-assisted navigation bronchoscopy as a paradigm shift in peripheral lung access. Lung. 2021 Apr;199((2)):177–186. doi: 10.1007/s00408-021-00421-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
The authors confirm that the data supporting the conclusions of this study are available within the manuscript, the online supplementary material. The data, study protocol, and statistical analysis plan are available at https://clinicaltrials.gov/ct2/show/NCT03603652. Further inquiries can be directed to the corresponding author.